There is growing interest in the use of inverse Diels–Alder tetrazine cycloadditions as rapid catalyst-free bioorthogonal reactions.[1–3] Fluorogenic tetrazines that increase in fluorescence after reaction with dienophiles are particularly useful for live-cell imaging applications.[4] Fluorogenic tetrazines have been recently used for live-cell imaging of small molecules, biomolecules tagged enzymatically with dienophiles, and proteins modified by reactive unnatural amino acids.[4,5] Although fluorogenic tetrazine probes hold great potential for intracellular imaging of small molecules, previous approaches are limited by requiring a large strained dienophile, such as trans-cyclooctene, cyclooctyne, or norbornene.[1,6] This situation is in contrast to Staudinger ligations or strain-promoted azide–cycloalkyne cycloadditions that utilize a small azide functional group.[7,8] This requirement has limited the use of tetrazine reactions in methods that require tags with minimal steric impact or nominal effect on the partition ratio.[2] The development of smaller dienophile partners capable of reacting rapidly with tetrazines would therefore represent a major advance. However, it has been unclear whether small dienophiles could be developed that react rapidly with tetrazines while maintaining their stability. Herein, we demonstrate the applicability of methylcyclopropene tags as dienophiles for reaction with fluorogenic tetrazines. Through systematic synthetic modifications we have optimized the stability, size, and reactivity of the cyclopropene scaffold. We have developed methylcyclopropene derivatives that react rapidly with tetrazines while retaining their aqueous stability and small size. These cyclopropene handles elicit fluorescent responses from quenched tetrazine dyes and are suitable for cellular imaging applications, which we demonstrate by imaging cyclopropene phospholipids distributed in live human breast cancer cells.
The use of cyclopropenes offers a possible approach to smaller dienophile partners for tetrazine cycloadditions. It has long been known that cyclopropenes react rapidly with tetrazines to form stable diazanorcaradienes (Figure 1a).[9] In seminal work, Sauer and co-workers demonstrated that unsubstituted cyclopropene reacts with dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate extremely rapidly, measuring a rate constant of 448M−1s−1 in dioxane at 20°C.[10] However, unsubstituted cyclopropene is highly unstable, dimerizing and polymerizing readily at room temperature.[11,12] Our challenge was to create a stable cyclopropene handle while maintaining rapid tetrazine reactivity and small size. The stability of cyclopropenes can be dramatically increased by substitution and we envisioned several substituted cyclopropenes that could be suitable for cycloaddition with tetrazines (Figure 1b).[11,13,14] Sauer and co-workers demonstrated that substitutions do not necessarily diminish the reactivity of cyclopropenes with tetrazine. In fact, the reaction of 3-methylcycloprop-1-ene with tetrazine proceeded with a slightly higher rate constant than the unsubstituted cyclopropene. However, 3,3-dimethylcycloprop-1-ene reacted with the same tetrazine approximately 5766-times slower, illustrating the importance of balancing stability with reactivity.[10]
Figure 1.
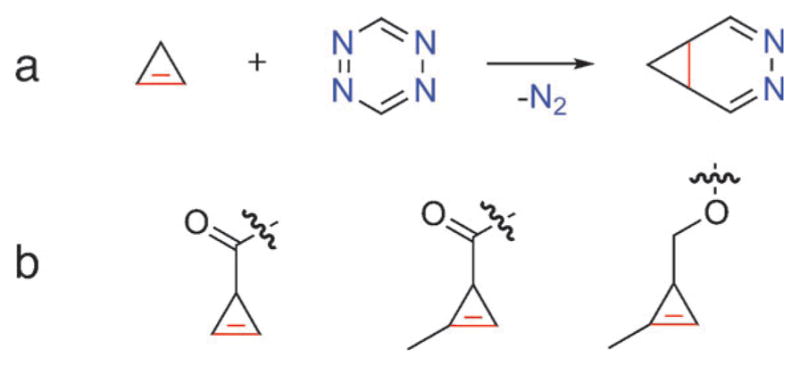
a) Inverse Diels–Alder reaction of cyclopropene with tetrazine results in loss of nitrogen gas and formation of a diazanorcaradiene. b) Examples of substituted cyclopropene handles that may be suitable dienophiles for tetrazine cycloaddition.
