Background: Accessory subunit Ac45 is an important regulator of the V-ATPase pump.
Results: Ac45 deletion mutants (involving its proteolytic cleavage site or luminal/cytoplasmic domains) affected Ac45 transport through the secretory pathway, V-ATPase trafficking, and Ca2+-dependent secretion.
Conclusion: Proper V-ATPase functioning requires Ac45 processing, and N- and C-terminal domains of Ac45.
Significance: Elucidation of structural requirements for Ac45 to act as V-ATPase regulator.
Keywords: Membrane Proteins, Protein Processing, Protein Secretion, Transgenic, Vacuolar ATPase, ATP6AP1, Xenopus, Neuroendocrine Cells, Regulated Secretory Pathway
Abstract
The vacuolar (H+)-ATPase (V-ATPase) is crucial for maintenance of the acidic microenvironment in intracellular organelles, whereas its membrane-bound V0-sector is involved in Ca2+-dependent membrane fusion. In the secretory pathway, the V-ATPase is regulated by its type I transmembrane and V0-associated accessory subunit Ac45. To execute its function, the intact-Ac45 protein is proteolytically processed to cleaved-Ac45 thereby releasing its N-terminal domain. Here, we searched for the functional domains within Ac45 by analyzing a set of deletion mutants close to the in vivo situation, namely in transgenic Xenopus intermediate pituitary melanotrope cells. Intact-Ac45 was poorly processed and accumulated in the endoplasmic reticulum of the transgenic melanotrope cells. In contrast, cleaved-Ac45 was efficiently transported through the secretory pathway, caused an accumulation of the V-ATPase at the plasma membrane and reduced dopaminergic inhibition of Ca2+-dependent peptide secretion. Surprisingly, removal of the C-tail from intact-Ac45 caused cellular phenotypes also found for cleaved-Ac45, whereas C-tail removal from cleaved-Ac45 still allowed its transport to the plasma membrane, but abolished V-ATPase recruitment into the secretory pathway and left dopaminergic inhibition of the cells unaffected. We conclude that domains located in the N- and C-terminal portions of the Ac45 protein direct its trafficking, V-ATPase recruitment and Ca2+-dependent-regulated exocytosis.
Introduction
The vacuolar (H+)-ATPase (V-ATPase)2 is a proton pump and its function is crucial for a broad range of biological processes such as membrane trafficking, receptor-mediated endocytosis, lysosomal protein degradation (1), embryonic left-right patterning (2), Wnt signaling during anterior-posterior patterning (3), and maintenance of the acid-base homeostasis (4). In intracellular organelles, such as lysosomes, secretory granules, and the yeast vacuole, the V-ATPase is the major regulator of the pH (2, 5). Moreover, the V-ATPase provides an electrochemical membrane potential that is required for yeast vacuole membrane fusion (6). In neuroendocrine cells, inhibition of the V-ATPase greatly affects neuroendocrine prohormone processing and regulated secretion (7, 8), and in neuronal cells evokes a deceleration in the kinetics of exocytosis and a reduction in the neurotransmitter content of the vesicles (9).
The V-ATPase consists of two sectors, namely the cytoplasmic V1-sector that takes care of ATP hydrolysis and the membrane-bound V0-sector that harbors the proteolipid by which protons are translocated (1). The V-ATPase complex displays V1-V0 sector dissociation, which most likely represents a universal mechanism for the regulation of its activity (10).
Apart from its function in proton pumping, also a V1-independent role for the V0-sector in post-SNARE membrane fusion has been found in yeast vacuoles (11), Drosophila neurons (12), mouse pancreatic β-cells (13), and during apical exosome secretion in Caenorhabditis elegans (14). The formation of the pore preceding membrane fusion is induced by the V0-sector and involves the small Ca2+-binding protein calmodulin (11, 15). The recent discovery of a direct interaction between the v-SNARE synaptobrevin and the V0-sector of the V-ATPase in rat neurons underscores the important role of V0 in post-SNARE membrane fusion and Ca2+-dependent neurotransmitter release (16). Furthermore, a separate function for the V0-sector in the biogenesis of dense-core granules in neuroendocrine cells has recently been proposed (17). Together, these studies show that the V0-sector is of great importance to the regulated secretory pathway, in particular for the process of Ca2+-dependent regulated secretion.
In specialized cell types such as osteoclasts (18–20), and neuroendocrine chromaffin and pituitary cells (21, 22) the V0-sector of the V-ATPase is equipped with an accessory subunit, namely the glycosylated type I transmembrane protein Ac45 (23). Using a transgenic approach in Xenopus neuroendocrine melanotrope cells, we recently identified the Ac45 protein as a crucial regulator of the V-ATPase in the regulated secretory pathway (24, 25). Extensive biosynthetic labeling studies revealed that in the early secretory pathway the intact 62-kDa Ac45 protein is proteolytically cleaved to a C-terminal Ac45 fragment of ∼40 kDa (21), representing the endogenous protein originally isolated from bovine chromaffin granules (26). The ∼20-kDa N-terminal cleavage fragment has been suggested to be degraded following its cleavage from the Ac45 precursor protein (27).
In this study, we explored the structural requirements for Ac45 to function as a regulator of the V-ATPase. We examined the importance of a number of domains within the Ac45 protein, including of its short cytoplasmic tail which harbors essential and autonomous routing information (28). Using the technique of stable Xenopus transgenesis (29), we expressed the mutants in their natural environment, namely specifically in Xenopus intermediate pituitary melanotrope cells. We found that the N-terminal as well as the C-terminal portion of the Ac45 protein is crucial for its functioning.
EXPERIMENTAL PROCEDURES
Animals
Xenopus laevis were reared in the Xenopus facility of the Department of Molecular Animal Physiology (Central Animal Facility, Radboud University Nijmegen). For transgenesis experiments, adult female X. laevis were directly obtained from South Africa (Africa Reptile Park, Muizenberg, South Africa). Experimental animals were adapted to a black background for at least 3 weeks with a light/dark cycle of 12 h. All animal experiments were carried out in accordance with the European Communities Council Directive 86/609/EEC for animal welfare, and permit RBD0166(H10) to generate and house transgenic X. laevis.
Generation of Xenopus Stably Transgenic for Ac45 Mutants Fused to GFP
Transgenic lines #530 (Ac45ΔCS: intact-Ac45 from which the proteolytic cleavage site has been deleted), #629 (Ac45ΔC: intact-Ac45 from which the C-tail has been removed), #651 (cleaved-Ac45ΔC: cleaved-Ac45 without its C-terminal tail), and #481 (Ac45Nterm: the naturally-occurring ∼20-kDa Ac45 N-terminal processing product) were generated by stable Xenopus transgenesis using the GFP-transgene constructs described below. The generation of transgenic Xenopus, F1 offspring and transgenic lines #452 (intact-Ac45) and #533 (cleaved-Ac45: the naturally-occurring C-terminal ∼40-kDa Ac45 protein), have been previously described and defined as GFP/intact-Ac45 and GFP/cleaved-Ac45, respectively (24). For the generation of the Ac45ΔCS mutant, SpeI restriction sites were first introduced into wild-type Xenopus Ac45 (clone X1311–4, Ref. 21) using the Quickchange Mutagenesis kit (Stratagene) and the primers: 5′-gaccaagcaattggacaaactagtagcacattaaagtcagagggtg-3′ and 5′-caccctctgactttaatgtgctactagtttgtccaattgcttggtc-3′ (mutation 1, nt 569–574 of clone X1311–4) and 5′-gcgccagttacttgccactactagtcctatgccaagctatcctcc-3′ and 5′-ggaggatagcttggcataggactagtagtggcaagtaact ggcgc-3′ (mutation 2, nt 695–700 of clone X1311–4). Following a SpeI digestion, the cDNA was re-ligated and amplified by PCR using forward primer 5′-gggggaattccagcaagtgcccgtgctg-3′ and reverse primer 5′-ggggtctagattactctgtctggggcacagc-3′. For obtaining cDNA encoding Ac45ΔC and cleaved-Ac45ΔC fused to GFP, the Xenopus Ac45 ORF (without the signal peptide) was amplified by PCR using the forward primer 5′-gggggaattccagcaagtgcccgtgctg-3′ or forward primer 5′-gggggaattccctatgccaagctatcctcc-3′, respectively, with reverse primer 5′-ggggtctagactaaaccatgtgcagtccatagg-3′ introducing a stop codon proceeding Val411 of Xenopus Ac45. The Ac45ΔCS, Ac45ΔC or cleaved-Ac45ΔC PCR products were cloned into EcoRI/XbaI restriction sites of the pPOMC(A)2+-SP-GFP vector (30). For fusing intact-Ac45 and Ac45Nterm C-terminally to GFP, the Xenopus Ac45 ORF (including the signal peptide) was amplified by PCR using the forward primer 5′-ggggggatccgaattcaacagagatggcagcgatgg-3′ and reverse primer 5′-gcgcggatccctctgtctggggcacagc-3′ or forward primer: 5′-ggggggatccaggctgctcagtggcaag-3′ and reverse primer 5′-gcgcggatccctctgtctggggcacagc-3′, respectively. The PCR fragments were cloned into the EcoRI/BamHI sites of N3delAUG-EGFP (Invitrogen). Next, the EcoRI/XbaI-digested inserts were subcloned into the pPOMC2+ vector (31) and used for stable Xenopus transgenesis.
