Background: Recently, we demonstrated that the glycoprotein opticin is anti-angiogenic. Here, the underpining mechanism is explored.
Results: By binding to collagen, opticin competitively inhibits integrin-mediated endothelial cell adhesion.
Conclusion: Opticin inhibits angiogenesis by weakening endothelial cell adhesion to the surrounding extracellular matrix.
Significance: Elucidating the regulatory mechanisms of angiogenesis is important for understanding pathology and drug discovery.
Keywords: Angiogenesis, Cell Adhesion, Endothelial Cell, Extracellular Matrix Proteins, Eye, Integrins, Collagen, Opticin, Vitreous
Abstract
Opticin is an extracellular matrix glycoprotein that we identified associated with the collagen network of the vitreous humor of the eye. Recently, we discovered that opticin possesses anti-angiogenic activity using a murine oxygen-induced retinopathy model: here, we investigate the underlying mechanism. Using an ex vivo chick chorioallantoic membrane assay, we show that opticin inhibits angiogenesis when stimulated by a range of growth factors. We show that it suppresses capillary morphogenesis, inhibits endothelial invasion, and promotes capillary network regression in three-dimensional matrices of collagen and MatrigelTM. We then show that opticin binds to collagen and thereby competitively inhibits endothelial cell interactions with collagen via α1β1 and α2β1 integrins, thereby preventing the strong adhesion that is required for proangiogenic signaling via these integrins.
Introduction
The vitreous humor is a transparent, avascular, virtually acellular gel that fills the space between the lens and the retina in the eye. To maintain its avascular state, vitreous possesses anti-angiogenic properties (1). Angiogenesis is the process whereby new vessels grow from preexisting blood vessels, and it is an important cause of pathology in the eye. In conditions such as proliferative diabetic retinopathy and retinopathy of prematurity, new blood vessels grow from the retina into the vitreous, a process called preretinal neovascularization. Recently, we discovered that the glycoprotein opticin inhibits preretinal neovascularization in a dose-dependent manner (2). Using the murine oxygen-induced retinopathy model, we showed that opticin null mice developed more extensive neovascularization than wild-type mice and the intravitreal injection of recombinant opticin into wild-type murine eyes decreased preretinal neovascularization compared with controls (2).
Opticin is a member of the extracellular matrix (ECM)3 SLRP (small leucine-rich repeat protein/proteoglycan) family and is glycosylated uniquely via a cluster of sialylated O-linked oligosaccharides (3). It is a 90-kDa homodimer in solution that dimerizes through its leucine-rich repeats (4). In the eye, opticin is secreted by the posterior non-pigmented ciliary epithelium (5) during development, and high level expression is maintained in the adult eye, which is unusual for a vitreous ECM macromolecule (6, 7). The secreted opticin enters the vitreous cavity where it co-localizes with the fine network of collagen fibrils that maintains the gel state of the vitreous and the inner-limiting lamina, a basement membrane on the inner surface of the retina (2, 8, 9). Opticin has also been identified in other tissues, including the brain, heart, and cartilage (5, 10, 11).
Vitreous collagen fibrils, which are heterotypic and composed of collagens II, IX, and V/XI (8), contribute to preretinal neovascularization in two ways. First, they provide a scaffold for the vascular invasion into the vitreous (8, 12); consequently, vitreous collagen always is present in preretinal neovascular complexes (13). Second, collagen stimulates angiogenesis through sustained signaling via the α1β1 and α2β1 integrins expressed on endothelial cells (ECs), leading to an initial and critical change in cell shape, contractility, and polymerization and arrangement of cytoskeletal actin into stress fibers (14–16). Here, we investigated the hypothesis that opticin interferes with the integrin-mediated interactions between ECs and collagen and thereby prevents angiogenesis.
EXPERIMENTAL PROCEDURES
Materials
Recombinant bovine opticin was expressed in 293-EBNA cells and purified as described previously (4, 17). FGF-2 and VEGF165 were obtained from R&D Systems (Abingdon, UK) and bovine brain extract (BBE) from Sigma. A bovine opticin peptide (VLSLDNYDEVIDPSNYDELIDYGDQLPQVK) was used to generate rabbit polyclonal antiserum, and an antibody was then affinity-purified using the same peptide. Recombinant α2β1 was made by Dr. Dimitra Valdramidou (18) and recombinant α5β1 and αvβ3 were made by Dr. Sarah Baggaley in Dr. M. J. Humphries' laboratory. A cyclic RGD inhibitory compound, a non-anti-functional anti-α2 integrin antibody and a 50-kDa fragment of fibronectin (19) were all generous gifts from Dr. Paul Mold (University of Manchester, Manchester, UK). Recombinant α1β1 integrin, all of the integrin function-blocking antibodies, including monoclonal anti-α5β1 and anti-αvβ3 integrin antibodies used for binding assays, mouse laminin isolated from Engelbreth-Holm-Swarm mouse sarcoma and bovine collagen type IV were obtained from Chemicon (Watford, UK). Collagen type I was obtained from Nutacon (Leimuiden, The Netherlands) and growth factor-reduced MatrigelTM from Pharmingen (BD Biosciences). Pepsinized collagen type II was purified as published previously (20). Recombinant decorin was a generous gift from Paul Scott (University of Alberta, Alberta, Canada). Fibronectin, vitronectin, a monoclonal anti-vinculin antibody, a monoclonal anti-paxillin antibody, an anti-actin antibody, HRP-conjugated secondary antibodies, HRP-conjugated streptavidin, rhodamine-labeled phalloidin, and staurosporin stock solution were all obtained from Sigma. Fluorescently labeled secondary antibodies were purchased from Molecular Probes (Invitrogen). Biotinylated anti-human integrin β1 antibody and an anti-cleaved caspase-3 antibody were purchased from R&D Systems (Abingdon, UK).
Chick Chorioallantoic Membrane (CAM) Assay
Fertilized hen eggs (provided by Midmoor Farms, Ltd., Frodsham, UK) were prepared and windowed to expose the CAM on day 4 (21). Proangiogenic growth factors, including FGF-2 (100 ng), VEGF165 (100 ng), or BBE (250 ng, which contains a mixture of growth factors), with or without recombinant bovine opticin (10 μg) were mixed with an equal volume (5 μl) of 1% sterile aqueous methylcellulose (4000 centipoises, Sigma) to produce a single pellet. Each 10-μl sterile sample was applied to a Teflon stub and allowed to dry to a thin 2-mm diameter disc, in a laminar flow cabinet. The samples were applied to the CAM on day 8, and the angiogenic response was photographed at day 10. The degree of vessel growth was scored as described previously (21). In some cases, immediately prior to photography, 2 ml of a 50% emulsion of liquid paraffin and 4% aqueous Tween 80 was injected under the membrane to highlight the vasculature.
Cell Culture
Human umbilical vein ECs (HUVECs) and human dermal microvascular ECs were obtained from TCS Cellworks (Buckingham, UK) and cultured in 2% FCS-macrovascular or 10% FCS-microvascular endothelial medium, respectively according to the manufacturer's instructions. They were used between passages 2–8. Donors human eyes were collected from the Manchester Royal Eye Hospital Eye Bank and human retinal ECs (HRECs) were isolated and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 20% FCS, 2 mm glutamine, 10 μg/ml insulin from bovine pancreas, 5.5 μg/ml human transferrin, 5 ng/ml sodium selenite (ITS supplement), 15 μg/ml EC growth supplement from bovine pituitary. Bovine aortic ECs (BAECs) were isolated using standard methodologies (22), and both HRECs and BAECs were characterized as endothelial by the presence of Von Willebrand factor and the uptake of Dil-labeled acetylated low-density lipoprotein, as described previously (22). All cell lines were incubated in a humidified incubator at 37 °C with 5% CO2.
EC Network Formation in Three-dimensional Matrices of Collagen, MatrigelTM, and Fibrin
Collagen and MatrigelTM gels were prepared as described previously (23). Fibrin matrices were formed using an in vitro angiogenesis kit according to the manufacturer's instructions (Chemicon, Watford, UK). ECs (HUVECS, BAECs, or HRECs) were starved in 0.1% FCS-containing medium for 48 h prior to the experiments. They were seeded at 1.5 × 106 cells/ml in 300 μg/ml collagen type I, MatrigelTM, or fibrinogen diluted with medium containing 0.1% FCS, 25 ng/ml FGF-2, with or without 250 nm opticin. Cyclic RGD compound was also used in the assays performed in fibrin as a positive control (24) at a final concentration of 100 μm. Drops of these suspensions were applied to six-well plates in triplicate, and thrombin solution was added to the fibrinogen drops to enable polymerization. All gels were allowed to polymerize at 37 °C for 1 h. Gels were then covered with culture medium containing 25 ng/ml FGF-2, with or without 250 nm opticin. Cells were then incubated for 24 h and visualized using phase contrast microscopy. The vascular network formed by HUVECs in all three studied matrices was quantified by measuring the total length of extended cells, whether single or aligned end-to-end in tube-like structures, observed in five fields of view in triplicate wells.
