Abstract
N,N-Dimethylformamide (DMF) is globally used as an organic solvent in the production of synthetic leather and resins because of its low volatility, making it an attractive industrial material. Despite its excellent property as a chemical solvent, utilization of DMF is somewhat controversial nowadays due to its hazardous effects on exposed workers in work places. Many toxification cases are being reported globally and the number of cases of liver damage is still increasing in developing countries. On account of this, a series of epidemiologic surveys are being conducted to understand the degrees of liver damage caused by DMF exposure. Furthermore, many investigations have been performed to clarify the mechanism of DMF-induced liver toxicity using both human and experimental animal models. This review summarizes the current occupational cases reported on liver damage from workers exposed to DMF in industrial work places and the research results that account for DMF-induced liver failure and possible carcinogenesis. The findings reviewed here show the synergistic toxicity of DMF exposure with other toxicants, which might occur through complicated but distinct mechanisms, which may extend our knowledge for establishing risk assessments of DMF exposure in industrial work places.
Keywords: Dimethylformamide, Hepatotoxicity, Occupational exposure, Cytochrome P-450 2E1
Introduction
N,N-Dimethylformamide [DMF, formula; (CH3)2NC(O)H] is a representative solvent used in factories handling polyurethane materials and acrylic fibers [1,2]. DMF is also utilized in the pharmaceutical industry in pesticide formulation and in the production of synthetic leathers, surface coatings, films, and fibers [1,2]. This colorless solvent is miscible with most organic liquids as well as water. DMF also exerts low volatility, which makes it popular as a representative chemical solvent in various industries. The worldwide consumption of DMF in 2001 was ~285,000 tons and most of it was used as an industrial solvent [3,4]. In Korea, the amount of DMF utilized in industries was 73,385 tons in 2004; more than 3,600 workers handled DMF in their work places [5,6]. Among the work places, chemical manufacturing factories accounted for 46% of total use, followed by textile-producing industries, which reached 16%. In 2007, the amount of DMF consumed was further increased up to 100,501 metric tons, indicating that DMF is still used in numerous industries worldwide [6].
Toxic Effects of Occupational Exposure to DMF
As the amount of DMF utilization has increased, its potential toxic effects have also gained attention. During the last decades, several toxification cases have been reported in work places that deal with DMF [7-11]. The most prevalent disorders found in the workers exposed to DMF were liver toxicities, including hepatitis, fibrosis, cirrhosis, and cancer [7-11]. Other symptoms, such as alcohol intolerance, possible embryotoxicity, and teratogenicity, were also found [12-15], raising the necessity of establishing strict instructions and regulations for the utilization of DMF. Based on its versatile applications and severity of toxic effects, DMF has been classified as one of the four major compounds of precedence for human field studies by the National Toxicology Program (NTP) of the US National Institute of Environmental Health Sciences (NIEHS) [16]. In the United States, the time weighted average threshold limit value (TWA-TLV) for DMF in ambient air is 10 ppm in the work places [1]. The American Conference of Governmental Industrial Hygienists (ACGIH) recommended 15 mg/L and 40 mg/L as the biological exposure indices (BEIs) for the concentrations of N-methylformamide (UNMF) and N-acetyl-S-(N-methylcarbamoyl) cysteine (U-AMCC) in urine, respectively, which are two major metabolites of DMF [1].
In Korea, since the first patient with acute hepatitis that was induced by DMF exposure was reported in 1993 [17-26], the extent of damage from DMF exposure has been on the rise in parallel with increases of the amount of DMF utilization. Although some regulations and guidelines for workers dealing with DMF were established by the Occupational Safety and Health Act [6], there are still many accidental cases due to insufficient education and negligence in management.
