Background: Acidosis is a tumor hallmark that correlates with therapeutic resistance.
Results: We find that extracellular acidosis regulates multiple Bcl-2 family members to prevent apoptosis, in part through acid-sensing GPR65.
Conclusion: Cancer cells adopt a pro-survival Bcl-2 family expression pattern in response to this external pH-dependent signal.
Significance: This microenvironmental-cellular cross-talk can inform the development of therapeutic strategies against cancer.
Keywords: Acidosis, Apoptosis, Bcl-2 Family Proteins, Cancer, ERK, ABT-737, GPR65, TDAG8
Abstract
Acidosis arises in solid and lymphoid malignancies secondary to altered nutrient supply and utilization. Tumor acidosis correlates with therapeutic resistance, although the mechanism behind this effect is not fully understood. Here we show that incubation of lymphoma cell lines in acidic conditions (pH 6.5) blocks apoptosis induced by multiple cytotoxic metabolic stresses, including deprivation of glucose or glutamine and treatment with dexamethasone. We sought to examine the role of the Bcl-2 family of apoptosis regulators in this process. Interestingly, we found that acidic culture causes elevation of both Bcl-2 and Bcl-xL, while also attenuating glutamine starvation-induced elevation of p53-up-regulated modulator of apoptosis (PUMA) and Bim. We confirmed with knockdown studies that these shifts direct survival decisions during starvation and acidosis. Importantly, the promotion of a high anti- to pro-apoptotic Bcl-2 family member ratio by acidosis renders cells exquisitely sensitive to the Bcl-2/Bcl-xL antagonist ABT-737, suggesting that acidosis causes Bcl-2 family dependence. This dependence appears to be mediated, in part, by the acid-sensing G protein-coupled receptor, GPR65, via a MEK/ERK pathway.
Introduction
Acidosis is a characteristic feature of the tumor microenvironment. In fact, in vivo tumor pH measurements in the range of pH 6.5 and below are commonly reported (1, 2). This phenomenon has been demonstrated in numerous solid tumor types as well as in hematologic malignancies (3). The development of tumor-associated acidity stems largely from enhanced production of lactic acid and carbon dioxide secondary to several factors including inadequate blood supply, nutrient limitation, and altered cellular metabolism.
Malignant cells must adapt to the stresses caused by this extracellular acidification to survive. Some mechanisms by which cancer cells adapt to this potentially cytotoxic stimulus have been described previously, including up-regulation of enzymes and transporters to handle the persistent acid load (4). These cellular changes serve primarily to maintain intracellular pH homeostasis. Interestingly, tumor acidity correlates with resistance to both chemo- and radiation therapy both in vitro and in vivo (5, 6). As a result, current efforts seek to target tumor acidity as a treatment strategy in cancer (7, 8). However, relatively little is known about the signaling events responsible for this unexpected protective effect that acidosis confers upon cancer cells.
The very conditions that lead to tumor acidification additionally result in significant metabolic stress on cancer cells. Increased demand for nutrients to support growth and proliferation is exacerbated by simultaneous decrease in fuel supply. Specifically, the two most prominent fuel sources for malignant tissues, glucose and glutamine, are known to become limiting in the tumor microenvironment (9–12). Deprivation of either molecule leads to death of many cancer cell types (13–16). As such, compensatory mechanisms to avoid starvation-induced cytotoxicity are critical for cancer progression. As one essential means by which cancerous cells achieve this end, evasion of apoptosis represents a hallmark of the disease.
Among the most important groups of apoptosis regulators in cancer is the B cell lymphoma-2 (Bcl-2)2 family of proteins (17). The Bcl-2 gene was discovered at a chromosomal breakpoint in follicular lymphoma, where it is expressed constitutively as a result of t(14;18) translocation downstream of the immunoglobulin heavy chain enhancer element (18). Many human malignancies have since been shown to express high levels of Bcl-2, including both acute and chronic lymphocytic leukemia, non-Hodgkin's lymphoma, small cell lung cancer, and breast cancer (19). For some malignancies, the reason for Bcl-2 overexpression is not well understood. A closely related family member, Bcl-xL, is associated with a variety of cancers as well, where it functions similarly to Bcl-2. Both proteins act primarily to inhibit pro-apoptotic Bcl-2 homologs, thereby shutting down the intrinsic (mitochondrial) apoptosis cascade and downstream caspase activation (20). The importance of these anti-apoptotic factors in cancer is underscored by the continued interest in targeting these cell survival proteins therapeutically (21). A more complete understanding of the regulation of these oncogenes will guide the application and discovery of such agents.
Factors that initiate pro-survival signaling in response to acidosis continue to be elucidated. One such recently discovered cancer-associated acid sensor is the G protein-coupled receptor (GPCR) GPR65 (also known as T cell death-associated gene 8) (22, 23). Whereas this Gαs-coupled surface receptor is normally restricted in its expression to lymphoid cells, it has been noted in multiple reports to be expressed in both lymphoid and nonlymphoid cancers and to function as an oncogene (24–26). GPR65 has been shown to enhance viability of multiple cell types upon activation in response to acidosis (27, 28). The activation of GPR65 only occurs below pH 7.4 and reaches maximal activity between pH 6.2 and 6.8, in line with in vivo tumor pH (22, 23). Additionally, it was recently revealed that the ability of GPR65 to promote tumor formation requires its acid-sensing capabilities (25). Whereas these reports provide evidence for the importance of GPR65 in cancer, exactly how the receptor exerts its oncogenic function is unknown.
Herein we show that extracellular acidity has a robust protective effect against apoptosis induced by multiple cytotoxic metabolic stresses. Focusing on glutamine starvation, we show that acidosis alters expression of several Bcl-2 family members, leading to an abundance of anti-apoptotic while reducing pro-apoptotic family member levels. Interestingly, these acidosis-induced changes render cells exquisitely sensitive to Bcl-2-targeted chemotherapy. Additionally, we show that the up-regulation of Bcl-2 observed in acidic conditions occurs through GPR65 in a manner dependent upon MEK/ERK signaling but independent of the cAMP-dependent protein kinase (PKA). This work describes a novel mechanism by which cancer cells evade apoptosis in response to stresses relevant to the tumor microenvironment. Additionally, it is the first illustration of downstream effectors for the cytoprotective effect of GPR65.
EXPERIMENTAL PROCEDURES
Reagents
HEPES was purchased from Amresco (Solon, OH). H-89, forskolin, Rp-cAMPS (Rp-adenosine 3′,5′-cyclic monophosphorothioate triethylammonium salt hydrate), EPPS (3-[4-(2-hydroxyethyl)-1-piperazinyl]propane-sulfonic acid), and MES were purchased from Sigma. U0126 was from Cell Signaling Technology (Danvers, MA) and PD184352 was from Selleck Chemicals (Houston, TX). ABT-737 was provided by Abbott Laboratories.
Cell Culture
WEHI7.2 cells were grown in DMEM containing 2 mm l-glutamine, 10% bovine calf serum, and 100 μm nonessential amino acids. Bcl-2-overexpressing WEHI7.2 cells were described previously (29). CEM-C7 cells were grown in RPMI supplemented as for DMEM, in 5% CO2 at 37 °C. S49 cells (wild type (24.3.2), PKA-(24.6.1) and CYC-(94.15.1)) were purchased from the University of San Francisco Cell Culture Facility. S49 were grown in a humidified 7% CO2 incubator at 37 °C in DMEM supplemented as for WEHI, with the exception of 10% fetal bovine serum instead of bovine calf serum. For experiments, cells were incubated in medium with HEPES, EPPS, and MES (7.5 mm each), an established means of buffering across a range of pH values (30). Media pH was adjusted using HCl or NaOH and measured on a pH meter (Radiometer America, Westlake, OH). For all studies, cells were seeded at 4–5 × 105 cells/ml. The pH range for experiments was as follows: control pH 7.5 ± 0.1 and samples labeled acidity pH 6.5 ± 0.2.
Western Blot Analysis
Cells were collected by centrifugation, washed with PBS, and lysed with radioimmune precipitation assay buffer (10 mm Tris-HCl (pH 7.5), 150 mm NaCl, 200 mm dithiothreitol, 1% Triton X-100, 0.1% sodium dodecyl sulfate, PhosSTOP phosphatase inhibitor mixture and Complete Mini protease inhibitor mixture (Roche Applied Science)). Protein concentration was determined using Bradford reagent (Bio-Rad) and equal amounts of protein lysate plus sample buffer (50 nm Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate, 100 mm dithiothreitol, 10% glycerol, and 0.05% bromphenol blue) were boiled for 10 min to denature protein. Total protein was then separated by SDS-polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membrane, and blocked in Tris-buffered saline containing Tween 20 (0.1%) and either milk or BSA (5%). Membranes were then incubated overnight with primary antibodies, washed with Tween 20 (0.1%) and incubated for 1–4 h with horseradish peroxidase-conjugated secondary antibodies. The following antibodies were used: anti-Bcl-2 (Santa Cruz Biotechnology), anti-Mcl-1 (BioVision), anti-Noxa (Novus), anti-X-linked inhibitor of apoptosis protein (XIAP) (Pharmingen), and anti-Actin (Sigma). Additionally, anti-Bcl-xL, anti-PUMA, anti-Bim, anti-cleaved and total poly (ADP-ribose) polymerase (PARP), anti-cleaved caspase-3, anti-phospho and total ERK1/2, and anti-phospho and total cAMP-response element binding protein (CREB) were purchased from Cell Signaling. Immunoblots are representative of at least three unique experiments.
