Abstract
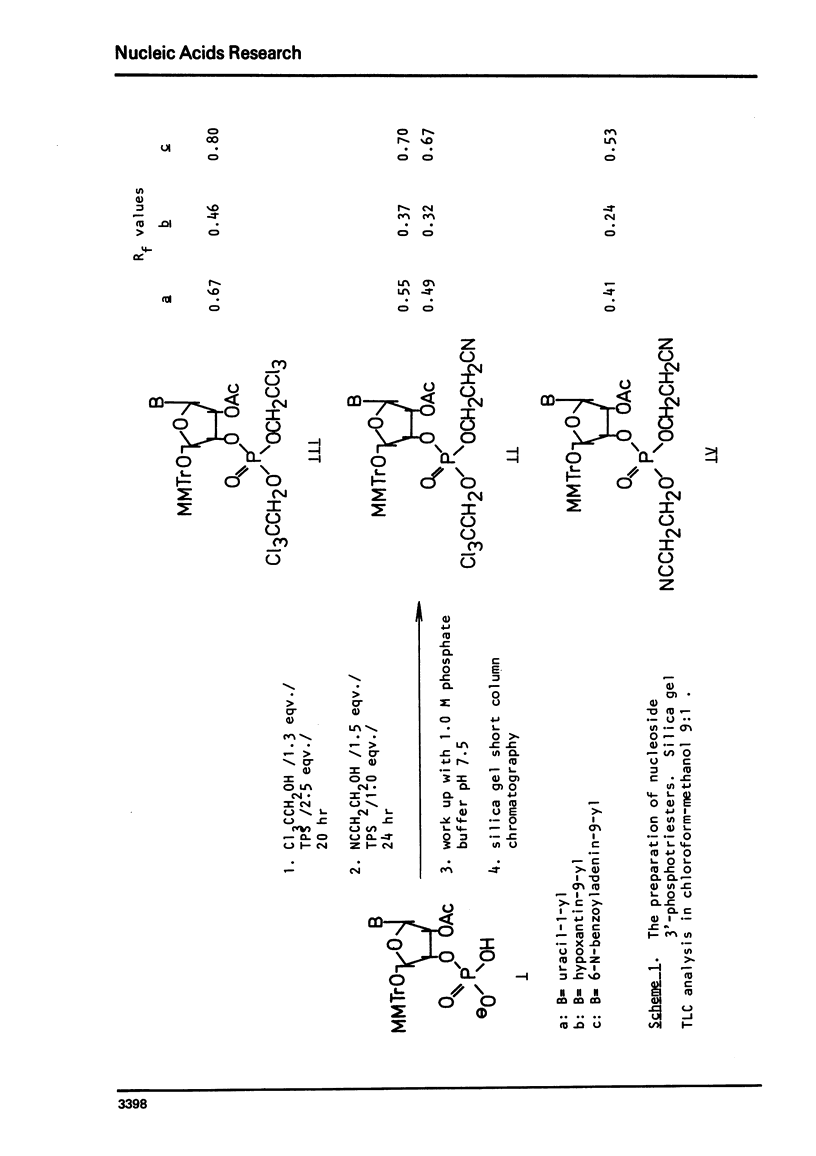
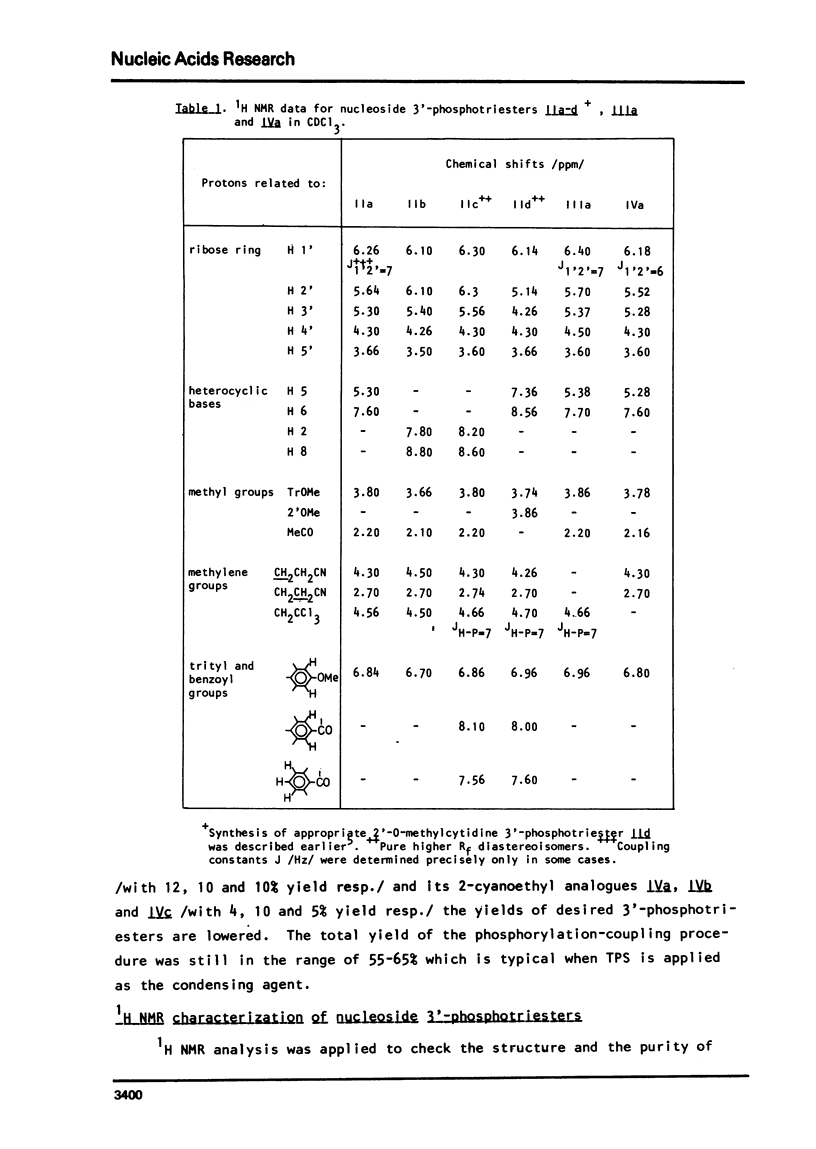
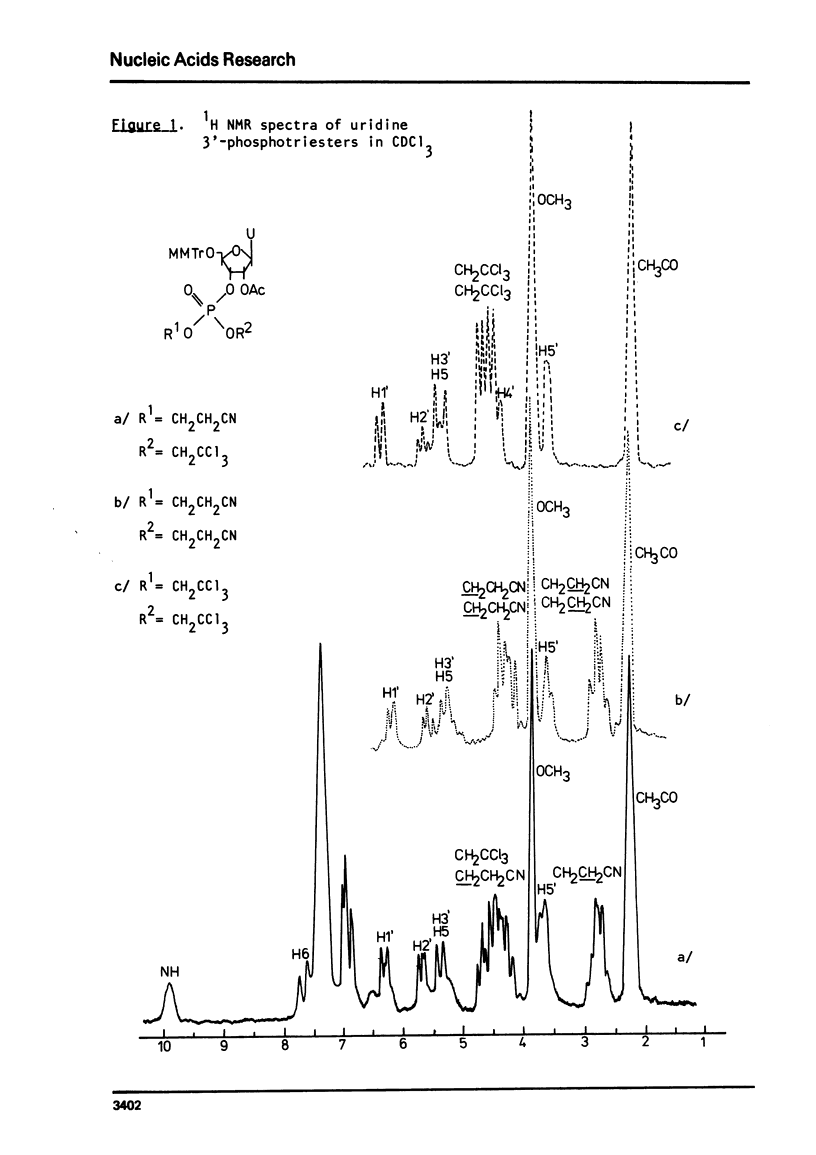
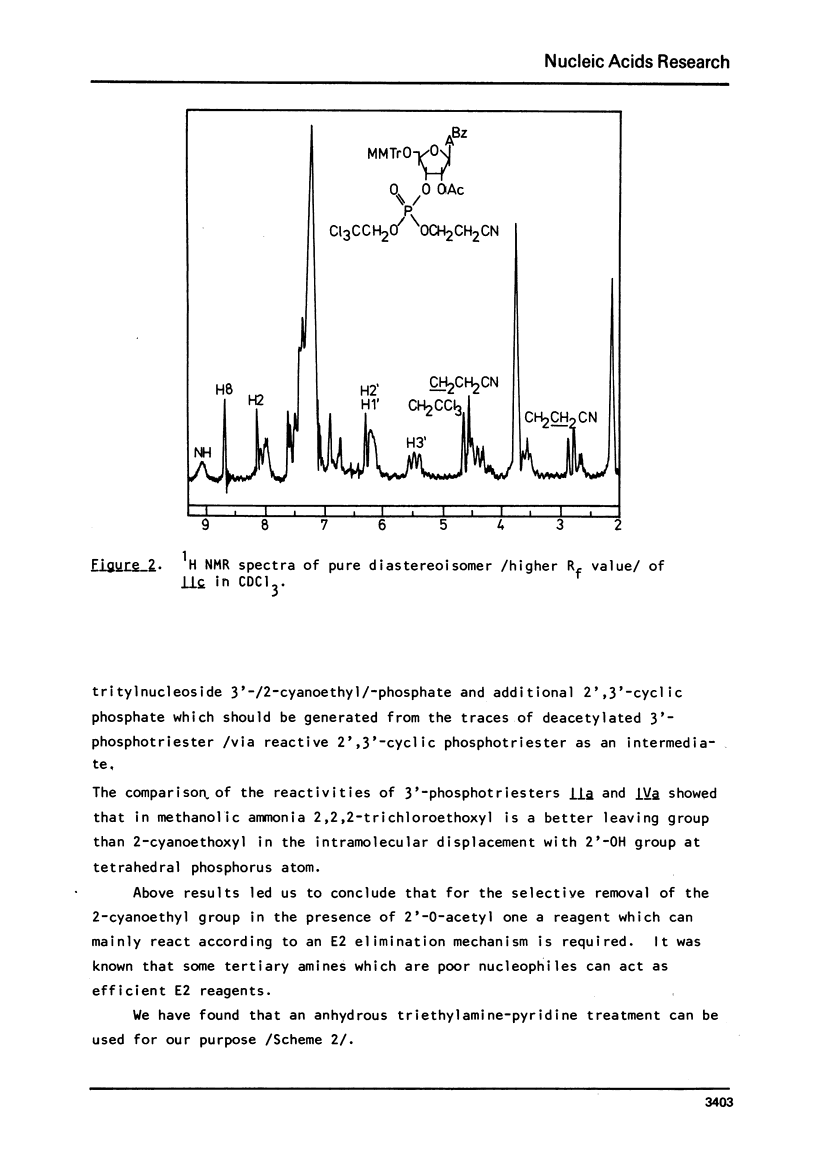
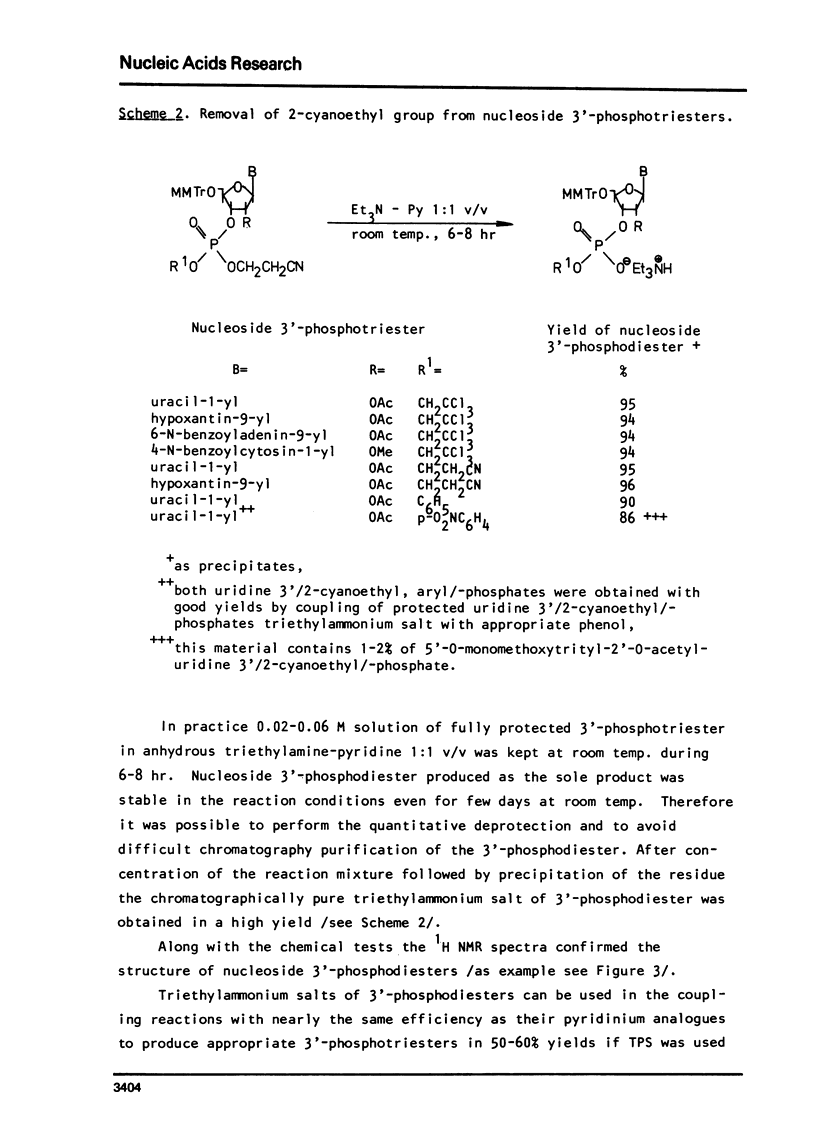
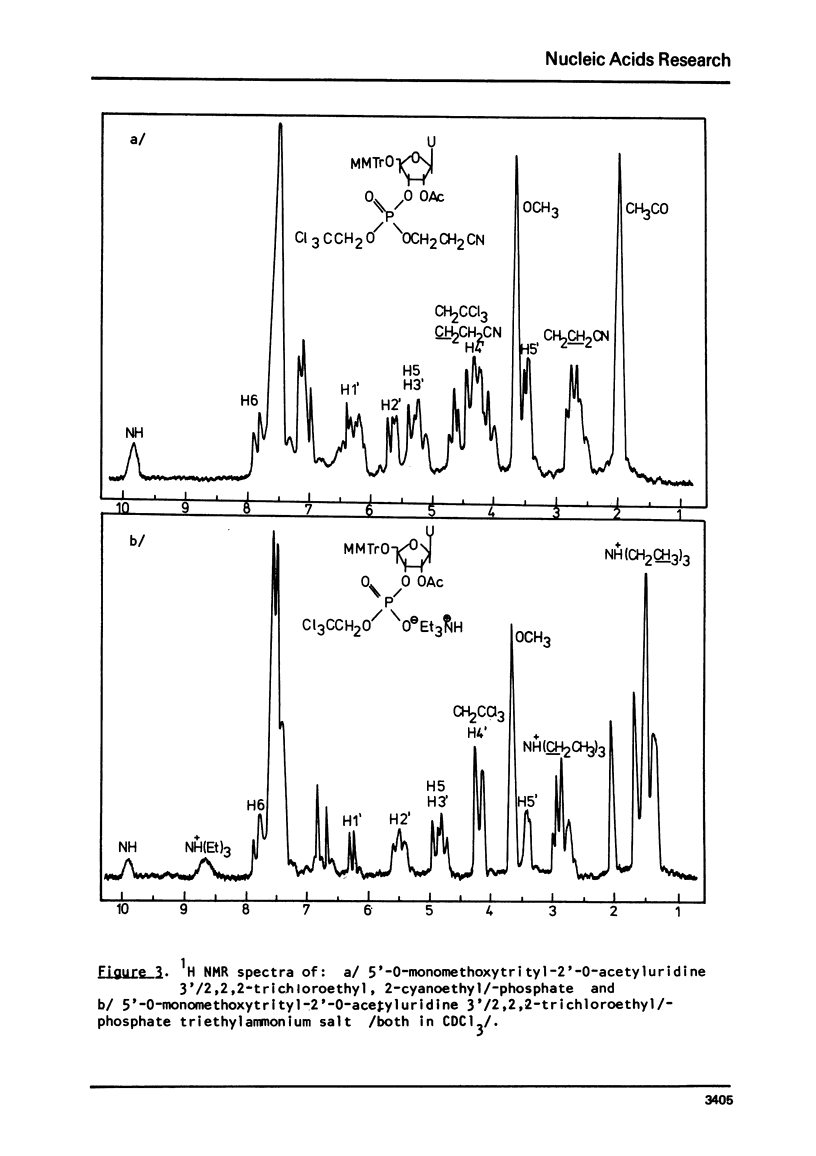
An improved procedure for the transformation of 