Abstract
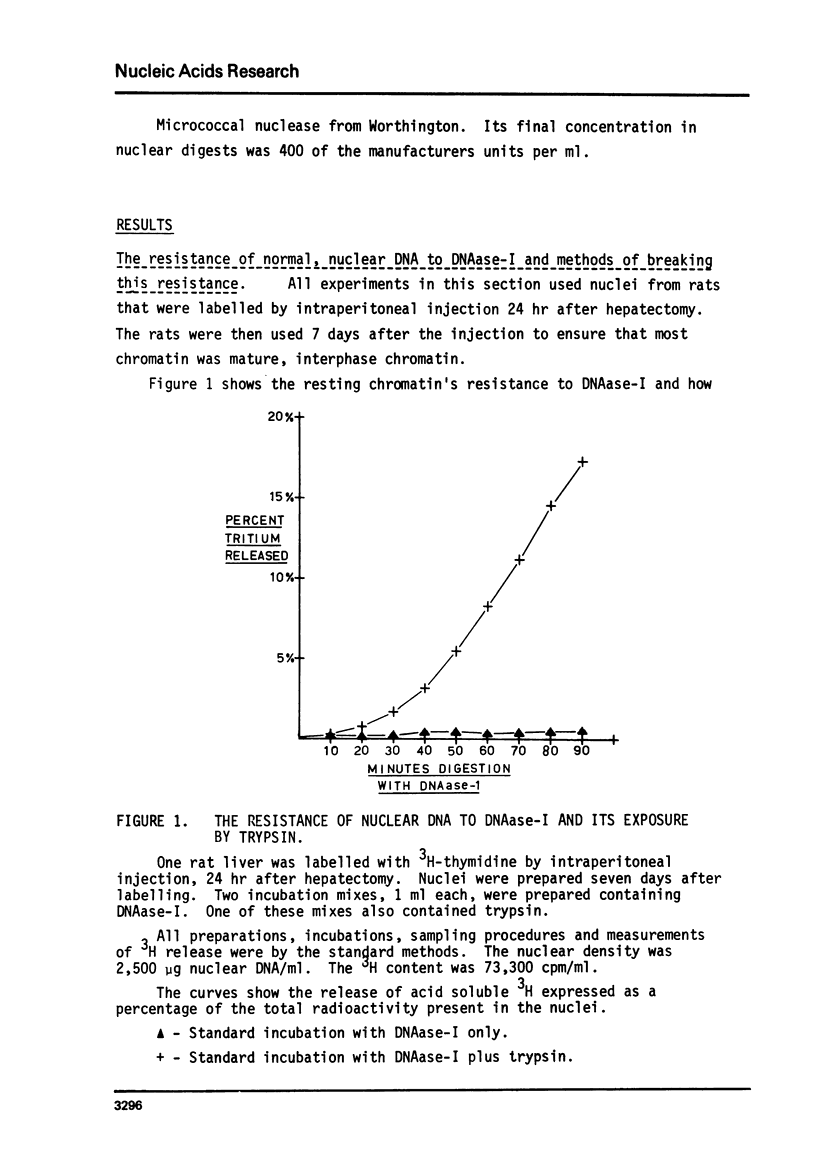
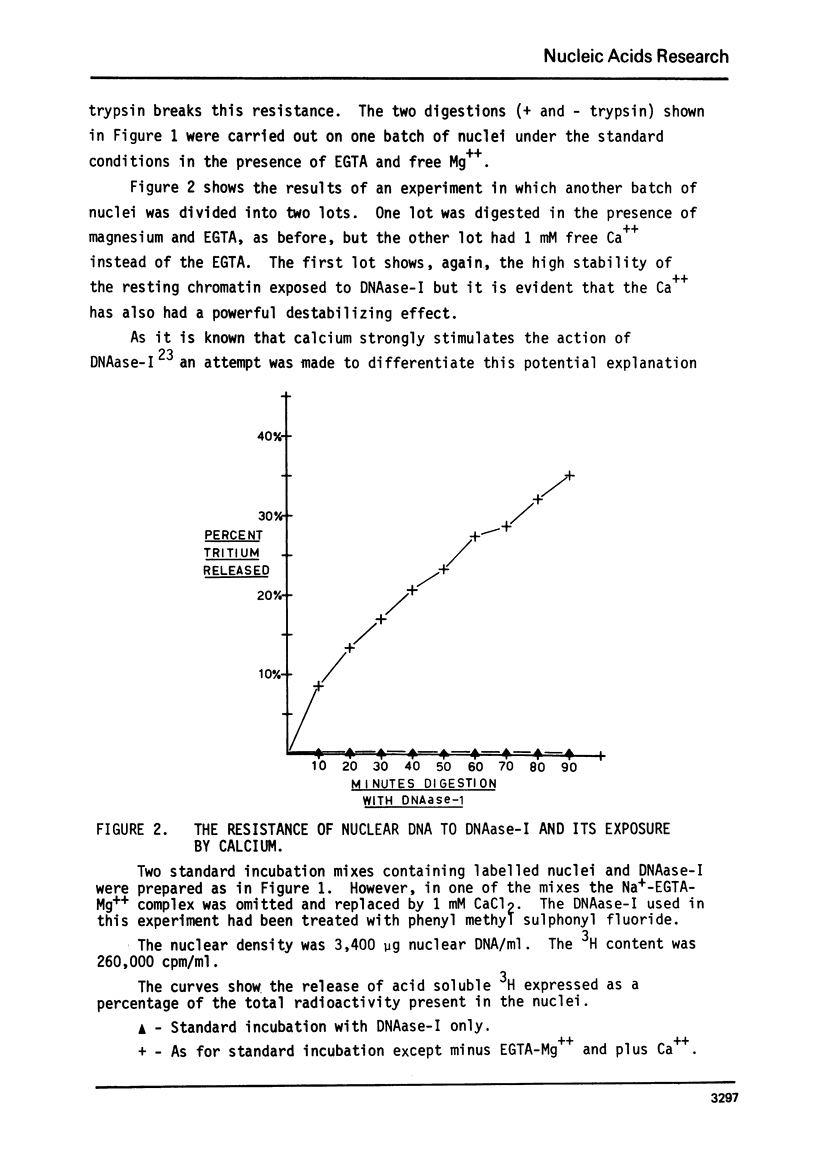
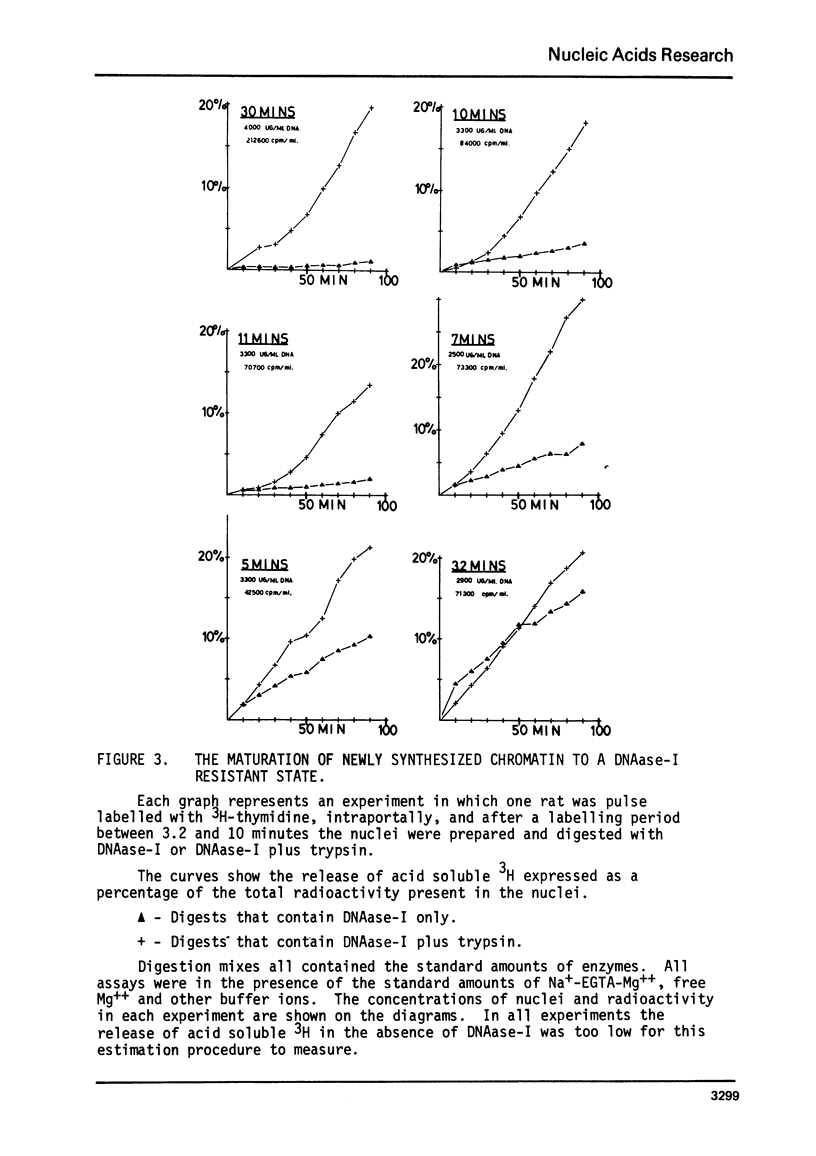
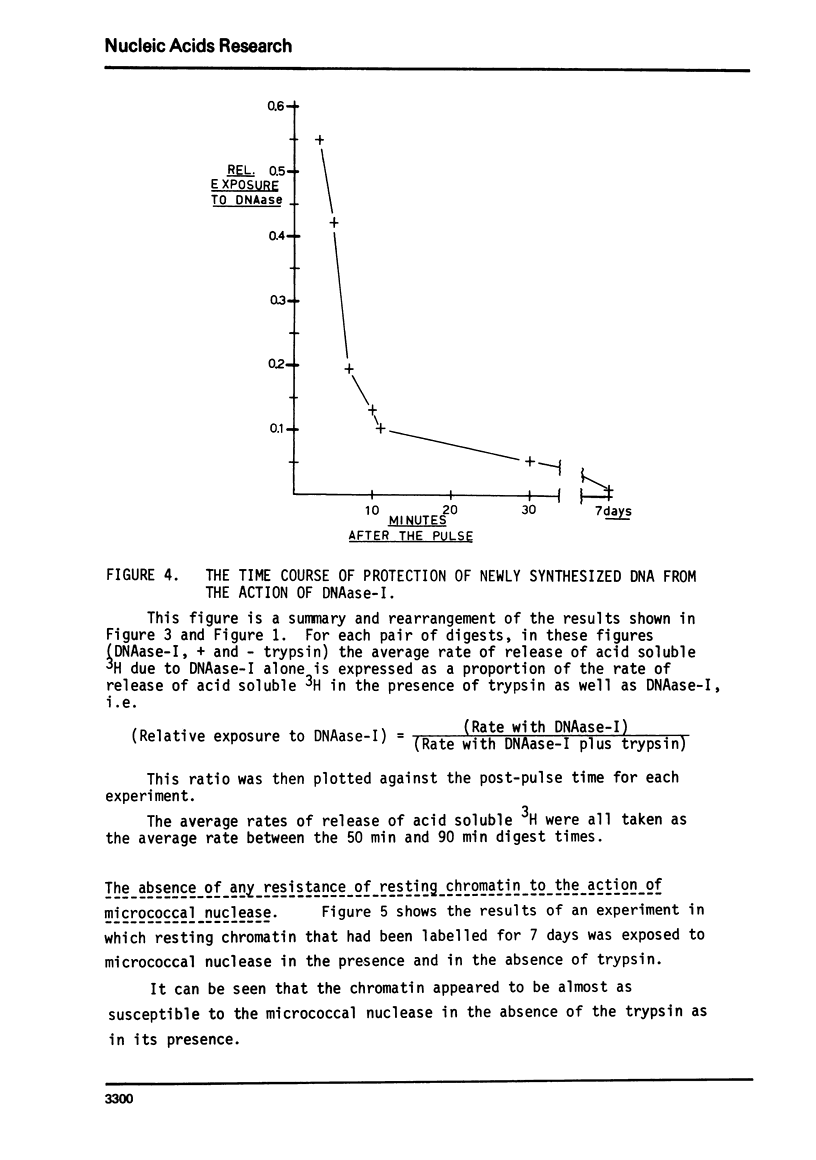
The action of a number of enzymes and metals on one nuclear preparation were interpreted in terms of the existence of a fragile but highly DNAase-I resistant feature of chromatin superstructure. The generation of this DNAase-I resistance feature of chromatin was then followed during normal DNA synthesis in the regenerating rat liver by following the disappearance of a transitory DNAase-I susceptible state. This transitory, DNAase-I susceptible state appears to be extremely similar to the post-synthetic, DNAase-I susceptible state that has been described in He La32.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axel R. Cleavage of DNA in nuclei and chromatin with staphylococcal nuclease. Biochemistry. 1975 Jul;14(13):2921–2925. doi: 10.1021/bi00684a020. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury E. M., Molgaard H. V., Stephens R. M., Bolund L. A., Johns E. W. X-ray studies of nucleoproteins depleted of lysine-rich histone. Eur J Biochem. 1972 Dec 18;31(3):474–482. doi: 10.1111/j.1432-1033.1972.tb02555.x. [DOI] [PubMed] [Google Scholar]
- Burgoyne L. A., Hewish D. R., Mobbs J. Mammalian chromatin substructure studies with the calcium-magnesium endonuclease and two-dimensional polyacrylamide-gel electrophoresis. Biochem J. 1974 Oct;143(1):67–72. doi: 10.1042/bj1430067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne L. A., Mobbs J. The reaction of the Ca-Mg endonuclease with the A-sites of rat nucleoprotein. Nucleic Acids Res. 1975 Sep;2(9):1551–1558. doi: 10.1093/nar/2.9.