Background: The rate-limiting step of cardiac muscle relaxation is not completely understood.
Results: We were able to measure two proposed rate-limiting steps of relaxation in ventricular myofibrils.
Conclusion: The rate of Ca2+ dissociation from troponin C may be rate-limiting during myofilament inactivation under physiological conditions.
Significance: Strategies that target both troponin C and myosin will be needed to treat diastolic dysfunction.
Keywords: ADP, Calcium, Calcium-binding Proteins, Cardiac Muscle, Kinetics, Myosin, Rate-limiting, Relaxation
Abstract
The rate-limiting step of cardiac muscle relaxation has been proposed to reside in the myofilament. Both the rates of cross-bridge detachment and Ca2+ dissociation from troponin C (TnC) have been hypothesized to rate-limit myofilament inactivation. In this study we used a fluorescent TnC to measure both the rate of Ca2+ dissociation from TnC and the rate of cross-bridge detachment from several different species of ventricular myofibrils. The fluorescently labeled TnC was sensitive to both Ca2+ dissociation and cross-bridge detachment at low Ca2+ (presence of EGTA), allowing for a direct comparison between the two proposed rates of myofilament inactivation. Unlike Ca2+ dissociation from TnC, cross-bridge detachment varied in myofibrils from different species and was rate-limited by ADP release. At subphysiological temperatures (<20 °C), the rate of Ca2+ dissociation from TnC was faster than the rate of cross-bridge detachment in the presence of ADP. These results support the hypothesis that cross-bridge detachment rate-limits relaxation. However, Ca2+ dissociation from TnC was not as temperature-sensitive as cross-bridge detachment. At a near physiological temperature (35 °C) and ADP, the rate of cross-bridge detachment may actually be faster than the rate of Ca2+ dissociation. This provides evidence that there may not be a simple, single rate-limiting step of myofilament inactivation.
Introduction
Cardiac function is dynamically regulated to meet the demands of the human body. However, in many cardiovascular diseases, relaxation becomes impaired, which can lead to diastolic dysfunction of the heart (1, 2). A more complete understanding of the molecular mechanisms that govern cardiac muscle relaxation will help to develop better treatment strategies for diastolic dysfunction. Biochemically, there are two main factors thought to control the rate of relaxation; the decline of the intracellular Ca2+ transient and the inactivation of the myofilament (3, 4). Although the removal of intracellular Ca2+ is a vital component to relaxation, it would appear that myofilament inactivation is equally important if not rate-limiting (4, 5). For the myofilament to relax, Ca2+ must dissociate from troponin C (TnC)2 to inactivate the thin filament and cross-bridges must detach from actin to alleviate the force (6). It is generally thought that the rate of cross-bridge detachment is substantially slower than the rate of Ca2+ dissociation from TnC and thus rate-limits myofilament inactivation (7, 8).
ATP binding to the actomyosin complex detaches myosin from actin. However, this ATP cannot complete the cross-bridge cycle and detach myosin until the previous hydrolysis products of ATP (phosphate (Pi) and ADP) are released (9). It is believed that Pi release is associated with either the transition of myosin from a weakly bound state to a strongly bound state or with the force-producing power stroke (10–12). In cardiac muscle, ADP release is thought to follow the power stroke and must dissociate before another ATP can bind and detach myosin (13, 14). The rate of ADP dissociation from myosin has been proposed to rate-limit cross-bridge detachment and potentially relaxation (15, 16). In support of this idea, increasing concentrations of ADP slowed the rate of cross-bridge detachment from actomyosin (15) and slowed the rate of relaxation of skeletal myofibrils and skinned cardiac muscle (17, 18). Furthermore, the rate of ADP dissociation from actomyosin and the rate of cardiac muscle relaxation were substantially slower when each system utilized β-myosin as compared with α-myosin (19). However, ADP dissociation has only been measured from unregulated actomyosin and was quantitatively one order of magnitude faster than the rate of cardiac muscle relaxation (15, 18, 20, 21). Either the rate of ADP dissociation does not rate-limit cardiac muscle relaxation or the actual rate of ADP dissociation is slower when measured from within the confines of the sarcomere.
The other component of myofilament relaxation is the inactivation of the thin filament, which is controlled by Ca2+ dissociation from TnC. Ca2+ dissociates from isolated TnC at least 2 orders of magnitude faster than the rate of myocardial relaxation (22–24). Because of this fact, Ca2+ dissociation from TnC was deemed an insignificant regulator of relaxation. However, TnC does not function in isolation but as an integral component of the thin filament system. Incorporation of TnC into the thin filament slowed the rate of Ca2+ dissociation by at least one order of magnitude (25). The binding of myosin-S1 to the thin filament further slowed the rate of Ca2+ dissociation from TnC one additional order of magnitude (25). These biochemical studies suggest that the rate of Ca2+ dissociation can be slowed to a rate that could influence the rate of relaxation. A recent study increased the complexity of the biochemical system even further by using ventricular myofibrils (21). This study concluded that the rate of Ca2+ dissociation from TnC in isolated cardiac myofibrils was still too rapid to rate-limit myofibril relaxation at 10 °C. However, the rate of Ca2+ dissociation from cardiac TnC is little affected by temperature (26), whereas cross-bridge function is highly temperature-sensitive (20). The actual rate of Ca2+ dissociation from TnC and the rate of cross-bridge detachment in ventricular myofibrils at physiological temperature are unknown.
To further investigate the rate-limiting steps of myofilament inactivation, we utilized a fluorescent TnC that was able to report the rate of Ca2+ dissociation from TnC and cross-bridge detachment in ventricular myofibrils over a wide range of temperatures. Myofibrils provided a unique biochemical system in which both Ca2+ dissociation and cross-bridge detachment could be measured from within the confines of the regulated sarcomere, which contained the myofilament proteins at a physiologically relevant geometry and stoichiometry.
Cross-bridge detachment in the absence of ADP was always faster than the rate of Ca2+ dissociation from TnC. However, the addition of ADP substantially slowed the rate of cross-bridge detachment across all temperatures. This would suggest that cross-bridge detachment is rate-limited by ADP dissociation. At temperatures below 20 °C, the rate of cross-bridge detachment in the presence of ADP was slower than the rate of Ca2+ dissociation. This would suggest that cross-bridge detachment rate-limits myofilament inactivation and potentially relaxation at cold temperatures. However, the rate of cross-bridge detachment was more sensitive to changes in temperature than was the rate of Ca2+ dissociation. At a more physiological temperature (35 °C) and ADP, the rate of cross-bridge detachment may actually be faster than the rate of Ca2+ dissociation. This provides evidence that there may not be a simple, single rate-limiting step of myofilament inactivation. Thus, Ca2+ dissociation and cross-bridge detachment may both influence the rate of relaxation and may be potential therapeutic targets for improving relaxation across a broad range of cardiomyopathies.
EXPERIMENTAL PROCEDURES
Materials
Phenyl-Sepharose, Tween 20, EGTA, ATP, and ADP were purchased from Sigma. 4-(N-(Iodoacetoxy)ethyl-N-methyl)amino-7-nitrobenz-2-oxa-1,3-diazole (IANBD) was purchased from Invitrogen. N-(3-dimethylaminopropyl)-N-ethyl-carbodiimide hydrochloride and N-hydroxysulfosuccinimide sodium salt were purchased from Fluka Analytical. All other chemicals were of analytical grade.
Protein Purification, Labeling, and Complex Formation
The human cardiac TnC with the C35S, T53C, and C84S mutations (herein designated as TnCT53C) was expressed and purified as previously described (25). The D65A mutation was inserted into the TnCT53C expression vector using techniques previously described (25). The mutations were confirmed by DNA sequence analysis. Expression and purification of D65A TnCT53C was conducted as previously described (27). Human cardiac TnI and TnT (isoform 3) were expressed and purified as previously described (25). TnCT53C was labeled with the environmentally sensitive thiol-reactive fluorescent probe IANBD (∼80% labeling efficiency) following a protocol previously described for IAANS labeling (28). The Tn complexes were reconstituted as previously described (27).
Myofibril Preparation
Ventricular cardiac muscle was obtained from male New Zealand White rabbits (2–3 months old, ∼2 kg in weight (29)), male LBN-F1 rats (175–225 g), heartworm free mongrel dogs (weighing 19.0 ± 0.4 kg; 2–3 years old (29)), and de-identified failing human tissue from ongoing studies with collaborators. All ventricular muscle was dissected in a Krebs-Henseleit solution containing 137 mm NaCl, 5 mm KCl, 1.2 mm MgSO4, 1.2 mm NaH2PO4, 20 mm NaHCO3, 10 mm glucose, 0.25 mm CaCl2, and 20 mm 2,3-butanedione monoxime to prevent contraction and damage to the cut tissue. The ventricular myofibrils were prepared as previously described (30). Briefly, the isolated ventricular tissue was minced with scissors in Krebs-Henseleit solution and then homogenized with 10-s bursts of a Polytron homogenizer. The suspension was filtered through cheesecloth to remove connective tissue and then further Dounce-homogenized. The myofibrils were collected by centrifugation and washed in rigor buffer before resuspension in glycerol rigor buffer. The myofibril stocks were stored at −20 °C. All animals and tissues were handled in accordance with the National Institutes of Health Guidelines and approved by the Institutional Laboratory Animal Care and Use Committee at The Ohio State University. All human tissue was obtained in accordance with a tissue acquisition protocol approved by the Biomedical Sciences Institutional Review Board of The Ohio State University.
