Background: Bone Morphogenetic Proteins (BMP) pathway defects and inflammation are hallmarks of pulmonary arterial hypertension (PAH).
Results: BMP signaling inhibits TNFα-induced activation of NF-κB by promoting an MRTF-A/NF-κB inhibitory complex in pulmonary smooth muscle cells.
Conclusion: BMPs binding to BMPR2 receptor play anti-inflammatory roles by inhibiting TNFα signaling via MRTF-A.
Significance: Defining how BMP pathway dysfunction promotes vascular pro-inflammatory state is critical to PAH therapy.
Keywords: Bone Morphogenetic Protein (BMP), Inflammation, Pulmonary Hypertension, Tumor Necrosis Factor (TNF), Vascular Smooth Muscle Cells, MRTF-A, NF-κB
Abstract
Pulmonary artery hypertension (PAH) patients exhibit elevated levels of inflammatory cytokines and infiltration of inflammatory cells in the lung. Concurrently, mutations of bmpr2, the gene encoding the type II receptor of bone morphogenetic proteins (BMP), are found in ∼75% of patients with familial PAH, but a possible nexus between increased inflammation and diminished BMP signaling has hitherto remained elusive. We previously showed that BMP4 triggers nuclear localization of the Myocardin-related transcription factor A (MRTF-A) in human pulmonary artery smooth muscle cells (PASMC), resulting in the induction of contractile proteins. Here we report the BMPR2-dependent repression of a set of inflammatory mediators in response to BMP4 stimulation of PASMC. Forced expression of MRTF-A precisely emulates the anti-inflammatory effect of BMP4, while MRTF-A depletion precludes BMP4-mediated cytokine inhibition. BMP4 and MRTF-A block signaling through NF-κB, the keystone of most pathways leading to inflammatory responses, at the level of chromatin recruitment and promoter activation. Moreover, MRTF-A physically interacts with RelA/p65, the NF-κB subunit endowed with a transcription activation domain. Interestingly, the MRTF-A-NF-κB interaction is mutually antagonistic: stimulation of NF-κB signaling by TNFα, as well as p65 overexpression, hinders MRTF-A activity and the expression of contractile genes. Thus, a molecular inhibitory pathway linking BMP4 signaling, activation of MRTF-A, and inhibition of NF-κB provides insights into the etiology of PAH and a potential focus of therapeutic intervention.
Introduction
Pulmonary artery hypertension (PAH)2 is a disease of the pulmonary vasculature associated with endothelial dysfunction, migration, and proliferation of PASMC into the small precapillary vessels, luminal narrowing, reduced dilation potential, and consequent increase in upstream pressure (1). For unknown reasons, a wide range of causes can lead to PAH, stemming from the genome, the environment, drugs and infectious diseases. Conceivably, a successful therapeutic outcome could be furthered by understanding the molecular and cellular underpinnings shared by these diverse etiologies. Among the various clinical features, two phenomena consistently underlie most forms of PAH: genetic and epigenetic changes in the bone morphogenetic protein type-II receptor (BMPR2) pathway (2, 3); and deregulation of immunity and inflammation (4).
BMPR2 is mutated in around 75% of familial PAH cases and in 20% of patients with non-familial PAH (5, 6). Mutations in other members of the transforming growth factor β (TGFβ) superfamily of receptors, such as endoglin and the activin-like receptor 1 (ALK-1), have also been observed. Even in the absence of genetic changes, down-regulation of BMPR2 or BMP type I receptor BMPR1A (ALK-3) expression has been documented in the lungs and cells of PAH patients without mutations in bmpr2 (7, 8), corroborating the suggestion that functional TGFβ and BMP signaling can hinder the progress of PAH. BMP signaling promotes SMC differentiation and growth arrest. It also has a role in vascular calcification, especially in senescent SMC, with BMP2/4 promoting osteoblastic transition and BMP7 inhibiting it (9, 10). It has been suggested that loss of BMP signaling following misexpression or inactivation of BMPR2 may predispose SMC to a phenotype switch in PAH (11), resulting in decreased expression of contractile proteins and increased proliferation and migration. We have previously discovered that the pro-contractile action of BMP signaling in smooth muscle is mediated by members of the MRTF family: MRTF-A and MRTF-B (11). Unlike myocardin, which localizes constitutively to nuclei and is restricted to cardiac and SMC, the MRTFs are expressed in various tissues and are regulated by signals that control actin polymerization. Upon receipt of a Rho GTPase-transduced signal, the pool of free monomeric actin declines. The MRTFs, which interact with unpolymerized actin, are subsequently released and activate transcription. Transcriptional activation by myocardin and MRTFs requires their interaction with Serum Response Factor (SRF) on promoter motifs called CArG boxes.
A role for inflammation, the second common feature of diverse injuries that trigger PAH, has been predicated on the finding of inflammatory cells, including macrophages and T or B lymphocytes, around the plexiform lesions of PAH (12). Levels of inflammatory cytokine and chemokine (ICC) expression, such as CCL2, CCL3, IL-1β, and interleukin-6 (IL-6), are also increased in severe PAH (13–15), with elevated serum levels of TNFα and higher levels of interleukins serving also as accurate predictors of reduced survival in PAH patients (16). In animal models, PAH can be induced by absence of T cells (17), repeated antigen exposure (18), or overexpression of IL-6 (19) and TNFα (20). Thus, in humans as in rodents, a functional immune system is required to prevent the occurrence of pulmonary hypertension.
Neither BMP signaling nor inflammatory dysfunction alone seems sufficient to trigger PAH, but in conjunction they may provide a two hits scenario that precipitates or worsens the disease (1). There is in vivo evidence for this synergism: a transgenic mouse expressing smooth muscle-restricted dominant-negative BMPR2 displays elevated IL-6 levels (21, 22), while inflammatory stimuli significantly increase PAH symptoms in heterozygote bmpr2-mutant mice (23). In the present study, we investigated whether these concurrent causes are molecularly connected and functionally interrelated. We find that BMP signaling inhibits the expression of inflammatory cytokines in PASMC. Surprisingly, MRTF-A acts as a transcriptional repressor, antagonizing the pro-inflammatory action of TNFα. MRTF-A interacts directly with, and inhibits the activity of, the primary transcriptional mediator of inflammatory signals, the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB). Conversely, acting via NF-κB, TNFα causes an inhibition of the BMP/MRTF-A axis, promoting smooth muscle dedifferentiation. Thus, through the mutually inhibitory effect between MRTF-A and NF-κB, smooth muscle cells integrate pro-differentiation stimuli by BMPs and pro-inflammatory stimuli by TNFα.
EXPERIMENTAL PROCEDURES
Cell Culture
Human primary pulmonary artery smooth muscle cells (PASMCs) were purchased from Lonza (#CC-14010) and were maintained in Sm-GM2 media (Lonza) containing 5% FBS. PAC-1 were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Sigma). 10T½ cells were obtained from ATCC.
Antibodies and Reagents
The antibodies used in this study are Myc epitope tag (clone 9E10, Tufts Core facility), Flag epitope tag (clone M2; Sigma), anti-smooth muscle actin (SMA) (clone 1A4, Sigma), anti-p65 (Cat# 3034, Cell Signaling Technology, for Western blot and immunoprecipitation; Cat# sc-372, Santa Cruz Biotechnology, for immunofluorescence), and anti-IκB (Cat# sc-203, Santa Cruz Biotechnology). Recombinant human BMP4 and TNFα were purchased from R&D Systems.