Antibodies
The anti-Xenopus Ac45-C and anti-Ac45-N rabbit antisera (1311N and 1311C, respectively) have been described previously (21). A polyclonal antiserum raised against GFP (32) was kindly provided by Dr. B. Wieringa (Radboud University Nijmegen, The Netherlands). Rabbit antisera against Xenopus POMC (ST62, Ref. 33) and Xenopus V1A (ST170, Ref. 24) were obtained from Dr. S. Tanaka (Shizuoka University, Japan) and the rabbit antiserum against Xenopus calnexin (34) was a generous gift of Dr. K. Geering (University of Lausanne, Switzerland). We generated a rabbit antiserum (ST201) against a synthetic peptide comprising 16 amino acid residues located between transmembrane domains 2 and 3 of the Xenopus NaK-ATPase α-subunit with an additional cysteine at the N terminus (CKVDNSSLTGESEPQTR).
Western Blot Analysis
Freshly dissected neurointermediate lobes (NILs) were homogenized in lysis buffer (140 mm NaCl, 0.1% Triton-X100, 1% Tween-20, 50 mm Hepes pH 7.4 supplemented with Complete protease inhibitor mix (Roche Diagnostics)). Lysates were denatured, separated on 10% SDS-PAGE, and transferred to nitrocellulose membrane. Blots were incubated with anti-Xenopus Ac45-C (1:5000) or anti-GFP (1:5000) rabbit antisera and with secondary peroxidase-conjugated goat-anti-rabbit antibody (1:5000) followed by chemoluminescence. Signals were detected and quantified using a BioImaging system with Labworks 4.0 software (UVP BioImaging systems, Cambridge, UK).
Cryosectioning and Immunohistochemistry
Cryosectioning, GFP imaging, and anti-POMC immunostainings on Xenopus brain-pituitary preparations were described previously (25).
Melanotrope Cell Isolation, Live Cell Imaging, and Immunofluorescence
Melanotrope cells were isolated from freshly dissected Xenopus NILs as described previously (21) and plated onto poly-l-lysine coated glass bottom dishes (WillcoWells, Amsterdam, The Netherlands). For determining the localization of the GFP fusion protein, cells were subjected to live cell imaging after 24 h of culturing using an Olympus FV1000 confocal laser scanning microscope. For immunostaining with marker antibodies, cells were fixed with 4% paraformaldehyde in Xenopus PBS (XPBS; 67% PBS) for 2 h, washed with 50 mm NH4Cl/XPBS, permeabilized with 0.05% Tween-20/XPBS and incubated with anti-POMC (ST-62, 1:5000), anti-NaK-ATPase (ST-201 1:1000), or anti-calnexin antibodies (1:1000) in blocking buffer (2% BSA, 0.05% Tween-20 in XPBS). After extensive washing with XPBS, cells were incubated for 1 h with a second antibody, Goat-anti-rabbit-Alexa Fluor 568 (1:100 in blocking buffer). Following additional washing steps, cells were mounted in Mowiol and imaged for GFP and Alexa Fluor 568 using an Olympus FV1000 confocal laser scanning microscope using the Image J software package.
Metabolic Cell Labeling and Immunoprecipitations
Radioactive labeling of newly synthesized proteins from freshly isolated Xenopus NILs was described previously (25). Chase incubations were in the absence or presence of 0.1 μm apomorphine. The gel migration positions of 37-kDa POMC, 18-kDa POMC, CPE, and the various PC2 forms corresponded to those previously observed (22, 24, 35). POMC represents more than 80% of all newly synthesized melanotrope proteins (22) allowing its direct analysis (i.e. no need for immunoprecipitation).
Immunoelectron Microscopy
Immunoelectron microscopy on Xenopus NILs using an anti-GFP or an anti-V1A antibody was described previously (24).
Fluorescence Measurements of Intracellular Ca2+
Dynamic video imaging to measure intracellular Ca2+ was essentially performed as described previously (36). Ca2+-oscillations were measured in a low-speed acquisition mode with a sample interval of 6 s. During the experiment, Ringer's solution without or with 0.1 μm apomorphine was continuously perfused at a flow rate of 0.7 ml/min; the apomorphine concentration was chosen on the basis of the results of our metabolic cell labeling studies.
Statistics
Data are presented as means ± S.E. Statistical evaluation was performed using an unpaired Student's t test.
RESULTS
Generation of Stable Transgenic Xenopus Expressing (Mutant) Ac45 Tagged with GFP in the Intermediate Pituitary Melanotrope Cells
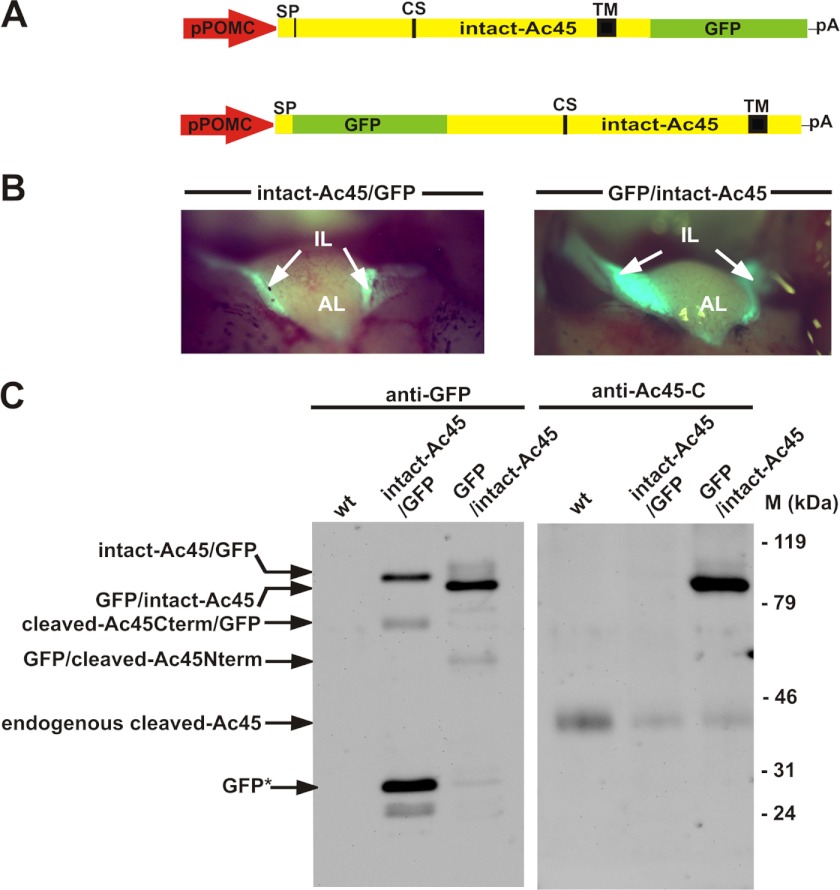
To study the functional domains within Ac45, we first expressed in the neuroendocrine Xenopus melanotrope cells intact-Ac45 containing a GFP tag at its N or C terminus (GFP/intact-Ac45 and intact-Ac45/GFP, respectively). The cDNAs were placed under the control of a POMC gene promoter fragment (31) (Fig. 1A) and stable transgenic Xenopus lines were established expressing the fusion proteins specifically in the intermediate pituitary melanotrope cells (Fig. 1B). To study the steady-state protein expression levels in the transgenic NILs, we performed Western blot analysis with anti-GFP and anti-Ac45-C antibodies. Analysis of the GFP/intact-Ac45 NIL lysate with the anti-GFP antibody showed a product of ∼90 kDa, representing the intact-Ac45 protein fused to GFP. The minor ∼50 kDa band corresponds to the size of the N-terminal Ac45 cleavage product fused to GFP. The GFP/intact-Ac45 protein was also recognized by the anti-Ac45-C antibody (Fig. 1C). In the intact-Ac45/GFP NIL lysate, an ∼90 kDa product corresponding to intact-Ac45/GFP and an ∼70 kDa representing the C-terminal Ac45 cleavage product fused to GFP (cleaved-Ac45/GFP) were observed. The ∼28 kDa product probably corresponds to the stable GFP moiety of the fusion protein, likely resulting from the degradation of the Ac45 portion. The anti-Ac45-C antibody did however not recognize the intact-Ac45/GFP product (Fig. 1C). This finding indicates that the GFP tag prevented detection of the C-terminal Ac45 epitope, presumably due to strong secondary structures being present even under the denaturing SDS-PAGE conditions. Since the C-tail of Ac45 contains important routing determinants (28), we decided to use for our mutational analysis the Ac45 protein fused to GFP at its N terminus.
