Abstract
Monomer chromatin subunit particles (nu1) have been isolated in gram quantities by large-scale zonal centrifugation of micrococcal nuclease digests of chicken erythrocyte nuclei. nu1 can be stored, apparently indefinitely, frozen in 0.2 mM EDTA (pH 7.0) at less than or equal to 25 degrees C. Aliquots of the stored monomers have been subfractionated by dialysis against 0.1 M KCl buffers into a soluble fraction containing equimolar amounts of H4, H3, H2A, H2B associated with a DNA fragment of approximately 130-140 nucleotide pairs, and a precipitated fraction containing all of the histones including H5 and H1 associated with DNA fragments. The total nu1 and the KCl-soluble fraction of nu1 have been examined by sedimentation, diffusion, sedimentation equilibrium ultracentrifugation, low-angle X-ray diffraction, and electron microscopy. Physical parameters from all of these techniques are presented and correlated in this study.
Full text
PDF




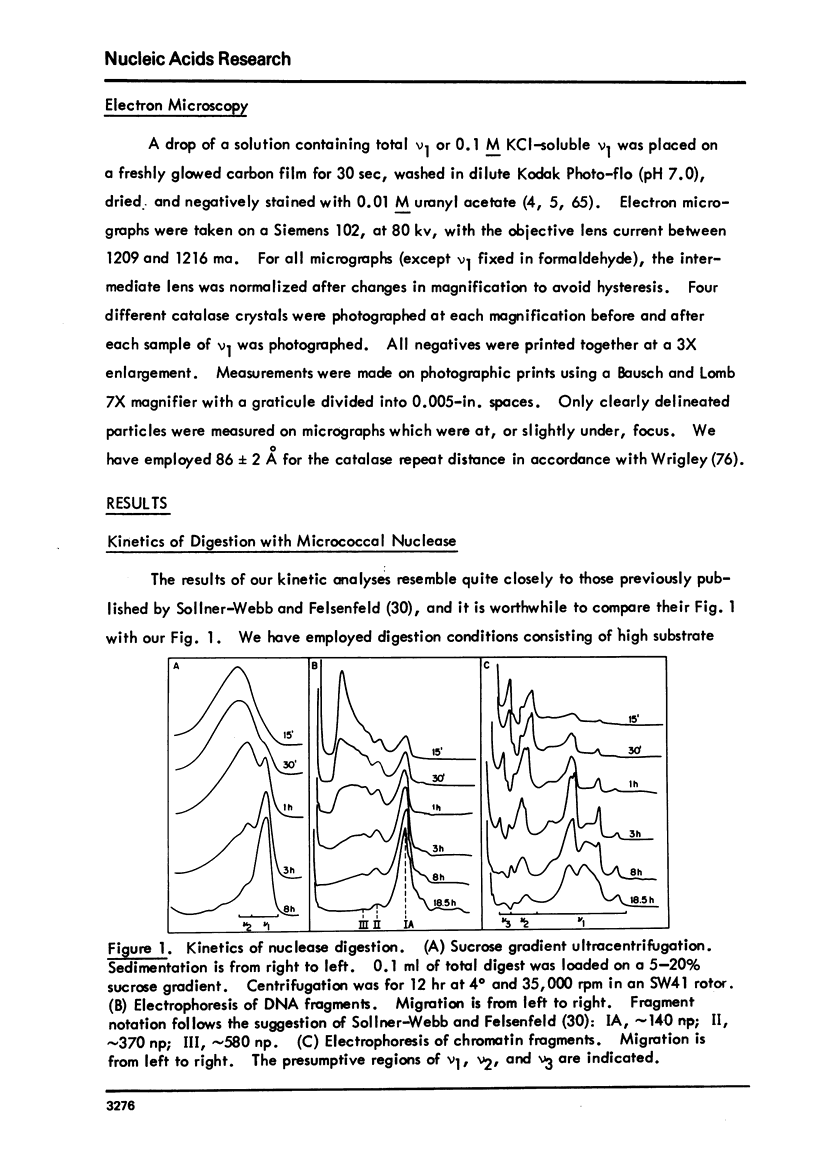

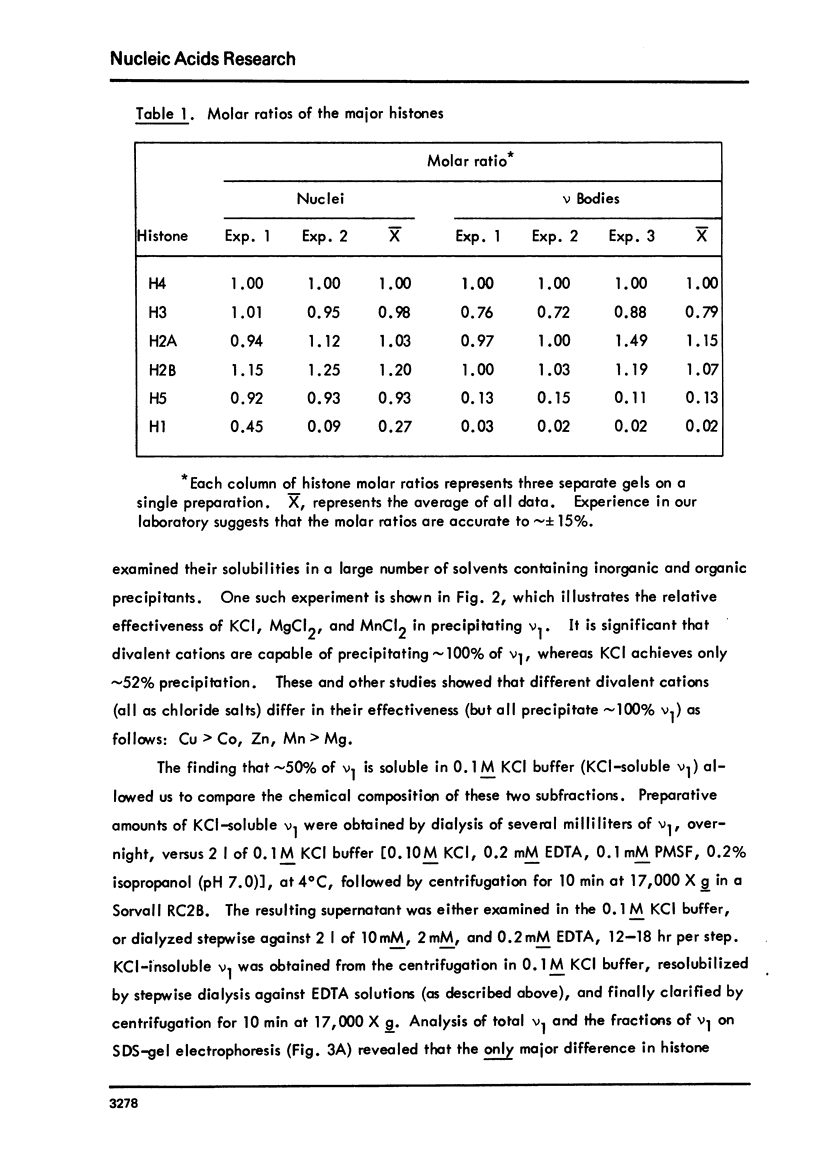
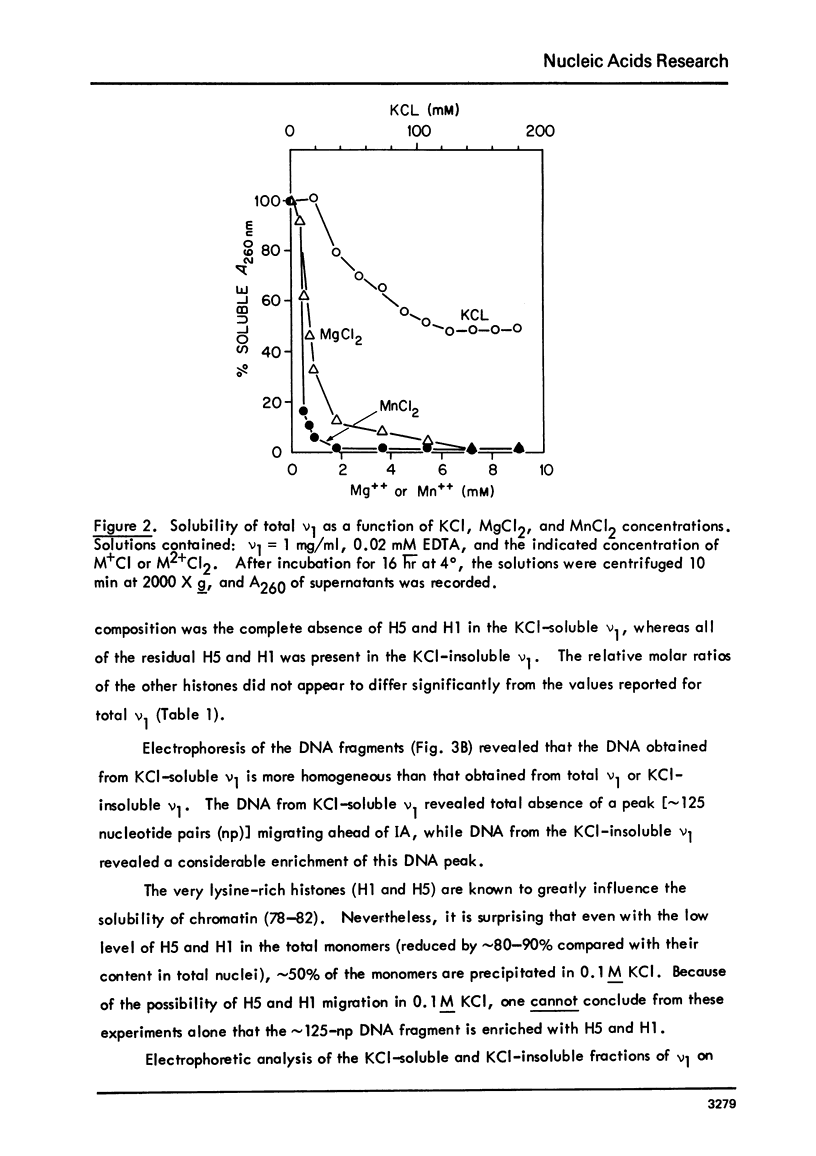
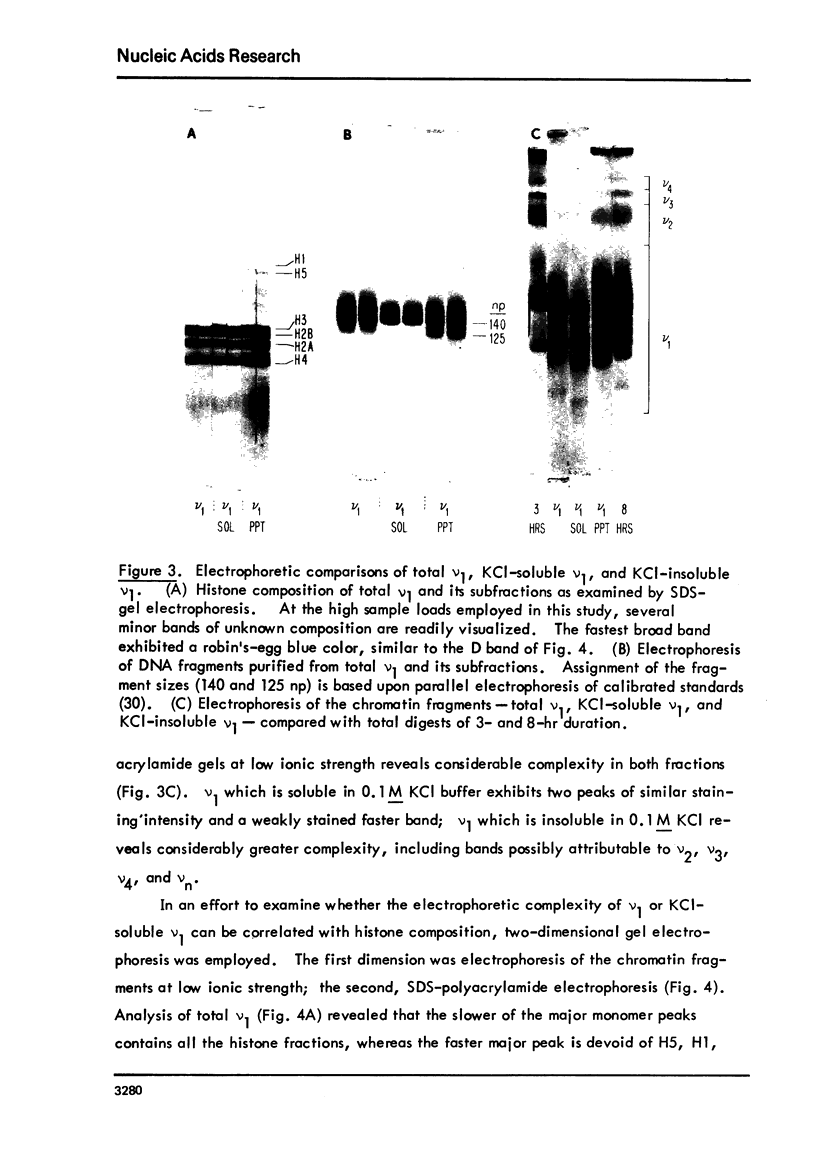

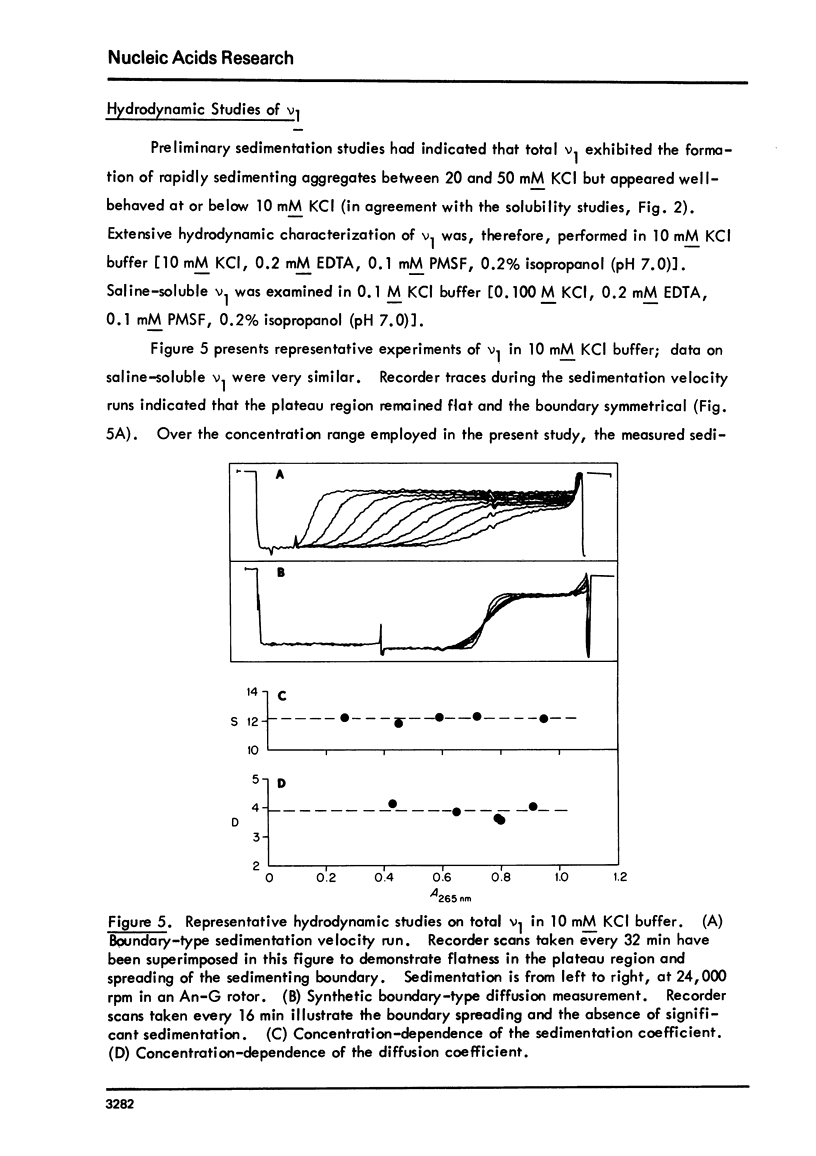
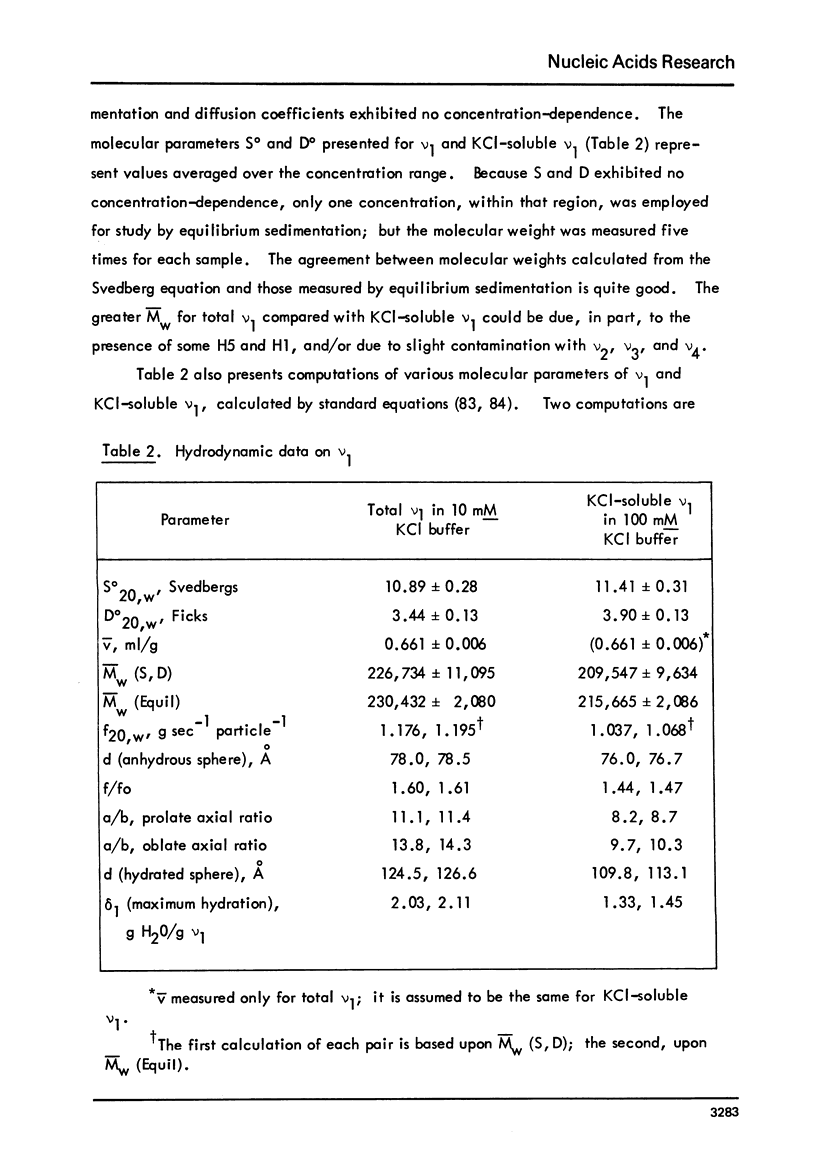

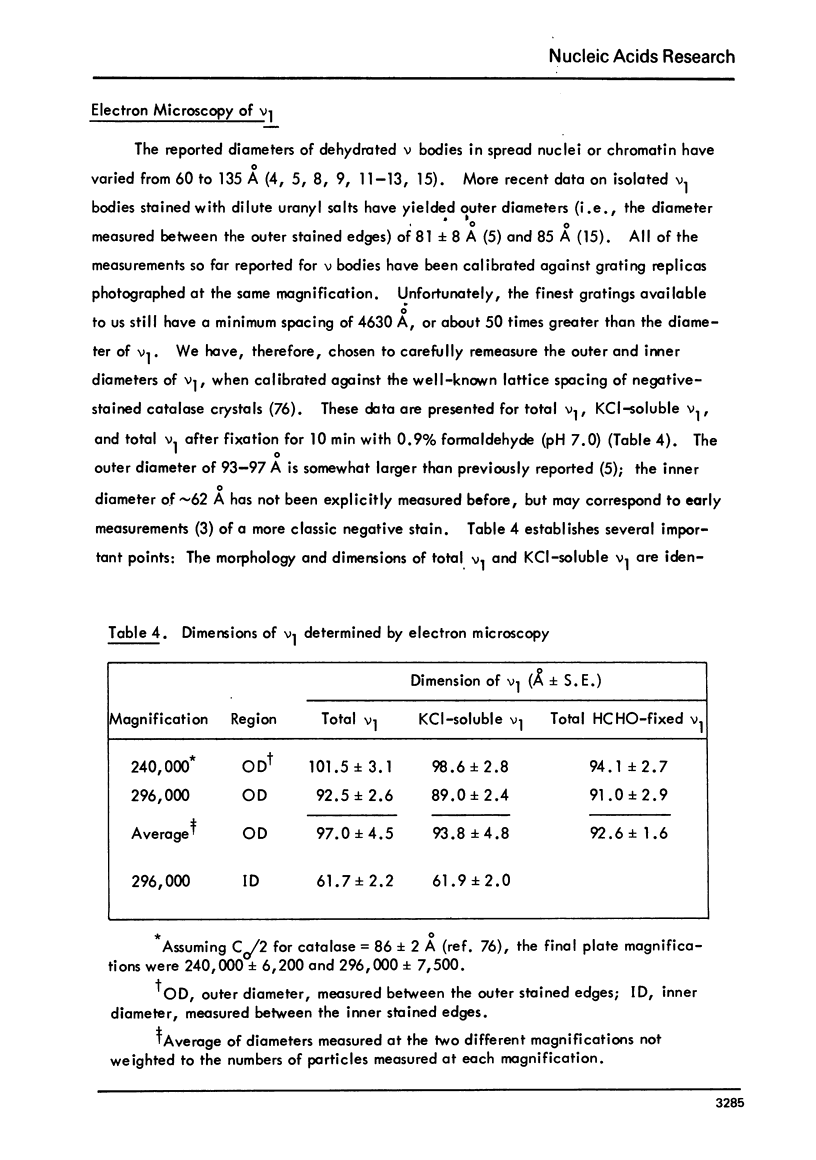






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augenlicht L. H., Lipkin M. Chromatin monomer: absence of non-histone proteins. Biochem Biophys Res Commun. 1976 May 17;70(2):540–544. doi: 10.1016/0006-291x(76)91080-9. [DOI] [PubMed] [Google Scholar]
- Axel R. Cleavage of DNA in nuclei and chromatin with staphylococcal nuclease. Biochemistry. 1975 Jul;14(13):2921–2925. doi: 10.1021/bi00684a020. [DOI] [PubMed] [Google Scholar]
- Axel R., Melchior W., Jr, Sollner-Webb B., Felsenfeld G. Specific sites of interaction between histones and DNA in chromatin. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4101–4105. doi: 10.1073/pnas.71.10.