Plasmid DNA Construct and Transfection
Flag-tagged NF-κB p65 plasmid was purchased from Addgene (Cat# 20012). Myc-tagged MRTF-A expression constructs were a gift of Dr. D.-Z. Wang. Cells were transfected using polyethylenimine (PEI) (Cat# 23966, Polysciences, Inc) according to the manufacturer's instructions.
Reverse Transcription Polymerase Chain Reaction (RT-PCR) Assay
Unless otherwise indicated, cells were treated with 3 nm BMP4 and/or 10 ng/ml TNFα in DMEM/0.2% FBS. Total RNA was extracted by TRIzol (Invitrogen) and subjected to reverse transcription using SuperScript II kit (Invitrogen), according to the manufacturer's instructions. Quantitative analysis was performed by real-time PCR (Bio-Rad). The sequences of the PCR primers are available upon request.
RNA Interference
Synthetic small interference RNA (siRNA) was purchased from Dharmacon. The siRNA sequences targeting human BMP receptor II (BMPRII), MRTF-A, MRTF-B and scrambled siRNA are available upon request. The siRNAs were transfected as described (11). Forty-eight hours after transfection, cells were treated and harvested.
Luciferase Assay
Luciferase reporter constructs containing the CXCL2 promoter (pSS1, −428/+76; pSS2, −80/+76; pSS4, a mutation within the NF-κB site at −76/−67) were a kind gift of Dr. Maheswaran; SM22 (445/1st exon) and SM22 with mutations in two CArG boxes were obtained from Dr. L. Li; After transfection in 6-well plates, the cells were re-seeded onto 12-well plates and treated with 3 nm BMP4 or 10 ng/ml TNFα or combination for 20 h in 0.2% FBS/DMEM. Luciferase assays were carried out as described (11).
Construction of Recombinant Adenovirus and Infection
Adenovirus carrying β-gal (control, Tet-Off), Flag-mMRTF-A (Tet-Off), ΔC-Flag-mMRTF-A (Tet-Off), and Tet activator (required to express TRE Adenovirus) were as described (24). Adenovirus carrying NF-κB subunit p65 (chicken) and dominant negative Iκb (Chicken IκBα S36–40A mutant) were provided by Dr. Blackwell. High titer stocks of recombinant viruses were grown in 293 cells and purified. Infection of recombinant adenoviruses was performed at a multiplicity of infection of <800 PFU/cell.
Immunoprecipitation and Immunoblot Assays
Cells were treated with 3 nm BMP4 and 10 ng/ml TNFα and then lysed in 100 mm Tris-1% Nonidet P-40–1 mm EDTA buffer. Immunoprecipitation and immunoblot analysis were performed as described previously (25)
Chromatin Immunoprecipitation Assay (ChIP)
ChIP assay was performed using EZ-Magna ChIP™ A Chromatin Immunoprecipitation Kit (Cat# 17-408, Millipore) according to the manufacturer's instructions. Briefly, after treatment, followed by cross-linking with formaldehyde, genomic DNA was sonicated to an average length of 500 bp. Soluble chromatin was then incubated with anti-p65 antibody. Immunoprecipitated DNA fragments were amplified and quantitated by real-time PCR with PCR primers specific for the human CXCL2 gene: 5′-GGCAGAAAGAGAACATCCCA-3′ and 5′-ACCCCTTTTATGCATGGTTG-3′ and IL-1β gene: 5′-TGGCCCTGTAACCTGGACCC-3′ and 5′-GCTGTGGCCTGAGCGGTCTC-3.
Immunofluorescence
After treatment for 48 h, PASMCs were fixed and permeabilized in 50% acetone/50% methanol solution and subjected to staining using anti-SMA, or anti NF-κB p65 antibodies. Secondary antibodies conjugated with Alexa Fluor 488 (Invitrogen) were used for detection. Nuclei were stained with 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI, Invitrogen).
Enzyme-linked Immunosorbent Assay (ELISA)
IL-1β and CCL8 protein levels in cell culture supernatants were determined using a commercially available ELISA kit specific for human IL-1β (Cat# DLB50, R&D System) and CCL8 (Cat# ELH-MCP2–001, RayBiotech, Inc). The lower limit for detection of IL-8 and CCL8 was <5 pg/ml. The manufacturer's instructions were followed to assay IL-1β and CCL8 concentrations. Absorbance was read at 450 nm using a Synergy HT Reader (BioTek). Concentrations of cytokines were calculated based on a standard curve for each cytokine.
Animal Study and Immunohistochemistry
All experiments were performed in accordance with the guidelines and regulations of the Institutional Animal Care and Use Committee at Tufts Medical Center. Adult male Sprague-Dawley rats were randomized to 17 days of normoxia or hypobaric hypoxia as described previously (26). At the end of the exposure period, rats were killed and PAs were processed for paraffin embedding. Paraffin-embedded tissue sections (5-μm) were immunostained with anti-SMA (clone 1A4, Sigma), anti-phospho-Smad1/5/8 (Cat# 9511, Cell Signaling), IL-1α (Cat# sc-9983, Santa Cruz Biotechnology), IL-1β (Cat# sc-7884, Santa Cruz Biotechnology) and IL-6 (Cat# AF506, R&D) antibodies.
Statistical Analysis
Statistical significance was calculated using analysis of variance (ANOVA) and Fisher's Projected Least Significant Difference (LSD) test, or by Student's t test analysis (p < 0.05), as appropriate. All data are plotted as the mean ± S.E.
RESULTS
BMP Signaling Inhibits Immune Cytokine and Chemokine Gene Expression
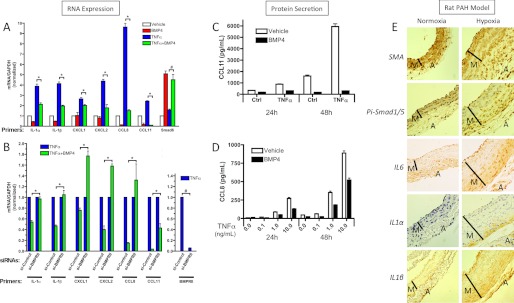
Preliminary microarray and qRT-PCR array analyses indicated an inhibitory effect of BMP4 on the expression of a number of ICC genes in human PASMC (data not shown). To further explore this finding, we measured by qRT-PCR the expression of a set of six ICC genes representing three major ICC structural families: IL-1α and IL-1β (of the interleukins family), CXCL1 and CXCL2 (CXC family) and CCL8 and CCL11 (CC family). BMP4 significantly inhibited [from 1.3-fold (CXCL1) to 24-fold (CCL11)] the TNFα-induced levels of all the ICCs tested, while it induced Smad6 [3-fold], a known transcriptional target of the BMP pathway (Fig. 1A), confirming our preliminary observations from the arrays.
FIGURE 1.
The BMP4/BMPR2 pathway is involved in the regulation of inflammation in human PASMCs (hPASMCs) and in a rat PAH model. A, hPASMCs were treated with or without 10 ng/ml TNFα in the presence or absence of 3 nm BMP4 for 24 h, followed by qRT-PCR analysis. Results were normalized to GAPDH expression, and the relative mRNA levels are presented as mean ± S.E., with each experiment conducted in triplicate (n = 3). The difference between two results indicated by asterisks is statistically significant; *, p < 0.05. B, hPASMCs were transfected with 1 nm control siRNA or 1 nm siBMPRII. Twenty-four hours later, cells were stimulated with 3 nm BMP4 or 10 ng/ml TNFα or combined BMP4 (3 nm) and TNFα (10 ng/ml) for 24 h and subjected to qRT-PCR analysis. Results are shown as relative mRNA levels normalized by the value of basal or TNF stimulation without BMP4 treatment. Mean values are expressed ± S.E. C and D, hPASMCs were treated with 3 nm BMP4 or 10 ng/ml TNFα or combined BMP4 (3 nm) and TNFα (10 ng/ml) for 48 h and medium was collected. Protein levels were examined by ELISA assay. The experiment was repeated three times and mean values are expressed ± S.E. E, histological examination of pulmonary arteries (PAs) from rats after 17-day hypoxia or normoxia treatment with anti-SMA, anti-phospho-Smad1/5 (Pi-Smad1/5), anti-IL-6, anti-IL-1α, or anti-IL-1β antibodies (×400) and by hematoxylin staining (×400). The media (M) and adventitia (A) of the PAs are indicated.