RNA Isolation and RT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. Isolated RNA was solvated in RNase-free water, and RNA concentration was determined by measuring absorbance at 260 and 280 nm. Total RNA was reverse transcribed using TaqMan Gold RT-PCR kit (Applied Biosystems). Universal TaqMan master mix and primer/probe sets specific for the genes of interest (Applied Biosystems) were used to amplify the reverse transcribed cDNAs in a Lightcycler 480 II (Roche Applied Science). Relative quantification was calculated using the ΔΔCt method with 18S rRNA and/or β-actin as reference genes using the manufacturer's software. Bcl-2, Bcl-xL, Bim, GPR65, GPR4, GPR68 (OGR1), GPR132 (G2A), β-actin, and 18S RNA TaqMan assays were purchased from Applied Biosystems. Assay for PUMA was generated using the Roche Universal probe library.
Cell Viability and Apoptosis Assays
Cell viability was quantified based on exclusion of trypan blue. Apoptosis was quantified based on Annexin V and propidium iodide (PI) staining using the Alexa Fluor 488 Annexin V/Dead Cell Apoptosis kit (Invitrogen) according to the manufacturer's protocol. Finally, cultured cells were harvested and suspended in cold PBS then fixed with ice-cold methanol. Fixed cells were incubated with 20 μg/ml RNase for 20–30 min at 37 °C, then with 50 μg/ml PI for an additional 45–60 min on ice. Both Annexin V/PI fluorescence from live cells and PI fluorescence of fixed cells were measured using an EPICS XL flow cytometer (Coulter, Miami, FL).
RNA Interference
To knock down the expression levels of genes of interest, 107 WEHI7.2 cells were transfected with ON-TARGETplus SMARTpool siRNA (Dharmacon, Lafayette, CO). Cells were washed with PBS and suspended in Opti-MEM (Invitrogen) within a 0.2-cm cuvette. To this cell suspension, siRNA either nontargeting or specific for murine Bcl-2, Bcl-xL, Bim, or GPR65 was added at a concentration of 1–2 μM. Cells were electroporated with a single 140-V, 10-ms2 wave pulse, transferred to fresh DMEM, and allowed to recover overnight. Alternatively, WEHI7.2 cells were transduced with one of 4–5 unique pLKO.1 lentiviral particles designed to express gene-specific shRNA against either mouse Bim (shBim) or PUMA (shPUMA) or empty vector control (Open Biosystems, Lafayette, CO). Antibiotic resistant cells were analyzed for knockdown efficiency by Western blotting, and the most effective isolates were chosen for further experimentation.
Statistical Analysis
A paired t test was used to compare two groups. A repeated measure ANOVA was applied to compare three or more groups. A Tukey-Kramer post test was used to compare pairs of group means. The highest level of significance for each comparison was reported. All statistical analyses were performed using GraphPad Prism. Densitometry was performed using ImageJ software. Error bars represent ± S.E. of at least three unique experiments.
RESULTS
Acidosis Protects Against Multiple Cytotoxic Metabolic Stresses
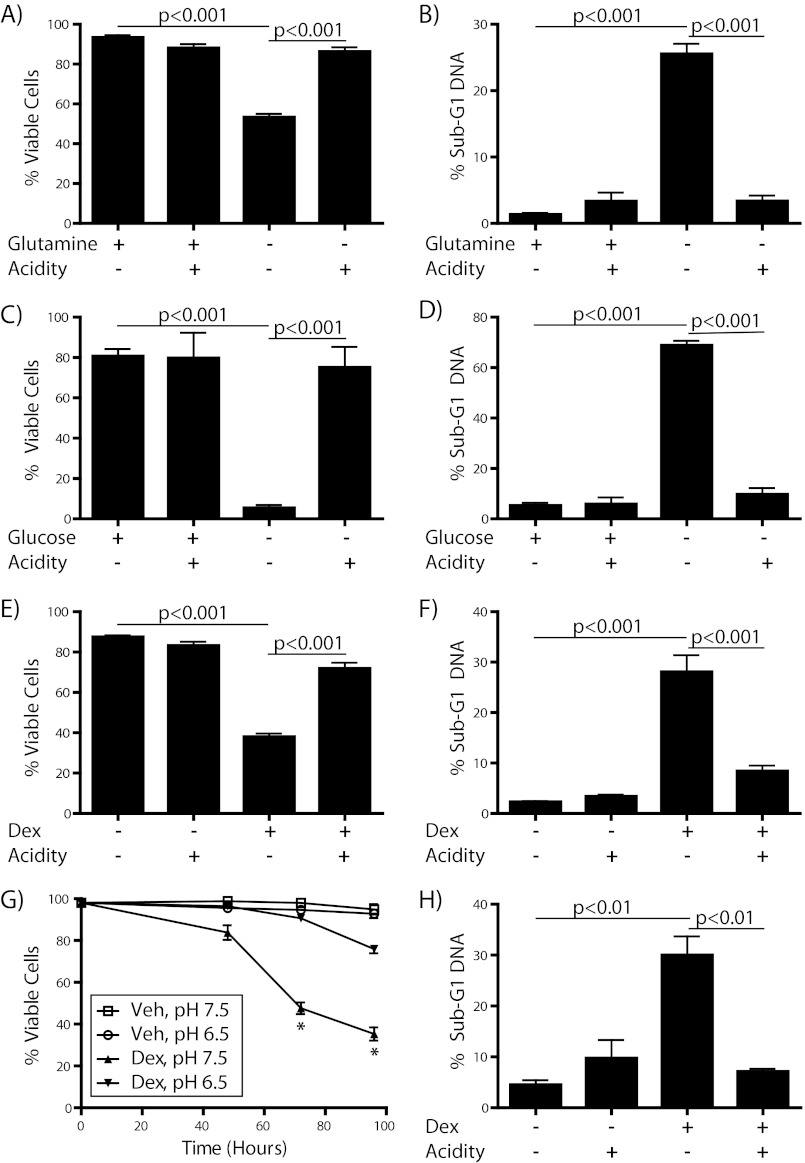
Abundant evidence confirms that extracellular acidity has a positive effect on the survival and malignant potential of many cancer cells (5, 6, 31). Although more intensively studied in solid tumors, acidosis is known to occur in the microenvironment of hematologic malignancies as well (3). Because these acidotic regions are simultaneously subject to nutrient limitation, we sought to investigate the influence of acidic culture conditions on survival of metabolically stressed lymphoma cells. For this purpose we used two T cell lymphoma cell lines, one murine (WEHI7.2) and one human (CEM-C7). These cell lines are well established models for the study of cell death (32, 33). Under normal culture conditions, acidosis had minimal effect on cell viability as determined by trypan blue exclusion or apoptosis as measured by quantification of sub-G1 DNA content via flow cytometry (Fig. 1). However, when cells were starved of either l-glutamine (Fig. 1, A and B) or d-glucose (Fig. 1, C and D) they underwent a robust increase in cell death that was prevented by acidification of the medium (to ∼pH 6.5). Additionally, acidosis blocked cell killing by dexamethasone, a synthetic glucocorticoid that is a vital component of therapy for a number of lymphoid malignancies and that acts in part by inhibiting glucose uptake (34), in both WEHI7.2 (Fig. 1, E and F) and CEM-C7 cells (Fig. 1, G and H). The combination of these data clearly shows that acidosis has a positive effect on the survival of lymphoma cells in response to numerous cytotoxic stimuli encountered by cancer cells in situ.
FIGURE 1.
Acidosis protects cells against multiple cell death stresses. A, C, E, and G, cell viability was measured by trypan blue exclusion assay. B, D, F, and H, cells were fixed with methanol and stained with PI, then sub-G1 DNA content was quantified by flow cytometry. WEHI7.2 cells were incubated either in the presence or absence of 2 mm l-glutamine for 12 h (A and B), in the presence or absence of 4.5 g/liter d-glucose for 48 h (C and D), or with vehicle (0.1% ethanol) or 100 nm dexamethasone for 24 h (E and F). CEM-C7 cells were incubated with vehicle or 100 nm dexamethasone for the times and pH indicated (G) or for 96 h in the presence or absence of acidification (H). *, p < 0.001.