Exchange of Labeled Tn into Myofibrils
0.5 ml of myofibril stock (∼20 mg/ml) was resuspended in 1 ml of Buffer A (10 mm MOPS, 150 mm KCl, 3 mm MgCl2, 1 mm DTT, and 0.02% Tween 20 (pH 7.0)). The myofibrils were washed 3 times in Buffer A to remove the cryogenic glycerol solution. Briefly, the myofibrils were pelleted at 2000 × g for 1 min, and the supernatant was removed and resuspended in 1 ml of Buffer A by gently pipetting. After the final centrifugation and removal of the supernatant, the myofibril pellet was resuspended in 1 ml of IANBD-labeled Tn (∼6 μm stock in Buffer A) and stored at 4 °C overnight. After the overnight Tn exchange, the myofibrils were washed three times with Buffer A to remove the un-exchanged Tn. After the last centrifugation, the myofibrils were resuspended in a total volume of 5.0 ml of Buffer A and filtered through 100-μm nylon mesh (Sefar Inc.) to remove any large pieces of myofibrils that could potentially clog the stopped-flow apparatus. For certain experiments the myofibrils were cross-linked for 90 min as previously described (31). Briefly, myofibrils in Buffer A without DTT were cross-linked with 2 mm N-(3-dimethylaminopropyl)-N-ethyl-carbodiimide hydrochloride and 5 mm N-hydroxysulfosuccinimide sodium salt at 4 °C for 90 min, and the reaction was quenched with the addition of 25 mm glycine and 10 mm DTT. The myosin concentration of the myofibrils was determined by weight per volume. An aliquot of myofibrils from the glycerol stock was washed in water three times. The myofibrils were then dried by vacuum centrifugation for 2 h at 3000 × g and weighed. Assuming that the myosin concentration is ∼43% that of the total myofibril protein by weight (32), the myosin head concentration of the stock was determined to be ∼18 μm, with a final concentration after mixing in the stopped-flow of ∼0.8 μm.
Determination of Ca2+ Dissociation and Cross-bridge Detachment Kinetics from Myofibrils
The kinetic values were measured using an Applied Photophysics Ltd. (Leatherhead, UK) model SX.18 MV stopped-flow instrument with a dead time of 1.4 ms at 15 °C. IANBD fluorescence was excited at 470 nm and monitored using a 500-nm long-pass interference filter from Newport (Irvine, CA). The experimental conditions for the Ca2+ dissociation and cross-bridge detachment kinetics are described in the figure legends. Both ATP and ADP contained an equimolar concentration of Mg2+ so as to maintain a constant concentration of free Mg2+. The data were fit using a program (by P. J. King, Applied Photophysics) that utilizes the nonlinear Levenberg-Marquardt algorithm. Each Ca2+ dissociation and cross-bridge detachment event represents an average of at least three separate experiments, each averaging at least five traces fit with either a single- or double-exponential equation. The total increase in fluorescence upon Ca2+ dissociation or the total decrease in fluorescence upon cross-bridge detachment was an ∼1.2-fold change in fluorescence intensity.
Determination of the S1 Detachment Rate from Reconstituted Thin Filaments
Regulated thin filaments were prepared and reconstituted as previously described using TnCIAANST53C Tn (27). IAANS fluorescence was excited at 330 nm and monitored using a 510-nm broad spectrum filter from Newport (Irvine, CA). Varying concentrations of rabbit skeletal myosin S1 were added to the thin filament and then rapidly mixed with 2 mm ATP to detach the S1 from the regulated actin. The experimental conditions are described in the figure legends.
Statistical Analysis
All data are expressed as the mean (±S.E.). Statistical significance of the data was determined by using one-way analysis of variance followed by a post-hoc least significance difference test using GraphPad Prism 4 (La Jolla, CA). Statistical significance was defined as p < 0.05.
RESULTS
Measuring Ca2+ Dissociation
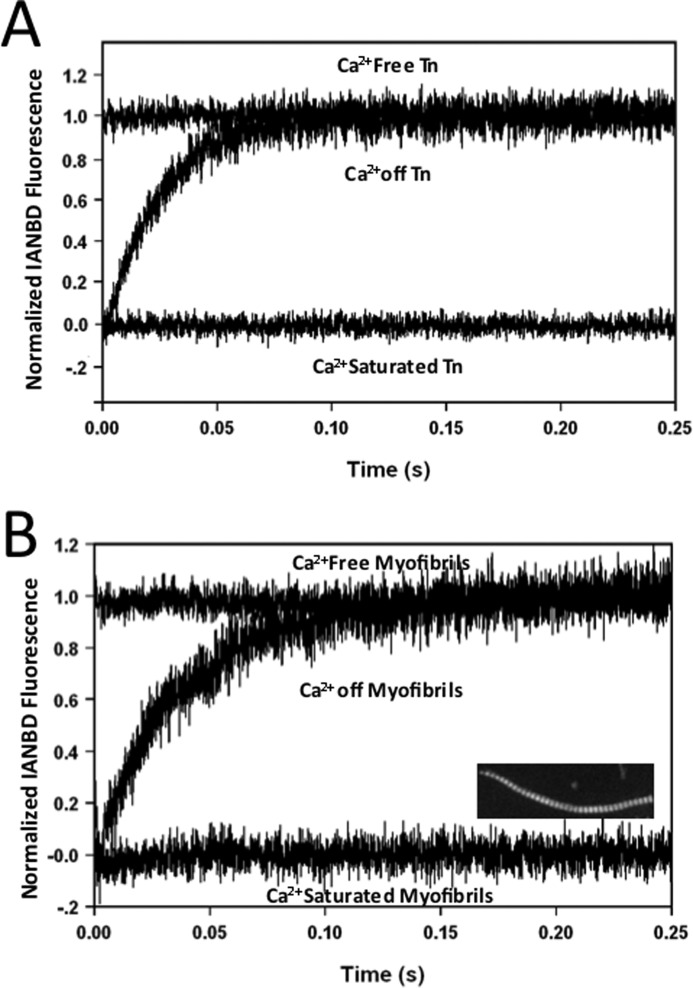
We have previously shown that TnCT53C labeled with IAANS reported the Ca2+ binding properties of reconstituted Tn and thin filaments with high fidelity (25). Although TnCT53C IAANS reported similar Ca2+ dissociation and cross-bridge detachment kinetics from myofibrils (data not shown), the signal-to-noise ratio was at least half that reported by TnCT53C labeled with IANBD (TnCIANBDT53C). Fig. 1A shows the apparent rate of Ca2+ dissociation from TnCIANBDT53C reconstituted into the Tn complex (40.8 ± 0.5/s) at 15 °C, which was nearly identical to that previously reported by the IAANS probe (25). Unlike the fluorescence signal from Tn labeled with IANBD at Cys-84 in TnC (21), the complete fluorescence change was observed between the Ca2+-saturated and Ca2+-free base lines of TnCIANBDT53C Tn. Similarly, upon exchange of the Tn complex containing TnCIANBDT53C into rabbit ventricular myofibrils in rigor, the complete fluorescence change also occurred between the Ca2+-saturated and Ca2+-free base lines, with an apparent rate of Ca2+ dissociation at 25.3 ± 0.7/s at 15 °C (Fig. 1B). The inset of Fig. 1B shows that TnCIANBDT53C Tn incorporated into the thin filaments of the myofibrils and replaced ∼60% of the endogenous Tn as determined by the Western blot ratio of endogenous to exogenous TnI (data not shown). A similar rate of Ca2+ dissociation was observed from the myofibrils when endogenous TnC was extracted and reconstituted with TnCIANBDT53C (21.1 ± 0.9/s; data not shown); however, the signal-to-noise ratio was approximately half that observed with Tn exchanged myofibrils. Thus, TnCIANBDT53C was capable of measuring the apparent rate of Ca2+ dissociation from the Tn complex, which slowed ∼2-fold upon incorporation into the rigor cardiac myofibrils.
FIGURE 1.
Ca2+ dissociation from IANBD-labeled TnC in Tn and Tn-exchanged rabbit rigor myofibrils. Panel A shows the time course of IANBD fluorescence as Ca2+ was chelated by EGTA and removed from the regulatory binding site of TnCIANBDT53C reconstituted into Tn. The Tn (0.3 μm) in Buffer A + 200 μm Ca2+ was rapidly mixed with an equal volume of Buffer A + 10 mm EGTA at 15 °C (Ca2+ off Tn trace, 40.8 ± 0.5/s). The Ca2+-saturated Tn base line was collected by mixing the Ca2+-saturated Tn with Buffer A + 200 μm Ca2+. The Ca2+-free Tn base line was acquired by rapidly mixing Tn in Buffer A + 5 mm EGTA against equal volumes of Buffer A + 5 mm EGTA. Panel B shows the time course of the IANBD fluorescence as Ca2+ was removed by EGTA from TnCIANBDT53C Tn-exchanged rabbit rigor ventricular myofibrils. TnCIANBDT53C myofibrils in Buffer A + 200 μm Ca2+ were rapidly mixed with an equal volume of the Buffer A + 10 mm EGTA at 15 °C (Ca2+ off Myofibrils trace, 25.3 ± 0.7/s). The Ca2+-saturated and Ca2+-free myofibril base lines were acquired from TnCIANBDT53C myofibrils under same buffer conditions as described for TnCIANBDT53CTn in panel A.
To verify that the observed rate of Ca2+ dissociation occurred from the regulatory domain of TnC, Ca2+ binding to the N-terminal domain of TnCT53C was abolished by the D65A mutation (33). The fluorescence intensity of reconstituted D65A TnCIANBDT53C Tn and Tn-exchanged myofibrils was insensitive to changes in Ca2+ (data not shown). Thus, the observed kinetics of TnCIANBDT53C Tn and TnCIANBDT53C myofibrils can be attributed to the apparent rate of Ca2+ dissociation from the N-terminal, regulatory domain of TnC.
Effect of Myofibril Species on the Rigor Ca2+ Dissociation Rate
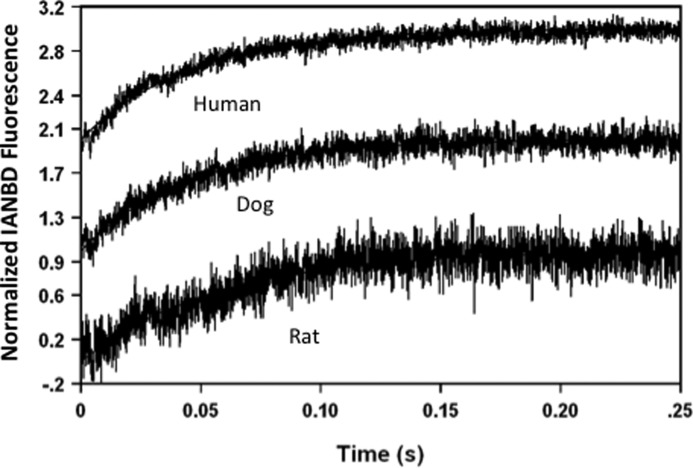
It is well established that isoforms of the thick and thin filament proteins vary across large and small species of mammals (9, 19, 34, 35). It is currently unknown whether these different protein isoforms can affect the rate of Ca2+ dissociation from TnC. Fig. 2 shows the apparent rates of Ca2+ dissociation from ventricular myofibrils in rigor from small, medium, and large sized mammals at 15 °C. Compared with the Ca2+ dissociation rate from rabbit myofibrils, the rates from rigor rat (16.7 ± 0.5/s) and dog (21.6 ± 0.7/s) were moderately, but statistically slower, whereas the rate from failing human ventricular myofibrils (22.9 ± 0.9/s) was not significantly different. Thus, the rigor Ca2+ dissociation rate from human cardiac TnCIANBDT53C in different species of myofibrils was similar.