BMPRII Is Required for ICC Repression
As BMPRII is frequently mutated or repressed in PAH patients, we tested whether BMPRII is required for regulation of ICCs by BMP4. In cells in which endogenous BMPRII levels are reduced 90–95% by small inhibitory RNA (siRNA), we observed a general reduction of the inhibitory activity of BMP4 on the TNFα-induced expression of all ICC tested, yet there were differences among the responses of individual ICC genes. BMP4 inhibition of IL-1α and IL-1β was abolished by the BMPRII siRNA, while the reduction of CCL11 was blunted from 25-fold to 2.3-fold in the presence of siBMPRII. Interestingly, expression of CXCL1, CXCL2 and CCL8 was augmented (1.3–1.8-fold) by BMP4 treatment when BMPRII was down-regulated (Fig. 1B), suggesting that the knock down of BMPRII and inhibition of BMP signaling pathway can partially switch the BMP4 signal from anti-inflammatory to pro-inflammatory. Notwithstanding these gene-specific features, BMPRII appears an essential mediator of the BMP4 anti-inflammatory signal in smooth muscle, and may also prevent BMP4 from inducing a subset of inflammatory cytokines.
BMP4 Reduces Chemokines Secretion
Next, we examined whether BMP signaling reduces the secretion of ICC proteins induced by TNFα. We observed a reduction of CCL8 (1.7∼2.5-fold) and CCL11 [3-fold (24 h) to 20-fold (48 h)] in TNFα-stimulated PASMC culture media upon BMP4 addition, at different doses of TNFα (0.1–10 ng/ml) and stimulation periods (24–48 h) (Fig. 1, C and D). Thus, BMP4 treatment reduces ICCs secretion upon TNFα treatment in smooth muscle cells.
Reduced BMP Signaling and Increased Cytokine Expression Correlate in PAH Model
To test whether changes in BMP signaling correlate with ICC expression in vivo, we employed a rat hypoxia-induced PAH model (Fig. 1E). The medial layer of pulmonary vessels is thicker and displays reduced smooth muscle α actin (SMA) immunohistochemical staining in hypoxia-treated rats compared with rats under normoxia (control) (Fig. 1E). This is due to increased proliferation of vascular smooth muscle cells, which also occurs in the pulmonary arteries of patients with PAH. We and others reported a reduced presence of phosphorylated Smad1/5 in hypoxic samples (Pi-Smad1/5) (8, 27), which is consistent with a role of BMP signaling in the maintenance of a contractile phenotype in SMC. In this study, concurrent with a decrease of Pi-Smad1/5, we observed an increase in expression of IL-6, IL-1α, and IL-1β in hypoxic lung vasculature. Therefore, a PAH animal model reveals an inverse relationship between intensity of BMP signaling as revealed by Pi-Smad1/5 and ICC expression as revealed by IL-6, IL-1α, and IL-1β expression (Fig. 1E and supplemental Fig. S1). Taken together, these data suggest that BMP4-BMPRII signaling represses ICC expression and secretion from vascular SMC both in vivo and in vitro.
BMP Stimulation Hinders NF-κB Signaling
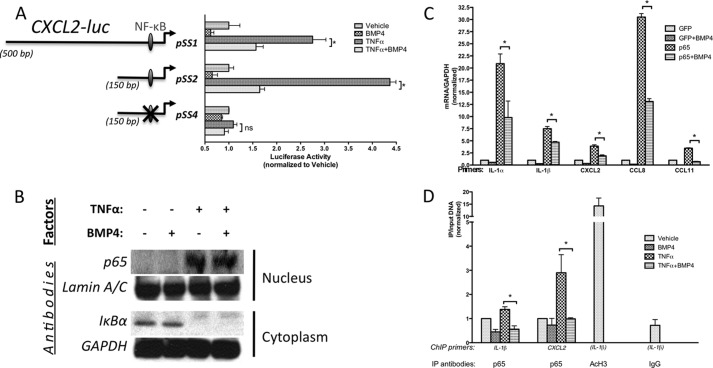
The expression of pro-inflammatory cytokines in the vasculature is primarily regulated through signaling cascades that converge on and cross-talk with the NF-κB pathway (28). To investigate the mechanism of ICC inhibition by BMP4, we tested whether a transcriptional regulatory element of an ICC gene could be inhibited by BMP signaling. Promoter-luciferase constructs (29) containing 500 bp and 150 bp of the CXCL2 gene: [pSS1 (−428 to +76, relative to the transcription start site) and pSS2 (−80 to +76), respectively] were transfected in PASMC (Fig. 2A). Both pSS1 and pSS2 contain a consensus NF-κB site located between −76 and −67 (29). As expected, both constructs were induced by TNFα in SMC, confirming the involvement of the NF-κB pathway in regulating CXCL2 transcription in SMC. The NF-κB site is essential for TNFα-mediated expression since a 150bp construct harboring a mutated NF-κB site (29) (pSS4, Fig. 2A) was not activated by TNFα (Fig. 2A). Co-treatment with TNFα and BMP4 did not significantly alter pSS4 expression compared with the TNFα treatment alone (Fig. 2A). Thus, BMP4 inhibits the transcriptional activation of CXCL2 by TNFα via the NF-κB binding site.
FIGURE 2.
BMP4 antagonizes the TNFα-activated NF-κB pathway. A, PAC-1 cells were transfected with wild-type (PSS1, −428/+76; PSS2, −80/+76) or mutant CXCL2 reporter constructs, which are mutated in the NF-κB binding site as indicated (PSS4, a mutation within the NF-κB site at −76/−67), treated with with or without TNFα (10 ng/ml) in the presence or absence of BMP4 (3 nm) for 18 h and followed by luciferase assay. Luciferase activities were normalized to the β-galactosidase activities. B, hPASMCs were pretreated with or without BMP4 (3 nm) for 1 h, following stimulated by TNFα (10 ng/ml) for 5 min. Nucleus was separated from cytoplasm and NF-κB p65 and IκBα expression levels were examined by immunoblot analysis. C, hPASMCs were infected with adenovirus carrying NF-κB p65 (Adeno-p65) or GFP (control) cDNA, followed by treatment with 3 nm BMP4 for 24 h, and then subjected to qRT-PCR assay. D, recruitment of NF-κB p65 to the IL-1β or CXCL2 promoter was examined by CHIP with an anti-NF-κB p65 in hPASMCs, followed by quantitative realtime-PCR analysis using primers specific for the IL-1β or CXCL2 promoter. Immunoprecipitation with AcH3 or nonspecific IgG were used as positive or negative control. Results are shown as relative enrichment of the IL-1β or CXCL2 promoter after IP and the basal level of binding without treatment was set to 1. Mean values are expressed ± S.E.