EC Invasion and Network Regression in Collagen and MatrigelTM
To determine whether opticin promoted vascular regression, EC networks were formed in collagen or MatrigelTM matrices containing 0.1% FCS and 25 ng/ml FGF-2 as described above for 24 or 36 h. After formation of the vascular network, the gels were incubated with medium containing 0.1% FCS and 25 ng/ml FGF-2, with or without 250 nm opticin, for a further 36 h. The total length of the tube-like structures observed in five fields of view was measured in triplicate wells.
To determine the effects of opticin on EC invasion into collagen or MatrigelTM matrices, a vascular network within MatrigelTM was formed in a similar manner as described above. After 24 h of incubation of the cells in MatrigelTM, a second layer of either collagen or MatrigelTM containing 25 ng/ml FGF-2, with or without 250 nm opticin, was added around the MatrigelTM gels and left to polymerize for 1 h at 37 °C. After polymerization, medium containing 0.1% FCS and 25 ng/ml FGF-2 was added, and the cells were incubated for a further 24 h. The wells were then fixed with 4% paraformaldehyde, and the extent of cell invasion into the collagen or MatrigelTM layer was quantified by visualizing the border with the MatrigelTM using phase contrast microscopy and counting the number of invading cells in three different areas. All experiments were performed in triplicate wells.
Cell Attachment Assays
Wells of 96-well plates were coated with collagen type I diluted at 100 μg/ml in phosphate-buffered saline, pH 7.4 (PBS), and nonspecific binding sites were blocked using 1% heat-denatured BSA in PBS. After washing with PBS, 50 μl of HUVECs resuspended at 1.5 × 106 cells/ml were added to each well. Inhibition of attachment was performed by adding opticin (250 nm), an anti-α2 integrin antibody raised against the A-domain (clone BHA2.1, 10 μg/ml) or a nonspecific mouse IgG (10 μg/ml) to the cell suspension and incubating for 45 min in a 37 °C, 5% CO2 humidified incubator. Detachment assays were performed by adding the HUVEC suspension to collagen type I-coated wells and incubating for 3 h in a 37 °C, 5% CO2 humidified incubator. Recombinant opticin used at 250 nm was then added to these wells for 10 min. In both assays, non-attached cells were removed by washing carefully with PBS, and attached cells were fixed with 5% glutaraldehyde and stained with 0.1% crystal violet for 1 h. After extensive wash with PBS, the dye was resuspended with 10% acetic acid, and the absorbance at 570 nm was measured using a Multiscan plate reader. The absorbance was converted into a percentage of attachment by using wells in which 25, 50, and 75% of HUVEC suspension was allowed to adhere to uncoated wells in triplicate.
Cell Proliferation Assays Using Bromodeoxyuridine (BrdU) Incorporations
The effects of recombinant opticin on EC proliferation were tested using BrdU incorporation assays (Calbiochem®, Nottingham, UK). Briefly, 2.5 × 103 cells/well were plated in 96-well plates. Increasing concentrations of opticin ranging from 0–1.2 μm were added for 48 h prior to the BrdU reagent. After fixation and denaturation, BrdU labeling was detected using a mouse anti-BrdU antibody followed by an HRP-conjugated anti-mouse antibody. After addition of the substrate solution, the absorbance was read at both 450 and 570 nm, and the background values were subtracted.
Apoptosis Assays
To investigate whether opticin induces apoptosis, HUVECs were allowed to spread for 3 h on collagen type I immobilized at 100 μg/ml as described previously. Recombinant opticin at 250 nm or staurosporin at 1 μm (used as a positive control) (25) were added to the wells and incubated at 37 °C, 5% CO2 for 16 h. Equal amounts of cell lysate were then subjected to 4–12% Bis-Tris gradient SDS-PAGE and Western blot analysis using anti-cleaved caspase-3 antibody followed by an HRP-conjugated secondary antibody and a chemiluminescence detection system (PerkinElmer Life Science). Equal protein loading was checked by probing with an anti-actin antibody.
Live Cell Imaging
Collagen gels were formed by mixing 300 μg/ml collagen type I, 10× PBS, and 10 mg/ml NaHCO3 with or without 250 nm of opticin in 12-well plates. These mixtures were allowed to polymerize at 37 °C for 1 h. After washing with PBS, HUVEC suspension at the density of 2 × 104 cells/ml in 2% FCS endothelial medium, with or without 250 nm opticin, was added to the wells and placed under a microscope that was installed under 37 °C, 5% CO2 conditions. After 1 h (to allow cell attachment to the collagen gels), images were taken every 10 min for 4 h. Images were acquired on AS MDW live cell imaging system (Leica DM IRE microscope body with motorized Z) using a 20×/0.5 HC Plan Fluotar objective. Point visiting was used to allow multiple positions to be imaged within the same time course. The images were collected using a Coolsnap HQ CCD camera (Photometrics). Images were then processed into a movie using ImageJ software.
Cell Spreading Assays
Eight-well slides were coated with collagen type I, II, IV, and laminin at 100 μg/ml and vitronectin and fibronectin at 1 μg/ml and blocked with 1% BSA in PBS. After washing with PBS, 250 μl of HUVEC or HMDEC suspension at 2 × 104 cells/ml was added to the wells and incubated at 37 °C, 5% CO2 for 3 h. Recombinant opticin or decorin were then added to the medium of some wells for 10 min prior to fixation. The opticin and decorin concentrations used in these assays were 250 nm unless stated otherwise. For visualization of the cytoskeletal components by fluorescent microscopy, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100/PBS. After blocking with 3% BSA/PBS, actin was stained using rhodamine-labeled phalloidin. Cells were stained with a monoclonal anti-vinculin or anti-paxillin antibodies followed by Alexa Fluor® 488 nm-conjugated anti-mouse antibody. Nuclei were counterstained with DAPI. Images were collected on an Olympus BX51 upright microscope and captured using a Coolsnap ES camera (Photometrics) through Metavue software (Molecular Devices). Images were then processed and analyzed using ImageJ software. Quantification of the effects of opticin was performed by counting the number of cells with an organized actin cytoskeleton per 100 cells in triplicate (26). To identify the integrins involved in EC spreading on ECM substrates, 96-well plates were coated with collagen type I, II, IV, laminin, a 50-kDa fragment of fibronectin and vitronectin, and blocked as described previously. After washing with PBS, ECs resuspended at 2 × 104 cells/ml were seeded into each well. Integrin function-blocking antibodies were added to the medium at the final concentration of 10 μg/ml. An anti-α2 integrin antibody not raised against the A-domain (clone 10A4) and a nonspecific mouse IgG were also used in these assays at 10 μg/ml. The cells were incubated at 37 °C, 5% CO2 for 90 min. To preclude spreading on endogenously produced matrix, BSA-coated wells were always used as negative controls. The cells were then fixed with 5% glutaraldehyde. The quantification was performed by counting the number of spread cells per 100 cells in triplicate for each well using phase contrast microscopy, and the percentage of spreading was then normalized to absence of inhibitors or mouse IgG control.
Biotinylation of Collagen Type I
Collagen type I was biotinylated using BiotinTagTM Micro Biotinylation kit according to the manufacturer's instructions (Sigma). After the biotinylation reaction, free biotin was removed by gel filtration chromatography using a 5-ml desalting column (GE Healthcare), and biotinylated collagen type I-containing fractions were identified by Western blotting analysis using HRP-conjugated streptavidin and pooled. Final protein concentration was determined using Bio-Rad Protein Assay (Bio-Rad Laboratories GmbH).