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakayev V. V., Melnickov A. A., Osicka V. D., Varshausky A. J. Studies on chromatin. II. Isolation and characterization of chromatin subunits. Nucleic Acids Res. 1975 Aug;2(8):1401–1419. doi: 10.1093/nar/2.8.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin J. P., Boseley P. G., Bradbury E. M., Ibel K. The subunit structure of the eukaryotic chromosome. Nature. 1975 Jan 24;253(5489):245–249. doi: 10.1038/253245a0. [DOI] [PubMed] [Google Scholar]
- Billett M. A., Barry J. M. Role of histones in chromatin condensation. Eur J Biochem. 1974 Dec 2;49(3):477–484. doi: 10.1111/j.1432-1033.1974.tb03852.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Pollard H. B. The presence of F3-F2a1 dimers and F1 oligomers in chromatin. Biochem Biophys Res Commun. 1975 May 5;64(1):282–288. doi: 10.1016/0006-291x(75)90250-8. [DOI] [PubMed] [Google Scholar]
- Boseley P. G., Bradbury E. M., Butler-Browne G. S., Carpenter B. G., Stephens R. M. Physical studies of chromatin. The recombination of histones with DNA. Eur J Biochem. 1976 Feb 2;62(1):21–31. doi: 10.1111/j.1432-1033.1976.tb10093.x. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Carpenter B. G., Rattle H. W. Magnetic resonance studies of deoxyribonucleoprotein. Nature. 1973 Jan 12;241(5385):123–126. doi: 10.1038/241123a0. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Danby S. E., Rattle H. W., Giancotti V. Studies on the role and mode of operation of the very-lysine-rich histone H1 (F1) in eukaryote chromatin. Histone H1 in chromatin and in H1 - DNA complexes. Eur J Biochem. 1975 Sep 1;57(1):97–105. doi: 10.1111/j.1432-1033.1975.tb02280.x. [DOI] [PubMed] [Google Scholar]
- Burgoyne L. A., Hewish D. R., Mobbs J. Mammalian chromatin substructure studies with the calcium-magnesium endonuclease and two-dimensional polyacrylamide-gel electrophoresis. Biochem J. 1974 Oct;143(1):67–72. doi: 10.1042/bj1430067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M., Goldblatt D., Sperling R. Chromatin structure visualization by immunoelectron microscopy. Cell. 1976 Feb;7(2):297–304. doi: 10.1016/0092-8674(76)90029-5. [DOI] [PubMed] [Google Scholar]
