Background: Apolipoprotein E4 (apoE4) is associated with inflammatory metabolic diseases.
Results: Human APOE4 gene replacement mice displayed elevated tissue inflammation. APOE4 macrophages showed impaired efferocytosis, increased apoptosis, and endoplasmic reticulum stress.
Conclusion: ApoE4 structural abnormalities induce ER stress to promote inflammation.
Significance: Reducing ER stress and/or apoE4 structure correctors may reduce inflammatory metabolic disease risk in human apoE4 subjects.
Keywords: Adipose Tissue, ApoE, Apoptosis, Atherosclerosis, ER Stress, Inflammation, Macrophages
Abstract
Apolipoprotein (apo) E4 is a major genetic risk factor for a wide spectrum of inflammatory metabolic diseases, including atherosclerosis, diabetes, and Alzheimer disease. This study compared diet-induced adipose tissue inflammation as well as functional properties of macrophages isolated from human APOE3 and APOE4 mice to identify the mechanism responsible for the association between apoE4 and inflammatory metabolic diseases. The initial study confirmed previous reports that APOE4 gene replacement mice were less sensitive than APOE3 mice to diet-induced body weight gain but exhibited hyperinsulinemia, and their adipose tissues were similarly inflamed as those in APOE3 mice. Peritoneal macrophages isolated from APOE4 mice were defective in efferocytosis compared with APOE3 macrophages. Increased cell death was also observed in APOE4 macrophages when stimulated with LPS or oxidized LDL. Western blot analysis of cell lysates revealed that APOE4 macrophages displayed elevated JNK phosphorylation indicative of cell stress even under basal culturing conditions. Significantly higher cell stress due mainly to potentiation of endoplasmic reticulum (ER) stress signaling was also observed in APOE4 macrophages after LPS and oxidized LDL activation. The defect in efferocytosis and elevated apoptosis sensitivity of APOE4 macrophages was ameliorated by treatment with the ER chaperone tauroursodeoxycholic acid. Taken together, these results showed that apoE4 expression causes macrophage dysfunction and promotes apoptosis via ER stress induction. The reduction of ER stress in macrophages may be a viable option to reduce inflammation and inflammation-related metabolic disorders associated with the apoE4 polymorphism.
Introduction
Apolipoprotein E (apoE) is a 34-kDa protein originally discovered to be associated with several classes of plasma lipoproteins (1). Unlike other apolipoproteins that are expressed only in liver and intestine, apoE is not expressed in intestine but is expressed in liver and other tissues, including the central nervous system, vascular smooth muscle cells, adrenals, macrophages, and adipocytes. Although the main function of apoE has been attributed to cholesterol transport, accumulating evidence indicates that apoE also modulates metabolic disease progression through lipid transport-independent mechanisms (2). The human APOE gene exists with three major polymorphic alleles (ϵ2, ϵ3, and ϵ4) encoding the apoE2, apoE3, and apoE4 isoforms with cysteine/cysteine, cysteine/arginine, and arginine/arginine residues at positions 112 and 158 of the 299-residue protein, respectively. The various apoE isoforms have different metabolic properties with apoE2 transporting lipids to the LDL receptor less efficiently, thereby conferring ϵ2 carriers with increased risk of type III hyperlipoproteinemia (3). Although apoE3 and apoE4 bind to LDL receptor and other LDL receptor family proteins with similar affinity, apoE3 appears to protect against metabolic disorders, whereas carriers of the ϵ4 allele have increased risk of Alzheimer disease as well as cardiovascular and metabolic diseases (2–4).
The exact mechanism by which apoE3 retards and apoE4 accelerates the onset and progression of a wide spectrum of metabolic disorders is not completely understood (2, 5). Previous studies have shown that apoE3 may protect against metabolic diseases via its anti-oxidation and anti-inflammatory properties (2, 6), including its attenuation of toll-like receptor signaling and converting the pro-inflammatory M1 macrophages to the anti-inflammatory M2 macrophage phenotype (7, 8). In the vessel wall, apoE3 also induces anti-inflammatory signaling events that limit injury-induced neointimal hyperplasia and hypercholesterolemia-induced atherosclerosis (9–13). In contrast, apoE4 is pro-inflammatory and has been shown to accelerate neurodegeneration by promoting neuronal cell death (14–16). Paradoxically, apoE4 is associated with lower body mass index compared with apoE3 carriers (17), but apoE4 carriers are more susceptible to diet-induced diabetes and coronary artery disease (4, 18–20). Recent data examining mice in which the endogenous mouse apoe gene has been replaced with the human APOE3 or APOE4 gene also showed the increased susceptibility of APOE4 gene replacement mice to diet-induced atherosclerosis and diabetes despite less adiposity and body weight gain compared with APOE3 mice (21–23). Atherosclerosis enhancement observed in APOE4 mice is at least in part due to the apoE4 expressed in macrophages (24).
The innate immune system plays a major contributory role toward the pathogenesis of cardiovascular and metabolic diseases (25–27). Accordingly, the association between the ϵ4 allele with increased risk of cardiometabolic diseases may be due to abnormal functions of apoE4-expressing macrophages compared with macrophages expressing the normal apoE3 protein. However, how the cysteine-to-arginine substitution in residue 112 converts the anti-inflammatory apoE3 to the pro-inflammatory apoE4 has not been resolved. The goal of this study is to compare functional properties of macrophages isolated from human APOE3 versus APOE4 gene replacement mice and to identify the mechanism responsible for the abnormal functional properties of apoE4-expressing macrophages.
EXPERIMENTAL PROCEDURES
Mice and Dietary Studies
Genetically modified C57BL/6 mice in which the endogenous mouse apoe gene has been replaced with the human APOE3 or APOE4 gene at the same locus (28, 29), hereafter designated as APOE3 and APOE4 mice, were purchased from Taconic (Hudson, NY) and housed in our institutional animal care facility under a controlled environment with free access to food and water. All animal protocols were approved by the University of Cincinnati Institutional Animal Use and Care Committee. The mice were maintained on standard chow (Teklad, Madison, WI) or fed a Western-type high fat, high cholesterol diet containing 21.2% fat by weight (41% by calories) and 0.2% cholesterol (TD88137, Teklad) for 4 weeks. Body weights were measured with a Denver 300K scale, and adiposity was determined using an EchoMRI Whole Body Composition Analyzer (Echo Medical Systems, Houston, TX) as described previously (30).
Blood Chemistry
Blood was obtained from animals fasted overnight. Fasting blood glucose was determined with an Accu-Chek Active Glucometer (Roche Applied Science), and plasma was obtained after centrifugation to measure plasma lipid levels with Infinity Kits (ThermoFisher Scientific) or insulin levels with an Ultra Sensitive Rat Insulin ELISA kit (Crystal Chem, Chicago). Insulin sensitivity and resistance were estimated by homeostasis model assessment index calculated from fasting glucose and insulin levels as described previously (31). Postprandial lipid clearance was determined by feeding fasted mice with a bolus lipid-rich meal (15 μl of olive oil/g body weight) and then measuring plasma triglyceride levels over a 3-h period.
