Background: It has been claimed that the regenerative potential of pancreatic beta cells is lost with advanced age.
Results: We demonstrate compensatory replication of beta cells in very old mice, albeit at low levels.
Conclusion: The potential for compensatory replication above base line is retained in beta cells of old mice.
Significance: We present mouse evidence for retention of regenerative potential in old beta cells.
Keywords: Beta Cell, Diabetes, Glucose Metabolism, Islet, Regeneration
Abstract
Recent studies suggested that in old mice, beta cells lose their regenerative potential and cannot respond to mitogenic triggers. These studies examined beta cell replication in aged mice under basal conditions and in response to specific stimuli including treatment with the glucagon-like peptide-1 analog exenatide, streptozotocin injection, partial pancreatectomy, and high fat diet. However, it remains possible that the ability to mount a compensatory response of beta cells is retained in old age, but depends on the specific stimulus. Here, we asked whether partial ablation of beta cells in transgenic mice, using doxycycline-inducible expression of diphtheria toxin, triggers a significant compensatory proliferative response in 1–2-year-old animals. Consistent with previous reports, the basal rate of beta cell replication declines dramatically with age, averaging 0.1% in 2-year-old mice. Transient expression of diphtheria toxin in beta cells of old mice resulted in impaired glucose homeostasis and disruption of islet architecture (ratio of beta to alpha cells). Strikingly, the replication rate of surviving beta cells increased 3-fold over basal rate, similarly to the -fold increase in replication rate of beta cells in young transgenic mice. Islet architecture and glucose tolerance slowly normalized, indicating functional significance of compensatory beta cell replication in this setting. Finally, administration of a small molecule glucokinase activator to old mice doubled the frequency of beta cell replication, further showing that old beta cells can respond to the mitogenic trigger of enhanced glycolysis. We conclude that the potential for functionally significant compensatory proliferation of beta cells is retained in old mice, despite a decline in basal replication rate.
Introduction
Regenerative therapy for diabetes will depend on the ability to enhance beta cell mass, either in patients or in isolated islets, hence it is critical to understand the dynamics and determinants of beta cell turnover. It has become clear that infrequent replication of differentiated beta cells is the major mechanism accounting for the maintenance of beta cell mass during healthy postnatal life in mice (1–3) and humans (4) (hereafter called “basal replication”). Moreover, we and others have shown that under certain injury or stress conditions in mice, the rate of beta cell replication increases and facilitates the regeneration of normal beta cell mass and recovery from diabetes (5, 6) (hereafter called “compensatory replication”). Both basal and compensatory replication appear to be driven to a large extent by the rate of glucose metabolism in beta cells (7). These findings point to basal and compensatory beta cell replication as key targets for regenerative strategies in diabetes. However, this view is complicated by the fact that the basal rate of beta cell replication declines dramatically with age, in both rodents (8, 9) and humans (10–12). The molecular basis for age-related decline in beta cell replication is under intense investigation, with evidence for enhanced expression of cyclin kinase inhibitors due to Polycomb-related chromatin remodeling (13, 14) and a role for p38/MAPK (15) and PDGF receptor signaling (16). Regardless of the mechanism of age-related decline, it has been suggested that studies of beta cell replication in mice, typically done on young animals (5–6 weeks of age) overestimate the significance of this process for human diabetes. To realistically model adult human beta cell dynamics in mice, it was proposed that one has to study very old animals, aged 18 months or older, where basal beta cell replication is extremely low. More recently, two studies have examined compensatory beta cell replication in old mice (17, 18). Using partial pancreatectomy, high fat diet, or administration of either exenatide or streptozotocin as triggers for replication, these studies reported that compensatory replication, and hence the regenerative capacity of beta cells, is severely restricted with age. If validated and shown to be general, this view questions the prospects for development of regenerative therapies for diabetes in adult humans.
We have previously developed a transgenic mouse system for conditional ablation of beta cells, using doxycycline-induced expression of diphtheria toxin in beta cells of transgenic Insulin-rtTA;TET-DTA3 mice (5). Following administration of doxycycline to mice, approximately 80% of beta cells are eliminated, and animals become severely diabetic. Surprisingly, we found that drug withdrawal led to a slow but consistent recovery of normoglycemia. The underlying mechanism was accelerated replication of surviving beta cells, due to increased glucose level causing increased glycolysis in beta cells (7). Our initial studies were carried out using 5-week-old mice and therefore were not informative regarding the potential for beta cell regeneration in old mice. Here, we report on compensatory replication of beta cells following genetic ablation in very old mice. We find that although the basal rate of beta cell replication drops with age as described previously, injury triggers a ∼3-fold increase in the fraction of replicating beta cells, regardless of age, which suffices for recovery of normoglycemia and proper islet architecture even in old mice. Thus, compensatory replication of beta cells is a function of the basal rate of replication and is not directly affected by age.
EXPERIMENTAL PROCEDURES
Mice
Genotyping, doxycycline administration of Insulin-rtTA;TET-DTA mice, and glucose tolerance tests were as described previously (5). BrdU (Sigma-Aldrich) was injected intraperitoneally (100 mg/kg) every 24 h, starting 3 days before sacrificing, three injections in total. Glucokinase activator (19) was diluted in saline containing 20% dimethyl sulfoxide and 1% Tween 80 and injected intraperitoneally at 50 mg/kg body weight, 16 h before sacrifice. The joint ethics committee (IACUC) of the Hebrew University and Hadassah Medical Center approved the study protocol for animal welfare. The Hebrew University is an AAALAC International accredited institute.