FIGURE 1.
C-terminal fusion of GFP to Ac45 interferes with C-terminal antibody recognition. A, transgenes used to generate transgenic Xenopus with expression of intact-Ac45 C- or N-terminally fused to GFP specifically in the intermediate pituitary melanotrope cells. B, direct GFP fluorescence was observed only in the IL and not in the AL of the pituitary. C, intact-Ac45/GFP fusion protein was recognized by the anti-GFP antibody but not by the anti-Ac45-C antibody. The GFP/intact-Ac45 fusion protein was recognized by both antibodies. SP, signal peptide; CS, cleavage site; TM, transmembrane domain; pA, SV40 polyadenylation site.
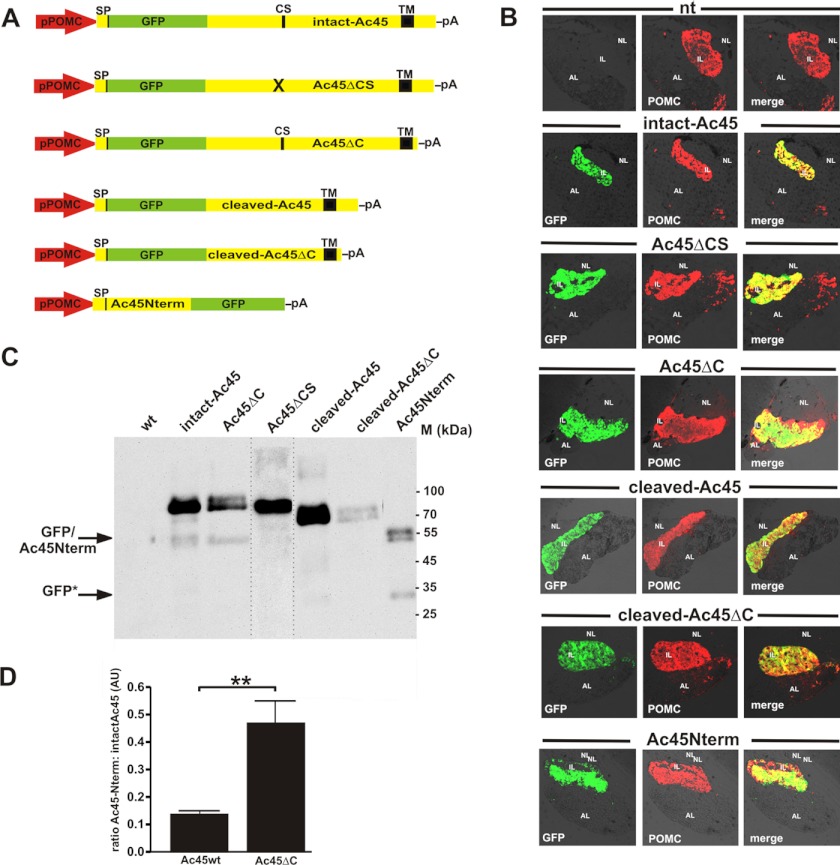
To gain insight into the significance of the Ac45 cleavage event and the function of the protein domains within Ac45, we generated transgenic Xenopus lines expressing intact-Ac45 (Ac45wt) or a variety of Ac45 mutants fused to GFP, namely Ac45ΔCS, Ac45ΔC, cleaved-Ac45, cleaved-Ac45ΔC, and Ac45Nterm (Fig. 2A). The mutant fusion proteins were colocalized with the main melanotrope cargo protein proopiomelanocortin (POMC) exclusively in the melanotrope cells of the intermediate lobe (IL) and not in the anterior lobe (AL) of the pituitaries (Fig. 2B).
FIGURE 2.
Transgene expression of GFP-Ac45 mutant proteins specifically in the Xenopus melanotrope cells. A, overview of transgenes used to express Ac45 mutant proteins fused to GFP in the Xenopus melanotrope cells. B, sagital cryosections of transgenic Xenopus pituitaries. Transgenic Ac45 mutant/GFP expression was directly viewed under a fluorescence microscope (green). Sections were stained with an anti-POMC antibody (red) showing coexpression of GFP and POMC in the intermediate pituitary melanotrope cells. C, Western blot analysis of NIL lysates with an anti-GFP showing the expression levels of the respective transgene products. Ten percent of a total NIL lysate was analyzed. The lane with the Ac45ΔCS NIL lysate was taken from a separate Western blot. GFP*, stable GFP moiety, probably resulting from Ac45Nterm mutant fusion protein breakdown. D, endoproteolytic processing efficiencies of the Ac45wt and Ac45ΔC proteins are presented as the ratio of the amount of Ac45N-term relative to the amount of intact-Ac45 and the ratio of the amount of Ac45N-term relative to the amount of Ac45ΔC, respectively. Shown are the means ± S.E. (n = 4). Significant difference is indicated by ** (p < 0.01).
Western blot analysis of a Ac45ΔCS NIL lysate using the anti-GFP antibody revealed only an ∼ 90-kDa product corresponding to the expected size of non-cleaved Ac45 fused to GFP, indicating that this Ac45 mutant indeed prevents Ac45 cleavage (Fig. 2C). In the Ac45ΔC NIL lysate, we detected a major ∼90-kDa protein corresponding to the non-cleaved C-terminally truncated Ac45 fusion protein (Fig. 2C). The slightly slower migrating diffuse product likely represents a more extensively glycosylated form of Ac45ΔC. Furthermore, we found a significantly higher processing efficiency of Ac45ΔC compared with Ac45wt. The increased processing efficiency of Ac45ΔC was reflected by an increased ratio of Nterm 50-kDa fragment to intact protein (0.463 ± 0.090 for Ac45ΔC and 0.131 ± 0.011, for Ac45wt; p < 0.01, n = 4).
Analysis of the cleaved-Ac45 NIL lysate revealed the expression of an ∼70-kDa transgene product corresponding to the cleaved-Ac45 product. The ∼70- and 72k-Da cleaved-Ac45ΔC products most likely correspond to two glycosylation states of the transgene product. The steady-state expression level of this transgene was relatively low. Finally, in the Ac45Nterm NIL lysate two ∼50-kDa products were detected, presumably also corresponding to differentially glycosylated forms of the N-terminal cleavage product of Ac45 fused to GFP.
