Abstract
Objective
To demonstrate the high efficiency and rapidity of allotopic expression of a normal human ND4 subunit of complex I in the vertebrate retina using a self-complementary adeno-associated virus (scAAV) vector for ocular gene delivery to treat acute visual loss in Leber hereditary optic neuropathy (LHON).
Methods
The nuclear-encoded human ND4 subunit fused to the P1 isoform of subunit C of adenosine tri-phosphate synthase (ATPc) mitochondrial targeting sequence and FLAG epitope was packaged in scAAV2 capsids or single-stranded (ss) AAV2 capsids. These constructs were injected into the vitreous cavities of mice. The contralateral eyes were injected with scAAV–green fluorescent protein (GFP). One week later, pattern electroretinograms and gene expression of the human ND4 subunit and GFP were evaluated. Quantitative analysis of ND4FLAG-injected eyes was assessed relative to Thy1.2-labeled retinal ganglion cells (RGCs).
Results
Pattern electroretinogram amplitudes remained normal in eyes inoculated with scAAV-ND4FLAG, ssAAV-ND4FLAG, and GFP. Confocal microscopy revealed the typical perinuclear mitochondrial expression of scAAV-ND4FLAG in almost the entire retinal flat mount. In contrast, scAAV-GFP expression was cytoplasmic and nuclear. Relative to Thy1.2-positive RGCs, quantification of scAAV-ND4FLAG–positive RGCs was 91% and that of ssAAV-ND4FLAG–positive RGCs was 51%.
Conclusion
Treatment of acute visual loss due to LHON may be possible with a normal human ND4 subunit gene of complex I, mutated in most cases of LHON, when delivered by an scAAV vector.
Clinical Relevance
Unlike most retinal degenerations that result in slowly progressive loss of vision over many years, LHON due to mutated mitochondrial DNA results in apoplectic, bilateral severe and usually irreversible visual loss. For rescue of acute visual loss in LHON, a highly efficient and rapid gene expression system is required.
Leber hereditary optic neuropathy (LHON) is a maternally inherited disease characterized by acute bilateral loss of central vision.1 In approximately 95% of the cases worldwide, LHON is caused by 3 pathogenic point mutations in mitochondrial DNA (mtDNA) coding for the respiratory chain subunits of complex I genes (the reduced form of nicotinamide adenine dinucleotide–ubiquinone-oxidoreductase), namely m.3460G>A in ND1, m.11778G>A in ND4, and m.14484T>C in ND6.2 Of these 3 mutations, 11778G>A, resulting in an arginine to histidine substitution at amino acid 340, is responsible for half of all LHON cases. Patients with this mutation exhibit the poorest prognosis for spontaneous visual improvement, and there is no effective therapy.3 Most patients with LHON carry the mtDNA mutations in homoplasmic condition, that is, they have no normal ND4 mtDNA. Thus, an effective therapeutic approach would be introduction of normal mtDNA into the affected cells (ganglion cells of the retina) in these patients. One of the major limitations in this aspect is that few practical methods for delivering genes to the mitochondria are available.4,5 To address this, we and other groups adapted an approach termed allotopic expression, in which the mutant mitochondrial gene (in this case ND4) is expressed in the nucleus, translated on cytoplasmic polyribosomes, and transported into mitochondria with the addition of an N-terminal mitochondrial targeting sequence.6-8 Allotopic expression of the normal ND4 gene normalized the defective adenosine triphosphate (ATP) synthesis of LHON cells and improved cell survival.9
Recombinant adeno-associated virus (AAV) vectors have been used safely for ocular gene delivery in a disorder affecting photoreceptors, Leber congenital amaurosis.10,11 Adeno-associated virus is derived from the nonpathogenic virus belonging to the Dependovirus genus of the Parvoviridae family and contains a linear single-stranded DNA genome enclosed in a capsid composed of 3 proteins: VP1, VP2, and VP3.12,13 Studies on the hybrid vectors or the pseudotyped vectors containing the promoter and the trans-gene flanked by the 145–base pair (bp) AAV inverted terminal repeats packaged into different capsid serotypes not only reveal cellular tropism in different organs but also show an increased efficiency for specific tissues.14,15 In a previous study, we showed that the human ND4 subunit was expressed in only one-third of retinal ganglion cells (RGCs) 1 month after intravitreal injections in mice. For treatment of acute visual loss in LHON, a more efficient and rapid gene expression delivery system is needed.
Strategies to improve the efficiency of transgene expression are being continuously explored by manipulating the AAV genome. One such development is the self-complementary AAV (scAAV) vector with a double-stranded vector genome. This vector was generated by deleting the terminal resolution site from one of the inverted terminal repeats of recombinant AAV (rAAV), which prevents the initiation of replication at the mutated end.16 This strategy allows packaging of a self-complementary form of vector DNA that has the ability of annealing to itself to form a double-stranded DNA immediately on vector delivery to the target cell nucleus. This overcomes the rate-limiting step of replication of the single-stranded viral genome into the double-stranded DNA within the cell nucleus, thereby increasing the efficiency and speed of transgene expression manyfold, as observed in the liver,17,18 brain,19 eye,20,21 and heart.22 Whether scDNA is single or double stranded while inside the AAV vector capsid is unclear at present. In this study, we compared expression of the human ND4 subunit gene when delivered by scAAV relative to that when delivered by the single-stranded AAV (ssAAV).
