Background: Ricin is reported to be a bioterrorism agent.
Results: Reconstituted fat-free milk removes ricin from a glycoprotein-ricin complex and competitively binds to ricin and inhibits its biological effect in Vero cells.
Conclusion: The protective effect was at the same level as that of the anti-ricin antibody.
Significance: The competitive inhibition by milk compounds reduces the amount of toxin uptake by the cells.
Keywords: Bacterial Toxins, Cell Surface Receptor, Galactose, Glycoprotein, Lectin, Toxins, Biological Activity, Inhibition, Ricin, Vero Cells
Abstract
Ricin is a highly toxic protein produced by the castor plant Ricinus communis. The toxin is relatively easy to isolate and can be used as a biological weapon. There is great interest in identifying effective inhibitors for ricin. In this study, we demonstrated by three independent assays that a component of reconstituted powdered milk has a high binding affinity to ricin. We discovered that milk can competitively bind to and reduce the amount of toxin available to asialofetuin type II, which is used as a model to study the binding of ricin to galactose cell-surface receptors. Milk also removes ricin bound to the microtiter plate. In parallel experiments, we demonstrated by activity assay and by immuno-PCR that milk can bind competitively to 1 ng/ml ricin, reducing the amount of toxin uptake by the cells, and thus inhibit the biological activity of ricin. The inhibitory effect of milk on ricin activity in Vero cells was at the same level as by anti-ricin antibodies. We also found that (a) milk did not inhibit ricin at concentrations of 10 or 100 ng/ml; (b) autoclaving 10 and 100 ng/ml ricin in DMEM at 121 °C for 30 min completely abolished activity; and (c) milk did not affect the activity of another ribosome inactivating protein, Shiga toxin type 2 (Stx2), produced by pathogenic Escherichia coli O157:H7. Unlike ricin, which is internalized into the cells via a galactose-binding site, Stx2 is internalized through the cell surface receptor glycolipid globotriasylceramides Gb3 and Gb4. These observations suggest that ricin toxicity may possibly be reduced at room temperature by a widely consumed natural liquid food.
Introduction
The castor bean plant Ricinus communis, which is harvested worldwide, is a major source of castor oil that can be used industrially for many purposes, including as a source for an inexpensive biodiesel fuel (1). The poisonous plant seeds are a ubiquitous and inexpensive source of the ricin toxin that can be used as a biological bioterrorism agent (2).
In the plant, ricin is translated as a single 66-kDa polypeptide chain protein that is activated intracellularly by proteolytic cleavage to form the active 32-kDa A chain containing enzymatic activity (3, 4). The B chain is essential for the toxin's entry into the cell. The A chain is linked by a disulfide bond to the 34-kDa B chain lectin, which has an affinity to bind to cell surface carbohydrates such as galactose, galactosamine, or N-acetylgalactosamine present in glycoproteins and glycolipids. The toxin enters the cell by endocytosis in membrane vesicles and is transported to endosomes and then into the cytosol. After the disulfide bond is reduced, the ricin A chain inactivates ribosomes by removing the 28 S ribosomal RNA in the 60 S ribosomal subunit at the adenine nucleotide (A4324) near the 3′ end of the polynucleotide chain (5). This deletion results in the failure of elongation factor-2 to bind to the ribosome and thus inhibits protein synthesis, resulting in cell death (3, 6). Because ricin has already been proven to be an effective bioterrorism agent, there is interest in identifying and designing effective inhibitors of ricin to counteract the bioterrorism threat.
Previous studies indicate the following: (a) anti-ricin antibodies can block the binding of the toxin to cells by interfering with galactose-binding sites (7, 8), thus conferring protective immunity against ricin; (b) ricin toxicity can be inhibited by synthetic carbohydrate and peptide compounds (9–11); and (c) ricin is detected readily in milk (12). Because synthetic anti-ricin compounds might not be safe to consume or be food-compatible, the objective of our studies is to explore the potential of foods and their bioactive constituents to inhibit the biological activity of ricin and other toxins. In the present study, we show that commercial fat-free milk has the potential to inhibit the biological activity of ricin, presumably because milk contains galactose and lactose, which are known to inhibit the toxin by attachment to sugar binding domains of the ricin B chain, thus competitively inhibit the entry of the toxin into the cytosol and abolish its activity (13–15).
EXPERIMENTAL PROCEDURES
Materials
Monoclonal antibodies clone number 1642 targeting the ricin A chain (RTA) were provided kindly by Dr. David L. Brandon (U. S. Department of Agriculture, Agricultural Research Service, Western Regional Research Center, Foodborne Contaminants Research Unit, Albany, CA) (16). Shiga toxin Stx22 was purchased from List Biological Laboratories, Inc. (Campbell, CA). Purified ricin (5 mg/ml) was obtained from Vector Laboratories (Burlingame, CA); type I and type II asialofetuin and glycoproteins from fetal calf serum were from Sigma-Aldrich; goat anti-mouse IgG-horseradish peroxidase conjugate was from Calbiochem (La Jolla, CA); human kidney cell line (HEK293) transformed with adenovirus 5 DNA (ATCC CRL-1573) was from the American Type Culture Collection (ATCC, Manassas, VA); Vero cells and African Green Monkey adult kidney cells (17) were from ATCC; and instant nonfat dry milk powder fortified with vitamins A and D (Nestle/Carnation Brand), designated in this paper as “milk,” was from a local store.
Reconstituted Powdered Milk
Fat-free commercial milk powder was dissolved in distilled water to form 5, 1, 0.5, and 0.15% milk stock solutions. To determine whether milk will interfere with ricin attachment to 96-well microplate or whether milk will remove bound ricin from plates, these stock milk solutions (100 μl) were each added in separate experiments. To determine whether milk will have the same effect in vitro and whether it will remove ricin from cell surfaces, each milk stock solution (15 μl) was added to the media (85 μl). The final concentration of milk in each well was thus reduced from 1, 0.5, and 0.1% to 0.15, 0.075, and 0.0225%, respectively. Aliquots of this solution were added to ricin-containing samples, which were then evaluated for bioactivity as described below.