FIGURE 2.
Effect of myofibril species on the rate of Ca2+ dissociation from Tn-exchanged rigor myofibrils. TnCIANBDT53C Tn was exchanged into rat, dog, and failing human ventricular myofibrils to acquire the apparent rates of Ca2+ dissociation for the respective myofibrils. The rigor Ca2+ dissociation rates for the different species were; rat (16.7 ± 0.5/s), dog (21.6 ± 0.7/s), and failing human ventricular myofibrils (22.9 ± 0.9/s). Buffer conditions were identical to those described for panel B of Fig. 1. All traces were acquired at 15 °C. The data traces were normalized and staggered for clarity.
Effect of ATP on the Observed Fluorescence Changes of TnC
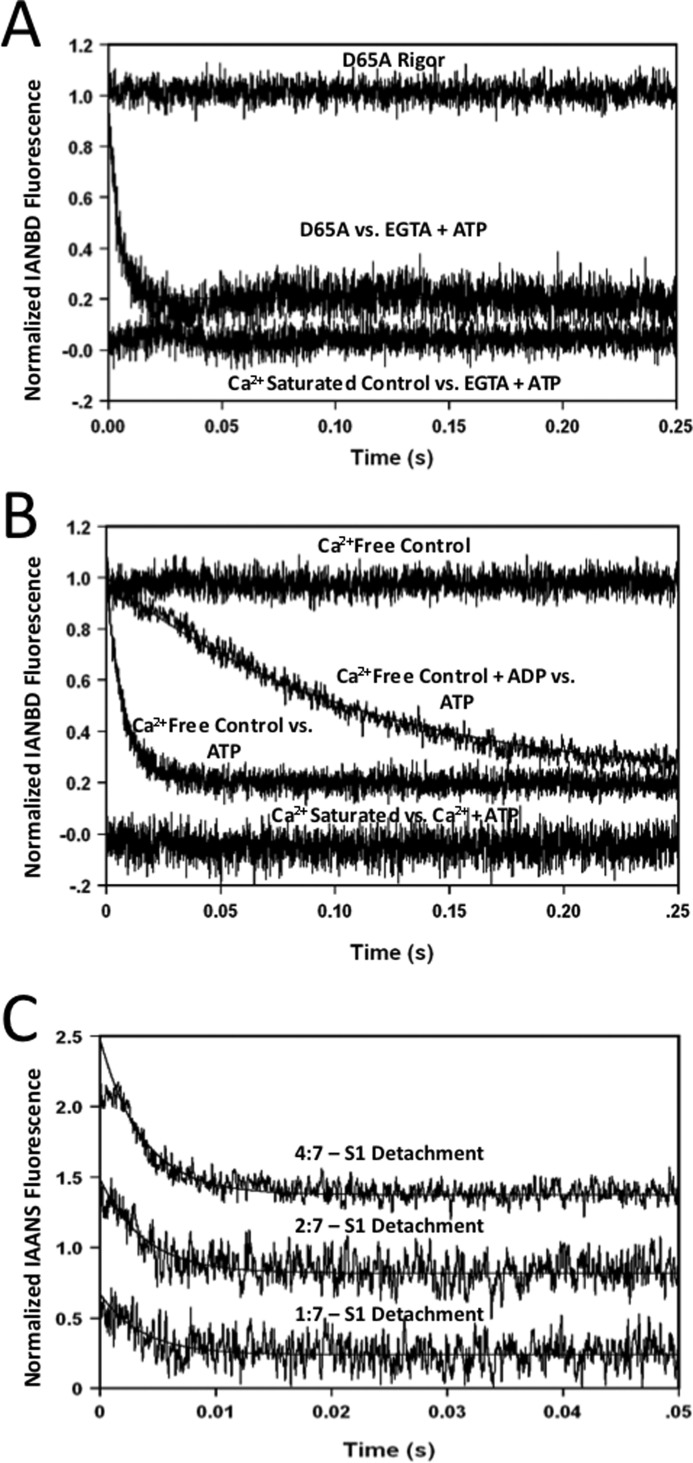
In a normal relaxing heart, Ca2+ dissociates from TnC, whereas cross-bridges actively cycle rather than in rigor. Unfortunately, the fluorescence intensity remained at the Ca2+-saturated base line and did not change when Ca2+ was removed from the rabbit myofibrils by EGTA that were also simultaneously mixed with 2 mm ATP to allow cross-bridge cycling (Fig. 3A, Ca2+-saturated control versus EGTA + ATP trace). This phenomenon was also observed when ATP was added to the Ca2+-saturated myofibrils before mixing with EGTA. However, when D65A TnCIANBDT53C myofibrils were rapidly mixed with 2 mm ATP, a rapid decrease in fluorescence intensity was observed at a rate of 203 ± 11/s at 15 °C (Fig. 3A, D65A versus EGTA + ATP trace). This ATP-dependent fluorescence decrease occurred from D65A TnCIANBDT53C myofibrils in the absence or presence of Ca2+ and originated from the rigor base-line fluorescence level (Fig. 3A, D65A Rigor trace). These data suggest that there are at least two processes influencing the fluorescence level of TnCIANBDT53C myofibrils during Ca2+ dissociation in the presence of cycling cross-bridges.
FIGURE 3.
Effect of ATP on rabbit ventricular myofibrils and reconstituted thin filaments + S1. Panel A shows the time course of IANBD fluorescence as Ca2+ was removed from TnCIANBDT53C and D65A TnCIANBDT53C rabbit myofibrils in the presence of ATP. TnCIANBDT53C rabbit myofibrils (Ca2+ Saturated Control versus EGTA + ATP trace, no observed rate) and D65A TnCIANBDT53C rabbit myofibrils (D65A versus EGTA + ATP trace, 203 ± 11/s) in Buffer A + 200 μm Ca2+ were mixed with an equal volume of the Buffer A + 10 mm EGTA and 2 mm ATP at 15 °C. The D65A TnCIANBDT53C Ca2+-free myofibril base line was acquired by rapidly mixing the D65A myofibrils in Buffer A + 5 mm EGTA against equal volumes of Buffer A + 5 mm EGTA (D65A Rigor). Panel B shows the time course of IANBD fluorescence decay from Ca2+-free TnCIANBDT53C rabbit myofibrils by mixing with ATP in the presence or absence of ADP. TnCIANBDT53C myofibrils in Buffer A + 5 mm EGTA ± 2 mm ADP were rapidly mixed with equal volumes of the Buffer A + 5 mm EGTA + 2 mm ATP (Ca2+-free Myofibrils versus ATP trace, 163 ± 8/s, and Ca2+-free Myofibrils + ADP versus ATP trace, 6.9 ± 0.6/s, respectively). To examine the effect of ATP on the Ca2+-saturated state, TnCIANBDT53C myofibrils in Buffer A + 200 μm Ca2+ were mixed with an equal volume of Buffer A + 200 μm Ca2+ + 2 mm ATP (Ca2+ Saturated versus Ca2+ + ATP trace, no observed rate). The Ca2+-free base line (Ca2+-free Control trace) from Fig. 1B was included as a reference point for the fluorescence decay induced by ATP. Panel C shows the time course of IAANS fluorescence decay from Ca2+-free reconstituted thin filaments containing TnCIAANST53C Tn upon dissociation of myosin-S1. TnCIAANST53C reconstituted thin filaments with a myosin S1 to actin subunit ratio of 1:7, 2:7 and 4:7 in Buffer A (without Tween 20) + 5 mm EGTA were rapidly mixed with equal volumes of the Buffer A (without Tween 20) + 5 mm EGTA + 2 mm ATP. The rates of S1 detachment reconstituted at the ratios of 1:7, 2:7, and 4:7 concentrations were ∼300/s, 268 ± 11/s, and 265 ± 10/s, respectively. All data were collected at 15 °C.
Sensing Cross-bridge Detachment through TnC
Unlike D65A TnCIANBDT53Cmyofibrils, the fluorescence intensity did not change when Ca2+-saturated TnCIANBDT53C myofibrils were mixed with 2 mm ATP (Fig. 3B, Ca2+ Saturated versus Ca2+ + ATP). However, the addition of 2 mm ATP to Ca2+-free TnCIANBDT53C myofibrils decreased the fluorescence at a rate of 163 ± 8/s at 15 °C (Ca2+ Free Control versus ATP trace). Similar to D65A TnCIANBDT53C myofibrils, the ATP-dependent fluorescence decrease originated from the Ca2+-free rigor baseline (Ca2+ Free Control trace). If this ATP-dependent decrease in fluorescence was related to cross-bridge detachment, then the addition of ADP to the myofibrils would be expected to slow the rate (15). As expected, the addition of 2 mm ADP to the Ca2+-free TnCIANBDT53C myofibrils substantially slowed the rate of the ATP-dependent fluorescence decrease to 6.9 ± 0.6/s (Ca2+ Free Control + ADP versus ATP trace). 2 mm ADP also slowed the ATP-dependent fluorescence decrease from D65A TnCIANBDT53C myofibrils to 6.4 ± 0.2/s (data not shown). Thus, TnCIANBDT53C fluorescence was sensitive to both Ca2+ binding and cross-bridge dissociation, each of which decreased IANBD fluorescence.
