Abstract
The ability to measure tumor determinants of response to nucleoside analog (NA) chemotherapy agents such as gemcitabine and related compounds could significantly affect the management of several types of cancer. Previously we showed that the accumulation in tumors of the new PET tracer 1-(2′-deoxy-2′-18F-fluoro-β-D-arabinofuranosyl)cytosine (18F-FAC) is predictive of responses to gemcitabine. 18F-FAC retention in cells requires deoxycytidine kinase (dCK), a rate-limiting enzyme in the deoxyribonucleoside salvage metabolism and in gemcitabine conversion from an inactive prodrug to a cytotoxic compound. The objectives of the current study were to determine whether 18F-FAC tumor uptake is also influenced by cytidine deaminase (CDA), a determinant of resistance to gemcitabine; to develop a new PET assay using 18F-FAC and the related probe 1-(2′-deoxy-2′-18F-fluoro-β-L-arabinofuranosyl)-5-methylcytosine (L-18F-FMAC) to profile tumor lesions for both dCK and CDA enzymatic activities; and to determine whether this PET assay can identify the most effective NA chemotherapy against tumors with differential expression of dCK and CDA.
Methods
Isogenic murine leukemic cell lines with defined dCK and CDA activities were generated by retroviral transduction. A cell viability assay was used to determine the sensitivity of the isogenic cell lines to the dCK-dependent NA prodrugs gemcitabine and clofarabine. In vitro enzymatic and cell-based tracer uptake assays and in vivo PET with 18F-FAC and L-18F-FMAC were used to predict tumor responses to gemcitabine and clofarabine.
Results
dCK and CDA activities measured by kinase and tracer uptake assays correlated with the sensitivity of isogenic cell lines to gemcitabine and clofarabine. Coexpression of CDA decreased the sensitivity of dCK-positive cells to gemcitabine treatment in vitro by 15-fold but did not affect responses to clofarabine. Coexpression of CDA decreased 18F-FAC but not L-18F-FMAC, phosphorylation, and uptake by dCK-positive cells. 18F-FAC and L-18F-FMAC PET estimates of the enzymatic activities of dCK and CDA in tumor implants in mice were predictive of responses to gemcitabine and clofarabine treatment in vivo.
Conclusion
These findings support the utility of PET-based phenotyping of tumor nucleoside metabolism for guiding the selection of NA prodrugs.
Keywords: deoxycytidine kinase (dCK), cytidine deaminase (CDA), deoxyribonucleoside salvage metabolism, 18F-FAC and L-18F-FMAC PET, personalized cancer therapy
Nucleoside analog (NA) prodrugs are indicated in many types of cancer but generally have low response rates and can induce significant side effects. For example, the response rates to gemcitabine in pancreatic, ovarian, and lung cancers rarely exceed 20% (1,2), whereas grade 3 or 4 toxicity occurs in up to 38% of patients. Nonetheless, for each malignancy a subset of patients responds well to gemcitabine. The ability to identify likely responders and nonresponders before treatment would be important in the management of cancers treated with NAs. In previous work, we have developed a new PET probe that may enable patient stratification in malignancies in which gemcitabine is indicated as the first or second line of treatment. This PET probe, designated 1-(2′-deoxy-2′-fluoroarabinofuranosyl)cytosine (18F-FAC), closely resembles the chemical structure of gemcitabine (3). 18F-FAC has a high affinity for deoxycytidine kinase (dCK), the rate-limiting enzyme in the activation of gemcitabine and related chemotherapeutic agents (Table 1). Pretreatment 18F-FAC PET of a murine leukemia or lymphoma tumor model identified dCK-positive and -negative tumors and predicted responses to gemcitabine (4). However, in addition to decreased dCK activity, other mechanisms of resistance to gemcitabine have been identified. Examples include downregulation of nucleoside transporters and overexpression of the ribonucleotide reductase subunit M1 (RRM1) and of cytidine deaminase (CDA) (5). CDA catalyzes the deamination of cytidine and deoxycytidine to uridine and deoxyuridine, respectively. In humans, CDA activity is primarily found in the liver, spleen, and plasma, whereas in mice it is mainly in the