Background: Non-receptor protein-tyrosine phosphatases (PTP) modulate the activity of K+ channels.
Results: Cortical neurons from PTPϵ knock-out (EKO) mice exhibit reduced IK and increased BK currents, enhanced excitability, and tyrosine phosphorylation of potassium channel subsets.
Conclusion: In EKO mice, the lack of PTPϵ modulation of potassium channels leads to increased excitability.
Significance: Modulation of K+ channels by PTPϵ tunes neuronal excitability.
Keywords: Neurophysiology, Patch Clamp Electrophysiology, Phosphotyrosine Signaling, Potassium Channels, Protein Kinases, Protein Phosphatase, PTK, PTP, Excitability
Abstract
Non-receptor-tyrosine kinases (protein-tyrosine kinases) and non-receptor tyrosine phosphatases (PTPs) have been implicated in the regulation of ion channels, neuronal excitability, and synaptic plasticity. We previously showed that protein-tyrosine kinases such as Src kinase and PTPs such as PTPα and PTPϵ modulate the activity of delayed-rectifier K+ channels (IK). Here we show cultured cortical neurons from PTPϵ knock-out (EKO) mice to exhibit increased excitability when compared with wild type (WT) mice, with larger spike discharge frequency, enhanced fast after-hyperpolarization, increased after-depolarization, and reduced spike width. A decrease in IK and a rise in large-conductance Ca2+-activated K+ currents (mBK) were observed in EKO cortical neurons compared with WT. Parallel studies in transfected CHO cells indicate that Kv1.1, Kv1.2, Kv7.2/7.3, and mBK are plausible molecular correlates of this multifaceted modulation of K+ channels by PTPϵ. In CHO cells, Kv1.1, Kv1.2, and Kv7.2/7.3 K+ currents were up-regulated by PTPϵ, whereas mBK channel activity was reduced. The levels of tyrosine phosphorylation of Kv1.1, Kv1.2, Kv7.3, and mBK potassium channels were increased in the brain cortices of neonatal and adult EKO mice compared with WT, suggesting that PTPϵ in the brain modulates these channel proteins. Our data indicate that in EKO mice, the lack of PTPϵ-mediated dephosphorylation of Kv1.1, Kv1.2, and Kv7.3 leads to decreased IK density and enhanced after-depolarization. In addition, the deficient PTPϵ-mediated dephosphorylation of mBK channels likely contributes to enhanced mBK and fast after-hyperpolarization, spike shortening, and consequent increase in neuronal excitability observed in cortical neurons from EKO mice.
Introduction
Reversible protein phosphorylation/dephosphorylation of tyrosine residues are key mechanisms for the control of ion channel function and membrane excitability (1, 2). This process is controlled by the opposing actions of protein-tyrosine kinases and protein-tyrosine phosphatases (PTPs).3 In the brain, protein-tyrosine kinases and PTPs affect synaptic plasticity, as illustrated by the role of Src-family kinases, PTPδ, and PTPα in hippocampal long term potentiation (3–6). Many lines of evidence suggest that one major function of protein-tyrosine kinases and PTPs is to regulate the activity of ion channels in the CNS. Thus, modulation of ligand-gated ionotropic receptors and voltage-gated cation channels by tyrosine phosphorylation/dephosphorylation play an important role in regulating neuronal excitability and in mediating synaptic plasticity (7–11). By up-regulating the function of N-methyl-d-aspartate (NMDA) receptors, Src kinase gates the production of NMDA receptor-dependent synaptic potentiation and plasticity (11, 12). Fyn kinase is associated with sodium channels in rat brain and phosphorylates residues in the inactivation gate, thereby modulating the fast inactivation (7). Nav1.2 sodium channels also associate with RPTPβ through interactions with both α and β subunits (9). RPTPβ dephosphorylates the sodium channel α subunit, slows the decay of the sodium current, and shifts the voltage dependence of fast inactivation to more positive membrane potentials (9), which reflects the opposite effects produced by Fyn kinase. Significant evidence indicates that voltage-gated K+ channels (Kv) are also substrates of protein-tyrosine kinase and PTP activities (8, 10). The M1 muscarinic acetylcholine receptor was shown to stimulate a cellular tyrosine kinase activity that leads to the direct tyrosine phosphorylation of Kv1.2 and to suppression of its activity (13). Dephosphorylation of Kv1.1 and Kv1.2 by RPTPα up-regulates the activity of the channel (14). Src family kinases have been shown to phosphorylate Kv1.3 and to down-regulate its activity in heterologous expression systems as well as in Jurkat T cells and in rat olfactory bulb neurons (8, 15–17). Src binding and tyrosine phosphorylation are each able to modulate independently both Kv1.4 and Kv1.5 channel currents (16, 18). Kv7.2/Kv7.3 subunits encoding the neuronal M-current are also modulated by Src and by the EGF receptor via tyrosine phosphorylation (19, 20). It was shown that Src acts both on heterologously expressed cloned channels and on native M-channels of cultured sympathetic neurons. Src kinase strongly suppresses current amplitudes and slows activation kinetics of Kv7.3, Kv7.4, and Kv7.5 homomultimers as well as Kv7.2/3 heteromultimers but spares Kv7.1 and Kv7.2 homomultimers (21). We previously showed that Kv1.5 and Kv2.1α subunits are constitutively tyrosine-phosphorylated and physically associated with Fyn kinase in Schwann cells, resulting in enhanced K+ channel activity (22, 23). We also found a down-regulation of Kv2.1 and Kv1.5 channel activity by PTPϵ, which antagonizes channel activation produced by the protein-tyrosine kinases, Src and Fyn (24). Kv2.1 was found to interact with the active site of PTPϵ in vitro. Lack of PTPϵ in mice causes hypomyelination of sciatic nerve axons in newborn mice together with increased Kv channel activity and hyperphosphorylation of the Kv1.5 and Kv2.1 α subunits in cultured schwann cells and sciatic nerve tissue (24, 25). Here we show cultured cortical neurons from PTPϵ knock-out (EKO) mice to exhibit increased excitability compared with wild type (WT) with reduced IK current density, enhanced large-conductance Ca2+-activated K+ currents (mBK), larger evoked spike discharge frequency, increased after-depolarization (ADP), enhanced fast after-hyperpolarization (fAHP), and reduced spike width. Kv1.1, Kv1.2, Kv7.3 and the mouse large conductance Ca2+-activated K+ (mBK or mSlo) channel proteins were hyperphosphorylated at tyrosine residues in the brain cortices from neonatal and adult EKO mice compared with WT. We suggest the increased excitability of cortical neurons from EKO mice to be accounted for by the versatile modulation of K+ channels by PTPϵ.
EXPERIMENTAL PROCEDURES
Mice
Gene targeted mice lacking all forms of PTPϵ (EKO mice, C57BL/6 × 129 genetic background) were described previously (24). The procedures followed for experimentation and maintenance of the animals were approved by the animal research ethics committee of the Weizmann Institute and Tel Aviv University in accordance with Israeli law and in accordance with the Guide for the Care and Use of Laboratory Animals (1996, National Academy of Sciences, Washington, D. C.).
Electroshock-induced Seizure Model
The sensitivity of EKO mice to electrical stimulations was compared with that of WT mice using the electroshock-induced seizure model. Electro-convulsions were produced by means of alternative current delivered (60 Hz for 0.2 s) via two transcorneal electrodes positioned on the eyes of ∼2-month-old mice (each test with a specific current intensity) using a rodent shocker (Hugo Sachs Electronik, type 221). The response of the animal was considered positive when a tonic seizure was observed. Tonic seizures were defined as such by the presence of full tonic hind limb extension. The number of positive responses in the EKO group was statistically compared with that of the WT group using Student's t test for two independent samples (p < 0.05 two-tailed).
Primary Cultures of Cortical Neurons
WT and EKO neonate mice (1–2 days old) were sacrificed by cervical dislocation, and their cortices were dissected out. The tissue was digested with papain (200 units, Sigma) for 40 min, triturated to a single-cell suspension, and plated at a density of 300,000 cells/ml on a substrate of bovine collagen type IV and a 10 μg/ml poly-l-lysine in 13-mm diameter glass coverslips of a 24-multiwell plate. The culture medium consisted of modified Eagle's medium containing 5% horse serum (Biological Industries, Beit Haemek, Israel), B-27 neuronal supplement (Invitrogen), 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mm glutamine. d-Glucose was supplemented to a final concentration of 6 g/liter. All cultures were maintained at 37 °C in humidified air containing 5% CO2. Half of the medium volume in each well was changed after 3 days to fresh medium with the addition of 5 μm cytosine arabinoside to limit the growth of glial cells.
Immunoprecipitation and Western Blotting
Cortical tissues were isolated from 2-day-old EKO and WT mice and kept frozen in liquid nitrogen until use. Cortical tissue was homogenized in ice-cold immunoprecipitation buffer (50 mm Tris-HCl, pH 7.5, 100 mm NaCl, 1% Nonidet P-40, 1 mm phenylmethylsulfonyl fluoride (PMSF), 0.5 mm sodium pervanadate, and a protease inhibitor mixture 10 μm/ml (Sigma)) and rotated at 4 °C for 1 h. Unsolubilized material was removed by centrifugation (10, 000 × g). Lysates were then incubated with the relevant immunoprecipitating antibody overnight at 4 °C followed by incubation with protein A-Sepharose (GE Healthcare ) for 2 h. Immunoprecipitating antibodies used in this study included polyclonal anti-Kv1.2 (Alomone Labs), polyclonal anti-Kv7.3 (Alomone Labs), monoclonal anti-Kv2.1 (Upstate Biotechnology), polyclonal anti-BK (Alomone Labs), and monoclonal anti-Kv1.1 (Upstate Biotechnology). Immune complexes were washed 3 times in washing buffer (50 mm Tris-HCl, pH 7.5, 100 mm NaCl, 1% Nonidet P-40, 1 mm PMSF, and 0.5 mm sodium pervanadate), then electrophoresed and Western-blotted with the monoclonal anti-phosphotyrosine antibody (clone 4G10; Upstate Biotechnology). In some cases we immunoprecipitated phosphotyrosine-containing proteins, where the cortical brain lysates were incubated with anti-Tyr(P)-agarose (clone PY20; Sigma) overnight at 4 °C followed by three washes with washing buffer and resolved by 8% SDS-PAGE. For preparation of human embryo kidney 293 (HEK 293) cell lysates, cells were washed in phosphate-buffered saline (PBS) containing 5 mm EDTA, 1 mm PMSF, and 0.5 mm sodium pervanadate, solubilized in ice-cold immunoprecipitation buffer, and rotated at 4 °C for 1 h. Unsolubilized material was removed by centrifugation (10,000 × g). Samples were electrophoresed on 8% SDS-PAGE polyacrylamide gel along with standard molecular weight markers and run at 50 mA for 1–2 h at room temperature. Proteins were then electro-transferred to nitrocellulose membranes at 200 mA for 1.5 h. Blots were blocked in PBS containing 10% nonfat milk and 0.05% Tween 20 (Sigma) for 1 h at room temperature under shaking, then incubated with the relevant primary antibody overnight at 4 °C. After three 5-min washes in PBS at room temperature, blots were incubated for 1 h with the relevant HRP-conjugated secondary antibodies followed by a 2-min wash with 3% Tween 20 in PBS and three 5-min washes in PBS at room temperature. To compare the total protein inputs in each lane, blots were incubated overnight at 4 °C with the respective anti-channel antibodies. Labeled proteins were detected by enhanced chemiluminescence (Pierce). Band signals corresponding to immunoreactive proteins were measured and scanned by image densitometry using Tina software. Densitometric data were normalized to the respective protein input as assessed by the channel probing of the blots.
