Background: Paxillin, a focal adhesion (FA) adaptor, functions in migration, although how Ser-85 phosphorylation affects migration is unknown.
Results: Phosphorylation of paxillin at Ser-85 is critical for its association with talin, which regulates FA formation along the cell boundary and haptotactic migration.
Conclusion: Paxillin Ser-85 phosphorylation regulates FA formation and migration.
Significance: Paxillin Ser-85 phosphorylation coordinates FA dynamics for migration.
Keywords: Adhesion, Invasion, Migration, Protein Phosphorylation, Signal Transduction, Focal Adhesion, Morphology, Paxillin, Talin
Abstract
Integrin-mediated adhesion to extracellular matrix proteins is dynamically regulated during morphological changes and cell migration. Upon cell adhesion, protein-protein interactions among molecules at focal adhesions (FAs) play major roles in the regulation of cell morphogenesis and migration. Although tyrosine phosphorylation of paxillin is critically involved in adhesion-mediated signaling, the significance of paxillin phosphorylation at Ser-85 and the mechanism by which it regulates cell migration remain unclear. In this study, we examined how Ser-85 phosphorylation of paxillin affects FA formation and cell migration. We found that paxillin phosphorylation at Ser-85 occurred during HeLa cell adhesion to collagen I and was concomitant with tyrosine phosphorylation of both focal adhesion kinase and talin. However, the non-phosphorylatable S85A mutant of paxillin impaired cell spreading, FA turnover, and migration toward collagen I but not toward serum. Furthermore, whereas the (presumably indirect) interaction between paxillin and the C-terminal tail of talin led to dynamic FAs at the cell boundary, S85A paxillin did not bind talin and caused stabilized FAs in the central region of cells. Together, these observations suggest that cell adhesion-dependent Ser-85 phosphorylation of paxillin is important for its interaction with talin and regulation of dynamic FAs and cell migration.
Introduction
Cell adhesion causes the tyrosine phosphorylation and activation of diverse signaling or adaptor molecules at focal adhesions (FAs),2 which play important roles in the regulation of cell adhesion, spreading, migration, and invasion (1). Among known FA molecules, focal adhesion kinase (FAK) and c-Src (or the FAK·c-Src complex) are involved in modulating diverse signaling pathways after cell adhesion (2). FAK is autophosphorylated at Tyr-397 upon cell engagement to extracellular matrix (ECM) proteins. Phosphorylated Tyr-397 recruits SH2 (Src homology 2) domain-containing molecules such as c-Src, which phosphorylates other Tyr residues in FAK (3). Another FA molecule, paxillin, has also been shown to be phosphorylated in vitro by FAK and is known to be involved in protein-protein interactions upon cell adhesion (4). Such cell adhesion-dependent protein interactions involving paxillin can lead to the dynamic regulation of downstream signaling activities for different cellular functions such as actin reorganization and morphological changes that are involved in both cell migration and invasion (5). Integrins on the cell surface interact with the ECM at FAs, where cells sense rigidity and dimensionality from the underlying ECM (6). Specialized focal contacts or adhesions such as invadopodia or invasive protrusions also dynamically sense the ECM, and their formation is regulated by cell adhesion signaling activity during invasion (7, 8).
The function of paxillin as an adhesion-dependent adaptor molecule can be attributed to its phosphorylation state. Phosphorylation of paxillin at Tyr-31 and Tyr-118 occurs in a FAK- and c-Src-dependent manner (9). These phosphorylation events allow recruitment of SH2 domain-containing molecules such as CrkII (a homologue of CT10 Regulator of Kinase) which leads to Rac1 signaling via the CrkII-Dock-ELMO complex (10), or p120RasGAP (RASA1), which leads to RhoA inactivation (11). Thus, cell adhesion-dependent tyrosine phosphorylation of paxillin regulates actin dynamics (12).
Paxillin also has many Ser/Thr phosphorylation sites that are targeted by diverse kinases, including MAPKs (12). Among them, JNK-mediated Ser-178 phosphorylation of paxillin plays a role in the migration of bladder tumor epithelial cells (13), and p38MAPK-mediated Ser-85 phosphorylation of paxillin regulates NGF-induced neurite outgrowth of PC-12 cells (14). Interestingly, post-translational modification of paxillin Ser-85 in rat insulinoma cells can occur via either O-GlcNAcylation or phosphorylation; Ser-85 O-GlcNAcylation of paxillin mediated by hyperglycemic conditions is counteracted by cell adhesion-dependent phosphorylation (15). O-GlcNAcylation of Ser-85 limits cellular protrusions, whereas phosphorylation or blocking of this O-GlcNAcylation enhances cellular protrusions (15). Because paxillin phosphorylation at Ser-85 is important for cell morphology, it is possible that Ser-85 can regulate metastatic potential, including cell migration and invasion capacity, although the mechanisms by which Ser-85 phosphorylation of paxillin affects such cellular functions are not clear.
In this study, we investigated how Ser-85 phosphorylation of paxillin is involved in both migration and invasion. We found that the phosphorylation of Ser-85 upon cell adhesion is required for the association of paxillin with the C-terminal tail domain of talin, which in turn plays an important role in the regulation of FA dynamics required for efficient migration and invasion.
EXPERIMENTAL PROCEDURES
Cell Cultures
HeLa cervical cancer cells (American Type Culture Collection, Manassas, VA) were maintained in DMEM-H (WelGENE, Inc., Daegu, Korea) supplemented with 10% FBS (Invitrogen) and gentamycin/streptomycin (Invitrogen).