METHODS
CONSTRUCTION OF HUMAN ND4FLAG AND AAV VECTORS
To construct the fusion gene containing the mitochondrial targeting sequences and epitope tag, synthetic 80-mer oligonucleo-tide pairs were created in the nuclear genetic code and codons prevalent in highly expressed nuclear genes to conserve the amino acid sequence. The synthetic oligonucleotides were overlapped by approximately 20 complementary nucleotides serving as primers for polymerase chain reaction with the high fidelity of pfu turbo DNA polymerase (Stratagene, La Jolla, California) until the entire 1377-nucleotide nuclear-encoded ND4 gene was constructed. Using this technique, the ND4 gene was then fused in-frame to the ATP1 mitochondrial targeting sequence and FLAG epitope tags. To complete generation of the wild-type ND4, base deletions and substitutions in the reading frame were corrected using an in vitro mutagenesis kit (QuickChange; Stratagene). Flanking XbaI restriction sites were added for cloning the human P1ND4FLAG gene into AAV vectors. The entire reading frame of the human P1ND4FLAG fusion gene was cloned in the XbaI sites of ssAAV plasmid vector pTR-UF22 (regulated by the 381-bp cytomegalovirus (CMV) immediate early gene enhancer/ 1352-bp chicken β-actin promoter-exon 1–intron 1 woodchuck posttranscriptional regulatory element) or scAAV (regulated by short hybrid CMV immediate early gene enhancer/chicken β-actin promoter).
The ssAAV-ND4FLAG, scAAV type 2 (scAAV2), scAAVND4FLAG, or a control scAAV2(mut444)–GFP were produced by the plasmid cotransfection method.23 The plasmids were amplified and purified by means of cesium chloride gradient centrifugation and then packaged into AAV2 capsids by transfection into human 293 cells using standard procedures. In brief, the crude iodixanol fractions were purified using a fast protein liquid chromatography system (AKTA; Amersham Pharmacia, Piscataway, New Jersey), the vector was then eluted from the column using 215mM sodium chloride (pH, 8.0), and the rAAV peak was collected. Vector-containing fractions were then concentrated and buffer exchanged in balanced salt solution (Alcon Laboratories, Fort Worth, Texas) with 0.014% polysorbate 20 (Tween 20; Jiangsu Haian Petrochemical Plant, Jiangsu, China), using a centrifugation concentrator (Biomax 100 K; Millipore, Billerica, Massachusetts). Vector was then titered for DNase-resistant vector genomes by real-time polymerase chain reaction relative to a standard. Finally, the purity of the vector was validated by silver-stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis, assayed for sterility and lack of endotoxin, divided into aliquots, and stored at –80°C.
INTRAOCULAR INJECTIONS
All animal procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. For the intraocular injection of rAAV, DBA/1J mice were sedated by inhalation with 1.5% to 2% isoflurane. A local anesthetic (proparacaine hydrochloride) was applied topically to the cornea, and a 32-gauge needle attached to a Hamilton syringe was inserted through the pars plana under the dissecting microscope. One microliter of scAAVND4FLAG (1.01 × 1012 particles/mL) (n = 6) or ssAAV-ND4FLAG (2.35×1012 particles/mL) (n=6) was injected into one eye and the scAAV-GFP (1.03×1012 particles/mL) was injected in the contralateral eye as an internal control.
FLAT MOUNTS, CRYOSECTIONS, AND IMMUNOLABELING
One week after the viral injections, the mice were humanely killed, and the eyes were removed and fixed with 4% paraformalde-hyde in phosphate-buffered saline solution (PBS) for 1 hour. The eyes were then transferred to 0.4% paraformaldehyde overnight. To make flat mounts, the cornea and crystalline lens were removed, and the entire retina was carefully dissected from the eyecup. Four radial cuts were made from the edge to the equator of the retina to make it flat. After 3 washes in PBS, retinas were permeablized with 0.5% Triton X-100 (Dow Chemical Corporation, Midland, Michigan) in PBS for 1 hour followed by incubation with a blocking solution of the 0.5% Triton X-100 and 10% goat serum for 1 hour. The flat-mounted retinas were then rinsed in PBS and incubated with a mixture of mouse monoclonal anti-FLAG M2 antibodies conjugated to Cy3 (1:100) (Sigma-Aldrich Corp, St Louis, Missouri) or polyclonal rabbit anti-GFP antibodies (1:400) (Abcam, Cambridge, Massachusetts) along with monoclonal rat Thy1.2 (1:200) (Abcam) overnight at 4°C. Primary antibody solution was contained in 10% goat serum and 0.2% Triton X-100 in PBS (pH, 7.4). After 3 washes with PBS, the retinas were treated with the secondary antibody, goat anti-rabbit Cy2 (Jackson Immunoresearch Laboratories, Inc, West Grove, Pennsylvania), goat antimouse Cy2 (Jackson Immunore-search Laboratories, Inc), goat antimouse Cy3 (Jackson Immunoresearch Laboratories, Inc), or goat antimouse IgG (Alexa Fluor 488; Invitrogen, San Diego, California) and 4′,6-diamidino-2-phenylindole (2μg/mL) (Santa Cruz Biotechnology, Inc, Santa Cruz, California), in 1:500 dilution contained in 10% goat serum and 0.2% Triton X-100 and incubated at 4°C overnight. The retinal tissues were finally washed 3 times in PBS. The whole mounts were then placed on a glass slide (RGC layer facing up), coverslipped, and observed for fluorescence with a confocal microscope (Leica TCS SP5; Leica, Wetzlar, Germany). For cryo-sections, the retinas were incubated overnight in 30% sucrose and then embedded in optimal cutting temperature embedding compound (Sakura Finetek, Torrance, California). Retinal sections, 8 μm in thickness, were then mounted with fluorescent mounting medium (Vectashield; Vector Laboratories, Burlingame, California) and examined for fluorescence using the confocal microscope.
QUANTITATIVE ANALYSIS
The FLAG-positive cells (in ssAAV2-injected and in double-stranded AAV2–injected) GFP and Thy1.2 immunopositive RGCs were counted on the longitudinal sections. The total cell count was an average of the counting data from the longitudinal sections of the retina per 40-mm2 area. The images were captured with a video camera mounted on a fluorescent microscope at a magnification of ×400 for counting the cells.