In Vitro Experiments with Peritoneal Macrophages
Peritoneal macrophages were obtained 3 days after intraperitoneal injection of a 4% thioglycollate solution as described (32). Peritoneal macrophages were plated overnight before use. Cells were maintained in basal conditions or incubated with either 50 ng/ml LPS or 100 μg/ml oxLDL2 for 1 h. For analysis of cell death, treated macrophages were removed using Accutase (Invitrogen) and labeled with annexin V and propidium iodide (eBioscience) according to the manufacturer's protocol. Flow cytometry was performed on Guava EasyCyte 8HT System and analyzed using Guava InCyte (Millipore, Hayward, CA). For efferocytosis experiments, peritoneal macrophages from wild type C57BL/6 mice were labeled with carboxyfluorescein diacetate-succinimidyl ester (CFDA-SE, from Invitrogen) for 30 min and then treated overnight with staurosporine (Sigma) to induce apoptosis. The CFDA-labeled apoptotic cells were then added onto cultures of APOE3 or APOE4 macrophages for a 2-h incubation. The macrophages were washed thoroughly with phosphate-buffered saline, removed using Accutase, and then analyzed by flow cytometry. When indicated, tauroursodeoxycholic acid (Sigma) was used at a concentration of 2 mm.
Western Blot Analysis
Peritoneal macrophage lysates were prepared in buffer containing 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 0.5% sodium deoxycholate, 1% Nonidet P-40, 0.1% SDS, 1 mm EDTA, and protease inhibitor mixture (Roche Applied Science) and phosphatase inhibitor mixtures (Sigma). Protein concentration was determined using BCA kit (Pierce) prior to PAGE and immunoblot analysis. Antibodies against PERK, phospho-PERK, and CHOP were obtained from Santa Cruz Biotechnology and used at 1:500 dilution. Antibodies against XBP1 and IRE-1α were obtained from Abcam and used at 1:1000 dilutions. Antibodies against phospho-IRE-1α were obtained from Pierce and used at 1:1000 dilution. JNK and phospho-JNK antibodies from Cell Signaling were used at 1:1000 dilution. Primary antibodies were detected using horseradish peroxidase-conjugated secondary antibodies (Cell Signaling) and visualized using enhanced chemiluminescence reagents (Amersham Biosciences). Quantitative measurements were performed using Image J software (National Institutes of Health).
Quantitative PCR Determination of mRNA Levels
Total RNA was extracted from adipose tissues and peritoneal macrophages in culture using TRIzol reagent (Invitrogen). cDNA was made using iScript cDNA synthesis kit (Bio-Rad), and RT-PCR was performed on iCyclerIQ (Bio-Rad) using sequence-specific primers (Table 1). For analysis of XBP1 splicing, the XBP1 mRNA-amplified product was digested with PstI (New England Biolabs) and then analyzed by electrophoresis on 1.7% agarose gels. For quantification, all qRT-PCR analyses were made using ΔΔCt measurements against cyclophilin mRNA as control.
TABLE 1.
RT-quantitative PCR primer sequences for experimental and reference genes
| Gene | Common name | Sense primer | Antisense primer |
|---|---|---|---|
| Cypa | Cyclophilin A | TCATGTGCCAGGGTGGTGAC | CCATTCAGTCTTGGCAGTGC |
| Emr1 | F4/80 | TGTCTGACAATTGGGATCTGCCCT | ATACGTTCCGAGAGTGTTGTGGCA |
| Nos2 | NOS2 | TTCACCCAGTTGTGCATCGACCTA | TCCATGGTCACCTCCAACACAAGA |
| Tnf | TNF-α | ATCCGCGACGTGGAACTG | ACCGCCTGGAGTTCTGGAA |
| Ddit3 | CHOP | ACAGAGCCAGAATAACAGCCGGAA | TCTGCTTTCAGGTGTGGTGGTGTA |
| Xbp1 | XBP1 | AAACAGAGTAGCAGCGCAGACTGC | TCCTTCTGGGTAGACCTCTGGGAG |
Statistics
Values were expressed as mean ± S.E. Student's t test or analysis of variance for multiple group comparisons was used to determine significance between samples (SigmaPlot version 11.0). A p < 0.05 difference between groups was considered to be statistically significant.
RESULTS
Resistance to Diet-induced Adiposity and Body Weight Gain in APOE4 Mice
Mice in which the endogenous mouse apoe gene has been replaced at the same locus by the human APOE3 or APOE4 gene express human apoE3 and apoE4 in a tissue-specific and physiological manner similar to apoE expression in humans (21). Previously, Maeda and co-workers (21) have reported that the APOE3 and APOE4 mice have similar food intake and absorb dietary lipids to a similar extent, but the APOE4 mice gained significantly less weight than APOE3 mice when challenged with a Western-type high fat, high cholesterol diet. The APOE4 mice were also reported to be more glucose intolerant compared with the APOE3 mice (21). Our initial experiments in characterizing inflammatory responses in APOE3 and APOE4 mice recapitulated these data. In our experiments, 10-week-old male APOE4 mice maintained on a basal chow diet displayed higher body weight compared with 10-week-old male APOE3 mice on a similar diet (Fig. 1A), but analysis of body composition by nuclear magnetic resonance revealed similar adiposity between chow-fed APOE3 and APOE4 mice (Fig. 1B). In contrast, when the mice were fed a Western-type high fat, high cholesterol diet for 4 weeks, significant body weight gain was observed only in the APOE3 mice, and the APOE4 mice did not show body weight difference after 4 weeks of feeding the hypercaloric Western-type diet (Fig. 1A). These results confirmed results reported previously, and our data additionally showed that body fat mass was also significantly lower in Western diet-fed APOE4 mice compared with APOE3 mice (Fig. 1B). The differences in body fat accounted for the differences in body weights between APOE3 and APOE4 mice with no statistically significant differences observed in lean body mass between APOE3 and APOE4 mice fed either chow or Western-type diets (Fig. 1C). No difference in food intake, activity, or fat absorption efficiency was observed between these animals; thus their differences in diet-induced body weight gain and adiposity were due to metabolic differences between the APOE3 and APOE4 mice. These results also established that APOE3 and APOE4 gene replacement mice are suitable models to identify the mechanism underlying the differences between ϵ3 and ϵ4 carriers in sensitivity to diet-induced metabolic diseases despite lower body mass index.
FIGURE 1.