A Generalized Pathway of DMF Metabolism in the Liver
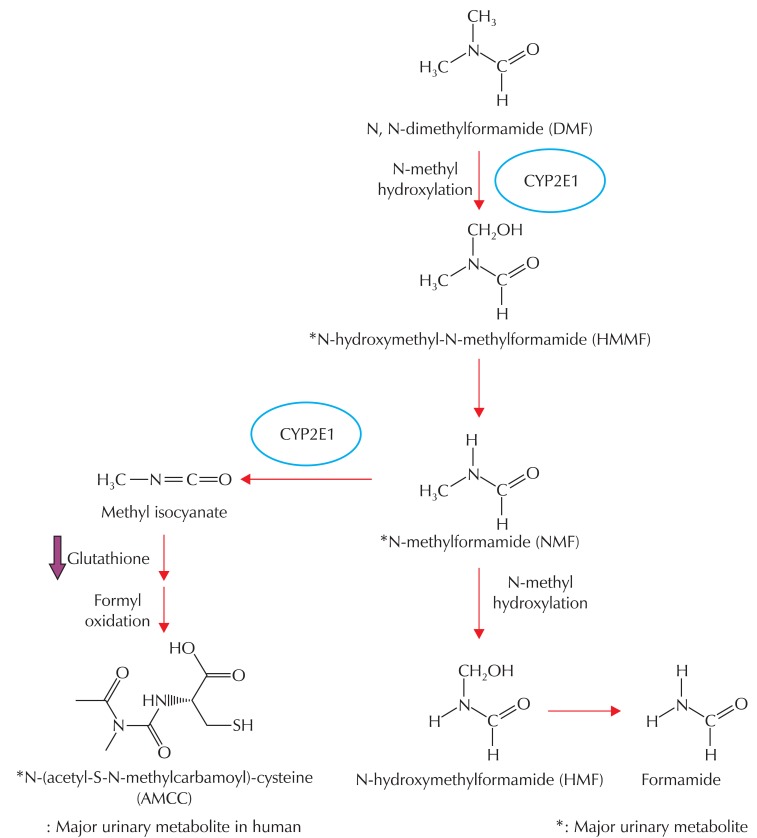
Since toxic effects from workers inflicted by occupational exposure to DMF had drawn attention worldwide, a number of studies have been performed to clarify the molecular mechanism of DMF-induced toxicity. DMF can be absorbed easily through oral, dermal, or inhalation exposure [2,7,9,10]. Following absorption, DMF is evenly distributed, metabolized mainly in the liver, and rapidly excreted in the form of metabolites through urine (Fig. 1) [7,10,27-31]. A series of studies have shown that DMF is N-methylated by microsomal enzymes in the liver, and that cytochrome P450s plays a role in the biotransformation of DMF [27,32-34]. Thus, the toxic effect elicited by DMF exposure is much more severe in the liver than in any other organ, presumably because its metabolism is mainly catalyzed by hepatic cytochrome P450s [27,32-34]. The hydroxylation of methyl moieties is the preceding step of DMF metabolism, resulting in the formation of N-(hydroxymethyl)-N-methylformamide (HMMF), the major urinary metabolite in both humans and animals. Following hydroxylation, HMMF decomposes to N-methylformamide (NMF). Enzymatic oxidation of the N-methyl moiety of NMF yields N-(hydroxymethyl)formamide (HMF), which then degenerates into formamide. However, confusion arose because HMMF, which is stable in an aqueous phase, was decomposed to NMF upon exposure to high temperature in a gas chromatography column [29,33,35]. Oxidation of the formyl group is another pathway for NMF metabolism to proceed, resulting in the production of N-acetyl-S-(N-methylcarbamoyl)cysteine (AMCC), which has been demonstrated to be a urinary metabolite in humans and rodents [29,32-34]. Mráz et al. [34] compared three DMF metabolites (i.e., HMMF, NMF, and AMCC) in these species. AMCC is a primary DMF metabolite in humans, but it is only a minor metabolite in rodents, with the mechanism of DMF metabolism remaining unclear. A reactive intermediate (presumably methyl isocyanate; a more reactive carbamoylating metabolite) is produced by the same pathway. Although indisputable supporting experimental data have not been reported yet, AMCC may be the putatively toxic metabolite [33,34].
Fig. 1.
A schematic cascade of DMF metabolism in vivo. Asterisk represents a major urinary metabolite of DMF. DMF: Dimethylformamide, CYP2E1: Cytochrome P-450 2E1.
Case Reports on the Outcomes of Occupational Exposure to DMF
Clinical reports on liver toxicity
The toxic effect of DMF has been investigated in a number of species following several routes of administration. DMF is generally absorbed into the body through dermal contact or inhalation [2,7,9,10]. Hepatotoxicity caused by DMF exposure has been studied in humans and a variety of animals upon both acute and subchronic exposure. The acute toxicity of DMF exposure through inhalation has also been considerably well studied [2,9,10].
Chronic liver disease was found in workers exposed to a DMF level of < 30 mg/m3, which is the threshold limit value (TLV) recommended by ACGIH. No significant liver dysfunction was observed in workers exposed to DMF levels of 0-47.7 mg/m3 (0.3-15.5 ppm) in the air, according to the report by Lauwerys et al. [36]. Wang et al. found hepatic dysfunction in workers that were chronically exposed to air levels of DMF of 77-186 mg/m3 or 25-60 ppm [37] and, in an epidemiologic study of chronically exposed workers with long-term follow-up, Redlich et al. studied both acute and chronic effects of exposure to DMF, showing fat accumulation in the liver in workers exposed for several years [38]. According to the report by Wang et al., there was a considerable association between a higher occurrence of liver abnormalities, as determined by elevated serum transaminase levels, and a higher degree of DMF exposure in 183 workers that were examined [37]. Another study performed by volunteers in an exposure chamber showed that ~40% of overall DMF exposure was due to dermal absorption [39]. Mráz and Nohová [40] studied the effect of skin penetration of DMF liquid or vapor in workers by using two methods: a "dipping experiment" (i.e., dipping one hand up to the wrist in DMF solution for 2 to 20 min and a "patch experiment" (i.e., applying 2 mM of DMF to the skin to be absorbed). The study showed that DMF exposure through skin contact contributes to a great degree with respect to DMF-induced total body burden, as shown by both plasma parameters of liver injury (i.e., ALT, AST, and γ-GT) and the content of DMF metabolites detected in urine. Therefore, protection of skin contact from exposure to DMF might be a critical issue in occupational health [36,39,40].