- Carlson R. D., Olins D. E. Chromatin model calculations: Arrays of spherical nu bodies. Nucleic Acids Res. 1976 Jan;3(1):89–100. doi: 10.1093/nar/3.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkley R. Histone propinquity using imidoesters. Biochem Biophys Res Commun. 1975 May 19;64(2):587–594. doi: 10.1016/0006-291x(75)90362-9. [DOI] [PubMed] [Google Scholar]
- Chalkley R., Hunter C. Histone-histone propinquity by aldehyde fixation of chromatin. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1304–1308. doi: 10.1073/pnas.72.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church R. B., McCarthy B. J. Related base sequences in the DNA of simple and complex organisms. II. The interpretation of DNA-RNA hybridization studies with mammalian nucleic acids. Biochem Genet. 1968 Jun;2(1):55–73. doi: 10.1007/BF01458451. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Jr, Isenberg I. A complex of histones IIb2 and IV. Biochemistry. 1973 Mar 13;12(6):1035–1043. doi: 10.1021/bi00730a003. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Jr, Isenberg I. A histone cross-complexing pattern. Biochemistry. 1974 Nov 19;13(24):4992–4997. doi: 10.1021/bi00721a019. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Jr, Isenberg I. Interactions of histone LAK (f2a2) with histones KAS (f2b) and GRK (f2a1). Biochemistry. 1974 May 7;13(10):2098–2104. doi: 10.1021/bi00707a016. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Klug A. Solenoidal model for superstructure in chromatin. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1897–1901. doi: 10.1073/pnas.73.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J. T., Noll M., Kornberg R. D. Electron microscopy of defined lengths of chromatin. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3320–3322. doi: 10.1073/pnas.72.9.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D. Chromatin structure: deduced from a minichromosome. Science. 1975 Mar 28;187(4182):1202–1203. doi: 10.1126/science.187.4182.1202. [DOI] [PubMed] [Google Scholar]