Cell Extract Preparation and Western Blotting
Subconfluent cells were harvested or transiently transfected with HA3-tagged WT, S85A, or S85D paxillin with or without GFP-talin, the GFP-tagged N-terminal talin head domain (amino acids (aa) 1–433), or the GFP-tagged C-terminal talin tail domain (aa 434–2541) for 36 h. In some cases, cells were microporated with control siRNA (sc-37007, Santa Cruz Biotechnology) or siRNA against paxillin (sc-29439) using the Neon® transfection system, and 1 day later, the cells were transfected with HA3-tagged WT or S85A mutant paxillin using FuGENE® HD (Promega) for 24 h prior to replating and harvesting. The transfected cells were trypsinized, washed twice with PBS, and pelleted. Pellets were resuspended in RPMI 1640 medium containing 1% BSA and rolled over (60 rpm) at 37 °C for 1 h to eliminate basal signaling activity. Resuspended cells were kept in suspension or replated onto dishes or coverslips precoated with fibronectin, collagen I, or laminin I (10 μg/ml; Trevigen, Gaithersburg, MD) in the absence or presence of 10% FBS. After incubation for the indicated times, cells were processed for whole cell lysate preparation, indirect immunofluorescence, or actin staining. Whole cell lysates were prepared using radioimmunoassay buffer (50 mm Tris (pH 7.5), 150 mm NaCl, 50 mm NaF, 1 mm sodium pyrophosphate, 0.1% SDS, 0.1% sodium deoxycholate, 1% Nonidet P-40, and protease inhibitors). To some samples, the pharmacological p38MAPK inhibitor SB202190 (10 μm; LC Laboratories, Woburn, MA) was directly added in serum-free replating medium 30 min before replating. The lysates were quantitated using the BCA method (Thermo Scientific Pierce), normalized, and used in standard Western blotting with antibodies against phospho-Ser-83 paxillin for rat paxillin (Ser-85 paxillin for human paxillin) (16); paxillin (BD Biosciences); phospho-Tyr-31/Tyr-118 paxillin, phospho-Tyr-397 FAK, phospho-Tyr-577 FAK, phospho-Tyr-925 FAK, FAK, and HA tag (Santa Cruz Biotechnology); phospho-ERK1/2, ERK1/2, phospho-Ser-425 talin, vinculin, and α-tubulin (Cell Signaling Technology, Danvers, MA); and talin (Sigma).
cDNA Constructs
pCMV-HA3-human paxillin (WT and S85A) and GST-paxillin (WT and S85A) have been described previously (15). Constructs for the head or tail domain of human talin tagged with GFP were kind gifts from Dr. Juliano Rudy (University of North Carolina, Chapel Hill, NC). Other phosphomimetic mutants of HA3-S85D or HA3-S85E paxillin were engineered using the QuikChange site-directed mutagenesis kit (Stratagene), and their sequences were confirmed. Fragments of the tail domain of talin tagged with GST were engineered by PCR using primers with linker oligonucleotides containing sequences for the EcoRI restriction site at the 5′-end and the NotI restriction site at the 3′-end to generate four fragments from the talin tail domain (aa 433–2541). The C-terminal tail domain 1 (D1) fragment (aa 434–1060), the C-terminal tail D2 fragment (aa 952–1372), the C-terminal tail D3 fragment (aa 1373–1948), or the C-terminal tail D4 fragment (aa 1949–2541) was subcloned into pGEX4T-1 (Amersham Biosciences), and their sequences were confirmed. mCherry-conjugated human WT and S85A paxillin constructs were engineered, and their sequences were confirmed by direct sequence analyses.
Co-immunoprecipitation
Whole cell lysates containing at least 500 μg of protein were mixed with anti-HA antibody-precoated beads (30 μl of 50% slurry/condition; Sigma) or anti-GFP antibody (0.5 μg/condition; Santa Cruz Biotechnology) and rotated overnight at 4 °C. Protein A/G-Sepharose beads (30 μl of 50% slurry; Upstate, Billerica, MA) were added to the mixture and rotated for an additional 2 h at 4 °C. The immunoprecipitates were collected, washed, and eluted prior to standard Western blotting using anti-talin, anti-FAK, anti-HA, anti-vinculin, or anti-GFP antibody.
In Vitro GST-Protein Pulldown Assay
Recombinant GST fusion proteins were prepared as described previously (15). Purified recombinant GST fusion proteins on glutathione-agarose beads (Cell Signaling Technology) were mixed with HeLa cell extracts (300 μg of proteins) by rotation (60 rpm) for 1 h at 4 °C and washed twice with ice-chilled lysis buffer and twice with PBS. Beads with attached proteins were mixed with 2× SDS-PAGE sample buffer and boiled for 5 min prior to immunoblotting using anti-GST, anti-talin, anti-FAK, or anti-paxillin antibody.
Immunofluorescence
Cells were transiently transfected with the various constructs described above, control or paxillin siRNA alone, or paxillin siRNA and S85A paxillin for 36 h. The cells were replated on coverslips precoated with collagen I in the absence of serum for 15 or 30 min prior to staining for actin using phalloidin (Invitrogen) and subsequent immunostaining using anti-HA, anti-phospho-Tyr-397 FAK, anti-paxillin, or anti-vinculin antibody overnight at 4 °C. Cells were also immunostained with anti-GFP-talin (green) and anti-HA3-paxillin (red) antibodies and the appropriate secondary antibodies. Cells were washed three times with PBS and once with pure H2O and mounted with a mounting solution (DakoCytomation). Visualization was performed using a BX51TR fluorescent microscope (Olympus, Tokyo, Japan).
Transwell Migration or Invasion Assay
Cells were transiently transfected with mock constructs, HA3-WT paxillin, or HA3-S85A paxillin for 36 h and analyzed for migration using Transwell chambers with 8-μm pores (Corning, Costar, NY) as described previously (17). The assay measured migration toward 10% FBS or collagen I (10 μg/ml) for 6 or 12 h. In some cases, SB202190 (10 μm) was added to cells just before loading into the upper chambers. Migration toward BSA was used as a negative control. The migrated cells on the filter were randomly imaged (at least five images/condition), and the numbers of migrated cells were counted independently by two investigators to obtain means ± S.D. for graphs.
Silver Staining of Immunoprecipitates
Cells transiently transfected with mock, HA3-WT paxillin, or HA3-S85A paxillin constructs for 36 h were harvested as described above and immunoprecipitated with anti-HA antibody prior to silver staining (Bio-Rad).
Invasive Protrusion Analysis
Cells transfected with HA3 (mock) or HA3-paxillin (WT or S85A) were analyzed for invasive protrusions by culturing the cells on Oregon Green® 488-conjugated gelatin (Invitrogen) and detecting the black spots produced due to the degradation of gelatin. The number of cells with degraded ECM among at least 100 cells was counted for each condition, and the percentage of the total area that showed ECM degradation was calculated by ImageJ analysis to give the mean ± S.D. for graphic presentation.
Statistical Analysis
Student's t test was performed for comparison of means to determine whether the differences were significant. p values <0.05 were considered significant.