Numerous research groups investigated the effects of other factors on liver damage induced by DMF exposure. Chivers reported that simultaneous exposure to DMF with alcohol consumption caused intolerance to alcohol, termed as 'disulfiram-like effect', possibly resulting from the accumulation of acetaldehyde following the blocking of aldehyde dehydrogenase activity [41]. This effect was observed in humans, although the extent of exposure to DMF was relatively small [42]. Another study conducted by Luo et al. [43] revealed that the hepatitis B virus infection and higher BMI score had a synergistic effect with DMF exposure in causing liver failure and that the viral hepatitis infection intensified the degree of liver damage in workers exposed to DMF.
Studies on DMF toxicity in animal models
Several research groups studied the functional and morphological changes in the livers of animals administered with DMF by various routes. The doses of DMF reported in the literature to cause liver toxicity vary significantly depending on species and routes of administration [7]. In an acute exposure model, the livers of animals exposed to DMF exhibited fatty degeneration and necrotic changes in rabbits and cats after administration of either lethal or sub-lethal doses of DMF orally [44]. Rats receiving single i.p. administration of DMF (0.9-1.2 ml/kg) exhibited inflammatory infiltration and centrilobular necrosis in the liver. At the same dose, necrotic cells around the centrilobular vein and a scattered distribution of inflammatory cells (granulomas) were observed 24 h after injection [7]. In a subchronic exposure model, Craig et al. [9] performed animal experiments using rats and mice, and after inhalation exposure to DMF at the concentrations of 300 and 600 ppm for 12 weeks, the liver toxicity was examined, the results showed a significant increase in serum transaminase activity, hepatomegaly, and tumor lesion formation in the liver [45].
Potential Mechanism of DMF-induced Liver Toxicity
To clarify the mechanism of liver damage induced by DMF exposure, many investigations have been conducted by several research groups for decades. Since mechanistic investigation is essential for the understanding of toxicology, studies were also performed using animals in conjunction with clinical surveys from workers exposed to DMF. Although several hypotheses on the mechanism of DMF-induced liver toxicity have been raised, no clear biological pathway has been demonstrated yet [7,27-29,43].
CYP2E1-mediated metabolism of DMF
The hypothesis that the liver toxicity of DMF involves biotransformation by metabolic pathways was proposed by several groups. Kestell et al. [45] reported that biotransformation is a crucial determinant of formamide toxicity since a series of formamides and acetamides that underwent metabolic oxidation in the formyl moiety were found to have hepatotoxic properties. Based on the findings that 1) DMF is isoelectronic with N,N-dimethylnitrosamine, a well-characterized carcinogenic substrate of CYP2E1 [46], and 2) the microsomal oxidation of NMF is catalyzed by CYP2E1 [47], Mráz et al. [40] proposed the possibility that DMF is a putative substrate of CYP2E1. The metabolism of DMF into HMMF was increased upon treatment of rats with acetone, a CYP2E1 inducer, which could be inhibited when DMF was co-incubated with a CYP2E1 inhibitor [34]. Since CYP2E1 plays a role in the promotion of oxidative stress as it metabolizes many hepatotoxicants with low molecular weights (for example, acetaminophen, alcohol, and carbon tetrachloride), it is highly likely that DMF metabolism in the liver and subsequent changes in cellular redox capacity account for DMF-induced toxicity. Apparently, NMF, one of the major metabolites of DMF, was more toxic than DMF in rodents because it depleted cellular their GSH content [33].
Potential mechanisms of DMF-induced toxicity
In spite of the reports on the prooxidant effects of DMF metabolites, the mechanism of DMF-induced liver toxicity is yet unclear. Given the close link between CYP2E1-mediated oxidative stress and hepatotoxicity, our laboratory examined whether DMF in combination with other hepatotoxicant might enhance toxicity. To determine the possible synergism of DMF toxicity with other toxicants, several hepatotoxicants, including CCl4, lipopolysaccharide (LPS), acetaminophen (APAP), and galactosamine (GalN), at subtoxic doses were administered to rats [48]. Among them, simultaneous treatment of DMF and a low dose of CCl4 significantly enhanced liver toxicity compared to each treatment alone, as shown by increases in plasma transaminase activities. We further assessed the dose-dependent effect of DMF treatment on the blood biochemistry in rats administered with CCl4 to confirm the synergism of liver toxicity. As expected, plasma ALT and AST activities increased as the dose of DMF escalated, but the DMF treatment alone (500 mg/kg) weakly changed them. Synergistic hepatotoxicity due to the combined treatment of DMF and CCl4 was also verified by histopathological examinations, which showed swelling, fatty degeneration and death of hepatocytes, inflammatory cell infiltration of the liver lobule, and necrosis [48].