- Hardison R. C., Eichner M. E., Chalkley R. An approach to histone nearest neighbours in extended chromatin. Nucleic Acids Res. 1975 Oct;2(10):1751–1770. doi: 10.1093/nar/2.10.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Honda B. M., Baillie D. L., Candido E. P. Properties of chromatin subunits from developing trout testis. J Biol Chem. 1975 Jun 25;250(12):4643–4647. [PubMed] [Google Scholar]
- Howze G. B., Hsie A. W., Olins A. L. nu-Bodies in mitotic chromatin. Exp Cell Res. 1976 Jul;100(2):424–428. doi: 10.1016/0014-4827(76)90173-7. [DOI] [PubMed] [Google Scholar]
- Hyde J. E., Walker I. O. Covalent cross-linking of histones in chromatin. FEBS Lett. 1975 Feb 1;50(2):150–154. doi: 10.1016/0014-5793(75)80477-7. [DOI] [PubMed] [Google Scholar]
- Ilyin Y. V., Bayev A. A., Jr, Zhuze A. L., Varshavsky A. J. Histone-histone proximity in chromatin as seen by imidoester cross-linking. Mol Biol Rep. 1974 Mar;1(6):343–348. doi: 10.1007/BF00309568. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., Thomas J. O. Chromatin structure; oligomers of the histones. Science. 1974 May 24;184(4139):865–868. doi: 10.1126/science.184.4139.865. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., Thomas J. O. Chromatin structure; oligomers of the histones. Science. 1974 May 24;184(4139):865–868. doi: 10.1126/science.184.4139.865. [DOI] [PubMed] [Google Scholar]
- Lacy E., Axel R. Analysis of DNA of isolated chromatin subunits. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3978–3982. doi: 10.1073/pnas.72.10.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langmore J. P., Wooley J. C. Chromatin architecture: investigation of a subunit of chromatin by dark field electron microscopy. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2691–2695. doi: 10.1073/pnas.72.7.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littau V. C., Burdick C. J., Allfrey V. G., Mirsky S. A. The role of histones in the maintenance of chromatin structure. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1204–1212. doi: 10.1073/pnas.54.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr D., Van Holde K. E. Yeast chromatin subunit structure. Science. 1975 Apr 11;188(4184):165–166. doi: 10.1126/science.1090006. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Martinson H. G., McCarthy B. J. Histone-histone associations within chromatin. Cross-linking studies using tetranitromethane. Biochemistry. 1975 Mar 11;14(5):1073–1078. doi: 10.1021/bi00676a030. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Engel J. D. Subunit structure of chromatin is the same in plants and animals. Nature. 1975 Apr 3;254(5499):449–450. doi: 10.1038/254449a0. [DOI] [PubMed] [Google Scholar]
- Noll M. Internal structure of the chromatin subunit. Nucleic Acids Res. 1974 Nov;1(11):1573–1578. doi: 10.1093/nar/1.11.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll M. Subunit structure of chromatin. Nature. 1974 Sep 20;251(5472):249–251. doi: 10.1038/251249a0. [DOI] [PubMed] [Google Scholar]
- Noll M., Thomas J. O., Kornberg R. D. Preparation of native chromatin and damage caused by shearing. Science. 1975 Mar 28;187(4182):1203–1206. doi: 10.1126/science.187.4182.1203. [DOI] [PubMed] [Google Scholar]
- Olins A. L., Carlson R. D., Olins D. E. Visualization of chromatin substructure: upsilon bodies. J Cell Biol. 1975 Mar;64(3):528–537. doi: 10.1083/jcb.64.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins A. L., Olins D. E. Spheroid chromatin units (v bodies). Science. 1974 Jan 25;183(4122):330–332. doi: 10.1126/science.183.4122.330. [DOI] [PubMed] [Google Scholar]
- Olins A. L., Senior M. B., Olins D. E. Ultrastructural features of chromatin nu bodies. J Cell Biol. 1976 Mar;68(3):787–793. doi: 10.1083/jcb.68.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterhof D. K., Hozier J. C., Rill R. L. Nucleas action on chromatin: evidence for discrete, repeated nucleoprotein units along chromatin fibrils. Proc Natl Acad Sci U S A. 1975 Feb;72(2):633–637. doi: 10.1073/pnas.72.