Immunostaining
Pancreas was fixed with 4% buffered formaldehyde for 4 h. Paraffin sections (5 μm thick) were rehydrated, and antigen retrieval was performed using a Biocare pressure cooker and citrate buffer. The following primary antibodies were used: guinea pig anti-insulin (1:200; DAKO), mouse anti-glucagon (1:800; Beta Cell Biology Consortium), rabbit anti-Glut2 (1:300; Millipore), rabbit anti-Ki67 (1:200; Neo markers), rabbit anti-pHH3 (1:100; Cell Signaling), and mouse anti-BrdU (1:300; Amersham Biosciences kit). For DNA counterstain we used either DAPI (Sigma) or Toto3 (1:1,000; Molecular Probes). Secondary antibodies were from Jackson ImmunoResearch: anti-guinea pig Cy2 (1:200), anti-mouse Cy3 (1:500), anti-rabbit Cy5 (1:500). Immunofluorescence images were captured using a Nikon C1 or Olympus FV1000 confocal microscope. For replication analysis at least 30 islets or 1,500 beta cells were counted per 1-month-old animal and 50 islets or 8,000–10,000 cells in old animals.
Analysis
To determine the alpha to beta cell ratio, slices of pancreas were stained for insulin and glucagon. At least 20 islets (all islets in the slice) were photographed using the same magnification. Threshold values were set to eliminate background using the NIS Elements program and were applied equally across all slides. The alpha to beta ratio was determined using NIS Elements software by calculating the area occupied by glucagon (after thresholding) and dividing it by the area occupied by insulin (after thresholding). Values are given as percent area. Beta cell mass analysis was done as described previously (5). Statistical analyses were performed using a two-tailed Student's t test. A p value < 0.05 was considered significant. Data are presented as mean ± S.E.
RESULTS
Beta Cell Ablation in Old Mice
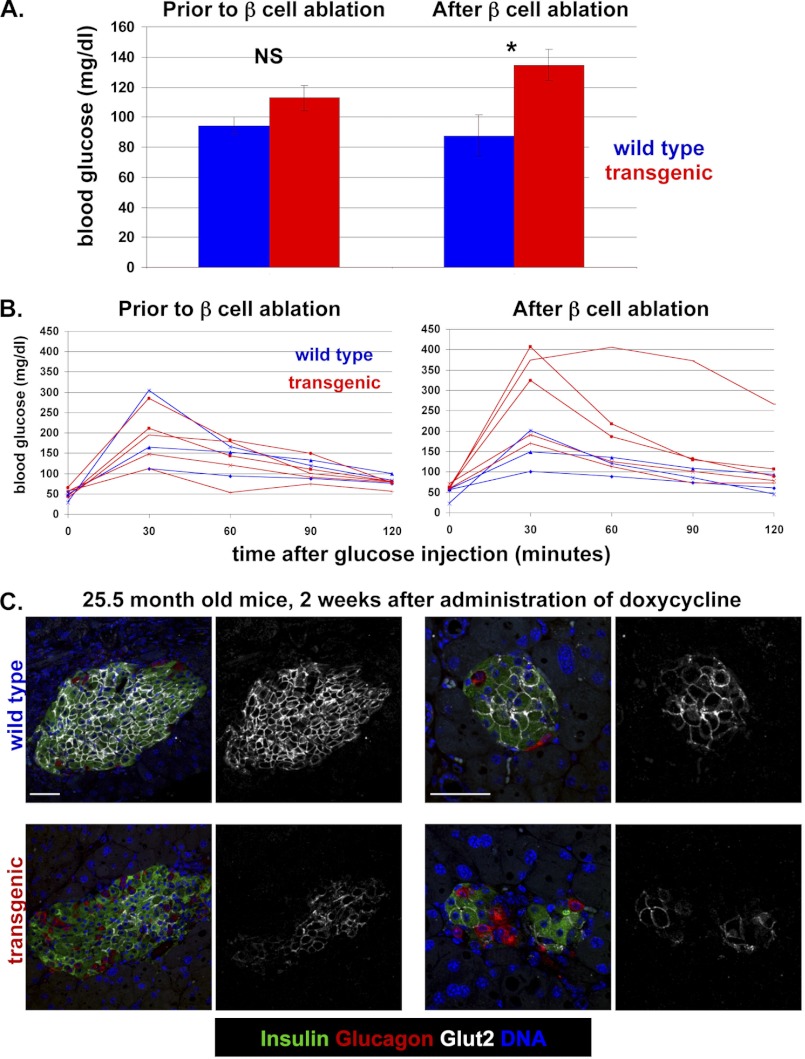
To characterize the dynamics of beta cell ablation in old mice, we prepared a cohort of transgenic mice aged 1–2 years, on ICR background. In the absence of doxycycline, 25-month-old mice had normal body weight (data not shown), normal fed and fasted blood glucose levels, and normal glucose tolerance, indicating that expression of the diphtheria toxin transgene is tightly controlled (Fig. 1, A and B). Administration of doxycycline in the drinking water (0.4 mg/ml) led to a mild but significant elevation of fed and fasted blood glucose levels compared with wild-type littermates and caused impaired glucose tolerance (Fig. 1, A and B, and supplemental Fig. 1). Histological examination revealed the typical alterations in islet architecture seen in younger mice following beta cell ablation (5). Abundant glucagon-expressing alpha cells were seen in islets, often in atypical locations at the center of islets. In addition, the expression of Glut2 was greatly reduced in surviving beta cells, reflecting their chronic exposure to high glucose levels (Fig. 1C). The phenotypic consequences of beta cell ablation in old mice were consistently less severe than in young mice, due to a smaller fraction of beta cells that expressed diphtheria toxin and died. The reason for this diminished expressivity of the transgene in old age is not known. Notwithstanding the reduced efficiency of cell killing, these results show that the Insulin-rtTA;TET-DTA beta cell ablation system functions sufficiently well in old mice to disrupt glucose homeostasis.