ELECTROPHYSIOLOGY
Pattern electroretinograms (PERGs) were obtained in 9 mice before and 1 week after the viral injection. The PERG technique has been previously described.24 In brief, mice were weighed and anesthetized using intraperitoneal injections with a mixture of ketamine hydrochloride (80 mg per kilogram of body weight) and xylazine hydrochloride (10 mg per kilogram of body weight) and were restrained using a bite bar and a nose holder that allowed unobstructed vision. The animals were kept at a constant body temperature of 37.6°C with a feedback-controlled heating pad. In these conditions, the eyes of mice were wide open and in a stable position, with undilated pupils pointing laterally and upward. The PERG electrode, with a diameter of 0.25 mm and made of silver wire configured to a semicircular loop of a 2-mm radius, was placed on the corneal surface by means of a micro-manipulator and positioned to encircle the pupil without limiting the field of view. Reference and ground electrodes were stainless steel needles inserted under the skin of the scalp and tail, respectively. After setting the mice on the stage and before recording, a small drop of balanced salt solution was topically applied on the cornea to prevent drying. A visual stimulus of contrast-reversing bars (field area, 50°× 58°; mean luminance, 50 candela (cd)/m2; spatial frequency, 0.05 cycles per degree; contrast, 100%; and temporal frequency, 1 Hz) was aligned with the projection of the pupil at a distance of 20 cm. Eyes were not refracted for the viewing distance because the mouse eye has a large depth of focus due to the pinhole pupil. Retinal signals were amplified (10 000-fold) and band-pass filtered (1-30 Hz). Three consecutive responses to 600 contrast reversals each were recorded. The responses were superimposed to check for consistency and then averaged. The PERG is a light-adapted response. To have a corresponding index of outer retinal function, a light-adapted flash ERG (FERG) was also recorded with un-dilated pupils in response to strobe flashes of 20 cd/m2 per sec ond superimposed on a steady background light of 12 cd/m2 and presented within a Ganzfeld bowl. Under these conditions, rod activity is largely suppressed and cone activity is minimally suppressed. Averaged PERGs and FERGs were automatically analyzed to evaluate the major positive and negative waves using commercially available software (Sigma Plot; Systat Software Inc, San Jose, California).
STATISTICAL ANALYSIS
Values are expressed as mean(SD). Data were analyzed using the t test for paired or unpaired data. P<.05 was considered significant, and P<.01 was considered highly significant.
RESULTS
EFFECTS OF AAV ON TRANSGENE EXPRESSION: IMMUNOHISTOCHEMISTRY
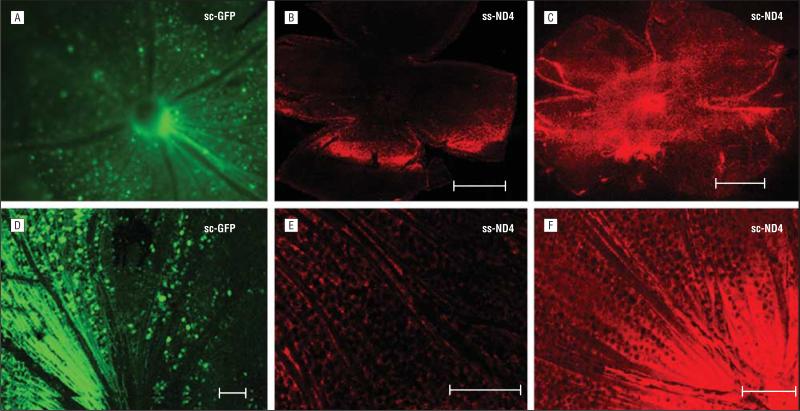
Retinal whole-mount analysis of DBA/1J mice examined 1 week after intravitreal injections showed a nuclear and cytoplasmic pattern of expression for GFP (Figure 1A), whereas ND4FLAG expression was seen in a perinuclear pattern (Figure 1B, C, E, and F) associated with mitochondrial localization. Qualitatively, transgene expression was substantially greater in eyes injected with scAAV2-GFP (Figure 1A and D) or scAAV2-ND4FLAG (Figure 1C and F), with expression evident throughout the entire retina in contrast to the ssAAV2-ND4FLAG (Figure 1B and E).
Figure 1.
Confocal microscopy of retinal whole mounts. A, Green fluorescent protein (GFP) cells surround the optic nerve head of an eye injected with self-complementary adeno-associated virus type 2 (scAAV2)–chicken β-actin (CBA)–GFP. B, Weak expression of ND4FLAG is seen in a single-stranded (ss) AAV2-CBA-ND4–injected eye. C, Strong ND4FLAG expression is evident in an scAAV2-CBA-ND4–injected eye. D, Retinal cells and nerve fibers show a cytoplasmic and nuclear pattern of GFP expression in scAAV2-CBA-GFP–injected eyes. E, Weak expression of ND4FLAG is evident in eyes injected with ssAAV-CBA-ND4FLAG. F, strong expression of ND4FLAG is seen in eyes injected with scAAV2-CBA-ND4FLAG. Scale bars equal 100 μm.