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne L. A., Wagar M. A., Atkinson M. R. Calcium-dependent priming of DNA synthesis in isolated rat liver nuclei. Biochem Biophys Res Commun. 1970 Apr 24;39(2):254–259. doi: 10.1016/0006-291x(70)90786-2. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Walker I. O. The modification of deoxyribonucleohistone by trypsin and chymotrypsin. Eur J Biochem. 1973 May 2;34(3):519–526. doi: 10.1111/j.1432-1033.1973.tb02789.x. [DOI] [PubMed] [Google Scholar]
- Chong M. T., Garrard W. T., Bonner J. Purification and properties of a neutral protease from rat liver chromatin. Biochemistry. 1974 Dec 3;13(25):5128–5134. doi: 10.1021/bi00722a012. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. The calcium dependent endonuclease activity of isolated nuclear preparations. Relationships between its occurrence and the occurrence of other classes of enzymes found in nuclear preparations. Biochem Biophys Res Commun. 1973 May 15;52(2):475–481. doi: 10.1016/0006-291x(73)90736-5. [DOI] [PubMed] [Google Scholar]
- Hyde J. E., Walker I. O. A model for chromatin sub-structure incorporating symmetry considerations of histone oligomers. Nucleic Acids Res. 1975 Mar;2(3):405–421. doi: 10.1093/nar/2.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U. Molecular weight and amino acid composition of deoxyribonuclease I. Biochemistry. 1967 Jan;6(1):335–342. doi: 10.1021/bi00853a050. [DOI] [PubMed] [Google Scholar]