Localization of the Mutant Ac45 Proteins in the Transgenic Melanotrope Cells
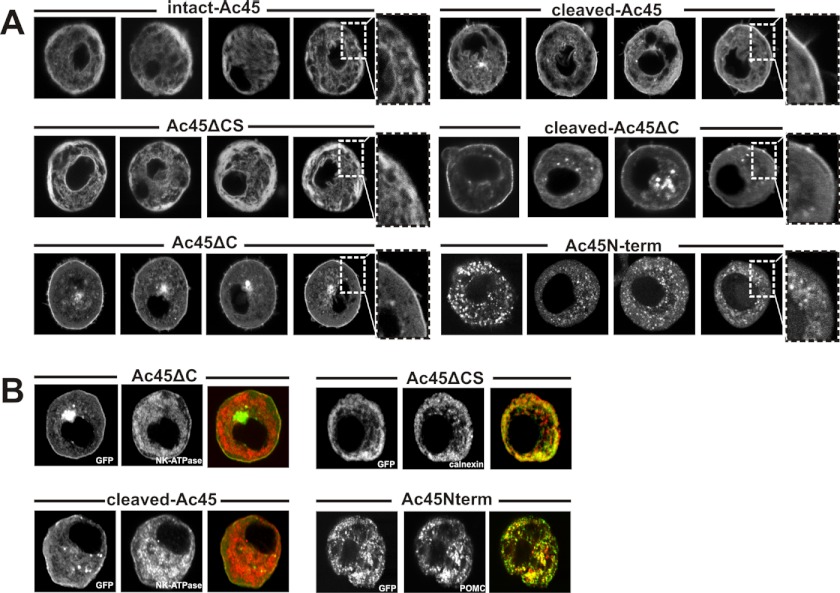
To examine the subcellular localization of the GFP-tagged mutant Ac45 products, we used confocal laser scanning microscopy (CLSM) on cultured live melanotrope cells. The intact-Ac45 and Ac45ΔCS transgene products were mainly found in a reticular network, most likely representing the endoplasmic reticulum (ER) (Fig. 3A). Surprisingly, besides in a perinuclear region, presumably representing the Golgi, Ac45ΔC was mainly localized to the plasma membrane, comparable to the localization of cleaved-Ac45 and cleaved-Ac45ΔC (Fig. 3A). The soluble Ac45Nterm product was found in granular structures in the melanotrope cells (Fig. 3A).
FIGURE 3.
Subcellular localization of GFP-Ac45mutant proteins in transgenic Xenopus melanotrope cells. A, Xenopus melanotrope cells were isolated from the transgenic pituitaries, cultured and examined with live imaging for GFP fluorescence. Note that in the active melanotrope cells the ER is situated near the plasma membrane (53). B, melanotrope cells were fixed and stained with the marker antibodies anti-NaK-ATPase (plasma membrane), anti-calnexin (for the ER) and anti-POMC (for secretory vesicles).
To confirm the observed intracellular localizations of the transgene products, isolated melanotrope cells were fixed and probed with specific marker antibodies. The localization of Ac45ΔCS in the ER was confirmed by the analysis using an antibody directed to the ER chaperone calnexin (34). At the plasma membrane, we found co-immunostaining of the plasma membrane marker NaK-ATPase and Ac45ΔC, and of NaK-ATPase and cleaved-Ac45. The localization of the soluble Ac45Nterm transgene product was in granules, as was evident from its colocalization with the main melanotrope prohormone proopiomelanocortin (POMC) (Fig. 3B). Thus, naturally occurring N- and C-terminal Ac45 fragments are transported through the secretory pathway, the cleavage of Ac45 is a prerequisite for its transport and the cytoplasmic tail of Ac45 plays a pivotal role in transporting the Ac45 protein from the ER to the later stages of the secretory pathway.
Differential Effects of the Ac45 Mutant Proteins on Endogenous V-ATPase Localization
Previous studies have shown that the cleaved-Ac45 transgene product directs the endogenous V-ATPase through the secretory pathway and to the plasma membrane of Xenopus melanotrope cells (24). We now wondered how the expression of the Ac45 mutant proteins would affect endogenous V-ATPase localization. We focused on the cleaved-Ac45, Ac45ΔC, and cleaved-Ac45ΔC transgene products since these were expressed at the plasma membrane. As expected, immuno-EM with an anti-GFP antibody showed no gold label in the wild-type cells, indicative of the specificity of our immunolabeling (Fig. 4A). Immuno-EM further revealed that the cleaved-Ac45 product was mainly localized to microvillar structures at the plasma membrane (Fig. 4C and Ref. 24), Ac45ΔC to both microvillar structures and smooth plasma membrane, and cleaved-Ac45ΔC to smooth, non-ruffled plasma membranes (Fig. 4, B and D, respectively). Next, we studied the intracellular localization of the endogenous V-ATPase A subunit. In wild-type cells, no V-ATPase was found at the plasma membrane of the cells (Fig. 4E). Cleaved-Ac45 co-localized with the endogenous V-ATPase A subunit in the microvillar structures (Fig. 4G and Ref. 24), and Ac45ΔC in both the microvillar structures and the non-ruffled plasma membranes (Fig. 4F). The localization of the endogenous V-ATPase A subunit in the cytoplasm and its partial association with vesicular structures in the cleaved-Ac45ΔC transgenic cells was similar to that in wild-type melanotrope cells (Fig. 4, E and H, respectively), suggesting that cleaved-Ac45ΔC did not recruit the endogenous V-ATPase to the plasma membrane.
FIGURE 4.
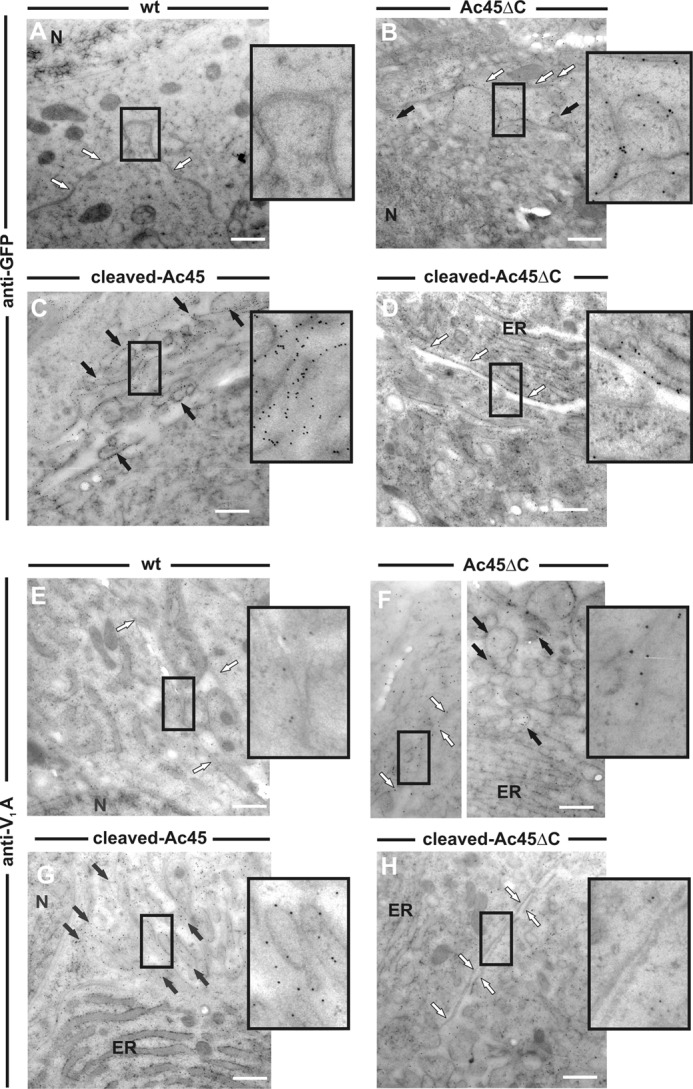
Ac45 mutant proteins differentially affect endogenous V-ATPase localization. A–D, immunogold labeling of ultra-thin pituitary cryosections with anti-GFP antibody confirmed the expression of the GFP/Ac45 mutant transgene products at the plasma membrane of the transgenic Xenopus melanotrope cells. No labeling was found in wild-type cells. E–H, immunogold labeling with an anti-V1A antibody showed that in the cleaved-Ac45 and Ac45 ΔC transgenic melanotrope cells the endogenous V-ATPase is recruited to the plasma membrane but not in wild-type cells nor in transgenic cells expressing cleaved-Ac45ΔC. White arrow, smooth plasma membrane; black arrow, microvillar plasma membrane; N, nucleus; ER, endoplasmic reticulum.