Detection of Ricin Toxin by ELISA
Asialofetuin type I or type II was diluted to 2 μg/ml in 0.5 m sodium carbonate buffer (pH 9.9). This dilution (50 μl) was added to wells of a 96-well microplate and incubated overnight at 4 °C. The wells were blocked with 200 μl of TBS (50 mm Tris/HCl, pH 7.5, 150 mm NaCl) containing 2% fetal calf serum (Hyclone, Logan, UT) for 1 h. After incubation, increasing concentrations of ricin diluted in blocking buffer containing 5, 1, 0.5, and 0.1% milk were added to 100 μl/well and then incubated for 1 h at room temperature. The plate was washed five times with TBS containing 0.1% Tween 20 to remove all unbound toxin. Mouse anti ricin IgG at concentration of 0.44 mg/ml was diluted 1:10,000 in TBS; and then, 100 μl of this dilution was added to the wells, and the plates were then incubated for 1 h at room temperature. Following incubation, the wells were washed five times with TBS-Tween. Next, 100 μl of goat anti-mouse IgG-horseradish peroxidase (HRP) conjugate (Calbiochem) diluted 1:5000 in TBS-Tween was added and incubated for 1 h at room temperature. Wells were again washed with TBS-Tween. 3,3′,5,5′-Tetramethybenzidine substrate (100 μl) was then added to each well and incubated for 30 min at room temperature. The reaction was stopped by adding 50 μl of 0.3 n HCl per well. Results were obtained by measuring the absorbance at 450 nm.
Cell Culture
Vero cells and HEK293 were cultured at in 75 cm2 flasks and maintained in DMEM containing 0.584 mg/ml of l-glutamine, 10% fetal bovine serum (FBS), and 100 units/ml of both penicillin and streptomycin. Cells were trypsinized when ready to harvest. To detach the cultured cells, flasks were rinsed with 10 ml of Dulbecco's phosphate-buffered saline (D-PBS), then trypsinized with 2 ml of 0.05% trypsin-EDTA solution (Invitrogen), and incubated for 3 min at 37 °C in a 5% CO2 incubator.
Generation of Adenoviral Vectors That Express Green Fluorescent Protein (GFP) Gene
To visualize and quantify the effect of ricin on living cells, we measured changes in the fluorescence intensity level of the GFP. The GFP gene was isolated from the Green Lantern vector (BRL) by digestion with the NotI restriction enzyme. The 750-bp fragment was purified from the gel using a Qiagen kit and was subcloned into the NotI site of the adenoviral shuttle plasmid between the cytomegalovirus (CMV) immediate-early promoter and the polyadenylation signal from bovine growth hormone. The plasmid pJM17 containing the full-length of the adenovirus genome including a 4.4-kb sequence of antibiotic-resistant gene, was co-transfected in HEK293 cells along with the shuttle plasmid containing the GFP gene flanked by the adenovirus E1 sequences. A cytopathic effect was observed after 10 days, and the transfected cells became round and detached from the plate. The cells were then analyzed by fluorescence microscopy to detect GFP gene expression. An individual plaque of the adenovirus vector that encoded and expressed the GFP gene (Ad-GFP) was amplified. The presence of GFP was confirmed by measuring the fluorescence signal intensity in transduced cells in a Synergy HT Multi-Detection Microplate Reader (BioTek, Winooki, VT) with a 485-nm excitation wavelength using a 485/20 excitation filter and a 528-nm emission wavelength using a 528/20 emission filter.
Plaque Assays for Purification and Titration of Adenovirus
Plaque assays depend on the ability of the adenovirus to propagate in HEK293 cells. Six 35-mm tissue culture plates were seeded with HEK293 cells. The cells were incubated at 37 °C in a 5% CO2 incubator until they were 90% confluent. Serial dilutions were made in DMEM supplemented with 2% FBS. The diluted virus was then added to the cells. After 2 h, the medium was removed and replaced with 1× DMEM and 1% SeaPlaqueTM agarose from Lonza Group Ltd. (Rockland, ME). The agar overlay was added to keep the virus localized after the cells had lysed. Plaques were visible after 5 days and counted for titer determination after 7 days.
Preparation of High-titer Viral Stocks
Because most of the virus remains associated with the infected cells until very late in the infection process, high-titer viral stocks were prepared by concentrating the infected cells. Tissue culture flasks (175 mm) were seeded with HEK293 cells in DMEM containing 10% FCS and 100 units/ml of penicillin/streptomycin. When cells reached 90% confluence, they were infected with the virus at a multiplicity of infection of 10. When the cytopathic effect was nearly completed (after 48–72 h) and most of the cells were rounded but not yet detached, the cells were harvested. (They were easily dislodged by tapping.) Cells were pelleted by centrifugation at 800 × g for 5 min at 4 °C. Both cell pellet and supernatant were collected. Because most progeny viruses remain cell-associated, infected cells were disrupted by freeze-thaw cycles followed by lysis as described below. To every cell pellet collected from 18 flasks (175 mm), 18 ml of PBS, 1 mm MgCl2, 0.1% Nonidet P-40, 1 mm CaCl2 were added for hypotonic lysis followed by three rounds of freeze thawing. Crude lysates were then pelleted to remove cellular debris by centrifugation at 9500 × g for 20 min at 4 °C. The supernatants that contained the crude viral lysates were removed carefully. The viral supernatants were loaded onto CsCl step gradients made by layering three densities of CsCl (1.25, 1.33, and 1.45 g/ml) and centrifuged at 50,000 × g for 2 h in a Beckman SW41 rotor at 14 °C. The lower band containing the intact packaged virus was removed, and a second step density gradient of 1.33 g/ml CsCl was centrifuged for 16 h at 48,000 × g at 14 °C. The lower band containing the packaged virus was collected and dialyzed against 10% glycerol in saline solution. Viral titer was determined by plaque assay. The stock of this virus contained 1011 plaque-forming units/ml.
