Abstract
One major morphological difference between chordates and annelids or arthropods is the opposite orientation of the nerve cord and heart. A long-standing proposal is that the chordate axis evolved by inverting the body of an ancestor with the annelid/arthropod orientation. However, the data can also be explained by a common ancestor with diffuse dorsoventral organization, followed by oppositely directed condensation of the nerve cord and relocation of the heart in the two lines.
In 1822 Geoffroy St. Hilaire suggested that arthropods have the inverse dorsoventral organization of chordates. After acceptance of Darwin's descent with modification, Dohrn (1) proposed in 1875 that the last common ancestor of these groups was an annelid worm-like animal with a ventral nerve cord, a dorsal heart, and a circulatory system with blood flowing anteriorly in dorsal vessels. This orientation was retained by evolving members of the 25–30 protostome phyla (such as annelids and arthropods) whereas, within the deuterostomes (chordates, hemichordates, and echinoderms), an ancestor of chordates inverted its body, sideways over, but retained the same relative organ placements. The nerve cord was now dorsal, the heart ventral, and the blood flowed anteriorly in ventral vessels (Fig. 1). In chordates, axial muscle blocks were dorsolateral and visceral mesoderm was ventrolateral whereas protostomes had the opposite arrangement. In the chordate line, the mouth eventually formed on the new ventral side and vanished from the old location. Thus, in the amended inversion hypothesis, the last common ancestor already had a complex differentiated dorsoventral axis. After protostomes and deuterostomes diverged, members of each group would have added organs along this axis. In chordates, for example, besides a dorsal hollow nerve cord and ventral heart, there evolved a dorsal notochord, ventrolateral gill slits, a ventral endostyle in the pharynx, and a dorsal postanal tail. None of these is found in protostomes, which would have added other organs.
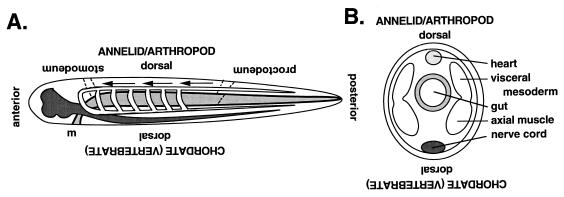
Figure 1.
The inversion hypothesis. (A) An annelid worm, side view. The mouth (m) and nerve cord (dark shading) are ventral. The gut (light shading) is midlevel. Arrows indicate the direction of blood flow. Inverted, it is a chordate, with the nerve cord dorsal, the gut ventral, and the blood flowing in the opposite direction. A new mouth (stomadeum) and anus (proctodeum) evolve in the chordate. Modified from ref. 2. (B) The dorsoventral axis in cross section, trunk level.
After denunciation of it by Cuvier in 1830, the hypothesis was reasserted and rejected every 50 years to the present (1). Recent data on regional gene expression in embryos of fruit flies, frogs, and mice have brought new credibility to the hypothesis and to deductions about the complexity of the last common ancestor of protostomes and deuterostomes. The significance of this ancestor is great in metazoan evolution, wrapped up as it is with the origins of bilateral animals. Modification of a radially symmetric or biradial body plan into a bilateral body plan with a dorsoventral axis is thought to have involved many changes, including appearance of mesoderm and coeloms, conversion of a closed gut to a through-gut, cephalization, and centralization of the nerve cord from a diffuse net. These modifications presumably occurred in steps between the time of the first simple bilateral animal, variously called the “urbilateria” (3) or “bilaterogastrea” (4), and the time of the last common ancestor of modern bilateral animals. Because the fossil record and modern phylogenies give few clues (see below) about the ancestor, it would be significant if comparative molecular and anatomical data could illuminate its character.
Recent Evidence for Inversion.