kidneys (6). The enzyme has been associated with resistance to various NA therapies such as gemcitabine (7). In this follow-up study, we focus on the development of a PET assay that can identify resistance to chemotherapy due to high tumor CDA activity. Although dCK and CDA act on the same substrates, the outcomes of their enzymatic activities are different: dCK phosphorylates and activates gemcitabine whereas CDA deaminates and inactivates this prodrug, thereby opposing the action of dCK (Supplemental Fig. 1A; supplemental materials are available online only at http://jnm.snmjournals.org) (5). The ability to identify tumors that coexpress dCK and CDA may enable chemotherapy stratification by indicating that such tumors will likely be resistant to gemcitabine but may still be sensitive to other dCK-dependent chemotherapeutics (Table 1). Among the dCK-dependent agents, clofarabine ranks highest as a potential alternative to gemcitabine in selected cancers that coexpress dCK and CDA. Clofarabine is indicated for pediatric acute lymphoblastic leukemia, has excellent metabolic stability, resists deamination by CDA, and has broad cytotoxicity in xenograft models of human colon, renal, non–small cell lung, and prostate cancers as well as leukemias (8). Identification of dCK-positive tumors that coexpress CDA would require PET probes that can be phosphorylated and trapped by dCK but, similar to clofarabine, resist deamination by CDA. We have recently developed a series of L-enantiomers of 18F-FAC with these properties (9). Among the L-enantiomers, 1-(2′-deoxy-2′-18F-fluoro-β-L-arabinofuranosyl)-5-methylcytosine (L-18F-FMAC) had the most desirable biodistribution (9). L-18F-FMAC biodistribution in mice was similar to that of 18F-FAC, with the added advantage of lower nonspecific retention in muscle for the L-enantiomer.
TABLE 1.
Panel of NA Prodrugs
| dCK-dependent drug | Nucleobase | CDA substrate |
|---|---|---|
| Cytarabine | Pyrimidine | Yes |
| Decitabine | Pyrimidine | Yes |
| Gemcitabine | Pyrimidine | Yes |
| Cladribine | Purine | No |
| Clofarabine | Purine | No |
| Fludarabine | Purine | No |
In the current study, we describe the utility of 18F-FAC and L-18F-FMAC PET to differentiate cell subtypes by their relative dCK and CDA activity levels. We analyzed the value of 18F-FAC and L-18F-FMAC PET in predicting differential tumor responses to gemcitabine and clofarabine, and we determined their ability to guide treatment decisions in murine cancer models. Our data support the use of PET to predict treatment responses to NA chemotherapeutics in murine models of cancer and potentially in cancer patients.
MATERIALS AND METHODS
Cell Lines
L1210 cell lines (positive for dCK and negative for CDA, designated WT cells) and 10K (negative for both dCK and CDA) (10) were a gift from Charles Dumontet (Université Claude Bernard Lyon I). Cells were cultured at 5% CO2 and 37°C in RPMI 1640, supplemented with 5% fetal calf serum and 2 mM L-glutamine. Murine stem cell virus–based helper-free retroviruses encoding human CDA (huCDA; gift from Dr. Margaret Black at Washington State University), an internal ribosomal entry site (IRES), and the yellow fluorescent protein (YFP) were produced by transient cotransfection of the amphotrophic retrovirus packaging cell line Phoenix (SD 3443; American Type Culture Collection) (11). WT cells underwent spinfection with the pMSCV-huCDA-IRES-YFP retrovirus with polybrene (2 μg/mL) (1,000g, 120 min, 37°C) and were sorted by flow cytometry to ensure a pure population of CDA-expressing cells.
Drugs and Half Maximal Inhibition Concentration Assays
Gemcitabine (570287; AK Scientific Inc.) stock solutions were prepared in water. Clofarabine (C7495; Sigma-Aldrich Co.) was prepared in dimethyl sulfoxide. Tetrahydrouridine (584222; EMD Chemicals) was prepared in water. Cells were seeded in 384-well plates (1 × 103 cells per well in 30 μL of culture medium) and allowed to settle for 4 h. Serial drug dilutions (4×) were performed in drug solvent to ensure equal concentrations of solvent for all dilutions and then diluted with culture medium; 10 μL of this dilution were added to cells. Results were normalized to the vehicle control.