Quantitative Measurement for Active Ricin or Stx2 Toxins
Vero cells were plated on black 96-well plates (Greiner 655090 obtained from Sigma) at 1 × 104 cells in 100 μl of medium per well. Cells were incubated overnight to allow time for cells to attach to the plate. The cells were then transduced with Ad-GFP at a multiplicity of infection of 100. After 1 h, samples (either 15 μl of milk sample in 85 μl of media, or 100 μl of media spiked with the toxin ricin or Stx2) were added to each well and incubated for 24–72 h at 37 °C in a 5% CO2 incubator. The sample was removed, and cells washed three times with PBS. The GFP fluorescence intensity was measured in a Synergy HT multidetection microplate reader (BioTek, Winooki, VT) with a 485-nm excitation wavelength using a 485/20 excitation filter and a 528-nm emission wavelength using a 528/20 emission filter (18, 19).
Immuno-PCR Assay
Immuno-PCR was performed as described for sandwich immuno-PCR for ricin (20) with minor modifications. Briefly, anti-ricin mAb (30 μl, 4 μg/ml) in borate buffer was used to coat USA Scientific v-bottom microtiter wells overnight at 4 °C. After blocking of non-adsorbed sites with 150 μl of 0.5% BSA/Tris-buffered-saline for 1 h at room temperature, ricin samples (30 μl) were added to each well. The plates were then incubated at room temperature for 1 h. The plates were washed with TBS-EDTA-Tween (Tris-buffered saline containing 5 mm EDTA and 0.05% Tween 20). This was followed by addition of streptavidin-conjugated anti-ricin polyclonal antibody (30 μl; 100 ng/ml) to each well and incubation at 37 °C for 1 h. After washing of the plates, the biotinylated DNA marker (30 μl; 0.5 ng/μl) was added to each well, and the plates were incubated at room temperature for 1 h. After thorough washing to remove unbound DNA, the bound DNA was detached from the immunocomplex by the restriction enzyme BamHI. Aliquots (6 μl) were used as template in a real-time PCR.
Effect of Heat on Ricin Activity
We carried out two experiments on the effect of heat on ricin activity. In the first experiment, milk or DMEM (500 μl) spiked with 1 ng/ml of ricin in tightly screw-capped Eppendorf tubes (1.5 ml) were inserted in an Eppendorf dry heating block (Government Scientific Source, Inc., Reston, VA) at 99 °C for 5 min. The cooled samples were then analyzed for ricin activity.
In the second experiment, three ricin concentrations (1, 10, and 100 ng/ml), each dissolved in the DMEM (500 μl) in loose screw-capped Eppendorf tubes (1.5 ml) were placed into a plastic container containing water. The container was then placed in an autoclave set at 121 °C for 30 min. The cooled samples were then analyzed for ricin activity.
RESULTS
Optimization of Galactose-binding Lectins as Model for Studying Binding of Ricin to Galactose Cell-surface Receptor
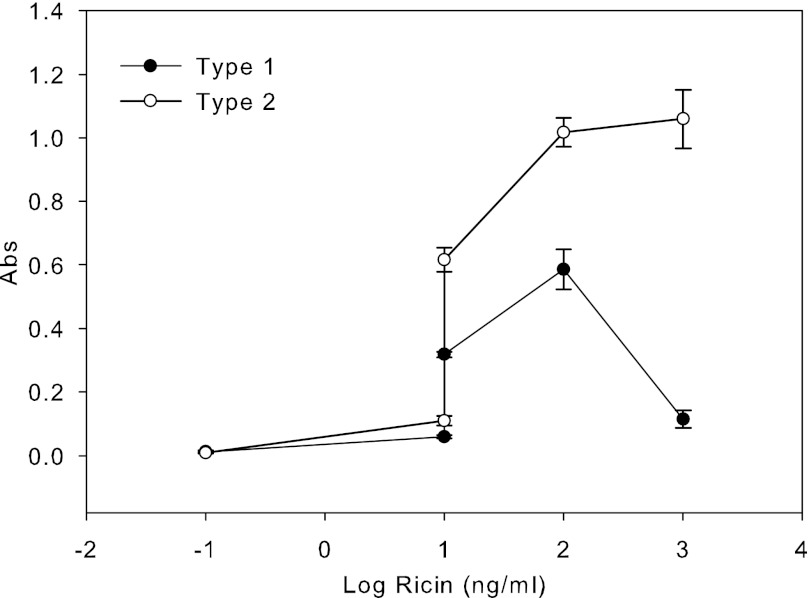
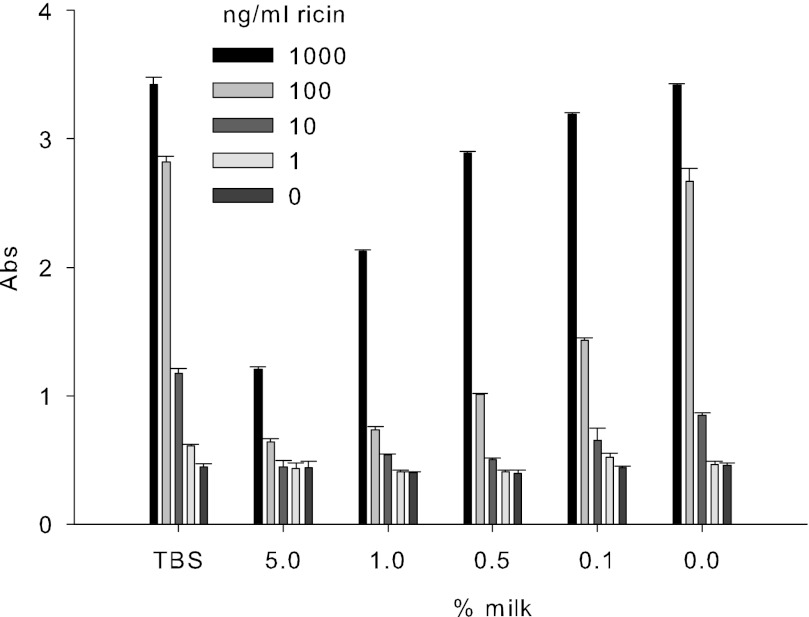