Body weights and adiposity of APOE3 and APOE4 mice. Ten-week-old male mice were maintained on basal chow diet (filled bars) or fed a Western-type high fat, high cholesterol diet for 4 weeks (open bars). A shows mean body weights; B shows average percent body fat, and C shows the average lean body mass of six APOE3 mice and six APOE4 mice ± S.D. * denotes p < 0.05 difference from APOE3 mice fed a similar diet.
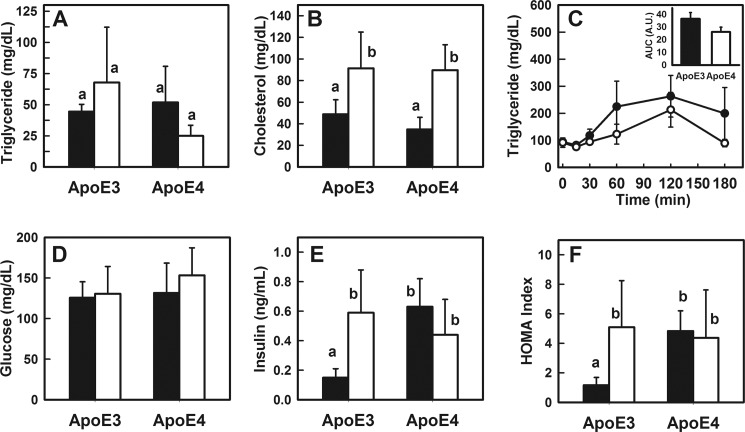
Similar Fasting Plasma Lipid and Glucose Levels but Differences in Fasting Insulin Levels between APOE3 and APOE4 Mice
Fasting plasma triglyceride and cholesterol levels were similar between APOE3 and APOE4 mice (Fig. 2, A and B). Both groups of mice responded to 4 weeks of Western diet feeding with elevated fasting plasma cholesterol levels, but no genotype-specific differences were observed (Fig. 2B). When the animals were fed a bolus lipid-rich meal by gastric gavage, a slight but statistically insignificant faster rate of triglyceride-rich lipoprotein clearance was observed in the APOE4 mice compared with APOE3 mice (Fig. 2C). These data are consistent with their similarities in food intake and dietary lipid absorption and the identical ability of apoE3 and apoE4 to interact with LDL receptor family proteins to mediate plasma lipoprotein clearance (33).
FIGURE 2.
Blood chemistry of APOE3 and APOE4 mice. Male APOE3 and APOE4 mice were fed either a chow diet (filled bars) or a Western-type diet (open bars) for 4 weeks. A shows fasting plasma triglyceride levels; B shows fasting cholesterol levels in APOE3 and APOE4 mice. C shows postprandial triglyceride levels in chow-fed APOE3 (solid symbols) and APOE4 (open symbols) mice after feeding a bolus lipid-rich meal (15 μl of olive oil/g body weight), and the inset shows area under the curve (AUC) analysis indicating no significant differences (p = 0.15) between the two groups. D and E show fasting glucose and insulin levels in APOE3 and APOE4 mice, respectively, and F shows the homeostasis model assessment index of insulin resistance calculated from these data. All data represent mean ± S.D. from six mice in each group. Bars with different letters in each graph are significantly different at p < 0.01.
Both APOE3 and APOE4 mice also displayed similar fasting glucose levels when maintained on chow diet, and both groups responded to the Western-type diet with a slight but statistically insignificant increase in fasting glucose levels after 4 weeks (Fig. 2D). The lack of significant hyperglycemia after 4 weeks of feeding the Western-type diet was consistent with previous observations that mice are resistant to short term diet-induced hyperglycemia and are similar to humans with onset of hyperinsulinemia preceding the hyperglycemia phenotype. Interestingly, measurements of fasting insulin levels revealed dramatic differences between APOE3 and APOE4 mice, with the APOE4 mice displaying ∼3-fold higher fasting insulin levels compared with APOE3 mice even under basal chow diet conditions (Fig. 2E). In fact, fasting insulin levels in chow-fed APOE4 mice were similar to that observed in APOE3 mice fed the Western-type diet (Fig. 2E). Western diet feeding did not promote an additional increase in plasma insulin levels in the APOE4 mice (Fig. 2E). Calculation of homeostasis model assessment index confirmed the insulin resistance phenotype of APOE4 mice under both basal and Western diet conditions, similar to that observed in Western diet-fed APOE3 mice (Fig. 2F). These results are consistent with those reported previously showing impaired glucose tolerance and insulin resistance in APOE4 mice (21).
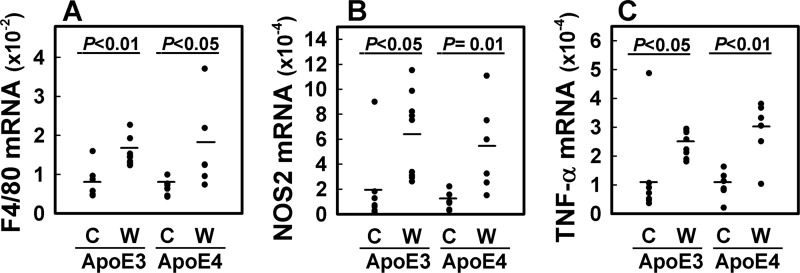
Diet-induced Inflammation in Adipose Tissues of Both APOE3 and APOE4 Mice
Despite the lower adiposity in APOE4 mice compared with APOE3 mice, the impaired glucose tolerance and insulin resistance observed in APOE4 mice suggested that they remain sensitive to diet-induced inflammation. To test this possibility, RNA was isolated from visceral adipose tissues of chow-fed and Western diet-fed APOE3 and APOE4 mice for quantitative PCR analysis. The results showed no difference in F4/80 mRNA levels between chow-fed APOE3 and APOE4 mice (Fig. 3A). Interestingly, despite their differences in adiposity in response to Western diet feeding, both groups of mice responded with similar elevation of F4/80 mRNA levels in their adipose tissues, indicative of macrophage infiltration after feeding the Western-type diet (Fig. 3A). Expression levels of inducible nitric-oxide synthase (NOS2) mRNA were also increased after Western diet feeding, thus indicating the pro-inflammatory states of the adipose tissues in both APOE3 and APOE4 adipose tissues (Fig. 3B). Additionally, both APOE3 and APOE4 mice displayed elevated expression levels of the inflammatory cytokine TNF-α after Western diet feeding (Fig. 3C). Because macrophages are responsible for the TNF-α produced in adipose tissues (34), these data indicated that despite the lower adiposity in APOE4 mice, their adipose tissues as well as their tissue macrophages were as inflamed as those observed in the more obese Western diet-fed APOE3 mice.
FIGURE 3.