In Vitro Kinase and Uptake Assays Using 18F-FAC and L-18F-FMAC
Kinase and cell-based uptake assays were performed as previously described (9) using 185 kBq (5 μCi) of 18F-FAC or L-18F-FMAC and without addition of a competing NA. The radiochemical purities of 18F-FAC and L-18F-FMAC were greater than 99%, and the specific activities were greater than 37,000 GBq (1,000 Ci)/mmol. Briefly, for kinase assays, 5 × 106 cells growing in exponential phase were lysed by 3 rounds of freeze-thaw. Supernatant containing purified protein was incubated with the radiolabeled probe for 20 min at 37°C and spotted on positively charged DE-61 Whatman filters, which bind negatively charged phosphorylated products. The filters were washed, allowed to dry, and analyzed for radioactivity. In uptake assays, cells were plated for 4–5 h in growth medium, followed by incubation with the radiolabeled probe. For 18F-based uptake assays, L1210 cells (2.5 × 105 cells per well in 24-well plates) were incubated in 1 mL of culture medium supplemented with 185 kBq (5 μCi) of 18F-labeled probe. After 1 h at 37°C and 5% CO2, samples were washed 3 times, and the cell pellet was resuspended in ice-cold phosphate-buffered saline. Samples were measured for radioactivity using a Wallac Wizard 3″ 1480 Automatic γ-Counter (PerkinElmer).
In Vivo Small-Animal PET/CT and Treatment Model
Animal studies were approved by the UCLA Animal Research Committee and were performed according to the guidelines of the Division of Laboratory Animal Medicine at UCLA. On day −7, severe combined immune-deficient mice were injected subcutaneously in the right flank with 1 × 106 cells resuspended in 50% phosphate-buffered saline and 50% Matrigel (354234; BD Biosciences). On day −2, mice underwent 18F-FAC small-animal PET/CT (Inveon [Siemens Medical Solutions USA Inc.] and microCAT [Imtek Inc.]). On day 0, before treatment, mice underwent L-18F-FMAC small-animal PET/CT. Mice were then randomized into treatment groups. Gemcitabine (360 mg/kg/dose) (10) was injected intraperitoneally on days 0 and 4. Clofarabine (60 mg/kg/dose) was administered intraperitoneally on days 0–4. Vehicle control mice received 5.4% dimethyl sulfoxide in saline on days 0–4. Mice were sacrificed when tumors reached an upper limit of 1.5 cm as required by regulations of the Division of Laboratory Animal Medicine. 18F-FAC and L-18F-FMAC were synthesized and used for small-animal PET/CT studies as described in the patent (12) and previously elsewhere (3,9). The radiochemical purity of the probes was greater than 99%, and the specific activity was greater than 37,000 GBq (1,000 Ci)/mmol. Static small-animal PET images were acquired for 600 s, converted into 3-dimensional histograms, and reconstructed with a zoom factor of 2.1 using 3-dimensional ordered-subset expectation maximization with 2 iterations and maximum a priori with 18 iterations and smoothing factor beta set at 0.1. Whole-body CT images were acquired using the microCAT scanner, with the x-ray source based at 70 kVp and 500 μA and an exposure time of 480 s. A Feldkamp reconstruction algorithm was applied. Images were analyzed using OsiriX Imaging Software (version 3.8; OsiriX).
Statistical Analyses
Data are presented as mean ± SD. All P values were determined with unpaired, 2-tailed t tests, and values less than 0.001 were considered to be statistically significant. Prism 5 (GraphPad Software) was used to calculate statistics and generate graphs.
RESULTS
Coexpression of dCK and CDA Confers Differential Sensitivity to NA Chemotherapeutics
To investigate the roles of dCK and CDA in resistance to NA chemotherapy, we generated a panel of L1210 isogenic cell lines that corresponds to 3 metabolic subtypes: dCK-positive, CDA-negative (WT); dCK-positive, CDA-positive (WT+CDA); and dCK-negative (10K, these cells also lack CDA expression). To validate the isogenic cell lines, we performed in vitro kinase assays using tritiated deoxycytidine (3H-dCyd), which is a substrate for both dCK and CDA. WT cells were 13-fold more efficient than WT+CDA cells at phosphorylating 3H-dCyd. This difference was abolished in the presence of tetrahydrouridine, a potent inhibitor of CDA (Supplemental Fig. 1B). 10K cells did not phosphorylate 3H-dCyd, as previously shown (4), and inability to phosphorylate the substrate was unaffected by tetrahydrouridine (Supplemental Fig. 1B). The results of the kinase assays were confirmed using a cell-based 3H-dCyd uptake assay (Supplemental Fig. 1C).