- Littau V. C., Burdick C. J., Allfrey V. G., Mirsky S. A. The role of histones in the maintenance of chromatin structure. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1204–1212. doi: 10.1073/pnas.54.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A. J., Burgoyne L. A. Interpretation of the properties of chromatin extracts from mammalian nuclei. Nucleic Acids Res. 1976 Apr;3(4):1101–1110. doi: 10.1093/nar/3.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll M. Internal structure of the chromatin subunit. Nucleic Acids Res. 1974 Nov;1(11):1573–1578. doi: 10.1093/nar/1.11.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll M. Subunit structure of chromatin. Nature. 1974 Sep 20;251(5472):249–251. doi: 10.1038/251249a0. [DOI] [PubMed] [Google Scholar]
- Oudet P., Gross-Bellard M., Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975 Apr;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- Prescott D. M. The syntheses of total macronuclear protein, histone, and DNA during the cell cycle in Euplotes eurystomus. J Cell Biol. 1966 Oct;31(1):1–9. doi: 10.1083/jcb.31.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffhill R., Itzhaki R. F. Accessibility of chromatin to DNA polymerase I and location of the F1 histone. Nucleic Acids Res. 1975 Jan;2(1):113–119. doi: 10.1093/nar/2.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahasrabuddhe C. G., Van Holde K. E. The effect of trypsin on nuclease-resistant chromatin fragments. J Biol Chem. 1974 Jan 10;249(1):152–156. [PubMed] [Google Scholar]
- Seale R. L. Assembly of DNA and protein during replication in HeLa cells. Nature. 1975 May 15;255(5505):247–249. doi: 10.1038/255247a0. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Modification of chromatin by trypsin. The role of proteins in maintainance of deoxyribonucleic acid conformation. Biochemistry. 1972 May 23;11(11):2003–2008. doi: 10.1021/bi00761a002. [DOI] [PubMed] [Google Scholar]