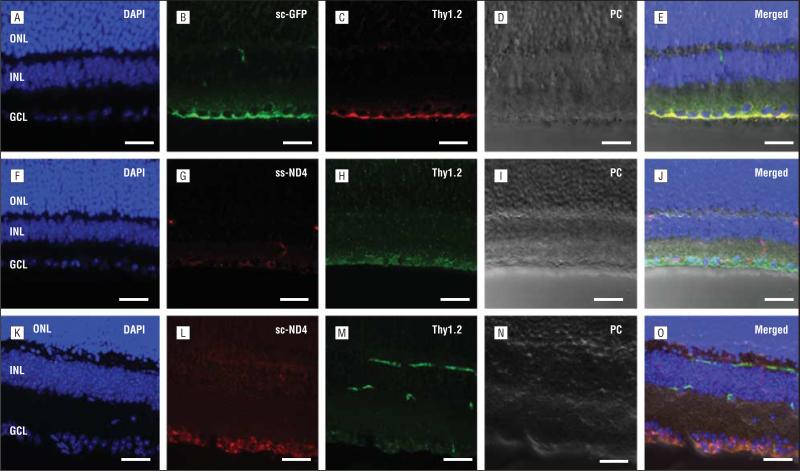
Thy1.2 staining (Figure 2C, H, and M) of the RGCs in the transverse retinal sections injected with scAAV2-GFP (Figure 2A-E), ssAAV2-ND4FLAG (Figure 2F-J), or scAAV2-ND4FLAG (Figure 2K-O) showed colocalization of GFP- or ND4FLAG-positive cells with the RGCs. Fluorescent microscopy of the transverse retinal sections showed scAAV2-GFP expression in the RGCs (Figure 2B). Similarly, ND4FLAG expression was observed in the RGC layer (Figure 2G and L). Merged images of the GFP-transduced (Figure 2E) or ND4FLAG-transduced (Figure 2J and O) cells with RGCs labeled by Thy1.2 antibody showed colocalization, thus indicating the transduced cells to be RGCs. The Thy1.2 signal observed at the junction of the outer boundary of the inner nuclear layer and the inner boundary of the outer plexiform layer is due to the staining of leukocytes in the retinal vasculature (Figure 2M).
Figure 2.
Confocal microscopy of retinal cryosections. A, Nuclei of an eye injected with self-complementary adeno-associated virus (scAAV)–chicken β-actin (CBA)–green fluorescent protein (GFP) and stained with 4′,6-diamidino-2-phenylindole (DAPI) (original magnification ×40). B and C, GFP fluorescence is evident in the retinal ganglion cell (RGC) layer labeled by Thy1.2. D, A phase contrast (PC) image of this retinal section is shown. E, A merged image of DAPI, GFP, and Thy1.2 indicates colocalization of GFP in the RGCs. F, DAPI-stained retinal nuclear layers of a single-strand (ss) AAV-CBA-ND4–injected eye. G and H, Expression of ND4FLAG is weak in RGCs labeled by Thy1.2. I, A PC image of the same section. J, A merged image of DAPI, ND4FLAG, and Thy1.2 indicates colocalization of ND4FLAG in the RGCs. K, DAPI-stained retinal nuclear layers of an scAAV-CBA-ND4–injected eye. L and M, Many RGCs express ND4FLAG and Thy1.2. Although Thy1.2 staining is also occasionally seen in the retinal vasculature, ND4FLAG expression is restricted to RGCs. N, A PC image of the retinal section in parts K through M. O, The merged image of DAPI, ND4FLAG, and Thy1.2 indicates colocalization with ND4FLAG-positive cells in the RGC layer. Scale bars equal 25 μm. GCL indicates ganglion cell layer; INL, inner nuclear layer; and ONL, outer nuclear layer.
To evaluate the efficiency of the single-stranded and double-stranded AAV2 vectors, we counted cells in the mouse retina that expressed ND4FLAG or GFP relative to the Thy1.2-labeled ganglion cells in the retinal trans-verse sections. Expression of GFP induced by scAAVGFP in RGCs of control eyes was 96%. Relative to the mean value of 1205 (344) cells per 40 mm2 for Thy1.2-positive RGCs, ND4FLAG-positive RGCs in the scAAV-ND4FLAG– injected eyes had a mean value of 1091 (316) cells per 40 mm2. Thus, ND4FLAG was expressed in almost 91% of RGCs. In contrast, relative to the mean value of 936 (183) cells per 40 mm2 for Thy1.2-positive RGCs, ND4FLAG-positive RGCs in the ssAAV-ND4FLAG–injected eyes had a mean value of 478 (101) cells per 40 mm2, which is only 51% of RGCs. The difference between scAAV-ND4FLAG– positive cells to ssAAV-ND4FLAG–positive cells was statistically significant (P=2.9×10–5, unpaired t test). Thus, the efficiency of allotopic ND4 expression using the self-complementary vector was approximately double that achieved by the single-stranded vector, even though we had injected almost double the dose of ssAAVND4FLAG. This level of allotopic ND4 expression achieved by the self-complementary vector was also comparable to the expression of GFP achieved in control eyes injected with scAAV-GFP.
EFFECTS OF GENE EXPRESSION ON RGCs: ELECTROPHYSIOLOGY
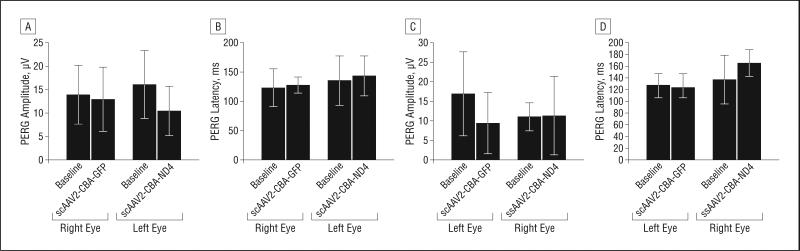
We recorded the PERG and FERG of DBA/1J mice (n=9) before and 1 week after rAAV injections. The mice were divided into 2 groups. The first group (n=5) received scAAV2-GFP in the right eyes and scAAV2-ND4 in the left eyes, whereas the second group (n = 4) received ssAAV2-ND4 in the right eyes and scAAV2-GFP in the left eyes. Our analysis of PERGs showed no significant difference in the average PERG amplitudes and latencies between the right and left eyes 1 week after the viral injection in the first and second groups (P>.05, paired t test). When the postinjection data of PERG amplitude and latencies in the respective eyes of both groups were compared with their preinjection (baseline) data, there was no significant difference, thus indicating no adverse effect on the functional status of RGCs with expression of human ND4 in almost all RGCs (scAAV2-ND4FLAG).