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudet P., Gross-Bellard M., Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975 Apr;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- Pardon J. F., Richards B. M., Skinner L. G., Ockey C. H. X-ray diffraction from isolated metaphase chromosomes. J Mol Biol. 1973 May 15;76(2):267–270. doi: 10.1016/0022-2836(73)90390-2. [DOI] [PubMed] [Google Scholar]
- Pardon J. F., Wilkins M. H. A super-coil model for nucleohistone. J Mol Biol. 1972 Jul 14;68(1):115–124. doi: 10.1016/0022-2836(72)90267-7. [DOI] [PubMed] [Google Scholar]
- Pardon J. F., Worcester D. L., Wooley J. C., Tatchell K., Van Holde K. E., Richards B. M. Low-angle neutron scattering from chromatin subunit particles. Nucleic Acids Res. 1975 Nov;2(11):2163–2176. doi: 10.1093/nar/2.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J., Gilmour R. S. Organ-specific restriction of transcription in mammalian chromatin. J Mol Biol. 1968 Jul 14;34(2):305–316. doi: 10.1016/0022-2836(68)90255-6. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Philippsen P., Streeck R. E., Zachau H. G. Defined fragments of calf, human, and rat DNA produced by restriction nucleases. Eur J Biochem. 1974 Jun 15;45(2):479–488. doi: 10.1111/j.1432-1033.1974.tb03573.x. [DOI] [PubMed] [Google Scholar]
- Rill R. L., Oosterhof D. K., Hozier J. C., Nelson D. A. Heterogeneity of chromatin fragments produced by micrococcal nuclease action. Nucleic Acids Res. 1975 Sep;2(9):1525–1538. doi: 10.1093/nar/2.9.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rill R., Van Holde K. E. Properties of nuclease-resistant fragments of calf thymus chromatin. J Biol Chem. 1973 Feb 10;248(3):1080–1083. [PubMed] [Google Scholar]
- Roark D. E., Geoghegan T. E., Keller G. H. A two-subunit histone complex from calf thymus. Biochem Biophys Res Commun. 1974 Jul 24;59(2):542–547. doi: 10.1016/s0006-291x(74)80014-8. [DOI] [PubMed] [Google Scholar]