Although changes in Trp fluorescence, light scatter, and various fluorescent nucleotides have been utilized to more directly observe cross-bridge detachment in other systems (15, 20, 36), none of these approaches were suitable with the ventricular myofibrils (data not shown). To further test the idea that the fluorescent TnC was sensitive to cross-bridge detachment, reconstituted thin filaments bound by myosin S1 were utilized. A fluorescent skeletal Tn was previously shown to be sensitive to myosin S1 binding and dissociation in reconstituted thin filaments (37). Similarly, Fig. 3C shows a decrease in TnCIAANST53C fluorescence as increasing amounts of myosin S1 were detached from the reconstituted thin filaments by 2 mm ATP. At a myosin S1 to actin subunit ratio of 1:7, the fluorescence decreased at a rate of ∼300/s. This rate, although substantially slower than the true rate of myosin S1 detachment from nucleotide free reconstituted thin filaments, was similar to that observed by a fluorescent skeletal Tn (37) and that observed in the ventricular myofibrils of this study. Increasing the myosin S1-to-actin ratio to 2:7 and then 4:7 had little effect on the apparent detachment rates (268 ± 11/s and 265 ± 10/s, respectively) but linearly increased the amplitude of the signals. Similar to the ventricular myofibrils, in the presence of Ca2+, the fluorescence intensity of TnCIAANST53C on the reconstituted thin filament was not sensitive to myosin S1 detachment (data not shown). Thus, it would appear that the fluorescent TnC in the ventricular myofibrils and reconstituted thin filament reported similar ATP-dependent decreases in fluorescence that are related to myosin detachment. Unfortunately, the addition of ADP to the reconstituted thin filaments caused myosin S1 detachment, complicating any further studies with ATP.
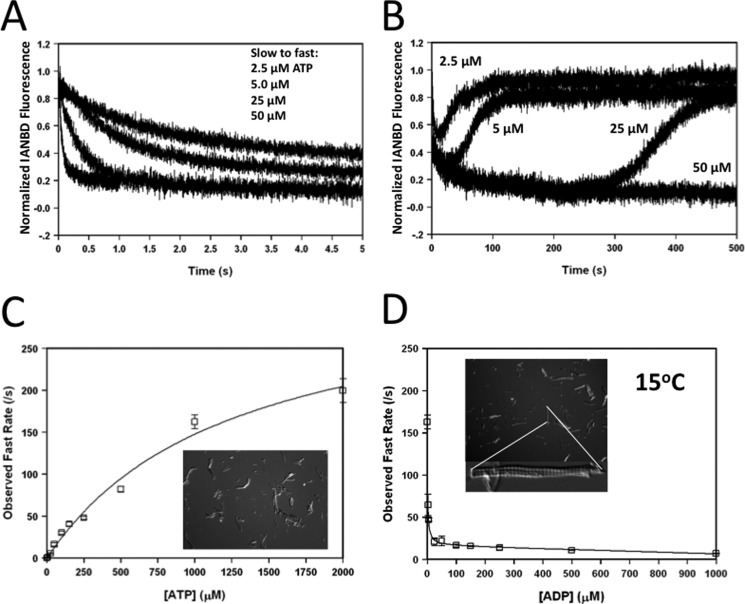
To further characterize the influence of ATP on the rabbit ventricular myofibrils, Fig. 4 shows the dependence of the concentration of ATP and ADP on the apparent rate of cross-bridge detachment from the Ca2+-free TnCIANBDT53C myofibrils at 15 °C. When the myofibrils were mixed with 5, 10, 50, and 100 μm ATP (concentration before mixing), the apparent rate of cross-bridge detachment occurred at 0.51 ± 0.02/s, 1.1 ± 0.2/s, 5.5 ± 0.6/s, and 16 ± 2/s, respectively (Fig. 4A). Over a longer time, there was an additional slow decrease in fluorescence that occurred at ∼0.02/s for all concentrations of ATP (Fig. 4B). Presumably as the ATP was depleted, the fluorescence slowly increased at ∼0.03/s back toward the Ca2+-free rigor base line as the cross-bridges went back into rigor (Fig. 4B). Increasing amounts of ATP delayed the reverse in fluorescence and rebinding of rigor cross-bridges (Fig. 4B). Fig. 4C shows the ATP dependence on the rate of the initial fast phase of cross-bridge detachment from Ca2+-free TnCIANBDT53C myofibrils. Previously, it was shown that increasing concentrations of ATP linearly accelerated the rate of rabbit cardiac myosin-S1 detachment from unregulated actin with an apparent second order rate constant of 2 × 106 m−1 s−1 at 15 °C (20). The apparent rate of cross-bridge detachment in Ca2+-free TnCIANBDT53C myofibrils increased linearly at an apparent second order rate constant of 2.8 × 105 ± 0.01 m−1s−1 for concentrations of ATP between 0 and 250 μm (Fig. 4C). At higher concentrations of ATP, the cross-bridge detachment rate hyperbolically plateaued at ∼330/s (Fig. 4C). To show that these ATP-dependent fluorescent changes were not due to myofibril shortening, the insets of Fig. 4, C and D, show the transmitted light microscopy images of Ca2+-free rabbit myofibrils taken from the stopped-flow after mixing with 50 μm ATP or 2 mm ATP, respectively. Thus, similar to skeletal myofibrils (38), the addition of ATP to Ca2+-free ventricular myofibrils did not cause shortening of the myofibrils.
FIGURE 4.
Dose dependence of ATP and ADP on the rate of cross-bridge detachment. Panel A shows the effect of increasing ATP on the apparent rate (fast phase) of cross-bridge detachment from Ca2+-free TnCIANBDT53C rabbit myofibrils. TnCIANBDT53C rabbit myofibrils in Buffer A + 5 mm EGTA were mixed against equal volumes of Buffer A + 5 (0.51 ± 0.02/s), 10 (1.1 ± 0.2/s), 50 (5.5 ± 0.6/s), or 100 (16 ± 2/s) μm ATP (traces shown top to bottom, respectively). Panel B shows the triphasic fluorescence change that occurs upon ATP addition to the Ca2+-free TnCIANBDT53C rabbit myofibrils with increasing ATP from 5 to 100 μm over an extended period of time. The Ca2+-free base line over these long times displayed a linear decrease in fluorescence that was due to either the bleaching of the signal or myofibril settling, which was subtracted from the experimental traces. Buffer conditions were identical to those described for panel A. Panel C shows the dose-dependent effect that increasing ATP had on the apparent fast rate of cross-bridge detachment from Ca2+-free TnCIANBDT53C rabbit myofibrils. The inset shows the transmitted light microscopy image of the myofibrils after being mixed with 50 μm ATP in the stopped flow. Panel D shows the effect that increasing ADP had on the apparent fast rate of cross-bridge detachment of Ca2+-free TnCIANBDT53C rabbit myofibrils. Increasing concentrations of ADP (0–2000 μm) were added to the Ca2+-free TnCIANBDT53C myofibrils in Buffer A + 5 mm EGTA and rapidly mixed against Buffer A + 5 mm EGTA + 2 mm ATP at 15 °C. Both the concentrations of ATP and ADP shown in panels C and D were the final concentration after mixing in the stopped-flow. The top inset shows the transmitted light microscopy image of the myofibrils after being mixed with 2 mm ATP in the stopped flow. The bottom inset shows an enlarged myofibril from this population.
In muscle, ATP cannot dissociate myosin from actin until ADP dissociates from the actomyosin complex (for review, see Refs. 14 and 13). As previously shown, the ADP dissociation rate limits rabbit cardiac myosin-S1 detachment from actin at ∼115/s at 15 °C (20). Increasing concentrations of ADP in Ca2+-free TnCIANBDT53C myofibrils hyperbolically decreased the apparent rate of cross-bridge detachment when the myofibrils were mixed with 2 mm ATP (Fig. 4D). The rate of cross-bridge detachment began to plateau at ∼25 μm ADP, signifying an ADP dissociation rate of 13 ± 1/s with an apparent affinity of 1.3 ± 0.1 μm. Further increases in ADP resulted in a mild linear decrease in the apparent rate of cross-bridge dissociation. At these high ADP concentrations, increasing the ATP concentration did not accelerate the apparent rate of cross-bridge detachment beyond the plateau rate of ∼13/s (data not shown). Although increasing the concentration of Pi can increase the rate of relaxation (17), increasing the Pi concentration up to 20 mm in the myofibrils had no effect on the rate of rigor Ca2+ dissociation or the rate of cross-bridge detachment with or without ADP (data not shown).
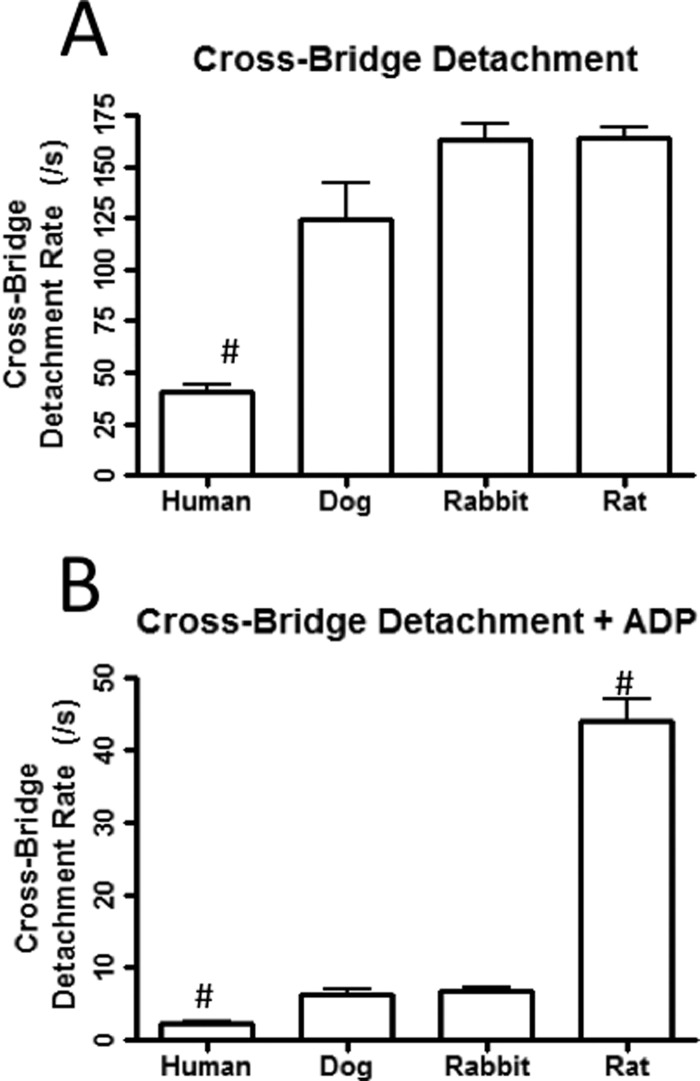
Species Dependence on the Cross-bridge Detachment Rate
It is well established that larger mammals have a higher percentage of the slow β-myosin, whereas smaller mammals have a greater percentage of the fast α-myosin (39, 40). Thus, the rate of cross-bridge detachment in myofibrils from large and small mammals would be expected to differ (15, 20). In the absence of ADP, only the failing human myofibrils (41 ± 4/s) had a rate of cross-bridge detachment (induced by 2 mm ATP) that was significantly slower than that of the rat (159 ± 6/s), dog (125 ± 17/s), and rabbit myofibrils (163 ± 8/s, Fig. 5A). However, compared with the rabbit (6.9 ± 0.6/s), the rate of cross-bridge detachment in the presence of 2 mm ADP was significantly faster for rat myofibrils (42 ± 3/s) and slower for the failing human myofibrils (2.3 ± 0.3/s) but similar to dog myofibrils (6.3 ± 0.6/s) (Fig. 5B). This data support the hypothesis that TnCIANBDT53C fluorescence is sensitive to cross-bridge detachment in ventricular myofibrils and that different myosin isoforms have different rates of cross-bridge detachment that appear rate-limited by ADP (41).