BMP4 Suppresses RelA/p65-mediated Transcription Activation
Canonical NF-κB signaling involves IκBα degradation in the cytoplasm, subsequent nuclear translocation of the RelA/p65 subunit, and dimerization with the DNA binding partner NF-κB1/p50 (30). Surprisingly, neither inhibition of IκBα degradation nor reduction of p65 nuclear localization was observed upon BMP4 treatment of TNFα-stimulated SMC (Fig. 2B, supplemental Fig. S2) Therefore, we postulated that BMP4 might be inhibiting NF-κB activity after p65 has already translocated to the nucleus. To test this hypothesis, we induced endogenous ICC genes by forced expression of exogenous p65 from an adenovirus vector (Adeno-p65), which leads to spontaneous nuclear localization (31–33) and inflammatory gene expression (Fig. 2C). Compared with infection with control GFP adenovirus (Adeno-GFP), Adeno-p65 strongly induced IL-1α, IL-1β, CXCL2, CCL8, and CCL11 mRNA levels between 3.5–30-fold in PASMC (Fig. 2C), similarly to TNFα treatment (see Fig. 1A). BMP4 treatment following Adeno-p65 infection significantly repressed (by 1.5–5-fold) the induction of all the ICCs tested (Fig. 2C). Next, we employed a chromatin immunoprecipitation (ChIP) assay to measure recruitment of p65 to the previously identified NF-κB sites (29, 34) in the IL-1β and CXCL2 promoters (Fig. 2D). BMP4 significantly inhibited (2.5–3-fold) the TNFα-induced p65 recruitment to both promoters (Fig. 2D). Taken together, these data suggest that BMP4 signaling inhibits p65/NF-κB function in the nucleus at the level of chromatin binding and/or transcriptional transactivation.
MRTF-A Is Required for NF-κB Inhibition by BMP Signaling
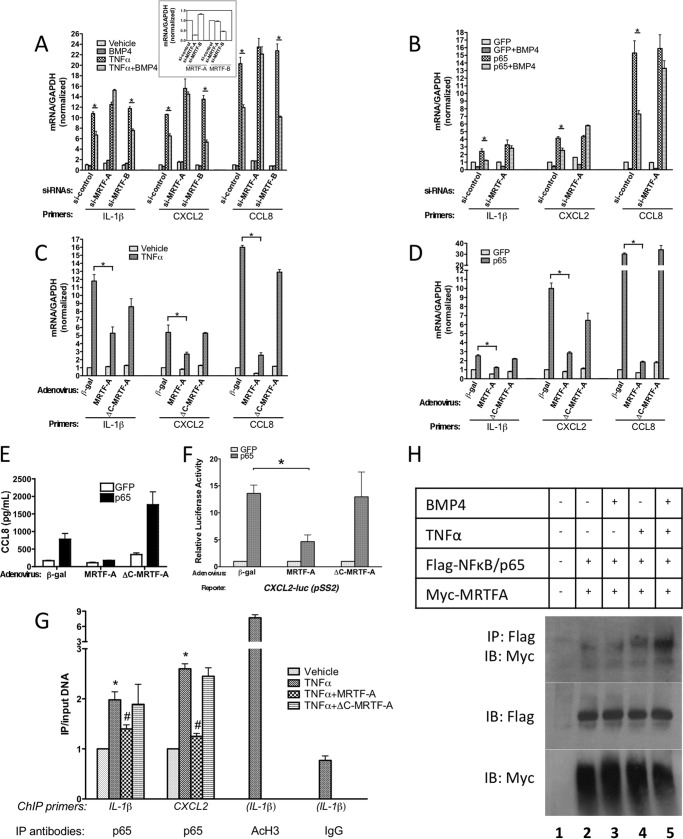
We previously reported that MRTF-A translocates to the nucleus and activates transcription of the contractile smooth muscle markers SMA, Calponin1 (CNN) and SM22α in response to BMP signaling (11). Thus, we hypothesized that MRTF-A and/or MRTF-B might also be involved in the negative regulation of ICC transcription upon BMP treatment. This model was tested in PASMC through knock down of MRTF-A or MRTF-B expression by siRNA (to less than 30 and 50% of control, respectively, Fig. 3A, inset). These siRNAs were selected from a pool of 4 siRNAs with qualitatively similar effects (not shown), followed by stimulation with TNFα and BMP4. Interestingly, knock down of MRTF-A, but not of MRTF-B, abolished the ability of BMP4 to inhibit the TNFα-mediated induction of IL-1β, CXCL2, and CCL8 (Fig. 3A). As knock down of MRTF-B has no apparent effect on ICC inhibition (Fig. 3A), we postulate that MRTF-B does not play a significant role in this process and focus all subsequent experiments on the role of MRTF-A. Since BMP4 antagonizes nuclear p65/NF-κB function (see Fig. 2C), we tested whether this effect requires MRTF-A. BMP4-mediated inhibition of IL-1β, CXCL2, and CCL8 induction by Adeno-p65 was blocked by the MRTF-A siRNA, but not control siRNA (Fig. 3B), demonstrating that MRTF-A is required for the inhibitory role of BMP signaling on TNFα- and p65-mediated induction of inflammatory cytokine genes.
FIGURE 3.
MRTF-A is essential for BMP4-mediated inhibition of the TNFα/NF-κB p65 pathway. A, hPASMCs were transfected with 40 nm siRNA against MRTF-A or MRTF-B or non-targeting (control) siRNA control for 24 h, followed by treatment with or without TNFα (10 ng/ml) in the presence or absence of BMP4 (3 nm) for another 24 h. Total RNAs were extracted and subjected to qRT-PCR analysis. B, hPASMCs were infected with Adeno-p65 or GFP (control) cDNA, followed by transfected with 40 nm siRNA against MRTF-A or non-targeting siRNA control for 24 h, then treated with or without BMP4 (3 nm) for another 24 h. Total RNAs were extracted and subjected to qRT-PCR analysis. C, hPASMCs were infected with adenovirus carrying β-gal (control, Tet-Off), FLAG-tagged-mMRTF-A (Tet-Off) and Tet activator for 24 h. followed by treatment with or without TNFα (10 ng/ml) for another 24 h. Total RNAs were extracted and subjected to qRT-PCR analysis. D, hPASMCs were infected with adenovirus carrying β-gal, FLAG-mMRTF-A, and Tet activator, and co-infected with Adeno-p65 or GFP (control) cDNA as indicated for 48 h. followed by qRT-PCR analysis. E, medium was collected from hPASMCs culture infected with adenovirus carrying β-gal, FLAG-mMRTF-A and Tet activator, and co-infected with Adeno-p65 or GFP cDNA as indicated for 48 h, subjected to ELISA assay. F, PASMCs were infected with adenovirus carrying β-gal, FLAG-mMRTF-A and Tet activator, followed by transfection with NF-κB p65 or control plasmid. All samples were co-transfected with CXCL2 promoter construct (PSS2, −80/+76), subjected to luciferase assay. Luciferase activities were normalized to the β-galactosidase activities. G, hPASMCs were infected with adenovirus carrying β-gal, FLAG-mMRTF-A and Tet activator, followed by treatment of TNFα (10 ng/ml). Recruitment of NF-κB p65 to the IL-1β or CXCL2 promoter was examined by ChIP assay. H, PAC-1 cells were transiently transfected with or without expression constructs encoding Myc-tagged MRTF-A and FLAG-NF-κB p65, then stimulated with or without BMP4 (3 nm) in the presence or absence of TNFα (10 ng/ml) for 4 h. Total cell lysates prepared from these cells were subjected to immunoprecipitation with anti-FLAG monoclonal antibody. Immunoprecipitates were separated by SDS-PAGE, followed by immunoblot with anti-Myc antibody. Total cell lysates were immunoblotted with anti-Flag or anti-Myc antibody to visualize the expression levels of NF-κB p65 and MRTF-A.