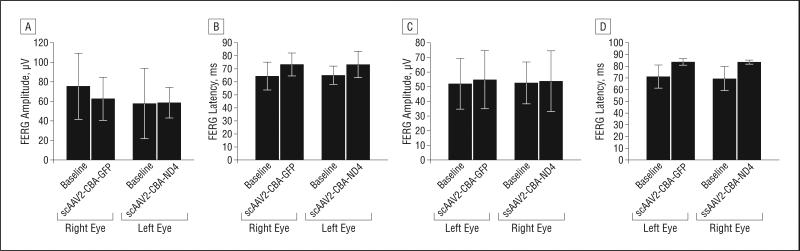
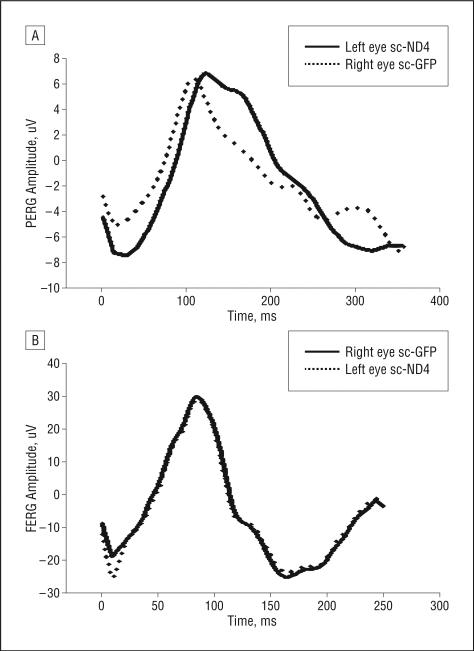
Similarly, the FERG analysis showed no significant difference between the right and left eyes before and after injections, indicating no adverse effect on overall retinal function. The Figure 3 and Figure 4 bar plots compare the average PERG and FERG amplitudes and latencies (baseline vs postinjection data) in the first and second groups of injected animals. Representative PERG and FERG waveforms are shown in Figure 5.
Figure 3.
Findings of pattern electoretinography (PERG). Bar plots show no significant difference in PERG amplitude (A) and latency (B) recorded 1 week after intravitreal injection of self-complementary adeno-associated virus type 2 (scAAV2)–chicken β-actin (CBA)–green fluorescent protein (GFP) (right eye) and scAAV2-CBA-ND4 (left eye) (group 1) (P>.05, paired t test). Similarly, there was no significant difference of the PERG amplitude (C) and latency (D) 1 week after intravitreal injection of single-stranded (ss) AAV2-CBA-ND4 (right eye) and scAAV2-CBA-GFP (left eye) (group 2) (P>.05, paired t test).
Figure 4.
Findings of flash electroretinography (FERG). Bar plots show no significant difference of FERG amplitude (A) and latency (B) recorded 1 week after intravitreal injection of self-complementary adeno-associated virus type 2 (scAAV2)–chicken β-actin(CBA)–green fluorescent protein (GFP) (right eye) and scAAV2-CBA-ND4 (left eye) (group 1) (P>.05, paired t test). Similarly, there was no significant difference of the FERG amplitude (C) and latency (D) 1 week after intravitreal injection of single-stranded (ss) AAV2-CBA-ND4 (right eye) and scAAV2-CBA-GFP (left eye) (group 2) (P>.05, paired t test).
Figure 5.
Electrophysiology findings. Representative waveforms of the postinjection pattern electroretinography (PERG) (A) and flash ERG (FERG) (B) of eyes inoculated with self-complementary adeno-associated virus (scAAV)–ND4FLAG or scAAV–green fluorescent protein (GFP) are shown.
COMMENT
In the present study, we demonstrated that, compared with the ssAAV2 vectors, an scAAV containing the normal human ND4 subunit gene of complex I expressed more rapidly and with much greater transduction efficiency, leading to expression in almost all ganglion cells of the murine retina. These are the cells affected exclusively in LHON. In a previous publication using the ssAAV, we found 38% ND4FLAG-positive RGCs relative to the controls and 65% GFP-positive RGCs.25 In that study, we examined transgene expression 1 month after intravitreal injection. In the present study, we examined transgene expression 1 week after intravitreal injection. Using the scAAV to deliver the transgene, we found that more than 90% of RGCs expressed the allotopic human ND4 or control GFP. We are in the process of evaluating the levels of ND4 expression in the first week and its persistence beyond 1 month.
The robust expression of allotopic ND4FLAG 1 week after vector injection into the vitreous with an scAAV vector rather than the standard ssAAV deserves comment. Deletion of 25 bases in the 3′ region of the forward terminal repeat of AAV results in generation of an AAV containing both complementary strands.16,26 Generation of the complementary strand of ssAAV is believed to be the rate-limiting step in expression from standard ssAAV vectors and leads to the several-week delay in transgene expression typically seen from ssAAV vectors in vivo. An scAAV like the one used herein resulted in transgene expression of ND4FLAG or GFP within 7 days after injection in rodents as also seen in other studies.22,27 Why would such rapidity of expression be important clinically? Increasing the speed of ND4 gene expression may be relevant to patients with LHON who have acute visual loss that must be treated quickly before RGC demise and development of optic atrophy. With scAAV-GFP or scAAV-ND4, the efficiency of RGC labeling was not only faster but far superior to that of ssAAV. Yokoi and coworkers21 compared transduction efficiency of ssAAV and scAAV vectors in the various murine retinas by means of vector dose-dilution experiments. They demonstrated GFP expression within 3 days of vector delivery using intravitreous injection of scAAV-CMV-GFP, whereas it took 1 week for expression of ssAAV-CMV-GFP. A lower dose of the scAAV-CMVGFP resulted in transduction of RGCs in a few cells of the inner nuclear layer, whereas the expression was restricted only to the RGC layer when a similar dose of ssAAV-CMV-GFP was used. Subretinal injection of a similar dose of scAAV-CMV-GFP transduced the retinal pigment epithelial cells rapidly and within 28 days transduced the photoreceptor cells to some extent. We also observed some photoreceptor expression in our GFP-injected eyes, even with intravitreal delivery that labeled mostly RGCs.