Quantitative PCR of mRNA in adipose tissues. Total mRNA were isolated from visceral (gonadal) adipose tissues of chow (C) and Western diet-fed (W) APOE3 and APOE4 mice for quantitative PCR analysis of F4/80 (A), NOS2 (B), and TNF-α mRNA (C) levels relative to the expression levels of cyclophilin mRNA. Each point in the display shows average of duplicate measurements in individual animals. The bars in each group represent mean from seven chow-fed APOE3 mice, seven chow-fed APOE4 mice, nine Western diet-fed APOE3 mice, and six Western diet-fed APOE4 mice with significant differences as denoted.
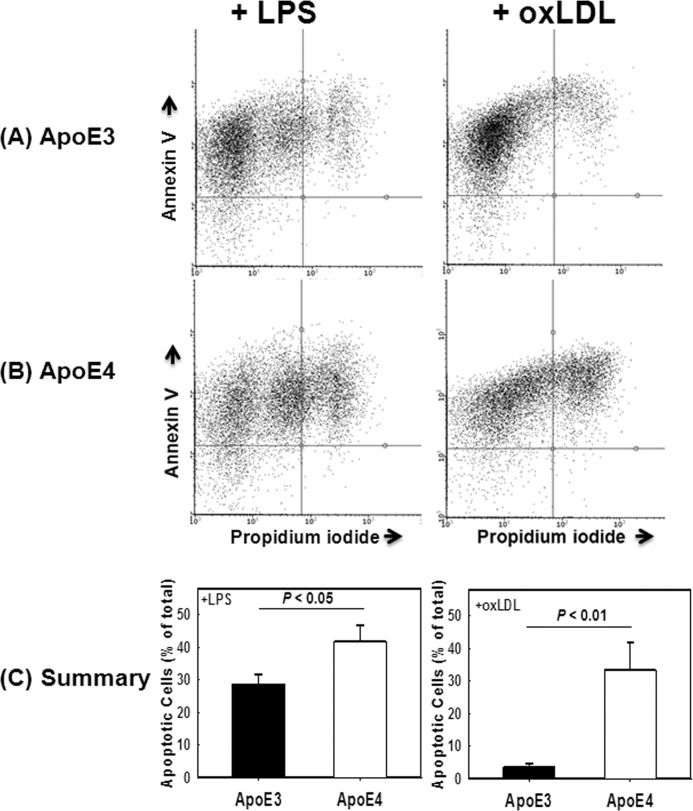
ApoE4 Expression Accelerates Inflammation-induced Cell Death and Decreases Efferocytosis in Macrophages
One key factor in modulating tissue inflammation is cell death and their phagocytic clearance by macrophages in a process termed efferocytosis (35, 36). Therefore, the elevated sensitivity of adipose tissue macrophages to diet-induced inflammation despite the lack of increased adiposity in APOE4 mice may be related to macrophage dysfunction and/or susceptibility for apoptosis. To test these possibilities, peritoneal macrophages obtained from APOE3 and APOE4 mice were incubated in the presence or absence of LPS (50 ng/ml) or oxLDL (100 μg/ml) at doses that are known to promote inflammation and apoptosis (37–39). The percentage of cells undergoing apoptosis was assessed by flow cytometry analysis of annexin V- and propidium iodide-positive cells. Results from four separate experiments, each performed with cells obtained from at least three mice per group plated separately, showed that in comparison with APOE3 macrophages, a significantly higher percentage of APOE4 macrophages were apoptotic with double annexin V- and propidium iodide-positive staining when incubated with LPS or oxLDL (Fig. 4).
FIGURE 4.
Agonist-induced death of APOE3 and APOE4 macrophages. Peritoneal macrophages from APOE3 (A) and APOE4 (B) mice were incubated with 50 ng/ml LPS (left panels) or 100 μg/ml oxLDL. Cell death was assessed by flow cytometry based on expression of annexin V and propidium iodide. Representative flow cytometry data are shown in A and B. C shows the mean ± S.D. from four separate experiments each performed with cells obtained from at least three mice per group. Differences as noted were evaluated by Student's t test.
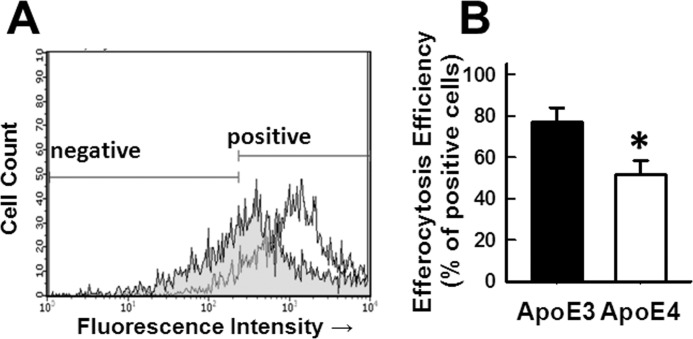
In the next series of experiments, peritoneal macrophages from C57BL/6 wild type mice were fluorescently labeled with CFDA-SE and then made apoptotic by incubation with staurosporine in serum-free media overnight. The CFDA-SE-labeled apoptotic cells (1 × 106 cells) were then added to freshly plated APOE3 and APOE4 macrophages in 24-well dishes for a 2-h incubation period. Efferocytosis efficiency of apoptotic cells assessed by flow cytometry showed ∼77% of the APOE3 macrophages were positive for apoptotic cell engulfment compared with ∼51% positive engulfment observed with APOE4 macrophages (Fig. 5). Taken together, these results demonstrated impaired efferocytosis and increased apoptosis susceptibility of APOE4 macrophages compared with APOE3 macrophages.
FIGURE 5.
Macrophage efferocytosis of apoptotic cells. Peritoneal macrophages from wild type C57BL/6 mice were fluorescently labeled with CFDA-SE and then made apoptotic by incubation with staurosporine in serum-free medium overnight. The fluorescently labeled apoptotic cells (1 × 106) were then added to freshly plated APOE3 and APOE4 macrophages in 24-well dishes for a 2-h incubation period. The phagocytes were fixed, and efferocytosis efficiency based on uptake of fluorescently labeled apoptotic cells by APOE3 (open tracings) and APOE4 (shaded tracings) macrophages was assessed by flow cytometry, using cells with or without fluorescent labels as positive and negative markers for gating purposes as indicated. A shows representative results from one experiment, and B shows data collected from four experiments ± S.E. * denotes difference from APOE3 macrophages at p < 0.01.