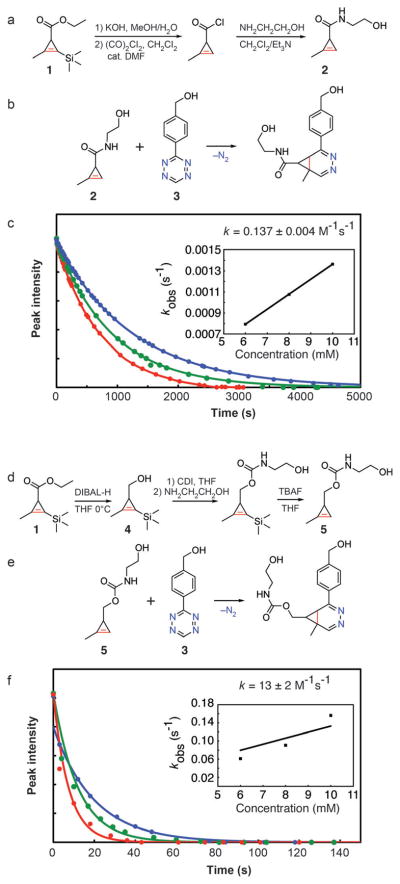
Based on studies from Sauer and co-workers, we initially synthesized carboxamide derivatives of cycloprop-2-enecar-boxylic acid without substitution of the double bond. These dienophiles reacted very rapidly with monoaryl tetrazines, but the substituted cyclopropenes proved to be highly unstable and could not be stored overnight at −20°C without degradation. Recently, Fox and co-workers have elegantly demonstrated that N-acyloxazolidinone derivatives of cycloprop-2-enecarboxylic acid are unusually stable and therefore valuable cyclopropene synthons.[15] Unfortunately, these modifications require a significant increase in the size of the reactive moiety and would defeat the purpose of using a small cyclopropene tag. The addition of a methyl substituent on the alkene offers an alternative method to improve stability with a less-dramatic steric impact.[14] Protected methylcyclopropene 1 was generated by rhodium-catalyzed cyclopropenation and used to synthesize 2-methylcycloprop-2-enecarboxamide (2).[16] The synthesis of 2 required four steps from commercially available starting materials and was completed in 21% overall yield (Figure 2a). As expected, the addition of a methyl group dramatically improved cyclopropene stability, and 2 could be stored for extended periods of time at −20°C without degradation. However, these derivatives reacted sluggishly with benzylalcohol tetrazine (3; Figure 2b).[17] By monitoring the disappearance of the characteristic tetrazine absorption band at 520 nm, we measured a second-order rate constant of 0.137 ±0.004M−1s−1 at 37°C in a solution of water/ DMSO (12% DMSO by volume; Figure 2c). Although this rate constant is comparable to previous bioorthogonal labeling strategies,[8] it is much slower than the reaction of other strained alkenes with tetrazine, such as trans-cyclooctene and even norbornene.[1] Faster kinetics would improve coupling yields, particularly for applications where it is not possible to flood the target with a large excess of reactant, for example in live-cell intracellular labeling or in vivo.
Figure 2.
a) Synthesis of 2-methylcycloprop-2-enecarboxamide (2). DMF =dimethylformamide. b) Reaction of 2 with monoaryl tetrazine 3 leads to formation of diazanorcaradiene isomers (only one regioisomer is depicted). d) Synthesis of 2-methylcyclopropene carbamate (5). DIBAL-H =diisobutylaluminum hydride; CDI =carbonyldiimidazole; THF =tetrahydrofuran; TBAF =tetra-n-butylammonium fluoride. e) Reaction of 5 with monoaryl tetrazine 3 leads to formation of diazanorcaradiene isomers (only one regioisomer is depicted). c, f) Plots of tetrazine absorbance versus time during the reaction between 0.6 mM tetrazine 3 and 6 (blue), 8 (green), or 10 mM (red) of cyclopropene 2 (c) or cyclopropene 5 (f). Data was fit to a first-order exponential decay. Inset: kobs plotted against concentration with the slope taken as the second-order rate constant.