Acidosis Exerts Its Cytoprotective Effect through Inhibition of Intrinsic Apoptosis
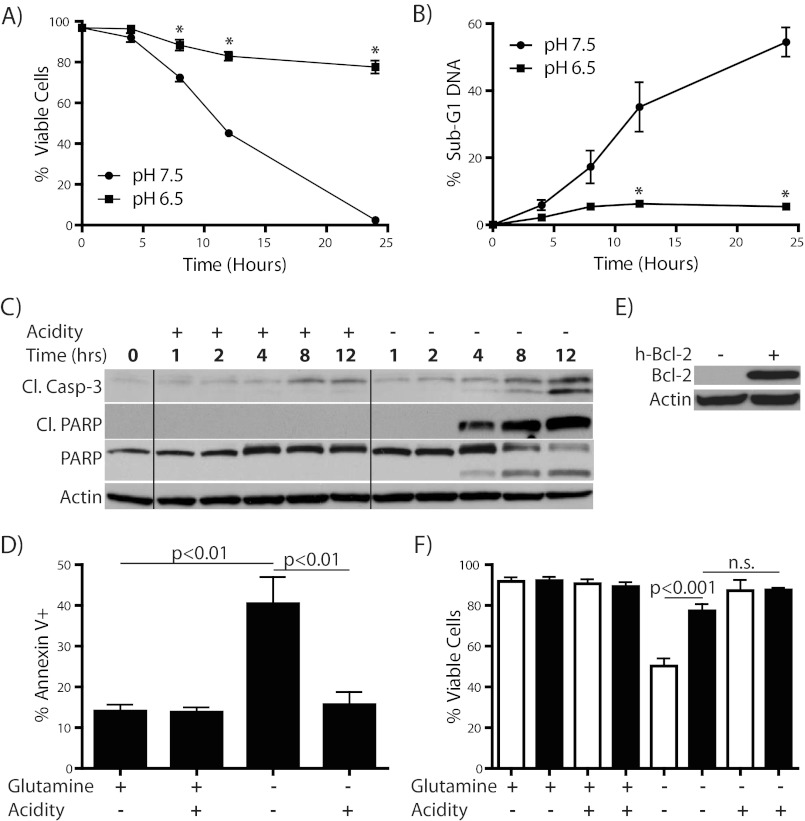
Having observed such a striking protective effect of acidosis against lethal metabolic stresses, we next wanted to further characterize the mechanism of cytoprotection in the context of glutamine starvation. We chose glutamine starvation because of its relevance to the tumor microenvironment and because its biology is less well described than glucose starvation or glucocorticoid treatment. Additionally, there has been a recent surge of interest in blocking glutamine uptake and/or metabolism as a therapeutic strategy in cancer, making an increased understanding of potential resistance mechanisms a priority (35, 36). To first examine the kinetics of cell death, we followed a time course of WEHI7.2 cell killing by glutamine starvation with or without acidosis and found a time-dependent decrease in cell viability with a concomitant increase in sub-G1 DNA content for glutamine-starved cells that was strongly attenuated by incubation at acidic pH (Fig. 2, A and B). In fact, by 24 h almost all starved cells incubated at pH 7.5 were dead, whereas nearly 80% of cells in acidified media remained viable. To characterize the mode of cell death more definitively, we probed for activation of the apoptotic cascade during glutamine starvation and found robust cleavage of caspase-3 and poly(ADP-ribose) polymerase (PARP), both of which increase in a time-dependent fashion (Fig. 2C). We additionally measured surface phosphatidylserine exposure, another hallmark of apoptosis, by flow cytometric analysis of Annexin V positivity at 8–10 h (Fig. 2D). These data led us to conclude that the cells are dying by intrinsic apoptosis. Importantly, acidification reduced apoptosis to near the levels observed in nutrient replete conditions in all of these experiments. Glutamine starvation-induced apoptosis has been shown to be inhibited by overexpression of Bcl-2 (15), which we confirmed in this system (Fig. 2, E and F). In the Bcl-2-overexpressing cells, acidosis did not add any significant protective effect against starvation-induced cell death. The strong inhibition of cell death by Bcl-2 further supports the conclusion that cell death upon glutamine starvation occurs through the intrinsic apoptotic cascade.
FIGURE 2.
Acidosis-mediated protection from glutamine starvation is due to a reduction in apoptosis. A–C, WEHI7.2 cells were incubated in the absence of 2 mm l-glutamine with or without acidification of the medium for the time indicated and then either tested for viability by trypan blue exclusion (A), fixed and stained with PI, then assayed for sub-G1 DNA content by flow cytometry (B), or analyzed by immunoblotting for appearance of apoptosis markers (C). D, WEHI7.2 cells were incubated in the presence or absence of l-glutamine for 8–10 h and then analyzed by flow cytometry for surface exposure of phosphatidylserine by assessment of Annexin V positivity. E and F, WEHI7.2 cells, either wild type (white bars) or Bcl-2 overexpressing (black bars) were incubated for 12 h in the presence or absence of l-glutamine with or without acidification of the medium, then viability was assessed by trypan blue exclusion assay. Overexpression of Bcl-2 is shown by Western blotting in E. *, p < 0.001.
Acidosis Elevates Bcl-2 and Bcl-xL mRNA and Protein Levels
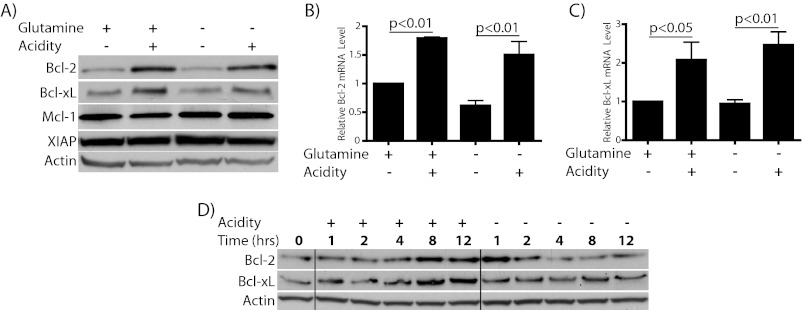
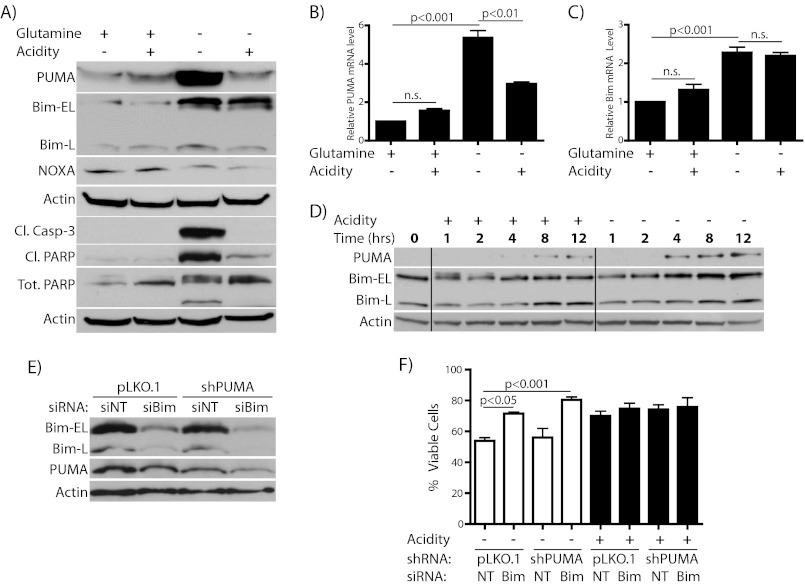
Having established that acidosis preserves survival of glutamine-starved cells through inhibition of apoptosis we next wanted to investigate the regulation of genes, which could be responsible for this cytoprotection. Because glutamine starvation is inhibited by Bcl-2 overexpression, we focused first on anti-apoptotic members of the Bcl-2 family. Among this group, Bcl-2, Bcl-xL, and Mcl-1 in particular are considered to be important for survival decisions in T cells (37). Therefore, we assessed expression of these anti-apoptotic Bcl-2 family members in cells cultured with or without acidification in the presence or absence of glutamine. In cells incubated under acidic conditions, both Bcl-2 and Bcl-xL protein and mRNA were found to be significantly elevated (Fig. 3, A–C). The up-regulation of Bcl-2 and Bcl-xL occurred similarly in both glutamine-deficient and -replete conditions, although Bcl-2 levels were lower in starved cells regardless of pH. This fits with previous evidence that glutamine can positively regulate Bcl-2 expression in lymphocytes (38). Furthermore, the extent of this increase depended on the degree of acidification, and Bcl-2 elevation was found to be reversible upon return to physiologic pH and to additionally occur in CEM-C7 cells (supplemental Fig. S1). Interestingly, Mcl-1, another pro-survival Bcl-2 family member thought to be important in lymphoid cells, was not altered by acidosis at the time points tested (Fig. 3A). As an additional control, we show that X-linked inhibitor of apoptosis protein (XIAP) level, a non-Bcl-2 family anti-apoptotic factor, is unaltered by acidosis. A time course for elevation of Bcl-2 and Bcl-xL protein by acidosis during glutamine starvation is shown in Fig. 3D. Surprisingly, there was an early burst of Bcl-2 protein elevation at pH 7.5 that rapidly dissipated. Whereas the signal for this event is unclear, the decay illustrates the short half-life of the protein in these cells. These data suggest a specific induction of Bcl-2 and Bcl-xL by acidosis which follows the pattern of protection from apoptosis seen in cells exposed to acidic culture.
FIGURE 3.
Acidosis regulates multiple anti-apoptotic Bcl-2 family members. A–C, WEHI7.2 cells were incubated for the times indicated in the presence or absence of 2 mm l-glutamine with or without acidification of the medium. A, immunoblot to show alterations in protein levels at 8–10 h. Similar results were found at all time points tested 8–24 h. B and C, RT-PCR was used to assess the expression level for Bcl-2 (B) and Bcl-xL (C) at 12 h. D, Western blot shows time course for changes in protein abundance during glutamine starvation with or without acidity.