ApoE4 Potentiates Endoplasmic Reticulum Stress in Macrophages
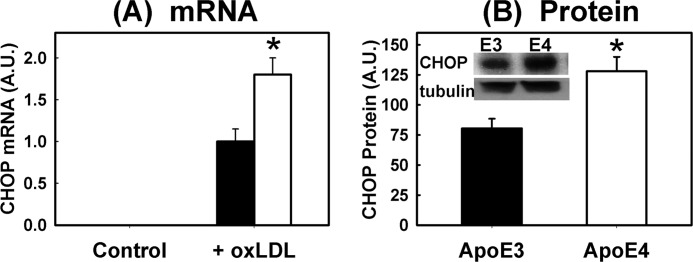
Differences between APOE3 and APOE4 macrophages in response to LPS and oxLDL were further characterized by immunoblot analysis of cell signaling pathways after incubation. Interestingly, the APOE4 macrophages displayed higher levels of JNK phosphorylation indicative of metabolic stress even under basal culturing conditions (Fig. 6A). When the macrophages were incubated with LPS or oxLDL, elevated JNK phosphorylation along with the phosphorylation of inositol-requiring protein-1 (IRE-1α) and PERK were observed in both APOE3 and APOE4 macrophages. Importantly, significantly higher levels of IRE-1α and PERK phosphorylation were observed in APOE4 macrophages compared with APOE3 macrophages under both LPS- and oxLDL-stimulated conditions (Fig. 6, B and C). Additional analysis of ER stress by monitoring XBP1 mRNA splicing also revealed the elevated presence of the alternatively spliced XBP1 mRNA as well as the accumulation of both cytosolic and nuclear spliced forms of XBP1 (40, 41) in APOE4 macrophages compared with APOE3 macrophages after oxLDL treatment (Fig. 6, D and E). Taken together, these results indicated that apoE4 expression promotes basal cell stress and provides the “second hit” to exacerbate agonist-induced ER stress and apoptosis. This conclusion was further supported by results showing that neither APOE3 nor APOE4 macrophages expressed CHOP mRNA under basal conditions, but oxLDL-induced CHOP expression was significantly higher in APOE4 macrophages compared with APOE3 macrophages (Fig. 7).
FIGURE 6.
Analysis of cell stress signals in APOE3 and APOE4 macrophages. Peritoneal macrophages isolated from APOE3 (filled bars) and APOE4 (open bars) mice were incubated in the absence or presence of 50 ng/ml LPS or 100 μg/ml oxLDL. Cell proteins were analyzed by Western blot analysis of total and phospho-JNK (A), total and phospho-IRE-1α (B), and total and phospho-PERK (C). Alternative splicing of XBP1 was analyzed based on appearance of alternatively spliced XBP1 mRNA (XBP1s) compared with XBP1 mRNA (D) and alternatively spliced forms of XBP1 protein in cytosol (C) and nucleus (N) (E). The insets in each graph show representative data of n = 3 experiments ± S.E. * denotes significant difference from APOE3 macrophages at p < 0.05.
FIGURE 7.
Elevated CHOP expression in APOE4 macrophages. A shows CHOP mRNA expression levels relative to cyclophilin mRNA in APOE3 (filled bars) and APOE4 (open bars) peritoneal macrophages incubated in the absence (control) or presence of 100 μg/ml oxLDL. Note that no CHOP mRNA was detected in samples incubated under control conditions. B shows CHOP protein levels in APOE3 (solid bar) and APOE4 (open bar) macrophages after incubation with oxLDL. The inset shows representative Western blot of CHOP protein with tubulin as loading control. The data are reported as mean ± S.E. from three separate experiments. * denotes difference from the APOE3 macrophages at p < 0.05.
Tauroursodeoxycholic Acid Improves APOE4 Macrophage Survival and Function
The relationship between the impairment of efferocytosis and elevated apoptosis sensitivity of APOE4 macrophages with apoE4-induced ER stress was explored by preincubating APOE3 and APOE4 macrophages with tauroursodeoxycholic acid for 24 h prior to the addition of LPS and oxLDL. Results showed that this ER chaperone significantly improved the efferocytosis efficiency of APOE4 macrophages to levels observed in APOE3 macrophages (Fig. 8). The ER chaperone tauroursodeoxycholic acid also attenuated LPS- and oxLDL-induced apoptosis of APOE4 macrophages to levels observed in APOE3 macrophages. Taken together, these results documented that the impaired macrophage function and elevated susceptibility to agonist-induced apoptosis of APOE4 macrophages are due to apoE4 potentiation of ER stress.
FIGURE 8.
Tauroursodeoxycholic acid improves efferocytosis and reduces death of APOE4 macrophages. A shows APOE3 macrophages (filled bars) and APOE4 macrophages (open bars) incubated either with 50 ng/ml LPS or 100 μg/ml oxLDL. A separate group of APOE4 macrophages was incubated in the presence of 2 mm tauroursodeoxycholic acid (hatched bars). Cell death was assessed by flow cytometry based on expression of annexin V and propidium iodide. B shows analysis of efferocytosis efficiency in APOE3 and APOE4 macrophages and in APOE4 macrophages treated with tauroursodeoxycholic acid (TUDCA). The data are reported as mean ± S.D. of three separate experiments. * denotes differences from the APOE3 macrophages at p < 0.05. # denotes differences from APOE4 macrophages incubated in the absence of TUDCA at p < 0.05.
DISCUSSION
Previous studies have shown a more robust innate immune response in macrophages from APOE4 mice compared with that observed in APOE3 macrophages, with elevated expression of inducible nitric-oxide synthase, TNF-α, and IL-6 after LPS activation (42). In this study, we showed that, in comparison with APOE3 macrophages, the APOE4 macrophages are also dysfunctional with impaired efferocytosis of apoptotic cells. The APOE4 macrophages are also more sensitive to LPS- and oxLDL-induced cell death. Our data revealed that one consequence of these defects is hyperinsulinemia due to the elevation of adipose tissue inflammation in response to feeding a Western-type high fat, high cholesterol diet, even in the absence of excessive adiposity. Because defective efferocytosis and macrophage apoptosis are also contributing factors to atherosclerosis (43, 44), the impairment of efferocytosis along with increased sensitivity to oxLDL-induced cell death observed in this study suggested that apoE4 instead of apoE3 expression in macrophages also provides a second hit to exacerbate atherosclerosis. Indeed, exaggerated atherosclerosis has been reported previously in diabetic Ldlr−/− mice expressing human APOE4 instead of the APOE3 gene (23). Taken together, our results indicate that apoE4-induced macrophage dysfunction is at least one underlying cause for the elevated risk of atherosclerosis and the prevalence of diabetes, independent of obesity, in human subjects carrying the ϵ4 allele.