The ricin B chain mediates entry of the A-B protein complex into the cytosol and has two sugar-binding domains that attach to galactose residues on the surfaces of target cells. We used commercial glycoproteins containing terminal galactose residues as a model to study the binding of ricin to the galactose cell-surface receptor. To optimize ricin binding to microtiter plates, we first determined the relative affinities of ricin to two types of asialofetuin, type I and type II. Our ELISA shows that both types are capable of binding to ricin. We were able to detect ricin at 1 ng/ml. The maximum binding capacity of ricin with both types of asialofetuin reached 100 ng/ml. Fig. 1 shows that the plate coated with type II asialofetuin has a higher binding affinity to ricin than type I. We therefore selected type II for the additional experiments. To determine whether milk will capture and interfere with ricin binding, we added increasing concentrations of ricin with different concentrations of milk to a microtiter plate coated with type II asialofetuin. As shown in Fig. 2, milk interfered with ricin attachment to the coated plates in a concentration-dependent manner. For example, milk at concentrations of 0.1, 0.5, 1, and 5% reduced the attachment of 100 ng/ml ricin by 46, 62, 72, and 76%, respectively.
FIGURE 1.
Assay optimization. Comparison between binding of ricin to asialofetuin type I and II using an ELISA. The figure demonstrates that asialofetuin type II produced a higher signal than did type I, suggesting that it has a stronger binding affinity to ricin than the corresponding type I. The maximum ricin binding capacity was 100 ng/ml. Absorbance (Abs) was read at 450 nm. Error bars represent S.E. (n = 3).
FIGURE 2.
Milk competitively inhibits attachment of ricin to asialofetuin type II in a concentration-dependent response. Increasing concentrations of ricin were added together with different concentrations of milk to microtiter plate coated with type II asialofetuin. After incubation, the plate was washed, and anti-ricin IgG was added, followed by addition of HRP-conjugated goat anti-rabbit IgG. The plates were developed, and the results were obtained by measuring the absorbance (Abs) at 450 nm. Error bars represent S.E. (n = 3).
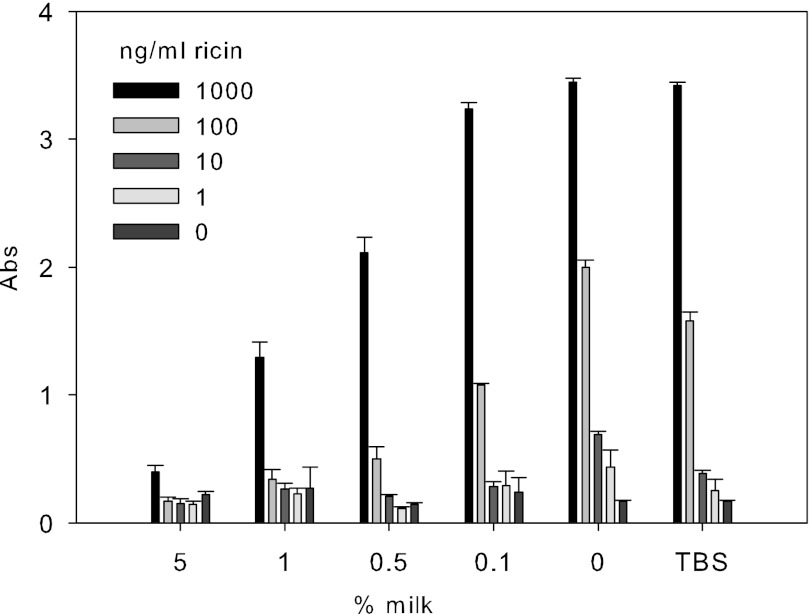
To mimic post-exposure to ricin and to determine whether milk will capture and remove bound ricin from microtiter plates, we added increasing concentrations of milk to different ricin concentrations that had been bound on the microtiter plate for 15 min. As shown in Fig. 3, milk has a higher binding affinity to ricin than does asialofetuin type II. It removed ricin from the plate in a concentration-dependent manner. Milk at concentrations of 0.1, 0.5, 1, and 5% completely removed bound ricin at concentrations of 1 ng/ml and 10 ng/ml and reduced the attachment of 1000 ng/ml ricin by 6.2, 38.5, 62.4, and 88.36%, respectively.
FIGURE 3.
Milk removes bound ricin from asialofetuin type II-coated plate in concentration-dependent response. Increasing concentrations of milk were added to different ricin concentrations that had been bound on the microtiter plate. The plate was washed, and anti-ricin IgG was added, followed by more washing and then addition of HRP-conjugated goat anti-rabbit IgG. The plates were developed, and the results were obtained by measuring the absorbance (Abs) at 450 nm. Error bars represent S.E. (n = 3).