Modern proponents of inversion, who have taken gene expression data as evidence, include Arendt and Nübler-Jung (5–9), De Robertis and Sasai (2), and Holley and Ferguson (10). Data are striking on the similar but inverted domains of expression of several orthologous genes in embryos of chordates (mostly frogs and mice) and protostomes (mostly Drosophila). In the nerve cord, three genes, vnd/Nk2, ind/Gsh1,2, and Msh/Msx1,3, are expressed in three columns of nerve cells aligned medial to lateral (11). Also, the netrin gene(s) is expressed in the medial floor plate of the chordate nerve cord and the ventral midline mesectoderm of Drosophila. Because the similarities seem too complex to have arisen by evolutionary convergence, the data imply a complex nerve cord in the last common ancestor. Results with the heart are also striking. The tinman/csk (also called Nkx2-5) gene is expressed very early in the prospective heart region in chordates (ventrally) and arthropods (dorsally) (12). The common ancestor's complexity is also implied by complex anteroposterior expression domains (emx, otx, Hox genes) in the chordate and arthropod nervous systems (8), and by expression similarities in segmentation, eyes, and limbs (13, 14).
Furthermore, the embryonic ectoderm in flies and the embryonic mesoderm in frogs segregate into two dorsoventral domains, inversely related in the two cases (3, 10). Cells of one domain secrete certain type β transforming growth factor signals (bone morphogenetic proteins 2, 4, and 7 in chordates; Dpp in Drosophila), and cells of the other do not but do secrete antagonists of these signals (Chordin in chordates; Sog in Drosophila). These genes seem particularly significant because their domains may serve as dorsoventral compartments of the body axis (like Hox compartments of the anteroposterior axis). Hence, they might reveal global body organization rather than specific cell type differentiation or local organ development. The molecular data support the assertions from morphology that nerve cords, hearts, and dorsoventral organization of all modern bilateral animals derive from organs and organization of a complex common ancestor, with body inversion in one of the two lines.
When did inversion occur? Deuterostomes currently include chordates, hemichordates, and echinoderms (15, 16). Because the body axis of echinoderms is greatly (and intriguingly) modified, only the hemichordate-chordate comparison has been pursued. The worm-like enteropneust hemichordates differ from chordates in having a ventral nerve cord, a protostome-like direction of blood flow, a liver-like organ protruding dorsally from the gut (rather than ventrally as in chordates), a notochord-like pygocord located ventrally and posteriorly, a ventral postanal tail, and a dorsal endostyle-like organ (Table 1). From these differences, several authors (9, 17–19) have concluded that enteropneusts have protostome-like organization and that inversion must have occurred in the chordate line after they branched off. As noted later, other features can be used to argue for non-inversion.
Table 1.
Evidence for and against inversion: The dorsoventral location of anatomical elements and gene expression domains in protostomes, hemichordates, and chordates
| Anatomical element/gene | Location of
element and associated gene expression
|
||
|---|---|---|---|
| Protostomes | Hemichordates | Chordates | |
| Mouth | Ventral | Ventral | Ventral |
| Nerve cord | Ventral | Dorsal (hollow) | Dorsal (hollow) |
| Ventral (solid) | |||
| Neural genes | vnd/ind/msh, netrin | ?? | Nkx2/Gsh/Msx, Netrin |
| sog/chordin domain | Ventrolateral | ?? | Dorsal |
| dpp/bmp domain | Dorsal | ?? | Ventrolateral |
| Heart | Dorsal | Anterior, dorsal | Ventral |
| Heart genes | tinman | ?? | Csx/Nkx2-5 |
| Notochord | Absent | Dorsal stomacord | Dorsal notochord |
| Ventral pygocord | |||
| Post-anal tail | Absent | Posterior, ventral to anus | Posterior, dorsal to anus |
| Liver | Absent | Dorsal | Ventral |
| Endostyle | Absent | Dorsal? ventral? | Ventral |
| Gill slits | Absent | Lateral | Ventrolateral |
| Left-right asymmetry | Absent? | Left pore of anterior coelom | Left dominant Nodal, Shh, Pitx |
Alternatives to Inversion.