FIGURE 5.
Cross-bridge detachment rates from varying species of myofibrils. Panel A displays the rates of cross-bridge detachment in the absence of ADP at 15 °C from failing human (41 ± 4/s), dog (125 ± 17/s), rabbit (163 ± 8/s), and rat ventricular myofibrils (159 ± 6/s) that were exchanged with TnCIANBDT53C Tn. The TnCIANBDT53C myofibrils in Buffer A + 5 mm EGTA were rapidly mixed with equal volumes of the Buffer A + 5 mm EGTA + 2 mm ATP. Panel B displays the rates of cross-bridge detachment in the presence of 2 mm ADP from failing human (2.3 ± 0.3/s), dog (6.3 ± 0.6/s), rabbit (6.9 ± 0.6/s), and rat ventricular myofibrils (42 ± 3/s) that were exchanged with TnCIANBDT53C Tn at 15 °C. TnCIANBDT53C myofibrils in Buffer A + 5 mm EGTA + 2 mm ADP were rapidly mixed with equal volumes of Buffer A + 5 mm EGTA + 2 mm ATP.
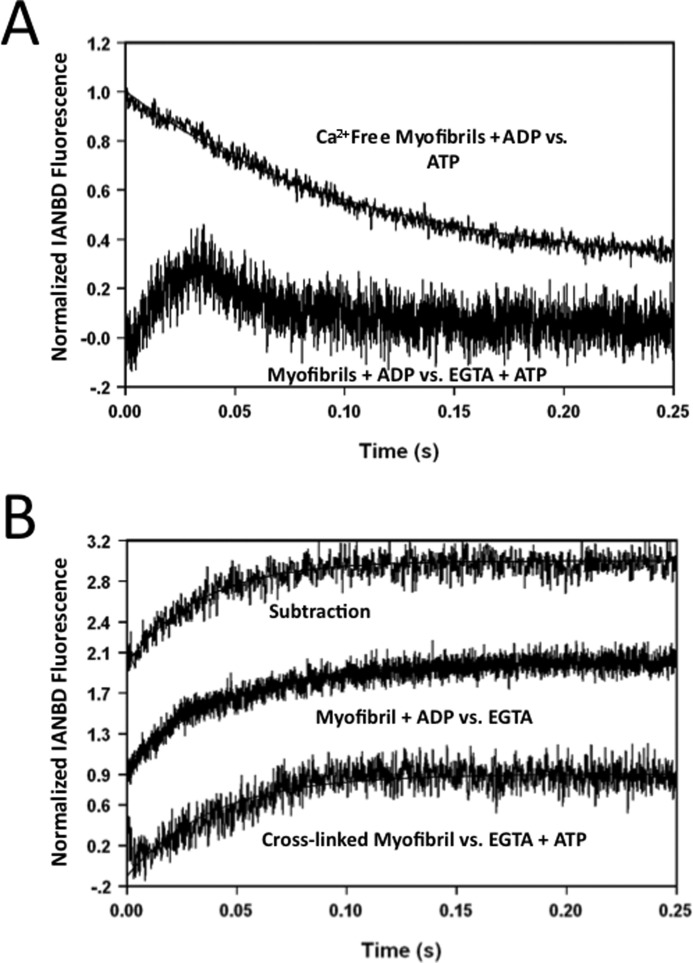
Recovery of the Ca2+ Dissociation Signal with Slowed Cross-bridge Detachment
In the presence of rapidly detaching cross-bridges, the Ca2+ dissociation event from TnCIANBDT53C myofibrils could not be observed (Fig. 3A, Control versus EGTA + ATP trace). However, when Ca2+ was rapidly chelated from TnCIANBDT53C rabbit myofibrils with slowly detaching cross-bridges due to 2 mm ADP, a biphasic fluorescence signal was observed (Fig. 6A, myofibrils + ADP versus EGTA + ATP trace). The signal originated from the Ca2+-saturated base line and then decayed back to the Ca2+-saturated base line. Under these conditions, the Ca2+ dissociation event (increase in fluorescence) and cross-bridge detachment event (decrease in fluorescence) were both observed. A monophasic increase in fluorescence (Fig. 6B, Subtraction trace) resulted from subtracting the cross-bridge detachment event (Fig. 6A, Ca2+-free Myofibrils + ADP versus ATP trace) from the biphasic fluorescence signal. The rate of this fluorescence increase (∼25/s) was nearly identical to that of rigor Ca2+ dissociation. A biphasic fluorescence signal was also observed when Ca2+ was dissociated from TnCIANBDT53Crabbit myofibrils, whereas cross-bridge detachment was slowed by decreasing the temperature (5 °C, data not shown). Similarly, mixing the Ca2+-saturated myofibrils with EGTA and a reduced amount of ATP (200 μm, before mixing) at 15 °C also caused a biphasic signal (data not shown).
FIGURE 6.
Effect of slowed cross-bridge detachment on the rate of Ca2+ dissociation from rabbit myofibrils. Panel A represents the biphasic kinetic trace for TnCIANBDT53C myofibrils in Buffer A + 200 μm Ca2+ + 2 mm ADP when mixed with an equal amount of Buffer A + 10 mm EGTA and 2 mm ATP at 15 °C (Myofibrils + ADP versus EGTA + ATP trace, biphasic). The Ca2+-free myofibrils + ADP versus ATP trace from Fig. 3, panel B (6.9 ± 0.6/s), was shown for a graphic comparison. Panel B shows the effect that ADP and cross-linking had on the rate of Ca2+ dissociation at 15 °C. The Ca2+-free myofibrils + ADP versus ATP trace (panel A) was subtracted from the biphasic myofibrils + ADP versus EGTA + ATP trace (panel A) to recover the Ca2+ dissociation signal (subtraction, ∼ 25/s). TnCIANBDT53C myofibrils in Buffer A + 200 μm Ca2+ + 2 mm ADP were rapidly mixed with an equal volume of the Buffer A + 10 mm EGTA at 15 °C (Myofibril + ADP versus EGTA trace, 24 ± 1/s). Cross-linked TnCIANBDT53C myofibrils in Buffer A + 200 μm Ca2+ were rapidly mixed with an equal volume of the Buffer A + 10 mm EGTA + 2 mm ATP (Cross-linked Myofibrils versus EGTA + ATP trace, 22 ± 1/s).
Additional studies were performed to examine the effect of ADP and cross-linking on the apparent rates of Ca2+ dissociation and cross-bridge detachment. The addition of 2 mm ADP to Ca2+-saturated TnCIANBDT53C rigor rabbit myofibrils did not alter the apparent rate of Ca2+ dissociation (24 ± 1/s; Fig. 6B, Myofibril + ADP versus EGTA) compared with that of nucleotide free rigor myofibrils. Although cross-linked myofibrils were no longer sensitive to ATP-dependent cross-bridge detachment events (no change in fluorescence observed upon mixing with ATP (data not shown)), the rate of Ca2+ dissociation from cross-linked myofibrils in the absence (21.3 ± 0.2/s; data not shown) or presence of ATP (22 ± 1/s; Fig. 6B, Cross-linked Myofibril versus EGTA + ATP) was also similar to that of rigor myofibrils. Thus, myofibrils with slowed or non-dissociating cross-bridges had similar apparent rates of Ca2+ dissociation. Furthermore, cross-bridge detachment could be prevented by cross-linking the myofibrils.