Effect of Milk on Biological Activity of Ricin
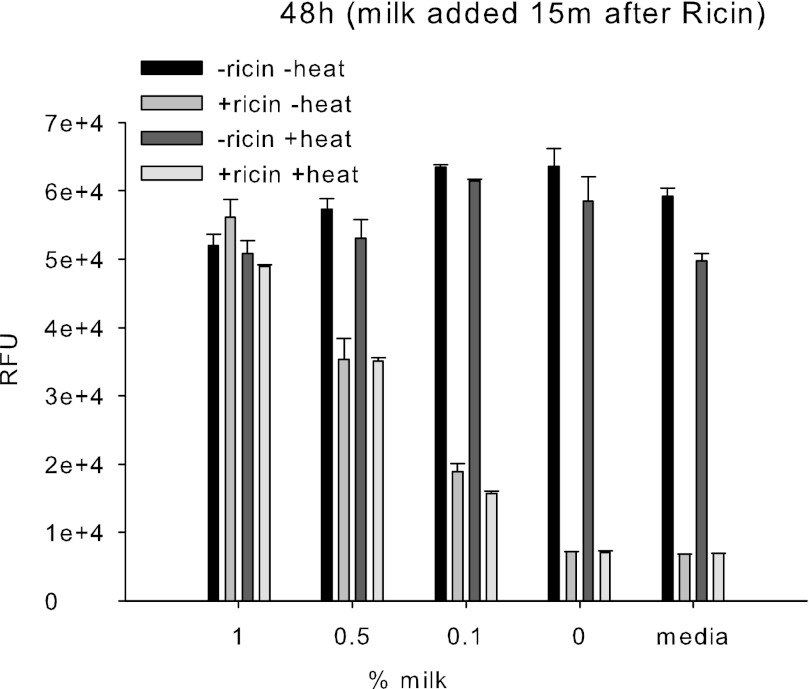
To investigate whether milk has the same effect in vitro as on cell-surface receptor model, and to determine whether thermal treatment of ricin inhibits biological activity, unheated ricin (1 ng/ml) or heat-treated ricin (99 °C for 5 min) was incubated for 15 min in 96-well microtiter plates seeded with GFP-transduced Vero cells. Increasing concentrations of milk were then added to the plates. After 48 h, cells were washed, and fluorescence emission intensity was measured. The quantitative measurement of active ricin was based on the inhibition of relative fluorescence intensity.
Fig. 4 shows that milk has high binding affinity to ricin. Indeed, after 15 min of exposure to ricin, milk reduced the availability of the toxin to cells and inhibited its biological activity in a concentration-dependent manner for both heated and unheated samples. Of the concentrations tested, 1% milk (0.15 mg milk powder/well) induced the highest reduction in biological activity of ricin compared with the other concentrations evaluated. It reduced the availability and the amount of toxin uptake by cells and protected Vero cell from protein inhibition effects, as indicated by reduction in the fluorescent expression of ricin-treated cells compared with control. We could not detect ricin in 1% milk at a concentration of 1 ng/ml. Therefore, 0.15 mg milk powder completely inactivated the toxicity of 1 ng of ricin. Additional studies also show that (a) milk was effective at 1 ng/ml; (b) only marginally (statistically not significant) effective at ricin concentration of 10 ng/ml; (c) milk was not effective at the ricin concentration of 100 ng/ml; (d) ricin at 1 ng/ml in DMEM was resistant to heat treatment at 99 °C for 5 min; and (e) ricin activity was abolished after autoclaving all three concentrations in DMEM at 121 °C for 30 min.
FIGURE 4.
Milk inhibits the biological activity of ricin. Ricin (1 ng/ml) in the absence and the presence of heat (99 °C, 5 min) was added to Vero cells transduced with Ad-GFP. After 15 min incubation, increasing concentrations of milk were added to the test samples. The inhibition of relative fluorescence intensity was quantified fluorometrically after 48 h. Error bars represent S.E. (n = 3). RFU, relative fluorescence units.
Milk Inhibits Uptake of Ricin by Cells
Our hypothesis is that milk inhibits ricin activity by capturing the toxin, thus reducing the number of toxin molecules that enter the cell. To demonstrate this possibility, we used the highly sensitive immuno-PCR assay, which combines the advantages of both ELISA and PCR, to measure the relative concentration of free (unbound) ricin in the supernatant that was not taken up by the cells. Fig. 5 shows that milk reduced the uptake of ricin by the cells and that there is a negative correlation between milk concentration and ricin uptake. The concentration of free ricin in the supernatant with 0% milk was less than in the samples treated with 0.1, 0.5, or 1% milk. These results confirm that milk prevents the uptake of ricin by the cells, thus preventing the toxin from inactivating the cell ribosomes, inhibiting protein synthesis, and reducing the GFP fluorescence intensity.
FIGURE 5.
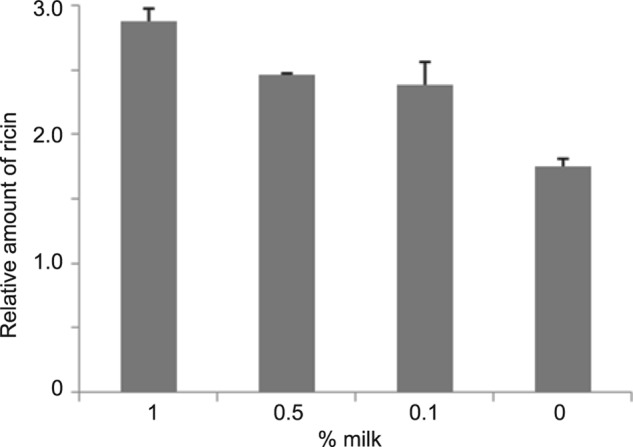
Milk captures ricin and reduces the number of toxin molecules that enter the cell. Ricin (1 ng/ml) was added to Vero cells. After 15 min of incubation, increasing concentrations of milk were added to the test samples. The relative concentration of free (unbound) ricin in the supernatant that was not taken up by the cells was quantified after 48 h by sandwich immuno-PCR assay. Error bars represent S.E. (n = 3).