MRTF-A Blocks ICC Expression and Secretion
MRTF-A appears to be sufficient to inhibit activation of ICC by TNFα and p65, since exogenous expression of adenovirus-transduced MRTF-A in PASMC reduced TNFα or p65-mediated activation of IL-1β, CXCL2, and CCL8 by 2–15-fold, compared with control virus (Fig. 3, C and D). Interestingly, a mutant MRTF-A harboring a deletion of the last 301 C-terminal amino acids (ΔC-MRTF-A, containing aa. 1–630) failed to inhibit TNFα and p65 in the same assays. Similarly, MRTF-A, but not ΔC-MRTF-A, inhibited CCL8 secretion (Fig. 3E, ELISA) and CXCL2-luc activation in PASMC (Fig. 3F, luciferase assay), upon infection with p65 adenovirus. This result suggests that the C-terminal region, which was previously identified as the transactivation domain of MRTF-A (35), is required for the inhibition of p65.
MRTF-A Impedes the Transcriptional Activity of RelA/p65 and Mimics BMP4 Effects
To investigate the inhibitory activity of MRTF-A on p65/NF-κB function, we tested whether MRTF-A directly influences the transactivation activity of the p65 subunit. A plasmid encoding the transactivation domain of RelA/p65 fused to the DNA binding domain of the yeast transcription factor Gal4 (GDB-RelA, supplemental Fig. S3) was transfected into COS7 cells along with 1) a luciferase reporter containing upstream Gal4 binding sites; and 2) wild type or mutant MRTF-A expression constructs. As expected, GDB-RelA potently activated the luciferase reporter compared with a control plasmid containing only the Gal4 DNA binding domain (GDB-Ctrl, supplemental Fig. S3). Wild type MRTF-A, as well as single-domain mutants including Rpel, B, Q, SAP, and LZ, blocked the transcriptional activity of the transactivation domain of RelA/p65 (between 68% to 88% inhibition). Surprisingly, two MRTF-A mutants, lacking 301 aa. (ΔC-630, a deletion identical to ΔC-MRTF-A in Fig. 3) and 208 aa. (ΔC-723) at the C terminus, not only failed to inhibit GDB-RelA, but also strongly synergized to induce the reporter to a level ∼10-fold higher than GDB-RelA alone (supplemental Fig. S3). Although this potent synergism between the GDB-RelA fusion and the truncated MRTF-A constructs was not detectable in PASMC, the loss of the RelA-inhibitory activity coinciding with the deletion of the MRTF-A C-terminal domain is in agreement with the effect of MRTF-A and ΔC-MRTF-A in PASMC (Fig. 3, A, C–F). Taken together, our results suggest that MRTF-A, through its C-terminal domain, inhibits the activation of NF-κB at least in part by inhibiting the transactivation activity of the RelA/p65 subunit. Additionally, MRTF-A may also inhibit the interaction of p65 with chromatin via the p50/p65 complex. As reported in Fig. 2D, a ChIP assay reveals that BMP4 inhibits the TNFα-induced recruitment of p65 to IL-1β enhancer and CXCL2 promoter (see Fig. 2D). Similarly, adenovirus-transduced MRTF-A effectively inhibited p65 binding to the NF-κB sites in both cytokine gene promoters (Fig. 3G). ΔC-MRTF-A was inactive (Fig. 3G). Therefore, MRTF-A overexpression mimics the anti-inflammatory effect of BMP4 in SMC, counteracting cytokine transcription and secretion, promoter activation and chromatin recruitment of p65 in response to TNFα stimulation. Furthermore, knock down of MRTF-A blunts the BMP4-mediated anti-inflammatory potential. These results suggest that BMP4 inhibits the TNFα/NF-κB axis in part via MRTF-A. The C-terminal domain of MRTF-A appears to be required for its inhibitory action.
MRTF-A Interacts with RelA/p65
Since BMP-mediated inhibition of NF-κB function occurs in the nucleus (see Fig. 2), we hypothesized that MRTF-A might interact with p65 to prevent p65 from binding DNA or activating transcription. This hypothesis was tested by co-immunoprecipitation of epitope-tagged human p65 and MRTF-A in rat pulmonary vascular smooth muscle PAC1 cells. In the absence of TNFα stimulation, only a weak interaction was detected (Fig. 3H, lane 2). Treatment with TNFα increased the interaction, while BMP4 alone had no significant effect (Fig. 3H, lanes 3 and 4), Simultaneous treatment with BMP4 and TNFα, however, potently induced p65-MRTF-A interaction (Fig. 3H, lane 5). p65 and MRTF-A co-localize in the nucleus upon concurrent BMP4 and TNFα stimulation (supplemental Fig. S4), suggesting that activation and nuclear localization of both proteins upon factor stimulation facilitates their interaction. Alternatively, a post-translational modification might facilitate the interaction. In summary, formation of a p65-MRTF-A complex induced by BMP4 and TNFα correlates with the ability of BMP4 to inhibit NF-κB signaling and ICC expression in SMC. To examine whether the domain of MRTF-A that interacts with p65 coincide with the domain that inhibits the p65 transcriptional activity, we examined the interaction between p65 and several MRTF-A mutants. The ΔC-630 and ΔC-723 mutants of MRTF-A fail to inhibit p65 activity (supplemental Fig. S3 and Fig. 3). Interestingly, co-immunoprecipitation experiment of epitope-tagged RelA/p65 and various domain-deletion mutants of MRTF-A showed that ΔC-630 and ΔC-723 strongly interact with p65 (supplemental Fig. S5). Therefore, it appears that the p65-inhibitory activity of MRTF-A resides in its carboxyl (C)-terminal end, while the domain(s) that interacts with p65 map to the amino (N)-terminus of MRTF-A.
TNFα/NF-κB Signaling Inhibits BMP/MRTF-A-mediated Induction of a Contractile Phenotype
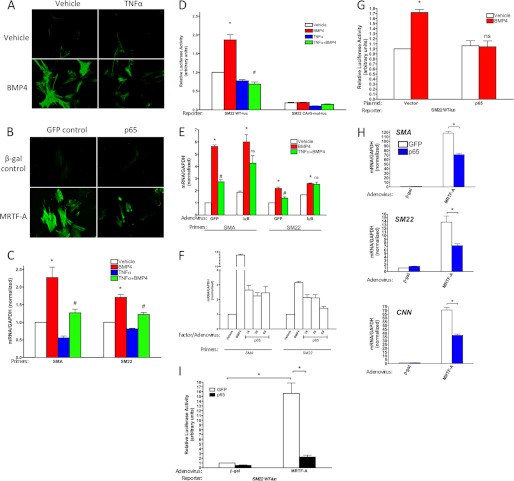
Since the interaction between p65 and MRTF-A inhibits p65 function, it is plausible that the transcriptional activity of MRTF-A might be affected by p65 or the TNFα pathway. As previously shown (11), BMP4 stimulation induces a contractile phenotype in SMC, as can be detected by an increase of SMA immunostaining in response to BMP4 (Fig. 4A, left panels) Co-treatment of TNFα and BMP4 inhibited by 50–70% the BMP4-induced phenotype switch (Fig. 4A, right panels). Interestingly, a similar result was obtained by infection of adenovirus carrying MRTF-A and/or p65 cDNA: MRTF-A-induced SMA expression was abrogated by p65 (Fig. 4B). Thus, BMP4 and MRTF-A promote SMC differentiation, while TNFα and p65 antagonize it. Likewise, BMP4 increased the expression of two SMC differentiation markers, SMA and SM22α, as measured by qRT-PCR, while TNFα repressed both basal and BMP-activated levels (Fig. 4C). TNFα also inhibits bmp4 gene expression, while BMP4 has no significant effect on the tnf gene (supplemental Fig. S6).