Bcl-2 and Bcl-xL Contribute to the Protective Effect of Acidosis against Starvation
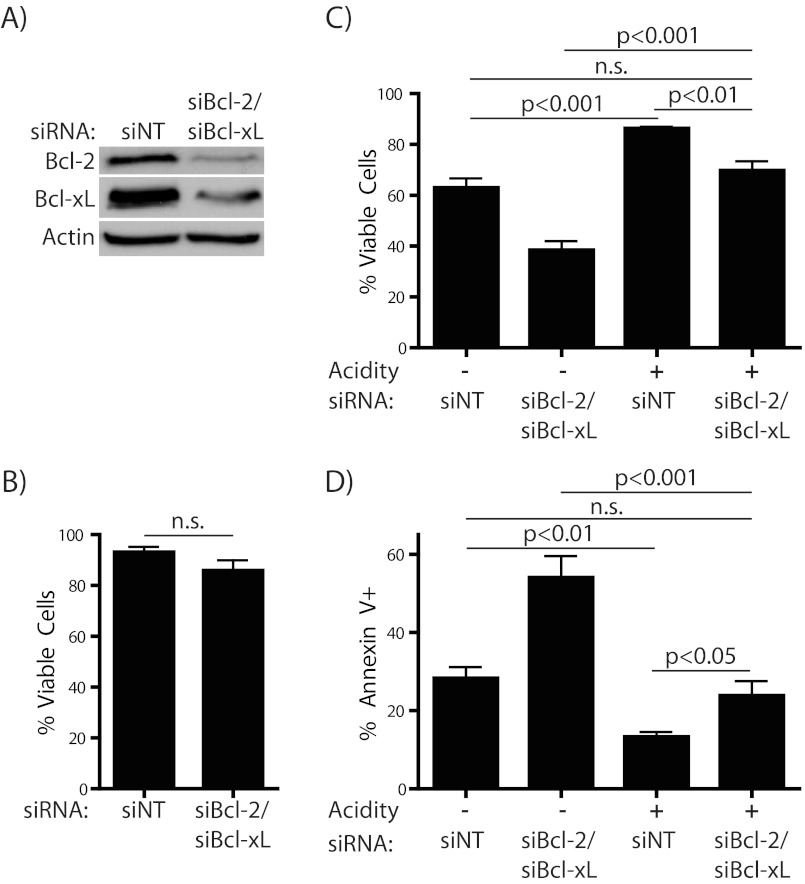
Because their positive regulation by acidosis correlates with the observed inhibition of apoptosis, we proposed that Bcl-2 and Bcl-xL act as important mediators of this protective effect. To assess this hypothesis, we blocked expression of Bcl-2 and Bcl-xL in combination using siRNA. The extent of the knockdown of the two proteins was confirmed by Western blotting after overnight recovery from electroporation (Fig. 4A), at which time there was minimal effect on survival (Fig. 4B). However, the double knockdown almost completely abrogated the protective effect of acidosis upon subsequent glutamine starvation (Fig. 4, C and D). We did observe an increased level of apoptosis due to glutamine starvation in Bcl-2/Bcl-xL knockdown cells under physiological pH conditions, consistent with the importance of these anti-apoptotic factors in WEHI7.2 cells undergoing stress. These data, as well as the Bcl-2 overexpression studies, led us to conclude that Bcl-2 and Bcl-xL are critical and possibly redundant mediators of the protection from apoptosis conferred by acidosis.
FIGURE 4.
Bcl-2 and Bcl-xL are required for the protective effect of acidosis on glutamine starvation. Cells were electroporated with either nontargeting or both Bcl-2- and Bcl-xL-specific SMARTpool siRNA. A, extent of knockdown was assessed by Western blotting and B, cell viability was determined by trypan blue exclusion after overnight recovery from electroporation. C and D, after recovery as in A and B, cells were incubated for 8–10 h in the absence of 2 mm l-glutamine, with or without acidification of the medium. Cells were then tested for cell death or apoptosis by trypan blue exclusion (C) or Annexin V (D) positivity, respectively.
Acidosis Negatively Regulates Glutamine Starvation-induced Expression of Pro-apoptotic PUMA and Bim
Despite effective knockdown of Bcl-2 and Bcl-xL, acidosis still had a considerable anti-apoptotic effect in these double knockdown cells (Fig. 4, C and D, compare columns 2 and 4). This observation raised the possibility that other genes are altered by acidosis in addition to Bcl-2 and Bcl-xL. Therefore, we went on to assess whether pro-apoptotic Bcl-2 family members are regulated by acidosis in a manner that would additionally support survival of starved cells. In lymphoid cells, PUMA, Bim, and Noxa are among the most important pro-apoptotic Bcl-2 relatives. We first probed for changes in protein levels of these three genes at 8 h, the earliest time point at which we detect significant cell death (Fig. 1 and note the extent of caspase-3 and PARP cleavage in Fig. 5A). By this time there was already a robust induction of both PUMA and Bim by glutamine starvation that was mirrored at the mRNA level (Fig. 5, A–C), whereas Noxa was little changed. Interestingly, incubation in acidic conditions markedly blocked PUMA induction at both the protein and mRNA levels (Fig. 5, A and B). However, whereas Bim protein elevation was somewhat attenuated in starved cells incubated at low pH, there was no concomitant change in mRNA levels (Fig. 5, A and C). Bim-S isoform was not readily detectable in these experiments. The time course for the elevation of these two proteins during glutamine starvation is shown in Fig. 5D. To assess whether PUMA and Bim are important for death of glutamine-starved cells, we employed a combination of stable and transient knockdown of these genes (Fig. 5, E and F and supplemental Fig. S2). First, we generated cells with stable expression of shRNA against PUMA. Knockdown of Bim by electroporation of Bim-specific siRNA in these PUMA knockdown cells or with stable shRNA expression in wild-type cells led to a strong decrease in Bim levels (Fig. 5E and supplemental Fig. S2). Whereas individual knockdown of either PUMA or Bim had some efficacy in blocking starvation-induced cell death (Fig. 5F and supplemental Fig. S2), the double knockdown strongly protected cells, in support of an essential role for these two factors in mediating apoptosis in response to glutamine deprivation. To our knowledge this is the first demonstration of effectors of this cytotoxicity. A similar role for these two factors has been described in glucose-starved leukemic cells (39).
FIGURE 5.
Acidosis attenuates starvation-induced increases in pro-apoptotic Bcl-2 family members. A–C, WEHI7.2 cells were incubated for the times indicated in the presence or absence of 2 mm l-glutamine with or without acidification of the media. A, immunoblot showing alterations in protein levels at 8–10 h. B and C, RT-PCR assessing expression level for PUMA (B) and Bim (C) at 12 h. D, Western blot showing time course for changes in protein abundance during glutamine starvation with or without acidity. E and F, cells transduced with pLKO.1 or pLKO.1-shPUMA lentivirus. Antibiotic-resistant cells were electroporated with either nontargeting (siNT/NT) or Bim-specific (siBim/Bim) siRNA. After overnight recovery, cells were analyzed by Western blotting for expression levels of Bim and PUMA (E). Finally, cells were incubated in the absence of l-glutamine with or without acidification of media for 12 h, then viability was assessed by trypan blue staining (F). White bars distinguish cells incubated at physiologic pH whereas acidic pH is shown in black bars.
Apoptosis Induction by ABT-737 Is Enhanced by Acidosis
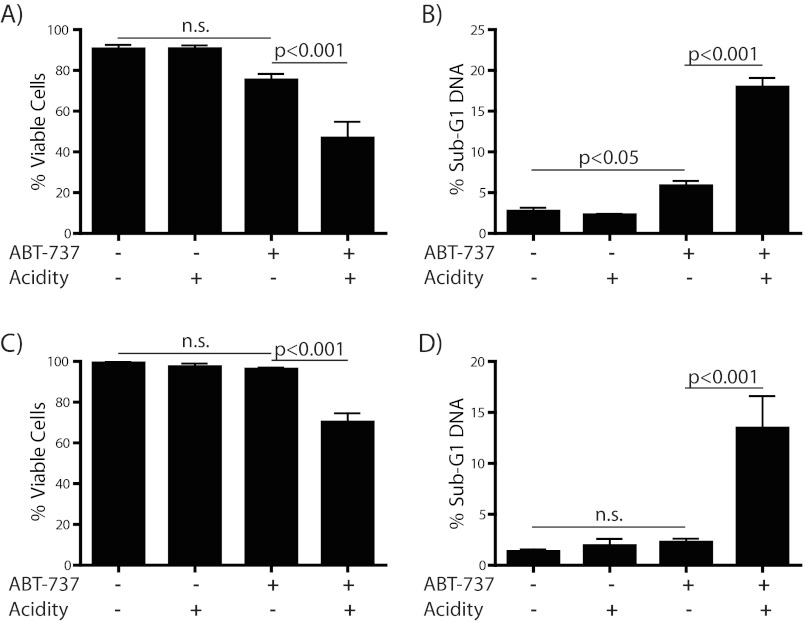
So far we have described the cytoprotective response to acidosis of T cell lymphoma cells. Our data show that the pro-survival effect of extracellular acidity is mediated by regulation of multiple Bcl-2 family genes. Acidosis dramatically increases the ratio of anti-apoptotic to pro-apoptotic family members in starved cells. However, pro-apoptotic members are still present to some degree in acidosis. Thus, a situation arises in acidosis where these cells require Bcl-2 and Bcl-xL to protect against their pro-apoptotic counterparts. This phenomenon is seen in numerous malignancies and has been dubbed “Bcl-2 addiction” (40). This state can be taken advantage of by targeting the interaction between anti- and pro-apoptotic Bcl-2 family members with the Bcl-2 targeted small molecule ABT-737. ABT-737 was rationally designed to inhibit the interaction between these two subsets of the Bcl-2 family, thereby freeing the pro-apoptotic proteins to activate the apoptotic cascade (41). An orally bioavailable derivative (ABT-263/Navitoclax) has since moved into clinical trials for the treatment of numerous malignancies, either as a single agent or as part of combination therapy (42). We hypothesized that although acidosis exerts a protective effect against the cytotoxic stresses representative of the tumor microenvironment, the mechanism by which this protection occurs would render these cells susceptible to killing by ABT-737. Therefore, we treated WEHI7.2 cells or CEM-C7 cells with ABT-737 and incubated in media with or without acidification. In support of the “addiction” hypothesis, we observed robustly increased apoptosis in cells grown under acidity compared with killing at physiological pH (Fig. 6). Notably, cells were treated in replete media. This paradoxical increase in killing at low pH supports the idea that these cells become dependent upon Bcl-2 and Bcl-xL for their survival in acidosis. It also represents a potential therapeutic advantage for the use of Bcl-2-targeted therapies in the treatment of acidotic tumors.
FIGURE 6.
Acidosis increases cell killing by ABT-737. WEHI7.2 (A and B) and CEM-C7 (C and D) cells were treated with vehicle or 1 μm ABT-737 and incubated for 24 h with or without acidification of the media. Cells were then tested for viability (A and C) by trypan blue exclusion or apoptosis (B and D) by measurement of sub-G1 DNA content via flow cytometry.