The differential uptake and phosphorylation of 3H-dCyd by the isogenic cell lines were consistent with their differential responses to the dCK-dependent NA prodrugs gemcitabine (which is deaminated by CDA) and clofarabine (which is resistant to deamination) (Table 1). WT cells were 15-fold more sensitive to gemcitabine than cells coexpressing dCK and CDA (WT+CDA). WT+CDA cells were more than 350 times more sensitive than the dCK-negative 10K cells (Table 2). In contrast, WT+CDA cells were marginally more sensitive than WT cells to clofarabine (not statistically significant). WT cells were greater than 290 times more sensitive to clofarabine than 10K cells (Table 2), reflecting the dependence of clofarabine activation on dCK activity.
TABLE 2.
Half Maximal Inhibition Concentration of NA Prodrugs
| Cell line | Gemcitabine (μM) | Clofarabine (μM) |
|---|---|---|
| WT | 0.00730 ± 0.00597 | 0.386 ± 0.202 |
| WT+CDA | 0.109 ± 0.0880 | 0.0894 ± 0.0516 |
| 10K | 38.5 ± 28.6 | 112 ± 68.1 |
Values are presented as mean ± SD. Results are representative of 3 independent experiments at 72 h of drug incubation.
New PET Assay Stratifies Tumor dCK and CDA Activities
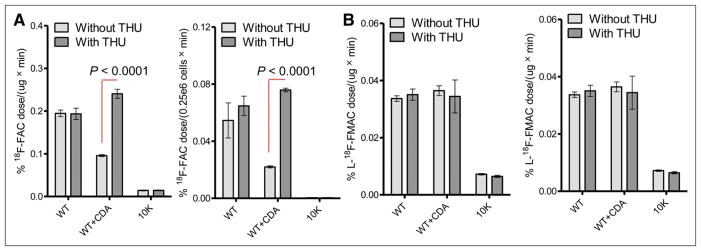
We previously reported on the ability of 18F-FAC and L-18F-FMAC (Supplemental Fig. 2) to differentiate dCK-positive and -negative tumors (9). Here, we investigated whether these 2 PET tracers may allow additional measurements of CDA activity. In cell-based uptake assays, both 18F-FAC and L-18F-FMAC showed high retention in WT cells and low retention in 10K cells, as previously reported (Fig. 1) (4,9). Relative to WT cells, WT+CDA cells showed drastically reduced 18F-FAC accumulation. 18F-FAC accumulation was restored in the presence of tetrahydrouridine (Fig. 1A). In contrast, L-18F-FMAC uptake was similar between WT and WT+CDA cells and was independent of tetrahydrouridine (Fig. 1B).
FIGURE 1.
Detection of dCK- and CDA-defined metabolic phenotypes. Graphs illustrate in vitro 18F-FAC (A) and L-18F-FMAC (B) enzymatic (left) and cell-based (right) uptake assays using L1210 cell lines, with or without tetrahydrouridine (100 μM). Results are normalized to protein concentration for kinase assays or cell number for uptake assays and are representative of 3 independent experiments. THU = tetrahydrouridine.
We next sought to determine whether PET with 18F-FAC and L-18F-FMAC could predict treatment responses in the L1210 tumor model. Before treatment, each tumor-bearing mouse was scanned with 18F-FAC and L-18F-FMAC on days −2 and 0, respectively. 18F-FAC uptake was significantly higher in WT tumors than in WT+CDA or 10K tumors, with WT+CDA or 10K tumors being indistinguishable by PET (Figs. 2A–2C). In contrast, L-18F-FMAC PET detected WT and WT+CDA tumors equally (Fig. 2A and 2B, respectively) and distinguished these from the dCK-deficient 10K tumors (Fig. 2C). The tumor-to-muscle ratio was approximately 5-fold higher for L-18F-FMAC than for 18F-FAC, as expected because of the higher nonspecific muscle uptake of 18F-FAC (Fig. 2D) (9).