FIGURE 1.
Characterization of beta cell ablation in 25.5-month-old Insulin-rtTA;TET-DTA transgenic mice. Mice were treated with doxycycline for 2 weeks before sacrifice. In all panels, control mice were single-transgenic littermates. A, blood glucose levels in the fed state before and after administration of doxycycline for 2 weeks. n = 3 wild-type mice, 5 transgenic mice. B, glucose tolerance before (left) and after (right) administration of doxycycline. Assay was performed after 12 days of doxycycline administration, 2 days prior to sacrifice. C, islet morphology after doxycycline administration. Images are of islets from wild-type and transgenic mice. For each genotype two images are shown in two different magnifications. Transgenic mice show the hallmarks of beta cell ablation observed in young mice: abundant and mislocalized alpha cells, and reduced levels of Glut2 in surviving beta cells. Scale bars, 50 μm. In each pair of images, the left panel is a composite of insulin, glucagon, Glut2, and DNA staining, and the right panel shows only the Glut2 channel.
Age Dependence of Basal and Compensatory Beta Cell Replication
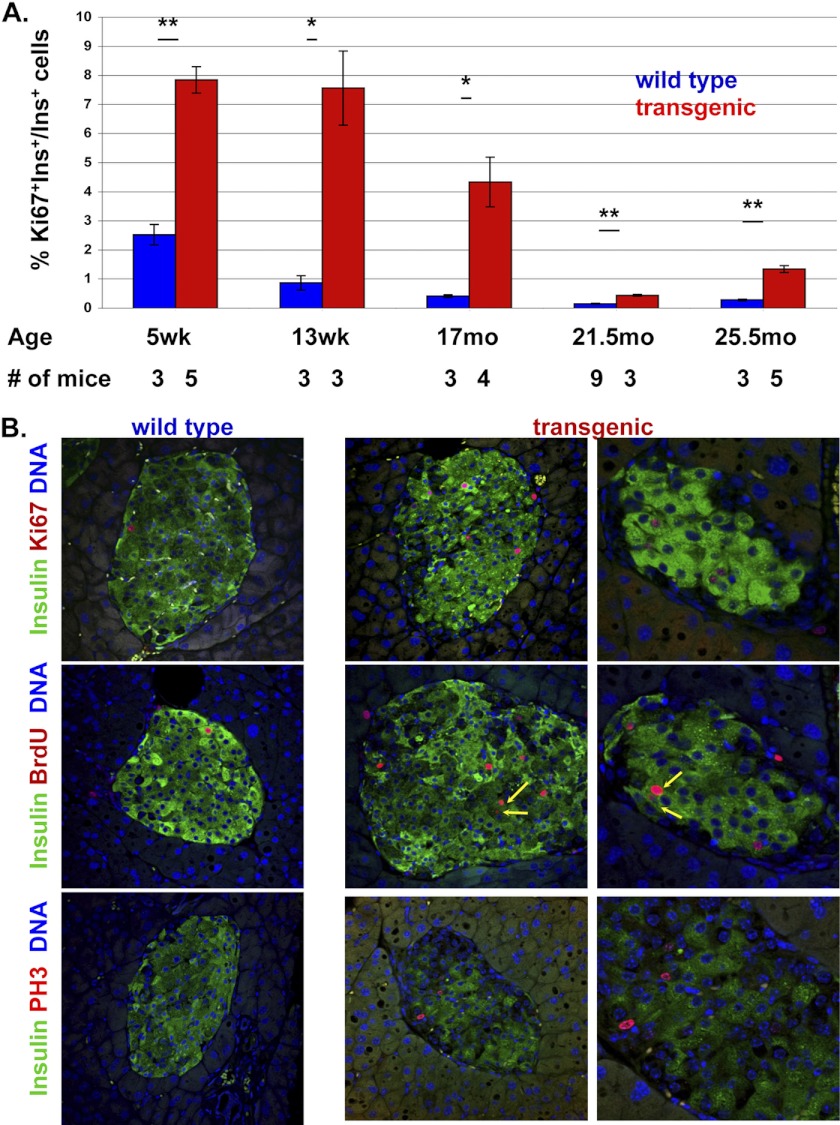
To examine how age affects beta cell replication in normal mice and in diabetic mice after beta cell ablation, we determined the fraction of Ki67+ cells in transgenic mice and control littermates of different ages, following treatment with doxycycline. As shown in Fig. 2A, the basal rate of beta cell replication (in control animals) gradually declined with age, from 2.5% Ki67+ beta cells in 5-week-old mice to ∼0.2% in 25-month-old mice. These results are in agreement with previous studies (8, 9) and reflect the age-dependent decline of beta cell replication. The fraction of Ki67+ beta cells in Insulin-rtTA; TET-DTA mice also declined with age. In 5-week-old mice, beta cell ablation caused the replication of ∼7.5% of beta cells that survived ablation. In 25-month-old mice, ∼1% of surviving beta cells in such mice were Ki67+. Thus, both basal and compensatory beta cell replication rates decline with age. However, at any given age the fraction of replicating beta cells following injury was 2–4-fold higher than the basal rate of replication. In other words, the ability to mount a compensatory proliferative response, as defined by -fold increase over basal, is age-independent. We note that due to the difficulty in obtaining very old animals, each age group contains a relatively small number of mice. However, in total we have analyzed 17 transgenic and 18 wild-type mice aged 15 months or older, which together provide strong support to the view that the potential for compensatory proliferation is retained in old age (Fig. 2 and supplemental Table 1).