Recent molecular phylogenies (18S RNA sequences) have deepened the uncertainty about the complexity of the last common ancestor (15, 16). Bilateral metazoa now fall into three large groups of phyla: the ecdysozoa, lophotrochozoa, and deuterostomes. Deuterostomes are the sister group of the other two protostome groups. In this phylogeny, few, if any, intermediate phyla exist between radially or biradially symmetric animals (coelenterates, ctenophores) and bilateral animals, to reveal steps of the evolution of the dorsoventral axis (15). In previous morphology-based phylogenies, flatworms, nemertines, and nematodes occupied intermediate positions, implying intermediate anatomies such as ones with mesoderm but no coelom or with a blind gut and diffuse nervous system. Development had protostome-like traits. However, flatworms and nemertines now fall within lophotrochozoa, and nematodes fall within ecdysozoa. They are probably secondarily simplified. Deuterostomes are now basal (and some deuterostome-like groups such as chaetognaths scatter among protostomes). With regard to the nervous system of the last common ancestor of modern bilateral animals, we are left with a wide range of possibilities. Did it have a diffuse nerve net or multiple nerve bundles with no dorsoventral differentiation, as do modern radial and biradial animals, or did it have a single concentrated cord, situated at a pole of the dorsoventral axis, as do many modern bilateral animals? Did it have a mouth separate from the anus, by which to assign dorsoventral orientation? In the absence of intermediate phyla, the possibility of a complex, well organized ancestor cannot be excluded, but it gains no additional support.
Critics of inversion have usually favored a less complex common ancestor and a different path of chordate evolution. Some of these hypotheses can accommodate the morphological and molecular data as well as does the inversion hypothesis. In the Auricularia Hypothesis (20), hemichordates and chordates evolved from a bilateral larval ancestor. Through a series of intermediates, bilateral ciliary rows and the associated nerve net moved dorsally, fused at the midline, and sank inside to form a new dorsal cord, without inversion (paedomorphosis followed). To include inversion, Nielsen (19) suggested that protostomes and hemichordates evolved from a ciliated larva by a ventral convergence of ciliary rows to generate a new ventral nerve cord in an animal already having a mouth (ventral by definition). Then, inversion occurred later in the chordate line to make it a dorsal cord, and the mouth relocated to the opposite side. Lacalli (21) suggested that a larval ancestor formed the nerve cord from ciliary rows at a time when it had only one terminal gut opening, and, hence, its dorsoventral axis was ambiguous. Descendents on the deuterostome branch perforated a mouth on the side opposite the nerve cord, making it dorsal. Those on the protostome branch formed a mouth on the same side as the nerve cord, making it ventral. Inversion did not really occur because the mouth arose after the nerve cord. In a related idea (22), a ctenophore-like ancestor with a concentrated nerve cord had two opposing openings (anal pores) in addition to a terminal gut opening, also precluding a definition of the dorsoventral axis. One of these pores was retained as a mouth by protostomes and the other by deuterostomes. These hypotheses have in common that the dorsoventral axis of the last common ancestor of modern bilateral animals was relatively undifferentiated and ambiguous, and, hence, inversion from one orientation to another is not an issue. As discussed later, these alternatives must still rationalize the striking inverted gene expression patterns. Related hypotheses involving an ancestor such as a bilaterogastrea (4) or a larva-like bilateral micrometazoan (23) as the platform for the diversification of bilateral metazoa address early steps of evolving bilaterality more so than they do questions of inversion and the complexity of the last common ancestor.
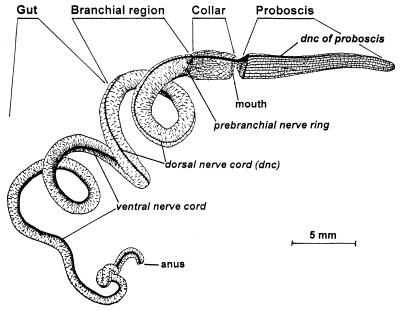
von Salvini-Plawen (24) raises another kind of alternative, that of a bilateral ancestor with a two-part nervous system: one part a plexus of the apical organ and the other a diffuse nerve net in the body. Descendents in the protostome line may have condensed a nerve cord from the apical organ complex whereas those of the deuterostome line may have done so from the diffuse net. Important to his argument (also see ref. 25) is the fact that hemichordates actually have two nerve cords (Fig. 2), perhaps reflecting an intermediate step in the condensation of nerve tracts from the ancestor's diffuse net. The dorsal cord is hollow and subepithelial in the animal's mesosomal (collar) region (27, 28). The collar portion of the cord develops by a chordate-like neurulation process in some species: that is, by inrolling of a sheet of ectoderm cells. The cord extends anteriorly into the proboscis and posteriorly to the anus. In contrast, the larger ventral cord is epithelial and solid. It extends from the collar to the anus, but not into the proboscis. At the pharynx/collar boundary, it bends to meet the dorsal cord. It develops separately from the dorsal cord. Neither cord resembles a ganglion or brain (29). von Salvini-Plawen proposes that the ventral cord eventually disappeared in the chordate line whereas the collar cord was retained in a series of evolutionary intermediates, as its neurulating mode of formation extended posteriorly to the blastopore, becoming the chordate neural plate. Hence, inversion never took place because, with two nerve cords, the ancestor was dorsoventrally ambiguous. The mouth was already ventral. As noted above, Nübler-Jung and Arendt (9) instead propose that the ventral cord was preserved and the dorsal cord became the chordate brain, with inversion and mouth relocation.