Binding Studies
Binding assays were performed using ELISA-based techniques. Briefly, 96-well plates were coated with collagen type I, II, IV, laminin, vitronectin, and fibronectin at 50 μg/ml in PBS or with recombinant α1β1 and α2β1 integrins at 5 μg/ml in Tris-buffered saline, pH 7.4 (TBS), containing 2 mm Mn2+ (TBS/Mn2+). Each experimental well was repeated in triplicate. After washing with TBS containing 0.1% Tween 20 (TBS-T) or TBS-T/Mn2+, the wells were blocked with 5% BSA. Increasing concentrations of recombinant opticin or biotinylated collagen type I were prepared in blocking buffer. These ligands were added to wells for 2 h. After washing, bound opticin was detected using an affinity-purified anti-bovine opticin antibody followed by an HRP-conjugated anti-rabbit antibody. Bound biotinylated collagen type I was detected using HRP-conjugated streptravidin. After extensive washing, TMB substrate was added to the wells, and the absorbance was measured at 620 nm using a Multiscan plate reader. Absorbances obtained from wells coated only with BSA were subtracted from the experimental wells. All these experiments were performed in triplicate. The apparent Kd values (Kd(app)) were determined using SigmaPlot software (version 11.0).
Inhibition Studies between Collagen Type I and Integrins
96-Well plates were coated with recombinant α1β1 or α2β1 integrins at 5 μg/ml. (Each experimental well was repeated in triplicate.) After washing, the wells were blocked with 5% BSA in TBS/Mn2+. A fixed concentration (0.5 μg/ml) of biotinylated collagen type I prepared in BSA/TBS/Mn2+ was pre-incubated with increasing concentrations of opticin for 2 h before being added to wells containing immobilized recombinant α1β1 and α2β1 integrins. After TBS/Mn2+ washes, biotinylated collagen type I was detected with HRP-conjugated streptavidin.
Statistical Analysis
All experiments were repeated at least three times, and results shown in the figures were expressed as means ± S.E. The statistical analyses were undertaken using Mann-Whitney U test for the CAM assay, and one-way analysis of variance for all in vitro assays, with p < 0.05 being considered significant.
RESULTS
Opticin Inhibits Growth Factor-induced Angiogenesis in CAM Assay
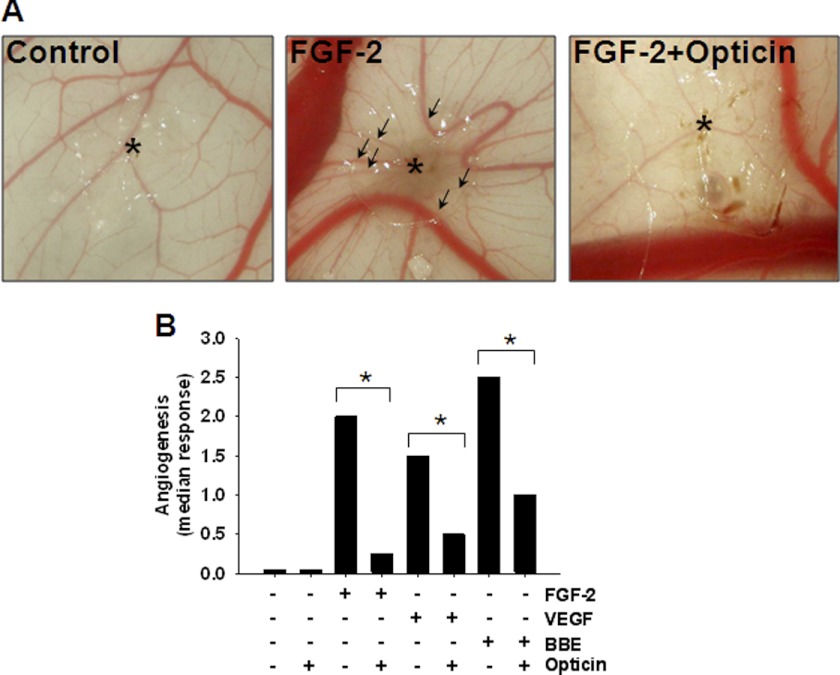
This assay was used to determine whether the anti-angiogenic activity of opticin is growth factor-specific. An angiogenic response was induced by various growth factors, including FGF-2, VEGF165, and BBE (which contains several proangiogenic growth factors). We found that, in each case, all new vessels converged toward the site of application of growth factor-containing pellets, but if opticin was present in the pellets, the neovascular response was inhibited significantly (Fig. 1, A and B). Therefore, the anti-angiogenic activity of opticin is not growth factor-specific.
FIGURE 1.
Effects of opticin in CAM ex vivo model. A, representative images showing the effects of a pellet without FGF-2 or opticin (left image), and pellets containing FGF-2 with or without opticin. Asterisk indicates the site of application of the pellet. Under FGF-2 stimulation (middle image), newly formed vessels converge toward where the growth factor-containing pellet was placed (arrows). B, pellets containing growth factors i.e. FGF-2, VEGF165, and BBE, with or without opticin, were applied on the CAM, and the angiogenic response was quantified at day 10. Addition of opticin in combination with FGF-2 (n = 8), VEGF165 (n = 12), and BBE (n = 12) significantly reduced their angiogenic drive on the CAM (*, p = 0.01, p = 0.04, and p < 0.035 for FGF-2, VEGF165, and BBE, respectively).
Opticin Inhibits Capillary Morphogenesis in Three-dimensional Collagen and MatrigelTM Matrices
The effects of opticin on capillary morphogenesis by HUVECs were studied in various matrices, including collagen type I, MatrigelTM, and fibrin. Opticin significantly decreased FGF-2-induced capillary morphogenesis in collagen type I (Fig. 2A) and MatrigelTM (Fig. 2B). However, opticin did not inhibit HUVEC morphogenesis in fibrin, whereas cyclic RGD peptide did (Fig. 2C). Together, these data demonstrate that the anti-angiogenic effects of opticin are matrix-specific.
FIGURE 2.
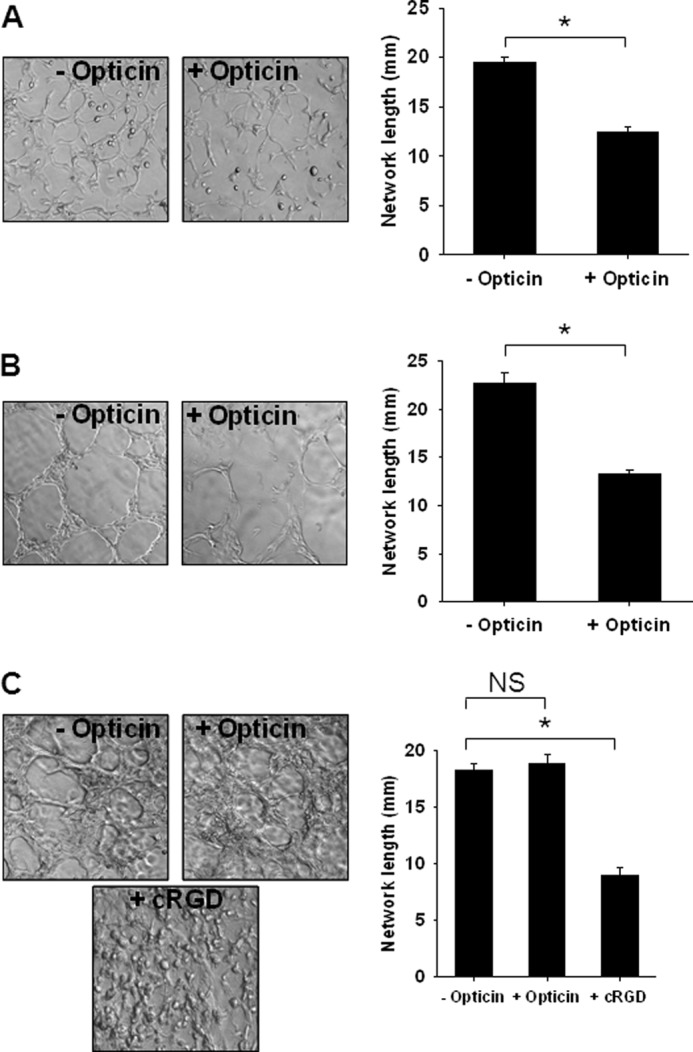
Opticin inhibits collagen type I and MatrigelTM-stimulated angiogenesis in vitro. Opticin (250 nm) significantly decreased FGF-2-stimulated HUVEC capillary morphogenesis in collagen type I (A) (*, p < 0.001) and in MatrigelTM (B) (*, p = 0.002). However, opticin had no significant effect (NS) on capillary morphogenesis in fibrin matrices (C), whereas cyclic RGD (cRGD), used as a positive control, produced a significant decrease in network length (*, p < 0.001).