FIGURE 2.
In vivo detection of dCK- and CDA-dependent metabolic phenotypes using 18F-FAC and L-18F-FMAC small-animal PET/CT. Shown are representative small-animal PET/CT scans of L1210 tumor subtypes (A–C) and quantification of tumor small-animal PET signals normalized to muscle (D). Same mouse was imaged on day −2 with 18F-FAC and day 0 with L-18F-FMAC, before day 0 treatment. Images have been scaled differently between probes to offset higher muscle background of 18F-FAC. CT-only images (panels below small-animal PET/CT images) are displayed with volume rendering. Each tumor group consisted of at least 5 mice. Results are representative of 3 independent experiments. %ID/g = percentage injected dose per gram.
PET with 18F-FAC and L-18F-FMAC Predicts Treatment Responses In Vivo
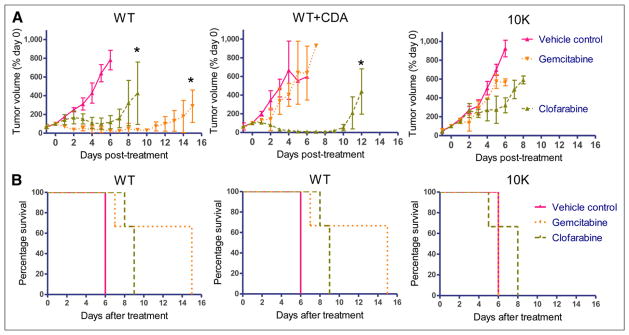
To determine whether the 18F-FAC and L-18F-FMAC PET assay is predictive of tumor responses in vivo, severe combined immune-deficient mice bearing established subcutaneous tumors were treated with gemcitabine, clofarabine, or vehicle control. Daily caliper measurements were performed to determine tumor growth, and animals were sacrificed when tumors reached 1.5 cm in the largest diameter. Growth curves for each tumor subtype are shown in Figure 3A. WT tumor volumes decreased significantly in response to both gemcitabine and clofarabine, compared with vehicle control–treated mice. Clofarabine-treated WT tumors relapsed earlier (~day +8) than the gemcitabine-treated WT tumors (~day +12), which parallels the in vitro sensitivities of WT cells to these drugs (Fig. 3A and Table 2, respectively). Whereas CDA overexpression in WT+CDA tumors significantly diminished the response to gemcitabine, it enhanced the response to clofarabine. Compared with vehicle controls, neither drug significantly affected the growth of dCK-negative 10K tumors. Tumor growth profiles paralleled differences in survival (Fig. 3B).
FIGURE 3.
dCK- and CDA-defined metabolic phenotypes corresponding to differential responses to NA prodrugs in vivo. (A) In vivo tumor growth curves in response to treatment as determined by caliper measurements and normalized to tumor volume before start of treatment. (B) Kaplan–Meier survival curves as defined by regulatory restrictions on tumor size of tumor-bearing mice treated with gemcitabine, clofarabine, or vehicle control. n = 3 for all groups. Results are representative of 2 independent experiments. *Statistically significant P < 0.001, compared with vehicle control.
DISCUSSION
Measurements of tumor nucleoside metabolism are clinically relevant for cancer diagnosis, prognosis, and assessment of therapy response (13–15). The ability to noninvasively estimate tumor dCK and CDA activities in vivo has therapeutic implications. Several NA prodrugs, including gemcitabine, require activation by dCK and are susceptible to inactivation by CDA, whereas others such as clofarabine are phosphorylated and activated by dCK but are not susceptible to deamination (5). Here we show that PET with 18F-FAC and L-18F-FMAC can be used to estimate dCK and CDA activities in tumor lesions and that these measurements can guide treatment stratification. Low 18F-FAC uptake in tumors indicates several possibilities, such as poor tumor vascularization, inefficient transport across the cell membrane, low dCK phosphorylation, and high levels of CDA activity. Subsequent imaging of these tumors with L-18F-FMAC PET may identify tumors in which CDA-mediated deamination represents the main mechanism of resistance to gemcitabine. These tumors are good candidates for treatment with dCK-dependent, CDA-insensitive prodrugs such as clofarabine.