Acidosis-induced Elevation of Bcl-2 and Bcl-xL Occurs through Acid-sensing GPR65
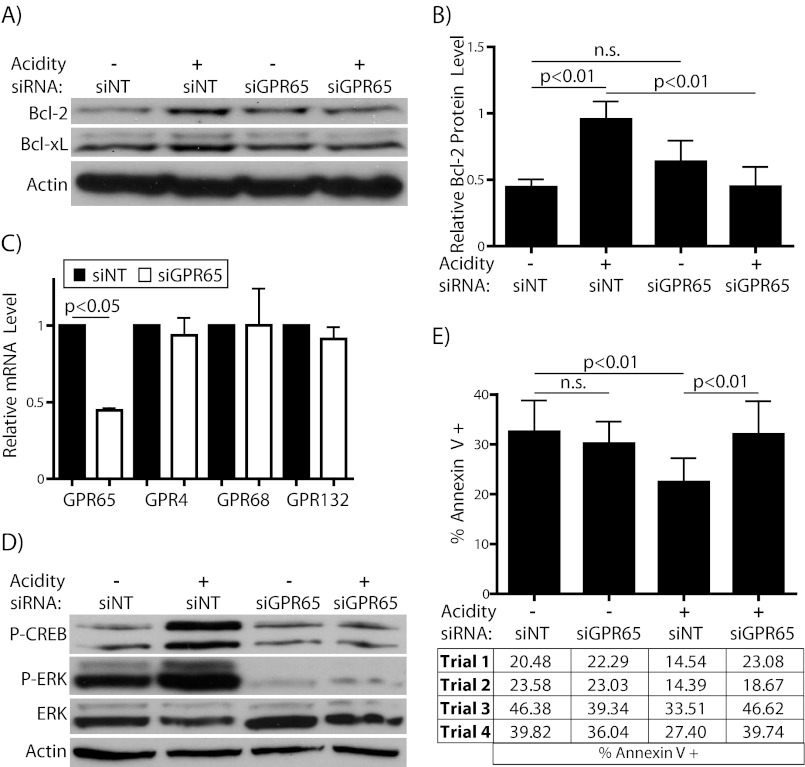
Our next goal was to explore the mechanism by which Bcl-2 and Bcl-xL are elevated under acidic conditions. Therefore, we tested whether the up-regulation of Bcl-2 and Bcl-xL are triggered by activation of the proton-sensing GPCR GPR65, which is expressed in WEHI7.2 cells (43). In support of this hypothesis, knockdown of GPR65 prevented the elevation of Bcl-2 and Bcl-xL in response to acidic conditions (Fig. 7, A and B). There was a small but not statistically significant increase in Bcl-2 protein in GPR65 knockdown cells compared with cells transfected with nontargeting siRNA when grown at physiologic pH, as assessed by densitometry. We show in Fig. 7C the effectiveness of knockdown at the transcript level. Additionally, the levels of related acid-sensing GPCRs were tested and showed little change in response to GPR65 siRNA treatment, supporting the specificity of these oligonucleotides. Transcript for GPR68 (also known as ovarian cancer G protein-coupled receptor 1) was barely detectable in these cells, which likely accounts for the variability in measurement of this gene. GPR4 and GPR132/G2A (from G2 accumulation) were both detectable by RT-PCR at threshold values similar to GPR65 (data not shown). Still, the essential parameter by which to assess the effectiveness of receptor knockdown is the blockade of its activity. For this reason we tested whether GPR65 knockdown led to inhibition of its downstream signaling. In fact, knockdown of GPR65 almost completely prevented the phosphorylation of CREB and ERK1/2 upon short term incubation in acidic pH (Fig. 7D). This follows from previous work by other groups demonstrating that pH-activated GPR65 signals through the Gαs/cAMP pathway and additionally through ERK1/2 phosphorylation (22, 23, 25). The ablation of both downstream signals indicates that our knockdown protocol effectively blunts the activity of GPR65. The absence of pH-dependent CREB and ERK1/2 phosphorylation in the GPR65-knockdown cells also suggests that other family members (especially Gαs-coupled GPR4) do not contribute to these signals. Thus we propose that the elevation of both Bcl-2 and Bcl-xL in acidosis is GPR65-dependent. Importantly, GPR65 knockdown also reduced the protective effect of acidosis on glutamine starvation (Fig. 7E). Of note, the knockdown had no effect on starvation-induced apoptosis at normal pH. This fits with previous work showing that the tumor-promoting and cytoprotective effect of GPR65 overexpression requires intact pH-sensing ability (25). Thus, from these data we conclude that GPR65 is an acidosis-specific survival factor that contributes to the anti-apoptotic effect of acidosis through the elevation of Bcl-2 and Bcl-xL.
FIGURE 7.
Acidosis-induced elevation of Bcl-2 and Bcl-xL requires GPR65. Cells were electroporated with either nontargeting (siNT) or GPR65-specific (siGPR65) SMARTpool siRNA and allowed to recover overnight. A, Western blot for Bcl-2 and Bcl-xL in cells incubated with or without acidification for 4–5 h. B, densitometric analysis of Bcl-2 protein level normalized to actin intensity for blots from panel A. C, RT-PCR for GPR65 and its family members after overnight recovery from electroporation. Nontargeting siRNA treated cells are in black bars, and GPR65-specific siRNA treated cells are in white. D, immunoblot of phosphorylated CREB and ERK1/2 proteins after 30–50 min incubation as in A. E, cells were incubated for 8–10 h in the absence of 2 mm l-glutamine, with or without acidification of the media. Apoptosis was then assessed by flow cytometric quantification of Annexin V positivity. The results of each individual experiment are shown below the graph.
Acidosis-induced Bcl-2 Elevation Is Independent of PKA Activity, but Dependent upon MEK/ERK Signaling
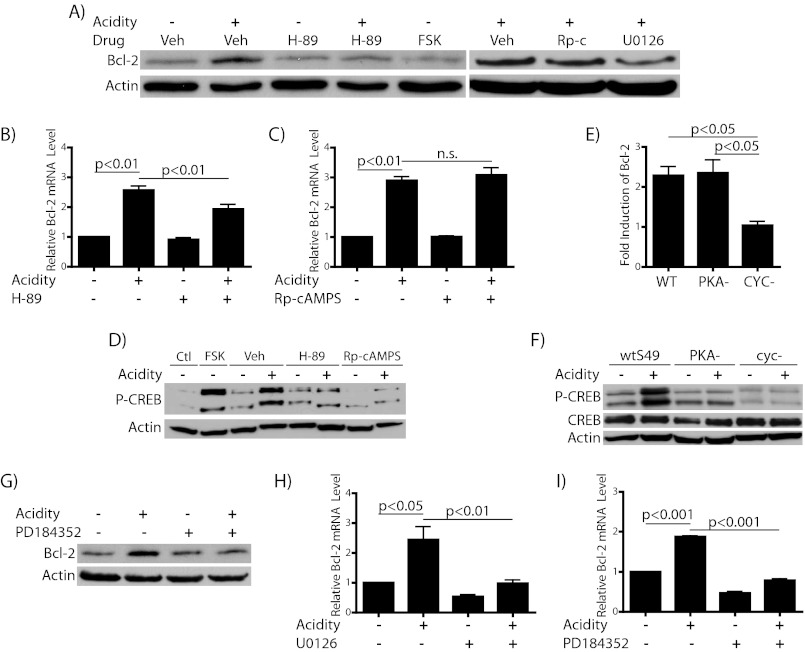
Finally, we wanted to elucidate further the mechanism of Bcl-2 elevation by investigating the signaling downstream of GPR65 responsible for the elevation of Bcl-2. As described above, GPR65 is known to activate the Gαs/cAMP/PKA pathway, and we have confirmed GPR65-dependent CREB phosphorylation in response to acidic conditions in our cells (Fig. 7D), suggesting the possibility that this pathway could mediate the elevation of Bcl-2 in response to acidosis. Furthermore, the Bcl-2 promoter contains a functional cAMP response element (44). To probe the role of this signaling cascade in our system, we first tested the effect of several pharmacological modulators of this pathway. First, the classical PKA inhibitor H-89, a competitive inhibitor for ATP binding to the catalytic subunit of PKA, both blocks the acidosis-mediated phosphorylation of CREB protein and partially inhibits the elevation of Bcl-2 in response to acidic pH at both the mRNA and protein level (Fig. 8, A, B, and D). Surprisingly, however, the adenylyl cyclase activator forskolin was unable to cause elevation of Bcl-2 despite its ability to increase the phosphorylation of CREB robustly (Fig. 8, A and D). Therefore, we next tested a more selective inhibitor of PKA, Rp-cAMPS, a nonhydrolyzable analog of cAMP, which competes for cAMP binding to the regulatory subunit of PKA. As shown in Fig. 8, A, C, and D, Rp-cAMPS at a concentration sufficient to block CREB phosphorylation did not inhibit the up-regulation of Bcl-2 under acidic pH. Unlike H-89, treatment with this drug revealed a lack of correlation between the inhibition of PKA activity and the inhibition of Bcl-2 elevation. Because of the conflicting effects of these two PKA inhibitors on preventing acidosis-induced Bcl-2 elevation and because forskolin alone was not sufficient to cause Bcl-2 induction, we sought to examine more definitively the role of PKA in this process. For this purpose we utilized the S49 murine T cell line, which we know to express GPR65 (43) and which has available mutant variants that lack either functional PKA (PKA−) or Gαs (CYC−, historical naming) (38). We observed elevation of Bcl-2 by acidosis in wild-type cells (Fig. 8E). These cells also exhibited CREB phosphorylation in response to acidic culture (Fig. 8F). Interestingly, the extent of elevation of Bcl-2 transcript was not affected by loss of PKA (PKA−) but was completely abolished in CYC- cells. The phenotype of these cells was confirmed in Fig. 8F, where both the PKA− and CYC−cells failed to phosphorylate CREB in response to acidosis. We represent the data as -fold induction of Bcl-2 due to the differing basal levels of Bcl-2 in these cell types. Because Bcl-2 elevation in acidosis occurs in the absence of PKA function based on small molecule and genetic inhibition of the kinase, we conclude that Bcl-2 up-regulation in acidic conditions occurs independently of PKA/CREB, although there appears to be a role for Gαs.