Figure 2.
Two nerve cords of an enteropneust hemichordate, Saccoglossus cambrensis. The animal is shown in semitransparent view. Nerves and nerve bundles, drawn in black, have been silver-stained. Modified from ref. 26.
What about the inversion evidence cited above: namely, the locations of the hemichordate tail, heart, endostyle, and pygocord? The tail and heart may also be ambiguous on the dorsoventral axis because they are at the posterior or anterior terminus, respectively. The tail is ventral to the anus, but a small displacement of the blastopore in development would suffice to make the tail dorsal, without body inversion. In the chordate gastrula, the blastopore/anus indeed moves ventrally through posterior mesoderm until the tail rudiment is dorsal to it (30). The hemichordate heart is located in the anterior coelom of the proboscis, anterior to the mouth and slightly dorsal. In chordate evolution, the anterior and middle coeloms have shrunk greatly, and the heart has come to lie in a ventral position posterior to the mouth (31). This displacement might not require inversion but only local changes of archenteron morphogenesis in the spherical gastrula. The ventral midline of the hemichordate pharynx was previously thought from light microscopy to resemble the ventral endostyle of chordates, but, from recent electron microscopy (32), the dorsal midline appears more endostyle-like, implying inversion. However, iodine incorporation was not done to localize the endostyle by function. As a speculation against inversion, perhaps the endostyle is double or circumferential in the hemichordate pharynx and has been narrowed down to a single ventral endostyle in chordates.
Finally, is the ventral pygocord of hemichordates (of the ptychoderids) the homolog of the dorsal notochord of chordates (implying inversion)? The alternative is the dorsal stomacord (no inversion). Both homolog candidates contain vacuolated cells like the notochord (9, 33). The stomacord does not express the brachyury gene, which the notochord does, and bra is expressed posteriorly and ventrally in hemichordates, perhaps including the pygocord (34). This would favor inversion (9). However, the role of bra expression in notochord evolution is unknown. It may not signify notochord differentiation but, rather, convergent extension morphogenesis. Perhaps such morphogenesis was only added late in notochord evolution: hence, its absence from the hemichordate's short stomacord. But, then, maybe neither candidate is the homolog, which might have arisen in the chordate line as an independent endoderm modification.
What Do the Gene Expression Patterns Reveal?
The strikingly similar but inverted gene expression patterns of protostomes and deuterostomes provide strong evidence that the common ancestor possessed certain organs (heart, nerve cord) in which certain genes were expressed, and from which homologous organs of modern bilateral animals are derived, expressing modern orthologs. Although this may be true, expression patterns in modern animals need not reveal details of the body organization of the last common ancestor. The ancestor may have had diffuse organization, and then centralization and placement of organs on the dorsoventral axis occurred independently in the two lines. Gene expression patterns of modern organs could still come out the same but inversely related in the two lines, without body inversion (Fig. 3).
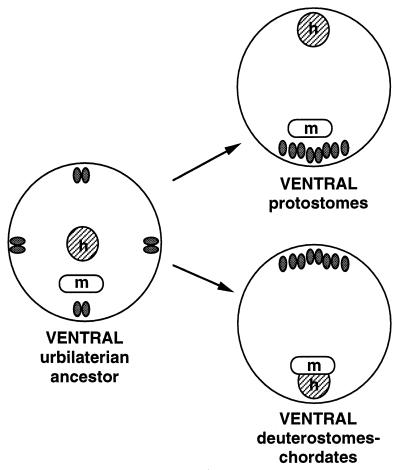
Figure 3.