FIGURE 2.
Basal and compensatory beta cell replication as a function of age. A, quantification of beta cell replication rate in transgenic Insulin-rtTA;TET-DTA and control littermates after administration of doxycycline at different ages. Values are mean ± SE. *, p < 0.05; **, p < 0.01; NS, not significant. B, representative images of beta cell replication in 25-month-old mice using different replication markers, co-stained with insulin. Mice were injected with BrdU for 3 days prior to sacrifice. Arrows point to doublets of BrdU+ beta cells in DTA mice, suggesting productive mitosis. Original magnification, ×400 in left and middle panels, ×800 in right panels. See supplemental Table 1 for detailed information on the mice analyzed.
To confirm that Ki67 staining reflected true beta cell replication, we injected mice with the thymidine analog BrdU (once per day in the last 3 days before sacrifice) and stained sections for BrdU. In addition, we stained for the mitotic marker phosphohistone H3. As shown in Fig. 2B, multiple beta cells in 25-month-old transgenic mice were stained for Ki67, phosphohistone H3, and BrdU, supporting the view that these cells were undergoing normal DNA synthesis and cell division. These results show that whereas basal beta cell replication drops with age, the potential to triple replication rate in response to injury remains intact.
Functional and Morphologic Evidence for Regeneration of Beta Cells
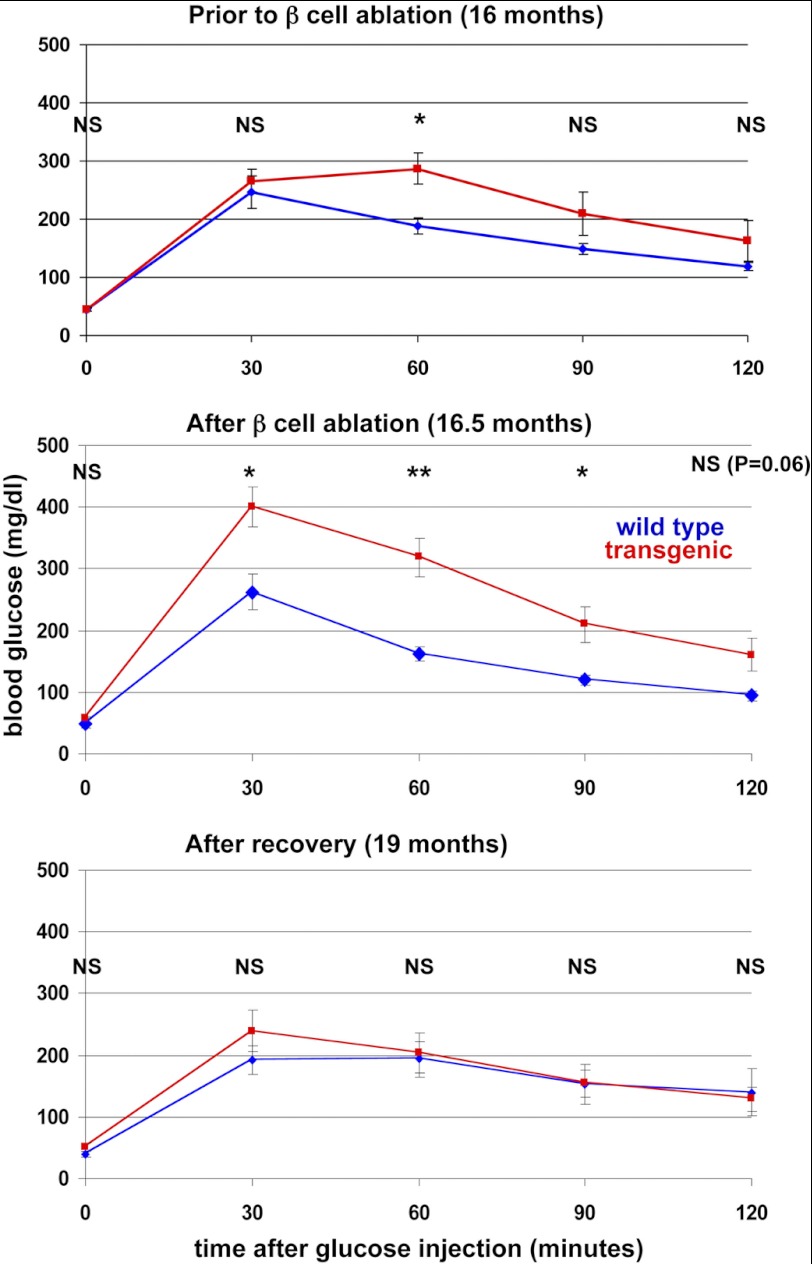
The observed compensatory replication in old mice could in principle lead to regeneration of beta cell function, as with young mice following a pulse of beta cell ablation. Alternatively, the absolute low rate of compensatory beta cell proliferation in old mice could be of no functional significance. To distinguish between these possibilities, we monitored glucose tolerance in a cohort of 16-month-old transgenic mice for 3 months after beta cell ablation. As shown in Fig. 3, administration of doxycycline led to a clear impairment of glucose tolerance in transgenic but not control littermates. Strikingly, 3 months after doxycycline withdrawal, glucose tolerance normalized and was indistinguishable between control and transgenic littermates. These results show that old mice can regain control of blood glucose after beta cell ablation and strongly suggest that the observed compensatory replication of beta cells in old age has functional significance.
FIGURE 3.