- Sahasrabuddhe C. G., Van Holde K. E. The effect of trypsin on nuclease-resistant chromatin fragments. J Biol Chem. 1974 Jan 10;249(1):152–156. [PubMed] [Google Scholar]
- Senior M. B., Olins A. L., Olins D. E. Chromatin fragments resembling v bodies. Science. 1975 Jan 17;187(4172):173–175. doi: 10.1126/science.1111096. [DOI] [PubMed] [Google Scholar]
- Shaw B. R., Corden J. L., Sahasrabuddhe C. G., Van Holde K. E. Chromatographic separation of chromatin subunits. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1193–1198. doi: 10.1016/s0006-291x(74)80410-9. [DOI] [PubMed] [Google Scholar]
- Shaw B. R., Herman T. M., Kovacic R. T., Beaudreau G. S., Van Holde K. E. Analysis of subunit organization in chicken erythrocyte chromatin. Proc Natl Acad Sci U S A. 1976 Feb;73(2):505–509. doi: 10.1073/pnas.73.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T., Whitlock J. P., Jr Chemical evidence that chromatin DNA exists as 160 base pair beads interspersed with 40 base pair bridges. Nucleic Acids Res. 1976 Jan;3(1):117–127. doi: 10.1093/nar/3.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner-Webb B., Felsenfeld G. A comparison of the digestion of nuclei and chromatin by staphylococcal nuclease. Biochemistry. 1975 Jul;14(13):2915–2920. doi: 10.1021/bi00684a019. [DOI] [PubMed] [Google Scholar]
- Spadafora C., Geraci G. The subunit structure of sea urchin sperm chromatin: a kinetic approach. FEBS Lett. 1975 Sep 1;57(1):79–82. doi: 10.1016/0014-5793(75)80156-6. [DOI] [PubMed] [Google Scholar]
- Sperling L. The mass per unit length of chromatin by low-angle x-ray scattering. FEBS Lett. 1976 Apr 15;64(1):89–91. doi: 10.1016/0014-5793(76)80256-6. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lente F., Jackson J. F., Weintraub H. Identification of specific crosslinked histones after treatment of chromatin with formaldehyde. Cell. 1975 May;5(1):45–50. doi: 10.1016/0092-8674(75)90090-2. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Georgiev G. P. Heterogeneity of chromatin subunits in vitro and location of histone H1. Nucleic Acids Res. 1976 Feb;3(2):477–492. doi: 10.1093/nar/3.