Clinical studies have demonstrated the prognostic significance of low dCK or high CDA activities for poor patient outcome (16–18). The current study assesses these enzymes at the upper and lower ranges of expression. Our data in a panel of 50 human lymphoma cell lines, compared with control, indicate that dCK messenger RNA levels vary as much as 40-fold (Supplemental Fig. 3A) and correlate with dCK enzymatic activity (Supplemental Fig. 3B). These findings are further supported by the variable dCK activities across human ovarian cancer cell lines (Supplemental Fig. 3C). Collectively, these data suggest cancer cells are metabolically distinct from one another in regard to the activity of the deoxyribonucleoside salvage pathway. It will be important to profile the panel of lymphoma and ovarian cancer cell lines for CDA activity and determine whether the 18F-FAC and L-18F-FMAC PET assay developed using the murine L1210 leukemia model can be generally applicable to human tumors of different histologic types. Ongoing clinical studies are evaluating the relationship between dCK activity measured on tumor biopsies and corresponding 18F-FAC and L-18F-FMAC PET signals in lymphoma, ovarian, and pancreatic cancer patients. It might be possible to estimate phosphorylation versus deamination activities with dynamic 18F-FAC and L-18F-FMAC PET studies, and we are, therefore, developing a tracer kinetic model to better describe these parameters. In addition to low dCK activity and increased deamination, reduced expression of nucleoside transporters such as SLC29A1 (19) and overexpression of RRM1 (20) have also been associated with NA chemoresistance. We have previously demonstrated that 18F-FAC is a substrate for SLC29A1 (3). The order-of-magnitude difference in probe uptake between 18F-FAC and L-18F-FMAC may reflect differences in transport between natural D- and unnatural L-enantiomers, and transporters other than SLC29A1 may also be involved. The contribution of RRM1 activity to the uptake of 18F-FAC and analogs remains to be determined. In theory, over-expression of RRM1 in tumors should expand their dCTP pools, which in turn may reduce the activity of dCK by feedback inhibition. Furthermore, extrinsic factors such as poor tissue perfusion may also contribute to a small therapeutic index of gemcitabine (21) and may also limit PET probe delivery. It is likely that PET alone will be insufficient to identify all mechanisms of resistance and that complementary imaging modalities such as contrast-enhanced endoscopic ultrasound or MRI have to be used.
CONCLUSION
Our findings indicate that PET using 18F-FAC and L-18F-FMAC may be useful for guiding the selection of NA chemotherapeutic agents. A more in-depth understanding of the advantages and limitations of the 18F-FAC and L-18F-FMAC PET probes together with other imaging modalities such as MRI will further the role of imaging in personalized, predictive medicine.
Supplementary Material
Acknowledgments
We thank Larry Pang and Dr. Liu Wei for animal and imaging expertise; the cyclotron group for the production of PET probes; Drs. Oliver Dorigo and Sven de Vos for the human ovarian and lymphoma cell lines, respectively; Dr. David Gjertson for review of biostatistics; Dr. Chintda Santiskulvong for preparation of ovarian cancer samples; and Amanda Armijo, Gerald Toy, Evan Shih, Michelle Tom, and Jeremy Work for general assay assistance. This work was supported by the In Vivo Cellular and Molecular Imaging Centers Developmental Project Award (NIH P50 CA86306) and the Dana Foundation. Caius G. Radu, Nagichettiar Satyamurthy, and Johannes Czernin are among the inventors of the national and PCT patent applications for the FAC technology referred to in the article. A group of UCLA faculty members including Caius G. Radu and Johannes Czernin are involved in Sofie Biosciences, a startup company that has licensed this intellectual property.
Footnotes
No other potential conflict of interest relevant to this article was reported.