The scAAV2-CBV-GFP viral vector that we used as an internal control had a 444 tyrosine to phenylalanine mutation, but the scAAV2-CBV-ND4 and ssAAV2-CBVND4 viral vectors lacked the mutation. Hence, a limitation in this study could have been an overestimation of GFP expression when compared with ND4 expression. Still, with more than 90% expression, the differences were slight. Only 5% more RGCs expressed GFP than ND4. According to a previous study,28 tyrosine phosphorylation of the viral capsids greatly decreases the transduction efficiency through the proteasome-mediated degradation pathways (ssAAV and scAAV) even before the virus enters the nucleus. Mutations in the capsid tyrosine residues in the viral particles allow these vectors to escape such degradation pathways, promoting a faster and greater transgene expression. A recent study compared the different tyrosine mutants Y444F and Y730F in AAV2, Y733F in AAV8, and Y446F in AAV9 by means of an intravitreal in jection and showed higher transduction efficiency in the RGC layer.27 The efficiency of gene therapy could be slowed down because of the existing neutralizing anti-AAV2 antibodies.29,30 This response can be a function of the route of AAV administration. Evidence suggests that the antibody response is observed with intravitreal delivery of viral vector, whereas it is absent with a subretinal injection.31 The transduction efficiency of 10 000-fold reduced vector particles with Y444F mutation was much better than the wild-type AAV2 virus particles. Thus, with the use of such tyrosine mutants in the capsid proteins, the viral-evoked immunological responses may be minimized by using a minimum dose and also with a maximum efficiency.27 Such vectors may be advantageous in gene therapy for LHON because they can be used at lower doses, thus minimizing immunological responses against the viral capsid that could prevent expression of ND4 in the contra-lateral eye after a previous injection in the first eye.
The rapidity of gene expression is clinically relevant in treating patients with LHON who have bilateral simultaneous onset of acute visual loss. However, oxidative injury and apoptosis may already be irreversible at this time. Gene therapy with a normal human ND4 is also highly relevant to those with acute visual loss in one eye followed by visual loss in the fellow eye within a mean duration of approximately 2 months. Gene therapy to rescue RGCs before visual loss of the second eye may be possible in these patients during this window period, if introduction of the normal ND4 subunit gene in those with acute optic disc edema after visual loss proves ineffective. Rescue of the less symptomatic fellow eye was attempted by Newman and coinvestigators,3 who used topical brimonidine purite (Alphagan) before optic nerve degeneration. Patients with severe loss of RGCs and optic atrophy may be the least desirable subgroup for rescue by a normal human ND4 gene therapy. Still, the Leber congenital amaurosis trial showed that partial return of visual function can occur even in eyes with severe photoreceptor loss. Perhaps insertion of the normal ND4 gene into remaining ganglion cells may restore some level of visual function to patients with LHON who have severe retinal nerve fiber layer loss. Whether successful rescue in symptomatic patients will support intervention in asymptomatic LHON carriers is unclear. The slight risks of an intraocular injection may or may not be acceptable to G11778A carriers. Only 50% of men and 10% of women who harbor the mtDNA mutation will develop visual loss.
We have also shown herein that expression of the wild-type human ND4 subunit in almost all RGCs by scAAV appears safe. Findings on the PERG that provide a sensitive measure of ganglion cell dysfunction as well as the FERG remained normal 1 week after ocular injections of the human ND4 gene. However, we did not see that the human ND4 had a measurable biological effect on mouse RGCs at this short time interval. When studied 1 month after intraocular injections, human ND4 expression shortened the PERG latency.25 Therefore, we conclude that, as gauged by this and our previous study, ssAAV expression of human ND4 gene had no adverse effect on ganglion cell function or survival, as also gauged by stable Thy1.2-labeled RGC counts.25 This is in sharp contrast to injections of the mutant version of human ND4 (R340H) into the mouse visual system that recapitulated in an animal model with histopathological resemblance to the human disease, that is, initial swelling of the optic nerve head followed by optic atrophy.32 These findings with scAAV-ND4FLAG and ssAAV-ND4FLAG support our previous study with ssAAV-ND4FLAG,25 in which we found that the normal human ND4 labeled by immunogold decorated the interior of the organelle, thus confirming trans-location of the FLAG-tagged ND4 into the mitochondria. This confirmed that the allotopic ND4 protein did not remain in the mitochondrial import channels as suggested by Oca-Cossio and coworkers.33 In addition, the cellular demise predicted by the studies of Oca-Cossio et al was not seen in our mice injected with scAAV-ND4 or ssAAV-ND4, which exhibited normal physiological characteristics and counts of the RGC population relative to controls injected with AAV-GFP. Moreover, we had found that, after immunoprecipitation of the murine complex I, Western blotting with the anti-FLAG antibody revealed the 52-kDa band of human ND4, thus indicating that it hadintegratedintothemousecomplexI.Theslightlyhigher molecular weight of uncleaved P1ND4FLAG fusion gene seen in mitochondrial isolates was not evident,32 suggesting that the nuclear-encoded human ND4 complex I subunit imported into the mitochondria was processed by cleavage of the ATPc targeting sequence from the mature human ND4 fusion protein that was then incorporated into the mouse complex I we had immunoprecipitated. For the normal human ND4 subunit, the incorporation into the mouse optic nerve mitochondria was greater than that seen for the retina. This was in contrast to the mutant ND4, in which integration into the retinal cells was greater than that seen for the optic nerve. These differences in the patterns of ND4 integration may have been due to the optic disc edema associated with the mutant ND4 interfering with mitochondrial movement from the retina into the nerve.