On the other hand, combinatorial treatment of DMF with other hepatotoxicants (for example, LPS, APAP, GalN) did not alter the blood biochemical parameters when compared with animal groups treated with a vehicle or DMF alone [48]. 1) Since LPS-induced toxicity notably targets the liver, it has been used in the studies for cytokine-induced liver toxicity. In our animal experiments, administration of DMF did not enhance liver toxicity in rats treated with LPS, suggesting that DMF might not cause liver injury by inducing inflammatory cytokines (e.g., TNF-α) [48]. APAP, one of the frequently prescribed analgesic and antipyretic agents, is used as a hepatotoxicant in in vivo models. APAP, when administered at high dose, causes hepatic GSH depletion, resulting in oxidative stress within hepatocytes. In this process, CYP2E1 plays a key role and may facilitate the production of reactive metabolites. The failure of DMF in increasing APAP-induced hepatotoxicity suggested that sulfhydryl pools in hepatocytes might not be notably altered by DMF treatment, at least at the doses examined [48]. Furthermore, Kelava et al. [49] reported that administration of DMF to mice 1 h prior to APAP treatment abolished APAP toxicity, possibly through their competitive inhibition of CYP2E1, thereby preventing GSH depletion by APAP. GalN, which is well characterized to potentiate LPS-induced toxicity, causes liver toxicity through inflammation and free radical formation in the liver. DMF did not result in enhanced liver toxicity in rats treated with GalN [48], indicating that the mechanism of DMF-induced toxicity might not involve the production of proinflammatory cytokines.
The endoplasmic reticulum (ER) plays a role in synthesis, folding, and maturation cascade of both membrane and soluble proteins. When several stressful conditions (for example, oxidative stress, inflammation, and viral infection) that raise the workload of protein folding occur, cells trigger specified programs to cope with those stimuli, and the programs are called ER stress. In cases when the stress maintains beyond the cellular adaptive capacity, ER stress elicits apoptosis [50-53]. Since hepatocyte death triggered by the ER stress may be a major mechanism of liver disease, we examined the effect(s) of DMF and/or CCl4 on the ER stress response by assessing ER-associated chaperones and other parameters. Our finding indicated that treatment with DMF and CCl4 up-regulated the expression of ER stress markers (for example, Grp78/94, p-PERK, and CHOP), suggesting that severe hepatotoxicity may be associated in part with ER stress caused by DMF and CCl4.
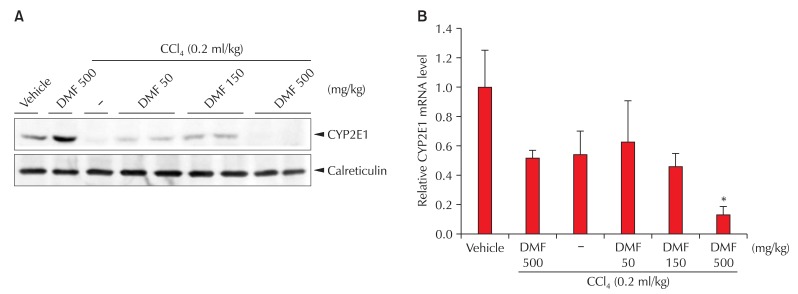
CYP2E1 metabolizes various endogenous and exogenous substrates to reactive metabolites, and thereby produces reactive oxygen species (ROS). Furthermore, it is well characterized that sustained ROS challenge aggravated cell viability when the activity of CYP2E1 was increased by chemical inducers or enzyme overexpression [54]. In an effort to find the link of hepatocyte death to CYP2E1-dependent metabolism, we measured CYP2E1 protein and mRNA levels after DMF and/or CCl4 treatment; DMF treatment at the daily dose of 500 mg/kg for 3 days increased the expression of the CYP2E1 level (~1.5-fold), which is consistent with the report that DMF induced CYP2E1 expression [55,56]. CCl4 is known to reduce CYP2E1 levels by producing reactive metabolites and the consequent suicide substrate inhibition [57]. As expected, CCl4 treatment decreased CYP2E1 expression. Interestingly, DMF at doses of 50 or 150 mg/kg/day prevented reductions in the CYP2E1 level by CCl4, presumably because of its competitive inhibition of CYP2E1-mediated biotransformation [57]. However, the combined treatment of 500 mg/kg DMF and CCl4 further repressed the CYP2E1 level (Fig. 2).
Fig. 2.
CYP2E1 repression by DMF and/or CCl4 treatment. (A) Immunoblottings for CYP2E1. CYP2E1 was immunoblotted on the liver homogenates prepared from five randomly selected animals per treatment. (B) Real-time RT-PCR assays. PCR assays were performed to assess CYP2E1 mRNA levels (significantly different from vehicle treatment alone, *p < 0.01). DMF: Dimethylformamide, CYP2E1: Cytochrome P-450 CYP2E1.