Cell Line Culture and Transfection
For electrophysiology, Chinese hamster ovary (CHO) cells were grown in Dulbecco's modified Eagle's medium supplemented with 2 mm glutamine, 10% fetal calf serum, and antibiotics. Briefly, 40,000 cells seeded on poly-d-lysine-coated glass coverslips in a 24-multiwell plate were transfected with pIRES-CD8 (0.5 μg) as a marker for transfection and with the various channel DNA constructs (0.5 μg of either Kv1.2/Kv1.1/Kv7.2 + Kv7.3 or 0.1 μg of mouse BK) as well as 0.5 μg of constitutively active Src (Y527FSrc) and 1 μg of PTPϵ. For electrophysiology, transfected cells were visualized ∼40 h after transfection using anti-CD8 antibody-coated beads. Transfection was performed using FuGENE 6 (Roche Applied Science) according to the manufacturer's protocol. For biochemistry, HEK 293 cells were grown as for CHO cells and transfected using the calcium phosphate method. Briefly, 1.5 × 106 cells were seeded on a 10-cm plate and incubated overnight in 7 ml of growth medium. Then, expression vectors encoding the channel (10 μg) + Y527FSrc (10 μg) + PTPϵ (10 μg) were mixed with the calcium phosphate solution and added dropwise to the plates. The mixture was swirled gently and incubated for ∼9 h. The medium was replaced with a fresh growth medium, and the cells were incubated for an additional 24 h until they were harvested and collected for cell lysate preparation.
Electrophysiology
Voltage-clamp recordings in CHO cells were performed using the whole-cell configuration of the patch clamp technique (26). Signals were amplified using an Axopatch 200A patch clamp amplifier (Molecular Devices), sampled at 2 kHz, and filtered at 800 Hz via a 4-pole Bessel low pass filter. Data were acquired using pClamp 9.2 software (Molecular Devices) and an IBM compatible Pentium IV computer in conjunction with a DigiData 1322A interface (Molecular Devices). The patch pipettes were pulled from borosilicate glass (Warner Instrument Corp.) with a resistance of 4–6 MΩ. For K+ current recordings in CHO cells, the intracellular pipette solution contained 130 mm KCl, 1 mm MgCl2, 5 mm K2ATP, 5 mm EGTA, 10 mm HEPES, adjusted with KOH to pH 7.4 (290 mosm). The extracellular solution contained 140 mm NaCl, 4 mm KCl, 1.8 mm CaCl2, 1.2 mm MgCl2, 11 mm glucose, 5.5 mm HEPES, adjusted with NaOH to pH 7.4 (310 mosm). Series resistances (5–13 MΩ) were compensated (75–90%) and periodically monitored. For voltage-clamp measurements of mouse cortical pyramidal-like neurons, the patch pipettes were filled with 90 mm potassium acetate, 40 mm KCl, 3 mm MgCl2, 2 mm K2ATP, and 20 mm HEPES, adjusted with KOH to pH 7.4 (310–315 mosm). The external solution contained 150 mm NaCl, 2.5 mm KCl, 15 mm glucose, 0.001 mm tetrodotoxin (to block voltage-gated sodium currents), 0.2 mm CdCl2 (to block voltage-gated calcium currents), 10 mm HEPES and adjusted with NaOH to pH 7.4 (325 mosm). To isolate the fast transient current IA from the delayed rectifier K+ currents (IK), an inactivating prepulse two-stage protocol was applied and subtracted one from the other. First, a series of depolarizing steps from −60 mV to +30 mV in +15-mV increments was applied to record both IA and IK (holding potential = −85 mV). Second, a similar protocol as above, but with a 1.5-s inactivating prepulse step of −40 mV to inactivate IA and isolate IK. Subtraction of the outward currents from the two-stage protocol yielded IA. To record BK currents, the patch pipettes were filled with mm 135 KCl, 1 mm K2ATP, 1 mm MgATP, 5 mm glucose, 10 mm Hepes (pH 7.25) and intracellular free Ca2+ of 10 μm (CaCl2 with EGTA). The extracellular solution included 160 mm NaCl, 2.5 mm KCl, 1.2 mm MgCl2, 1.8 mm CaCl2, 10 mm glucose, 10 mm Hepes (pH 7.3) where 1 mm 4-aminopyridine and 1 μm tetrodotoxin were added to block IA and Na+ currents, respectively. For current-clamp measurements of mouse cortical pyramidal-like neurons, recordings were performed 9–10 days after plating. The patch pipettes were filled with 135 mm KCl, 1 mm K2ATP, 1 mm MgATP, 2 mm EGTA, 1.1 mm CaCl2, 5 mm glucose, and 10 mm HEPES, adjusted with KOH to pH 7.4 (315 mosm) and corresponding to a free-Ca2+ concentration of about 86 nm. The external solution contained 140 mm NaCl, 4 mm KCl, 2 mm CaCl2, 2 mm MgCl2, 5 mm glucose, and 10 mm HEPES, adjusted with NaOH to pH 7.4 (325 mosm). The signals were sampled at 5 kHz and filtered at 2 kHz via a 4-pole Bessel low pass filter. Single channel recordings were performed in CHO cells transfected with mBK alone or either with Y527Src or both Y527FSrc + PTPϵ. The recordings were performed using either the cell-attached or the inside-out configurations of the patch clamp technique. For the cell-attached recording, cells were continuously perfused with a solution containing 150 mm KCl, 1 mm CaCl2, 1 mm MgCl2, 10 mm glucose, and 10 mm HEPES, adjusted with NaOH to pH 7.2 (320–325 mosm). Pipettes were pulled from borosilicate glass, coated with Sylgard (8–10 MΩ), and filled with 140 mm NaCl, 4 mm KCl, 1 mm CaCl2, 1 mm MgCl2, 11 mm glucose, and 5 mm HEPES, adjusted with NaOH to pH 7.4 (325 mosm). For inside-out recording, a symmetrical high K+ solution was used that consisted of 130 mm KCl, 10 mm HEPES (pH 7.3), 2 mm MgCl2, and mm CaCl2 and EGTA in appropriate concentrations to give a free Ca2+ concentration of 10 μm. The free Ca2+ concentration in the bath solution was estimated using MaxChelator. Signals were sampled at 20 kHz and low-pass-filtered at 1 kHz.
Data Analysis
Data analysis was performed using the Clampfit program (pClamp 9.2; Molecular Devices), Microsoft Excel (Microsoft), and Prism 5.0 (GraphPad Software). Leak subtraction was performed off-line using the Clampfit program of the pClamp 9.2 software. Chord conductance (G) was calculated by using the equation G = I/(V − Vrev) where I corresponds to the current amplitude, and Vrev is the calculated reversal potential of potassium (-90 mV). G was estimated at various test voltages V and then normalized to a maximal conductance value, Gmax. Activation curves were fitted by one Boltzmann distribution: G/Gmax = 1/{1 + exp[(V50 − V)/s]}, where V50 is the voltage at which the current is half-activated, and s is the slope factor. For single channel analysis, patches with a maximum of three active channels were used for analysis, and at least 7000 events were acquired for each record. Event detection was performed using the algorithm built-in pClamp10.2 software. All events were checked visually before being accepted. Conductance levels were derived from amplitude histograms built up from pClamp10.2-generated event list. The histograms were fitted with a multicomponential Gaussian probability function using Levenberg-Marquardt method (precision criterion for convergence was set to 10−6) with the sum of square errors minimization technique. NPo (number of functional channels X single-channel open probability) was calculated. To calculate Po, NPo was divided by the maximum number of channels present in the patch, which was estimated from the maximum number of simultaneously open channels at +100 mV in 10 μm free Ca2+. All data were expressed as the mean ± S.E. Statistically significant differences were assessed by unpaired two-tailed Student's t test assuming equal variances.
RESULTS
Intrinsic Excitability Properties of Cultured Cortical Pyramidal-like Neurons from EKO and WT Mice
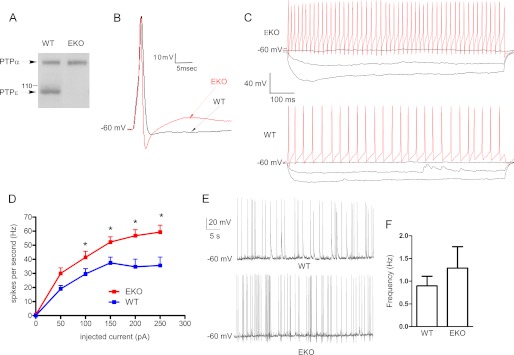
The expression of PTPϵ in brain lysates from WT and EKO mice was examined by Western blotting. Blots were exposed to anti-PTPϵ antibodies, which also cross-react with PTPα (24) (Fig. 1A). Although the antibodies showed a similar level of expression of PTPα in both WT and EKO mice brains, they revealed that the brain lysates of EKO mice do not express detectable levels of PTPϵ. Then we examined the intrinsic excitability properties of cultured cortical neurons with pyramidal-like morphology from EKO and WT mice. The resting membrane potential and the input resistance were similar in cortical neurons from EKO and WT mice (Table 1). Using a protocol that evoked a single action potential (AP), we analyzed the various parameters of solitary spikes. The cells were injected with a series of depolarizing current steps starting from 0.2 nA with 0.05-nA increments steps for a 2-ms pulse duration until a single spike was elicited that yielded the rheobase current (Fig. 1B and Table 1). The rheobase current was markedly reduced in cortical neurons from EKO mice compared with WT (253 ± 10 and 408 ± 40 pA, respectively; n = 14–20, p < 0.05; Table 1). Although the threshold potential and the AP amplitude were unaffected, the ADP and the fAHP were significantly enhanced in neurons from EKO compared with WT (ADP = 284 ± 73 and 59 ± 6 ms·mV, respectively; n = 11–15, p < 0.05; fAHP = −15.0 ± 1.8 and −6.0 ± 1.6 mV, respectively; n = 14–17, p < 0.05; Fig. 1B and Table 1). In addition, the AP width was significantly reduced in cortical neurons from EKO compared with WT (2.0 ± 0.1 ms versus 3.3 ± 0.2 ms, respectively; n = 14–20, p < 0.05; Table 1). When trains of AP discharge were evoked by depolarizing step current injection of 800-ms duration, a significantly larger spike frequency was obtained in neurons from EKO mice compared with WT (at 200 pA current injection, 57 ± 4 and 35 ± 5 Hz, respectively; n = 10, p < 0.05; Fig. 1, C and D). Synaptically driven spontaneous firing was examined in high density cultured cortical neurons in the absence of synaptic blockers. No significant difference in spontaneous firing frequency was observed between WT and EKO mice (Fig. 1, E and F; f = 1.3 ± 0.3 Hz and f = 1.1 ± 0.3 Hz for WT and EKO, respectively, n = 19–24). Interestingly, the spontaneous spiking discharge in EKO neurons was always accompanied with a pronounced fAHP, which was rarely observed in WT neurons (see Fig. 1E).
FIGURE 1.