Impairment of glucose tolerance after beta cell ablation in 16-month-old mice and normalization 3 months after doxycycline withdrawal. n = 7 wild-type and 5 transgenic mice prior to and after ablation; 6 wild-type and 4 transgenic mice after recovery. Values are mean ± SE. *, p < 0.05; **, p < 0.01; NS, not significant.
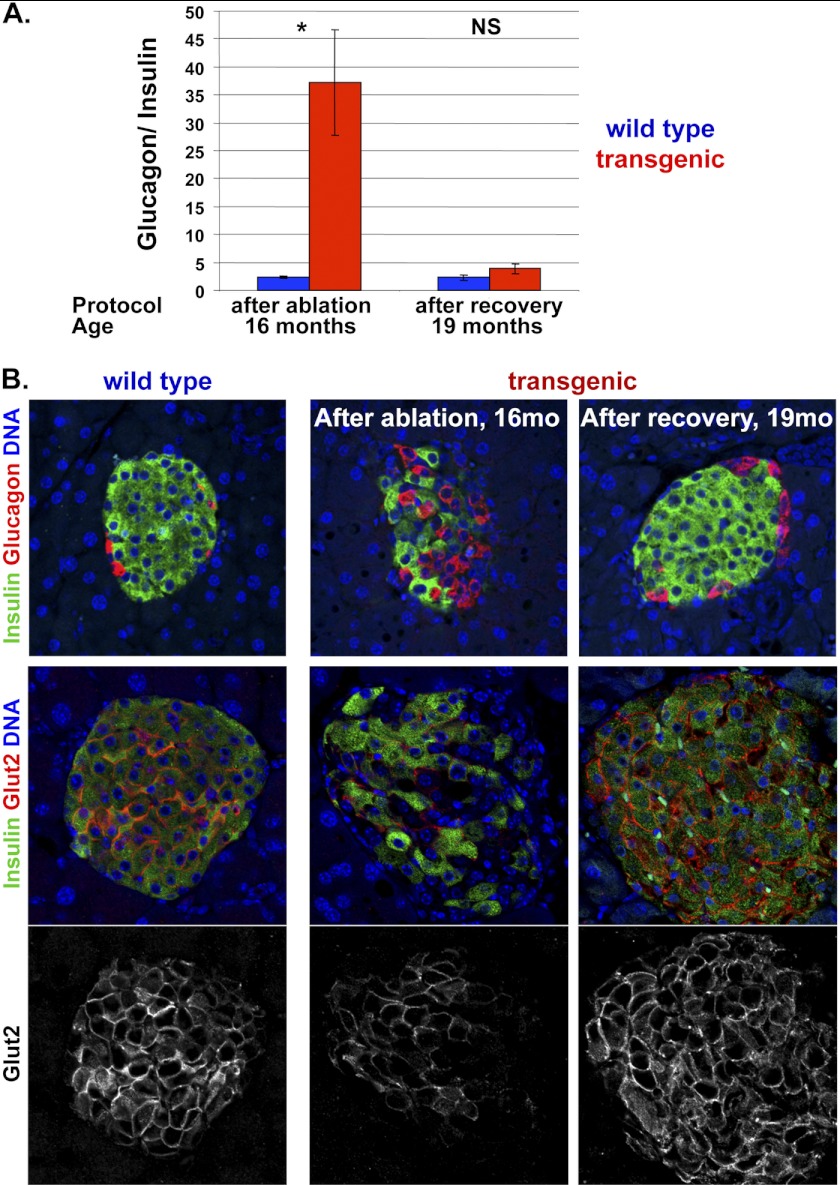
To further study the impact of increased beta cell replication, we examined islet morphology. Morphometric measurements of total beta cell mass proved highly variable in old mice, such that the natural variation in beta cell mass in normal old mice exceeded the extent of beta cell ablation (data not shown). As an alternative, we examined the ratio of alpha cells to beta cells within individual mice. In normal mice aged 16 months, there were about 30 times more beta cells than alpha cells in islets. This ratio was reverted after beta cell ablation. Strikingly, 3 months after doxycycline withdrawal the alpha to beta cell ratio normalized, providing a quantitative evidence for normalization of islet morphology (Fig. 4A). Fig. 4B provides a visual demonstration of these results, highlighting in addition the localization of each cell type within islets. Whereas in normal islets beta cells are located at the center and alpha cells are organized in the periphery, beta cell ablation led to a disruption of this architecture and to the present of numerous alpha cells at the center of islets. Strikingly, doxycycline withdrawal in 16-month-old mice led to restoration of normal islet architecture (Fig. 4B), as reported previously for beta cell regeneration in young mice (5).
FIGURE 4.
Recovery following beta cell ablation in old age. A, quantification of alpha to beta ratio in islets of transgenic Insulin-rtTA;TET-DTA and control littermates after administration of doxycycline for 2 weeks (starting at 16 months of age) and upon 3 months of recovery. n = 3 wild-type, 4 transgenic mice after ablation; n = 4 wild-type, 4 transgenic mice after recovery. Values are mean ± SE. *, p < 0.05; NS, not significant. B, representative images of islets co-stained for insulin (green) and glucagon (red) in 16-month-old mice after beta cell ablation for 2 weeks and in 19-month-old mice after 3 months of recovery.