Milk Inhibits Ricin but Not Shiga Stx2 Toxin
To demonstrate that inhibition of toxin activity is specific to ricin, we also evaluated the effect of milk on the Stx2 produced by enterohemorrhagic strain Escherichia coli O157:H7. This bacterial toxin acts similarly to ricin in inactivating ribosome proteins, and similar to ricin, is composed of two subunits. However, in contrast to ricin, the Stx2 B chain binds specifically to the cell surface receptor glycolipid globotriasylceramides Gb3 and Gb4. In addition, the two toxins seem to use different endocytic pathways (21).
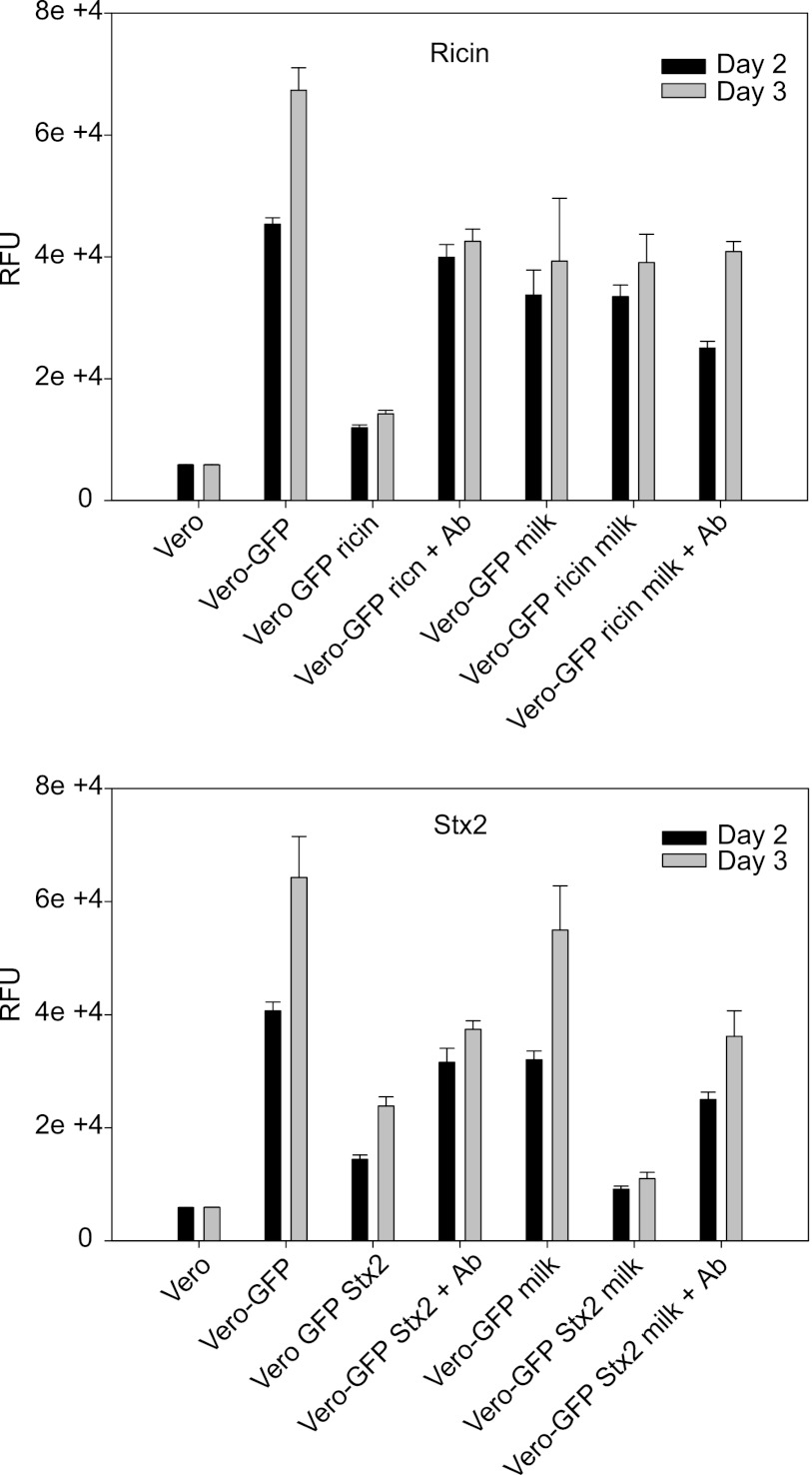
To test the specificity of milk inhibition and antibody neutralization, we compared the inhibition of GFP expression in Vero cells by Stx2 (1 ng/ml) and ricin (1 ng/ml) in the presence and absence of milk or antibodies. Fig. 6 shows that milk had the same protective effect in inhibiting ricin as did the anti-ricin antibody, as indicated by GFP fluorescence intensity levels. The results were nearly the same for the milk samples with and without ricin, indicating that 1% milk was fully effective at inhibiting ricin toxicity.
FIGURE 6.
Milk inhibits toxicity of ricin but not Shiga toxin. Vero cells grown in 96-well microtiter plates transduced with Ad-GFP and treated with ricin (1 ng/ml) or Stx2 (1 ng/ml) followed by addition of 1% milk or anti-ricin antibody. After 48 h, the inhibition of relative fluorescence intensity was quantified fluorometrically. Error bars represent S.E. RFU, relative fluorescence units.
Fig. 6 also shows that, as expected, anti-Stx2 antibody reduced Stx2 biological activity. It seems that because milk does not competitively bind to Stx2, it did not inhibit the toxic effects of Stx2. By contrast, milk component(s) did competitively bind to ricin, making the toxin unavailable to galactoside receptors.