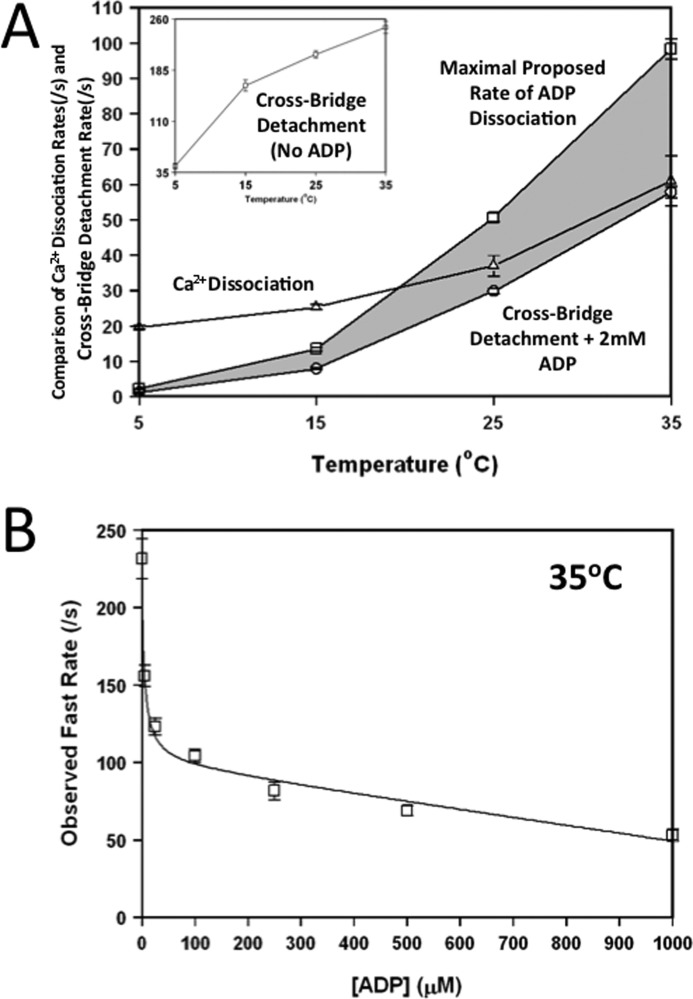
Physiological Significance of Ca2+ Dissociation and Cross-Bridge Detachment
Controversy abounds in the literature regarding the rate-limiting step of cardiac muscle relaxation. It is commonly thought that the rate of Ca2+ dissociation from TnC is too rapid, and therefore, cross-bridge detachment would rate-limit relaxation (6, 16, 21, 42). Consistent with this hypothesis, Fig. 7A shows that the apparent rate of cross-bridge detachment in the presence of 2 mm ADP was significantly slower than the rate of Ca2+ dissociation from TnCIANBDT53C rabbit myofibrils at temperatures less than 25 °C. However, at a more physiological temperature (35 °C), the two rates were nearly identical.
FIGURE 7.
Effect of temperature on the rates of Ca2+ dissociation and cross-bridge detachment as reported by TnC in rabbit myofibrils. Panel A shows the comparison between the rates of rigor Ca2+ dissociation (△), cross-bridge detachment + 2 mm ADP (○), and maximal proposed rate of ADP dissociation (□) at increasing temperatures (* denotes calculated value). Rigor Ca2+ dissociation (△) was determined by mixing TnCIANBDT53C myofibrils in Buffer A + 200 μm Ca2+ with an equal volume of the Buffer A + 10 mm EGTA. The Cross-Bridge Detachment (No ADP) plot (inset, □) represents cross-bridge detachment from ADP free myofibrils. Cross-bridge detachment in presence of ADP was determined by mixing TnCIANBDT53C myofibrils in Buffer A + 5 mm EGTA + 2 mm ADP (○) with an equal volume of the Buffer A + 5 mm EGTA + 2 mm ATP. The shaded area is used to highlight the difference between the maximal proposed rate of ADP dissociation to that with 2 mm ADP. Panel B shows the effect that increasing ADP had on the apparent fast rate of cross-bridge detachment of Ca2+-free TnCIANBDT53C rabbit myofibrils at 35 °C. Increasing concentrations of ADP (0–2000 μm) were added to the Ca2+-free TnCIANBDT53C myofibrils in Buffer A + 5 mm EGTA and rapidly mixed against Buffer A + 2 mm ATP at 35 °C. The concentrations of ADP shown were the final concentration after mixing in the stopped-flow.
Physiologically, the concentration of cytosolic ADP in the cardiac myocyte is thought to be ∼30 μm and can increase to mm levels during ischemia (43). Thus, ADP dependence on the apparent rate of cross-bridge detachment in TnCIANBDT53C myofibrils was determined at 35 °C. Similar to the results at 15 °C, at 35 °C (in Fig. 7B) ADP hyperbolically decreased the apparent rate of cross-bridge detachment when the myofibrils were mixed with 2 mm ATP. The rate of cross-bridge detachment began to plateau at ∼100 μm ADP, signifying an ADP dissociation rate of 104 ± 5/s with an apparent affinity of 4 ± 1 μm. At concentrations of ADP that were supraphysiological, there was a more pronounced linear drop-off in the rate of cross-bridge detachment at 35 °C (Fig. 7B) than was observed at 15 °C (Fig. 4D). At these supraphysiological ADP concentrations, increasing the ATP concentration increased the apparent rate of cross-bridge detachment to that comparable to the plateau rate of ∼100/s (data not shown). We suggest that this plateau rate is the rate of ADP dissociation (Fig. 7A, Maximal Proposed Rate of ADP Dissociation), which rate-limits cross-bridge detachment. This rate was measured for 15 and 35 °C and calculated from the 2 mm ADP curve for 5 and 25 °C. The rate previous measured in the calculated plateau rate for 5 and 25 °C was based on the 1.7-fold increase of the detachment rate (as compared with the detachment rate at 2 mm ADP) that was observed for both 15 and 35 °C. Thus, the apparent rate of cross-bridge detachment may actually be slightly faster than the rate of Ca2+ dissociation at 35 °C in the presence of ADP and saturating ATP but not at temperatures below ∼20 °C (Fig. 7A). The data suggest that there may not be a simple, single rate-limiting step for myofilament inactivation. Furthermore, both the rates of Ca2+ dissociation and cross-bridge detachment may be slow enough to influence ventricular muscle relaxation.
DISCUSSION
The fluorescent TnCIANBDT53C was able to report Ca2+ dissociation as well as ATP-dependent events. We hypothesized that the fluorescent TnC was capable of measuring the rate of cross-bridge detachment. This hypothesis was supported by several lines of evidence in regard to the measured rate of cross-bridge detachment in the presence of EGTA (low Ca2+): 1) increasing ATP hyperbolically increased the apparent rate (Fig. 4C); 2) upon ATP depletion the cross-bridges rebound and reversed the fluorescence signal, which took longer for increased concentrations of ATP; 3) in the absence of ADP (Fig. 4C), the maximal rate was similar to the rate of rigor cardiac muscle relaxation induced by ATP (44); 4) increasing concentrations of ADP hyperbolically decreased the apparent rate (Fig. 4D); 5) in the presence of ADP, the rate varied in a manner expected for different species of myofibrils (i.e. fastest in rat and slowest with the failing human; Fig. 5B); 6) TnC fluorescence was sensitive to S1 detachment from the reconstituted thin filaments; 7) the addition of ADP slowed the cross-bridge detachment rate in ventricular myofibrils to that comparable to the rate of relaxation for different cardiac muscles at similar temperatures (44–46); 8) cross-linking the myofibrils abolished the rate.
In the absence of ADP, ATP-induced detachment of myosin from reconstituted skeletal and cardiac actomyosin is extremely fast (>500/s) (15, 47). This may suggest that cross-bridge detachment observed by TnCIANBDT53C is sensed and limited by movement of another myofilament protein (tropomyosin (Tm), TnI, or TnT) or that the rate of cross-bridge detachment from the nucleotide free myofibrils is slower than that measured from reconstituted actomyosin. The idea that Tm movement may limit the observed rate of cross-bridge detachment is consistent with skeletal thin filament studies where myosin detachment was >500/s as measured by light scatter but sensed at ∼200/s by a fluorescent Tm (47). However, by following a change in Trp fluorescence, ATP also hyperbolically accelerated the rate of cross-bridge detachment in skeletal myofibrils (in the absence of ADP) (36). The maximal rate observed in skeletal myofibrils was similar to the maximal rate of cross-bridge detachment observed by TnCIANBDT53C in cardiac myofibrils. Furthermore, the rate of rigor relaxation induced by caged ATP (in the absence of ADP (44)) was also similar to the maximal rate of cross-bridge detachment sensed by TnCIANBDT53C. Thus, cross-bridge detachment in the sarcomere may be slower than that from a reconstituted filament. In any regard, it would appear that the other myofilament proteins (Tm, TnI, and TnT) can move on the thin filament at a rate that is significantly faster than either Ca2+ dissociation from TnC or cross-bridge detachment in the presence of ADP.
The actual mechanism behind the ability of TnCIANBDT53C to sense cross-bridge detachment at low Ca2+ (presence of EGTA) is unknown. However, there is ample evidence that the binding of myosin to actin in reconstituted thin filaments, myofibrils, and muscle can influence the fluorescence of multiple TnC constructs (21, 48–50). It has been shown that cross-bridges can alter the orientation of various helices within TnC exchanged into cardiac trabeculae (51). These changes in TnC structure are not thought to be caused by a direct interaction of myosin with TnC but through myosin ability to alter Tm position on actin (14). Thus, the structure of TnC could be directly altered by the movement of Tm or indirectly through the movement modifying TnC interactions with TnI and TnT. Regardless, TnCIANBDT53C was only able to report cross-bridge detachment in the absence of Ca2+. One explanation for this phenomenon is that Ca2+-bound TnC exists in a structural conformation that makes the fluorescent probe insensitive to cross-bridge detachment. However, in the presence of Ca2+ and ATP, the myofibrils rapidly and irreversibly shortened, which may have compromised the fluorescence change.
Our data are consistent with the hypothesis that the rate of cross-bridge detachment is limited by the rate of ADP dissociation. Previously, the rates of ADP dissociation from different cardiac myosins were inferred from the study of unregulated actomyosin in solution (20), laser trap experiments (44), and sinusoidal analysis on skinned cardiac muscle (52–54). The apparent rate of ADP dissociation measured by our fluorescent TnC is nearly one order of magnitude slower than that measured from actomyosin in solution for the different fast and slow myosins (20). This result may not be surprising considering that previous studies have shown that myosin S1 and single-headed myosin have different properties than even heavy meromyosin (55). In agreement with our results, the apparent rates of ADP dissociation measured from the rabbit, rat, and human myofibrils were similar to the calculated rates of ADP dissociation measured by the laser trap assay (rabbit and rat) (41, 44) and from the sinusoidal length perturbation analysis of cross-bridge detachment (human, rabbit, and rat) (52–54).
There is evidence that the rate of ADP dissociation from various non-muscle and smooth muscle myosins is load-dependent (56–59). This phenomenon has recently been suggested to be true for cardiac myosin (60). It may be that the cross-bridges in the myofibrils are under some sort of load that would affect the rate of ADP dissociation. Consistent with this idea, it is striking that the apparent rate of ADP dissociation from the myofibrils from different species in this study was similar to the reported rates of cardiac muscle relaxation from the respective species. Again, these data would suggest that in more complex physiological systems the rate of ADP dissociation is slowed and that cross-bridge detachment rate-limits relaxation at subphysiological temperatures.