- Taniuchi H., Anfinsen C. B., Sodja A. The amino acid sequence of an extracellular nuclease of Staphylococcus aureus. 3. Complete amino acid sequence. J Biol Chem. 1967 Oct 25;242(20):4752–4758. [PubMed] [Google Scholar]
- Van Holde K. E., Sahasrabuddhe C. G., Shaw B. R. A model for particulate structure in chromatin. Nucleic Acids Res. 1974 Nov;1(11):1579–1586. doi: 10.1093/nar/1.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Georgiev G. P. Heterogeneity of chromatin subunits in vitro and location of histone H1. Nucleic Acids Res. 1976 Feb;3(2):477–492. doi: 10.1093/nar/3.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIBERG J. S. On the mechanism of metal activation of deoxyribobuclease I. Arch Biochem Biophys. 1958 Feb;73(2):337–358. doi: 10.1016/0003-9861(58)90280-7. [DOI] [PubMed] [Google Scholar]
- Waqar M. A., Burgoyne L. A., Atkinson M. R. Deoxyribonucleic acid synthesis in mammalian nuclei. Incorporation of deoxyribonucleotides and chain-terminating nucleotide analogues. Biochem J. 1971 Mar;121(5):803–809. doi: 10.1042/bj1210803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. The assembly of newly replicated DNA into chromatin. Cold Spring Harb Symp Quant Biol. 1974;38:247–256. doi: 10.1101/sqb.1974.038.01.028. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Van Lente F. Dissection of chromosome structure with trypsin and nucleases. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4249–4253. doi: 10.1073/pnas.71.10.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]