FIGURE 8.
Acidosis-induced Bcl-2 elevation is independent of PKA activity, dependent on MEK/ERK. Cells were incubated with or without acidification for the indicated times after 30–45 min pretreatment with dimethyl sulfoxide (Veh), 20 μm H-89, 10 μm forskolin, 20 μm Rp-cAMPS (Rp-c), 10 μm U0126, or 5 μm PD184352. A, immunoblot showing effect of inhibitors on acidosis-mediated Bcl-2 elevation after 5 h. B, C, H, and I, RT-PCR for Bcl-2 mRNA levels after 4 h. D, Western blot for CREB phosphorylation after 30 min (compared with base-line (Ctl)) to confirm effectiveness of PKA inhibitors (and forskolin). E and F, S49 cells, either wild type (wt), PKA−, or CYC− were incubated in medium with or without acidification for time indicated. E, -fold induction of Bcl-2 mRNA by acidosis as measured by RT-PCR after 5 h with or without acidification. F, immunoblot for CREB phosphorylation after 30 min to show the loss of acidosis-induced CREB phosphorylation in mutant S49 cells compared with wild type. G, Western blot showing inhibition of acidosis-induced Bcl-2 elevation by PD184352 at 5 h.
Whereas H-89 gave a confounding result, we propose that this finding represents an off-target effect. There is a host of literature describing the effects of H-89 not mediated by its ability to block PKA activity (45). Among the numerous PKA-independent effects of H-89 is its ability to inhibit phosphorylation of ERK1/2, suggesting that it may inhibit those kinases or upstream MAPKs such as MEK1/2, whose only known targets are ERK1/2 (46). As detailed previously, ERK1/2 phosphorylation is a known downstream target of GPR65 activation that is functional in WEHI7.2 cells (Fig. 7D). Additionally, ERK1 has been demonstrated previously to be essential for the activation of the Bcl-2 gene in response to certain stimuli (47). Therefore, we next explored whether the MEK inhibitor U0126 could block the elevation of Bcl-2 in response to low pH. In fact, U0126 strongly blocked Bcl-2 protein and transcript accumulation in response to acidic conditions (Fig. 8, A and H). We additionally tested the efficacy of a more selective second generation MEK inhibitor, PD184352, which does not inhibit the alternative MEK5/ERK5 pathway (48). This more selective inhibitor fully blocked Bcl-2 elevation by acidosis at both the mRNA and protein level (Fig. 8, G and I). Therefore, we conclude that Bcl-2 elevation by acidosis occurs through activation of the MEK/ERK signaling cascade. Our data support a model where GPR65 activation leads to Bcl-2 elevation through MEK/ERK via a mechanism that does not require the activity of PKA.
DISCUSSION
The tumor microenvironment plays a well established role in modulating the survival of cancer cells (5, 6, 49). Yet, the mechanistic basis for this phenomenon is not completely understood. Advances in this area promise to facilitate the discovery of novel therapeutic strategies that will succeed beyond in vitro models. In this work we examined the underpinnings for the cytoprotective effect that is exerted upon cancerous cells by extracellular acidity and found a multifaceted alteration in the ratio of Bcl-2 family members that supported cell survival. In fact, cells became dependent upon Bcl-2 family members when exposed to acidic conditions, thereby rendering them exquisitely sensitive to Bcl-2 targeted therapeutics. Furthermore, we discovered that GPR65, a cancer-associated GPCR, contributed to the survival effect of acidosis through regulation of Bcl-2 and Bcl-xL. Finally, the regulation of Bcl-2 was shown to involve MEK/ERK activation downstream of GPR65.
Nutrient deprivation and acidosis are two cellular stresses that must be overcome for tumor formation and progression to occur (50). In fact, acidosis is cytotoxic to some cell types (51–53). Cancer cells have established mechanisms to maintain pH homeostasis, yet the enhanced survival associated with extracellular acidity has not been fully explained to date. Others have shown that acidosis inhibits the accumulation or uptake of some chemotherapeutic agents by altering efflux transporter activity or by causing protonation of weakly basic sites on drug molecules (31, 54). Our model of nutrient deprivation, as opposed to addition of a cytotoxic compound, takes away these potentially confounding mechanisms of pH-mediated effects. Additionally, in our system preincubation with dexamethasone for 6 h prior to media acidification had the same effect as introducing the two variables simultaneously (data not shown).
Whereas other processes are certain to be modulated by acidosis, we uncovered a role for the Bcl-2 family in its cytoprotective effect. Among anti-apoptotic members, we observed elevation of both mRNA and protein for Bcl-2 and Bcl-xL. Transcription of each can be activated downstream of MEK/ERK signaling (47, 55). This pathway has also been implicated in post-transcriptional regulation of Bcl-xL (56). Bcl-xL additionally has a protective role in acidic preconditioning of endothelium, although independent of MEK/ERK (57). Both Bcl-2 and Bcl-xL are described to have internal ribosomal entry sites in their 5′ promoter region (58). However, the lack of elevation of XIAP by acidosis (Fig. 3A), another internal ribosomal entry site-containing anti-apoptotic gene (59), renders this possibility less likely. Finally, Bcl-2 and Bcl-xL transcripts are reported to have a short half-life in some systems (60). Thus, the regulation of mRNA decay could play a major role in regulating these survival factors, and several RNA-binding proteins could be involved in this process (61–63).
As for the pro-apoptotic Bcl-2 family members, both PUMA and Bim have been implicated in cell death due to glucose starvation (39). Noxa has also been implicated in this process, but we found no role for this pro-apoptotic member in our glutamine starvation model (64). The similar regulation of these pro-apoptotic factors by starvation of either glutamine or glucose suggests that cells may respond to these stresses through shared pathways. As for the effect of acidosis on these pro-apoptotic factors, phosphorylation of Bim-EL by ERK can direct its proteasomal degradation (reviewed in Ref. 65). In support of this potential mechanism, early time points of glutamine-starved cells grown in acidic culture show a second Bim-EL band that may represent a phosphorylation event (Fig. 5D). PUMA, on the other hand, is primarily regulated at the level of transcription, although post-transcriptional alterations are now being uncovered (66, 67). Neither p53 protein nor other p53 target gene mRNA levels were increased by glutamine starvation, nor were they repressed by acidosis, arguing against a role for this transcription factor in the regulation of PUMA (data not shown). MEK inhibition has been shown to induce cell death involving PUMA in cancer cells (68, 69). However, no demonstration of PUMA repression by MEK/ERK has been reported and therefore represents an important area for further inquiry.
Another important area that merits additional inquiry concerns GPR65 signaling. As mentioned above, GPR65 has been studied primarily as a Gαs-coupled GPCR. Increases in cAMP levels (22), CRE- and CREB-specific luciferase activity (24) and CREB phosphorylation (Fig. 7C) have all been demonstrated downstream of GPR65 activation. Others have shown that low pH activates the small GTPase RhoA in GPR65-expressing cells (23). Finally, GPR65 activation of MAPK signaling has been described (24, 25). Exactly how these multiple signals are integrated by a cell is unclear. Whereas PKA can activate MEK/ERK in some cell types (70), the results herein suggest that GPR65 induction of Bcl-2 requires ERK activity but is independent of PKA activity. Several pathways could mediate this signal, including RhoA-dependent activation of MEK/ERK or signaling through β-arrestin. β-Arrestin 1 has been shown to drive Bcl-2 expression in CD4+ T cells (71), although a role of MEK/ERK in this process has not been explored. Additionally, the exchange protein activated by cAMP, or EPAC, can cause PKA-independent ERK activation (72). A functional role for EPAC has been demonstrated downstream of the GPR65 homolog GPR4 (73). A more complete study of the activation cascade of GPR65 could provide valuable information regarding its role in normal cellular processes and tumorigenesis alike.
Whereas further investigation into signaling by GPR65 stands to be a fruitful effort, a greater appreciation of its role in human tumors is of the utmost importance. Several reports indicate that GPR65 is ectopically expressed in a variety of human malignancies (24–26). Furthermore, it is known to have a critical role in promoting cell survival and tumor growth. Yet, a GPR65 knock-out mouse has no significant phenotype (74), highlighting the potential selectivity of targeting this receptor in cancer.
Whereas the therapeutic potential of targeting GPR65 is an important future direction, this work additionally highlights the need for appropriate models of cancer at all levels of preclinical study. In this regard, on one hand we observe a role for acidosis in promoting resistance to metabolic stresses and dexamethasone. On the other, we have uncovered its enhancement of ABT-737 efficacy. Interestingly, a similar effect was recently reported for neuroblastoma cells under hypoxia (75). These results bring hope for the translational success of Bcl-2-targeted therapies. Yet, at the same time the disparate effect of acidosis toward different cytotoxic stresses illustrates the importance of considering this among the multitude of other variables that affect this complex disease.
Supplementary Material
Acknowledgments
We thank Arlene Forte at Abbott Laboratories for approving our experimental use of ABT-737. Additionally, we thank members of the Distelhorst lab for critical assessment of the work.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 CA42755, R01 CA085804, and T32 GM08803. This work was also supported by a Leukemia and Lymphoma Society Translational Research Program grant and by the Flow Cytometry Core Facility of the Case Comprehensive Cancer Center Grant P30 CA43703.