Rescue of optic neuropathy will be proof that allotopic gene therapy may be effective in patients with LHON. This is the goal of the LHON Gene Therapy Clinical Trial in its second of 5 years, funded by the National Eye Institute and currently enrolling patients at the Bascom Palmer Eye Institute. That allotopic expression can rescue complex I deficiency was first proved in a murine model of Parkinson disease. Rather than complementing the defective 8-kDa complex I subunit34 with a human gene, the investigators used the AAV vector to deliver the single-subunit reduced form of nicotinamide adenine dinucleotide dehydrogenase (NDI1) of yeast (Saccharomyces cerevisiae).35 Despite the marked mismatch in the amino acid sequence and the size of the yeast relative to the murine complex I, a 50% rescue of complex I activity was seen in their mice. However, these investigators injected AAV-NDI1 4 months before induction of Parkinson disease by toxin. Ellouze and coworkers36 used the COX10 mitochondrial targeting sequence to drive import of human ND4 that was also optimized for mitochondrial expression with the 3′ untranslated region of the SOD2 gene for allotopic expression; they averted visual loss and RGC demise in rat eyes that were also injected with the mutant ND4 gene to induce optic neuropathy. Because they did not perform immunoprecipitation experiments needed to demonstrate incorporation of the human ND4 subunit into the rat complex I, we do not know whether there was a difference in incorporation of the mutant or wild-type versions of human ND4 in the retina relative to the optic nerve as we had seen in our previous immunoprecipitation experiments.25,32 Nevertheless, their work supports our observations that allotopic ND4 gene therapy is safe and not harmful, thus further contradicting the studies of Oca-Cossio et al.33 For ocular gene delivery, Ellouze and coworkers36 used electroporation of plasmid DNA injected into the vitreous. This resulted in labeling of one-quarter of the ganglion cells of the rat. Unlike electroporation of plasmid DNA that provides transient low-efficiency ocular gene expression and thus is not suitable for treatment of patients with LHON, gene delivery with the AAV vector is highly efficient and stable for years, and AAV has a proven track record in human clinical trials in the eye.10,11,32,37-39 Their work, together with ours, paves the way for allotopic gene therapy for LHON.
Of the other experimental approaches, such as mitochondrial gene replacement in embryonic stem cells,5 protofection,4 importing genes from other species, changing the ratio of heteroplasmy with specific restriction endonucleases,40 and selecting for respiratory function or regeneration (in muscle), none of these techniques is directly applicable to the treatment of LHON, which is caused by 100% mutated mtDNA.41,42 In summary, we report herein important clinical implications in gene therapy for patients with LHON by using self-complementary vectors to augment the ND4 gene expression rapidly and efficiently. Only specific retinal layers can be targeted by optimizing the vector dose and by choosing the route of vector administration. However, care must be taken in extrapolating the results in mice to humans, particularly under pathological conditions.
Acknowledgments
Funding/Support: This study was supported by grants R01EY017141 and R24EY018600 (Dr Guy), core grant P30EY14801 (Dr Porciatti), and an unrestricted departmental grant from Research to Prevent Blindness.
Footnotes
Financial Disclosure: None reported.
Additional Contributions: Mabel Wilson edited the manuscript.
REFERENCES
- 1.Riordan-Eva P, Sanders MD, Govan GG, Sweeney MG, Da Costa J, Harding AE. The clinical features of Leber's hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain. 1995;118(pt 2):319–337. doi: 10.1093/brain/118.2.319. [DOI] [PubMed] [Google Scholar]
- 2.Harding AE, Sweeney MG, Govan GG, Riordan-Eva P. Pedigree analysis in Leber hereditary optic neuropathy families with a pathogenic mtDNA mutation. Am J Hum Genet. 1995;57(1):77–86. [PMC free article] [PubMed] [Google Scholar]
- 3.Newman NJ, Biousse V, David R, et al. Prophylaxis for second eye involvement in Leber hereditary optic neuropathy: an open-labeled, nonrandomized multicenter trial of topical brimonidine purite. Am J Ophthalmol. 2005;140(3):407–415. doi: 10.1016/j.ajo.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 4.Keeney PM, Quigley CK, Dunham LD, et al. Mitochondrial gene therapy augments mitochondrial physiology in a Parkinson's disease cell model. Hum Gene Ther. 2009;20(8):897–907. doi: 10.1089/hum.2009.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tachibana M, Sparman M, Sritanaudomchai H, et al. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009;461(7262):367–372. doi: 10.1038/nature08368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glick B, Schatz G. Import of proteins into mitochondria. Annu Rev Genet. 1991;25:21–44. doi: 10.1146/annurev.ge.25.120191.000321. [DOI] [PubMed] [Google Scholar]
- 7.Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- 8.Manfredi G, Fu J, Ojaimi J, et al. Rescue of a deficiency in ATP synthesis by transfer of MTATP6, a mitochondrial DNA–encoded gene to the nucleus. Nat Genet. 2002;30(4):394–399. doi: 10.1038/ng851. [DOI] [PubMed] [Google Scholar]
- 9.Guy J, Qi X, Pallotti F, et al. Rescue of a mitochondrial deficiency causing Leber hereditary optic neuropathy. Ann Neurol. 2002;52(5):534–542. doi: 10.1002/ana.10354. [DOI] [PubMed] [Google Scholar]
- 10.Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19(10):979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358(21):2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonçalves MA. Adeno-associated virus: from defective virus to effective vector. Virol J. 2005;2:43. doi: 10.1186/1743-422X-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie Q, Bu W, Bhatia S, et al. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci U S A. 2002;99(16):10405–10410. doi: 10.1073/pnas.162250899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabinowitz JE, Rolling F, Li C, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76(2):791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muzyczka N, Warrington KH., Jr Custom adeno-associated virus capsids: the next generation of recombinant vectors with novel tropism. Hum Gene Ther. 2005;16(4):408–416. doi: 10.1089/hum.2005.16.408. [DOI] [PubMed] [Google Scholar]