Real-time PCR analysis showed that the levels of CYP2E1 mRNA were not notably affected by treatment of either DMF or CCl4 alone, which is in line with the previous reports that many exogenous low-molecular-weight substrates for CYP2E1 (for example, pyrazole, 4-methylpyrazole, and acetone) induce CYP2E1 mainly through posttranscriptional regulation, which is not accompanied by an increase in its mRNA level [58]. However, the combined treatment of 500 mg/kg DMF and CCl4 substantially decreased the mRNA level, which might result from transcriptional repression due to hepatocyte death (Fig. 2).
Conclusions and Implications
1) DMF, a representative industrial solvent that is still being used in a number of developing countries, has been reported to cause severe liver toxicities from workers who are exposed to DMF at work places. Although several research institutes provided regulations for using DMF in work places based on the accumulated accidental reports, more definite countermeasures are still required to reduce the risks resulting from DMF utilization. Since toxic chemicals have their distinct mechanisms for inducing liver toxicity, it is essential to identify the molecular mechanism of toxicity to minimize liver damage caused by DMF exposure [58]. Nevertheless, the knowledge for the mechanistic basis of DMF-induced liver toxicity is still somewhat limited; it would be insufficient to define the degree of liver damage upon DMF exposure and adequate therapy without any profound biomarker, which should be identified by mechanistic studies.
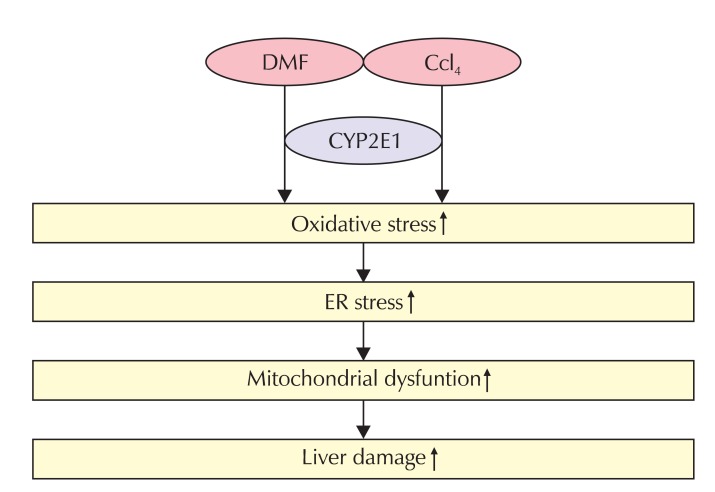
2) In chronic animal models, DMF had a toxic effect on the liver [2,9,10,33]. In an acute animal model, exposing rats to DMF + CCl4 increased their ER stress response, as indicated by the increased expression of ER stress markers. So, DMF induction of ER stress was promoted by a low dose of CCl4 treatment, which might be associated with enhanced liver toxicity. The profile of dose-dependency and synergism in liver injury induced by DMF was similar to that of the ER stress responses (Fig. 3). Furthermore, ROS elicit cell death upon toxicant exposure partly through ER stress, indicating the link between oxidative stress and ER stress [59]. Biotransformation of DMF to more reactive metabolites may cause CYP2E1 induction as an adaptive response [54]. In our study, CYP2E1 induction by DMF might enhance the metabolism of CCl4, resulting in increases of reactive metabolites from the toxicants. In parallel with this toxicity, we found a comparable change in ER stress markers, also supporting their synergism. The reactive metabolites generated from DMF evoke oxidative stress, which may cause hepatocyte injury. Radical scavengers attenuated the oxidation of DMF, supporting the hypothesis that oxidative stress contributes to the toxicity of DMF [60].
Fig. 3.
A scheme illustrating the possible mechanism of the synergistic liver toxicity of DMF with CCl4. DMF: Dimethylformamide, CYP2E1: Cytochrome P-450 2E1, ER: endoplasmic reticulum.
3) The in vivo results using two-chemical models allowed us to discover the possible mechanism of DMF-induced liver toxicity. The papers reviewed here demonstrate the toxicity of DMF exposure with other toxicants, which might occur through complicated but distinct mechanisms. These findings may be of help in understanding the hazardous effect exhibited in workers exposed to DMF, especially the susceptibility of individuals who are simultaneously exposed to other toxicants. This information may enable us to build up proper guidelines with respect to handling DMF in order to decrease the risk of liver toxicity for workers exposed to DMF at work places.