Glycolysis Triggers Beta Cell Replication in Old Mice
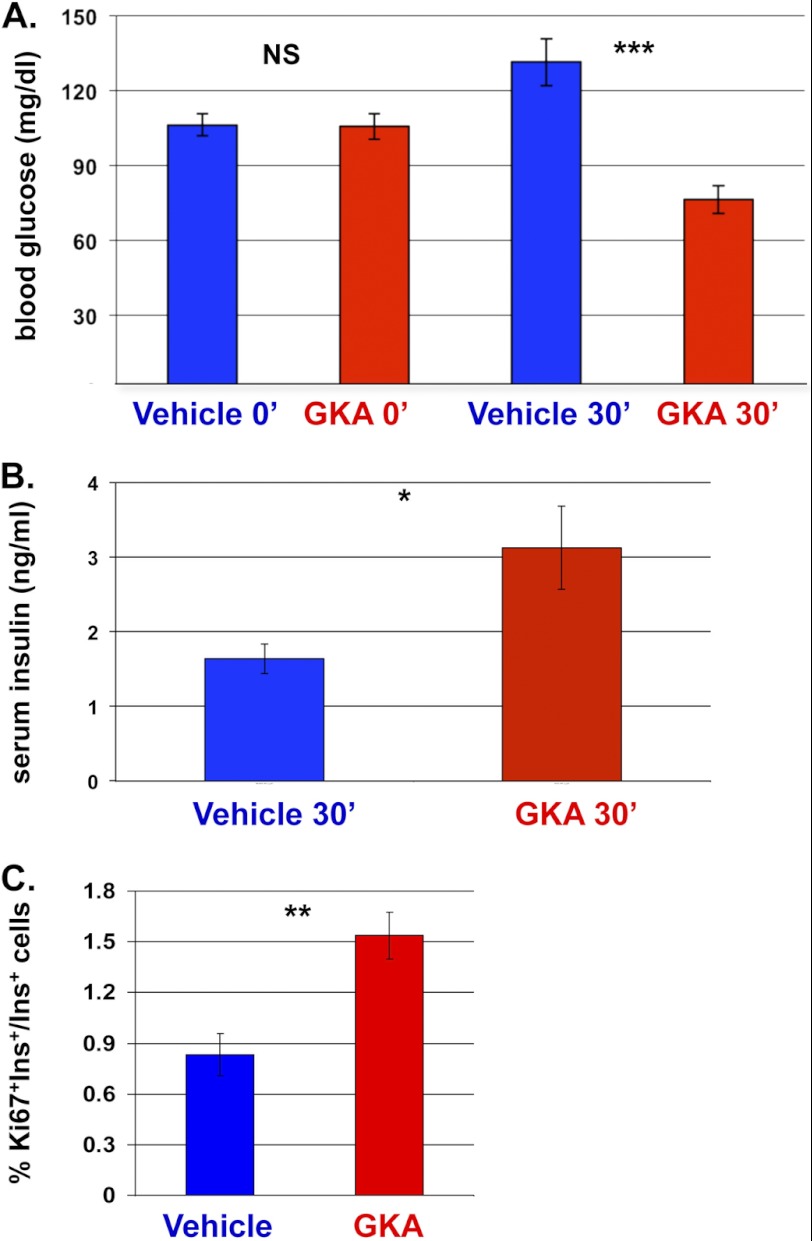
In a parallel work, we have studied the molecular basis for beta cell regeneration in young mice and found that the key driver for compensatory beta cell replication in the DTA system is blood glucose, acting via the rate of glucose metabolism in beta cells (7). Administration of a small molecule Glucokinase activator (GKA) mimics the effect of high blood glucose and boosts both insulin secretion and beta cell replication. To test whether increased glucose metabolism can enhance similarly the rate of beta cell replication in old mice, we treated 6-month-old mice with GKA. As expected, this led to a transient decrease in blood glucose levels, due to enhanced insulin secretion (Fig. 5, A and B). As seen with young mice, the fraction of replicating beta cells in these mice doubled, showing that beta cells in old age retain the ability to increase replication in response to the physiological trigger of enhanced glucose metabolism (Fig. 5C). Taken together, the results show that in old mice, beta cells retain the capacity to enter the cell division cycle in response to physiological triggers, most notably increased blood glucose levels and increased glycolysis. The magnitude of this response appears to be limited by the gradual decline of basal replication rate, but our results suggest that this low replication rate does have functional significance in normalizing glycemia and islet architecture.
FIGURE 5.
Mitogenic response of beta cells to GKA in 6-month-old wild-type mice. A, blood glucose levels, prior to and 30 min after GKA or vehicle administration. n = 12 vehicle-injected mice, 6 GKA-injected mice. B, serum insulin levels, 30 min after administration of GKA or vehicle. n = 6 mice per group. C, quantification of the fraction of replicating beta cells, 16 h following GKA or vehicle injection. n = 5 vehicle-injected mice, 4 GKA-injected mice. Values are mean ± SE. *, p < 0.05; **, p < 0.01; ***, p < 0.001; NS, not significant.
DISCUSSION
We show here that injury triggers compensatory replication of beta cells even in very old mice, up to an age representing two-thirds the life span of a mouse. Moreover, aged mice retain the ability to spontaneously recover glucose homeostasis and to normalize islet architecture following beta cell ablation, likely as a consequence of continuous enhanced beta cell replication. Treatment with GKA supports the idea that old beta cells remain sensitive to glucose-induced replication, the central determinant of beta cell mass homeostasis. Thus, the fundamental potential of beta cells for regeneration and functional recovery remains largely intact in old age.