In an attempt to improve the kinetics of cycloaddition, we reduced the ester of precursor 1 to form [2-methyl-3-(tri-methylsilyl)cycloprop-2-en-1-yl]methanol (4; Figure 2d). Compound 4 is a highly convenient synthon that can be made in two steps from commercially available starting materials. Methylcyclopropene 4 can be further conjugated to primary amines by carbamate formation, followed by deprotection of the trimethylsilyl protecting group to afford 2-methylcyclopropene carbamate (5). The synthesis of 5 required five steps from commercially available starting materials and was completed in 33% overall yield. Unlike the synthesis of trans-cyclooctene, the synthesis of cyclopropene 5 does not require the use of an ultraviolet reactor, silver nitrate trapping agent, or a metering pump.[18] We speculated that 5 would possess the stability afforded by the methyl derivatized alkene but display increased inverse Diels-Alder reactivity compared to 2 by elimination of the electron-withdrawing carbonyl. Indeed, carbamate 5 was highly reactive with tetrazine 3 (Figure 2e), and cycloaddition proceeded with a second-order rate constant of 13 ±2M−1s−1 at 37°C in a solution of water/DMSO (12% DMSO by volume; Figure 2f). This is an improvement of approximately two orders of magnitude compared to cyclopropene carboxamide 2. At 20°C, we measured a rate constant of 7 ±1M−1s−1 (12% DMSO by volume; Supporting Information, Figure S1). Methylcyclopropene 5 could be stored at −20°C without degradation and displayed excellent stability in aqueous solutions at 37°C for over 24 h (Figure S2). Furthermore, 5 was stable in aqueous solutions containing L-cysteine, both at room temperature and after heating at 60°C (Figure S3), in agreement with previous studies on related methylcyclopropenes.[19] Interestingly, when heated to 60°C in the presence of L-cysteine, we found that trans-cyclooctenol [rel-(1R,4E,pR)-cyclooct-4-enol] isomerized slowly to cis-cyclooctenol (Figure S4). Although further work will be needed to determine the relevance of this phenomenon for bioconjugation, it does illustrate that, under certain conditions, methylcyclopropenes may possess superior stability to trans-cyclooctenes, because cyclopropenes are not subject to this cis/trans isomerization.
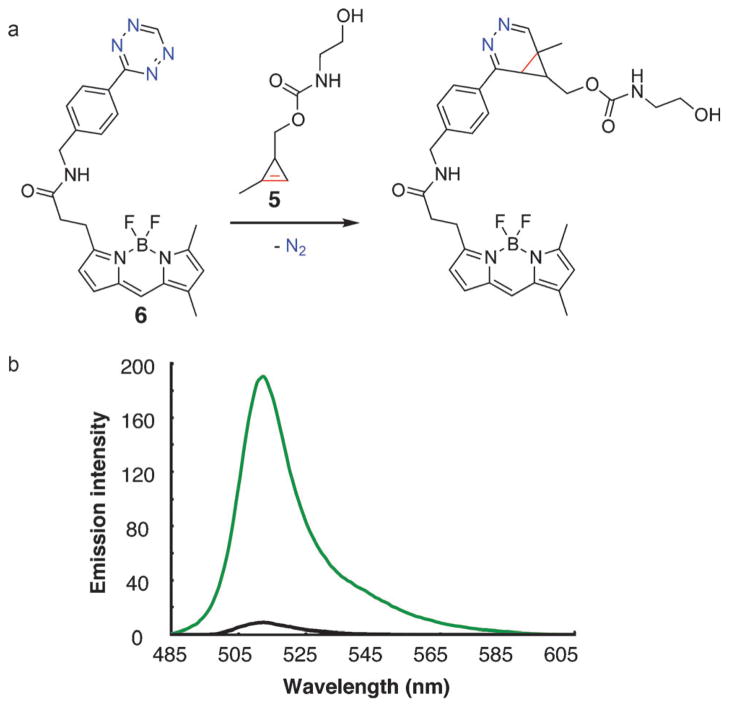
Fluorogenic probes are highly valuable in live-cell imaging applications owing to an inherently lower background fluorescence from nonspecific binding or accumulation.[4,20] This is particularly relevant for imaging intracellular molecules as washout can be problematic. Recent work has demonstrated that tetrazines are capable of significantly quenching several bright fluorescent probes, potentially through a resonant energy transfer mechanism.[21] These probes show significant fluorescent “turn-on” after reaction with dienophiles, can be synthesized from commercially available reactive precursors, and use bright, conveniently excited fluorophores that are commonly used in cellular imaging, such as boron dipyrromethane (BODIPY) and Oregon Green. The combination of recently discovered high-quality fluorogenic tetrazine probes, such as tetrazine-BODIPY FL, with small dienophile tags would advance bioorthogonal live-cell imaging. Tetrazine-BODIPY FL probe (6) reacts rapidly with 5, with a concomitant increase in fluorescence (Figure 3).[4] In phosphate-buffered saline at 20°C, there is a 22-fold increase in fluorescence intensity at 512 nm after addition of excess 5, similar to previous measurments of fluorescence increase after reaction with other strained dienophiles.[4,5] The reaction can also be monitored by liquid chromatography/mass spectrometry. Addition of one equivalent of 5 results in complete reaction of the tetrazine-BODIPY FL and, as expected, the formation of multiple overlapping peaks all with the molecular mass of the diazanorcaradiene isomers (Figure S5).
Figure 3.
Reaction of fluorogenic tetrazine-BODIPY FL (6) with 5. a) Reaction scheme. b) Emission spectra of tetrazine-BODIPY FL before reaction with 5 (black) and after reaction (green).