An alternative to inversion. The hypothetical ancestor (Left, cross section) has little dorsoventral differentiation except for the mouth on the ventral side. Anterior is toward the reader. The body has multiple nerve cords and a centrally located anterior heart. In the protostome line (Upper Right), the cords coalesce toward the mouth side (ventral) and the heart shifts dorsally whereas, in the deuterostome line (Lower Right), the opposite occurs. Two body plans thus arise with inverse dorsoventral organization, without inversion.
As noted above, the most compelling evidence for inversion may be the complementary gene expression domains of bmp and chordin in chordates and dpp and sog in Drosophila because they seem to define dorsoventral compartments of the body plan (3, 10). In both chordates and Drosophila, the entire ectoderm possesses a potential for neural development, which is repressed via a type β transforming growth factor signal [bone morphogenetic protein (BMP) or Dpp]. The antagonists (Chordin or Sog) are then released locally, and neural development is derepressed at that site (35). Although the last common ancestor probably had this default mode of neural development, we cannot conclude that the antagonist was released at only one place on the dorsoventral axis. What determines nowadays the location of the Sog/Chordin compartment? Drosophila uses a gradient of activated Dorsal transcription factor to locate two bilateral domains of sog expression in the ectoderm whereas frogs use a quite different means, a combination of β-catenin and nodal-related proteins to activate chordin expression in midline mesoderm. Secreted Chordin protein then antagonizes BMP of the mesoderm and ectoderm (3, 10, 36). These different means for localizing the Sog/Chordin compartment may indicate separate evolutionary paths from a less localized ancestor. For example, perhaps the default mechanism predates bilaterality and was used in radial or biradial animals to generate their 4–8 nerve tracts (regions receiving Chordin/Sog) spaced between epidermal zones (regions not receiving Chordin/Sog). Perhaps chordin/sog expression was only unified into a contiguous domain in the separate protostome and deuterostome lines. Presumably, a hemichordate with two cords has two locations in the embryo in which Sog/Chordin releases neural development. And, presumably, both nerve cords express a full array of neural genes.
In conclusion, the hypothesis of body inversion has been vitalized recently by data on inverted patterns of orthologous gene expression in insects and vertebrates. Results are consistent with the hypothesis and with the existence of a complex ancestor with a highly differentiated dorsoventral axis. However, the data may also be explained by alternatives in which the last common ancestor of protostomes and deuterostomes had ambiguous dorsoventral organization, such as a diffuse nerve net or multiple nerve cords, and perhaps a heart at an anterior location. From this ancestor, protostome descendents may have further differentiated the dorsoventral axis in one direction, and deuterostome descendents in the opposite direction, generating inversely related gene expression patterns and coalesced organs without an actual inversion of the body.
References
- 1.Nübler-Jung K, Arendt D. Roux's Arch Dev Biol. 1994;203:357–366. doi: 10.1007/BF00188683. [DOI] [PubMed] [Google Scholar]
- 2.Romer A. The Vertebrate Body. Philadelphia: Saunders; 1962. [Google Scholar]
- 3.De Robertis E M, Sasai Y. Nature (London) 1996;380:37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- 4.Jägersten G. Evolution of the Metazoan Life Cycle: A Comprehensive Theory. New York: Academic; 1972. [Google Scholar]