Intrinsic excitability properties of cultured cortical pyramidal-like neurons from EKO and WT mice. A, a Western blot shows the expression of PTPϵ in brain lysate from the adult brains of WT and EKO mice. Blots containing lysates of brains from WT and EKO mice were reacted with anti-PTPϵ antibodies, which also cross-react with RPTPα. Molecular mass standards are in kilodaltons. B, shown are representative superimposed traces of a single action potential from EKO (red) and WT (black) cultured cortical pyramidal-like neurons. Cells were injected with a series of current steps starting from 0.2 nA followed by 0.05-nA incremental steps for 2-ms pulse duration until a single spike was elicited. See Table 1 for a detailed comparison of the various parameters measured. C, evoked spike discharge of EKO (upper panel) and WT (lower panel) cultured cortical pyramidal-like neurons are shown. Spiking discharge was evoked by a series of current steps (−0.1 nA up to 0.25 nA for 800 ms). D, spike frequency-injected current plot shows that the firing frequency in cortical neurons from EKO mice is higher than that from WT (*, evoked spike frequency significantly different between WT and EKO p < 0.05; n = 10; unpaired two-tailed Student's t test). E, shown are representative traces of spontaneous firing discharge in WT and EKO cortical neurons; notice the presence of frequent fAHP in EKO firing. F, shown is a summary of spontaneous firing expressed as frequency discharge in WT and EKO neurons (no significant differences were found between WT and EKO; n = 19–24).
TABLE 1.
Intrinsic excitability parameters of cultured cortical pyramidal-like neurons from EKO and WT mice
Neurons were injected with a series of current steps starting from 200 pA followed by 50-pA increments steps for a 2-ms pulse duration until a single action potential (AP) was elicited.
| Parameter | Threshold potentiala | Resting potential | Input resistanceb | AP amplitude | AP width at threshold | fAHPc | ADPd | Rheobasee |
|---|---|---|---|---|---|---|---|---|
| mV | mV | MΩ | mV | ms | mV | m·mV | pA | |
| WT mice | −34.0 ± 1.8 | −60.0 ± 0.2 | 485 ± 70 | 95 ± 4 | 3.3 ± 0.2 | −6.0 ± 1.6 | 59 ± 6 | 408 ± 40 |
| EKO mice | −33.0 ± 1.8 | −57.5 ± 0.8 | 515 ± 58 | 93 ± 4 | 2.0 ± 0.1f | −s15.0 ± 1.8f | 284 ± 73f | 253 ± 10f |
a Measured as the potential at the beginning of the AP upstroke.
b Calculated from the voltage change after a −50 pA current injection for 800 ms (Rin = V/I in MΩ).
c fAHP, calculated as (resting potential − potential measured immediately after AP).
d ADP, calculated as the area under the curve from the time where the trace crosses the resting potential up to the end of the recording time.
e Calculated from a series of 50-pA current steps of 2-mc duration and expressed as the minimal current necessary to elicit an action potential.
f Statistical significance of at least p < 0.05 (assessed by two-tailed unpaired Student's t test; n = 11–20).
Sensitivity of EKO Mice to Electroshock-induced Seizures in Vivo
Cortical neurons from EKO mice exhibit increased excitability; however, it is important to emphasize that EKO mice did not exhibit spontaneous epileptic seizures. Thus, we examined whether EKO mice are more prone to develop epileptic attacks after electrical stimulation using the electroshock-induced seizure model. We checked the number of tonic seizures developed by EKO mice as compared with WT mice when subjected to a fixed alternative current delivered via transcorneal electrodes (at 60 Hz for 0.2 s). The response of the animal was considered positive when a tonic seizure was observed. Tonic seizures were defined by the presence of full tonic hind limb extension. There was no significant difference in the number of mice developing tonic seizures in the EKO group (12 of 21) in response to 20-mA current delivered, as compared with that of the WT group (12 of 20). Also, no significant difference was observed when the current intensity was decreased to 17 mA (3 of 8 mice developed tonic seizures as compared with 3 of 8 mice in the WT group). Decreasing the current intensity further (below 15 mA) did not produce tonic seizures in both WT and EKO mice.
Delayed Rectifier (IK) and Transient (IA) Outward K+ Currents in Cultured Cortical Neurons from EKO and WT Mice
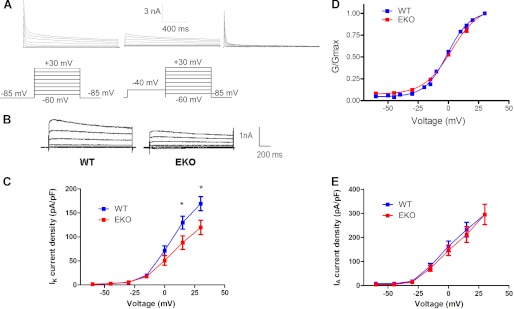
The enhanced excitability of cortical neurons from EKO mice may potentially result from the loss of PTPϵ modulation of voltage-gated sodium, calcium, or potassium currents or a combination of them. It is well known that voltage-gated potassium channels (Kv) channels are crucial for controlling membrane electrical excitability. We and others previously showed that Kv channels are substrates of protein-tyrosine kinases and protein-tyrosine phosphatases activities, which affect channel characteristics (22–25, 27). Thus, we hypothesized that PTPϵ may modulate subtypes of Kv channels in cortical neurons, which may account for the increased excitability found in EKO mice. The voltage-dependent K+ currents exhibit two components: the inactivating transient K+ current, IA, and the delayed-rectifier K+ current IK. IA was isolated from IK by an inactivating prepulse two-stage protocol (see “Experimental Procedures”; Fig. 2A). Results show that the delayed-rectifier K+ current density is significantly reduced (30% decrease at +30 mV) in EKO mice compared with WT mice (Fig. 2, B and C; WT IK = 169 pA/pF, n = 40, and EKO IK = 119 pA/pF, n = 32; p < 0.05). The conductance-voltage relations (G/Gmax) indicate that the voltage dependence of IK activation is very similar in EKO mice compared with WT animals (Fig. 2D; V50 = −0.8 ± 0.7 mV, and V50 = 2.4 ± 1.2 mV for WT and EKO mice; n = 32–40). No significant differences were found in the current density of the inactivating transient K+ current IA (Fig. 2E). These results suggest that under normal conditions PTPϵ positively modulates the delayed-rectifier current IK without affecting the transient component IA of voltage-dependent K+ currents in cultured cortical pyramidal-like neurons.
FIGURE 2.
IK but not IA is decreased in EKO cultured cortical neurons compared with WT. A, shown are representative traces of outward K+ currents from WT cortical neurons before (IA+IK) (left), after a 1.5-s inactivating prepulse to −40 mV yielding IK (middle), and after subtraction of traces obtained after the inactivating prepulse, yielding IA (right). From a holding potential of −85 mV, cells were stepped from −60 to +30 mV in +15-mV increments for 1.5-s pulse duration. B, shown are representative traces of IK from cortical neurons of EKO and WT mice, obtained after a 1.5-s inactivating prepulse to −40 mV as described in A. C, IK current density-voltage relations of WT (n = 40, blue squares) and EKO (n = 32, red squares) cortical pyramidal neurons (*, IK density significantly different between WT and EKO p < 0.05; unpaired two-tailed Student's t test). D, the normalized conductance-voltage relations (G/Gmax) indicate that the voltage dependence of IK activation is very similar in EKO mice compared with WT. The curves were fitted using a single Boltzmann distribution (V50 = −0.8 ± 0.7 mV and V50 = 2.4 ± 1.2 mV, respectively for WT and EKO mice; n = 32–40). E, no significant differences were found between WT (blue squares) and EKO (red squares) neurons in the current density-voltage relations of the inactivating transient K+ current IA, obtained after subtraction of traces after the inactivating prepulse.
Plausible Molecular Correlates of IK That Are Modulated by PTPϵ
To establish which plausible delayed-rectifier Kv channels are primarily modulated by PTPϵ, we expressed several Kv channels in CHO cells with and without PTPϵ and measured the currents using the whole-cell configuration of the patch clamp technique. Because we do not know what the basal tyrosine phosphorylation levels of the expressed Kv channels are in CHO cells, we systematically stimulated the tyrosine phosphorylation of Kv channels by co-expressing them with the constitutively active tyrosine kinase Y527F Src in the absence and presence of PTPϵ. In principle, Kv1.1, Kv1.2, Kv1.6, Kv2.1, Kv3.1, Kv3.2, and K7.2/3 channels are potential contributors to the delayed-rectifier potassium currents. So far, no tyrosine kinase modulation was observed for Kv1.6 and Kv3 channels, the latter being regulated by PKA, PKC, and casein kinase (10). Essentially, we checked Kv1.1, Kv1.2, Kv2.1, and Kv7.2/7.3.