The N-terminal Ac45 Cleavage Product Is Secreted via the Regulated Secretory Pathway
We next performed biosynthetic labeling studies to study the fate of the Ac45Nterm protein in the melanotrope cells. In addition to the biosynthetically active melanotrope cells (in the intermediate lobe), the Xenopus NIL consists of nerve terminals of hypothalamic origin (the neural lobe) which are biosynthetically inactive. The radiolabeled proteins are therefore synthesized solely by the melanotrope cells. During the 30-min pulse period, in both wild-type and transgenic cells 37-kDa POMC was clearly the major newly synthesized protein that during the 180-min chase incubation was processed to 18-kDa POMC (Fig. 5A). This protein represents the N-terminal portion of the Xenopus POMC molecule, contains the only N-glycosylation site present in the POMC molecule and is the result of the first endoproteolytic cleavage step during POMC processing (37). The biosynthesis of newly synthesized proteins in the Ac45Nterm-transgenic NILs was similar to that in wild-type NILs (Fig. 5A). Immunoprecipitations from the NIL lysates with an anti-Ac45-N antibody revealed the specific expression of two protein products of ∼50 and ∼55 kDa in the transgenic NILs, representing Ac45Nterm transgene products. Since immunoprecipitations from the incubation medium only detected the ∼55-kDa Ac45Nterm form, the 50-kDa protein most likely represents its nonglycosylated form and the 55-kDa product the glycosylated form (Fig. 5B). Since the Ac45Nterm protein was secreted by the transgenic melanotrope cells, we wondered whether this secretion occurred in a regulated manner. Under physiological conditions, secretion by the melanotrope cells is under strict control of neurotransmitters of hypothalamic origin, with dopamine, acting through the dopamine D2-receptor, as one of the main inhibitors of peptide release (38). We applied the dopamine D2-receptor agonist apomorphine during the 180-min chase incubations and found that in addition to the secretion of the well-defined regulated secretory proteins 18-kDa POMC, prohormone convertase 2 (PC2), and carboxypeptidase E (CPE), also the secretion of Ac45Nterm was blocked (Fig. 5A), showing that the transgene product was secreted in a regulated fashion.
FIGURE 5.
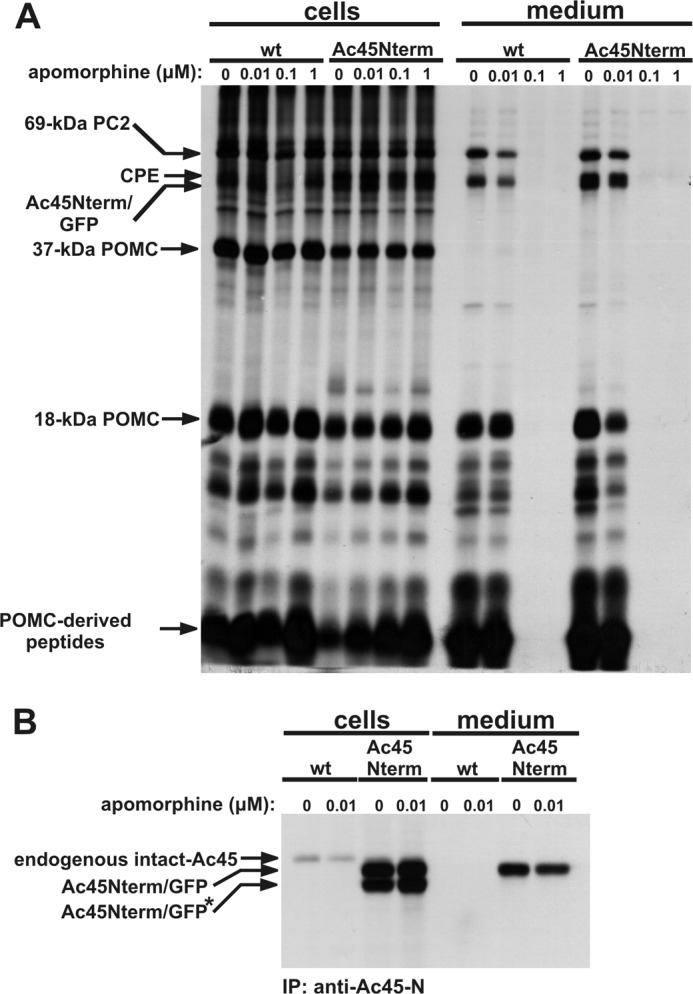
Ac45Nterm is secreted via the regulated secretory pathway. Wild-type (wt) and Ac45Nterm-transgenic NILs were pulse labeled for 30 min and chased for 180 min in the presence of various concentrations of apomorphine, as indicated. A, five percent of the total labeled NIL lysates and 20% of the incubation media were directly resolved by SDS-PAGE. B, NIL lysates and incubation media were incubated with an anti-Ac45-N antibody, and the immune complexes were immunoprecipititated using protein A-Sepharose and resolved by SDS-PAGE. Radioactive signals were visualized by autoradiography.
The Dopaminergic Inhibition of Peptide Secretion Is Differentially Affected by the Ac45 Mutant Proteins
Since Ac45 is associated with the V0-sector of the V-ATPase (18, 26, 39) and given the involvement of V0 in Ca2+-dependent exocytosis (11, 12), we next wondered how the various Ac45 mutants affect Ca2+-dependent secretion by the transgenic cells. We therefore performed biosynthetic labeling studies in the presence or absence of apomorphine. During the 30-min pulse and 180-min chase period in the absence of apomorphine, the NILs secreted regulated secretory proteins, including 18-kDa POMC, PC2 and CPE into the incubation medium. In the presence of 0.1 μm apomorphine, peptide secretion from the wild-type NILs was completely blocked. Similarly, peptide secretion from the transgenic NILs expressing intact-Ac45, Ac45ΔCS, Ac45Nterm or cleaved-Ac45ΔC was inhibited. Surprisingly, in the presence of apomorphine transgenic NILs expressing cleaved-Ac45 or Ac45ΔC still secreted substantial amounts of the regulated secretory proteins into the incubation medium (Fig. 6). Only ∼25% inhibition of secretion was found for the cleaved-Ac45 transgenic melanotrope cells (data not shown). Thus, an Ac45 variant localized to the ER (intact-Ac45 or Ac45ΔCS), localized to the plasma membrane without affecting V-ATPase localization (cleaved-Ac45ΔC) or secreted by the cells (Ac45Nterm) does not affect dopaminergic inhibition of peptide release. In contrast, the Ac45 mutants that travel through the secretory pathway and recruit the V-ATPase (namely cleaved-Ac45 and Ac45ΔC, see above) affect regulated peptide release by the melanotrope cells.
FIGURE 6.
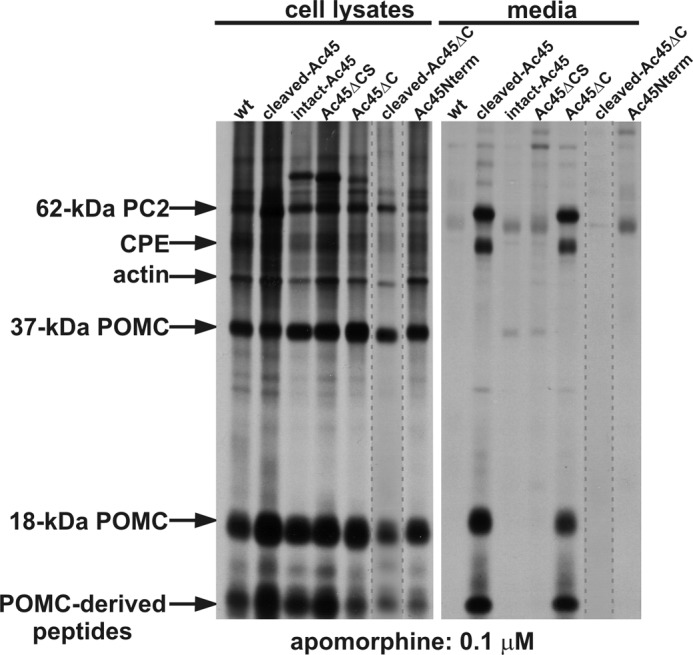
Dopaminergic inhibition of peptide release is affected in cleaved-Ac45- and Ac45 ΔC-transgenic Xenopus melanotrope cells. Wild-type (wt) and transgenic NILs were pulse-labeled for 30 min and chased for 180 min in the presence of 0.1 μm apomorphine. NILs were lysed and 5% of the total lysates and 20% of the incubation media was directly analyzed by SDS-PAGE. The lane with the cleaved-Ac45 ΔC NIL lysate was taken from a separate gel. Radioactive signals were visualized by autoradiography.