DISCUSSION
The present study aimed to establish whether reconstituted liquid milk prepared from a commercial milk powder could inhibit the toxic effects of ricin. With the aid of a cell culture model, we demonstrated that milk is capable of protecting cultured monkey epithelial cells from the inhibition of the ribosome inactivating effects of ricin in a dose-dependent manner. The following findings lead us to believe that the mode of action of milk in inhibiting the toxicity of ricin seems to take place by competitively binding to ricin, making the toxin unavailable to galactoside receptors sites on the cell surfaces. (a) Milk decreased ricin uptake into the cells as evidenced by increased levels of ricin in the supernatant measured by immuno-PCR. (b) Milk bound competitively to and reduced the amount of ricin available to immobilized asialofetuin type II, which is used as a model to study the binding of ricin to galactose cell-surface receptors. (c) Milk has a high binding affinity to ricin. It removes bound ricin from the microplate in a concentration-dependent manner. (d) Milk contains galactose (hydrolysis product of lactose), galactosamine, N-acetyl galactosamine-containing oligosaccharides as well as glycoproteins (22–27) and has the same protective effect in removing bound ricin from cells as the anti-ricin antibody. (e) Milk did not bind competitively to or inhibit the toxic effects of the Stx2 toxin, presumably because Stx2 does not attach to galactoside receptor sites, as does ricin. Because pH and presence of digestive enzymes in the digestive tract may cause release of ricin rendering the protein toxic in vivo, we do not know whether the in vitro assays that were used to estimate toxicity would apply to oral exposure to ricin in animals and humans.
Whether milk could be used to prevent and treat ricin intoxication shortly after exposure merits further study. It is relevant to also note that human milk secretory IgA is reported to bind to and limit botulinum toxin adherence to intestinal eptithelial T84 cells (28). By contrast, human milk oligosaccharides did not significantly inhibit the cytotoxic effects of two bacterial toxins produced by Clostridium difficile (29).
The results of the present study show that milk prevented the inhibition of protein synthesis in Vero cells by 1 ng/ml of ricin as indicated by GFP fluorescence intensity levels. The protective effect of milk was at the same level as that of the anti-ricin antibody. We could not detect any ricin activity in milk (1%) with an initial concentration of 1 ng/ml. Although the effective inhibitory dose of 1 ng of ricin/ml of milk seems low, ricin is toxic at very low concentrations, e.g. inhalation LD50 dose equals 0.24 ng/g in rats (30, 31). It is relevant to note that only ∼1% of Shiga toxin fed orally to mice was absorbed into the blood circulation (19).
By contrast, exposure of mouse macrophage cells to infant milk formula containing ricin at 100 μg/ml, compared with the maximum concentration of 1 μg/ml used in the present study, did not inhibit protein synthesis in the cells (32). This result is consistent with our finding that milk did not inactivate ricin at concentrations of 10 or 100 ng/ml. Evidently, milk and milk-based products might not inhibit the adverse biological effects of very high concentrations of ricin at room temperature.
Acknowledgments
We thank Stephanie McMahon for technical assistance, Carol E. Levin for assistance with the preparation of the manuscript, and Daphne Tamar and Sharon Abigail for helpful suggestions.
Footnotes
- Stx2
- Shiga toxin type 2
- Ad
- adenovirus.
REFERENCES
- 1. Wang M. L., Morris J. B., Tonnis B., Pinnow D., Davis J., Raymer P., Pederson G. A. (2011) Screening of the entire USDA castor germplasm collection for oil content and fatty acid composition for optimum biodiesel production. J. Agric. Food Chem. 59, 9250–9256 [DOI] [PubMed] [Google Scholar]
- 2. Poore C., Clark P., Emanuel P. A. (2009) An evaluation of suspicious powder screening tools for first responders. J. Hazard. Mater. 172, 559–565 [DOI] [PubMed] [Google Scholar]
- 3. Lord M. J., Jolliffe N. A., Marsden C. J., Pateman C. S., Smith D. C., Spooner R. A., Watson P. D., Roberts L. M. (2003) Ricin. Mechanisms of cytotoxicity. Toxicol. Rev. 22, 53–64 [DOI] [PubMed] [Google Scholar]
- 4. Jasheway K., Pruet J., Anslyn E. V., Robertus J. D. (2011) Structure-based design of ricin inhibitors. Toxins 3, 1233–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barbieri L., Ciani M., Girbés T., Liu W. Y., Van Damme E. J., Peumans W. J., Stirpe F. (2004) Enzymatic activity of toxic and non-toxic type 2 ribosome-inactivating proteins. FEBS Lett. 563, 219–222 [DOI] [PubMed] [Google Scholar]
- 6. Hartley M. R., Lord J. M. (2004) Cytotoxic ribosome-inactivating lectins from plants. Biochim. Biophys. Acta 1701, 1–14 [DOI] [PubMed] [Google Scholar]
- 7. Yermakova A., Mantis N. J. (2011) Protective immunity to ricin toxin conferred by antibodies against the toxin's binding subunit (RTB). Vaccine 29, 7925–7935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McGuinness C. R., Mantis N. J. (2006) Characterization of a novel high-affinity monoclonal immunoglobulin G antibody against the ricin B subunit. Infect. Immun. 74, 3463–3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mantis N. J. (2005) Vaccines against the category B toxins: Staphylococcal enterotoxin B, epsilon toxin and ricin. Adv. Drug Deliv. Rev. 57, 1424–1439 [DOI] [PubMed] [Google Scholar]