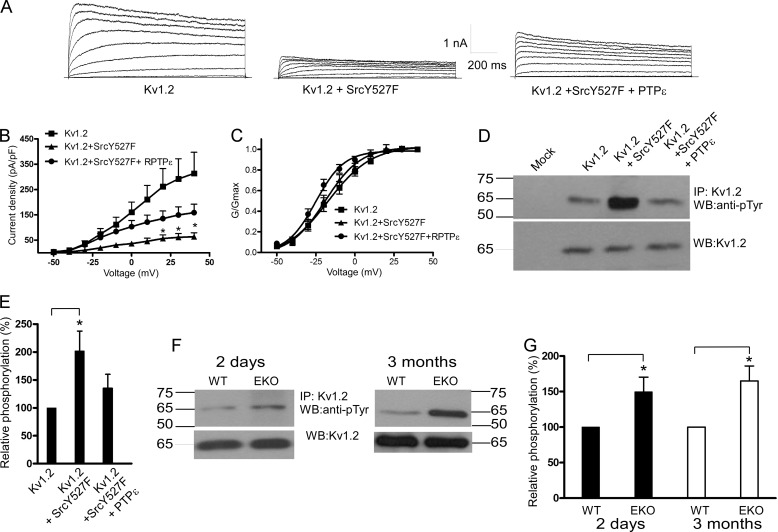
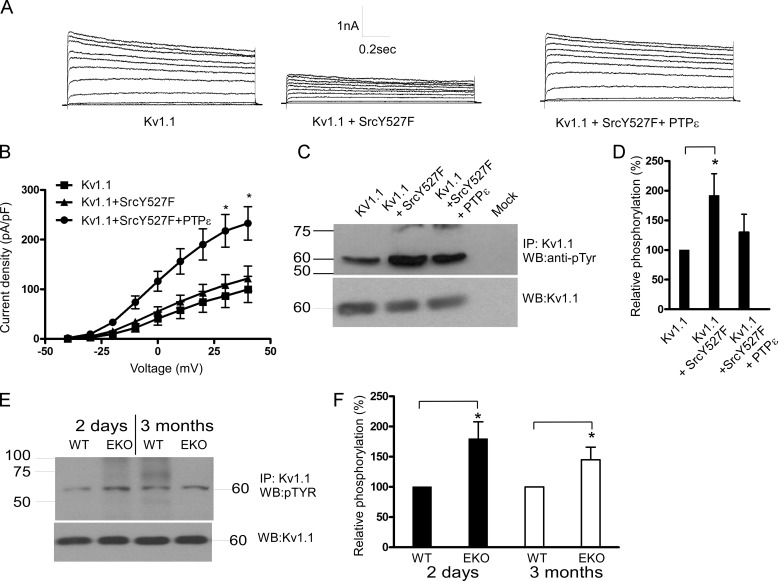
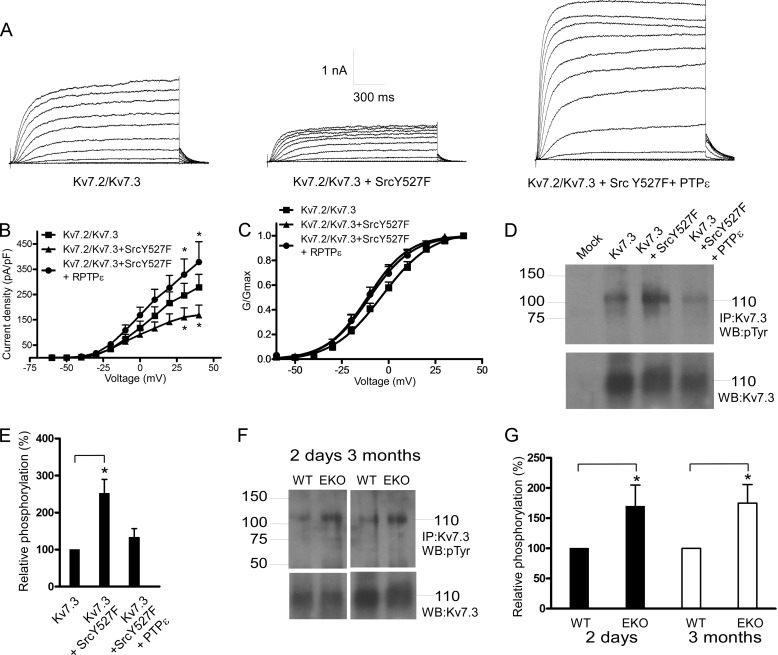
Y527F Src kinase markedly reduced Kv1.2 current density (79 ± 5% inhibition at +40 mV; n = 15–18, p < 0.01) (Fig. 3, A and B), and cotransfection with PTPϵ significantly relieved the Src kinase-induced inhibition (51 ± 11% of control at +40 mV; n = 15–18, p < 0.01) (Fig. 3, A and B). The voltage dependence of channel activation was slightly left-shifted, although not significantly, and the gating kinetics were not affected (Fig. 3, A and C). We examined whether the positive modulation of Kv1.2 by PTPϵ could be also detected in the absence of stimulation by Y527F Src. In the absence of co-transfected Y527F Src, PTPϵ increased by more than 2-fold Kv1.2 current density (n = 13–21, p < 0.01). This result indicates that in CHO cells there is a significant basal level of Kv1.2 tyrosine phosphorylation. In addition, the inactive mutant of PTPϵ (R340M) failed to increase the Kv1.2 currents and even produced some inhibition (data not shown). We also checked the impact of constitutively active Y527F Src in the absence and presence of PTPϵ on Kv1.2 tyrosine phosphorylation in transfected HEK 293 cells. Y527F Src significantly increased (2-fold) the basal tyrosine phosphorylation of Kv1.2 (Fig. 3, D and E; n = 4, p < 0.05), and cotransfection with PTPϵ prevented this Src-induced increase in tyrosine phosphorylation of Kv1.2. Taken together these data suggest that Kv1.2 channels are reciprocally modulated by Src kinase and PTPϵ. Thus, in cortical neurons Kv1.2 channels may be modulated by PTPϵ via dephosphorylation of Kv1.2 tyrosine residues. If so, one would expect different levels of Kv1.2 tyrosine phosphorylation between the brain cortices of EKO and those of WT mice. Crude lysates from cortical tissues were prepared from neonatal (2-day-old) and adult (3-month-old) EKO and WT mice, subjected to immunoprecipitation with anti-Kv1.2 antibodies, Western-blotted, and probed with anti-phosphotyrosine antibody. Western blots were also probed with anti-Kv1.2 antibodies to compare the different inputs (Fig. 3, F and G). The tyrosine phosphorylation level of Kv1.2 channels was significantly increased in cortices from both neonatal and adult EKO mice compared with that from WT (50 ± 20 and 65 ± 21% increase for neonatal and adult, respectively; n = 4, p < 0.05) (Fig. 3, F and G). For Kv1.1 channels, Y527F Src kinase weakly reduced the expressed currents (∼20% inhibition), probably due to a significant level of basal Kv1.1 tyrosine phosphorylation. However, cotransfection with PTPϵ not only relieved the Src kinase-induced inhibition but considerably increased the Kv1.1 current density (1.9-fold current increase; n = 5–11, p < 0.01) (Fig. 4, A and B). Similar to Kv1.2, Y527F Src significantly increased (1.9-fold) the tyrosine phosphorylation of Kv1.1 (Fig. 4, C and D; n = 4, p < 0.05), and cotransfection with PTPϵ prevented this Src-induced increase in tyrosine phosphorylation of Kv1.1 in HEK 293 cells. The tyrosine phosphorylation level of Kv1.1 channels was significantly increased in cortices from both neonatal and adult EKO mice compared with that from WT (80 ± 28 and 45 ± 21% increase for neonatal and adult, respectively; n = 4, p < 0.05) (Fig. 4, E and F). For Kv7.2/3 heteromeric channels, Y527F Src moderately reduced the currents (39 ± 13% reduction at +40 mV; n = 11–13, p < 0.05), and cotransfection with PTPϵ not only relieved the Src kinase-induced inhibition but also increased the current density (36% increase at +40 mV; n = 11–13, p < 0.01) (Fig. 5, A and B). The voltage dependence of channel activation was slightly left-shifted, although not significantly, by Y527F Src co-expression (Fig. 5C). Gamper et al. (19) show that Src kinase strongly suppressed the current amplitude of Kv7.3 homomers as well as that of Kv7.2/7.3 heteromers but spared that of Kv7.2 homomers. Furthermore, they showed Src-dependent phosphotyrosine signals associated with Kv7.3 but not with Kv7.2 (19, 21). In HEK 293 cells we found that Y527F Src significantly increased (2.5-fold) tyrosine phosphorylation of Kv7.3 (Fig. 5 D and E; n = 4, p < 0.05), and cotransfection with PTPϵ prevented the Src-induced increase in tyrosine phosphorylation of Kv7.3. We also checked whether Kv7.3 proteins exhibited tyrosine hyperphosphorylation in the brain cortices from neonatal and adult EKO mice in a similar manner to Kv1.2 and Kv1.1 channels. Results show that the tyrosine phosphorylation level of Kv7.3 subunits was significantly increased in cortices from neonatal and adult EKO mice compared with that from WT (70 ± 35 and 75 ± 31% increase for neonatal and adult, respectively; n = 4, p < 0.05) (Fig. 5, F and G).
FIGURE 3.
Effects of PTPϵ and constitutively active Y527F Src on Kv1.2 K+ currents expressed in CHO cells and Kv1.2 phosphotyrosine levels in transfected HEK 293 cells and in brain cortices of WT and EKO mice. A, shown are representative whole-cell K+ currents from cells transfected with Kv1.2 (left), Kv1.2 + Y527F Src (middle), Kv1.2+Y527F Src + PTPϵ (right). From a holding potential of −90 mV, cells were stepped from −50 to +40 mV in +10-mV increments for 1.5 s pulse duration. B and C, shown are current density-voltage relations and normalized conductance-voltage relations, respectively, of Kv1.2 (squares), Kv1.2+Y527F Src (triangles), and Kv1.2+Y527F Src + PTPϵ (circles) (*, the current densities of Kv1.2 and Kv1.2+Y527F Src were significantly different p < 0.01, n = 15–18; unpaired two-tailed Student's t test). RPTPα, receptor protein tyrosine phosphatase α. D, crude lysates of HEK 293 cells transfected with Kv1.2, Kv1.2+Y527F Src, and Kv1.2+Y527F Src + PTPϵ were subjected to immunoprecipitation (IP) with anti-Kv1.2 antibodies, and immune complexes were Western-blotted (WB) with anti-phosphotyrosine antibodies (top) and with anti-Kv1.2 antibodies for input comparison (bottom). E, a bar diagram shows the relative tyrosine phosphorylation of Kv1.2, Kv1.2+Y527F Src, and Kv1.2+Y527F Src+PTPϵ, expressed as % of phosphorylation of Kv1.2. (*, the tyrosine phosphorylation level of Kv1.2 channels was significantly increased in the presence of Y527F Src, n = 4; p < 0.05; unpaired two-tailed Student's t test). F, crude lysates of brain cortices from 2-day-old and 3-month-old WT and EKO mice were subjected to immunoprecipitation with anti-Kv1.2 antibodies, and immune complexes were Western-blotted with anti-phosphotyrosine antibodies (top) and with anti-Kv1.2 antibodies for input comparison (bottom). G, a bar diagram shows Kv1.2 relative phosphorylation in EKO mice relative to WT. (*, the tyrosine phosphorylation level of Kv1.2 channels was significantly increased in cortices from 2-day-old and 3-month-old EKO mice compared with that from WT; n = 4, p < 0.05; unpaired two-tailed Student's t test).
FIGURE 4.
Effects of PTPϵ and constitutively active Y527F Src on Kv1.1 K+ currents expressed in CHO cells and Kv1.1 phosphotyrosine levels in transfected HEK 293 cells and in brain cortices of WT and EKO mice. A, shown are representative whole-cell K+ currents from cells transfected with Kv1.1 (left), Kv1.1 + Y527F Src (middle), Kv1.1+Y527F Src + PTPϵ (right). From a holding potential of −90 mV, cells were stepped from −40 to +40 mV in +10-mV increments for 1.5-s pulse duration. B, shown are current density-voltage relations of Kv1.1 (squares), Kv1.1+Y527F Src (triangles), and Kv1.1+Y527F Src + PTPϵ (circles) (*, the current density of Kv1.1+Y527F Src + PTPϵ was significantly different from that of Kv1.1; n = 5–11, p < 0.01; unpaired two-tailed Student's t test). C, crude lysates of HEK 293 cells transfected with Kv1.1, Kv1.1+Y527F Src, and Kv1.1+Y527F Src + PTPϵ were subjected to immunoprecipitation (IP) with anti-Kv1.1 antibodies, and immune complexes were Western-blotted (WB) with anti-phosphotyrosine antibodies (top) and with anti-Kv1.1 antibodies for input comparison (bottom). D, a bar diagram shows the relative tyrosine phosphorylation of Kv1.1, Kv1.1+Y527F Src, and Kv1.1+Y527F Src+PTPϵ, expressed as % of phosphorylation of Kv1.1 (*, the tyrosine phosphorylation level of Kv1.1 channels was significantly increased in the presence of Y527F Src, n = 4; p < 0.05; unpaired two-tailed Student's t test). E, crude lysates of brain cortices from 2-day-old and 3-month-old WT and EKO mice were subjected to immunoprecipitation with anti-Kv1.1 antibodies, and immune complexes were Western-blotted with anti-phosphotyrosine antibodies (top) and with anti-Kv1.1 antibodies for input comparison (bottom). F, a bar diagram shows Kv1.1 relative phosphorylation in EKO mice relative to WT. (*, the tyrosine phosphorylation level of Kv1.1 channels was significantly increased in cortices from 2-day-old and 3-month-old EKO mice compared with that from WT; n = 4, p < 0.05; unpaired two-tailed Student's t test).