This article contains supplemental Figs. S1 and S2.
- Bcl-2
- B cell lymphoma-2
- Bim
- Bcl-2-interacting mediator of cell death
- GPCR
- G protein-coupled receptor
- PUMA
- p53-up-regulated modulator of apoptosis
- CREB
- cAMP-response element-binding protein
- PI
- propidium iodide
- XIAP
- X-linked inhibitor of apoptosis protein
- PARP
- poly-(ADP-ribose) polymerase.
REFERENCES
- 1. Evelhoch J. L. (2001) pH and therapy of human cancers. Novartis Found. Symp. 240, 68–80; discussion 80–4, 152–3 [DOI] [PubMed] [Google Scholar]
- 2. Vaupel P., Kallinowski F., Okunieff P. (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 49, 6449–6465 [PubMed] [Google Scholar]
- 3. Mortensen B. T., Jensen P. O., Helledie N., Iversen P. O., Ralfkiaer E., Larsen J. K., Madsen M. T. (1998) Changing bone marrow micro-environment during development of acute myeloid leukaemia in rats. Br. J. Haematol. 102, 458–464 [DOI] [PubMed] [Google Scholar]
- 4. Parks S. K., Chiche J., Pouyssegur J. (2011) pH control mechanisms of tumor survival and growth. J. Cell. Physiol. 226, 299–308 [DOI] [PubMed] [Google Scholar]
- 5. Reichert M., Steinbach J. P., Supra P., Weller M. (2002) Modulation of growth and radiochemosensitivity of human malignant glioma cells by acidosis. Cancer 95, 1113–1119 [DOI] [PubMed] [Google Scholar]
- 6. Thews O., Gassner B., Kelleher D. K., Schwerdt G., Gekle M. (2007) Impact of hypoxic and acidic extracellular conditions on cytotoxicity of chemotherapeutic drugs. Adv. Exp. Med. Biol. 599, 155–161 [DOI] [PubMed] [Google Scholar]
- 7. McCarty M. F., Whitaker J. (2010) Manipulating tumor acidification as a cancer treatment strategy. Altern. Med. Rev. 15, 264–272 [PubMed] [Google Scholar]
- 8. De Milito A., Fais S. (2005) Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol. 1, 779–786 [DOI] [PubMed] [Google Scholar]
- 9. Moyer G. H., Pitot H. C. (1974) Static and dynamic aspects of amino acid pools in rat liver and Morris hepatomas 9618A and 7800. Cancer Res. 34, 2647–2653 [PubMed] [Google Scholar]
- 10. DeBerardinis R. J., Cheng T. (2010) Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 29, 313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schroeder T., Yuan H., Viglianti B. L., Peltz C., Asopa S., Vujaskovic Z., Dewhirst M. W. (2005) Spatial heterogeneity and oxygen dependence of glucose consumption in R3230Ac and fibrosarcomas of the Fischer 344 rat. Cancer Res. 65, 5163–5171 [DOI] [PubMed] [Google Scholar]
- 12. Sauer L. A., Stayman J. W., 3rd, Dauchy R. T. (1982) Amino acid, glucose, and lactic acid utilization in vivo by rat tumors. Cancer Res. 42, 4090–4097 [PubMed] [Google Scholar]
- 13. Eagle H. (1955) Nutrition needs of mammalian cells in tissue culture. Science 122, 501–514 [DOI] [PubMed] [Google Scholar]
- 14. Fuchs B. C., Bode B. P. (2006) Stressing out over survival: glutamine as an apoptotic modulator. J. Surg. Res. 131, 26–40 [DOI] [PubMed] [Google Scholar]
- 15. Yuneva M., Zamboni N., Oefner P., Sachidanandam R., Lazebnik Y. (2007) Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J. Cell Biol. 178, 93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El Mjiyad N., Caro-Maldonado A., Ramírez-Peinado S., Muñoz-Pinedo C. (2011) Sugar-free approaches to cancer cell killing. Oncogene 30, 253–264 [DOI] [PubMed] [Google Scholar]
- 17. Hanahan D., Weinberg R. A. (2000) The hallmarks of cancer. Cell 100, 57–70 [DOI] [PubMed] [Google Scholar]
- 18. Tsujimoto Y., Finger L. R., Yunis J., Nowell P. C., Croce C. M. (1984) Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 226, 1097–1099 [DOI] [PubMed] [Google Scholar]
- 19. Hockenbery D. M. (2010) Targeting mitochondria for cancer therapy. Environ. Mol. Mutagen. 51, 476–489 [DOI] [PubMed] [Google Scholar]
- 20. Chipuk J. E., Moldoveanu T., Llambi F., Parsons M. J., Green D. R. (2010) The BCL-2 family reunion. Mol. Cell 37, 299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Letai A. (2005) Pharmacological manipulation of Bcl-2 family members to control cell death. J. Clin. Invest. 115, 2648–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang J. Q., Kon J., Mogi C., Tobo M., Damirin A., Sato K., Komachi M., Malchinkhuu E., Murata N., Kimura T., Kuwabara A., Wakamatsu K., Koizumi H., Uede T., Tsujimoto G., Kurose H., Sato T., Harada A., Misawa N., Tomura H., Okajima F. (2004) TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J. Biol. Chem. 279, 45626–45633 [DOI] [PubMed] [Google Scholar]
- 23. Ishii S., Kihara Y., Shimizu T. (2005) Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J. Biol. Chem. 280, 9083–9087 [DOI] [PubMed] [Google Scholar]
- 24. Sin W. C., Zhang Y., Zhong W., Adhikarakunnathu S., Powers S., Hoey T., An S., Yang J. (2004) G protein-coupled receptors GPR4 and TDAG8 are oncogenic and overexpressed in human cancers. Oncogene 23, 6299–6303 [DOI] [PubMed] [Google Scholar]
- 25. Ihara Y., Kihara Y., Hamano F., Yanagida K., Morishita Y., Kunita A., Yamori T., Fukayama M., Aburatani H., Shimizu T., Ishii S. (2010) The G protein-coupled receptor T-cell death-associated gene 8 (TDAG8) facilitates tumor development by serving as an extracellular pH sensor. Proc. Natl. Acad. Sci. U.S.A. 107, 17309–17314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li S., Huang S., Peng S. B. (2005) Overexpression of G protein-coupled receptors in cancer cells: involvement in tumor progression. Int. J. Oncol. 27, 1329–1339 [PubMed] [Google Scholar]
- 27. Kottyan L. C., Collier A. R., Cao K. H., Niese K. A., Hedgebeth M., Radu C. G., Witte O. N., Khurana Hershey G. K., Rothenberg M. E., Zimmermann N. (2009) Eosinophil viability is increased by acidic pH in a cAMP- and GPR65-dependent manner. Blood 114, 2774–2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McGuire J., Herman J. P., Ghosal S., Eaton K., Sallee F. R., Sah R. (2009) Acid-sensing by the T cell death-associated gene 8 (TDAG8) receptor cloned from rat brain. Biochem. Biophys. Res. Commun. 386, 420–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen R., Valencia I., Zhong F., McColl K. S., Roderick H. L., Bootman M. D., Berridge M. J., Conway S. J., Holmes A. B., Mignery G. A., Velez P., Distelhorst C. W. (2004) Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J. Cell Biol. 166, 193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Radu C. G., Nijagal A., McLaughlin J., Wang L., Witte O. N. (2005) Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc. Natl. Acad. Sci. U.S.A. 102, 1632–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thews O., Gassner B., Kelleher D. K., Schwerdt G., Gekle M. (2006) Impact of extracellular acidity on the activity of P-glycoprotein and the cytotoxicity of chemotherapeutic drugs. Neoplasia 8, 143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dowd D. R., Miesfeld R. L. (1992) Evidence that glucocorticoid- and cyclic AMP-induced apoptotic pathways in lymphocytes share distal events. Mol. Cell. Biol. 12, 3600–3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Molitoris J. K., McColl K. S., Distelhorst C. W. (2011) Glucocorticoid-mediated repression of the oncogenic microRNA cluster miR-17∼92 contributes to the induction of Bim and initiation of apoptosis. Mol. Endocrinol. 25, 409–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosen J. M., Milholland R. J., Rosen F. (1970) A comparison of the effect of glucocorticoids on glucose uptake and hexokinase activity in lymphosarcoma P1798. Biochim. Biophys. Acta 219, 447–454 [DOI] [PubMed] [Google Scholar]
- 35. Wise D. R., Thompson C. B. (2010) Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 35, 427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dang C. V. (2010) Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res. 70, 859–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marrack P., Kappler J. (2004) Control of T cell viability. Annu. Rev. Immunol. 22, 765–787 [DOI] [PubMed] [Google Scholar]
- 38. Chang W. K., Yang K. D., Chuang H., Jan J. T., Shaio M. F. (2002) Glutamine protects activated human T cells from apoptosis by up-regulating glutathione and Bcl-2 levels. Clin. Immunol. 104, 151–160 [DOI] [PubMed] [Google Scholar]
- 39. Coloff J. L., Mason E. F., Altman B. J., Gerriets V. A., Liu T., Nichols A. N., Zhao Y., Wofford J. A., Jacobs S. R., Ilkayeva O., Garrison S. P., Zambetti G. P., Rathmell J. C. (2011) Akt requires glucose metabolism to suppress PUMA expression and prevent apoptosis of leukemic T cells. J. Biol. Chem. 286, 5921–5933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Certo M., Del Gaizo Moore V., Nishino M., Wei G., Korsmeyer S., Armstrong S. A., Letai A. (2006) Mitochondria primed by death signals determine cellular addiction to antiapoptotic Bcl-2 family members. Cancer Cell 9, 351–365 [DOI] [PubMed] [Google Scholar]