Opticin Inhibits EC Capillary Morphogenesis and Invasion and Promotes Capillary Regression in Three-dimensional Collagen and MatrigelTM Matrices
We investigated further the effects of opticin on capillary morphogenesis in both collagen and MatrigelTM using various EC lines. Network formation in collagen (Fig. 3A) or MatrigelTM (supplemental Fig. 1A) gels was inhibited by opticin irrespective of whether HUVECs, HRECs, or BAECs were used.
FIGURE 3.
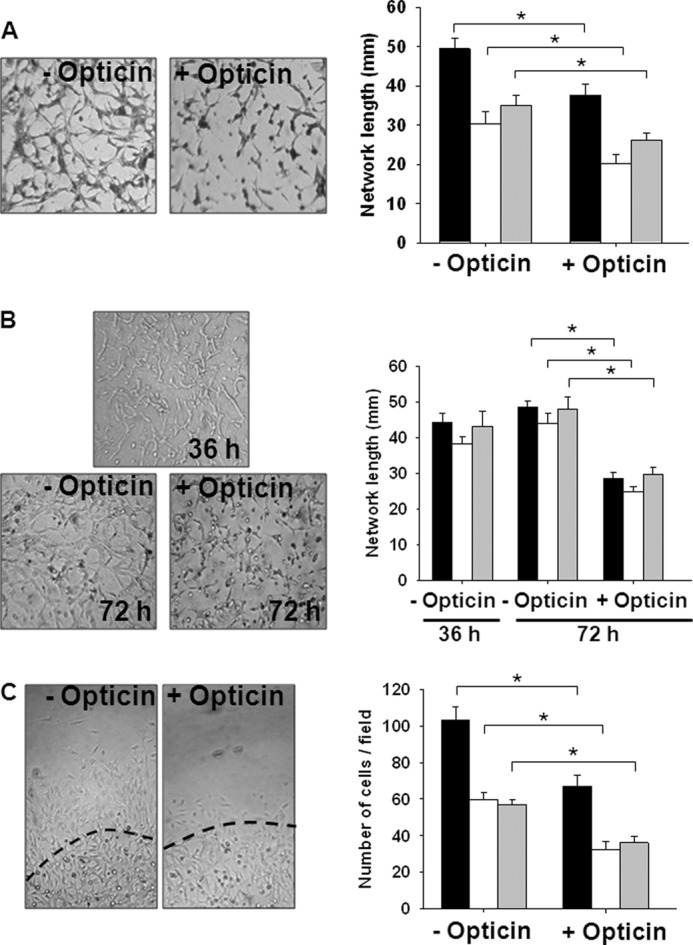
Opticin inhibits capillary morphogenesis, promotes capillary network regression, and inhibits EC invasion in collagen gels. Three different types of EC were used: BAECs (black bars), HRECs (white bars), and HUVECs (gray bars); exemplar images shown are of BAECs, and opticin concentration was 250 nm. A, measurements of the total network length formed by BAECs, HRECs, and HUVECs under FGF-2 stimulation in collagen type I showed a significant decrease in presence of opticin (*, p < 0.001). B, FGF-2-stimulated EC networks were initially formed in collagen type I for 36 h. The gels were then incubated in the presence or absence of opticin for a further 36 h. In the absence of opticin, FGF-2-stimulated networks were maintained between 36 and 72 h. However, opticin significantly decreased the total network length for BAECs, HRECs, and HUVECs (*, p < 0.001). C, an initial vascular network was formed in MatrigelTM, and then a layer of collagen gel containing opticin or not was polymerized around the MatrigelTM. The dashed line shows boundary between the two matrices on the images representing BAEC invasion. Quantification of the number of invading ECs revealed a significant decrease in presence of opticin for BAECs, HRECs, and HUVECs (*, p < 0.001).
We investigated whether opticin could induce the regression of preformed EC networks and found that opticin disrupted preformed networks in either collagen type I or MatrigelTM, irrespective of EC type (Fig. 3B and supplemental Fig. 1B). Next, we determined whether opticin could inhibit EC invasion into either collagen type I (Fig. 3C) or MatrigelTM (supplemental Fig. 1C) and found that opticin inhibited invasion with all three EC types investigated. As the effects of opticin on EC invasion, capillary morphogenesis, and network regression were independent of EC type, further in vitro studies focused mainly on one cell type, HUVECs.
Effects of Opticin on EC Attachment, Proliferation, and Apoptosis on Collagen Type I
There was no difference in the ability of HUVECs to attach to collagen in the presence or absence of opticin (supplemental Fig. 2A). An antibody against α2-A-domain integrin did decrease attachment, whereas a nonspecific IgG antibody did not. Furthermore, detachment assays showed that 10 min of exposure of HUVECs prespread on collagen type I to opticin did not induce cell detachment (data not shown). BrdU incorporation assays were performed using a range of concentrations of opticin. At opticin concentrations of 0.85 μm and above, BrdU incorporation was inhibited (supplemental Fig. 2B), suggesting that opticin had an anti-proliferative effect. However, these concentrations were much higher than those required to disrupt EC morphology, which were as low as 10 nm (see below). Therefore, we conclude that the anti-proliferative effects of opticin only make a minor contribution to its anti-angiogenic properties. Furthermore, we could not demonstrate opticin-induced EC apoptosis at a concentration of 250 nm by Western blotting analysis of cell extracts for cleaved caspase-3 (supplemental Fig. 2C). Equal volumes of cell lysates were used, and equal loading was confirmed by probing the Western blot with an antibody to actin.
Opticin Disrupts Morphology of HUVECs Spread on Collagens and Laminin
We examined, by live cell imaging, the behavior of HUVECs when placed on collagen gels polymerized in the presence or absence of opticin. In the absence of opticin, the HUVECs adopted a proangiogenic elongated morphology (supplemental Fig. 3A), but in the presence of opticin, they maintained a persistent rounded morphology (supplemental Fig. 3B).
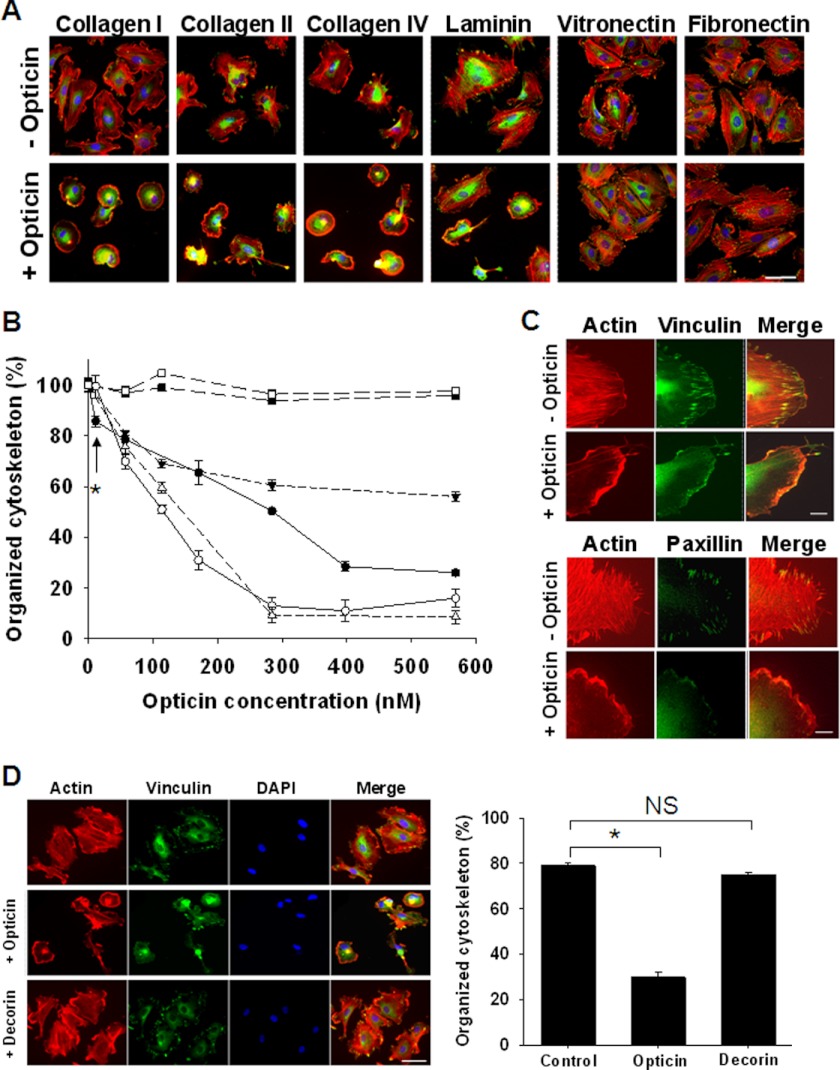
When HUVECs were allowed to spread on plates coated with collagen type I, II, IV, laminin, vitronectin, and fibronectin, they formed focal adhesions and actin stress fibers (Fig. 4A); however, after the addition of opticin, HUVEC morphology was disrupted in a concentration-dependent manner on the collagens and laminin, but not on vitronectin and fibronectin (Fig. 4, A and B). In further experiments, opticin was added to HUVECs spread on vitronectin and fibronectin at higher concentrations (up to 1.2 μm), and it again did not affect their morphology, whereas cyclic RGD peptide (used as a positive control) markedly affected the morphology of HUVECs spread on both vitronectin and fibronectin (data not shown). Opticin disrupted HUVEC morphology on collagen type I at concentrations as low as 10 nm (Fig. 4B). After exposure of HUVECs on collagen type I to opticin, vinculin- and paxillin-containing focal adhesions and actin stress fibers disappeared rapidly (Fig. 4C). We compared the effects of opticin with decorin (another member of the SLRP family) on HUVEC morphology on collagen type I. When tested under the same conditions, opticin disrupted HUVEC morphology, whereas decorin did not (Fig. 4D).