Acknowledgments
This research was supported by a grant (10182KFDA992) from the Korea Food & Drug Administration in 2011.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.American Conference of Govermental Industrial Hygienists (ACGIH) Documentation for DMF. Cincinnati (OH): ACGIH; 2001. [Google Scholar]
- 2.Lynch DW, Placke ME, Persing RL, Ryan MJ. Thirteen-week inhalation toxicity of N,N-dimethylformamide in F344/N rats and B6C3F1 mice. Toxicol Sci. 2003;72:347–358. doi: 10.1093/toxsci/kfg033. [DOI] [PubMed] [Google Scholar]
- 3.Johnson W, Yagi K. CEH Report: Dimethylformamide. Menlo Park (CA): SRI Consulting; 2002. [Google Scholar]
- 4.Shieh DB, Chen CC, Shih TS, Tai HM, Wei YH, Chang HY. Mitochondrial DNA alterations in blood of the humans exposed to N,N-dimethylformamide. Chem Biol Interact. 2007;165:211–219. doi: 10.1016/j.cbi.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Lee SW, Kim TH, Kim JM. A study on the necessity in establishment of STEL of dimethylformamide (DMF) -on the focus of the exposure in synthetic Leather factories. J Korean Soc Occup Environ Hyg. 2008;18:80–90. [Google Scholar]
- 6.Lee YJ. Annual reports on usage of chemical materials - Documentation for DMF. Incheon (Korea): Korea Occupational Safety & Health Agency (KOSHA); 2007. pp. 72–73. Korean. [Google Scholar]
- 7.Scailteur V, Lauwerys RR. Dimethylformamide (DMF) hepatotoxicity. Toxicology. 1987;43:231–238. doi: 10.1016/0300-483x(87)90082-5. [DOI] [PubMed] [Google Scholar]
- 8.Potter HP. Dimethylformamide-induced abdominal pain and liver injury. Arch Environ Health. 1973;27:340–341. doi: 10.1080/00039896.1973.10666392. [DOI] [PubMed] [Google Scholar]
- 9.Craig DK, Weir RJ, Wagner W, Groth D. Subchronic inhalation toxicity of dimethylformamide in rats and mice. Drug Chem Toxicol. 1984;7:551–571. doi: 10.3109/01480548409042819. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy GL, Jr, Sherman H. Acute and subchronic toxicity of dimethylformamide and dimethylacetamide following various routes of administration. Drug Chem Toxicol. 1986;9:147–170. doi: 10.3109/01480548608998272. [DOI] [PubMed] [Google Scholar]
- 11.Redlich CA, Beckett WS, Sparer J, Barwick KW, Riely CA, Miller H, Sigal SL, Shalat SL, Cullen MR. Liver disease associated with occupational exposure to the solvent dimethylformamide. Ann Intern Med. 1988;108:680–686. doi: 10.7326/0003-4819-108-5-680. [DOI] [PubMed] [Google Scholar]
- 12.Calvert GM, Fajen JM, Hills BW, Halperin WE. Testicular cancer, dimethylformamide, and leather tanneries. Lancet. 1990;336:1253–1254. doi: 10.1016/0140-6736(90)92870-n. [DOI] [PubMed] [Google Scholar]
- 13.Hansen E, Meyer O. Embryotoxicity and teratogenicity study in rats dosed epicutaneously with dimethylformamide (DMF) J Appl Toxicol. 1990;10:333–338. doi: 10.1002/jat.2550100505. [DOI] [PubMed] [Google Scholar]
- 14.Kang IC, Oh HM. Increased growth of human leukemic HL-60 cells by dimethyl sulfoxide and dimethylformamide. Korean J Immunol. 1999;21:285–289. [Google Scholar]
- 15.Kim KH, Seoh JY. Phenotypic and functional differentiation of promyelocytic cell Line HL-60 by N-N-dimethylformamide. J Korean Pediatr Soc. 1998;41:481–488. [Google Scholar]
- 16.Moorman WJ, Ahlers HW, Chapin RE, Daston GP, Foster PM, Kavlock RJ, Morawetz JS, Schnorr TM, Schrader SM. Prioritization of NTP reproductive toxicants for field studies. Reprod Toxicol. 2000;14:293–301. doi: 10.1016/s0890-6238(00)00089-7. [DOI] [PubMed] [Google Scholar]
- 17.Kim KW, Choi BS, Kang SK, Moon YH. A study on the N-methylformamide excretion rate of workers at synthetic leather factories in Korea. Korean J Occup Environ Med. 1999;11:106–112. [Google Scholar]
- 18.Lee SW, Kim TH, Kim JM, Kim JC. A valuation and improvement of industrial ventilation system of printing process in synthetic leather factory using dimethylformamide. J Korean Soc Occup Environ Hyg. 2009;19:113–126. [Google Scholar]
- 19.Kang SK, Jang JY, Rhee KY, Chung HK. A study on the liver dysfunction due to dimethylformamide. Korean J Occup Environ Med. 1991;3:58–64. [Google Scholar]
- 20.Kim SK, Lee SJ, Chung KC. A suspicious case of dimethylformamide induced fulminant hepatitis in synthetic leather workers. Korean J Occup Environ Med. 1995;7:186–190. [Google Scholar]