Results of our study also showed that expression of apoE4 instead of apoE3 causes macrophage cell stress even under basal conditions as indicated by increased JNK phosphorylation in APOE4 macrophages compared with that observed in APOE3 macrophages. Additionally, apoE4 also provides a second hit in exacerbating cell stress in LPS- and oxLDL-stimulated macrophages. The increase in IRE-1α and PERK phosphorylation along with elevated alternative splicing of XBP1 illustrated that the increased cell stress is due to apoE4 potentiation of ER stress response. Previously, endogenous apoE4 expression has been shown to alter cell functions via induction of ER stress and/or mitochondrial dysfunction in a cell type-specific manner. Specifically, apoE4-expressing astrocytes have elevated ER stress with increased expression of CHOP, ATF4, and XBP1 compared with apoE3-expressing astrocytes (45). In contrast, the apoE4-induced neuronal dysfunction is not related to ER stress but is a result of mitochondrial dysfunctions with reduced mitochondrial respiration activities (46). The ability of the ER chaperone tauroursodeoxycholic acid to improve efferocytosis efficiency and reduce apoptosis of APOE4 macrophages indicates that the apoE4-related macrophage dysfunction is due to its elevation of ER stress. It is important to note that although this study focused on the relationship between apoE4-induced ER stress and macrophage efferocytosis and apoptosis, mitochondrial dysfunction in macrophages has also been reported to promote inflammation (47). Additionally, mitochondrial dysfunction has also been associated with increased risk of atherosclerosis (48) and diabetes (49). Thus, it is possible that apoE4 expression may also induce mitochondrial abnormalities in macrophages and promote inflammation through this additional mechanism. Whether apoE4 expression in macrophages also causes mitochondrial abnormalities independent of ER stress needs to be explored further in future experiments.
Recent data comparing tertiary structures between apoE3 and apoE4 have provided valuable insights into potential underlying mechanisms by which apoE4 causes macrophage dysfunction. X-ray crystallographic analyses revealed the cysteine to arginine substitution at position 112 alters interactions between the N- and C-terminal domains of the protein, resulting in apoE4 having a more compact conformation (50–52) that can also exist in a molten globule state that favors unfolding (53). Thus, the elevated ER stress observed in APOE4 macrophages may be due to the abnormal conformation of apoE4, compared with the normal apoE3, in triggering the ER stress response.
In contrast to human apoE3, apoE in other species contains arginine 112 similar to human apoE4. However, native apoE expressed in macrophages of other species resembles human apoE3 without the compacted domain interaction (54). Interestingly, replacement of threonine 61 in mouse apoE with arginine resulted in a mutant mouse apoE protein with a compact domain interaction similar to human apoE4 but without the molten globule state (55). Interestingly, a recent report from Raffai and co-workers (56) showed that macrophages isolated from mutant mice expressing mouse apoE with Arg-61 did not have elevated ER stress compared with wild type macrophages. The difference between their observations and this study is that the APOE4 mice expressed human apoE4 with both the compact domain interaction as well as a molten globule state that is lacking in mouse Arg-61 mutant apoE. Additionally, macrophages used in the study by Raffai and co-workers (56) were obtained from hypomorphic mutant mice with dramatically reduced apoE expression, although this study used APOE4 gene replacement mice expressing physiological levels of apoE. The normal level of apoE expression together with the molten globule state of apoE4 may account for the elevated ER stress observed in APOE4 macrophages.
Efferocytosis efficiency and apoptosis sensitivity were not reported in the macrophages of mouse Arg-61 mutant mice. Nevertheless, the ability of tauroursodeoxycholic acid to improve efferocytosis function and to reduce apoptosis sensitivity of APOE4 macrophages indicates that these abnormalities are attributable to apoE4-induced ER stress. It is important to note, however, that even in the absence of elevated ER stress, the macrophages from hypomorphic Arg-61 mutant mice were also more inflamed, and the mice also displayed accelerated atherosclerosis in comparison with wild type mice (56). Thus, based on the results of our study together with reports from Raffai and co-workers (56), we propose that apoE4 may promote macrophage inflammation and dysfunction in accelerating atherosclerosis through multiple mechanisms. We speculate that expression of the molten globule state of apoE4 promotes ER stress leading to impaired efferocytosis and increased apoptosis sensitivity while the compacted domain interaction in apoE4 also contributes to elevated inflammation. Whether the molten globule- and ER stress-independent mechanism is similar to the apoE4-induced mitochondrial dysfunction in neurons requires additional studies.
In summary, this study shows that endogenous apoE4 expression in macrophages impairs efferocytosis and provides a second hit to accentuate agonist-induced apoptosis. The mechanism is related to apoE4 potentiating ER stress. Because abnormal macrophage functions and inflammation are important contributors to a wide spectrum of metabolic diseases, including atherosclerosis, diabetes, and Alzheimer disease, the association between apoE4 with the same spectrum of metabolic diseases may be due in large part to its role in promoting macrophage dysfunction. Moreover, apoE4 structure correctors that have been proposed for Alzheimer disease modulation (57, 58) may also be tested for efficacy in reducing the risk of atherosclerosis, diabetes, and other inflammatory diseases associated with the ϵ4 genotype.
This work was supported, in whole or in part, by National Institutes of Health Grant DK74932 (to D. Y. H.).
- oxLDL
- oxidized LDL
- NOS2
- inducible nitric-oxide synthase
- MCP-1
- monocyte chemotactic protein-1
- MIP-1α
- macrophage inflammatory protein-1α
- CHOP
- C/EBP homologous protein
- CFDA-SE
- carboxyfluorescein diacetate succinimidyl ester
- PERK
- protein kinase RNA-like ER kinase.