FIGURE 4.
TNFα/NF-κB p65 inhibit the BMP4/MRTF-A pathway. A, hPASMCs were treated with or without BMP4 (3 nm) in the presence or absence of TNFα (10 ng/ml) for 48 h. Cells were then subjected to immunofluorescence staining with FITC-conjugated anti-SMA antibody. B, hPASMCs were co-infected with adenovirus carrying β-gal or FLAG-mMRTF-A and NF-κB p65 or GFP control for 48 h. Cells were then subjected to immunofluorescence staining with FITC-conjugated anti-SMA antibody. C, hPASMCs were treated with or without 10 ng/ml TNFα in the presence or absence of 3 nm BMP4 for 24 h, followed by qRT-PCR analysis. D, PAC-1 cells were transfected with wild-type or mutant SM22α reporter constructs, which are mutated in the CArG box sequence as indicated, treated with or without TNFα in the presence or absence of BMP4 for 18 h, and assayed for luciferase activity. E, hPASMCs were infected with adenovirus carrying a degradation-resistant form of IκBα (IκB) or GFP control for 24 h, followed by treatment with or without TNFα (10 ng/ml) in the presence or absence of BMP4 (3 nm) for another 24 h. Total RNA was isolated and subjected to RT-PCR analysis. F, hPASMCs were infected with different doses of Adeno-p65 as indicated for 24 h, followed by treatment with BMP4 (3 nm) for another 24 h. Total RNA was isolated and subjected to RT-PCR analysis. G, PAC-1 cells were transfected with SM22-reporter constructs and NF-κB p65 expression plasmid, followed by BMP4 (3 nm) treatment and assayed for luciferase activity. H, hPASMCs were co-infected with adenovirus carrying β-gal or FLAG-mMRTF-A and Adeno-p65 or GFP control for 48 h, Total RNA was isolated and subjected to RT-PCR analysis. I, hPASMCs were infected with adenovirus carrying β-gal or FLAG-mMRTF-A, followed by transfection with SM22-reporter constructs and NF-κB p65 expression plasmid and assayed for luciferase activity.
TNFα and NF-κB Interfere with Transcription Activation by MRTF-A
MRTF-A, in a complex with SRF, binds and activates transcription through a consensus binding sequence named “CArG box,” which is present in the promoters of most SMC-specific genes, including SM22α. To examine whether the inhibitory effect of TNFα/p65 on BMP4/MRTF-A requires a CArG box, we used a SM22α promoter-luciferase reporter construct (Fig. 4D). As previously reported, BMP4 can activate the promoter through the action of MRTF-A (11). We observed a complete inhibition of MRTF-A-dependent activation of the reporter by concurrent treatment with TNFα (Fig. 4D), suggesting that MRTF-A activity is directly inhibited by TNFα. Several pathways propagate the TNFα signal inside the cell. Next, we measured the extent to which TNFα can inhibit BMP4-mediated induction of contractile genes when challenged with a NF-κB inhibitor, the degradation-resistant form of IκBα (DR-IκBα) (Fig. 4E). SMC markers SMA and SM22α were both induced by BMP4 and repressed by co-treatment with TNFα in the presence of a control GFP adenovirus (Fig. 4E). However, transduction of DR-IκBα reduced the TNFα effect to non-significant levels, implicating NF-κB in the suppression of phenotype switch by TNFα (Fig. 4E). Conversely, p65 overexpression was sufficient to inhibit BMP4-mediated induction of contractile genes. BMP4-induced SMA and SM22α expression was inhibited by increasing amounts of p65 (Fig. 4F). Furthermore, CArG-mediated induction of the SM22α-luc reporter by BMP4 was also blocked by p65 cotransfection in rat PASMC PAC1 cells (Fig. 4G), indicating that TNFα and p65/NF-κB inhibit SMC phenotype switch in response to BMP4 through a similar mechanism involving a CArG box-binding factor. To test inhibition of MRTF-A activity by NF-κB, we used the MRTF-A adenovirus to induce the expression of contractile gene mRNAs such as SMA, SM22α and CNN1, and opposed it by co-expressing p65, also by adenovirus infection in PASMC (Fig. 4H). p65 potently inhibited the MRTF-A-mediated induction of these genes, confirming the mutual functional antagonism between these two transcription factors (Fig. 4H). Finally, p65 can directly antagonize MRTF-A at the transcription level, as shown by the ability of p65 to block MRTF-A-mediated induction of the SM22α-luc reporter in PASMC (Fig. 4I).
DISCUSSION
In the present study, we demonstrated a cross-talk between the pro-inflammatory TNFα pathway and the pro-contractile BMP4 pathway in pulmonary SMC. These two pathways drive the activation and nuclear translocation of NF-κB and MRTF-A, respectively. Forming a complex, NF-κB and MRTF-A mutually inhibit their respective function, providing a stoichiometric integration of opposing extracellular stimuli to maintain SMC homeostasis.
While the anti-inflammatory role of TGF-β is well established, an anti-inflammatory role for BMP signaling has been proposed in different systems, such as the kidney (36, 37 and references therein). However, other reports have suggested that several BMP-family ligands can act as pro-inflammatory agents in atherosclerosis (Ref. 38 and references therein). Therefore, the relationship between BMP and inflammation appears complex and possibly dependent on the organ system. We provide evidence in the context of pulmonary artery SMC that BMP signaling potently represses signaling through the ubiquitous NF-κB inflammatory pathway. Since NF-κB repression by BMP4 requires MRTF-A, we postulate that in cells expressing low levels of MRTF-A, such as endothelial cells,3 BMP4 might be unable to antagonize NF-κB signaling through this mechanism.
In this study, we demonstrate that MRTF-A inhibits RelA/p65 in a BMP-dependent manner, providing for the first time a link between BMP signaling and NF-κB inhibition in SMC. Myocd has been shown to bind RelA/p65 and inhibit its function in SMC (39). Although Myocd appears to be constitutively active in SMC, it is possible that factors that modulate Myocd expression, such as TGF-β (40), might affect RelA/p65 activity. Furthermore, we demonstrate that the interaction between MRTF-A and RelA/p65 is not sufficient to inhibit NF-κB, but it requires an inhibitory domain located at the C terminus of MRTF-A. Further studies will be required to finely map the activation and inhibition domains in MRTF-A.
Our work uncovers a potential link between BMP signaling defects and the pro-inflammatory condition of PASMCs. In particular, we find that BMP signaling, through BMPR2, inhibits the activation of p65/NF-κB. Therefore, we predict that in PAH patients with a bmpr2 mutation, the level of p65/NF-κB activation may be increased, either at the basal level or in response to an inflammatory stimulus. Interestingly, there is genetic evidence linking NF-κB to PAH. A single nucleotide polymorphism found in the promoter of TRPC6, a gene previously implicated in the proliferation of PASMC from PAH patients (41), is ∼2.85 times more frequent in PAH patients than controls (42). This mutation creates a binding sequence for NF-κB, enhancing NF-κB-mediated promoter activity and stimulating TRPC6 expression in PASMC (42). Furthermore, an NF-κB inhibitor has been shown to ameliorate monocrotaline-induced pulmonary hypertension in rats (43). Thus, bmpr2 mutations might promote PAH by facilitating the induction of p65/NF-κB. In support of this notion, we observe that a reduction of BMPR2 expression in PASMCs by siRNA not only prevents the repression of cytokines expression by BMP4, but it leads to activation of some cytokine genes (such as CXCL1, CXCL2, and CCL8; Fig. 1B) in response to BMP4 stimulation. The mechanism for this response switch is under investigation, but may be related to the augmented signaling by BMP6 and BMP7 observed in cells lacking BMPR2 (44).