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V. Studies on chromatin. III. v-Bodies and free DNA in chromatin lacking histone H1. Mol Biol Rep. 1975 Oct;2(3):209–217. doi: 10.1007/BF00356990. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V. Studies on chromatin. IV. Evidence for a toroidal shape of chromatin subunits. Mol Biol Rep. 1975 Oct;2(3):247–254. doi: 10.1007/BF00356995. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Palter K., Van Lente F. Histones H2a, H2b, H3, and H4 form a tetrameric complex in solutions of high salt. Cell. 1975 Sep;6(1):85–110. doi: 10.1016/0092-8674(75)90077-x. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Van Lente F. Dissection of chromosome structure with trypsin and nucleases. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4249–4253. doi: 10.1073/pnas.71.10.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock C. L., Safer J. P., Stanchfield J. E. Structural repeating units in chromatin. I. Evidence for their general occurrence. Exp Cell Res. 1976 Jan;97:101–110. doi: 10.1016/0014-4827(76)90659-5. [DOI] [PubMed] [Google Scholar]
- Woodhead L., Johns E. W. The isolation of nucleosomes from saline-washed chromatin. FEBS Lett. 1976 Feb 15;62(2):115–117. doi: 10.1016/0014-5793(76)80031-2. [DOI] [PubMed] [Google Scholar]
- Wright E. B., Olins D. E. Histone stoichiometry in chicken erythrocyte nuclei. Biochem Biophys Res Commun. 1975 Apr 7;63(3):642–650. doi: 10.1016/s0006-291x(75)80432-3. [DOI] [PubMed] [Google Scholar]
- Wrigley N. G. The lattice spacing of crystalline catalase as an internal standard of length in electron microscopy. J Ultrastruct Res. 1968 Sep;24(5):454–464. doi: 10.1016/s0022-5320(68)80048-6. [DOI] [PubMed] [Google Scholar]
- van Bruggen E. F., Arnberg A. C., van Holde K. E., Sahasrabuddhe C. G., Shaw B. R. Electron microscopy of chromatin subunit particles. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1365–1370. doi: 10.1016/0006-291x(74)90348-9. [DOI] [PubMed] [Google Scholar]