RESULTS
Ser-85 Phosphorylation of Paxillin Depends on Cell Adhesion
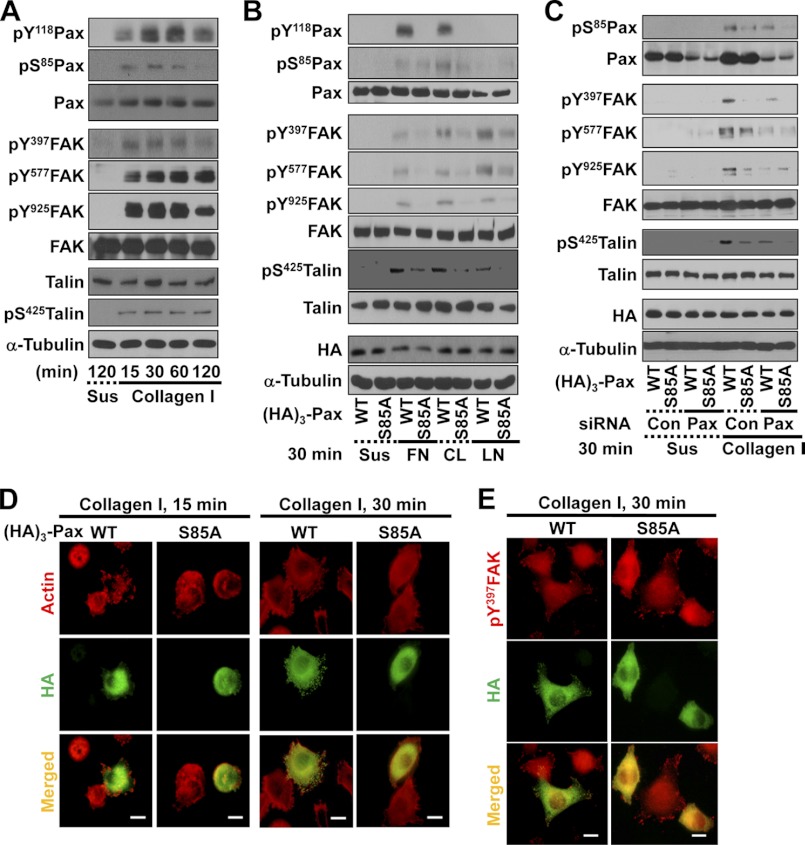
We previously reported that Ser-85 phosphorylation of paxillin upon cell adhesion could enhance membrane protrusions in insulinoma cells under hyperglycemic conditions and that this effect was antagonized by O-GlcNAcylation (i.e. post-translational modification of Ser/Thr residues with O-GlcNAc) (15). In this study, we used HeLa cervical cancer cells to reveal how paxillin Ser-85 phosphorylation is important for cellular functions such as FA dynamics and cell migration/invasion. We either kept HeLa cells in suspension or newly replated them onto collagen I-precoated culture dishes for different times. Through immunoblot analysis of diverse cell adhesion-related molecules, we found that cell adhesion caused not only tyrosine phosphorylation of FAK and paxillin but also Ser-85 phosphorylation of paxillin and Ser-425 phosphorylation of talin (Fig. 1A). Thus, the phosphorylation of paxillin Ser-85 also depended on the cell adhesion process. To further confirm this dependence on cell/ECM adhesion, we transfected cells with either WT or S85A mutant paxillin for 36 h and maintained cells in suspension or replated cells onto dishes precoated with different ECM components for 30 min. On fibronectin, collagen I, and laminin, adhesion signaling activities involving FAK and paxillin tyrosine phosphorylation were obvious when cells were transfected with WT paxillin but were reduced in cells transfected with S85A mutant paxillin (Fig. 1B). Moreover, transfection with WT paxillin did not result in the obvious formation of phospho-Ser-85 paxillin when the transfected cells were kept in suspension or replated on fibronectin or laminin. Furthermore, suppression of paxillin decreased phospho-Ser-85 paxillin, phospho-Tyr-397 FAK, phospho-Tyr-577 FAK, and phospho-Ser-425 talin in cells transiently transfected with WT or S85A paxillin (Fig. 1C). Therefore, it appears that the formation of phospho-Ser-85 paxillin may be linked to specific adhesion signaling contexts such as adhesion of HeLa cells onto collagen I.
FIGURE 1.
Ser-85 phosphorylation of paxillin is dependent on cell adhesion. HeLa cells without (A) or with transfection with HA3-paxillin (paxillin or S85A) for 36 h (C, D, and E) were collected, rolled over at 37 °C for 1 h, and maintained in suspension (Sus) for 2 h. Cells were reseeded onto culture dishes newly precoated with collagen I (10 μg/ml) for the indicated times prior to harvesting and immunoblot analysis of whole cell lysates with the indicated antibodies (A–C) or reseeded on collagen I-precoated coverslips for 15 or 30 min before processing for indirect immunostaining or actin staining (D and E). Anti-HA tag antibody (green; D) or anti-HA tag (green) and anti-phospho-Tyr-397 FAK (pY397FAK; red) primary antibodies and the appropriate secondary antibodies (E) were used. Scale bars = 20 μm (D and E). B, HeLa cells transfected with HA3-paxillin (WT or S85A) for 36 h were kept in suspension or reseeded onto culture dishes precoated with 10 μg/ml fibronectin (FN), collagen I (CL), or laminin I (LN) for 30 min as described above. The cells were then harvested, and whole cell lysates were subjected to immunoblot analysis for the indicated molecules. The data represent three independent experiments. pS85Pax, phospho-Ser-85 paxillin; Con, control.
Paxillin Ser-85 Phosphorylation Is Important for Adhesion Signaling and FA Formation
We next examined the significance of phospho-Ser-85 paxillin in actin remodeling and FA formation. By actin staining 15 or 30 min after replating on collagen I, we found that WT paxillin caused extensive spreading of cells with well developed actin stress fibers (94.7 ± 3.5 or 96.3 ± 3.8%, respectively), whereas the S85A mutant caused limited spreading with less significant actin remodeling (25.4 ± 6.8 or 12.5 ± 4.3%, respectively) (Fig. 1D). As for FA formation via immunostaining Tyr-397-phosphorylated FAK, cells expressing WT paxillin showed well developed FAs along cell boundaries and protrusions (91.7 ± 5.9%), whereas cells expressing the S85A mutant had limited FA formation (17.2 ± 4.7%) (Fig. 1E). These observations indicate that phospho-Ser-85 paxillin depends on cell adhesion and plays roles in FA formation and actin remodeling during cell adhesion.