These results seem at odds with two recent studies that reported on severe age-dependent restriction of compensatory, or adaptive, beta cell proliferation with advanced age in mice (17, 18). These studies examined the adaptive response to a number of challenges: high fat diet, partial pancreatectomy, injection of the beta cell-selective toxin streptozotocin, and injection of the glucagon-like peptide-1-mimetic exenatide. In all cases, young mice (aged ∼2 months) presented with a robust proliferative response of beta cells to challenge, whereas old mice (aged 8–19 months; notably younger than the oldest mice used in our studies) failed to mount such a response. The reasons for the different results are not known, but we speculate that selection of the specific mode of beta cell injury is crucial. In each of the models used in these studies, additional factors may have masked the inherent regenerative potential of beta cells. For example, streptozotocin is a DNA-alkylating agent that probably damages all beta cells, including those that survive killing; diminished DNA repair activity with old age (20) could thus preclude compensatory beta cell replication in old streptozotocin-treated mice. Likewise, high fat diet challenge involves peripheral insulin resistance, inflammation, and glucolipotoxicity (21); age-dependent diminution in coping with these stresses could negatively affect adaptive beta cell replication. By contrast, expression of diphtheria toxin has a binary mode of action: because a single molecule of the toxin is sufficient to kill a cell (22), beta cells in this system are either killed or remain completely intact, in a normal metabolic environment. Consequently, compensatory beta cell replication is triggered in the DTA system mostly via increased glucose metabolism (7). This view is supported by our finding that GKA can trigger beta cell replication in old mice and is consistent with recent papers that reported considerable beta cell replication in islets from old mice (23) or humans (24) grafted into hyperglycemic mouse recipients. The setting of DTA-mediated beta cell ablation likely provides optimal conditions for compensatory beta cell replication, exposing the regenerative potential that remains through old age. Obviously, metabolic derangements and genetic defects in the cell cycle machinery are the rule rather than the exception in human diabetes and could explain why spontaneous recovery from diabetes is not seen in old (as well as young) human patients. Nonetheless, the current study defines a “ground state” of regenerative potential of beta cells and suggests a positive outlook for addressing the adverse factors precluding human beta cell regeneration.
Our results expose a previously ignored disconnect between the determinants of basal and compensatory beta cell replication. Basal replication declines with age, as shown here and elsewhere, likely reflecting the combinatorial effects of changes in signal transduction pathways, chromatin remodeling, and additional factors. By contrast, the capacity for adaptive/compensatory replication (likely driven by enhanced glycolysis), defined as the -fold change of replication rate beyond basal levels, appears to be age-independent. We propose that the diminished absolute magnitude of adaptive beta cell replication in old mice reflects the reduced pool of beta cells that are replication-competent. Despite this limitation, increased beta cell replication in old mice does have functional consequences, as suggested by the slow but robust recovery of glycemic control as well as normalization of islet architecture in very old Insulin-rtTA;TET-DTA mice. We speculate that this distinction between basal and compensatory replication applies to age-related decline of tissue regeneration in other organs as well.
Why do so few beta cells in very old mice replicate, in the basal state and in response to injury? One possibility is that beta cells are intrinsically heterogeneous in their replicative capacity. For example, some beta cells could have been born recently from progenitor cells (neogenesis) (25, 26) or other types of nonbeta cells (27) and thus might be endowed with a higher capacity for replication compared with their “old” neighbors. Although we cannot exclude this possibility, we note that most evidence so far suggests that under normal conditions (1), as well during regeneration in the TET-DTA system (5), beta cells derive from beta cells, not from any type of progenitor. Moreover, there is ample evidence that all beta cells in adult mice have a similar likelihood to replicate (2, 28). The mechanism that underlies the gradual decrease in basal and compensatory beta cell replication with age is under intense investigation (13–16) and represents a major future challenge.
In summary, we show that beta cells in old mice retain a potential to mount a functionally significant compensatory proliferation in vivo. Further study is required to determine the regenerative potential of adult human beta cells, particularly in the context of adverse genetic and environmental conditions in diabetes.
Supplementary Material
Acknowledgment
We thank Jonatan Enk for assistance.
This work was supported, in whole or in part, by the National Institutes of Health Beta Cell Biology Consortium. This work was also supported by grants from Juvenile Diabetes Research Foundation, the Helmsley Foundation, and the Dutch friends of Hebrew University, the European Union Seventh Framework Programme Grant 241883 and European Research Council, the DON Foundation, the I-CORE Program of the Planning and Budgeting Committee, and The Israel Science Foundation Grant 41.11 (to Y. D.).

This article contains supplemental Fig. 1 and Table 1.
- TET-DTA
- tetracycline-diphtheria toxin A
- GKA
- glucokinase activator.