- 10. Bai Y., Monzingo A. F., Robertus J. D. (2009) The x-ray structure of ricin A chain with a novel inhibitor. Arch. Biochem. Biophys. 483, 23–28 [DOI] [PubMed] [Google Scholar]
- 11. Rainey G. J., Young J. A. (2004) Antitoxins: Novel strategies to target agents of bioterrorism. Nat. Rev. Microbiol. 2, 721–726 [DOI] [PubMed] [Google Scholar]
- 12. He L., Deen B., Rodda T., Ronningen I., Blasius T., Haynes C., Diez-Gonzalez F., Labuza T. P. (2011) Rapid detection of ricin in milk using immunomagnetic separation combined with surface-enhanced raman spectroscopy. J. Food Sci. 76, N49-N53 [DOI] [PubMed] [Google Scholar]
- 13. Zentz C., Frénoy J. P., Bourrillon R. (1978) Binding of galactose and lactose to ricin. Equilibrium studies. Biochim. Biophys. Acta 536, 18–26 [DOI] [PubMed] [Google Scholar]
- 14. Nagatsuka T., Uzawa H., Ohsawa I., Seto Y., Nishida Y. (2010) Use of lactose against the deadly biological toxin ricin. ACS Appl. Mater. Interfaces 2, 1081–1085 [DOI] [PubMed] [Google Scholar]
- 15. Labuza T. P., Diez-Gonzalez F., Lumor S. E., Deen B. D., Roningen I., Smith K. (2011) Effect of lactose on the biological activity of ricin, Annual Meeting of the International Association for Food Protection, Abstract P1–42, August 1–4, Milwaukee, WI [Google Scholar]
- 16. Brandon D. L. (2011) Detection of ricin contamination in ground beef by electrochemiluminescence immunosorbent assay. Toxins 3, 398–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blum J. S., Fiani M. L., Stahl P. D. (1991) Proteolytic cleavage of ricin A chain in endosomal vesicles. Evidence for the action of endosomal proteases at both neutral and acidic pH. J. Biol. Chem. 266, 22091–22095 [PubMed] [Google Scholar]
- 18. Rasooly R., Do P. M., Levin C. E., Friedman M. (2010) Inhibition of Shiga toxin 2 (Stx2) in apple juices and its resistance to pasteurization. J. Food Sci. 75, M296-M301 [DOI] [PubMed] [Google Scholar]
- 19. Rasooly R., Do P. M., Griffey S. M., Vilches-Moure J. G., Friedman M. (2010) Ingested Shiga toxin 2 (Stx2) causes histopathological changes in kidney, spleen, and thymus tissues and mortality in mice. J. Agric. Food Chem. 58, 9281–9286 [DOI] [PubMed] [Google Scholar]
- 20. He X., McMahon S., McKeon T. A., Brandon D. L. (2010) Development of a novel immuno-PCR assay for detection of ricin in ground beef, liquid chicken egg, and milk. J. Food Prot. 73, 695–700 [DOI] [PubMed] [Google Scholar]
- 21. Sandvig K., van Deurs B. (2000) Entry of ricin and Shiga toxin into cells: Molecular mechanisms and medical perspectives. EMBO J. 19, 5943–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mijan M. A., Lee Y. K., Kwak H. S. (2011) Classification, structure, and bioactive functions of oligosaccharides in milk. Korean J. Food Sci. An. 31, 631–640 [Google Scholar]
- 23. Casado B., Affolter M., Kussmann M. (2009) OMICS-rooted studies of milk proteins, oligosaccharides, and lipids. J. Proteomics 73, 196–208 [DOI] [PubMed] [Google Scholar]
- 24. Rao R. D., Wendorff W. L., Smith K. (2004) Changes in galactose and lactic acid content of sweet whey during storage. J. Food Prot. 67, 403–406 [DOI] [PubMed] [Google Scholar]
- 25. Forsum E. (1975) Whey proteins for food and feed supplement in Protein Nutritional Quality of Foods and Feeds. Part II. Quality Factors- Plant Breeding, Composition, Processing, and Antinutrients (Friedman M., ed) pp. 433–470, Mercel Dekker, New York [Google Scholar]
- 26. Horner T. W., Dunn M. L., Eggett D. L., Ogden L. V. (2011) β-Galactosidase activity of commercial lactase samples in raw and pasteurized milk at refrigerated temperatures. J. Dairy Sci. 94, 3242–3249 [DOI] [PubMed] [Google Scholar]
- 27. Brown-Esters O., Mc Namara P., Savaiano D. (2012) Dietary and biological factors influencing lactose intolerance. Int. Dairy J. 22, 98–103 [Google Scholar]
- 28. Matsumura T., Fujinaga Y., Jin Y., Kabumoto Y., Oguma K. (2007) Human milk SIgA binds to botulinum type B 16S toxin and limits toxin adherence on T84 cells. Biochem. Biophys. Res. Commun. 352, 867–872 [DOI] [PubMed] [Google Scholar]
- 29. El-Hawiet A., Kitova E. N., Kitov P. I., Eugenio L., Ng K. K., Mulvey G. L., Dingle T. C., Szpacenko A., Armstrong G. D., Klassen J. S. (2011) Binding of Clostridium difficile toxins to human milk oligosaccharides. Glycobiology 21, 1217–1227 [DOI] [PubMed] [Google Scholar]
- 30. Benson J. M., Gomez A. P., Wolf M. L., Tibbetts B. M., March T. H. (2011) The acute toxicity, tissue distribution, and histopathology of inhaled ricin in Sprague-Dawley rats and BALB/c mice. Inhal. Toxicol. 23, 247–256 [DOI] [PubMed] [Google Scholar]
- 31. Garber E. A. (2008) Toxicity and detection of ricin and abrin in beverages. J. Food Prot. 71, 1875–1883 [DOI] [PubMed] [Google Scholar]
- 32. Jackson L. S., Tolleson W. H., Chirtel S. J. (2006) Thermal inactivation of ricin using infant formula as a food matrix. J. Agric. Food Chem. 54, 7300–7304 [DOI] [PubMed] [Google Scholar]