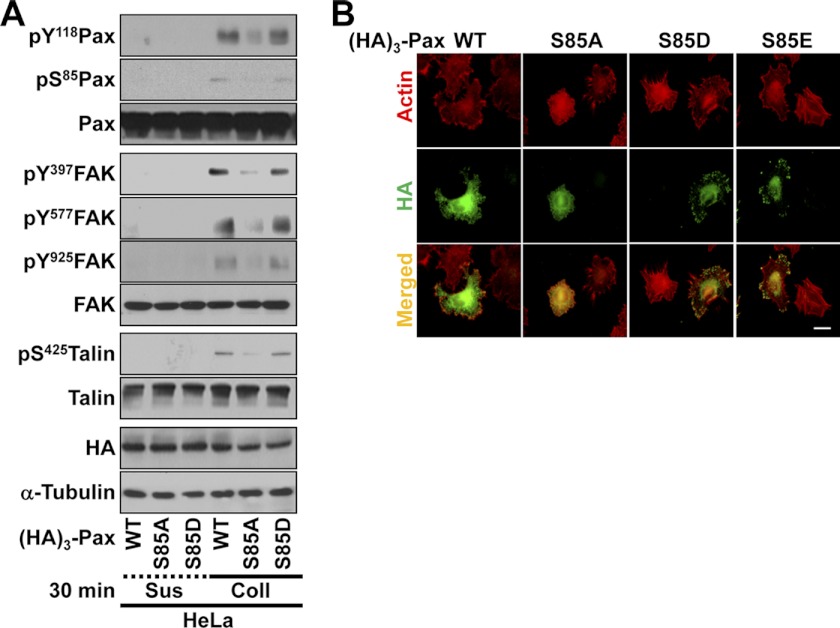
Given that paxillin Ser-85 can be modified by O-phosphorylation or O-GlcNAcylation (15), we mutated Ser-85 to either Asp (S85D) or Glu (S85E) to clarify how the S85A mutant-mediated effects occurred. Standard Western blotting using lysates from suspended or adherent cells transfected with WT, S85A, or S85D paxillin vector revealed that WT or S85D paxillin expression caused sufficient activation of cell adhesion-related signaling molecules, including FAK, paxillin, and talin, whereas the S85A mutant did not (Fig. 2A). Furthermore, when actin was stained in cells expressing WT, S85D, or S85E paxillin, efficient protrusive processes were observed, whereas cells expressing S85A paxillin formed less efficient protrusive processes (Fig. 2B, upper panels). Meanwhile, when the localization of exogenous paxillin was examined, WT, S85D, or S85E paxillin localized efficiently at FAs along the cell boundaries, but the S85A mutant did not (Fig. 2B, middle panels). Therefore, these observations indicate that Ser-85-phosphorylated paxillin affects cell adhesion and actin organization via localization at FAs along cell boundaries (Fig. 2B).
FIGURE 2.
Paxillin Ser-85 phosphorylation affects cell adhesion signaling activity, FA formation, and actin organization. A, cells were transfected with HA3-paxillin (WT, S85A, or S85D) for 36 h before being kept in suspension (Sus) or reseeded onto collagen I (Coll; 10 μg/ml)-precoated culture dishes for 30 min. Whole cell lysates were prepared for standard Western blots for the indicated molecules. pS85Pax, phospho-Ser-85 paxillin. B, HeLa cells were transfected with HA3-paxillin (WT, S85A, S85D, or S85E) for 36 h before replating on collagen I (10 μg/ml)-precoated coverslips for 30 min. The cells were stained for actin using phalloidin and immunostained for HA3-paxillin using anti-HA antibody. Scale bar = 10 μm. The data shown represent three independent experiments.
Paxillin Ser-85 Phosphorylation Is Important for Haptotactic Cell Migration and Invasion
We next investigated whether phospho-Ser-85 paxillin is involved in cell migration because it appears to be important for FA formation and actin remodeling (Fig. 1). A Transwell migration assay was performed using cells transfected with WT or S85A paxillin. Cells showed similar migration capacity toward 10% FBS in the lower chamber regardless of WT or mutant paxillin transfection (Fig. 3A, upper panels, and B, left panel). However, haptotactic migration toward collagen I depended on paxillin type: cells expressing WT paxillin showed efficient migration, whereas cells expressing the S85A mutant showed greatly reduced migration (Fig. 3A, lower panels, and B, right panel), indicating that phospho-Ser-85 paxillin is important for haptotaxis but not for serum-mediated chemotaxis. Consistent with the lack of difference in chemotactic migration, adhesion signaling activities of cells adhered to collagen I in the presence of 10% FBS showed no difference between WT or S85A paxillin cells. Tyrosine phosphorylation of FAK and paxillin and Ser-425 phosphorylation of talin were not attenuated by the mutation, whereas Ser-85 phosphorylation of paxillin was blocked by the S85A mutation, as expected (Fig. 3C). Meanwhile, replating in the absence of serum resulted in lower signaling activity in S85A mutant cells compared with WT cells (Fig. 1B), which might be correlated with the reduced migration toward collagen I.
FIGURE 3.
Paxillin Ser-85 phosphorylation is important for haptotactic cell migration. A and B, cells were transfected with mock or HA3-paxillin (WT or S85A) constructs for 36 h before a Transwell migration assay, in which the lower chamber was filled with 1% BSA and DMEM-H (negative control), 10% FBS, or serum-free medium containing 10 μg/ml collagen I (Coll) for 12 h. After incubation, migrated cells on the chamber filter were stained with Diff-Quik solution (Medion Diagnostics), and at least five random images for each condition (A) were imaged to calculate means ± S.D. (B). C, cells were transfected with HA3-paxillin (WT or S85A) for 36 h and kept in suspension (Sus) or replated onto collagen I-precoated culture dishes for 30 min in the presence of 10% FBS before harvesting and analysis of whole cell lysates by immunoblotting for the indicated molecules. D and E, assay for cell migration through the Transwell for 6 h was performed as described for A. Cells were pretreated with SB202190 (SB) 30 min before loading cells into the upper chambers. Migrated cells from representative images (D) were calculated for means ± S.D. (E). F, cells were transfected and manipulated as described for C in the absence (−) or presence of SB202190 pretreatment before harvesting and analysis of whole cell lysates by immunoblotting for the indicated molecules. The data shown represent three independent experiments. pS85Pax, phospho-Ser-85 paxillin.