REFERENCES
- 1. Mahley R. W. (1988) Apolipoprotein E. Cholesterol transport protein with expanding role in cell biology. Science 240, 622–630 [DOI] [PubMed] [Google Scholar]
- 2. Getz G. S., Reardon C. A. (2009) Apoprotein E as a lipid transport and signaling protein in the blood, liver, and artery wall. J. Lipid Res. 50, S156–S161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mahley R. W., Weisgraber K. H., Huang Y. (2009) Apolipoprotein E. Structure determines function, from atherosclerosis to Alzheimer disease to AIDS. J. Lipid Res. 50, S183–S188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Song Y., Stampfer M. J., Liu S. (2004) Meta-analysis. Apolipoprotein E genotypes and risk for coronary heart disease. Ann. Intern. Med. 141, 137–147 [DOI] [PubMed] [Google Scholar]
- 5. Bu G. (2009) Apolipoprotein E and its receptors in Alzheimer disease. Pathways, pathogenesis, and therapy. Nat. Rev. Neurosci. 10, 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jofre-Monseny L., Minihane A. M., Rimbach G. (2008) Impact of apoE genotype on oxidative stress, inflammation, and disease risk. Mol. Nutr. Food Res. 52, 131–145 [DOI] [PubMed] [Google Scholar]
- 7. Zhu Y., Kodvawala A., Hui D. Y. (2010) Apolipoprotein E inhibits Toll-like receptor (TLR)-3- and TLR-4-mediated macrophage activation through distinct mechanisms. Biochem. J. 428, 47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baitsch D., Bock H. H., Engel T., Telgmann R., Müller-Tidow C., Varga G., Bot M., Herz J., Robenek H., von Eckardstein A., Nofer J. R. (2011) Apolipoprotein E induces anti-inflammatory phenotype in macrophages. Arterioscler. Thromb. Vasc. Biol. 31, 1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sacre S. M., Stannard A. K., Owen J. S. (2003) Apolipoprotein E (apoE) isoforms differentially induce nitric oxide production in endothelial cells. FEBS Lett. 540, 181–187 [DOI] [PubMed] [Google Scholar]
- 10. Ishigami M., Swertfeger D. K., Granholm N. A., Hui D. Y. (1998) Apolipoprotein E inhibits platelet-derived growth factor-induced vascular smooth muscle cell migration and proliferation by suppressing signal transduction and preventing cell entry to G1 phase. J. Biol. Chem. 273, 20156–20161 [DOI] [PubMed] [Google Scholar]
- 11. Ishigami M., Swertfeger D. K., Hui M. S., Granholm N. A., Hui D. Y. (2000) Apolipoprotein E inhibition of vascular smooth muscle cell proliferation but not the inhibition of migration is mediated through activation of inducible nitric-oxide synthase. Arterioscler. Thromb. Vasc. Biol. 20, 1020–1026 [DOI] [PubMed] [Google Scholar]
- 12. Moore Z. W., Hui D. Y. (2005) Apolipoprotein E inhibition of vascular hyperplasia and neointima formation requires inducible nitric-oxide synthase. J. Lipid Res. 46, 2083–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moore Z. W., Zhu B., Kuhel D. G., Hui D. Y. (2004) Vascular apolipoprotein E expression and recruitment from circulation to modulate smooth muscle cell response to endothelial denudation. Am. J. Pathol. 164, 2109–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marques M. A., Crutcher K. A. (2003) Apolipoprotein E-related neurotoxicity as a therapeutic target for Alzheimer disease. J. Mol. Neurosci. 20, 327–337 [DOI] [PubMed] [Google Scholar]
- 15. Ji Z. S., Miranda R. D., Newhouse Y. M., Weisgraber K. H., Huang Y., Mahley R. W. (2002) Apolipoprotein E4 potentiates amyloid β peptide-induced lysosomal leakage and apoptosis in neuronal cells. J. Biol. Chem. 277, 21821–21828 [DOI] [PubMed] [Google Scholar]
- 16. Ji Z. S., Müllendorff K., Cheng I. H., Miranda R. D., Huang Y., Mahley R. W. (2006) Reactivity of apolipoprotein E4 and amyloid β peptide. Lysosomal stability and neurodegeneration. J. Biol. Chem. 281, 2683–2692 [DOI] [PubMed] [Google Scholar]
- 17. Volcik K. A., Barkley R. A., Hutchinson R. G., Mosley T. H., Heiss G., Sharrett A. R., Ballantyne C. M., Boerwinkle E. (2006) Apolipoprotein E polymorphisms predict low density lipoprotein cholesterol levels and carotid artery wall thickness but not incident coronary heart disease in 12,491 ARIC study participants. Am. J. Epidemiol. 164, 342–348 [DOI] [PubMed] [Google Scholar]
- 18. Sima A., Iordan A., Stancu C. (2007) Apolipoprotein E polymorphism. A risk factor for metabolic syndrome. Clin. Chem. Lab. Med. 45, 1149–1153 [DOI] [PubMed] [Google Scholar]
- 19. Anthopoulos P. G., Hamodrakas S. J., Bagos P. G. (2010) Apolipoprotein E polymorphisms and type 2 diabetes. A meta-analysis of 30 studies, including 5423 cases and 8192 controls. Mol. Genet. Metab. 100, 283–291 [DOI] [PubMed] [Google Scholar]
- 20. Vaisi-Raygani A., Rahimi Z., Nomani H., Tavilani H., Pourmotabbed T. (2007) The presence of apolipoprotein ϵ4 and ϵ2 alleles augments the risk of coronary artery disease in type 2 diabetic patients. Clin. Biochem. 40, 1150–1156 [DOI] [PubMed] [Google Scholar]
- 21. Arbones-Mainar J. M., Johnson L. A., Altenburg M. K., Maeda N. (2008) Differential modulation of diet-induced obesity and adipocyte functionality by human apolipoprotein E3 and E4 in mice. Int. J. Obes. 32, 1595–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arbones-Mainar J. M., Johnson L. A., Altenburg M. K., Kim H. S., Maeda N. (2010) Impaired adipogenic response to thiazolidinediones in mice expressing human apolipoprotein E4. FASEB J. 24, 3809–3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson L. A., Arbones-Mainar J. M., Fox R. G., Pendse A. A., Altenburg M. K., Kim H. S., Maeda N. (2011) Apolipoprotein E4 exaggerates diabetic dyslipidemia and atherosclerosis in mice lacking the LDL receptor. Diabetes 60, 2285–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Altenburg M., Johnson L., Wilder J., Maeda N. (2007) Apolipoprotein E4 in macrophages enhances atherogenesis in a low density lipoprotein receptor-dependent manner. J. Biol. Chem. 282, 7817–7824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ley K., Miller Y. I., Hedrick C. C. (2011) Monocyte and macrophage dynamics during atherogenesis. Arterioscler. Thromb. Vasc. Biol. 31, 1506–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olefsky J. M., Glass C. K. (2010) Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246 [DOI] [PubMed] [Google Scholar]
- 27. Chinetti-Gbaguidi G., Staels B. (2011) Macrophage polarization in metabolic disorders. Functions and regulation. Curr. Opin. Lipidol. 22, 365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sullivan P. M., Mezdour H., Aratani Y., Knouff C., Najib J., Reddick R. L., Quarfordt S. H., Maeda N. (1997) Targeted replacement of the mouse apolipoprotein E gene with the common human apoE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J. Biol. Chem. 272, 17972–17980 [DOI] [PubMed] [Google Scholar]