The Dopaminergic Inhibition of Ca2+ Oscillations Is Not Affected in the Cleaved Ac45 Transgenic Melanotrope Cells
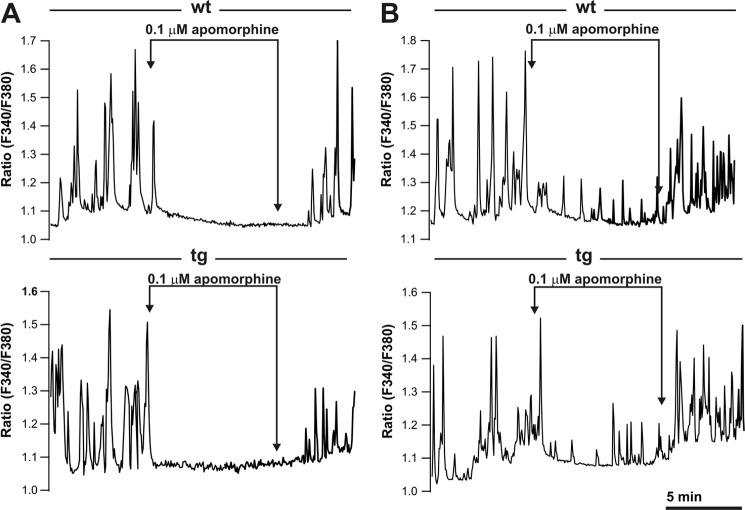
Since regulated peptide secretion by the cleaved-Ac45 and Ac45ΔC-transgenic melanotrope cells was not effectively inhibited by apomorphine, we wondered whether these transgenic cells would still display normal dopamine D2-receptor functioning. In wild-type melanotrope cells, Ca2+-oscillations regulate the secretory activity of the cells, as they are the driving force for regulated secretion and are effectively inhibited by dopamine (40–42). We choose to study receptor functioning in the cleaved-Ac45-transgenic cells, since this protein resembles the naturally occurring Ac45 protein that is found in secretory granules (26). Following loading of the cells with the Ca2+-probe fura-2 and in the absence of apomorphine, both wild-type and the cleaved-Ac45-transgenic melanotrope cells displayed spontaneous intracellular Ca2+-oscillations. Upon treatment of the cells with 0.1 μm apomorphine for 10 min, in ∼75% of both the wild-type cells (25 out of 34 cells, three independent experiments) as well as the transgenic cells (30 out of 41 cells, three independent experiments) showed severe inhibition of the Ca2+-oscillations, resulting in minimal, close-to-noise level, Ca2+-oscillations, whereas the remainder of the cells displayed low-amplitude Ca2+-oscillations within the 10-min incubation period (compare Fig. 7, A and B). Removal of the drug reversed the inhibition and Ca2+-oscillations were regained. Since in our peptide release studies we monitored the apomorphine effect following a 180-min chase period, we examined whether the inhibition of the Ca2+-oscillations sustained up to 180 min. Comparable with the 10-min measurements, ∼25% of the wild-type and transgenic cells gained low-amplitude Ca2+-oscillations while other cells remained largely inhibited by the drug for 180 min, and no difference was observed between wild-type and the transgenic cells (data not shown). Thus, in the cleaved-Ac45-transgenic melanotrope cells dopamine D2-receptor functioning was unaffected, but the inhibition of the Ca2+-oscillations did no longer lead to the inhibition of peptide secretion.
FIGURE 7.
Excess of cleaved-Ac45 does not affect the inhibition of the spontaneous Ca2+-oscillations in cleaved-Ac45-transgenic Xenopus melanotrope cells. A, Ca2+-oscillations in wild-type (wt) and cleaved-Ac45-transgenic (tg) cells loaded with fura-2 are inhibited in the presence of 0.1 μm apomorphine and reappear after removal of the drug. B, in the presence of 0.1 μm apomorphine low-frequency Ca2+-oscillations occur in ∼25% of the wt and tg cells.
DISCUSSION
In this study, we examined the structural domains within the Ac45 protein that are necessary for its function as the V-ATPase regulator in the regulated secretory pathway. We used transgene products representing the naturally occurring N- and C-terminal processing products of Ac45, namely Nterm-Ac45 and cleaved-Ac45, respectively. Microscopic analysis revealed that these transgene products were transported through the secretory pathway of the Xenopus melanotrope cells. The endogenous, soluble N-terminal Ac45 fragment appears to be degraded in vivo (27) however we cannot exclude that this fragment is secreted. In our transgenic melanotrope cells this fragment, apparently stabilized by its fusion to GFP, was released and in a regulated fashion, since its secretion was completely inhibited by the treatment of the cells with the D2-receptor agonist apomorphine.
We further observed that in the melanotrope cells the wild-type intact-Ac45 protein was proteolytically processed. Removal of the region harboring the putative cleavage site (Val164-Gln207 of Xenopus Ac45; (21) prevented its processing and caused its accumulation in the ER. This finding indicates that region Val164-Gln207, which includes a potential cleavage site for the endoprotease furin, contains the endoproteolytic processing site within the Xenopus Ac45 protein. In mouse pancreatic β-cells, furin indeed cleaves mouse Ac45 (43). However, the furin cleavage site of mouse Ac45 is not conserved in Xenopus Ac45 and the adjacent, conserved putative furin cleavage site in mouse Ac45 was not recognized by furin (43). Furthermore, since the treatment of Xenopus melanotrope cells with the Golgi-disrupting drug Brefeldin A did not interfere with the proteolytic processing of Xenopus Ac45 (27), Ac45 processing likely takes place in the early secretory pathway, i.e. before the site of furin action (44) has been reached. Thus, furin might not represent the Ac45 cleaving enzyme in Xenopus melanotrope cells. Alternatively, in these cells furin could already be activated in the early secretory pathway, as previously found in HEK293 cells (45).
Interestingly, our C-terminally truncated Ac45 mutant was more extensively glycosylated and processed in the secretory pathway than the wild-type Ac45 protein. Removal of the C-tail apparently allowed efficient transport of the Ac45 mutant through the secretory pathway, illustrated by its extensive glycosylation, a Golgi event (46), and its extensive endoproteolytic cleavage. We conclude that, in addition to its role in Ac45 internalization (28), the C-tail also affects Ac45 trafficking efficiency through the secretory pathway. Possibly, the transport of Ac45 resembles that of other type I transmembrane proteins, such as amyloid precursor protein (APP) and peptidylglycine α-amidating monooxygenase (PAM), in which cytoplasmic C-tail-binding proteins play a prominent role in trafficking and processing (47–50).
Besides being efficiently transported through the secretory pathway, our immuno-EM analysis showed that cleaved-Ac45 and the C-tail Ac45 mutant caused recruitment of endogenous V-ATPase to the plasma membrane. In osteoclasts, removal of the Ac45 C-tail still allowed, albeit less tightly, its interaction with the V0-sector of the pump (18). However, we found that removal of the C-tail from the N-terminally cleaved form of Ac45 abolished V-ATPase recruitment, despite of its transport to the plasma membrane. We conclude that domains within both the luminal N-terminal portion and the cytoplasmic C-tail of the Ac45 protein are necessary for interaction with and recruitment of the V-ATPase.
Cleaved-Ac45 and the C-tail Ac45 mutant also affected the dopaminergic inhibition of regulated peptide secretion. Intriguingly, despite of its localization at the plasma membrane the cleaved-Ac45 form lacking its C-tail did not influence the inhibition of regulated exocytosis, although one has to realize that this mutant was expressed at relatively low levels. Nevertheless, affecting dopaminergic inhibition of peptide release apparently necessitates the recruitment of the V-ATPase into the regulated secretory pathway.