FIGURE 4.
Opticin disrupts HUVEC morphology when spread on collagen types I, II, and IV and laminin. A, opticin was added at 250 nm in solution to HUVECs spread on plates coated with collagen types I, II, and IV, and laminin, vitronectin, or fibronectin. Visualization of stress fibers (actin in red), focal adhesions (vinculin in green), and nuclei (in blue) showed that in absence of opticin, HUVECs spread on all these matrices; focal adhesions and actin stress fibers were clearly visible (top panel). However, after exposure to opticin the cells retracted: the stress fibers and focal adhesion disappeared when spread on collagen types I, II, and IV and laminin (bottom panel). The cell morphology was unaltered by opticin on vitronectin or fibronectin (scale bar, 50 μm). B, quantification showed that opticin disrupted HUVEC cytoskeleton in a concentration-dependent manner on collagen type I (●), collagen type II (○), collagen type IV (Δ), and laminin (▾) but not on vitronectin (■) or fibronectin (□); significant effects were observed at concentrations as low as 10 nm on collagen type I (*, p = 0.017). C, visualization of actin stress fibers (in red) and focal adhesions (vinculin and paxillin in green) showed that the discrete localization of both vinculin (top panel) and paxillin (bottom panel) in focal adhesions was lost when HUVEC spread on collagen type I were exposed to opticin at 250 nm (scale bar, 10 μm). D, when the effects of opticin and another SLRP family member, decorin, were compared, decorin had no effect, whereas opticin significantly disrupted HUVEC morphology (*, p < 0.001; not significant (NS), p = 0.287; scale bar, 50 μm).
To identify the integrins involved in EC adhesion to the various ECM macromolecules used in this study, spreading experiments were performed in the presence of anti-functional anti-integrin antibodies. Inhibition of spreading revealed that HUVECs used mainly α2β1 integrin to spread on collagen types I, II, and IV and α2β1, α3β1, and α6β1 integrins to spread on laminin (Table 1). HUVECs used αvβ3 and α5β1 integrins to spread on vitronectin and on a 50-kDa fragment of fibronectin, respectively (Table 1). As HUVECs do not express α1β1 integrin, or do so at very low levels (Table 1), similar inhibitory experiments on both collagen types I and IV were undertaken with human dermal microvascular ECs, which do express α1β1 integrin. Inhibition of spreading showed that these cells mainly use the α2β1 integrin to spread on collagen type I but engage both the α1β1 and α2β1 integrins to spread on collagen type IV (supplemental Fig. 4A).
TABLE 1.
Identification of integrins involved in HUVEC spreading on collagen I, II, and IV, laminin, vitronectin, and a 50-kDa fragment of fibronectin
HUVECs were allowed to spread on immobilized ECM substrates, including collagen I, II, and IV, laminin, vitronectin, and a 50-kDa fragment of fibronectin in the presence of a range of anti-functional antibodies raised against several α and β integrin subunits. Quantification of spreading was performed by counting the number of cells spread over 100 cells, and the percentage was normalized to the control, i.e. addition of mouse IgG. Names of clones from which the anti-integrins antibodies were generated are listed in the table. Note that clone BHA2.1 for anti-α2 antibody is function-blocking, and clone 10A4 is not.
| Anti-integrin antibody |
ECM substrate |
||||||
|---|---|---|---|---|---|---|---|
| Integrin | Clone | Collagen I | Collagen II | Collagen IV | Laminin | Vitronectin | 50-kDa fragment |
| α1 | FB12 | 102 ± 1.2 | 91.9 ± 1.4 | 109.5 ± 1.5 | 88.8 ± 2.7 | 96.4 ± 2.5 | 87.4 ± 2.6 |
| α2 | BHA2.1 | 0.0 ± 0.0 | 6.8 ± 1.0 | 0.0 ± 0.0 | 41.6 ± 1.9 | 97.8 ± 1.8 | 77.4 ± 0.9 |
| α2 | 10A4 | 94.8 ± 1.4 | 91.9 ± 1.4 | 90.0 ± 0.5 | 95.7 ± 3.0 | 102.2 ± 0.8 | 92.1 ± 3.2 |
| α3 | P1B5 | 100.9 ± 0.5 | 94.6 ± 1.0 | 83.4 ± 2.1 | 59.2 ± 2.7 | 97.1 ± 1.4 | 77.9 ± 2.8 |
| α4 | HP1/2 | 95.3 ± 1.4 | 91.2 ± 1.2 | 109.5 ± 1.8 | 88.8 ± 0.7 | 111.5 ± 4.1 | 91.6 ± 3.3 |
| α5 | Mab16 | 99.5 ± 0.7 | 100 ± 2.2 | 108.9 ± 1.9 | 84.1 ± 1.9 | 115.1 ± 3.0 | 0.0 ± 0.0 |
| α6 | GoH3 | 103.3 ± 0.8 | 95.3 ± 1.4 | 104.7 ± 0.6 | 35.6 ± 1.9 | 115.8 ± 1.5 | 91.6 ± 1.8 |
| αv | 17E6 | 104.2 ± 1.0 | 98.6 ± 1.0 | 108.3 ± 1.0 | 85.4 ± 1.1 | 0.0 ± 0.0 | 78.9 ± 3.6 |
| β1 | Mab13 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 112.2 ± 1.9 | 0.0 ± 0.0 |
| αvβ3 | LM609 | 101.9 ± 1.9 | 95.9 ± 1.7 | 87.6 ± 0.3 | 96.6 ± 2.6 | 5.8 ± 1.5 | 100.5 ± 1.9 |
| αvβ5 | P1F6 | 100.9 ± 1.5 | 104.7 ± 3.2 | 106.5 ± 1.2 | 83.7 ± 2.2 | 82.7 ± 1.1 | 80.5 ± 3.3 |
To investigate the effects of opticin on α1β1 integrin engagement with ECM, we performed similar experiments as described before with human dermal microvascular ECs and found a similar pattern with spreading on collagen types I, II, and IV and laminin disrupted by the presence of opticin in a dose-dependent manner (supplemental Fig. 4, B and C). Together, the above data demonstrate that opticin disrupts EC morphology when spread on α1β1 and α2β1 integrin-specific ECM substrates, including collagen types I, II, and IV and laminin.