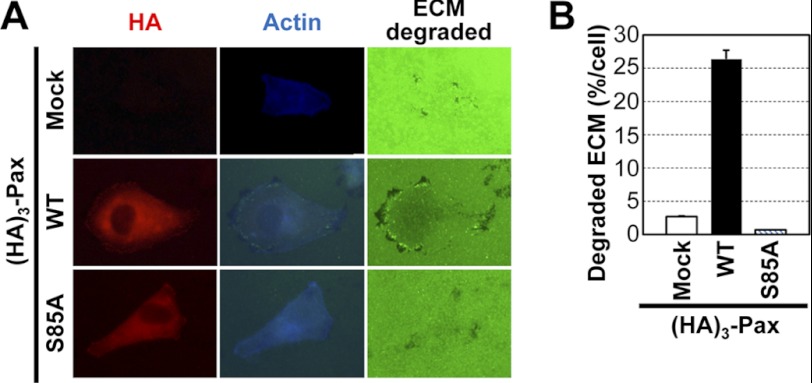
It was previously reported that Ser-85 phosphorylation of paxillin in PC-12 cells is mediated by p38MAPK (14). We therefore examined whether Ser-85 phosphorylation-mediated migration depends on p38MAPK activity by Transwell migration analysis using cells transfected with WT or S85A paxillin in the absence or presence of the p38MAPK inhibitor SB202190. SB202190 treatment decreased the WT paxillin-mediated migration toward collagen I to a similar level to that in S85A mutant cells (Fig. 3, D and E). As expected, SB202190 treatment also reduced phospho-Ser-85 paxillin but not phospho-Tyr-118 paxillin (Fig. 3F). Interestingly, although phospho-Tyr-118 paxillin was not inhibited (Fig. 3F, first and third lanes), WT paxillin-mediated signaling activities of phospho-Tyr-397 FAK and phospho-ERK1/2 were inhibited by SB202190 treatment (Fig. 3F). This observation suggests that phospho-Ser-85 paxillin might be involved in certain signaling activities during cell migration, presumably through protein-protein interactions among FA molecules. In addition to haptotactic migration, invasive protrusion formation was enhanced by WT paxillin but was abolished by S85A paxillin (Fig. 4, A and B). These observations indicate that phospho-Ser-85 paxillin is involved in cell migration and invasion, especially when the surrounding ECM is critically involved.
FIGURE 4.
Paxillin Ser-85 phosphorylation is important for invasive protrusion formation. Cells were either mock-transfected or transfected with HA3-paxillin (HA3-Pax; WT or S85A) for 36 h before reculturing on Oregon Green® 488-conjugated gelatin for 6 h. ECM degradation by invasive protrusions along the cell boundary was confirmed by actin staining concurrent with detection of black spots (degradation of Oregon Green 488-conjugated gelatin) (A). The numbers of cells with degraded ECM were counted among at least 100 cells for each condition, and the percentage of the ECM-degraded area out of the total area was calculated to obtain means ± S.D. from three different experiments (B).
WT but Not S85A Paxillin Binds to Talin
We examined how phospho-Ser-85 paxillin is involved in cell adhesion during migration and invasion. We hypothesized that paxillin might be involved in the formation of an efficient FA complex, whereas S85A paxillin might not perform this function. Therefore, we examined differential protein interactions of WT and S85A paxillin via silver staining of their immunoprecipitates. Exogenously transfected WT paxillin co-immunoprecipitated diverse proteins compared with S85A paxillin or the mock control (Fig. 5A), indicating that there might be differential protein complexes presumably depending on its Ser-85 phosphorylation. We thus examined the existence of FA molecules in in vitro pulldown experiments using recombinant GST-paxillin (WT or S85A) and HeLa extracts. Among FA molecules, we found that a small amount of talin was present in the GST-WT paxillin pulldown but not in the GST-S85A paxillin pulldown (Fig. 5B and supplemental Fig. S1). Interestingly, both GST-tagged WT and S85A paxillin bound FAK (supplemental Fig. S1A), indicating that the specificity of paxillin binding may depend on Ser-85 phosphorylation.
FIGURE 5.
WT (but not S85A) paxillin binds to talin. A, immunoprecipitates (IP) using anti-HA antibody from extracts of mock-transfected or HA3-paxillin (HA3-Pax; WT or S85A)-transfected cells were run in a gel and silver-stained. Arrowheads indicate certain proteins immunoprecipitated from WT paxillin-transfected cell extracts but not from mock-transfected or S85A (SA) paxillin-transfected cell extracts. IgG-h and IgG-l, IgG heavy and light chains, respectively. B, recombinant GST alone or GST-paxillin (WT or S85A) proteins bound to glutathione-agarose beads were mixed with whole cell lysates (WCL) from HeLa cells overnight at 4 °C, and the pulldown samples were analyzed by Western blotting using anti-GST or anti-talin antibody. C, HeLa cells were transfected with HA3-paxillin (WT or S85A) for 36 h and kept in suspension (Sus) or reseeded onto collagen I (Coll)-precoated culture dishes for 30 min prior to harvesting. Whole cell lysates were immunoprecipitated with anti-HA antibody as described under “Experimental Procedures.” The immunoprecipitates and whole cell lysates were analyzed in parallel by standard Western blotting with anti-HA, anti-FAK, or anti-talin antibody. D–F, HeLa cells were cotransfected with HA3-paxillin (WT or S85A) and the GFP-tagged N-terminal head (Head) or C-terminal tail (C-tail) domain of talin for 36 h. The cells were then kept in suspension or reseeded onto collagen I-precoated culture dishes for 30 min prior to harvesting. Whole cell lysates were subjected to standard Western blot (WB) analysis using the indicated antibodies (D) or co-immunoprecipitation using anti-GFP (E) or anti-HA (F) antibody prior to immunoblotting in parallel with lysates (Input) using antibodies for the indicated molecules. pS85Pax, phospho-Ser-85 paxillin. G, recombinant GST-talin tail fragments (D1–D4) on beads were mixed with HeLa cell extracts as described under “Experimental Procedures.” The proteins on beads were subjected to standard Western blotting for paxillin or GST. H, cells were mock-transfected or transfected with HA3-paxillin (WT or S85A) for 36 h before manipulating cells as described for C. Immunoprecipitates with anti-HA antibody were immunoblotted in parallel with lysates for the indicated molecules. The data are representative of three independent experiments.