FIGURE 5.
Effects of PTPϵ and constitutively active Y527F Src on Kv7.2/3 K+ currents expressed in CHO cells and Kv7.3 phosphotyrosine levels in transfected HEK 293 cells and in brain cortices of WT and EKO mice. A, shown are representative whole-cell K+ currents from cells transfected with Kv7.2/3 (left), Kv7.2/3 + Y527F Src (middle), Kv7.2/3+Y527F Src + PTPϵ (right). From a holding potential of −90 mV, cells were stepped from −60 to +40 mV in +10-mV increments for 1.5-s pulse duration. B and C, current density-voltage relations and normalized conductance-voltage relations, respectively, of Kv7.2/3 (squares), Kv7.2/3+Y527F Src (triangles), and Kv7.2/3+Y527F Src + PTPϵ (circles) (*, the current density of Kv7.3+Y527F Src was significantly different from that of Kv7.3, and the current density of Kv7.2/3+Y527F Src+PTPϵ was significantly different from that of Kv7.2/3+Y527F Src; n = 11–13, p < 0.01; unpaired two-tailed Student's t test). D, crude lysates of HEK 293 cells transfected with Kv7.3, Kv7.3+Y527F Src, and Kv7.3+Y527F Src + PTPϵ were subjected to immunoprecipitation (IP) with anti-Kv7.3 antibodies, and immune complexes were Western-blotted (WB) with anti-phosphotyrosine antibodies (top) and with anti-Kv7.3 antibodies for input comparison (bottom). E, a bar diagram shows the relative tyrosine phosphorylation of Kv7.3, Kv7.3+Y527F Src, and Kv7.3+Y527F Src+PTPϵ, expressed as % of phosphorylation of Kv7.3 (*, the tyrosine phosphorylation level of Kv7.3 channels was significantly increased in the presence of Y527F Src, n = 4; p < 0.05; unpaired two-tailed Student's t test). F, crude lysates of brain cortices from 2-day-old and 3-month-old WT and EKO mice were subjected to immunoprecipitation with anti-Kv7.3 antibodies, and immune complexes were Western-blotted with anti-phosphotyrosine antibodies (top) and with anti-Kv7.3 antibodies for input comparison (bottom). G, a bar diagram shows Kv7.3 relative phosphorylation in EKO mice relative to WT. (*, the tyrosine phosphorylation level of Kv7.3 channels was significantly increased in cortices from 2-day-old and 3-month-old EKO mice compared with that from WT; n = 4, p < 0.05; unpaired two-tailed Student's t test).
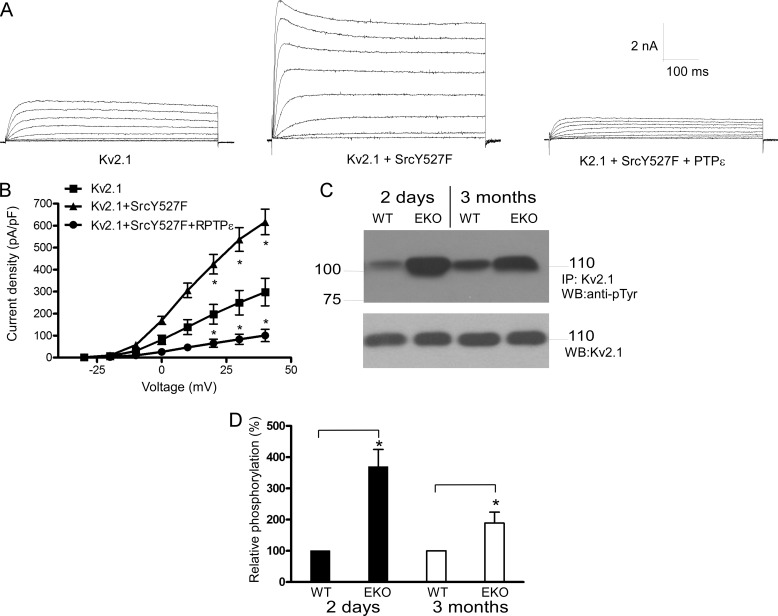
In contrast to Kv1.1, Kv1.2, and Kv7.2/3 channels, Y527F Src significantly enhanced Kv2.1 currents (206% increase at +40 mV; n = 12–15, p < 0.01) (Fig. 6, A and B). Cotransfection with PTPϵ not only counteracted the Src kinase-induced stimulation but also decreased the current density below the basal Kv2.1 levels (83% decrease at +40 mV; n = 12–15, p < 0.01) (Fig. 6, A and B). We previously showed that in HEK 293 cells Y527F Src significantly increases, whereas PTPϵ reduces the tyrosine phosphorylation of Kv2.1 (25, 27). Fig. 6, C and D, shows that the tyrosine phosphorylation level of Kv2.1 was markedly increased in cortices from neonatal and adult EKO mice compared with that from WT (3.7- and 1.9-fold increase for neonatal and adult, respectively; n = 4, p < 0.05). Thus, these results suggest that Kv2.1 channels are also reciprocally modulated by Src and PTPϵ but in an opposite manner to that of Kv1.1, Kv1.2, and Kv7.2/3 channels.
FIGURE 6.
Effects of PTPϵ and constitutively active Y527F Src on Kv2.1 K+ currents expressed in CHO cells and Kv2.1 phosphotyrosine levels in brain cortices of WT and EKO mice. A, shown is a representative whole-cell K+ currents from cells transfected with Kv2.1 (left), Kv2.1 + Y527F Src (middle), Kv2.1+Y527F Src + PTPϵ, (right). From a holding potential of −90 mV, cells were stepped from −30 to +40 mV in +10-mV increments for 1.5-s pulse duration. B, current density-voltage relations of Kv2.1 (squares), Kv2.1+Y527F Src (triangles), and Kv2.1+Y527F Src + PTPϵ (circles) (*, the current density of Kv2.1+Y527F Src was significantly different from that of Kv2.1, and the current density of Kv2.1+Y527F Src+PTPϵ was significantly different from that of Kv2.1+Y527F Src; n = 12–15, p < 0.01; unpaired two-tailed Student's t test). C, crude lysates of brain cortices from 2-day-old and 3-month-old WT and EKO mice were subjected to immunoprecipitation with anti-Kv2.1 antibodies, and immune complexes were Western blotted with anti-phosphotyrosine antibodies (top) and with anti-Kv2.1 antibodies for input comparison (bottom). D, bar diagram showing Kv2.1 relative phosphorylation in EKO mice relative to WT. *, the tyrosine phosphorylation level of Kv2.1 channels was significantly increased in cortices from 2-day-old and 3-month-old EKO mice compared to that from WT; n = 4, p < 0.05; unpaired two-tailed Student t-test.
Plausible Molecular Correlate of the Fast AHP and Spike Shortening
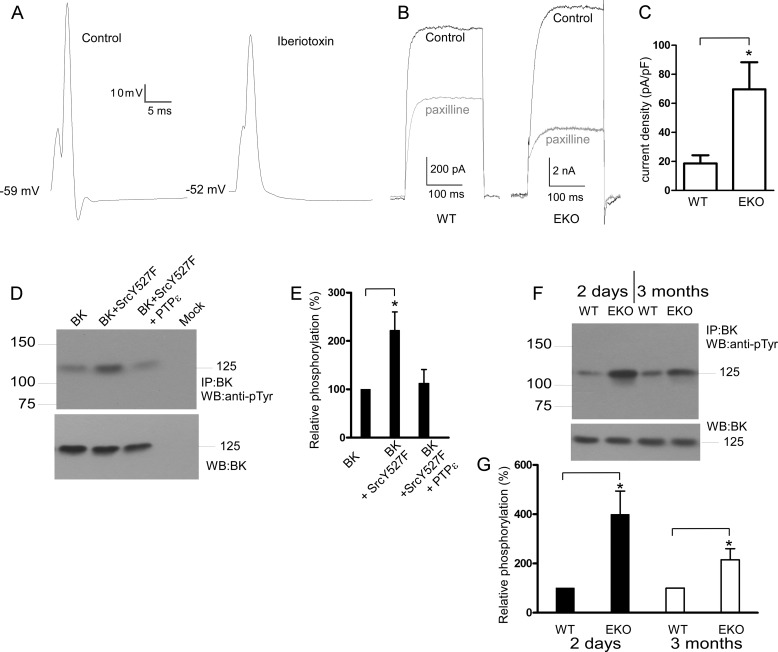
The large conductance Ca2+-activated K+ (BK) channels play an important role in controlling neuronal excitability (28, 29). The BK current is both Ca2+- and voltage-dependent and is thought to cause final spike repolarization of the action potential and fAHP (28, 30–32). BK channels are known targets of intracellular kinases and phosphatases (33, 34). Several studies have implicated tyrosine phosphorylation-dependent regulation of BK channels (35–38). However, the functional impact of BK channel tyrosine phosphorylation is diverse and species-dependent. Co-expression of active c-Src kinase with the mouse BK (mSlo or mBK) resulted in channel activation, whereas its expression with the human BK homolog (hSlo) produced an inhibition of BK channel activity (35–37). We hypothesized that the enhanced fAHP and the significant AP shortening observed in cultured cortical neurons from EKO mice (Fig. 1B and Table 1) may arise from a PTPϵ-dependent negative modulation of BK channels. If so, we expect the fAHP to be sensitive to BK channel blockers and an up-regulation of BK currents in the cortical neurons from EKO mice compared with WT. Fig. 7A shows that the fAHP of an evoked single spike in cortical neurons from EKO mice was suppressed by 100 nm of iberiotoxin (n = 6). Similar results were obtained with 1 μm paxilline (n = 6). In the voltage-clamp mode, we applied a step depolarization to +20 mV (with extracellular 1 mm 4-AP and 1 μm tetrodotoxin and 10 μm intracellular free Ca2+) in the absence and presence of 1 μm paxilline to measure BK currents, which were defined as paxilline-sensitive currents. The BK current density was markedly increased in cortical neurons from EKO mice compared with WT (70 ± 18 versus 19 ± 6 pA/pF for EKO and WT, respectively; n = 9, p < 0.03) (Fig. 7, B and C). In HEK 293 cells, Y527F Src increased by 2.2-fold tyrosine phosphorylation of mBK (Fig. 7, D and E; n = 4, p < 0.05), and cotransfection with PTPϵ inhibited the Src-induced increase in tyrosine phosphorylation of mBK. The tyrosine phosphorylation level of mBK was significantly increased in cortices from neonatal and adult EKO mice compared with that from WT (4- and 2.1-fold increase for neonatal and adult, respectively; n = 4, p < 0.05) (Fig. 7, F and G).
FIGURE 7.