Opticin Inhibits Binding of α1β1 and α2β1 Integrins to Collagen
The binding of opticin to various ECM ligands was investigated using ELISA-based assays: opticin bound collagen type I (Kd(app) = 60 nm), collagen type II and laminin (Kd(app) = 40 nm for both) and collagen type IV (Kd(app) = 500 nm), but not to vitronectin and fibronectin (Fig. 5A). In control experiments, it was confirmed that the immobilized vitronectin and fibronectin bound known ligands, i.e. recombinant αvβ3 and α5β1 integrins, respectively (data not shown). Therefore, opticin directly binds to the same substrates on which it disrupts EC morphology. ECs interact with collagen via the α1β1 and α2β1 integrins, and both integrins are also receptors for laminin. Furthermore angiogenesis is driven by interaction of ECs with fibrillar collagens via these integrins. Therefore, we investigated whether opticin perturbs the interaction between the α1β1 and α2β1 integrins and collagen. First, we determined whether these integrins bound to opticin and collagen. We could not demonstrate an interaction between opticin and either integrin (Fig. 5B). However, there was strong binding between both α1β1 and α2β1 integrins and collagen type I (Kd(app) = 2.9 nm and 1.5 nm, respectively) (Fig. 5B). Inhibition assays were then performed, revealing that opticin competed the interactions between collagen type I and these two integrins in a concentration-dependent manner, with significant inhibition at a 1:1 molar ratio between the collagen and opticin (Fig. 5C).
FIGURE 5.
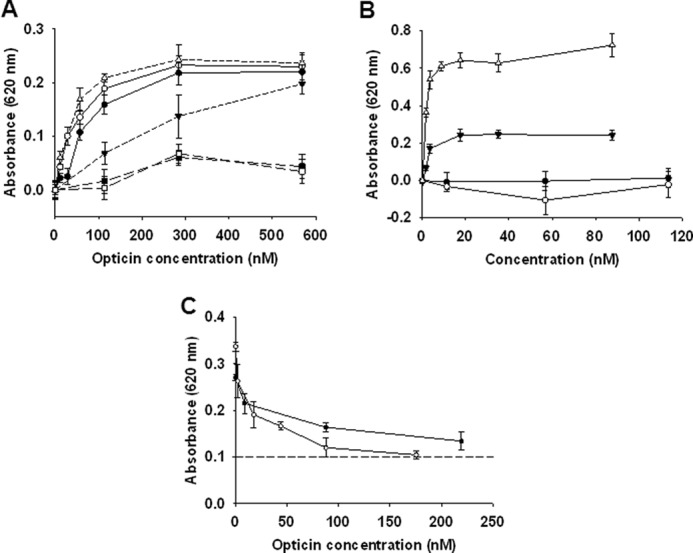
Opticin inhibits interactions between integrins and collagens/laminin. A, binding studies between opticin and various substrates revealed that it interacted strongly with collagen type I (●), collagen type II (○), and laminin (Δ), and more weakly to collagen type IV (▾). However, no binding was observed to vitronectin (■) or fibronectin (□). B, binding assays showed that both α1β1 and α2β1 integrins interacted with collagen type I strongly (▾ and Δ, respectively), but no binding to opticin was observed for either integrins (● and ○, respectively). C, inhibition studies using a fixed concentration of collagen type I preincubated with increasing opticin concentrations revealed that opticin inhibited collagen type I binding to both α1β1 and α2β1 integrins (● and ○, respectively). This inhibition was significant for both integrins at opticin concentrations as low as 1.75 nm (p = 0.02 and p = 0.002 for α1β1 and α2β1 integrins, respectively), corresponding to a 1:1 molar ratio between collagen type I and opticin; background absorbance level is indicated by a dashed line.
DISCUSSION
We have shown recently that opticin inhibits pathological angiogenesis into the vitreous (preretinal neovascularization) using the murine oxygen-induced retinopathy model. Other molecules that normally are present in the vitreous when injected to create an excess have been shown to inhibit preretinal neovascularization using the oxygen-induced retinopathy model, including pigment epithelial-derived factor, thrombospondin-1, and endostatin (27–29). Collectively, the anti-angiogenic components of the vitreous are thought to normally maintain it in an avascular state, but their relative importance is unknown. However, it is of note that opticin is the only one of these molecules where the null mice show increased preretinal neovascularization using the oxygen-induced retinopathy model (2, 30–32).
Here, we investigate how opticin inhibits angiogenesis. We found that opticin decreased the EC network formation in collagen and MatrigelTM, a matrix rich in collagen type IV and laminin (33) but not in fibrin, an αvβ3 and α5β1 integrin-dependent proangiogenic matrix (34). Furthermore, opticin disrupted the morphology of ECs on collagen and laminin, but not on vitronectin or fibronectin. However, opticin did not prevent adhesion of ECs to collagen, and although opticin disrupted EC focal adhesions and actin stress fibers when added in solution to cells attached to collagen, they did not detach. Next, we demonstrated that opticin binds to collagen and thereby inhibits the interactions between α1β1//α2β1 and collagen.
In our studies, decorin did not disrupt the morphology of ECs spread on collagen type I. A number of other studies have shown decorin to have either pro- or anti-angiogenic properties, depending upon the microenvironment in which the decorin is presented and the source of the decorin used (35). Indeed, it has been shown that decorin can interact with α2β1 integrin via its glycosaminoglycan chain, and it is proposed that decorin expression in angiogenic ECs promotes angiogenesis in a collagen type I-rich environment by signaling through insulin-like growth factor 1 receptor and influencing α2β1 integrin activity (36).
There is compelling evidence from in vivo and in vitro studies that collagen promotes capillary morphogenesis through integrin signaling (33, 37). It has been shown that collagen type I can initiate the early stages of angiogenesis, including initial EC retraction followed by shape change to a spindle-like morphology, alignment, and connection into precapillary cord-like structures; tubes then form by a process of vacuolation (33). Collagen type I exerts these effects via outside-in signaling through α1β1 and/or α2β1 integrins, leading to alterations in cell shape, contractility, and polymerization and arrangement of cytoskeletal actin into stress fibers (14–16, 38). The main collagen in vitreous is collagen type II (8), which also interacts with α1β1 and α2β1 integrins (39) and is likely therefore to induce similar signaling processes. Several studies have highlighted the importance of strong interactions between these integrins and their extracellular ligands in pathological angiogenesis (14, 38). Here, we show that opticin, by binding to collagen types I and II, hinders strong EC interactions with both α1β1 and α2β1 integrins, resulting in a failure of formation of focal adhesions and actin stress fibers.
Many of the known ECM inhibitors of angiogenesis exert their effects by perturbing integrin binding and signaling pathways, including endorepellin, arresten, tumstatin, endostatin, and canstatin (40–47). They generally act through integrin ligation, so endorepellin and arresten bind to the α2β1 and α1β1 integrins, respectively (41, 45), and tumstatin, endostatin, and canstatin interact with α5β1 or αVβ3 integrins (42–44, 46, 47). Opticin instead has a different mode of action, i.e. it binds to the ECM rather than the integrins themselves. There are parallels with the counteradhesive effects of tenascin-C, which binds to fibronectin and thereby inhibits its interaction with syndecan-4 and prevents focal adhesion and stress fiber formation (48). Another common feature shared by many of the endogenous anti-angiogenic molecules is that they are derived from proteolysis of larger ECM components such as proteoglycans and collagens: endorepellin is a C-terminal fragment of perlecan (40); arresten, tumstatin, and canstatin are all proteolytic fragments of collagen type IV (49–51), and endostatin corresponds to the C-terminal end of collagen type XVIII (52). However, opticin exerts its anti-angiogenic effects as an intact molecule.
The findings described in this study do not rule out the possibility that opticin has additional anti-angiogenic effects such as regulating metalloproteinase (MMP) expression. The SLRP lumican has been shown to down-regulate MMP-14 expression by ECs as well as binding α2β1 integrin and interfering with its activity (53). Decorin has been shown to both increase the expression of MMP-1, MMP-2, and MMP-14 by an EC-derived cell line (54), whereas in other studies, exogenous decorin was shown to decrease MMP-2 and MMP-9 expression by HeLa cells (55). In the light of these studies, we undertook a pilot study to investigate the effects of opticin on the expression of MMP-2, MMP-9, and MMP-14 and draw the tentative conclusion that opticin does not have a marked effect upon MMP expression (data not shown).
Collagen is the most abundant protein in the body and forms a structural framework for tissues. Cells can form firm adhesions to collagen through interactions using the α1β1 and α2β1 integrins. Our data suggest that opticin alters the surface characteristics of collagen, so it becomes less adhesive and can no longer support firm EC attachment. This work therefore develops the concept of the adhesive properties of collagen networks being modified by additional surface components, thereby altering cell-matrix interactions. As opticin binds several collagens and inhibits the interaction between collagen and the major collagen-binding integrins, it has potential as an anti-angiogenic therapeutic. Preclinical studies with α1β1 or α2β1 integrin antagonists suggest that blocking the function of these integrins individually may be an effective anti-angiogenic strategy (38), but a therapeutic that blocks the actions of both of these integrins simultaneously could be even more potent.