Previous data from skinned skeletal muscle and cardiac myofibrils suggest that the rate of Ca2+ dissociation from TnC can influence the duration and rate of relaxation (61, 62). This was primarily determined by utilizing TnC mutants that possessed slower rates of Ca2+ dissociation. To date, the rate of Ca2+ dissociation from TnC in intact muscle has not been measured. Current evidence suggests that the rate of Ca2+ dissociation from TnC may not be constant and is dependent upon strongly bound cross-bridges. For instance, the rate of Ca2+ dissociation is slowed nearly one order of magnitude upon myosin-S1 binding to the thin filament (25). Consistent with these studies, extra bursts of Ca2+ were released from the myofilament when myosin was forced to rapidly detach from skinned and intact cardiac muscle (63, 64). It is believed that the rapid detachment of myosin from actin weakened the affinity of TnC for Ca2+ and accelerated the rate of Ca2+ dissociation. Interestingly, the rate of Ca2+ dissociation from TnC in guinea pig ventricular myofibrils with detached cross-bridges was nearly one order of magnitude faster than the rate of relaxation (21). This study concluded that the rate of Ca2+ dissociation from TnC could not rate-limit relaxation as it was substantially faster than that of relaxation. However, the initiation of relaxation occurs in the presence of strongly bound cross-bridges. Thus, we set out to measure the rate of Ca2+ dissociation from TnC in Tn-exchanged ventricular myofibrils under conditions of strongly bound cross-bridges.
In rigor, the rate of Ca2+ dissociation from TnCIANBDT53C myofibrils was slightly influenced by the species of myofibrils (Fig. 2). The addition of ADP to the rigor myofibrils had little effect on the rate of Ca2+ dissociation from TnC (Fig. 6B). In the presence of ATP, the Ca2+ dissociation signal could not be observed unless ADP was present, the temperature was decreased, a minimal amount of ATP was used, or the myofibrils were cross-linked. This data suggest that the rates of Ca2+ dissociation from TnC in myofibrils with different strongly bound cross-bridge states are similar. However, even with a slower rate of cross-bridge detachment, the rate of Ca2+ dissociation appears too fast to rate-limit myofilament inactivation and relaxation at subphysiological temperatures.
It has been suggested that the myofilament rather than Ca2+ dynamics rate-limits relaxation at physiological temperature (4). It is clear that cross-bridge dynamics are highly temperature-sensitive with a Q10 as high as 5 (65). On the other hand, the rate of Ca2+ dissociation from cardiac TnC has been shown to have negligible temperature sensitivity (26). Consistent with these studies, the rate of cross-bridge detachment was substantially more affected by temperature than the rate of Ca2+ dissociation from TnC in the cardiac myofibrils. Interestingly, at physiological temperature, the rate of Ca2+ dissociation and cross-bridge detachment in the ventricular myofibrils were equivalent at 1 mm ADP (after mixing). However, at more physiological concentrations of ADP, the rate of cross-bridge detachment (∼100/s) was actually faster than the rate of Ca2+ dissociation (∼60/s). Thus, under physiological conditions the rate of Ca2+ dissociation may actually rate-limit myofilament inactivation. The maximal proposed rate of ADP dissociation (Fig. 7A) may be an overestimation of the rate as the myofibrils may be an unloaded system. Thus, in cardiac muscle the two proposed rate-limiting mechanisms for myofilament inactivation may actually be kinetically tuned with one another. This would suggest that slowing one of these two rates would slow relaxation, but to accelerate relaxation both rates would have to be increased. Therefore, therapeutic strategies designed to increase the rate of relaxation may need to target both the thin and thick filament.
Acknowledgments
We thank Dr. Jack Rall and Dr. Jianchao Zhang for critical reading of the manuscript.
This work was supported, in whole or in part, by National Center for Research Resources Award TL1RR025753 and Grants HL091986 (to J. P. D., HL091056 (to B. J. B.), and RC1 HL099538 (to P. M. L. J.).
- Tn
- human cardiac troponin (Tn)
- TnC
- human cardiac troponin C
- TnI
- human cardiac troponin I
- TnT
- human cardiac troponin T
- IANBD
- 4-(N-(iodoacetoxy)ethyl-N-methyl)amino-7-nitrobenz-2-oxa-1,3-diazole
- TnCIANBDT53C
- control human cardiac troponin C containing the C35S, T53C, C84S modifications that was labeled with IANBD
- D65A TnCIANBDT53C
- human cardiac troponin C containing C35S, T53C D65A, C84S modifications labeled with IANBD.
REFERENCES
- 1. Grossman W. (1990) Diastolic function and heart failure. An overview. Eur. Heart J. 11, 2–7 [DOI] [PubMed] [Google Scholar]
- 2. Janssen P. M., Periasamy M. (2007) Determinants of frequency-dependent contraction and relaxation of mammalian myocardium. J. Mol. Cell. Cardiol. 43, 523–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bers D. M. (2002) Cardiac excitation-contraction coupling. Nature 415, 198–205 [DOI] [PubMed] [Google Scholar]
- 4. Janssen P. M., Stull L. B., Marbán E. (2002) Myofilament properties comprise the rate-limiting step for cardiac relaxation at body temperature in the rat. Am. J. Physiol. Heart Circ. Physiol. 282, H499–H507 [DOI] [PubMed] [Google Scholar]
- 5. Backx P. H., Gao W. D., Azan-Backx M. D., Marban E. (1995) The relationship between contractile force and intracellular [Ca2+] in intact rat cardiac trabeculae. J. Gen. Physiol. 105, 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon A. M., Homsher E., Regnier M. (2000) Regulation of contraction in striated muscle. Physiol. Rev. 80, 853–924 [DOI] [PubMed] [Google Scholar]
- 7. Liang B., Chung F., Qu Y., Pavlov D., Gillis T. E., Tikunova S. B., Davis J. P., Tibbits G. F. (2008) Familial hypertrophic cardiomyopathy-related cardiac troponin C mutation L29Q affects Ca2+ binding and myofilament contractility. Physiol. Genomics 33, 257–266 [DOI] [PubMed] [Google Scholar]
- 8. Stehle R., Solzin J., Iorga B., Poggesi C. (2009) Insights into the kinetics of Ca2+-regulated contraction and relaxation from myofibril studies. Pflugers Arch. 458, 337–357 [DOI] [PubMed] [Google Scholar]
- 9. Bloemink M. J., Geeves M. A. (2011) Shaking the myosin family tree. Biochemical kinetics defines four types of myosin motor. Semin. Cell Dev. Biol. 22, 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brenner B., Chalovich J. M., Yu L. C. (1995) Distinct molecular processes associated with isometric force generation and rapid tension recovery after quick release. Biophys. J. 68, 106S–111S [PMC free article] [PubMed] [Google Scholar]
- 11. Eisenberg E., Hill T. L. (1985) Muscle contraction and free energy transduction in biological systems. Science 227, 999–1006 [DOI] [PubMed] [Google Scholar]
- 12. Hibberd M. G., Dantzig J. A., Trentham D. R., Goldman Y. E. (1985) Phosphate release and force generation in skeletal muscle fibers. Science 228, 1317–1319 [DOI] [PubMed] [Google Scholar]
- 13. Nyitrai M., Geeves M. A. (2004) Adenosine diphosphate and strain sensitivity in myosin motors. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 359, 1867–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon A. M., Regnier M., Homsher E. (2001) Skeletal and cardiac muscle contractile activation. Tropomyosin “rocks and rolls.” News Physiol. Sci. 16, 49–55 [PubMed] [Google Scholar]
- 15. Siemankowski R. F., White H. D. (1984) Kinetics of the interaction between actin, ADP, and cardiac myosin-S1. J. Biol. Chem. 259, 5045–5053 [PubMed] [Google Scholar]
- 16. Poggesi C., Tesi C., Stehle R. (2005) Sarcomeric determinants of striated muscle relaxation kinetics. Pflugers Arch. 449, 505–517 [DOI] [PubMed] [Google Scholar]
- 17. Simnett S. J., Johns E. C., Lipscomb S., Mulligan I. P., Ashley C. C. (1998) Effect of pH, phosphate, and ADP on relaxation of myocardium after photolysis of diazo 2. Am. J. Physiol. 275, H951–H960 [DOI] [PubMed] [Google Scholar]
- 18. Tesi C., Piroddi N., Colomo F., Poggesi C. (2002) Relaxation kinetics following sudden Ca2+ reduction in single myofibrils from skeletal muscle. Biophys. J. 83, 2142–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fitzsimons D. P., Patel J. R., Moss R. L. (1998) Role of myosin heavy chain composition in kinetics of force development and relaxation in rat myocardium. J. Physiol. 513, 171–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siemankowski R. F., Wiseman M. O., White H. D. (1985) ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc. Natl. Acad. Sci. U.S.A. 82, 658–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Solzin J., Iorga B., Sierakowski E., Gomez Alcazar D. P., Ruess D. F., Kubacki T., Zittrich S., Blaudeck N., Pfitzer G., Stehle R. (2007) Kinetic mechanism of the Ca2+-dependent switch-on and switch-off of cardiac troponin in myofibrils. Biophys. J. 93, 3917–3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davis J. P., Rall J. A., Alionte C., Tikunova S. B. (2004) Mutations of hydrophobic residues in the N-terminal domain of troponin C affect calcium binding and exchange with the troponin C-troponin I96–148 complex and muscle force production. J. Biol. Chem. 279, 17348–17360 [DOI] [PubMed] [Google Scholar]
- 23. Dong W., Rosenfeld S. S., Wang C. K., Gordon A. M., Cheung H. C. (1996) Kinetic studies of calcium binding to the regulatory site of troponin C from cardiac muscle. J. Biol. Chem. 271, 688–694 [DOI] [PubMed] [Google Scholar]