Contribution of BK channels to fAHP, BK current density in cortical neurons and mBK phosphotyrosine levels in transfected HEK 293 cells and in brain cortices of WT and EKO mice. A, a typical current-clamp trace shows that the fAHP of an evoked single spike in cortical neurons from EKO mice was suppressed by 100 nm iberiotoxin (n = 6). B, shown is a typical voltage-clamp trace evoked by a step depolarization to +20 mV (with extracellular 1 mm 4-AP and 1 μm tetrodotoxin and 10 μm intracellular free Ca2+) in the absence and presence of 1 μm paxilline to measure BK currents in cortical neurons from WT and EKO mice. BK currents were defined as paxilline-sensitive currents. C, a bar graph shows that the BK current density was significantly increased in cortical neurons from EKO mice compared with WT (*, significantly different, n = 9, p < 0.03; unpaired two-tailed Student's t test). D, crude lysates of HEK 293 cells transfected with BK, BK+Y527F Src, and BK+Y527F Src + PTPϵ were subjected to immunoprecipitation (IP) with anti-mBK antibodies, and immune complexes were Western-blotted (WB) with anti-phosphotyrosine antibodies (top) and with anti-mBK antibodies for input comparison (bottom). E, a bar diagram shows the relative tyrosine phosphorylation of BK, BK+Y527F Src, and BK+Y527F Src+PTPϵ, expressed as % of phosphorylation of BK (*, the tyrosine phosphorylation level of BK channels was significantly increased in the presence of Y527F Src, n = 4; p < 0.05; unpaired two-tailed Student's t test). F, crude lysates of brain cortices from 2-day-old and 3-month-old WT and EKO mice were subjected to immunoprecipitation with anti-mBK antibodies, and immune complexes were Western-blotted with anti-phosphotyrosine antibodies (top) and with anti-mBK antibodies for input comparison (bottom). G, a bar diagram shows BK relative phosphorylation in EKO mice relative to WT (*, the tyrosine phosphorylation level of BK channels was significantly increased in cortices from 2-day-old and 3-month-old EKO mice compared with that from WT; n = 4, p < 0.05; unpaired two-tailed Student's t test).
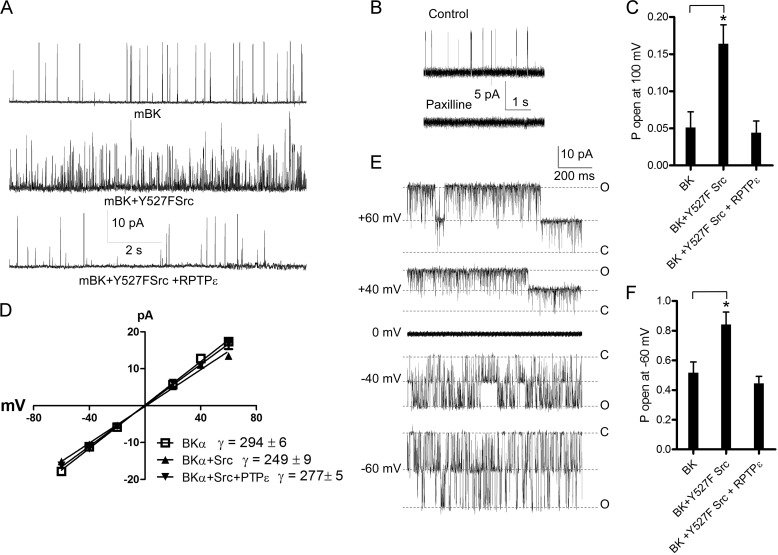
Then we investigated the effect of PTPϵ on the open probability of mBK channels in transfected CHO cells. Using the cell-attached and inside-out configurations of the patch clamp technique, we measured in CHO cells the unitary currents and open probability of mBK channels cotransfected with Y527F Src in the absence and presence of PTPϵ (Fig. 8). In the cell-attached patches, Y527F Src kinase stimulated by 3.2-fold the Po of mBK measured at +100 mV, a feature in line with previous studies (36–39) (Fig. 8, A and C; from Po = 0.052 ± 0.020 to Po = 0.164 ± 0.025; n = 5–6, p < 0.05). Interestingly, PTPϵ coexpression completely reversed the Src kinase-induced increase in Po and even reduced it below the control level, suggesting that mBK channels exhibit some basal level of tyrosine phosphorylation. Single BK channel activity was totally suppressed by 1 μm paxilline (Fig. 8B). However, these cell-attached recordings were measured under quasi-physiological K+ gradient and at low Po. Thus, we repeated the same experiments using inside-out patches under symmetrical high K+ solutions containing 10 μm free Ca2+ to increase the Po and observe the currents in both directions. As for cell-attached patches, Y527F Src kinase stimulated by more than 1.6-fold the Po of mBK measured at −60 mV (from Po = 0.52 ± 0.07 to Po = 0.84 ± 0.08; n = 6–7, p < 0.05), and PTPϵ coexpression completely reversed the Src kinase-induced increase in Po (Fig. 8, E and F). Comparable unitary current-voltage relations and especially unitary slope conductances were observed after Src kinase and PTPϵ coexpression as compared with control conditions (Fig. 8D; γ = 294 ± 6 picosiemens (pS), γ = 249 ± 9 pS, and γ = 277 ± 5 pS for mBK, mBK+Y527F Src, and mBK+Y527F Src +PTPϵ, respectively; n = 6–7).
FIGURE 8.
Effects of PTPϵ and constitutively active Y527F Src on mBK K+ currents expressed in CHO cells. A, shown are representative single channel current traces recorded from cell-attached patches of CHO cells transfected with mBK (top), mBK+Y527F Src (middle), and mBK +Y527F Src + PTPϵ, (bottom). The traces are displayed at a potential of +100 mV evoked from cell resting potential of 0 mV. The K+ concentrations in the pipette and the bath were 4 and 150 mm, respectively. B, the single channel activity in a CHO cell expressing mBK was totally suppressed by 1 μm paxilline. C, open probability at +100 mV was obtained from cell-attached patches of CHO cells expressing mBK, mBK + Y527F Src, and mBK +Y527F Src + PTPϵ (*, the open probability of cell-attached patches expressing mBK + Y527F Src was significantly higher than that of cell-attached patches expressing mBK; n = 5–6; p < 0.05; unpaired two-tailed Student's t test). D, shown are unitary current-voltage relations produced by inside-out patches from CHO cells transfected with mBK (empty squares), mBK + Y527FSrc (upward triangles), and Kv2.1+ Y527FSrc + PTPϵ (downward triangles) (n = 6–7). The straight line corresponds to the fit of data points. E, shown are representative single channel current traces recorded from an inside-out patch containing two channels in a CHO cell transfected with mBK. The inward and outward traces correspond to currents obtained at the indicated potentials. The closed (C) and open (O) channel levels are indicated. F, shown is open probability at −60 mV obtained from inside-out patches of CHO cells expressing mBK, mBK + Y527F Src, and mBK +Y527F Src + PTPϵ (*, the open probability of inside-out patches expressing mBK + Y527F Src was significantly higher than that of inside-out patches expressing mBK; n = 6–7; p < 0.05; unpaired two-tailed Student's t test).
DISCUSSION
This study describes the multi-faceted modulation of K+ channels by PTPϵ that affects cortical neuron excitability. Cultured cortical neurons from EKO mice exhibit increased excitability compared with WT, with reduced rheobase current and IK density, larger evoked spike discharge frequency and BK current density, increased ADP and fAHP and spike shortening.
The current density of IK but not that of IA was significantly reduced, suggesting that PTPϵ positively modulates delayed-rectifier Kv channels. Thus, it is reasonable to assume that the decreased IK density detected in EKO cortical neurons contributes to the enhanced excitability. Along this line, our results in transfected CHO cells show that Kv1.1, Kv1.2, and Kv7.2/7.3 are negatively modulated by the constitutively active mutant of Src kinase (Y527F Src), whereas Kv2.1 is positively regulated, in agreement with previous studies (13, 16, 19, 21–25, 27, 40). PTPϵ counteracts the modulation seen with Y527F Src in CHO cells and thereby positively regulates Kv1.1, Kv1.2, and Kv7.2/7.3 channel activities while depressing Kv2.1 currents. Although Kv2.1 subunits are a major component of the somatodendritic IK in cortical and hippocampal pyramidal neurons (41–43), Kv1.1, Kv1.2, and Kv7.2/7.3 are the plausible Kv channel correlates accounting for the decreased IK density observed in EKO cortical pyramidal neurons. In fact, the net 30% decrease in IK density likely corresponds to the result of two opposite modulations: a PTPϵ-mediated positive modulation of Kv1.1, Kv1.2, and Kv7.2/7.3 and a PTPϵ-mediated negative modulation of Kv2.1. This dual modulation of PTPϵ implies that in EKO mice the decreased activity (∼30%) involving Kv1.1, Kv1.2, and Kv7.2/7.3 channels is probably underestimated because of the PTPϵ-mediated negative modulation of Kv2.1.
The regulatory mechanisms of PTPs and protein-tyrosine kinases are complex. In fact, tyrosine phosphatases can regulate tyrosine kinases by activating or inhibiting them. For example, PTPϵ activates Src kinase via dephosphorylation, thereby promoting a Src-related phenotype of mammary epithelial tumor and in osteoclasts (44, 45). This mechanism, however, does not operate in cortical neurons and in CHO cells used in this study, because PTPϵ and Src exerted opposite effects on the channels examined. The mechanisms of K+ channel modulation by PTPϵ are not clear yet. The effect of phosphorylation may involve changes in channel trafficking. For example, it is known that the phosphorylation status of Kv2.1 channels affect their trafficking and localization in neurons (42). PTPϵ could modulate the channel activity by an indirect mechanism involving dephosphorylation of an unknown regulatory protein. Alternatively, the modulation of Kv1.1, Kv1.2, Kv7.2/7.3, and mBK by PTPϵ may be mediated via direct channel dephosphorylation of tyrosine residues. Interestingly, we found the levels of tyrosine phosphorylation of Kv1.1, Kv1.2, Kv7. 3, and mBK channel proteins to be significantly increased in the brain cortices of both neonatal and adult EKO mice as compared with WT. Our data suggest that the lack of PTPϵ in EKO mice leads to channel hyperphosphorylation, and this effect is retained into adulthood. This channel hyperphosphorylation may in turn decrease the current density of Kv.1, Kv1.2, and Kv7.2/3 and enhance the mBK channel activity. Yet further studies are needed to clarify the mechanisms involved.
The action potentials generated by cultured EKO cortical neurons show enhanced ADP. The ADP is a depolarizing envelope that follows the action potential repolarization (31). ADP is a robust mechanism for driving bursting (46–48). Experimental and modeling analyses have shown that in adult CA1 pyramidal neurons, the persistent Na+ current (INaP) in the perisomatic region is the predominant inward current generating the active ADP (46, 49–51). R-type Ca2+ currents were also shown to contribute to the ADP both from the somatic compartment (48, 52) and from the apical dendrite of CA1 pyramidal neurons (53), where they can be activated by back-propagating action potentials. The ionic contribution to the ADP is not only determined by inward currents but is also shaped by outward K+ currents. The depolarizing action of INaP is counteracted primarily by perisomatic M-type K+ current, encoded by Kv7.2/Kv7.3 (KCNQ2/3) subunits (54–58). This current prevents the escalation of the ADP into a spike burst (57). Interestingly, in apical dendrites of CA1 pyramidal neurons, recruitment of D-type K+ currents encoded by delayed-rectifier Kv1 K+ channels were recently shown to inhibit action potential bursting by restricting the size of the ADP (59). In this study the enhanced ADP observed in EKO mice may arise from a PTPϵ-dependent modulating effect on one or more ionic conductances contributing to the ADP. The most obvious possibility is that under normal conditions PTPϵ counteracts the depressing effect of tyrosine kinases such as Src on M-type and D-type delayed-rectifier K+ currents encoded, respectively, by Kv7.2/Kv7.3 and by Kv1 potassium channels. In the absence of PTPϵ, these channels are hyperphosphorylated at tyrosine residues and show reduced activity. This scenario would then weaken the outward currents and thereby lead to enhanced ADP.