In conclusion, we provide evidence that opticin binds collagen and thereby inhibits α1β1 and α2β1 integrin binding to the collagen. Opticin thereby decreases the strength of EC adhesion to collagen, and the weakened adhesion is insufficient to promote angiogenesis.
Supplementary Material
Acknowledgment
We acknowledge Dr. David West for help with the CAM assay.
This work was supported by Manchester National Institute of Health Research Biomedical Research Centre and Manchester Royal Eye Hospital and Guide Dogs. This work was also supported by Wellcome Trust Grants 045225 and 074941 (to M. J. H.) and 057940 (to P. N. B.).

This article contains supplemental data and supplemental videos for Fig. 3, A and B.
- ECM
- extracellular matrix
- BBE
- bovine brain extract
- CAM
- chick chorioallantoic membrane
- HUVEC
- human umbilical vein EC
- HREC
- human retinal EC
- BAEC
- bovine aortic EC
- MMP
- metalloproteinase.
REFERENCES
- 1. Lutty G. A., Thompson D. C., Gallup J. Y., Mello R. J., Patz A., Fenselau A. (1983) Vitreous: An inhibitor of retinal extract-induced neovascularization. Invest. Ophthalmol. Vis. Sci. 24, 52–56 [PubMed] [Google Scholar]
- 2. Goff M. M., Lu H., Ugarte M., Henry S., Takanosu M., Mayne R., Bishop P. N. (2012) The vitreous glycoprotein opticin inhibits preretinal neovascularization. Invest. Ophthalmol. Vis. Sci. 53, 228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reardon A. J., Le Goff M., Briggs M. D., McLeod D., Sheehan J. K., Thornton D. J., Bishop P. N. (2000) Identification in vitreous and molecular cloning of opticin, a novel member of the family of leucine-rich repeat proteins of the extracellular matrix. J. Biol. Chem. 275, 2123–2129 [DOI] [PubMed] [Google Scholar]
- 4. Le Goff M. M., Hindson V. J., Jowitt T. A., Scott P. G., Bishop P. N. (2003) Characterization of opticin and evidence of stable dimerization in solution. J. Biol. Chem. 278, 45280–45287 [DOI] [PubMed] [Google Scholar]
- 5. Takanosu M., Boyd T. C., Le Goff M., Henry S. P., Zhang Y., Bishop P. N., Mayne R. (2001) Structure, chromosomal location, and tissue-specific expression of the mouse opticin gene. Invest. Ophthalmol. Vis. Sci. 42, 2202–2210 [PubMed] [Google Scholar]
- 6. Wistow G., Bernstein S. L., Ray S., Wyatt M. K., Behal A., Touchman J. W., Bouffard G., Smith D., Peterson K. (2002) Expressed sequence tag analysis of adult human iris for the NEIBank Project: Steroid-response factors and similarities with retinal pigment epithelium. Mol. Vis. 8, 185–195 [PubMed] [Google Scholar]
- 7. Halfter W., Dong S., Dong A., Eller A. W., Nischt R. (2008) Origin and turnover of ECM proteins from the inner limiting membrane and vitreous body. Eye 22, 1207–1213 [DOI] [PubMed] [Google Scholar]
- 8. Bishop P. N. (2000) Structural macromolecules and supramolecular organization of the vitreous gel. Prog. Retin. Eye Res. 19, 323–344 [DOI] [PubMed] [Google Scholar]
- 9. Ramesh S., Bonshek R. E., Bishop P. N. (2004) Immunolocalisation of opticin in the human eye. Br. J. Ophthalmol. 88, 607–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frolova E. I., Fokina V. M., Beebe D. C. (2004) The expression pattern of opticin during chicken embryogenesis. Gene Expr. Patterns 4, 335–338 [DOI] [PubMed] [Google Scholar]
- 11. Monfort J., Tardif G., Roughley P., Reboul P., Boileau C., Bishop P. N., Pelletier J. P., Martel-Pelletier J. (2008) Identification of opticin, a member of the small leucine-rich repeat proteoglycan family, in human articular tissues: A novel target for MMP-13 in osteoarthritis. Osteoarthritis Cartilage 16, 749–755 [DOI] [PubMed] [Google Scholar]
- 12. Wong H. C., Sehmi K. S., McLeod D. (1989) Abortive neovascular outgrowths discovered during vitrectomy for diabetic vitreous haemorrhage. Graefes Arch. Clin. Exp. Ophthalmol. 227, 237–240 [DOI] [PubMed] [Google Scholar]
- 13. Hosoda Y., Okada M., Matsumura M., Ogino N., Honda Y., Nagai Y. (1992) Intravitreal neovascular tissue of proliferative diabetic retinopathy: An immunohistochemical study. Ophthalmic Res. 24, 260–264 [DOI] [PubMed] [Google Scholar]
- 14. Davis G. E., Senger D. R. (2005) Endothelial extracellular matrix: Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 97, 1093–1107 [DOI] [PubMed] [Google Scholar]
- 15. Whelan M. C., Senger D. R. (2003) Collagen I initiates endothelial cell morphogenesis by inducing actin polymerization through suppression of cyclic AMP and protein kinase A. J. Biol. Chem. 278, 327–334 [DOI] [PubMed] [Google Scholar]
- 16. Hoang M. V., Whelan M. C., Senger D. R. (2004) Rho activity critically and selectively regulates endothelial cell organization during angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 101, 1874–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hindson V. J., Gallagher J. T., Halfter W., Bishop P. N. (2005) Opticin binds to heparan and chondroitin sulfate proteoglycans. Invest. Ophthalmol. Vis. Sci. 46, 4417–4423 [DOI] [PubMed] [Google Scholar]
- 18. Valdramidou D., Humphries M. J., Mould A. P. (2008) Distinct roles of beta1 metal ion-dependent adhesion site (MIDAS), adjacent to MIDAS (ADMIDAS), and ligand-associated metal-binding site (LIMBS) cation-binding sites in ligand recognition by integrin α2β1. J. Biol. Chem. 283, 32704–32714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mould A. P., Koper E. J., Byron A., Zahn G., Humphries M. J. (2009) Mapping the ligand-binding pocket of integrin α5β1 using a gain-of-function approach. Biochem. J. 424, 179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bishop P. N., Crossman M. V., McLeod D., Ayad S. (1994) Extraction and characterization of the tissue forms of collagen types II and IX from bovine vitreous. Biochem. J. 299, 497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. West D. C., Thompson W. D., Sells P. G., Burbridge M. F. (2001) in Methods in molecular medicine–Angiogenesis: Reviews and Protocols (Murray J. C., ed) pp. 107–130, Humana Press, Totowa, NJ: [DOI] [PubMed] [Google Scholar]
- 22. Sattar A., Kumar S., West D. C. (1992) Does hyaluronan have a role in endothelial cell proliferation of the synovium? Semin Arthritis Rheum. 22, 37–43 [DOI] [PubMed] [Google Scholar]
- 23. Montesano R., Orci L., Vassalli P. (1983) In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J. Cell Biol. 97, 1648–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laurens N., Engelse M. A., Jungerius C., Löwik C. W., van Hinsbergh V. W., Koolwijk P. (2009) Single and combined effects of αvβ3- and α5β1-integrins on capillary tube formation in a human fibrinous matrix. Angiogenesis 12, 275–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thuret G., Chiquet C., Herrag S., Dumollard J. M., Boudard D., Bednarz J., Campos L., Gain P. (2003) Mechanisms of staurosporine-induced apoptosis in a human corneal endothelial cell line. Br. J. Ophthalmol. 87, 346–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galaup A., Cazes A., Le Jan S., Philippe J., Connault E., Le Coz E., Mekid H., Mir L. M., Opolon P., Corvol P., Monnot C., Germain S. (2006) Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc. Natl. Acad. Sci. U.S.A. 103, 18721–18726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dawson D. W., Volpert O. V., Gillis P., Crawford S. E., Xu H., Benedict W., Bouck N. P. (1999) Pigment epithelium-derived factor: A potent inhibitor of angiogenesis. Science 285, 245–248 [DOI] [PubMed] [Google Scholar]