- 21.Kim HR, Kim TW. Occupational hepatic disorders in Korea. J Korean Med Sci. 2010;25(Suppl):S36–S40. doi: 10.3346/jkms.2010.25.S.S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joo MD, Sohn YD, Choi WI. A case of toxic hepatitis after the exposure of dimethylformamide. J Korean Soc Emerg Med. 2006;17:515–518. [Google Scholar]
- 23.Roh JR, Sohn JG, Kim JH, Park SJ. A case of acute toxic hepatitis induced by brief exposure to Dimethylformamide. Korean J Occup Environ Med. 2005;17:144–148. [Google Scholar]
- 24.Lee KY, Byeon JH, Song HR, Kim JH, Ko KW, Lee YH. Seasonal variations of the urinary N-Methylformamide concentration among workers at a synthetic leather factory. Korean J Occup Environ Med. 2003;15:162–172. [Google Scholar]
- 25.Kim SA, Kim JS, Jeon HR, Jung SJ, Kim SW, Lee CY, Ham JO, Yoo JY, Choi TS, Goo HB, Cho MH, Woo KH. Surveillance of work-related diseases in Kumi. Korean J Occup Environ Med. 2003;15:95–110. [Google Scholar]
- 26.Heo JH, Lee KL, Han SG, Kim HJ, Pai YM, Whang YH, Kang PJ, Kim CH, Cho SL. A case of fulminant hepatitis due to dimethylformamide. Korean J Gastroenterol. 1999;34:547–550. [Google Scholar]
- 27.Hantson P, Villa A, Galloy AC, Negri S, Esabon G, Lambiotte F, Haufroid V, Garnier R. Dimethylformamide metabolism following self-harm using a veterinary euthanasia product. Clin Toxicol (Phila) 2010;48:725–729. doi: 10.3109/15563650.2010.498790. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy GL., Jr Biological effects of acetamide, formamide, and their mono and dimethyl derivatives: an update. Crit Rev Toxicol. 2001;31:139–222. doi: 10.1080/200140911116861. [DOI] [PubMed] [Google Scholar]
- 29.Koh SB, Cha BS, Park JK, Chang SH, Chang SJ. The metabolism and liver toxicity of N,N-dimethylformamide in the isolated perfused rat liver. Yonsei Med J. 2002;43:491–499. doi: 10.3349/ymj.2002.43.4.491. [DOI] [PubMed] [Google Scholar]
- 30.Chung IS, Kim JG, Choi SK, Bae JY, Lee MY. Influencing factors that affect the biological monitoring of workers exposed to N,N-dimethylformamide in textile coating factories. J Prev Med Public Health. 2006;39:171–176. [PubMed] [Google Scholar]
- 31.Chung HK, Kang SK, Rhee KY, Jang JY. Evaluation of biological metabolites among the workers exposed todimethylformanide. Korean J Occup Environ Med. 1992;4:144–150. [Google Scholar]
- 32.Amato G, Grasso E, Longo V, Gervasi PG. Oxidation of N,N-dimethylformamide and N,N-diethylformamide by human liver microsomes and human recombinant P450s. Toxicol Lett. 2001;124:11–19. doi: 10.1016/s0378-4274(01)00324-1. [DOI] [PubMed] [Google Scholar]
- 33.Scailteur V, Lauwerys R. In vivo metabolism of dimethylformamide and relationship to toxicity in the male rat. Arch Toxicol. 1984;56:87–91. doi: 10.1007/BF00349077. [DOI] [PubMed] [Google Scholar]
- 34.Mráz J, Jheeta P, Gescher A, Hyland R, Thummel K, Threadgill MD. Investigation of the mechanistic basis of N,N-dimethylformamide toxicity. Metabolism of N,N-dimethylformamide and its deuterated isotopomers by cytochrome P450 2E1. Chem Res Toxicol. 1993;6:197–207. doi: 10.1021/tx00032a009. [DOI] [PubMed] [Google Scholar]
- 35.Koh SB, Cha BS, Kang MG, Koh SY, Lee JW, Kwon SO. The metabolism and liver toxicity of N, N-dimethylformamide in the isolated perfused liver. Korean J Occup Environ Med. 1997;9:217–229. [Google Scholar]
- 36.Lauwerys RR, Kivits A, Lhoir M, Rigolet P, Houbeau D, Buchet JP, Roels HA. Biological surveillance of workers exposed to dimethylformamide and the influence of skin protection on its percutaneous absorption. Int Arch Occup Environ Health. 1980;45:189–203. doi: 10.1007/BF00380783. [DOI] [PubMed] [Google Scholar]
- 37.Wang JD, Lai MY, Chen JS, Lin JM, Chiang JR, Shiau SJ, Chang WS. Dimethylformamide-induced liver damage among synthetic leather workers. Arch Environ Health. 1991;46:161–166. doi: 10.1080/00039896.1991.9937444. [DOI] [PubMed] [Google Scholar]
- 38.Redlich CA, West AB, Fleming L, True LD, Cullen MR, Riely CA. Clinical and pathological characteristics of hepatotoxicity associated with occupational exposure to dimethylformamide. Gastroenterology. 1990;99:748–757. doi: 10.1016/0016-5085(90)90964-3. [DOI] [PubMed] [Google Scholar]