Moreover, immunoprecipitation of HA3-paxillin showed co-immunoprecipitation of talin from adherent cells transfected with WT paxillin but not from adherent cells transfected with S85A paxillin or from suspended cells (Fig. 5C), indicating that paxillin but not the S85A mutant associates with talin upon adhesion. However, FAK was constitutively found in the HA3-paxillin immunoprecipitates whether paxillin was mutated or not (Fig. 5C and supplemental S1A). We next cotransfected cells with HA3-paxillin (WT or S85A) together with either the head or tail domain of talin and performed immunoblot analysis of FAK and paxillin phosphorylation. Although tyrosine phosphorylation of FAK and paxillin was generally inhibited by cotransfection of S85A paxillin and the C-terminal tail domain of talin, tyrosine phosphorylation was not altered by the paxillin mutant when it was cotransfected with the N-terminal head domain of talin (Fig. 5D). Furthermore, phospho-Ser-85 paxillin was obvious in adherent cells that were cotransfected with WT paxillin and the C-terminal tail of talin, whereas it was insignificant in cells transfected with either S85A paxillin or the head domain of talin or in suspended cells (Fig. 5D). In the reverse co-immunoprecipitation experiment, GFP-talin immunoprecipitates showed a slight co-immunoprecipitation of HA3-paxillin only in cells that were cotransfected with GFP-talin and WT paxillin but not the S85A mutant adhered on collagen I (Fig. 5E). Furthermore, immunoprecipitates of HA3-WT paxillin but not HA3-S85A paxillin co-immunoprecipitated the C-terminal tail domain of talin conjugated with GFP but not the head domain (Fig. 5F). These data suggest that Ser-85 phosphorylation of paxillin upon cell adhesion is important for its association with talin, especially with the C-terminal tail domain. Furthermore, a fragment (aa 434–1060) of the C-terminal tail domain of talin bound paxillin when recombinant GST-talin tail fragments were incubated with HeLa cell extracts, whereas other fragments (aa 951–2451) did not (Fig. 5G). Because paxillin and talin are components of FAs, we next examined whether paxillin binds to other FA components like vinculin. However, vinculin was not detected in HA3-paxillin immunoprecipitates (Fig. 5H). It may thus be likely that there is a certain specificity for the binding between Ser-85-phosphorylated paxillin and FA molecules.
Regulation of FA Formation via Association between Paxillin and Talin
Because we observed that the S85A paxillin mutant did not bind to talin, we examined whether the cellular localization of paxillin is affected by its binding to talin. To this end, we cotransfected cells with HA3-paxillin (WT or S85A) and GFP-WT talin (the N-terminal head domain or the C-terminal tail domain) prior to indirect immunofluorescence microscopy. Following cotransfection with GFP-WT talin, WT paxillin co-localized with talin along cell boundaries (82.3 ± 7.3%), whereas S85A paxillin did not (23.5 ± 3.9%) (Fig. 6A), indicating that phospho-Ser-85 paxillin might be required for efficient FAs during cell adhesion and spreading. When cotransfected with the GFP-tagged N-terminal head domain of talin, WT paxillin caused more efficient formation of protrusive processes rather than FAs along the boundaries (79.6 ± 8.5%) than did the S85A mutant, which did not co-localize with talin (18.8 ± 6.8%) (Fig. 6B). When cotransfected with the GFP-tagged C-terminal tail of talin, however, WT paxillin efficiently formed FAs along the cell boundaries (86.2 ± 9.6%), whereas S85A paxillin caused either quite stable FAs or the accumulation of the GFP-talin tail domain in the central area of cells (24.8 ± 4.3%) (Fig. 6C), indicating altered formation of FAs with paxillin when S85A paxillin and the C-terminal domain of talin are cotransfected. Furthermore, another FA marker, vinculin, was not peripherally localized in the cells cotransfected with S85A paxillin and the C-terminal domain of talin (75.2 ± 5.8%) (Fig. 6D). As shown in Fig. 6E, transient transfection of paxillin siRNA could suppress expression efficiently in most transfection-positive cells (97.3 ± 3.8%). In the cells in which endogenous paxillin was suppressed (at least partially) by paxillin siRNA and then reconstituted with S85A paxillin, S85A paxillin and vinculin appeared not to localize at FAs (84.3 ± 4.9%), whereas cells without S85A paxillin introduction (but presumably with a residual endogenous paxillin after paxillin siRNA transfection) showed well established vinculin-positive FAs along the cell peripheries (96.9 ± 3.8%) (Fig. 6F).
FIGURE 6.
FA formation is regulated by WT (but not S85A) paxillin together with the talin C-terminal tail domain. Cells were cotransfected with HA3-paxillin (HA3-Pax; WT or S85A) and GFP-WT talin (A), the N-terminal head domain (B), or the C-terminal tail domain (C and D) for 36 h. Alternatively, cells were transiently transfected with control siRNA (siControl) or siRNA against paxillin (siPaxillin) (E) or cotransfected with paxillin siRNA and HA3-S85A paxillin (F) for 36 h. The cells were then replated onto collagen I (10 μg/ml)-precoated coverslips for 30 min before staining with anti-HA3 tag (red, A–C; and green, F), anti-actin (red, E), anti-paxillin (green, E), or anti-vinculin (red, D and F) antibody. Scale bars = 10 μm (A–D and F) and 20 μm (E). The data shown represent three independent experiments.
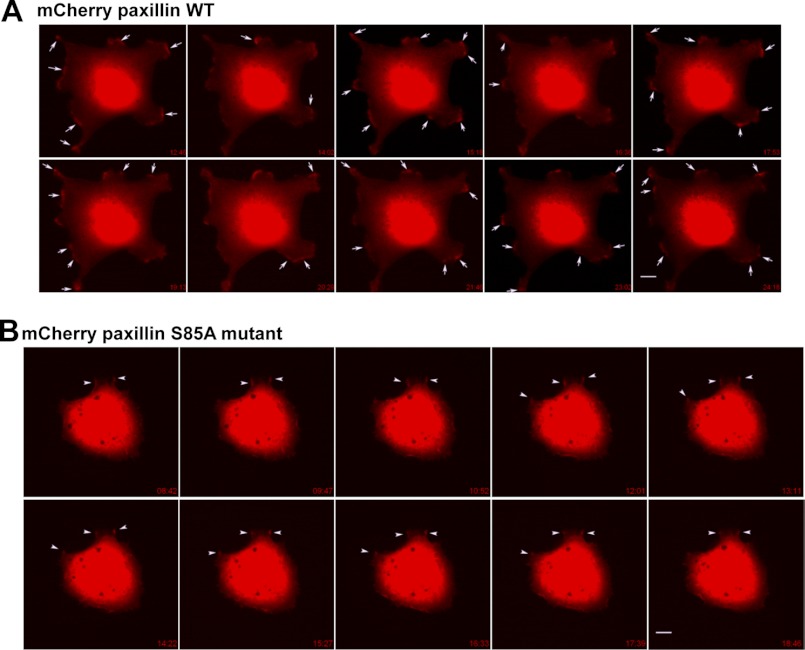
We next analyzed the dynamic changes in FAs and protrusive process formation via time-lapse microscopy after transfection of cells with mCherry-paxillin (WT or S85A). Cells transfected with mCherry-WT paxillin showed very active dynamic protrusions and FAs along cell boundaries as time progressed, whereas cells transfected with mCherry-S85A paxillin showed very stable boundaries without a dynamic alteration of paxillin localization (Fig. 7 and supplemental Movies 1 and 2). This observation indicates that phospho-Ser-85 paxillin is required for dynamic cell adhesions during cell migration.