- 28. Sheibani N., Sorenson C. M., Cornelius L. A., Frazier W. A. (2000) Thrombospondin-1, a natural inhibitor of angiogenesis, is present in vitreous and aqueous humor and is modulated by hyperglycemia. Biochem. Biophys. Res. Commun. 267, 257–261 [DOI] [PubMed] [Google Scholar]
- 29. Määttä M., Heljasvaara R., Pihlajaniemi T., Uusitalo M. (2007) Collagen XVIII/endostatin shows a ubiquitous distribution in human ocular tissues and endostatin-containing fragments accumulate in ocular fluid samples. Graefes Arch. Clin. Exp. Ophthalmol. 245, 74–81 [DOI] [PubMed] [Google Scholar]
- 30. Wiegand S. J., Song H., Renard R., Kraus P., Gale N. W., Noguera I., Qian Y., Holash J., Yancopoulos G. D., Cao J. (2004) Genetic modulation of pigment epithelium–derived factor (PEDF) expression does not alter normal or pathological angiogenesis in the eye, or tumor growth. Invest. Ophthalmol. Vis. Sci. 45, E-Abstract 1884 [Google Scholar]
- 31. Wang S., Wu Z., Sorenson C. M., Lawler J., Sheibani N. (2003) Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev. Dyn. 228, 630–642 [DOI] [PubMed] [Google Scholar]
- 32. Hurskainen M., Eklund L., Hägg P. O., Fruttiger M., Sormunen R., Ilves M., Pihlajaniemi T. (2005) Abnormal maturation of the retinal vasculature in type XVIII collagen/endostatin deficient mice and changes in retinal glial cells due to lack of collagen types XV and XVIII. FASEB J. 19, 1564–1566 [DOI] [PubMed] [Google Scholar]
- 33. Vernon R. B., Sage E. H. (1995) Between molecules and morphology. Extracellular matrix and creation of vascular form. Am. J. Pathol. 147, 873–883 [PMC free article] [PubMed] [Google Scholar]
- 34. Bayless K. J., Salazar R., Davis G. E. (2000) RGD-dependent vacuolation and lumen formation observed during endothelial cell morphogenesis in three-dimensional fibrin matrices involves the αvβ3 and α5β1 integrins. Am. J. Pathol. 156, 1673–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fiedler L. R., Eble J. A. (2009) Decorin regulates endothelial cell-matrix interactions during angiogenesis. Cell Adh. Migr. 3, 3–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fiedler L. R., Schönherr E., Waddington R., Niland S., Seidler D. G., Aeschlimann D., Eble J. A. (2008) Decorin regulates endothelial cell motility on collagen I through activation of insulin-like growth factor I receptor and modulation of α2β1 integrin activity. J. Biol. Chem. 283, 17406–17415 [DOI] [PubMed] [Google Scholar]
- 37. Kamei M., Saunders W. B., Bayless K. J., Dye L., Davis G. E., Weinstein B. M. (2006) Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 442, 453–456 [DOI] [PubMed] [Google Scholar]
- 38. Senger D. R., Perruzzi C. A., Streit M., Koteliansky V. E., de Fougerolles A. R., Detmar M. (2002) The α1β1 and α2β1 integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis. Am. J. Pathol. 160, 195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guidetti G. F., Greco F., Bertoni A., Giudici C., Viola M., Tenni R., Tira E. M., Balduini C., Torti M. (2003) Platelet interaction with CNBr peptides from type II collagen via integrin α2β1. Biochim. Biophys. Acta 1640, 43–51 [DOI] [PubMed] [Google Scholar]
- 40. Mongiat M., Sweeney S. M., San Antonio J. D., Fu J., Iozzo R. V. (2003) Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J. Biol. Chem. 278, 4238–4249 [DOI] [PubMed] [Google Scholar]
- 41. Nyberg P., Xie L., Sugimoto H., Colorado P., Sund M., Holthaus K., Sudhakar A., Salo T., Kalluri R. (2008) Characterization of the anti-angiogenic properties of arresten, an α1β1 integrin-dependent collagen-derived tumor suppressor. Exp. Cell Res. 314, 3292–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hamano Y., Kalluri R. (2005) Tumstatin, the NC1 domain of α3 chain of type IV collagen, is an endogenous inhibitor of pathological angiogenesis and suppresses tumor growth. Biochem. Biophys. Res. Commun. 333, 292–298 [DOI] [PubMed] [Google Scholar]
- 43. Wickström S. A., Alitalo K., Keski-Oja J. (2002) Endostatin associates with integrin α5β1 and caveolin-1 and activates Src via a tyrosyl phosphatase-dependent pathway in human endothelial cells. Cancer Res. 62, 5580–5589 [PubMed] [Google Scholar]
- 44. Magnon C., Galaup A., Mullan B., Rouffiac V., Bouquet C., Bidart J. M., Griscelli F., Opolon P., Perricaudet M. (2005) Canstatin acts on endothelial and tumor cells via mitochondrial damage initiated through interaction with αvβ3 and αvβ5 integrins. Cancer Res. 65, 4353–4361 [DOI] [PubMed] [Google Scholar]
- 45. Bix G., Fu J., Gonzalez E. M., Macro L., Barker A., Campbell S., Zutter M. M., Santoro S. A., Kim J. K., Höök M., Reed C. C., Iozzo R. V. (2004) Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through α2β1 integrin. J. Cell Biol. 166, 97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maeshima Y., Colorado P. C., Kalluri R. (2000) Two RGD-independent αvβ3 integrin binding sites on tumstatin regulate distinct anti-tumor properties. J. Biol. Chem. 275, 23745–23750 [DOI] [PubMed] [Google Scholar]
- 47. Sudhakar A., Sugimoto H., Yang C., Lively J., Zeisberg M., Kalluri R. (2003) Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by αvβ3 and α5β1 integrins. Proc. Natl. Acad. Sci. U.S.A. 100, 4766–4771 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 48. Van Obberghen-Schilling E., Tucker R. P., Saupe F., Gasser I., Cseh B., Orend G. (2011) Fibronectin and tenascin-C: Accomplices in vascular morphogenesis during development and tumor growth. Int. J. Dev. Biol. 55, 511–525 [DOI] [PubMed] [Google Scholar]
- 49. Colorado P. C., Torre A., Kamphaus G., Maeshima Y., Hopfer H., Takahashi K., Volk R., Zamborsky E. D., Herman S., Sarkar P. K., Ericksen M. B., Dhanabal M., Simons M., Post M., Kufe D. W., Weichselbaum R. R., Sukhatme V. P., Kalluri R. (2000) Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 60, 2520–2526 [PubMed] [Google Scholar]
- 50. Maeshima Y., Colorado P. C., Torre A., Holthaus K. A., Grunkemeyer J. A., Ericksen M. B., Hopfer H., Xiao Y., Stillman I. E., Kalluri R. (2000) Distinct antitumor properties of a type IV collagen domain derived from basement membrane. J. Biol. Chem. 275, 21340–21348 [DOI] [PubMed] [Google Scholar]
- 51. Kamphaus G. D., Colorado P. C., Panka D. J., Hopfer H., Ramchandran R., Torre A., Maeshima Y., Mier J. W., Sukhatme V. P., Kalluri R. (2000) Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J. Biol. Chem. 275, 1209–1215 [DOI] [PubMed] [Google Scholar]
- 52. O'Reilly M. S., Boehm T., Shing Y., Fukai N., Vasios G., Lane W. S., Flynn E., Birkhead J. R., Olsen B. R., Folkman J. (1997) Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell 88, 277–285 [DOI] [PubMed] [Google Scholar]
- 53. Niewiarowska J., Brézillon S., Sacewicz-Hofman I., Bednarek R., Maquart F. X., Malinowski M., Wiktorska M., Wegrowski Y., Cierniewski C. S. (2011) Lumican inhibits angiogenesis by interfering with α2β1 receptor activity and down-regulating MMP-14 expression. Thromb. Res. 128, 452–457 [DOI] [PubMed] [Google Scholar]
- 54. Schönherr E., Schaefer L., O'Connell B. C., Kresse H. (2001) Matrix metalloproteinase expression by endothelial cells in collagen lattices changes during co-culture with fibroblasts and upon induction of decorin expression. J. Cell Physiol. 187, 37–47 [DOI] [PubMed] [Google Scholar]
- 55. Neill T., Painter H., Buraschi S., Owens R. T., Lisanti M. P., Schaefer L., Iozzo R. V. (2012) Decorin antagonizes the angiogenic network: Concurrent inhibition of Met, hypoxia inducible factor 1α, vascular endothelial growth factor A, and induction of thrombospondin-1 and TIMP3. J. Biol. Chem. 287, 5492–5506 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.