- 5.Arendt D, Nübler-Jung K. Nature (London) 1994;371:26. doi: 10.1038/371026a0. [DOI] [PubMed] [Google Scholar]
- 6.Arendt D, Nübler-Jung K. BioEssays. 1996;18:255–259. doi: 10.1002/bies.950180314. [DOI] [PubMed] [Google Scholar]
- 7.Arendt D, Nübler-Jung K. Mech Dev. 1997;61:7–21. doi: 10.1016/s0925-4773(96)00620-x. [DOI] [PubMed] [Google Scholar]
- 8.Arendt D, Nübler-Jung K. Development (Cambridge, UK) 1999a;126:2309–2325. doi: 10.1242/dev.126.11.2309. [DOI] [PubMed] [Google Scholar]
- 9.Nübler-Jung K, Arendt D. J Zool Syst Evol Res. 1999;37:93–100. [Google Scholar]
- 10.Holley S A, Ferguson E L. BioEssays. 1997;19:281–284. doi: 10.1002/bies.950190404. [DOI] [PubMed] [Google Scholar]
- 11.Weiss J B, Von Ohlen T, Mellerick D M, Dressler G, Doe C Q, Scott M P. Genes Dev. 1998;12:3591–3602. doi: 10.1101/gad.12.22.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka M, Wechsler S B, Lee I W, Yamasaki N, Lawitts J A, Izumo S. Development (Cambridge, UK) 1999;126:1439–1450. doi: 10.1242/dev.126.7.1439. [DOI] [PubMed] [Google Scholar]
- 13.Christ B, Schmidt C, Huang R, Wilting J, Brand-Saberi B. Anat Embryol. 1998;197:1–8. doi: 10.1007/s004290050116. [DOI] [PubMed] [Google Scholar]
- 14.Gehring W J, Ikeo K. Trends Genet. 1999;15:371–377. doi: 10.1016/s0168-9525(99)01776-x. [DOI] [PubMed] [Google Scholar]
- 15.Adoutte A, Balavoine G, Lartillot N, de Rosa R. Trends Genet. 1999;15:104–108. doi: 10.1016/s0168-9525(98)01671-0. [DOI] [PubMed] [Google Scholar]
- 16.Aguinaldo A M A, Turbeville J M, Linford L S, Rivera M C, Garey J R, Raff R A, Lake J A. Nature (London) 1997;387:489–493. doi: 10.1038/387489a0. [DOI] [PubMed] [Google Scholar]
- 17.Bergstrom J. Paleontol Res. 1997;1:1–14. [Google Scholar]
- 18.Malakov V V. Zh Obsch Biol. 1977;38:485–499. [Google Scholar]
- 19.Nielsen C. Dev Genes Evol. 1999;209:198–205. doi: 10.1007/s004270050244. [DOI] [PubMed] [Google Scholar]
- 20.Garstang W. Q J Microbiol Sci. 1928;72:51–187. [Google Scholar]
- 21.Lacalli T C. BioEssays. 1996;18:251–254. [Google Scholar]
- 22.Martindale M Q, Henry J Q. Am Zool. 1988;38:672–684. [Google Scholar]
- 23.Peterson K J, Cameron R A, Davidson E H. BioEssays. 1997;19:623–631. doi: 10.1002/bies.950190713. [DOI] [PubMed] [Google Scholar]
- 24.von Salvini-Plawen L. J Zool Syst Evol Res. 1998;36:129–145. [Google Scholar]
- 25.Peterson K J. Nature (London) 1995;373:111–112. doi: 10.1038/373111a0. [DOI] [PubMed] [Google Scholar]
- 26.Knight-Jones E W. Philos Trans R Soc London B. 1952;236:315–354. [Google Scholar]
- 27.Hyman L H. The Invertebrates. Vol. 5. New York: McGraw–Hill; 1959. pp. 72–207. [Google Scholar]
- 28.Van ter Horst C J. In: Klassen und Ordnungen des Tierreichs. Bronn H G, editor. Vol. 4. Leipzig, Germany: C. F. Winter; 1939. pp. 1–739. [Google Scholar]
- 29.Cameron C B, Mackie G O. Can J Zool. 1996;74:15–19. [Google Scholar]
- 30.Keller R E. Dev Biol. 1976;51:118–137. doi: 10.1016/0012-1606(76)90127-5. [DOI] [PubMed] [Google Scholar]
- 31.De Beer G. Q J Microbiol Sci. 1955;96:279–283. [Google Scholar]
- 32.Ruppert E E, Cameron C B, Frick J E. Invertebr Biol. 1999;118:202–212. [Google Scholar]
- 33.Balser E J, Ruppert E E. Acta Zool. 1990;71:235–249. [Google Scholar]
- 34.Peterson K J, Cameron R A, Tagawa K, Satoh N, Davidson E H. Development (Cambridge, UK) 1999;126:85–95. doi: 10.1242/dev.126.1.85. [DOI] [PubMed] [Google Scholar]
- 35.Weinstein D C, Hemmati-Brivanlou A. Annu Rev Cell Dev Biol. 1999;15:411–433. doi: 10.1146/annurev.cellbio.15.1.411. [DOI] [PubMed] [Google Scholar]
- 36.Harland R, Gerhart J. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]