- 41. Petros A. M., Dinges J., Augeri D. J., Baumeister S. A., Betebenner D. A., Bures M. G., Elmore S. W., Hajduk P. J., Joseph M. K., Landis S. K., Nettesheim D. G., Rosenberg S. H., Shen W., Thomas S., Wang X., Zanze I., Zhang H., Fesik S. W. (2006) Discovery of a potent inhibitor of the antiapoptotic protein Bcl-xL from NMR and parallel synthesis. J. Med. Chem. 49, 656–663 [DOI] [PubMed] [Google Scholar]
- 42. Vogler M., Dinsdale D., Dyer M. J., Cohen G. M. (2009) Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ. 16, 360–367 [DOI] [PubMed] [Google Scholar]
- 43. Malone M. H., Wang Z., Distelhorst C. W. (2004) The glucocorticoid-induced gene tdag8 encodes a pro-apoptotic G protein-coupled receptor whose activation promotes glucocorticoid-induced apoptosis. J. Biol. Chem. 279, 52850–52859 [DOI] [PubMed] [Google Scholar]
- 44. Wilson B. E., Mochon E., Boxer L. M. (1996) Induction of Bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol. Cell. Biol. 16, 5546–5556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Murray A. J. (2008) Pharmacological PKA inhibition: all may not be what it seems. Sci. Signal 1, re4. [DOI] [PubMed] [Google Scholar]
- 46. Pearson G., Robinson F., Beers Gibson T., Xu B. E., Karandikar M., Berman K., Cobb M. H. (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22, 153–183 [DOI] [PubMed] [Google Scholar]
- 47. Liu Y. Z., Boxer L. M., Latchman D. S. (1999) Activation of the Bcl-2 promoter by nerve growth factor is mediated by the p42/p44 MAPK cascade. Nucleic Acids Res. 27, 2086–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Squires M. S., Nixon P. M., Cook S. J. (2002) Cell-cycle arrest by PD184352 requires inhibition of extracellular signal-regulated kinases (ERK) 1/2 but not ERK5/BMK1. Biochem. J. 366, 673–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meads M. B., Hazlehurst L. A., Dalton W. S. (2008) The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin. Cancer Res. 14, 2519–2526 [DOI] [PubMed] [Google Scholar]
- 50. Gatenby R. A., Gillies R. J. (2004) Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4, 891–899 [DOI] [PubMed] [Google Scholar]
- 51. Kubasiak L. A., Hernandez O. M., Bishopric N. H., Webster K. A. (2002) Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc. Natl. Acad. Sci. U.S.A. 99, 12825–12830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jeong D., Kim T. S., Lee J. W., Kim K. T., Kim H. J., Kim I. H., Kim I. Y. (2001) Blocking of acidosis-mediated apoptosis by a reduction of lactate dehydrogenase activity through antisense mRNA expression. Biochem. Biophys. Res. Commun. 289, 1141–1149 [DOI] [PubMed] [Google Scholar]
- 53. Ding D., Moskowitz S. I., Li R., Lee S. B., Esteban M., Tomaselli K., Chan J., Bergold P. J. (2000) Acidosis induces necrosis and apoptosis of cultured hippocampal neurons. Exp. Neurol. 162, 1–12 [DOI] [PubMed] [Google Scholar]
- 54. Raghunand N., He X., van Sluis R., Mahoney B., Baggett B., Taylor C. W., Paine-Murrieta G., Roe D., Bhujwalla Z. M., Gillies R. J. (1999) Enhancement of chemotherapy by manipulation of tumour pH. Br. J. Cancer 80, 1005–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jazirehi A. R., Vega M. I., Chatterjee D., Goodglick L., Bonavida B. (2004) Inhibition of the Raf-MEK1/2-ERK1/2 signaling pathway, Bcl-xL down-regulation, and chemosensitization of non-Hodgkin's lymphoma B cells by rituximab. Cancer Res. 64, 7117–7126 [DOI] [PubMed] [Google Scholar]
- 56. Pardo O. E., Arcaro A., Salerno G., Raguz S., Downward J., Seckl M. J. (2002) Fibroblast growth factor-2 induces translational regulation of Bcl-xL and Bcl-2 via a MEK-dependent pathway: correlation with resistance to etoposide-induced apoptosis. J. Biol. Chem. 277, 12040–12046 [DOI] [PubMed] [Google Scholar]
- 57. Flacke J. P., Kumar S., Kostin S., Reusch H. P., Ladilov Y. (2009) Acidic preconditioning protects endothelial cells against apoptosis through p38- and Akt-dependent Bcl-xL overexpression. Apoptosis 14, 90–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Willimott S., Wagner S. D. (2010) Post-transcriptional and post-translational regulation of Bcl2. Biochem. Soc. Trans. 38, 1571–1575 [DOI] [PubMed] [Google Scholar]
- 59. Holcik M., Yeh C., Korneluk R. G., Chow T. (2000) Translational up-regulation of X-linked inhibitor of apoptosis (XIAP) increases resistance to radiation induced cell death. Oncogene 19, 4174–4177 [DOI] [PubMed] [Google Scholar]
- 60. Reed J. C., Tsujimoto Y., Epstein S. F., Cuddy M., Slabiak T., Nowell P. C., Croce C. M. (1989) Regulation of Bcl-2 gene expression in lymphoid cell lines containing normal #18 or t(14;18) chromosomes. Oncogene Res. 4, 271–282 [PubMed] [Google Scholar]
- 61. Ishimaru D., Zuraw L., Ramalingam S., Sengupta T. K., Bandyopadhyay S., Reuben A., Fernandes D. J., Spicer E. K. (2010) Mechanism of regulation of Bcl-2 mRNA by nucleolin and A+U-rich element-binding factor 1 (AUF1). J. Biol. Chem. 285, 27182–27191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lapucci A., Lulli M., Amedei A., Papucci L., Witort E., Di Gesualdo F., Bertolini F., Brewer G., Nicolin A., Bevilacqua A., Schiavone N., Morello D., Donnini M., Capaccioli S. (2010) ζ-Crystallin is a bcl-2 mRNA binding protein involved in Bcl-2 overexpression in T-cell acute lymphocytic leukemia. FASEB J. 24, 1852–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sengupta T. K., Bandyopadhyay S., Fernandes D. J., Spicer E. K. (2004) Identification of nucleolin as an AU-rich element binding protein involved in Bcl-2 mRNA stabilization. J. Biol. Chem. 279, 10855–10863 [DOI] [PubMed] [Google Scholar]
- 64. Wensveen F. M., Alves N. L., Derks I. A., Reedquist K. A., Eldering E. (2011) Apoptosis induced by overall metabolic stress converges on the Bcl-2 family proteins Noxa and Mcl-1. Apoptosis 16, 708–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Balmanno K., Cook S. J. (2009) Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 16, 368–377 [DOI] [PubMed] [Google Scholar]
- 66. Fricker M., O'Prey J., Tolkovsky A. M., Ryan K. M. (2010) Phosphorylation of PUMA modulates its apoptotic function by regulating protein stability. Cell Death Dis. 1, e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yu J., Zhang L. (2008) PUMA, a potent killer with or without p53. Oncogene 27, Suppl, 1, S71–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang W., Konopleva M., Burks J. K., Dywer K. C., Schober W. D., Yang J. Y., McQueen T. J., Hung M. C., Andreeff M. (2010) Blockade of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase and murine double minute synergistically induces apoptosis in acute myeloid leukemia via BH3-only proteins PUMA and Bim. Cancer Res. 70, 2424–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang Y. F., Jiang C. C., Kiejda K. A., Gillespie S., Zhang X. D., Hersey P. (2007) Apoptosis induction in human melanoma cells by inhibition of MEK is caspase-independent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin. Cancer Res. 13, 4934–4942 [DOI] [PubMed] [Google Scholar]
- 70. Gerits N., Kostenko S., Shiryaev A., Johannessen M., Moens U. (2008) Relations between the mitogen-activated protein kinase and the cAMP-dependent protein kinase pathways: comradeship and hostility. Cell. Signal. 20, 1592–1607 [DOI] [PubMed] [Google Scholar]
- 71. Shi Y., Feng Y., Kang J., Liu C., Li Z., Li D., Cao W., Qiu J., Guo Z., Bi E., Zang L., Lu C., Zhang J. Z., Pei G. (2007) Critical regulation of CD4+ T cell survival and autoimmunity by β-arrestin 1. Nat. Immunol. 8, 817–824 [DOI] [PubMed] [Google Scholar]
- 72. Laroche-Joubert N., Marsy S., Michelet S., Imbert-Teboul M., Doucet A. (2002) Protein kinase A-independent activation of ERK and H,K-ATPase by cAMP in native kidney cells: role of Epac I. J. Biol. Chem. 277, 18598–18604 [DOI] [PubMed] [Google Scholar]
- 73. Chen A., Dong L., Leffler N. R., Asch A. S., Witte O. N., Yang L. V. (2011) Activation of GPR4 by acidosis increases endothelial cell adhesion through the cAMP/Epac pathway. PLoS One 6, e27586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Radu C. G., Cheng D., Nijagal A., Riedinger M., McLaughlin J., Yang L. V., Johnson J., Witte O. N. (2006) Normal immune development and glucocorticoid-induced thymocyte apoptosis in mice deficient for the T-cell death-associated gene 8 receptor. Mol. Cell. Biol. 26, 668–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Klymenko T., Brandenburg M., Morrow C., Dive C., Makin G. (2011) The novel Bcl-2 inhibitor ABT-737 is more effective in hypoxia and is able to reverse hypoxia-induced drug resistance in neuroblastoma cells. Mol. Cancer Ther. 10, 2373–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.