FIGURE 7.
Time-lapse images of mCherry-paxillin (WT or S85A)-expressing cells. Cells were transiently transfected with mCherry-tagged WT (A) or S85A mutant (B) paxillin for 36 h and replated onto collagen I (2 μg/ml)-precoated coverslips for 30 min within 0.2% BSA and DMEM-H before analysis with time-lapse microscopy. Images were saved over 30 min, and snap pictures in series over at least 10 min are represented. A, in images of mCherry-WT paxillin-transfected cells, dynamic turnover of paxillin-positive spots (arrows) is evidenced by newly formed spots compared with the immediately preceding snap picture. B, in images of mCherry-S85A paxillin-transfected cells, the positive staining spots (arrowheads) were less dynamic, and the staining was sustained for longer times. Images shown represent cells from several analyses (mCherry-WT paxillin, n = 9; and mCherry-S85A paxillin, n = 11).
DISCUSSION
This study demonstrates that paxillin is phosphorylated at Ser-85 in a cell adhesion-dependent manner and that Ser-85 phosphorylation appears to be important for the signaling activities of FA molecules such as FAK and talin. Ser-85 phosphorylation of paxillin appears to be important for FA formation and actin organization and is also required for its binding to the C-terminal tail domain of talin in an adhesion-dependent manner. Phospho-Ser-85 paxillin-dependent binding to talin correlates with obvious FAs along cell boundaries such that the S85A mutant caused abnormal formation of vinculin- or paxillin-positive FAs in central regions of cells rather than around the cell peripheries. Furthermore, phospho-Ser-85 paxillin is critical for haptotactic but not serum-induced migration and invasion. Therefore, the mechanisms by which phospho-Ser-85 paxillin regulates cell migration/invasion may be attributed to the regulation of FAs and invasive protrusion formation via binding to the C-terminal tail domain of talin.
Most previous studies on paxillin have focused on the effects of phosphorylation at Tyr-51 and Tyr-118, which are critically involved in cellular functions such as migration and invasion (12, 18). However, serine phosphorylation of paxillin is also involved in the regulation of cellular functions (19); for example, phosphorylation of Ser-273 by p21-activated kinase enhances migration, protrusion, and FA turnover via the localization of a paxillin·GIT1·PIX·p21-activated kinase complex at the leading edges of Chinese hamster ovary cells (20), and Ser-85 of human paxillin (Ser-83 of rat paxillin) is phosphorylated either by p38MAPK during NGF-induced neurite outgrowth of PC-12 cells or by ERK1/2 during hepatocyte growth factor-mediated morphogenesis of mouse inner medullary collecting duct epithelial cells (mIMCD-3) (14, 16). Ser-85 of human paxillin (and the corresponding Ser-83 of rat paxillin) is post-translationally modified with O-GlcNAc under hyperglycemic conditions, which competes with cell adhesion-dependent phosphorylation and correlates with the formation of protrusive processes (15). In this study, we also showed that HeLa cell adhesion induced Ser-85 phosphorylation of paxillin and that this effect was inhibited by the pharmacological inhibition of p38MAPK.
In addition to NGF-mediated neurite outgrowth in PC-12 cells, phospho-Ser-85 paxillin is involved in the modulation of FA length, although how phospho-Ser-85 paxillin regulates the FAs remains unknown (14). During the hepatocyte growth factor-mediated morphogenesis of mIMCD-3 cells, the association between phospho-Ser-85 paxillin and FAK appears to be important for morphogenesis because the S85A mutation inhibits both the association and morphological effects (16). Similarly, the results of this study indicate that cell adhesion-dependent phospho-Ser-85 paxillin regulates the tyrosine phosphorylation of FA molecules (FAK, paxillin, and talin), FA formation, actin organization, migration, and invasion, given that the S85A paxillin mutation reduced phosphorylation of FAK Tyr-397 and paxillin Tyr-118, FA formation at the periphery, haptotactic migration, and invasive protrusion formation. Interestingly, in contrast to WT paxillin, the S85A mutant could not associate with the C-terminal tail (rod) domain of talin, leading to abnormal intracellular formation of stabilized FAs, including phospho-Tyr-397 FAK (Fig. 1E), HA3-paxillin (Figs. 2 and 6), and vinculin (Fig. 6, D and F). These observations indicate that the efficient dynamics of FA turnover (supported by either WT talin or its C-terminal tail domain) are not possible when phospho-Ser-85 paxillin is unavailable. Furthermore, no phospho-Ser-85 paxillin resulted in an abnormal formation of FAs with vinculin, paxillin, and FAK. Given that paxillin and talin are components of FAs, which are quite complex structures and signaling hubs, phospho-Ser-85 paxillin can lead to changes in phosphorylation events of certain FA molecules. Furthermore, the association of a C-terminal region (aa 434–1060) of talin with paxillin, as shown by co-immunoprecipitation and GST-talin fusion fragment pulldown of paxillin in cell extracts, might be an indirect association mediated by other component(s) such as actin or another FA molecule. It is known that the N-terminal LD [repeated leucine-rich sequences that begin with a leucine (L) and aspartate (D)] domains of paxillin bind directly to the tail domain of vinculin (21), of which the head domain associates with the stretched tail domain of talin (22). It is known that cryptic vinculin-binding sites in the talin tail undergo conformational changes to be stretched on adhesion-mediated force transduction (22). However, under certain experimental conditions in this study, the association between talin and paxillin appeared not to be mediated by vinculin because paxillin immunoprecipitates did not include vinculin but rather talin in a Ser-85 phosphorylation-dependent manner. It may be that the talin tail domain is not stretched enough to associate with vinculin in our particular system. Although analyses to test the direct binding between paxillin and talin are still required, it is likely that this binding can still be mediated via another FA component or actin.
Talin is an adaptor molecule at FAs (18). The N-terminal head domain of talin is responsible for integrin activation presumably during inside-out signaling, whereas the C-terminal tail domain is required for linkage of cell adhesion to actin rearrangement (23). Because talin is recruited to FAs early after cell adhesion, it has been suggested that talin plays a scaffolding function in recruiting FA molecules and enzymes (24). Both talin and paxillin are known to be recruited at the earlier FA or contacts (25). Furthermore, although the C-terminal tail domain of talin locates at FAs, the N-terminal head domain does not (23). During time-lapse imaging in this study, cells expressing the S85A paxillin mutant showed less efficient turnover of paxillin-positive staining, whereas cells expressing WT paxillin showed dynamic turnover of paxillin-positive staining along cell boundaries and invasive process formation. Therefore, this study revealed that Ser-85 phosphorylation of paxillin regulates FAs for efficient migration and invasion via its involvement in protein-protein interactions with FA molecules such as talin. Taken together, our findings suggest that the Ser-85 phosphorylation of paxillin upon cell adhesion regulates its interaction with talin and the signaling activities of FA molecules, leading to efficient turnover of FAs along cell boundaries during cell migration and invasion in ECM-rich environments.