The action potentials produced by cultured EKO cortical neurons show enhanced fAHP and spike shortening. The fAHP, which activates rapidly and typically lasts 1–10 ms, is mainly mediated by BK-type calcium-activated potassium channels, which are also responsible for action potential repolarization (60). We showed that BK channel blockers such as iberiotoxin and paxilline suppress the fAHP in cortical neurons from EKO. Our data suggest that the enhanced fAHP observed in EKO mice is due to the lack of a PTPϵ-dependent negative modulation of mBK channels. Consistent with this assumption, PTPϵ significantly opposes the Src-mediated increase in the Po of mBK channels expressed in CHO cells. In addition, the tyrosine phosphorylation level of mBK channels is significantly higher in the brain cortices from EKO mice compared with WT. This result suggests that the observed PTPϵ-dependent negative modulation of mBK channels is at least partially mediated by a channel dephosphorylation mechanism. Thus, it seems likely that the mBK channel activity is higher in cortical neurons lacking PTPϵ, which leads to enhanced fAHP and spike shortening. Consistent with this idea, we found that the BK current density is increased in cortical neurons from EKO mice compared with WT. Previous reports already showed that the activity of BK channels could be modulated via phosphorylation by the prototypical tyrosine kinase c-Src (35–37). Notably, depending on the BK channel species, the Src-tyrosine kinase was found to modulate the channel activity in opposite direction. Thus, it was shown that c-Src kinase can enhance mBK channel activity in a calcium-dependent manner and that mBK channels undergo tyrosine phosphorylation (37). In contrast, it was found that agonist-induced vasoconstriction by serotonin, angiotensin II, and phenylephrine involves inhibition of human BK channels (hSlo) by c-Src via direct phosphorylation of the channel protein (35).
How can both reduction of IK density and enhanced fAHP contribute to increased firing frequency? It is not difficult to envision why a decreased density of IK would lead to an increased neuronal excitability. Indeed, genes encoding for typical IK such as Kv1.1 or Kv1.2 are known to contribute to low threshold voltage-activated potassium currents, which powerfully limits excitability. Kcna1-null mice lacking Kv1.1 exhibit seizure susceptibility and hyperexcitability (61). Similarly, Kcna2-null mice lacking Kv1.2 have reduced lifespans and exhibit spontaneous generalized seizures (62). In contrast, the notion that BK channels can lead to increased excitability may appear counterintuitive knowing the classical function of potassium channels in reducing membrane excitability. Neuronal BK channels are activated by coincident depolarization and increase in intracellular Ca2+ generated by opening of voltage-gated Ca2+ channel during the action potential spike (60). Depending on the neuronal circuit, BK channels are shown to either increase or decrease excitability (63, 64). According to their gating kinetics, Ca2+ dynamics, and pharmacological profile, two types of BK channels could be distinguished, fast-gated type I and slow-gated type II BK channels (28, 65). Recent experimental and simulation studies indicate that the fast-gated type I (α subunits) BK channels can better follow the time course of the action potential and are associated with the lack of β4 accessory subunits, thereby leading to spike shortening and large fAHP. The slower kinetics provided by the β4 subunit that endows the type II BK channel (αβ4 subunits) leads to broadening of action potentials and, secondarily, to greater recruitment of SK channels and reduction of neuronal excitability (65). Thus, fast-gated type I BK channels both repolarizes the action potential and quickly resets the membrane potential, supporting higher firing rates. Interestingly, type I BK channels were found responsible for epileptic bursting behavior in cortical neurons, which was blocked by iberiotoxin (66). Similarly, a gain-of-function mutation of the α subunit of BK channels in humans was found to be linked to a syndrome of generalized epilepsy with paroxysmal movement disorders (67). More recently, β4 KO mice showed epileptic seizures emanating from the temporal cortex. In these β4 KO mice, hippocampal granule cells demonstrate a gain-of-function for BK channels that sharpens action potentials and supports higher firing rates (68). In line with this channel behavior, our data showing enhanced fAHP, AP shortening, and higher spike discharge rate suggest that in EKO cortical neurons BK channels are of type I, probably lacking β4 subunits. Furthermore, in EKO cortical neurons the lack of a PTPϵ-mediated negative channel modulation likely leads to a gain of type I BK channel function.
Although cortical neurons from EKO mice exhibit increased excitability, it is important to emphasize that EKO mice neither exhibit spontaneous epileptic seizures nor show an increased sensitivity to electroshock-induced seizures in vivo. It is possible that the increased excitability observed in EKO cortical neurons is such that at the level of the whole brain circuitry, it is not drastic enough to produce an epileptic phenotype. In addition, unknown compensatory mechanisms may also prevent the EKO mice from developing seizures. Nevertheless, our data indicate that PTPϵ plays a role in fine-tuning cortical neuron excitability through its multifaceted modulation of K+ channels.
Acknowledgments
We thank Dr. David L. Armstrong for the gift of the mBK channel clone, Dr. Thomas Jentsch for the human clones of Kv7.2 and Kv7.3, and Dr. Nataly Menaker for excellent technical assistance.
This work was supported by the Deutsch-Israelische Projektkooperation DIP fund to B.A.
- PTP
- non-receptor protein-tyrosine phosphatase
- IK
- delayed-rectifier K+ channels
- IA
- transient A-type K+ channel
- EKO
- PTPϵ knockout
- ADP
- after-depolarization
- fAHP
- fast after-hyperpolarization
- Kv
- voltage-gated K+ channels
- mBK
- mouse large conductance Ca2+-activated K+ channels
- AP
- action potential
- RPTP
- receptor protein-tyrosine phosphatase
- pF
- picofarads
- MΩ
- megaohms.
REFERENCES
- 1. Jonas E. A., Kaczmarek L. K. (1996) Regulation of potassium channels by protein kinases. Curr. Opin. Neurobiol. 6, 318–323 [DOI] [PubMed] [Google Scholar]
- 2. Levitan I. B. (1994) Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu. Rev. Physiol. 56, 193–212 [DOI] [PubMed] [Google Scholar]
- 3. Grant S. G., O'Dell T. J., Karl K. A., Stein P. L., Soriano P., Kandel E. R. (1992) Impaired long term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science 258, 1903–1910 [DOI] [PubMed] [Google Scholar]
- 4. Lu Y. M., Roder J. C., Davidow J., Salter M. W. (1998) Src activation in the induction of long term potentiation in CA1 hippocampal neurons. Science 279, 1363–1367 [DOI] [PubMed] [Google Scholar]
- 5. Petrone A., Battaglia F., Wang C., Dusa A., Su J., Zagzag D., Bianchi R., Casaccia-Bonnefil P., Arancio O., Sap J. (2003) Receptor protein-tyrosine phosphatase α is essential for hippocampal neuronal migration and long term potentiation. EMBO J. 22, 4121–4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uetani N., Kato K., Ogura H., Mizuno K., Kawano K., Mikoshiba K., Yakura H., Asano M., Iwakura Y. (2000) Impaired learning with enhanced hippocampal long term potentiation in PTPδ-deficient mice. EMBO J. 19, 2775–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahn M., Beacham D., Westenbroek R. E., Scheuer T., Catterall W. A. (2007) Regulation of Na(v)1.2 channels by brain-derived neurotrophic factor, TrkB, and associated Fyn kinase. J. Neurosci. 27, 11533–11542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fadool D. A., Holmes T. C., Berman K., Dagan D., Levitan I. B. (1997) Tyrosine phosphorylation modulates current amplitude and kinetics of a neuronal voltage-gated potassium channel. J. Neurophysiol. 78, 1563–1573 [DOI] [PubMed] [Google Scholar]
- 9. Ratcliffe C. F., Qu Y., McCormick K. A., Tibbs V. C., Dixon J. E., Scheuer T., Catterall W. A. (2000) A sodium channel signaling complex. Modulation by associated receptor protein-tyrosine phosphatase β. Nat. Neurosci. 3, 437–444 [DOI] [PubMed] [Google Scholar]
- 10. Rudy B., McBain C. J. (2001) Kv3 channels. Voltage-gated K+ channels designed for high frequency repetitive firing. Trends Neurosci. 24, 517–526 [DOI] [PubMed] [Google Scholar]
- 11. Wang Y. T., Salter M. W. (1994) Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature 369, 233–235 [DOI] [PubMed] [Google Scholar]
- 12. Wang Y. T., Yu X. M., Salter M. W. (1996) Ca2+-independent reduction of N-methyl-d-aspartate channel activity by protein-tyrosine phosphatase. Proc. Natl. Acad. Sci. U.S.A. 93, 1721–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsai W., Morielli A. D., Cachero T. G., Peralta E. G. (1999) Receptor protein-tyrosine phosphatase α participates in the m1 muscarinic acetylcholine receptor-dependent regulation of Kv1.2 channel activity. EMBO J. 18, 109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Imbrici P., Tucker S. J., D'Adamo M. C., Pessia M. (2000) Role of receptor protein-tyrosine phosphatase α (RPTPα) and tyrosine phosphorylation in the serotonergic inhibition of voltage-dependent potassium channels. Pflugers Arch. 441, 257–262 [DOI] [PubMed] [Google Scholar]
- 15. Fadool D. A., Levitan I. B. (1998) Modulation of olfactory bulb neuron potassium current by tyrosine phosphorylation. J. Neurosci. 18, 6126–6137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holmes T. C., Fadool D. A., Ren R., Levitan I. B. (1996) Association of Src-tyrosine kinase with a human potassium channel mediated by SH3 domain. Science 274, 2089–2091 [DOI] [PubMed] [Google Scholar]
- 17. Szabò I., Gulbins E., Apfel H., Zhang X., Barth P., Busch A. E., Schlottmann K., Pongs O., Lang F. (1996) Tyrosine phosphorylation-dependent suppression of a voltage-gated K+ channel in T lymphocytes upon Fas stimulation. J. Biol. Chem. 271, 20465–20469 [DOI] [PubMed] [Google Scholar]
- 18. Nitabach M. N., Llamas D. A., Thompson I. J., Collins K. A., Holmes T. C. (2002) Phosphorylation-dependent and phosphorylation-independent modes of modulation of shaker family voltage-gated potassium channels by SRC family protein-tyrosine kinases. J. Neurosci. 22, 7913–7922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gamper N., Stockand J. D., Shapiro M. S. (2003) Subunit-specific modulation of KCNQ potassium channels by Src-tyrosine kinase. J. Neurosci. 23, 84–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jia Q., Jia Z., Zhao Z., Liu B., Liang H., Zhang H. (2007) Activation of epidermal growth factor receptor inhibits KCNQ2/3 current through two distinct pathways. Membrane PtdIns(4,5)P2 hydrolysis and channel phosphorylation. J. Neurosci. 27, 2503–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Y., Langlais P., Gamper N., Liu F., Shapiro M. S. (2004) Dual phosphorylations underlie modulation of unitary KCNQ K+ channels by Src-tyrosine kinase. J. Biol. Chem. 279, 45399–45407 [DOI] [PubMed] [Google Scholar]