MRTF-A plays a versatile role in response to BMP activation: it both directly promotes contractile gene expression and indirectly represses inflammatory cytokine and chemokine gene expression through antagonism with NF-κB. Reciprocally, NF-κB both induces inflammatory mediators and promotes SMC de-differentiation through action on MRTF-A. Thus, an activity that interrupts the MRTF-A/NF-κB cross-talk in PASMC would be expected to be beneficial to patients with PAH.
Supplementary Material
Acknowledgments
We thank members of the Lagna and Hata laboratories for stimulating conversations and sharing of reagents. We acknowledge Dr. Shyamala Maheswaran for the CXCL2 promoter-luciferase constructs, Dr. Li Li for the SM22 promoter plasmid, Dr. Timothy S. Blackwell for NF-κB p65 and IκBα adenovirus, and Drs. Eric Olson and Da-Zhi Wang for the Myc-tagged MRTF-A expression constructs.
This work was supported, in whole or in part, by National Institutes of Health Grants HL086572 (to G. L.), HL082854 (to A. H. and G. L.), HL093154 (to A. H.), and HL078869 (to M. D. L.). Further support was provided by the American Heart Association and the Molecular Cardiology Research Institute at Tufts University.

This article contains supplemental Figs. S1–S6.
D. Wang and G. Lagna, unpublished observations.
- PAH
- pulmonary arterial hypertension
- BMP
- bone morphogenetic protein
- MRTF
- myocardin-related transcription factor
- PASMC
- pulmonary artery smooth muscle cells
- SMC
- smooth muscle cells
- ICC
- inflammatory cytokine and chemokine.
REFERENCES
- 1. Toshner M., Tajsic T., Morrell N. W. (2010) Pulmonary hypertension: advances in pathogenesis and treatment. Br. Med. Bullet. 94, 21–32 [DOI] [PubMed] [Google Scholar]
- 2. Machado R. D., Eickelberg O., Elliott C. G., Geraci M. W., Hanaoka M., Loyd J. E., Newman J. H., Phillips J. A., 3rd, Soubrier F., Trembath R. C., Chung W. K. (2009) Genetics and genomics of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 54, S32–S42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eickelberg O., Morty R. E. (2007) Transforming growth factor β/bone morphogenic protein signaling in pulmonary arterial hypertension: remodeling revisited. Trends Cardiovasc. Med. 17, 263–269 [DOI] [PubMed] [Google Scholar]
- 4. Pullamsetti S. S., Savai R., Janssen W., Dahal B. K., Seeger W., Grimminger F., Ghofrani H. A., Weissmann N., Schermuly R. T. (2011) Inflammation, immunological reaction and role of infection in pulmonary hypertension. Clin. Microbiol. Infect 17, 7–14 [DOI] [PubMed] [Google Scholar]
- 5. Machado R. D., Aldred M. A., James V., Harrison R. E., Patel B., Schwalbe E. C., Gruenig E., Janssen B., Koehler R., Seeger W., Eickelberg O., Olschewski H., Elliott C. G., Glissmeyer E., Carlquist J., Kim M., Torbicki A., Fijalkowska A., Szewczyk G., Parma J., Abramowicz M. J., Galie N., Morisaki H., Kyotani S., Nakanishi N., Morisaki T., Humbert M., Simonneau G., Sitbon O., Soubrier F., Coulet F., Morrell N. W., Trembath R. C. (2006) Mutations of the TGF-β type II receptor BMPR2 in pulmonary arterial hypertension. Hum. Mutat. 27, 121–132 [DOI] [PubMed] [Google Scholar]
- 6. Thomson J. R., Machado R. D., Pauciulo M. W., Morgan N. V., Humbert M., Elliott G. C., Ward K., Yacoub M., Mikhail G., Rogers P., Newman J., Wheeler L., Higenbottam T., Gibbs J. S., Egan J., Crozier A., Peacock A., Allcock R., Corris P., Loyd J. E., Trembath R. C., Nichols W. C. (2000) Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-β family. J. Med. Genet. 37, 741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atkinson C., Stewart S., Upton P. D., Machado R., Thomson J. R., Trembath R. C., Morrell N. W. (2002) Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 105, 1672–1678 [DOI] [PubMed] [Google Scholar]
- 8. Yang X., Long L., Southwood M., Rudarakanchana N., Upton P. D., Jeffery T. K., Atkinson C., Chen H., Trembath R. C., Morrell N. W. (2005) Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ. Res. 96, 1053–1063 [DOI] [PubMed] [Google Scholar]
- 9. Hruska K. A., Mathew S., Saab G. (2005) Bone morphogenetic proteins in vascular calcification. Circ. Res. 97, 105–114 [DOI] [PubMed] [Google Scholar]
- 10. Burton D. G., Matsubara H., Ikeda K. (2010) Pathophysiology of vascular calcification: Pivotal role of cellular senescence in vascular smooth muscle cells. Exp. Gerontol. 45, 819–824 [DOI] [PubMed] [Google Scholar]
- 11. Lagna G., Ku M. M., Nguyen P. H., Neuman N. A., Davis B. N., Hata A. (2007) Control of phenotypic plasticity of smooth muscle cells by bone morphogenetic protein signaling through the myocardin-related transcription factors. J. Biol. Chem. 282, 37244–37255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nicolls M. R., Taraseviciene-Stewart L., Rai P. R., Badesch D. B., Voelkel N. F. (2005) Autoimmunity and pulmonary hypertension: a perspective. Eur. Respir. J. 26, 1110–1118 [DOI] [PubMed] [Google Scholar]
- 13. Humbert M., Monti G., Brenot F., Sitbon O., Portier A., Grangeot-Keros L., Duroux P., Galanaud P., Simonneau G., Emilie D. (1995) Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am. J. Respir. Crit. Care Med. 151, 1628–1631 [DOI] [PubMed] [Google Scholar]
- 14. Fartoukh M., Emilie D., Le Gall C., Monti G., Simonneau G., Humbert M. (1998) Chemokine macrophage inflammatory protein-1alpha mRNA expression in lung biopsy specimens of primary pulmonary hypertension. Chest 114, 50S-51S [DOI] [PubMed] [Google Scholar]
- 15. Sanchez O., Marcos E., Perros F., Fadel E., Tu L., Humbert M., Dartevelle P., Simonneau G., Adnot S., Eddahibi S. (2007) Role of endothelium-derived CC chemokine ligand 2 in idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 176, 1041–1047 [DOI] [PubMed] [Google Scholar]
- 16. Soon E., Holmes A. M., Treacy C. M., Doughty N. J., Southgate L., Machado R. D., Trembath R. C., Jennings S., Barker L., Nicklin P., Walker C., Budd D. C., Pepke-Zaba J., Morrell N. W. (2010) Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 122, 920–927 [DOI] [PubMed] [Google Scholar]
- 17. Taraseviciene-Stewart L., Nicolls M. R., Kraskauskas D., Scerbavicius R., Burns N., Cool C., Wood K., Parr J. E., Boackle S. A., Voelkel N. F. (2007) Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling. Am. J. Respir. Crit. Care Med. 175, 1280–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daley E., Emson C., Guignabert C., de Waal Malefyt R., Louten J., Kurup V. P., Hogaboam C., Taraseviciene-Stewart L., Voelkel N. F., Rabinovitch M., Grunig E., Grunig G. (2008) Pulmonary arterial remodeling induced by a Th2 immune response. J. Exp. Med. 205, 361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Steiner M. K., Syrkina O. L., Kolliputi N., Mark E. J., Hales C. A., Waxman A. B. (2009) Interleukin-6 overexpression induces pulmonary hypertension. Circ. Res. 104, 236–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fujita M., Mason R. J., Cool C., Shannon J. M., Hara N., Fagan K. A. (2002) Pulmonary hypertension in TNF-α-overexpressing mice is associated with decreased VEGF gene expression. J. Appl. Physiol. 93, 2162–2170 [DOI] [PubMed] [Google Scholar]