Supplementary Material
This work was supported by Ministry of Education & Human Resources Development (MOEHRD) Basic Research Promotion Fund Grant KRF-2008-013-C00054, National Research Foundation Grant 2012-0004891 from the Korean Ministry of Education, Science and Technology (to the Tumor Microenvironment Global Core Research Center), LEAP Research Senior Researchers Program Grant 2011-0016446/2012-0005606, Global Frontier Project Grant NRF-M1AXA002-2011-0028411, and Korean Health Technology R&D Project Grant A100727 from the Ministry for Health, Welfare and Family Affairs (MHWFA), Republic of Korea (to J. W. L.).

This article contains supplemental Fig. S1 and Movies 1 and 2.
- FA
- focal adhesion
- FAK
- focal adhesion kinase
- ECM
- extracellular matrix
- O-GlcNAc
- O-linked N-GlcNAc
- aa
- amino acids.
REFERENCES
- 1. Danen E. H. (2009) Integrin proteomes reveal a new guide for cell motility. Sci. Signal. 2, pe58. [DOI] [PubMed] [Google Scholar]
- 2. Mitra S. K., Schlaepfer D. D. (2006) Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18, 516–523 [DOI] [PubMed] [Google Scholar]
- 3. Schaller M. D. (2010) Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J. Cell Sci. 123, 1007–1013 [DOI] [PubMed] [Google Scholar]
- 4. Lock J. G., Wehrle-Haller B., Stromblad S. (2008) Cell-matrix adhesion complexes: master control machinery of cell migration. Semin. Cancer Biol. 18, 65–76 [DOI] [PubMed] [Google Scholar]
- 5. Carragher N. O., Frame M. C. (2004) Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 14, 241–249 [DOI] [PubMed] [Google Scholar]
- 6. Destaing O., Block M. R., Planus E., Albiges-Rizo C. (2011) Invadosome regulation by adhesion signaling. Curr. Opin. Cell Biol. 23, 597–606 [DOI] [PubMed] [Google Scholar]
- 7. Linder S. (2007) The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 17, 107–117 [DOI] [PubMed] [Google Scholar]
- 8. Albiges-Rizo C., Destaing O., Fourcade B., Planus E., Block M. R. (2009) Actin machinery and mechanosensitivity in invadopodia, podosomes, and focal adhesions. J. Cell Sci. 122, 3037–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schaller M. D., Parsons J. T. (1995) pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol. Cell. Biol. 15, 2635–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grimsley C. M., Kinchen J. M., Tosello-Trampont A. C., Brugnera E., Haney L. B., Lu M., Chen Q., Klingele D., Hengartner M. O., Ravichandran K. S. (2004) Dock180 and ELMO1 proteins cooperate to promote evolutionarily conserved Rac-dependent cell migration. J. Biol. Chem. 279, 6087–6097 [DOI] [PubMed] [Google Scholar]
- 11. Tsubouchi A., Sakakura J., Yagi R., Mazaki Y., Schaefer E., Yano H., Sabe H. (2002) Localized suppression of RhoA activity by Tyr-31/118-phosphorylated paxillin in cell adhesion and migration. J. Cell Biol. 159, 673–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deakin N. O., Turner C. E. (2008) Paxillin comes of age. J. Cell Sci. 121, 2435–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang C., Rajfur Z., Borchers C., Schaller M. D., Jacobson K. (2003) JNK phosphorylates paxillin and regulates cell migration. Nature 424, 219–223 [DOI] [PubMed] [Google Scholar]
- 14. Huang C., Borchers C. H., Schaller M. D., Jacobson K. (2004) Phosphorylation of paxillin by p38MAPK is involved in the neurite extension of PC-12 cells. J. Cell Biol. 164, 593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kwak T. K., Kim H., Jung O., Lee S. A., Kang M., Kim H. J., Park J. M., Kim S. H., Lee J. W. (2010) Glucosamine treatment-mediated O-GlcNAc modification of paxillin depends on adhesion state of rat insulinoma INS-1 cells. J. Biol. Chem. 285, 36021–36031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ishibe S., Joly D., Liu Z. X., Cantley L. G. (2004) Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol. Cell 16, 257–267 [DOI] [PubMed] [Google Scholar]
- 17. Lee S. A., Kim T. Y., Kwak T. K., Kim H., Kim S., Lee H. J., Kim S. H., Park K. H., Kim H. J., Cho M., Lee J. W. (2010) Transmembrane 4 L six family member 5 (TM4SF5) enhances migration and invasion of hepatocytes for effective metastasis. J. Cell Biochem. 111, 59–66 [DOI] [PubMed] [Google Scholar]
- 18. Desiniotis A., Kyprianou N. (2011) Significance of talin in cancer progression and metastasis. Int. Rev. Cell Mol. Biol. 289, 117–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown M. C., Turner C. E. (2004) Paxillin: adapting to change. Physiol. Rev. 84, 1315–1339 [DOI] [PubMed] [Google Scholar]
- 20. Nayal A., Webb D. J., Brown C. M., Schaefer E. M., Vicente-Manzanares M., Horwitz A. R. (2006) Paxillin phosphorylation at Ser-273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J. Cell Biol. 173, 587–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turner C. E., Glenney J. R., Jr., Burridge K. (1990) Paxillin: a new vinculin-binding protein present in focal adhesions. J. Cell Biol. 111, 1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. del Rio A., Perez-Jimenez R., Liu R., Roca-Cusachs P., Fernandez J. M., Sheetz M. P. (2009) Stretching single talin rod molecules activates vinculin binding. Science 323, 638–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang P., Ballestrem C., Streuli C. H. (2011) The C terminus of talin links integrins to cell cycle progression. J. Cell Biol. 195, 499–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Partridge M. A., Marcantonio E. E. (2006) Initiation of attachment and generation of mature focal adhesions by integrin-containing filopodia in cell spreading. Mol. Biol. Cell 17, 4237–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scales T. M., Parsons M. (2011) Spatial and temporal regulation of integrin signaling during cell migration. Curr. Opin. Cell Biol. 23, 562–568 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.