REFERENCES
- 1. Dor Y., Brown J., Martinez O. I., Melton D. A. (2004) Adult pancreatic beta cells are formed by self-duplication rather than stem cell differentiation. Nature 429, 41–46 [DOI] [PubMed] [Google Scholar]
- 2. Teta M., Rankin M. M., Long S. Y., Stein G. M., Kushner J. A. (2007) Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev. Cell 12, 817–826 [DOI] [PubMed] [Google Scholar]
- 3. Georgia S., Bhushan A. (2004) Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J. Clin. Invest. 114, 963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meier J. J., Butler A. E., Saisho Y., Monchamp T., Galasso R., Bhushan A., Rizza R. A., Butler P. C. (2008) Beta cell replication is the primary mechanism subserving the postnatal expansion of beta cell mass in humans. Diabetes 57, 1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nir T., Melton D. A., Dor Y. (2007) Recovery from diabetes in mice by beta cell regeneration. J. Clin. Invest. 117, 2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cano D. A., Rulifson I. C., Heiser P. W., Swigart L. B., Pelengaris S., German M., Evan G. I., Bluestone J. A., Hebrok M. (2008) Regulated beta cell regeneration in the adult mouse pancreas. Diabetes 57, 958–966 [DOI] [PubMed] [Google Scholar]
- 7. Porat S., Weinberg-Corem N., Tornovsky-Babaey S., Schyr-Ben-Haroush R., Hija A., Stolovich-Rain M., Dadon D., Granot Z., Ben-Hur V., White P., Girard C. A., Karni R., Kaestner K. H., Ashcroft F. M., Magnuson M. A., Saada A., Grimsby J., Glaser B., Dor Y. (2011) Control of pancreatic beta cell regeneration by glucose metabolism. Cell Metab. 13, 440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teta M., Long S. Y., Wartschow L. M., Rankin M. M., Kushner J. A. (2005) Very slow turnover of beta cells in aged adult mice. Diabetes 54, 2557–2567 [DOI] [PubMed] [Google Scholar]
- 9. Finegood D. T., Scaglia L., Bonner-Weir S. (1995) Dynamics of beta cell mass in the growing rat pancreas: estimation with a simple mathematical model. Diabetes 44, 249–256 [DOI] [PubMed] [Google Scholar]
- 10. Kassem S. A., Ariel I., Thornton P. S., Scheimberg I., Glaser B. (2000) Beta cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 49, 1325–1333 [DOI] [PubMed] [Google Scholar]
- 11. Perl S., Kushner J. A., Buchholz B. A., Meeker A. K., Stein G. M., Hsieh M., Kirby M., Pechhold S., Liu E. H., Harlan D. M., Tisdale J. F. (2010) Significant human beta cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J. Clin. Endocrinol. Metab. 95, E234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Clercq L., Delaere P., Remacle C. (1988) The aging of the endocrine pancreas of the rat. I. Parameters of cell proliferation. Mech. Ageing Dev. 43, 11–24 [DOI] [PubMed] [Google Scholar]
- 13. Chen H., Gu X., Su I. H., Bottino R., Contreras J. L., Tarakhovsky A., Kim S. K. (2009) Polycomb protein Ezh2 regulates pancreatic beta cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev. 23, 975–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dhawan S., Tschen S. I., Bhushan A. (2009) Bmi-1 regulates the Ink4a/Arf locus to control pancreatic beta cell proliferation. Genes Dev. 23, 906–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong E. S., Le Guezennec X., Demidov O. N., Marshall N. T., Wang S. T., Krishnamurthy J., Sharpless N. E., Dunn N. R., Bulavin D. V. (2009) p38MAPK controls expression of multiple cell cycle inhibitors and islet proliferation with advancing age. Dev. Cell 17, 142–149 [DOI] [PubMed] [Google Scholar]
- 16. Chen H., Gu X., Liu Y., Wang J., Wirt S. E., Bottino R., Schorle H., Sage J., Kim S. K. (2011) PDGF signalling controls age-dependent proliferation in pancreatic beta cells. Nature 478, 349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rankin M. M., Kushner J. A. (2009) Adaptive beta cell proliferation is severely restricted with advanced age. Diabetes 58, 1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tschen S. I., Dhawan S., Gurlo T., Bhushan A. (2009) Age-dependent decline in beta cell proliferation restricts the capacity of beta cell regeneration in mice. Diabetes 58, 1312–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grimsby J., Sarabu R., Corbett W. L., Haynes N. E., Bizzarro F. T., Coffey J. W., Guertin K. R., Hilliard D. W., Kester R. F., Mahaney P. E., Marcus L., Qi L., Spence C. L., Tengi J., Magnuson M. A., Chu C. A., Dvorozniak M. T., Matschinsky F. M., Grippo J. F. (2003) Allosteric activators of glucokinase: potential role in diabetes therapy. Science 301, 370–373 [DOI] [PubMed] [Google Scholar]
- 20. Gorbunova V., Seluanov A., Mao Z., Hine C. (2007) Changes in DNA repair during aging. Nucleic Acids Res. 35, 7466–7474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cai D., Yuan M., Frantz D. F., Melendez P. A., Hansen L., Lee J., Shoelson S. E. (2005) Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 11, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamaizumi M., Mekada E., Uchida T., Okada Y. (1978) One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell 15, 245–250 [DOI] [PubMed] [Google Scholar]
- 23. Chen X., Zhang X., Chen F., Larson C. S., Wang L. J., Kaufman D. B. (2009) Comparative study of regenerative potential of beta cells from young and aged donor mice using a novel islet transplantation model. Transplantation 88, 496–503 [DOI] [PubMed] [Google Scholar]
- 24. Levitt H. E., Cyphert T. J., Pascoe J. L., Hollern D. A., Abraham N., Lundell R. J., Rosa T., Romano L. C., Zou B., O'Donnell C. P., Stewart A. F., Garcia-Ocaña A., Alonso L. C. (2011) Glucose stimulates human beta cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia 54, 572–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu X., D'Hoker J., Stangé G., Bonné S., De Leu N., Xiao X., Van de Casteele M., Mellitzer G., Ling Z., Pipeleers D., Bouwens L., Scharfmann R., Gradwohl G., Heimberg H. (2008) Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132, 197–207 [DOI] [PubMed] [Google Scholar]
- 26. Inada A., Nienaber C., Katsuta H., Fujitani Y., Levine J., Morita R., Sharma A., Bonner-Weir S. (2008) Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc. Natl. Acad. Sci. U.S.A. 105, 19915–19919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thorel F., Népote V., Avril I., Kohno K., Desgraz R., Chera S., Herrera P. L. (2010) Conversion of adult pancreatic alpha cells to beta cells after extreme beta cell loss. Nature 464, 1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brennand K., Huangfu D., Melton D. (2007) All beta cells contribute equally to islet growth and maintenance. PLoS Biol. 5, e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.