- 29. Knouff C., Hinsdale M. E., Mezdour H., Altenburg M. K., Watanabe M., Quarfordt S. H., Sullivan P. M., Maeda N. (1999) ApoE structure determines VLDL clearance and atherosclerosis risk in mice. J. Clin. Invest. 103, 1579–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hofmann S. M., Zhou L., Perez-Tilve D., Greer T., Grant E., Wancata L., Thomas A., Pfluger P. T., Basford J. E., Gilham D., Herz J., Tschöp M. H., Hui D. Y. (2007) Adipocyte LDL receptor-related protein-1 expression modulates postprandial lipid transport and glucose homeostasis in mice. J. Clin. Invest. 117, 3271–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cash J. G., Kuhel D. G., Goodin C., Hui D. Y. (2011) Pancreatic acinar cell-specific overexpression of group 1B phospholipase A2 exacerbates diet-induced obesity and insulin resistance in mice. Int. J. Obes. 35, 877–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kodvawala A., Ghering A. B., Davidson W. S., Hui D. Y. (2005) Carboxyl ester lipase expression in macrophages increases cholesteryl ester accumulation and promotes atherosclerosis. J. Biol. Chem. 280, 38592–38598 [DOI] [PubMed] [Google Scholar]
- 33. Mahley R. W., Rall S. C. (1989) in The Metabolic Basis of Inherited Disease (Scriver C. R., Beaudet A. L., Sly W. S., Valle D., eds) pp. 1195–1213, McGraw-Hill, Inc., New York [Google Scholar]
- 34. Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Savill J., Dransfield I., Gregory C., Haslett C. (2002) A blast from the past. Clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2, 965–975 [DOI] [PubMed] [Google Scholar]
- 36. Elliott M. R., Ravichandran K. S. (2010) Clearance of apoptotic cells. Implications in health and disease. J. Cell Biol. 189, 1059–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xaus J., Comalada M., Valledor A. F., Lloberas J., López-Soriano F., Argilés J. M., Bogdan C., Celada A. (2000) LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-α. Blood 95, 3823–3831 [PubMed] [Google Scholar]
- 38. Terasaka N., Wang N., Yvan-Charvet L., Tall A. R. (2007) High density lipoprotein protects macrophages from oxidized low density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc. Natl. Acad. Sci. U.S.A. 104, 15093–15098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martinon F., Chen X., Lee A. H., Glimcher L. H. (2010) TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 11, 411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Uemura A., Oku M., Mori K., Yoshida H. (2009) Unconventional splicing of XBP1 mRNA occurs in the cytoplasm during the mammalian unfolded protein response. J. Cell Sci. 122, 2877–2886 [DOI] [PubMed] [Google Scholar]
- 41. Park S. W., Zhou Y., Lee J., Lu A., Sun C., Chung J., Ueki K., Ozcan U. (2010) The regulatory subunits of PI3K, p85α and p85β, interact with XBP-1 and increase its nuclear translocation. Nat. Med. 16, 429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vitek M. P., Brown C. M., Colton C. A. (2009) APOE genotype-specific differences in the innate immune response. Neurobiol. Aging 30, 1350–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thorp E., Li G., Seimon T. A., Kuriakose G., Ron D., Tabas I. (2009) Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of apoE−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 9, 474–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thorp E., Tabas I. (2009) Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J. Leukocyte Biol. 86, 1089–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhong N., Ramaswamy G., Weisgraber K. H. (2009) Apolipoprotein E4 domain interaction induces endoplasmic reticulum stress and impairs astrocyte function. J. Biol. Chem. 284, 27273–27280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen H. K., Ji Z. S., Dodson S. E., Miranda R. D., Rosenblum C. I., Reynolds I. J., Freedman S. B., Weisgraber K. H., Huang Y., Mahley R. W. (2011) Apolipoprotein E4 domain interaction mediates detrimental effects on mitochondria and is a potential therapeutic target for Alzheimer disease. J. Biol. Chem. 286, 5215–5221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vats D., Mukundan L., Odegaard J. I., Zhang L., Smith K. L., Morel C. R., Wagner R. A., Greaves D. R., Murray P. J., Chawla A. (2006) Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 4, 13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Madamanchi N. R., Runge M. S. (2007) Mitochondrial dysfunction in atherosclerosis. Circ. Res. 100, 460–473 [DOI] [PubMed] [Google Scholar]
- 49. Lowell B. B., Shulman G. I. (2005) Mitochondrial dysfunction and type 2 diabetes. Science 307, 384–387 [DOI] [PubMed] [Google Scholar]
- 50. Dong L. M., Weisgraber K. H. (1996) Human apolipoprotein E4 domain interaction. Arginine 61 and glutamic acid 255 interact to direct the preference for very low density lipoproteins. J. Biol. Chem. 271, 19053–19057 [DOI] [PubMed] [Google Scholar]
- 51. Dong L. M., Wilson C., Wardell M. R., Simmons T., Mahley R. W., Weisgraber K. H., Agard D. A. (1994) Human apolipoprotein E. Role of arginine 61 in mediating the lipoprotein preferences of the E3 and E4 isoforms. J. Biol. Chem. 269, 22358–22365 [PubMed] [Google Scholar]
- 52. Wilson C., Wardell M. R., Weisgraber K. H., Mahley R. W., Agard D. A. (1991) Three-dimensional structure of the LDL receptor binding domain of human apolipoprotein E. Science 252, 1817–1822 [DOI] [PubMed] [Google Scholar]
- 53. Morrow J. A., Hatters D. M., Lu B., Hochtl P., Oberg K. A., Rupp B., Weisgraber K. H. (2002) Apolipoprotein E4 forms a molten globule. A potential basis for its association with disease. J. Biol. Chem. 277, 50380–50385 [DOI] [PubMed] [Google Scholar]
- 54. Weisgraber K. H. (1994) Apolipoprotein E. Structure-function relationships. Adv. Protein Chem. 45, 249–302 [DOI] [PubMed] [Google Scholar]
- 55. Hatters D. M., Budamagunta M. S., Voss J. C., Weisgraber K. H. (2005) Modulation of apolipoprotein E structure by domain interaction. J. Biol. Chem. 280, 34288–34295 [DOI] [PubMed] [Google Scholar]
- 56. Eberlé D., Kim R. Y., Luk F. S., de Mochel N. S., Gaudreault N., Olivas V. R., Kumar N., Posada J. M., Birkeland A. C., Rapp J. H., Raffai R. L. (2012) Apolipoprotein E4 domain interaction accelerates diet-induced atherosclerosis in hypomorphic Arg-61 ApoE mice. Arterioscler. Thromb. Vasc. Biol. 32, 1116–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brodbeck J., McGuire J., Liu Z., Meyer-Franke A., Balestra M. E., Jeong D. E., Pleiss M., McComas C., Hess F., Witter D., Peterson S., Childers M., Goulet M., Liverton N., Hargreaves R., Freedman S., Weisgraber K. H., Mahley R. W., Huang Y. (2011) Structure-dependent impairment of intracellular apolipoprotein E4 trafficking is rescued by small molecule structure correctors. J. Biol. Chem. 286, 17217–17226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen H. K., Liu Z., Meyer-Franke A., Brodbeck J., Miranda R. D., McGuire J. G., Pleiss M. A., Ji Z. S., Balestra M. E., Walker D. W., Xu Q., Jeong D. E., Budamagunta M. S., Voss J. C., Freedman S. B., Weisgraber K. H., Huang Y., Mahley R. W. (2012) Small molecule structure correctors abolish detrimental effects of apolipoprotein E4 in cultured neurons. J. Biol. Chem. 287, 5253–5266 [DOI] [PMC free article] [PubMed] [Google Scholar]