5'-O-monomethoxytrityl-2'-O-acetyl-3'-phosphates of uridine la, inosine ib and 6-N-benzoyladenosine lc into corresponding 3'/2,2,2-trichloroethyl, 2-cyanoethyl/-phosphates iiaic is reported. H NMR characterization of nucleoside 3'-phosphotriesters is presented. New conditions i.e. anhydrous triethylamine-pyridine treatment have been found for the selective removal of 2-cyanoethyl group from nucleoside 3'-phosphotriesters in the presence of neighbouring 2'-O-acetyl one.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catlin J. C., Cramer F. Deoxy oligonucleotide synthesis via the triester method. J Org Chem. 1973 Jan 26;38(2):245–250. doi: 10.1021/jo00942a011. [DOI] [PubMed] [Google Scholar]
- Itakura K., Katagiri N., Bahl C. P., Wightman R. H., Narang S. A. Improved triester approach for the synthesis of pentadecathymidylic acid. J Am Chem Soc. 1975 Dec 10;97(25):7327–7332. doi: 10.1021/ja00858a020. [DOI] [PubMed] [Google Scholar]
- Lohrmann R., Söll D., Hayatsu H., Ohtsuka E., Khorana H. G. Studies on polynucleotides. LI. Syntheses of the 64 possible ribotrinucleotides derived from the four major ribomononucleotides. J Am Chem Soc. 1966 Feb 20;88(4):819–829. doi: 10.1021/ja00956a039. [DOI] [PubMed] [Google Scholar]