- 22. Peretz A., Sobko A., Attali B. (1999) Tyrosine kinases modulate K+ channel gating in mouse Schwann cells. J. Physiol. 519, 373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sobko A., Peretz A., Attali B. (1998) Constitutive activation of delayed-rectifier potassium channels by a src family tyrosine kinase in Schwann cells. EMBO J. 17, 4723–4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peretz A., Gil-Henn H., Sobko A., Shinder V., Attali B., Elson A. (2000) Hypomyelination and increased activity of voltage-gated K+ channels in mice lacking protein-tyrosine phosphatase ϵ. EMBO J. 19, 4036–4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tiran Z., Peretz A., Sines T., Shinder V., Sap J., Attali B., Elson A. (2006) Tyrosine phosphatases ϵ and α perform specific and overlapping functions in regulation of voltage-gated potassium channels in Schwann cells. Mol. Biol. Cell 17, 4330–4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. (1981) Improved patch clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391, 85–100 [DOI] [PubMed] [Google Scholar]
- 27. Tiran Z., Peretz A., Attali B., Elson A. (2003) Phosphorylation-dependent regulation of Kv2.1 channel activity at tyrosine 124 by Src and by protein-tyrosine phosphatase ϵ. J. Biol. Chem. 278, 17509–1751412615930 [Google Scholar]
- 28. Fakler B., Adelman J. P. (2008) Control of K(Ca) channels by calcium nano/microdomains. Neuron 59, 873–881 [DOI] [PubMed] [Google Scholar]
- 29. Latorre R., Brauchi S. (2006) Large conductance Ca2+-activated K+ (BK) channel. Activation by Ca2+ and voltage. Biol. Res. 39, 385–401 [DOI] [PubMed] [Google Scholar]
- 30. Adams P. R., Constanti A., Brown D. A., Clark R. B. (1982) Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature 296, 746–749 [DOI] [PubMed] [Google Scholar]
- 31. Storm J. F. (1987) Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J. Physiol. 385, 733–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Womack M. D., Khodakhah K. (2002) Characterization of large conductance Ca2+-activated K+ channels in cerebellar Purkinje neurons. Eur. J. Neurosci. 16, 1214–1222 [DOI] [PubMed] [Google Scholar]
- 33. Chung S. K., Reinhart P. H., Martin B. L., Brautigan D., Levitan I. B. (1991) Protein kinase activity closely associated with a reconstituted calcium-activated potassium channel. Science 253, 560–562 [DOI] [PubMed] [Google Scholar]
- 34. Reinhart P. H., Chung S., Martin B. L., Brautigan D. L., Levitan I. B. (1991) Modulation of calcium-activated potassium channels from rat brain by protein kinase A and phosphatase 2A. J. Neurosci. 11, 1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alioua A., Mahajan A., Nishimaru K., Zarei M. M., Stefani E., Toro L. (2002) Coupling of c-Src to large conductance voltage- and Ca2+-activated K+ channels as a new mechanism of agonist-induced vasoconstriction. Proc. Natl. Acad. Sci. U.S.A. 99, 14560–14565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ling S., Sheng J. Z., Braun A. P. (2004) The calcium-dependent activity of large conductance, calcium-activated K+ channels is enhanced by Pyk2- and Hck-induced tyrosine phosphorylation. Am. J. Physiol. Cell Physiol. 287, C698–C706 [DOI] [PubMed] [Google Scholar]
- 37. Ling S., Woronuk G., Sy L., Lev S., Braun A. P. (2000) Enhanced activity of a large conductance, calcium-sensitive K+ channel in the presence of Src-tyrosine kinase. J. Biol. Chem. 275, 30683–30689 [DOI] [PubMed] [Google Scholar]
- 38. Tian L., McClafferty H., Chen L., Shipston M. J. (2008) Reversible tyrosine protein phosphorylation regulates large conductance voltage- and calcium-activated potassium channels via cortactin. J. Biol. Chem. 283, 3067–3076 [DOI] [PubMed] [Google Scholar]
- 39. Zhou R., Liu L., Hu D. (2005) Involvement of BKCa α subunit tyrosine phosphorylation in vascular hyporesponsiveness of superior mesenteric artery following hemorrhagic shock in rats. Cardiovasc. Res. 68, 327–335 [DOI] [PubMed] [Google Scholar]
- 40. Huang X. Y., Morielli A. D., Peralta E. G. (1993) Tyrosine kinase-dependent suppression of a potassium channel by the G protein-coupled m1 muscarinic acetylcholine receptor. Cell 75, 1145–1156 [DOI] [PubMed] [Google Scholar]
- 41. Du J., Haak L. L., Phillips-Tansey E., Russell J. T., McBain C. J. (2000) Frequency-dependent regulation of rat hippocampal somato-dendritic excitability by the K+ channel subunit Kv2.1. J. Physiol. 522, 19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Misonou H., Mohapatra D. P., Trimmer J. S. (2005) Kv2.1, a voltage-gated K+ channel critical to dynamic control of neuronal excitability. Neurotoxicology 26, 743–752 [DOI] [PubMed] [Google Scholar]
- 43. Murakoshi H., Trimmer J. S. (1999) Identification of the Kv2.1 K+ channel as a major component of the delayed rectifier K+ current in rat hippocampal neurons. J. Neurosci. 19, 1728–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gil-Henn H., Elson A. (2003) Tyrosine phosphatase-ϵ activates Src and supports the transformed phenotype of Neu-induced mammary tumor cells. J. Biol. Chem. 278, 15579–15586 [DOI] [PubMed] [Google Scholar]
- 45. Granot-Attas S., Luxenburg C., Finkelshtein E., Elson A. (2009) Protein-tyrosine phosphatase ϵ regulates integrin-mediated podosome stability in osteoclasts by activating Src. Mol. Biol. Cell 20, 4324–4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Azouz R., Jensen M. S., Yaari Y. (1996) Ionic basis of spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J. Physiol. 492, 211–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Magee J. C., Carruth M. (1999) Dendritic voltage-gated ion channels regulate the action potential firing mode of hippocampal CA1 pyramidal neurons. J. Neurophysiol. 82, 1895–1901 [DOI] [PubMed] [Google Scholar]
- 48. Metz A. E., Jarsky T., Martina M., Spruston N. (2005) R-type calcium channels contribute to after depolarization and bursting in hippocampal CA1 pyramidal neurons. J. Neurosci. 25, 5763–5773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Golomb D., Yue C., Yaari Y. (2006) Contribution of persistent Na+ current and M-type K+ current to somatic bursting in CA1 pyramidal cells. Combined experimental and modeling study. J. Neurophysiol. 96, 1912–1926 [DOI] [PubMed] [Google Scholar]
- 50. Su H., Alroy G., Kirson E. D., Yaari Y. (2001) Extracellular calcium modulates persistent sodium current-dependent burst-firing in hippocampal pyramidal neurons. J. Neurosci. 21, 4173–4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yue C., Remy S., Su H., Beck H., Yaari Y. (2005) Proximal persistent Na+ channels drive spike after depolarizations and associated bursting in adult CA1 pyramidal cells. J. Neurosci. 25, 9704–9720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen S., Yue C., Yaari Y. (2005) A transitional period of Ca2+-dependent spike afterdepolarization and bursting in developing rat CA1 pyramidal cells. J. Physiol. 567, 79–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Christie B. R., Eliot L. S., Ito K., Miyakawa H., Johnston D. (1995) Different Ca2+ channels in soma and dendrites of hippocampal pyramidal neurons mediate spike-induced Ca2+ influx. J. Neurophysiol. 73, 2553–2557 [DOI] [PubMed] [Google Scholar]
- 54. Hu H., Vervaeke K., Storm J. F. (2007) M-channels (Kv7/KCNQ channels) that regulate synaptic integration, excitability, and spike pattern of CA1 pyramidal cells are located in the perisomatic region. J. Neurosci. 27, 1853–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Peretz A., Sheinin A., Yue C., Degani-Katzav N., Gibor G., Nachman R., Gopin A., Tam E., Shabat D., Yaari Y., Attali B. (2007) Pre- and postsynaptic activation of M-channels by a novel opener dampens neuronal firing and transmitter release. J. Neurophysiol. 97, 283–295 [DOI] [PubMed] [Google Scholar]
- 56. Shah M. M., Migliore M., Valencia I., Cooper E. C., Brown D. A. (2008) Functional significance of axonal Kv7 channels in hippocampal pyramidal neurons. Proc. Natl. Acad. Sci. U.S.A. 105, 7869–7874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yue C., Yaari Y. (2004) KCNQ/M channels control spike after depolarization and burst generation in hippocampal neurons. J. Neurosci. 24, 4614–4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yue C., Yaari Y. (2006) Axo-somatic and apical dendritic Kv7/M channels differentially regulate the intrinsic excitability of adult rat CA1 pyramidal cells. J. Neurophysiol. 95, 3480–3495 [DOI] [PubMed] [Google Scholar]
- 59. Metz A. E., Spruston N., Martina M. (2007) Dendritic D-type potassium currents inhibit the spike after depolarization in rat hippocampal CA1 pyramidal neurons. J. Physiol. 581, 175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Faber E. S., Sah P. (2003) Calcium-activated potassium channels. Multiple contributions to neuronal function. Neuroscientist 9, 181–194 [DOI] [PubMed] [Google Scholar]
- 61. Smart S. L., Lopantsev V., Zhang C. L., Robbins C. A., Wang H., Chiu S. Y., Schwartzkroin P. A., Messing A., Tempel B. L. (1998) Deletion of the K(V)1.1 potassium channel causes epilepsy in mice. Neuron 20, 809–819 [DOI] [PubMed] [Google Scholar]
- 62. Brew H. M., Gittelman J. X., Silverstein R. S., Hanks T. D., Demas V. P., Robinson L. C., Robbins C. A., McKee-Johnson J., Chiu S. Y., Messing A., Tempel B. L. (2007) Seizures and reduced life span in mice lacking the potassium channel subunit Kv1.2 but hypoexcitability and enlarged Kv1 currents in auditory neurons. J. Neurophysiol. 98, 1501–1525 [DOI] [PubMed] [Google Scholar]
- 63. Gu N., Vervaeke K., Storm J. F. (2007) BK potassium channels facilitate high frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J. Physiol. 580, 859–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Matthews E. A., Weible A. P., Shah S., Disterhoft J. F. (2008) The BK-mediated fAHP is modulated by learning a hippocampus-dependent task. Proc. Natl. Acad. Sci. U.S.A. 105, 15154–15159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jaffe D. B., Wang B., Brenner R. (2011) Shaping of action potentials by type I and type II large-conductance Ca2+-activated K+ channels. Neuroscience 192, 205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jin W., Sugaya A., Tsuda T., Ohguchi H., Sugaya E. (2000) Relationship between large conductance calcium-activated potassium channel and bursting activity. Brain Res. 860, 21–28 [DOI] [PubMed] [Google Scholar]
- 67. Du W., Bautista J. F., Yang H., Diez-Sampedro A., You S. A., Wang L., Kotagal P., Lüders H. O., Shi J., Cui J., Richerson G. B., Wang Q. K. (2005) Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat. Genet. 37, 733–738 [DOI] [PubMed] [Google Scholar]
- 68. Brenner R., Chen Q. H., Vilaythong A., Toney G. M., Noebels J. L., Aldrich R. W. (2005) BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat. Neurosci. 8, 1752–1759 [DOI] [PubMed] [Google Scholar]