References
- 1.Colucci G, Giuliani F, Gebbia V, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell’Italia Meridionale. Cancer. 2002;94:902–910. [PubMed] [Google Scholar]
- 2.Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946–3952. doi: 10.1200/JCO.2005.05.1490. [DOI] [PubMed] [Google Scholar]
- 3.Radu CG, Shu CJ, Nair-Gill E, et al. Molecular imaging of lymphoid organs and immune activation by positron emission tomography with a new [18F]-labeled 2′-deoxycytidine analog. Nat Med. 2008;14:783–788. doi: 10.1038/nm1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laing RE, Walter MA, Campbell DO, et al. Noninvasive prediction of tumor responses to gemcitabine using positron emission tomography. Proc Natl Acad Sci USA. 2009;106:2847–2852. doi: 10.1073/pnas.0812890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordheim LP, Dumontet C. Review of recent studies on resistance to cytotoxic deoxynucleoside analogues. Biochim Biophys Acta. 2007;1776:138–159. doi: 10.1016/j.bbcan.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Ho DH. Distribution of kinase and deaminase of 1-beta-D-arabinofuranosylcytosine in tissues of man and mouse. Cancer Res. 1973;33:2816–2820. [PubMed] [Google Scholar]
- 7.Lamba JK. Genetic factors influencing cytarabine therapy. Pharmacogenomics. 2009;10:1657–1674. doi: 10.2217/pgs.09.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonate PL, Arthaud L, Cantrell WR, Jr, Stephenson K, Secrist JA, 3rd, Weitman S. Discovery and development of clofarabine: a nucleoside analogue for treating cancer. Nat Rev Drug Discov. 2006;5:855–863. doi: 10.1038/nrd2055. [DOI] [PubMed] [Google Scholar]
- 9.Shu CJ, Campbell DO, Lee JT, et al. Novel PET probes specific for deoxycytidine kinase. J Nucl Med. 2010;51:1092–1098. doi: 10.2967/jnumed.109.073361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordheim LP, Cros E, Gouy MH, et al. Characterization of a gemcitabine-resistant murine leukemic cell line: reversion of in vitro resistance by a mononucleotide prodrug. Clin Cancer Res. 2004;10:5614–5621. doi: 10.1158/1078-0432.CCR-04-0506. [DOI] [PubMed] [Google Scholar]
- 11.Hawley RG, Lieu FH, Fong AZ, Hawley TS. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1994;1:136–138. [PubMed] [Google Scholar]
- 12.Radu CG, Witte ON, Nair-Gill E, Satyamurthy N, Shu CJ, Czernin J, inventors. The Regents of the University of California, assignee. US patent PCT WO 2009/038795 A2. Positron emission tomography probes for imaging immune activation and selected cancers. 2009 Mar 26;
- 13.Sreekumar A, Poisson LM, Rajendiran TM, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 14.Spratlin JL, Serkova NJ, Eckhardt SG. Clinical applications of metabolomics in oncology: a review. Clin Cancer Res. 2009;15:431–440. doi: 10.1158/1078-0432.CCR-08-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 16.Ueno H, Kiyosawa K, Kaniwa N. Pharmacogenomics of gemcitabine: can genetic studies lead to tailor-made therapy? Br J Cancer. 2007;97:145–151. doi: 10.1038/sj.bjc.6603860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sebastiani V, Ricci F, Rubio-Viqueira B, et al. Immunohistochemical and genetic evaluation of deoxycytidine kinase in pancreatic cancer: relationship to molecular mechanisms of gemcitabine resistance and survival. Clin Cancer Res. 2006;12:2492–2497. doi: 10.1158/1078-0432.CCR-05-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bengala C, Guarneri V, Giovannetti E, et al. Prolonged fixed dose rate infusion of gemcitabine with autologous haemopoietic support in advanced pancreatic adenocarcinoma. Br J Cancer. 2005;93:35–40. doi: 10.1038/sj.bjc.6602673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damaraju VL, Damaraju S, Young JD, et al. Nucleoside anticancer drugs: the role of nucleoside transporters in resistance to cancer chemotherapy. Oncogene. 2003;22:7524–7536. doi: 10.1038/sj.onc.1206952. [DOI] [PubMed] [Google Scholar]
- 20.Bergman AM, Eijk PP, Ruiz van Haperen VW, et al. In vivo induction of resistance to gemcitabine results in increased expression of ribonucleotide reductase subunit M1 as the major determinant. Cancer Res. 2005;65:9510–9516. doi: 10.1158/0008-5472.CAN-05-0989. [DOI] [PubMed] [Google Scholar]
- 21.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.