- 24. Hazard A. L., Kohout S. C., Stricker N. L., Putkey J. A., Falke J. J. (1998) The kinetic cycle of cardiac troponin C: calcium binding and dissociation at site II trigger slow conformational rearrangements. Protein Sci. 7, 2451–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davis J. P., Norman C., Kobayashi T., Solaro R. J., Swartz D. R., Tikunova S. B. (2007) Effects of thin and thick filament proteins on calcium binding and exchange with cardiac troponin C. Biophys. J. 92, 3195–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tikunova S. B., Davis J. P. (2004) Designing calcium-sensitizing mutations in the regulatory domain of cardiac troponin C. J. Biol. Chem. 279, 35341–35352 [DOI] [PubMed] [Google Scholar]
- 27. Tikunova S. B., Liu B., Swindle N., Little S. C., Gomes A. V., Swartz D. R., Davis J. P. (2010) Effect of calcium-sensitizing mutations on calcium binding and exchange with troponin C in increasingly complex biochemical systems. Biochemistry 49, 1975–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Putkey J. A., Liu W., Lin X., Ahmed S., Zhang M., Potter J. D., Kerrick W. G. (1997) Fluorescent probes attached to Cys-35 or Cys-84 in cardiac troponin C are differentially sensitive to Ca2+-dependent events in vitro and in situ. Biochemistry 36, 970–978 [DOI] [PubMed] [Google Scholar]
- 29. Xu Y., Monasky M. M., Hiranandani N., Haizlip K. M., Billman G. E., Janssen P. M. (2011) Effect of twitch interval duration on the contractile function of subsequent twitches in isolated rat, rabbit, and dog myocardium under physiological conditions. J. Appl. Physiol. 111, 1159–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Swartz D. R., Zhang D., Yancey K. W. (1999) Cross-bridge-dependent activation of contraction in cardiac myofibrils at low pH. Am. J. Physiol. 276, H1460–H1467 [DOI] [PubMed] [Google Scholar]
- 31. Lionne C., Iorga B., Candau R., Piroddi N., Webb M. R., Belus A., Travers F., Barman T. (2002) Evidence that phosphate release is the rate-limiting step on the overall ATPase of psoas myofibrils prevented from shortening by chemical cross-linking. Biochemistry 41, 13297-13308 [DOI] [PubMed] [Google Scholar]
- 32. Yates L. D., Greaser M. L. (1983) Quantitative determination of myosin and actin in rabbit skeletal muscle. J. Mol. Biol. 168, 123–141 [DOI] [PubMed] [Google Scholar]
- 33. Gillis T. E., Martyn D. A., Rivera A. J., Regnier M. (2007) Investigation of thin filament near-neighbor regulatory unit interactions during force development in skinned cardiac and skeletal muscle. J. Physiol. 580, 561–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. James J., Hor K., Moga M. A., Martin L. A., Robbins J. (2010) Effects of myosin heavy chain manipulation in experimental heart failure. J. Mol. Cell. Cardiol. 48, 999–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. VanBuren P., Harris D. E., Alpert N. R., Warshaw D. M. (1995) Cardiac V1 and V3 myosins differ in their hydrolytic and mechanical activities in vitro. Circ. Res. 77, 439–444 [DOI] [PubMed] [Google Scholar]
- 36. Candau R., Kawai M. (2011) Correlation between cross-bridge kinetics obtained from Trp fluorescence of myofibril suspensions and mechanical studies of single muscle fibers in rabbit psoas. J. Muscle Res. Cell Motil. 32, 315–326 [DOI] [PubMed] [Google Scholar]
- 37. Trybus K. M., Taylor E. W. (1980) Kinetic studies of the cooperative binding of subfragment 1 to regulated actin. Proc. Natl. Acad. Sci. U.S.A. 77, 7209–7213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lionne C., Iorga B., Candau R., Travers F. (2003) Why choose myofibrils to study muscle myosin ATPase? J. Muscle Res. Cell Motil. 24, 139–148 [DOI] [PubMed] [Google Scholar]
- 39. Liu J. X., Höglund A. S., Karlsson P., Lindblad J., Qaisar R., Aare S., Bengtsson E., Larsson L. (2009) Myonuclear domain size and myosin isoform expression in muscle fibres from mammals representing a 100,000-fold difference in body size. Exp. Physiol. 94, 117–129 [DOI] [PubMed] [Google Scholar]
- 40. Hamilton N., Ianuzzo C. D. (1991) Contractile and calcium regulating capacities of myocardia of different sized mammals scale with resting heart rate. Mol. Cell. Biochem. 106, 133–141 [DOI] [PubMed] [Google Scholar]
- 41. Palmiter K. A., Tyska M. J., Dupuis D. E., Alpert N. R., Warshaw D. M. (1999) Kinetic differences at the single molecule level account for the functional diversity of rabbit cardiac myosin isoforms. J. Physiol. 519, 669–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Belus A., Piroddi N., Tesi C. (2003) Mechanism of cross-bridge detachment in isometric force relaxation of skeletal and cardiac myofibrils. J. Muscle Res. Cell Motil. 24, 261–267 [PubMed] [Google Scholar]
- 43. Ingwall J. S. (2002) ATP and the Heart, pp. 21–38, Kluwer Academic Publishers, Norwell, MA [Google Scholar]
- 44. Han Y. S., Ogut O. (2011) Force relaxation and thin filament protein phosphorylation during acute myocardial ischemia. Cytoskeleton 68, 18–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Belus A., Piroddi N., Scellini B., Tesi C., Amati G. D., Girolami F., Yacoub M., Cecchi F., Olivotto I., Poggesi C. (2008) The familial hypertrophic cardiomyopathy-associated myosin mutation R403Q accelerates tension generation and relaxation of human cardiac myofibrils. J. Physiol. 586, 3639–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martin H., Barsotti R. J. (1994) Relaxation from rigor of skinned trabeculae of the guinea pig induced by laser photolysis of caged ATP. Biophys. J. 66, 1115–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Borrego-Diaz E., Chalovich J. M. (2010) Kinetics of regulated actin transitions measured by probes on tropomyosin. Biophys. J. 98, 2601–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hannon J. D., Martyn D. A., Gordon A. M. (1992) Effects of cycling and rigor cross-bridges on the conformation of cardiac troponin C. Circ. Res. 71, 984–991 [DOI] [PubMed] [Google Scholar]
- 49. Martyn D. A., Freitag C. J., Chase P. B., Gordon A. M. (1999) Ca2+ and cross-bridge-induced changes in troponin C in skinned skeletal muscle fibers: effects of force inhibition. Biophys. J. 76, 1480–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zot A. S., Potter J. D. (1989) Reciprocal coupling between troponin C and myosin cross-bridge attachment. Biochemistry 28, 6751–6756 [DOI] [PubMed] [Google Scholar]
- 51. Sun Y. B., Lou F., Irving M. (2009) Calcium- and myosin-dependent changes in troponin structure during activation of heart muscle. J. Physiol. 587, 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Suzuki T., Palmer B. M., James J., Wang Y., Chen Z., VanBuren P., Maughan D. W., Robbins J., LeWinter M. M. (2009) Effects of cardiac myosin isoform variation on myofilament function and cross-bridge kinetics in transgenic rabbits. Circ. Heart Fail. 2, 334–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maughan D., Moore J., Vigoreaux J., Barnes B., Mulieri L. A. (1998) Work production and work absorption in muscle strips from vertebrate cardiac and insect flight muscle fibers. Adv. Exp. Med. Biol. 453, 471–480 [DOI] [PubMed] [Google Scholar]
- 54. McCurdy D. T., Palmer B. M., Maughan D. W., LeWinter M. M. (2001) Myocardial cross-bridge kinetics in transition to failure in Dahl salt-sensitive rats. Am. J. Physiol. Heart Circ. Physiol. 281, H1390–H1396 [DOI] [PubMed] [Google Scholar]
- 55. Kad N. M., Rovner A. S., Fagnant P. M., Joel P. B., Kennedy G. G., Patlak J. B., Warshaw D. M., Trybus K. M. (2003) A mutant heterodimeric myosin with one inactive head generates maximal displacement. J. Cell Biol. 162, 481–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kovács M., Thirumurugan K., Knight P. J., Sellers J. R. (2007) Load-dependent mechanism of nonmuscle myosin 2. Proc. Natl. Acad. Sci. U.S.A. 104, 9994–9999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Laakso J. M., Lewis J. H., Shuman H., Ostap E. M. (2008) Myosin I can act as a molecular force sensor. Science 321, 133–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Veigel C., Schmitz S., Wang F., Sellers J. R. (2005) Load-dependent kinetics of myosin-V can explain its high processivity. Nat. Cell Biol. 7, 861–869 [DOI] [PubMed] [Google Scholar]
- 59. Veigel C., Molloy J. E., Schmitz S., Kendrick-Jones J. (2003) Load-dependent kinetics of force production by smooth muscle myosin measured with optical tweezers. Nat. Cell Biol. 5, 980–986 [DOI] [PubMed] [Google Scholar]
- 60. Greenberg M. J., Kazmierczak K., Szczesna-Cordary D., Moore J. R. (2010) Cardiomyopathy-linked myosin regulatory light chain mutations disrupt myosin strain-dependent biochemistry. Proc. Natl. Acad. Sci. U.S.A. 107, 17403–17408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee R. S., Tikunova S. B., Kline K. P., Zot H. G., Hasbun J. E., Minh N. V., Swartz D. R., Rall J. A., Davis J. P. (2010) Effect of Ca2+ binding properties of troponin C on rate of skeletal muscle force redevelopment. Am. J. Physiol. Cell Physiol. 299, C1091–C1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kreutziger K. L., Piroddi N., McMichael J. T., Tesi C., Poggesi C., Regnier M. (2011) Calcium binding kinetics of troponin C strongly modulate cooperative activation and tension kinetics in cardiac muscle. J. Mol. Cell. Cardiol. 50, 165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Allen D. G., Kentish J. C. (1988) Calcium concentration in the myoplasm of skinned ferret ventricular muscle following changes in muscle length. J. Physiol. 407, 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Housmans P. R., Lee N. K., Blinks J. R. (1983) Active shortening retards the decline of the intracellular calcium transient in mammalian heart muscle. Science 221, 159–161 [DOI] [PubMed] [Google Scholar]
- 65. de Tombe P. P., Stienen G. J. (2007) Impact of temperature on cross-bridge cycling kinetics in rat myocardium. J. Physiol. 584, 591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]