In wild-type and the cleaved-Ac45-transgenic melanotrope cells, cytoplasmic Ca2+-oscillations, the driving force for secretion (42, 51), were similarly inhibited upon apomorphine treatment, suggesting that D2-receptor activation and the resulting inhibition of high-voltage activated Ca2+-channels (36, 52) were unaffected. The low-amplitude Ca2+-oscillations observed in the transgenic cells apparently provide sufficient driving force for regulated Ca2+-dependent membrane fusion. We recently showed that in the cleaved-Ac45-transgenic cells the secretion efficiency (the direct link between influx of Ca2+ and exocytosis) was substantially increased (24). Furthermore, these transgenic cells displayed elevated levels of basal peptide release (25), showing that also under non-inhibitory conditions Ca2+-dependent secretion is enhanced. In addition, in the cleaved-Ac45-transgenic melanotrope cells the regulated secretory vesicles were more acidified (25). We hypothesize that an increased V-ATPase recruitment to the regulated vesicles provides a higher abundance of V0 in the vesicular membrane. V0 recruits via its V0a subunit the small Ca2+-binding protein calmodulin (15) and interacts with the SNARE fusion machinery via synaptobrevin (16) and therefore may facilitate Ca2+-dependent secretion.
Taking our results together, we conclude that domains within the N-terminal portion and in the C-tail of Ac45 play a key role in Ac45 transport, V-ATPase recruitment and the process of Ca2+-dependent regulated exocytosis.
Acknowledgments
We thank Drs. B. Wieringa (Radboud University, Nijmegen, The Netherlands), K.Geering (University of Lausanne, Switzerland), and S. Tanaka (Shizuoka University, Japan) for antibodies and Ron Engels for animal care. Microscopic imaging was performed at the Microscopic Imaging Centre (MIC) of the NCMLS.
Footnotes
- V-ATPase
- vacuolar (H+)-ATPase
- POMC
- proopiomelanocortin
- IL
- intermediate lobe
- AL
- anterior lobe
- NIL
- neurointermediate lobe
- CLSM
- confocal laser scanning microscopy
- PC
- prohormone convertase
- CPE
- carboxypeptidase E.
REFERENCES
- 1. Nishi T., Forgac M. (2002) The vacuolar (H+)-ATPases–nature's most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 3, 94–103 [DOI] [PubMed] [Google Scholar]
- 2. Adams D. S., Robinson K. R., Fukumoto T., Yuan S., Albertson R. C., Yelick P., Kuo L., McSweeney M., Levin M. (2006) Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development 133, 1657–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cruciat C. M., Ohkawara B., Acebron S. P., Karaulanov E., Reinhard C., Ingelfinger D., Boutros M., Niehrs C. (2010) Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327, 459–463 [DOI] [PubMed] [Google Scholar]
- 4. Wagner C. A., Finberg K. E., Breton S., Marshansky V., Brown D., Geibel J. P. (2004) Renal vacuolar H+-ATPase. Physiol. Rev. 84, 1263–1314 [DOI] [PubMed] [Google Scholar]
- 5. Paroutis P., Touret N., Grinstein S. (2004) The pH of the secretory pathway: measurement, determinants, and regulation. Physiology 19, 207–215 [DOI] [PubMed] [Google Scholar]
- 6. Ungermann C., Wickner W., Xu Z. (1999) Vacuole acidification is required for trans-SNARE pairing, LMA1 release, and homotypic fusion. Proc. Natl. Acad. Sci. U.S.A. 96, 11194–11199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schoonderwoert V. T., Holthuis J. C., Tanaka S., Tooze S. A., Martens G. J. (2000) Inhibition of the vacuolar H+-ATPase perturbs the transport, sorting, processing, and release of regulated secretory proteins. Eur. J. Biochem. 267, 5646–5654 [DOI] [PubMed] [Google Scholar]
- 8. Tanaka S., Yora T., Nakayama K., Inoue K., Kurosumi K. (1997) Proteolytic processing of pro-opiomelanocortin occurs in acidifying secretory granules of AtT-20 cells. J. Histochem. Cytochem. 45, 425–436 [DOI] [PubMed] [Google Scholar]
- 9. Camacho M., Machado J. D., Montesinos M. S., Criado M., Borges R. (2006) Intragranular pH rapidly modulates exocytosis in adrenal chromaffin cells. J. Neurochem. 96, 324–334 [DOI] [PubMed] [Google Scholar]
- 10. Beyenbach K. W., Wieczorek H. (2006) The V-type H+ ATPase: molecular structure and function, physiological roles, and regulation. J. Exp. Biol. 209, 577–589 [DOI] [PubMed] [Google Scholar]
- 11. Peters C., Bayer M. J., Bühler S., Andersen J. S., Mann M., Mayer A. (2001) Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature 409, 581–588 [DOI] [PubMed] [Google Scholar]
- 12. Hiesinger P. R., Fayyazuddin A., Mehta S. Q., Rosenmund T., Schulze K. L., Zhai R. G., Verstreken P., Cao Y., Zhou Y., Kunz J., Bellen H. J. (2005) The v-ATPase V0 subunit a1 is required for a late step in synaptic vesicle exocytosis in Drosophila. Cell 121, 607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun-Wada G. H., Toyomura T., Murata Y., Yamamoto A., Futai M., Wada Y. (2006) The a3 isoform of V-ATPase regulates insulin secretion from pancreatic beta-cells. J. Cell Sci. 119, 4531–4540 [DOI] [PubMed] [Google Scholar]
- 14. Liégeois S., Benedetto A., Garnier J. M., Schwab Y., Labouesse M. (2006) The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J. Cell Biol. 173, 949–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang W., Wang D., Volk E., Bellen H. J., Hiesinger P. R., Quiocho F. A. (2008) V-ATPase V0 sector subunit a1 in neurons is a target of calmodulin. J. Biol. Chem. 283, 294–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Di Giovanni J., Boudkkazi S., Mochida S., Bialowas A., Samari N., Lévêque C., Youssouf F., Brechet A., Iborra C., Maulet Y., Moutot N., Debanne D., Seagar M., El Far O. (2010) V-ATPase membrane sector associates with synaptobrevin to modulate neurotransmitter release. Neuron 67, 268–279 [DOI] [PubMed] [Google Scholar]
- 17. Sobota J. A., Bäck N., Eipper B. A., Mains R. E. (2009) Inhibitors of the V0 subunit of the vacuolar H+-ATPase prevent segregation of lysosomal and secretory pathway proteins. J. Cell Sci. 122, 3542–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feng H., Cheng T., Pavlos N. J., Yip K. H., Carrello A., Seeber R., Eidne K., Zheng M. H., Xu J. (2008) Cytoplasmic terminus of vacuolar type proton pump accessory subunit Ac45 is required for proper interaction with V(0) domain subunits and efficient osteoclastic bone resorption. J. Biol. Chem. 283, 13194–13204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu J., Cheng T., Feng H. T., Pavlos N. J., Zheng M. H. (2007) Structure and function of V-ATPases in osteoclasts: potential therapeutic targets for the treatment of osteolysis. Histol. Histopathol. 22, 443–454 [DOI] [PubMed] [Google Scholar]
- 20. Qin A., Cheng T. S., Lin Z., Pavlos N. J., Jiang Q., Xu J., Dai K. R., Zheng M. H. (2011) Versatile roles of V-ATPases accessory subunit Ac45 in osteoclast formation and function. PLoS One 6, e27155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holthuis J. C., Jansen E. J., Schoonderwoert V. T., Burbach J. P., Martens G. J. (1999) Biosynthesis of the vacuolar H+-ATPase accessory subunit Ac45 in Xenopus pituitary. Eur. J. Biochem. 262, 484–491 [DOI] [PubMed] [Google Scholar]
- 22. Holthuis J. C., Jansen E. J., van Riel M. C., Martens G. J. (1995) Molecular probing of the secretory pathway in peptide hormone-producing cells. J. Cell Sci. 108, 3295–3305 [DOI] [PubMed] [Google Scholar]