- 21. Hagen M., Fagan K., Steudel W., Carr M., Lane K., Rodman D. M., West J. (2007) Interaction of interleukin-6 and the BMP pathway in pulmonary smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 292, L1473–L1479 [DOI] [PubMed] [Google Scholar]
- 22. West J. (2010) Cross talk between Smad, MAPK, and actin in the etiology of pulmonary arterial hypertension. Adv. Exp. Med. Biol. 661, 265–278 [DOI] [PubMed] [Google Scholar]
- 23. Song Y., Coleman L., Shi J., Beppu H., Sato K., Walsh K., Loscalzo J., Zhang Y. Y. (2008) Inflammation, endothelial injury, and persistent pulmonary hypertension in heterozygous BMPR2-mutant mice. Am. J. Physiol. 295, H677–H690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luchsinger L. L., Patenaude C. A., Smith B. D., Layne M. D. (2011) Myocardin-related transcription factor-A complexes activate type I collagen expression in lung fibroblasts. J. Biol. Chem. 286, 44116–44125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hata A., Seoane J., Lagna G., Montalvo E., Hemmati-Brivanlou A., Massagué J. (2000) OAZ uses distinct DNA- and protein-binding zinc fingers in separate BMP-Smad and Olf signaling pathways. Cell 100, 229–240 [DOI] [PubMed] [Google Scholar]
- 26. Preston I. R., Hill N. S., Warburton R. R., Fanburg B. L. (2006) Role of 12-lipoxygenase in hypoxia-induced rat pulmonary artery smooth muscle cell proliferation. Am. J. Physiol. Lung Cell Mol. Physiol. 290, L367–L374 [DOI] [PubMed] [Google Scholar]
- 27. Chan M. C., Hilyard A. C., Wu C., Davis B. N., Hill N. S., Lal A., Lieberman J., Lagna G., Hata A. (2010) Molecular basis for antagonism between PDGF and the TGFβ family of signaling pathways by control of miR-24 expression. EMBO J. 29, 559–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sprague A. H., Khalil R. A. (2009) Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 78, 539–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gupta V., Yeo G., Kawakubo H., Rangnekar V., Ramaswamy P., Hayashida T., MacLaughlin D. T., Donahoe P. K., Maheswaran S. (2007) Mullerian-inhibiting substance induces Gro-β expression in breast cancer cells through a nuclear factor-κB-dependent and Smad1-dependent mechanism. Cancer Res. 67, 2747–2756 [DOI] [PubMed] [Google Scholar]
- 30. Karin M. (1999) How NF-κB is activated: the role of the IκB kinase (IKK) complex. Oncogene 18, 6867–6874 [DOI] [PubMed] [Google Scholar]
- 31. Beg A. A., Ruben S. M., Scheinman R. I., Haskill S., Rosen C. A., Baldwin A. S., Jr. (1992) IκB interacts with the nuclear localization sequences of the subunits of NF-κB: a mechanism for cytoplasmic retention. Genes Dev. 6, 1899–1913 [DOI] [PubMed] [Google Scholar]
- 32. Zabel U., Henkel T., Silva M. S., Baeuerle P. A. (1993) Nuclear uptake control of NF-κB by MAD-3, an IκB protein present in the nucleus. EMBO J. 12, 201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Agrawal A., Cha-Molstad H., Samols D., Kushner I. (2003) Overexpressed nuclear factor-κB can participate in endogenous C-reactive protein induction, and enhances the effects of C/EBPβ and signal transducer and activator of transcription-3. Immunology 108, 539–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Y., Saccani S., Shin H., Nikolajczyk B. S. (2008) Dynamic protein associations define two phases of IL-1β transcriptional activation. J. Immunol. 181, 503–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang D. Z., Li S., Hockemeyer D., Sutherland L., Wang Z., Schratt G., Richardson J. A., Nordheim A., Olson E. N. (2002) Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc. Natl. Acad. Sci. U.S.A. 99, 14855–14860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simic P., Vukicevic S. (2005) Bone morphogenetic proteins in development and homeostasis of kidney. Cytokine Growth Factor Rev. 16, 299–308 [DOI] [PubMed] [Google Scholar]
- 37. Dendooven A., van Oostrom O., van der Giezen D. M., Leeuwis J. W., Snijckers C., Joles J. A., Robertson E. J., Verhaar M. C., Nguyen T. Q., Goldschmeding R. (2011) Loss of endogenous bone morphogenetic protein-6 aggravates renal fibrosis. Am. J. Pathol. 178, 1069–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yao Y., Bennett B. J., Wang X., Rosenfeld M. E., Giachelli C., Lusis A. J., Boström K. I. (2010) Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circulation Research 107, 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang R. H., Zheng X. L., Callis T. E., Stansfield W. E., He J., Baldwin A. S., Wang D. Z., Selzman C. H. (2008) Myocardin inhibits cellular proliferation by inhibiting NF-κB(p65)-dependent cell cycle progression. Proc. Natl. Acad. Sci. U.S.A. 105, 3362–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Davis-Dusenbery B. N., Chan M. C., Reno K. E., Weisman A. S., Layne M. D., Lagna G., Hata A. (2011) Down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J. Biol. Chem. 286, 28097–28110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yu Y., Sweeney M., Zhang S., Platoshyn O., Landsberg J., Rothman A., Yuan J. X. (2003) PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am. J. Physiol. Cell Physiol. 284, C316–C330 [DOI] [PubMed] [Google Scholar]
- 42. Yu Y., Keller S. H., Remillard C. V., Safrina O., Nicholson A., Zhang S. L., Jiang W., Vangala N., Landsberg J. W., Wang J. Y., Thistlethwaite P. A., Channick R. N., Robbins I. M., Loyd J. E., Ghofrani H. A., Grimminger F., Schermuly R. T., Cahalan M. D., Rubin L. J., Yuan J. X. (2009) A functional single-nucleotide polymorphism in the TRPC6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation 119, 2313–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sawada H., Mitani Y., Maruyama J., Jiang B. H., Ikeyama Y., Dida F. A., Yamamoto H., Imanaka-Yoshida K., Shimpo H., Mizoguchi A., Maruyama K., Komada Y. (2007) A nuclear factor-κB inhibitor pyrrolidine dithiocarbamate ameliorates pulmonary hypertension in rats. Chest 132, 1265–1274 [DOI] [PubMed] [Google Scholar]
- 44. Yu P. B., Beppu H., Kawai N., Li E., Bloch K. D. (2005) Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J. Biol. Chem. 280, 24443–24450 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.