- 39.Nomiyama T, Nakashima H, Chen LL, Tanaka S, Miyauchi H, Yamauchi T, Sakurai H, Omae K. N,N-dimethylformamide: significance of dermal absorption and adjustment method for urinary N-methylformamide concentration as a biological exposure item. Int Arch Occup Environ Health. 2001;74:224–228. doi: 10.1007/s004200000207. [DOI] [PubMed] [Google Scholar]
- 40.Mráz J, Nohová H. Percutaneous absorption of N,N-dimethylformamide in humans. Int Arch Occup Environ Health. 1992;64:79–83. doi: 10.1007/BF00381473. [DOI] [PubMed] [Google Scholar]
- 41.Lyle WH, Spence TW, McKinneley WM, Duckers K. Dimethylformamide and alcohol intolerance. Br J Ind Med. 1979;36:63–66. doi: 10.1136/oem.36.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chivers CP. Disulfiram effect from inhalation of dimethylformamide. Lancet. 1978;1:331. doi: 10.1016/s0140-6736(78)90105-8. [DOI] [PubMed] [Google Scholar]
- 43.Luo JC, Kuo HW, Cheng TJ, Chang MJ. Abnormal liver function associated with occupational exposure to dimethylformamide and hepatitis B virus. J Occup Environ Med. 2001;43:474–482. doi: 10.1097/00043764-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Massmann W. Toxicological investigations on dimethylformamide. Br J Ind Med. 1956;13:51–54. doi: 10.1136/oem.13.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kestell P, Threadgill MD, Gescher A, Gledhill AP, Shaw AJ, Farmer PB. An investigation of the relationship between the hepatotoxicity and the metabolism of N-alkylformamides. J Pharmacol Exp Ther. 1987;240:265–270. [PubMed] [Google Scholar]
- 46.Yang CS, Yoo JS, Ishizaki H, Hong JY. Cytochrome P450IIE1: roles in nitrosamine metabolism and mechanisms of regulation. Drug Metab Rev. 1990;22:147–159. doi: 10.3109/03602539009041082. [DOI] [PubMed] [Google Scholar]
- 47.Hyland R, Gescher A, Thummel K, Schiller C, Jheeta P, Mynett K, Smith AW, Mráz J. Metabolic oxidation and toxification of N-methylformamide catalyzed by the cytochrome P450 isoenzyme CYP2E1. Mol Pharmacol. 1992;41:259–266. [PubMed] [Google Scholar]
- 48.Kim TH, Kim YW, Shin SM, Kim CW, Yu IJ, Kim SG. Synergistic hepatotoxicity of N,N-dimethylformamide with carbon tetrachloride in association with endoplasmic reticulum stress. Chem Biol Interact. 2010;184:492–501. doi: 10.1016/j.cbi.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 49.Kelava T, Cavar I, Culo F. Influence of small doses of various drug vehicles on acetaminophen-induced liver injury. Can J Physiol Pharmacol. 2010;88:960–967. doi: 10.1139/y10-065. [DOI] [PubMed] [Google Scholar]
- 50.Nolan JP. The role of endotoxin in liver injury. Gastroenterology. 1975;69:1346–1356. [PubMed] [Google Scholar]
- 51.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaufman RJ, Scheuner D, Schröder M, Shen X, Lee K, Liu CY, Arnold SM. The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol. 2002;3:411–421. doi: 10.1038/nrm829. [DOI] [PubMed] [Google Scholar]
- 53.Arduíno DM, Esteves AR, Cardoso SM, Oliveira CR. Endoplasmic reticulum and mitochondria interplay mediates apoptotic cell death: relevance to Parkinson's disease. Neurochem Int. 2009;55:341–348. doi: 10.1016/j.neuint.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 55.Wang RS, Nakajima T, Honma T. Different change patterns of the isozymes of cytochrome P450 and glutathione S-transferases in chemically induced liver damage in rat. Ind Health. 1999;37:440–448. doi: 10.2486/indhealth.37.440. [DOI] [PubMed] [Google Scholar]
- 56.Koh SB, Cha BS, Kim KJ, Kang SK, Joung HS. Induction of hepatic microsomal cytochrome P450 by N,N-dimethylformamide in sprague-dawley rats. Korean J Prev Med. 1999;32:88–94. [Google Scholar]
- 57.Dai Y, Cederbaum AI. Inactivation and degradation of human cytochrome P4502E1 by CCl4 in a transfected HepG2 cell line. J Pharmacol Exp Ther. 1995;275:1614–1622. [PubMed] [Google Scholar]
- 58.Song BJ, Gelboin HV, Park SS, Yang CS, Gonzalez FJ. Complementary DNA and protein sequences of ethanol-inducible rat and human cytochrome P-450s. Transcriptional and post-transcriptional regulation of the rat enzyme. J Biol Chem. 1986;261:16689–16697. [PubMed] [Google Scholar]
- 59.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 60.Scailteur V, Lauwerys R. In vivo and in vitro oxidative biotransformation of dimethylformamide in rat. Chem Biol Interact. 1984;50:327–337. doi: 10.1016/0009-2797(84)90040-1. [DOI] [PubMed] [Google Scholar]