- 23. Jansen E. J., Martens G. J. (2012) Novel insights into V-ATPase functioning: distinct roles for its accessory subunits ATP6AP1/Ac45 and ATP6AP2/(pro)renin receptor. Curr. Protein Pept Sci. 13, 124–133 [DOI] [PubMed] [Google Scholar]
- 24. Jansen E. J., Scheenen W. J., Hafmans T. G., Martens G. J. (2008) Accessory subunit Ac45 controls the V-ATPase in the regulated secretory pathway. Biochim. Biophys. Acta 1783, 2301–2310 [DOI] [PubMed] [Google Scholar]
- 25. Jansen E. J., Hafmans T. G., Martens G. J. (2010) V-ATPase-mediated granular acidification is regulated by the V-ATPase accessory subunit Ac45 in POMC-producing cells. Mol. Biol. Cell 21, 3330–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Supek F., Supekova L., Mandiyan S., Pan Y. C., Nelson H., Nelson N. (1994) A novel accessory subunit for vacuolar H(+)-ATPase from chromaffin granules. J. Biol. Chem. 269, 24102–24106 [PubMed] [Google Scholar]
- 27. Schoonderwoert V. T., Jansen E. J., Martens G. J. (2002) The fate of newly synthesized V-ATPase accessory subunit Ac45 in the secretory pathway. Eur. J. Biochem. 269, 1844–1853 [DOI] [PubMed] [Google Scholar]
- 28. Jansen E. J., Holthuis J. C., McGrouther C., Burbach J. P., Martens G. J. (1998) Intracellular trafficking of the vacuolar H+-ATPase accessory subunit Ac45. J. Cell Sci. 111, 2999–3006 [DOI] [PubMed] [Google Scholar]
- 29. Kroll K. L., Amaya E. (1996) Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development 122, 3173–3183 [DOI] [PubMed] [Google Scholar]
- 30. Collin R. W., Martens G. J. (2006) The coding sequence of amyloid-β precursor protein APP contains a neural-specific promoter element. Brain Res. 1087, 41–51 [DOI] [PubMed] [Google Scholar]
- 31. Jansen E. J., Holling T. M., van Herp F., Martens G. J. (2002) Transgene-driven protein expression specific to the intermediate pituitary melanotrope cells of Xenopus laevis. FEBS Lett. 516, 201–207 [DOI] [PubMed] [Google Scholar]
- 32. Cuppen E., Wijers M., Schepens J., Fransen J., Wieringa B., Hendriks W. (1999) A FERM domain governs apical confinement of PTP-BL in epithelial cells. J. Cell Sci. 112, 3299–3308 [DOI] [PubMed] [Google Scholar]
- 33. Berghs C. A., Tanaka S., Van Strien F. J., Kurabuchi S., Roubos E. W. (1997) The secretory granule and pro-opiomelanocortin processing in Xenopus melanotrope cells during background adaptation. J. Histochem. Cytochem. 45, 1673–1682 [DOI] [PubMed] [Google Scholar]
- 34. Beggah A. T., Geering K. (1997) α and β subunits of Na, K-ATPase interact with BiP and calnexin. Ann. N.Y. Acad. Sci. 834, 537–539 [DOI] [PubMed] [Google Scholar]
- 35. Braks J. A., Martens G. J. (1994) 7B2 is a neuroendocrine chaperone that transiently interacts with prohormone convertase PC2 in the secretory pathway. Cell 78, 263–273 [DOI] [PubMed] [Google Scholar]
- 36. Zhang H. Y., Langeslag M., Voncken M., Roubos E. W., Scheenen W. J. (2005) Melanotrope cells of Xenopus laevis express multiple types of high-voltage-activated Ca2+ channels. J. Neuroendocrinol. 17, 1–9 [DOI] [PubMed] [Google Scholar]
- 37. Martens G. J. (1986) Expression of two proopiomelanocortin genes in the pituitary gland of Xenopus laevis: complete structures of the two preprohormones. Nucleic Acids Res. 14, 3791–3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roubos E. W. (1997) Background adaptation by Xenopus laevis: a model for studying neuronal information processing in the pituitary pars intermedia. Comp Biochem. Physiol. A. Physiol. 118, 533–550 [DOI] [PubMed] [Google Scholar]
- 39. Getlawi F., Laslop A., Schägger H., Ludwig J., Haywood J., Apps D. (1996) Chromaffin granule membrane glycoprotein IV is identical with Ac45, a membrane-integral subunit of the granule's H(+)-ATPase. Neurosci. Lett. 219, 13–16 [DOI] [PubMed] [Google Scholar]
- 40. Scheenen W. J., Jenks B. G., Roubos E. W., Willems P. H. (1994) Spontaneous calcium oscillations in Xenopus laevis melanotrope cells are mediated by omega-conotoxin sensitive calcium channels. Cell Calcium 15, 36–44 [DOI] [PubMed] [Google Scholar]
- 41. Shibuya I., Douglas W. W. (1993) Spontaneous cytosolic calcium pulsing detected in Xenopus melanotrophs: modulation by secreto-inhibitory and stimulant ligands. Endocrinology 132, 2166–2175 [DOI] [PubMed] [Google Scholar]
- 42. Jenks B. G., Roubos E. W., Scheenen W. J. (2003) Ca2+ oscillations in melanotropes of Xenopus laevis: their generation, propagation, and function. Gen. Comp. Endocrinol. 131, 209–219 [DOI] [PubMed] [Google Scholar]
- 43. Louagie E., Taylor N. A., Flamez D., Roebroek A. J., Bright N. A., Meulemans S., Quintens R., Herrera P. L., Schuit F., Van de Ven W. J., Creemers J. W. (2008) Role of furin in granular acidification in the endocrine pancreas: identification of the V-ATPase subunit Ac45 as a candidate substrate. Proc. Natl. Acad. Sci. U.S.A. 105, 12319–12324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thomas G. (2002) Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 3, 753–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bass J., Turck C., Rouard M., Steiner D. F. (2000) Furin-mediated processing in the early secretory pathway: sequential cleavage and degradation of misfolded insulin receptors. Proc. Natl. Acad. Sci. U.S.A. 97, 11905–11909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roth J. (2002) Protein N-glycosylation along the secretory pathway: relationship to organelle topography and function, protein quality control, and cell interactions. Chem. Rev. 102, 285–303 [DOI] [PubMed] [Google Scholar]
- 47. Alam M. R., Caldwell B. D., Johnson R. C., Darlington D. N., Mains R. E., Eipper B. A. (1996) Novel proteins that interact with the COOH-terminal cytosolic routing determinants of an integral membrane peptide-processing enzyme. J. Biol. Chem. 271, 28636–28640 [DOI] [PubMed] [Google Scholar]
- 48. Mains R. E., Alam M. R., Johnson R. C., Darlington D. N., Bäck N., Hand T. A., Eipper B. A. (1999) Kalirin, a multifunctional PAM COOH-terminal domain interactor protein, affects cytoskeletal organization and ACTH secretion from AtT-20 cells. J. Biol. Chem. 274, 2929–2937 [DOI] [PubMed] [Google Scholar]
- 49. Burgos P. V., Mardones G. A., Rojas A. L., daSilva L. L., Prabhu Y., Hurley J. H., Bonifacino J. S. (2010) Sorting of the Alzheimer's disease amyloid precursor protein mediated by the AP-4 complex. Dev. Cell 18, 425–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kuan Y. H., Gruebl T., Soba P., Eggert S., Nesic I., Back S., Kirsch J., Beyreuther K., Kins S. (2006) PAT1a modulates intracellular transport and processing of amyloid precursor protein (APP), APLP1, and APLP2. J. Biol. Chem. 281, 40114–40123 [DOI] [PubMed] [Google Scholar]
- 51. Lieste J. R., Koopman W. J., Reynen V. C., Scheenen W. J., Jenks B. G., Roubos E. W. (1998) Action currents generate stepwise intracellular Ca2+ patterns in a neuroendocrine cell. J. Biol. Chem. 273, 25686–25694 [DOI] [PubMed] [Google Scholar]
- 52. Zhang H., Jenks B. G., Ciccarelli A., Roubos E. W., Scheenen W. J. (2004) Dopamine D2-receptor activation differentially inhibits N- and R-type Ca2+ channels in Xenopus melanotrope cells. Neuroendocrinology 80, 368–378 [DOI] [PubMed] [Google Scholar]
- 53. Van Herp F., Coenen T., Geurts H. P., Janssen G. J., Martens G. J. (2005) A fast method to study the secretory activity of neuroendocrine cells at the ultrastructural level. J. Microsc. 218, 79–83 [DOI] [PubMed] [Google Scholar]