- 16.McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10(26):2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 17.Nathwani AC, Gray JT, Ng CY, et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107(7):2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao GP, Lu Y, Sun X, et al. High-level transgene expression in nonhuman primate liver with novel adeno-associated virus serotypes containing self-complementary genomes. J Virol. 2006;80(12):6192–6194. doi: 10.1128/JVI.00526-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu H, Muenzer J, Samulski RJ, et al. Self-complementary adeno-associated virus serotype 2 vector: global distribution and broad dispersion of AAV-mediated transgene expression in mouse brain. Mol Ther. 2003;8(6):911–917. doi: 10.1016/j.ymthe.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Borrás T, Xue W, Choi VW, et al. Mechanisms of AAV transduction in glaucoma-associated human trabecular meshwork cells. J Gene Med. 2006;8(5):589–602. doi: 10.1002/jgm.886. [DOI] [PubMed] [Google Scholar]
- 21.Yokoi K, Kachi S, Zhang HS, et al. Ocular gene transfer with self-complementary AAV vectors. Invest Ophthalmol Vis Sci. 2007;48(7):3324–3328. doi: 10.1167/iovs.06-1306. [DOI] [PubMed] [Google Scholar]
- 22.Andino LM, Conlon TJ, Porvasnik SL, Boye SL, Hauswirth WW, Lewin AS. Rapid, widespread transduction of the murine myocardium using self-complementary adeno-associated virus. Genet Vaccines Ther. 2007;5:13. doi: 10.1186/1479-0556-5-13. doi:10.1186/1479-0556-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauswirth WW, Lewin AS, Zolotukhin S, Muzyczka N. Production and purification of recombinant AAV vectors. In: Palczewski K, editor. Vertebrate Phototransduction and the Visual Cycle. Academic Press Inc; Orlando, FL: 2000. pp. 743–761. [Google Scholar]
- 24.Nagaraju M, Saleh M, Porciatti V. IOP-dependent retinal ganglion cell dysfunction in glaucomatous DBA/2J mice. Invest Ophthalmol Vis Sci. 2007;48(10):4573–4579. doi: 10.1167/iovs.07-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guy J, Qi X, Koilkonda RD, et al. Efficiency and safety of AAV-mediated gene delivery of the human ND4 complex I subunit in the mouse visual system. Invest Ophthalmol Vis Sci. 2009;50(9):4205–4214. doi: 10.1167/iovs.08-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8(16):1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 27.Petrs-Silva H, Dinculescu A, Li Q, et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther. 2009;17(3):463–471. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong L, Li B, Mah CS, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A. 2008;105(22):7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaiss AK, Muruve DA. Immunity to adeno-associated virus vectors in animals and humans: a continued challenge. Gene Ther. 2008;15(11):808–816. doi: 10.1038/gt.2008.54. [DOI] [PubMed] [Google Scholar]
- 30.Peden CS, Burger C, Muzyczka N, Mandel RJ. Circulating anti–wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J Virol. 2004;78(12):6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Miller R, Han PY, et al. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol Vis. 2008;14:1760–1769. [PMC free article] [PubMed] [Google Scholar]
- 32.Qi X, Sun L, Lewin AS, Hauswirth WW, Guy J. The mutant human ND4 subunit of complex I induces optic neuropathy in the mouse. Invest Ophthalmol Vis Sci. 2007;48(1):1–10. doi: 10.1167/iovs.06-0789. [DOI] [PubMed] [Google Scholar]
- 33.Oca-Cossio J, Kenyon L, Hao H, Moraes CT. Limitations of allotopic expression of mitochondrial genes in mammalian cells. Genetics. 2003;165(2):707–720. doi: 10.1093/genetics/165.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keeney PM, Xie J, Capaldi RA, Bennett JP., Jr Parkinson's disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci. 2006;26(19):5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seo BB, Nakamaru-Ogiso E, Flotte TR, Matsuno-Yagi A, Yagi T. In vivo complementation of complex I by the yeast Ndi1 enzyme: possible application for treatment of Parkinson disease. J Biol Chem. 2006;281(20):14250–14255. doi: 10.1074/jbc.M600922200. [DOI] [PubMed] [Google Scholar]
- 36.Ellouze S, Augustin S, Bouaita A, et al. Optimized allotopic expression of the human mitochondrial ND4 prevents blindness in a rat model of mitochondrial dysfunction. Am J Hum Genet. 2008;83(3):373–387. doi: 10.1016/j.ajhg.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dudus L, Anand V, Acland GM, et al. Persistent transgene product in retina, optic nerve and brain after intraocular injection of rAAV. Vision Res. 1999;39(15):2545–2553. doi: 10.1016/s0042-6989(98)00308-3. [DOI] [PubMed] [Google Scholar]
- 38.Guy J, Qi X, Muzyczka N, Hauswirth WW. Reporter expression persists 1 year after adeno-associated virus–mediated gene transfer to the optic nerve. Arch Ophthalmol. 1999;117(7):929–937. doi: 10.1001/archopht.117.7.929. [DOI] [PubMed] [Google Scholar]
- 39.Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28(1):92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 40.Bacman SR, Williams SL, Hernandez D, Moraes CT. Modulating mtDNA heteroplasmy by mitochondria-targeted restriction endonucleases in a “differential multiple cleavage-site” model. Gene Ther. 2007;14(18):1309–1318. doi: 10.1038/sj.gt.3302981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DiMauro S, Hirano M, Schon EA. Approaches to the treatment of mitochondrial diseases. Muscle Nerve. 2006;34(3):265–283. doi: 10.1002/mus.20598. [DOI] [PubMed] [Google Scholar]
- 42.Bayona-Bafaluy MP, Blits B, Battersby BJ, Shoubridge EA, Moraes CT. Rapid directional shift of mitochondrial DNA heteroplasmy in animal tissues by a mitochondrially targeted restriction endonuclease. Proc Natl Acad Sci U S A. 2005;102(40):14392–14397. doi: 10.1073/pnas.0502896102. [DOI] [PMC free article] [PubMed] [Google Scholar]