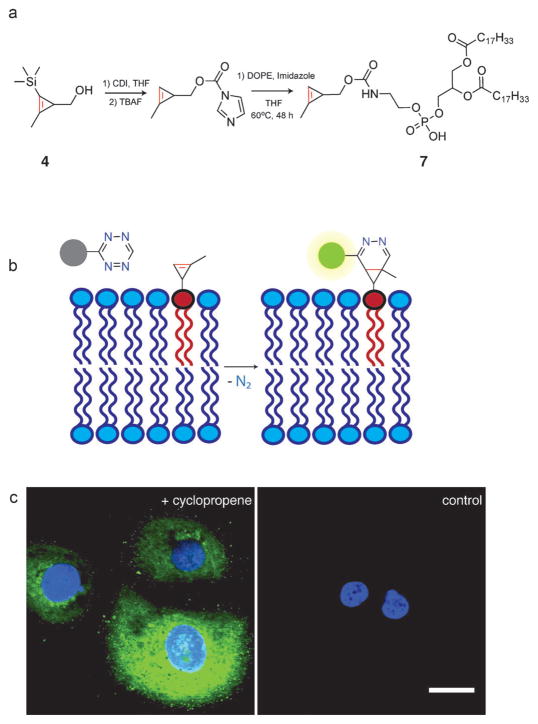
Live-cell labeling of bioorthogonal functional groups has emerged as a powerful tool for analyzing small molecule distributions in cells.[22] To demonstrate the applicability of cyclopropene tags for live-cell imaging using fluorogenic tetrazine cycloadditions, we synthesized a cyclopropene tagged phospholipid 7 (Figure 4a), which we characterized by NMR spectroscopy and high resolution mass spectrometry. Bioorthogonal reactions are increasingly used for lipid imaging and labeling, and there have been several exciting applications, such as metabolic labeling of choline phospholipids, high-throughput analysis of protein lipidation, and monitoring the trafficking of soluble lipids.[23] Cyclopropene–tetrazine cycloadditions would allow lipophilic fluorogenic tetrazines to be used, offering the important advantage of intracellular imaging in live cells (Figure 4b). Additionally, in vitro applications would benefit from improved reaction kinetics and the lack of redox-active copper catalysts, which can potentially damage biomolecules while adding an extra layer of complexity.[24]
Figure 4.
a) Synthesis of cyclopropene phospholipid 7. b) Reaction of fluorogenic tetrazine-BODIPY FL with membrane-bound 7. The BODIPY chromophore is initially quenched by tetrazine with the fluorescence recovered after cycloaddition and formation of the coupling adduct. DOPE =1,2-dioleoyl-sn-glycero-3-phosphoethanol-amine. c) Live-cell confocal imaging of 7 distribution in SKBR3 cells with the fluorogenic probe tetrazine-BODIPY FL. Left: cells incubated (t =1 h) with 50 μM 7 followed by 10 μM tetrazine-BODIPY FL probe (t =1 h; green). Right: control in which SKBR3 cells were treated with 10 μM tetrazine-BODIPY FL (t =1 h; green). Cells were treated with 300 nM DAPI to visualize the nuclei (blue). Scale bar =20 μm.
To image the distribution of cyclopropene phospholipids in human cells, we incubated SKBR3 breast cancer cells in media (cDMEM) containing 50 μM 7 for one hour. After washing with media, the cells were subsequently incubated with 10 μM of tetrazine-BODIPY FL for one hour. Cells were then washed and imaged using confocal fluorescence microscopy (Figure 4c). Staining of membrane structures could be readily observed with a notable absence of staining within the nucleus (Figure S10). In contrast, control cells that were not exposed to 7 but were treated with the tetrazine-BODIPY FL probe showed relatively negligible background staining, demonstrating the benefit of using a fluorogenic cycloaddition, which significantly mitigates the signal from nonspecific binding. Images obtained with 7 were similar to images obtained with a trans-cyclooctene phospholipid (Figure S11), indicating that, under the conditions tested, the cyclopropene tag is of comparable quality for live-cell imaging with respect to previously introduced dienophiles. We are presently utilizing 7 to visualize phospholipid uptake in several model systems. Additionally, we are synthesizing additional cyclopropene lipid tags to image and detect specific lipid distribution and lipid post-translational modifications in live cells.
In conclusion, we have studied the suitability of methyl-cyclopropene tags as small dienophiles for tetrazine bioconjugation chemistry. Modulating the substituents of cyclopropenes can have a dramatic effect on their stability and on the kinetics of tetrazine cycloaddition. Through synthetic modification of the cyclopropene scaffold, we have developed a methylcyclopropene derivative that is stable in aqueous solution but retains high reactivity with tetrazines. Cyclopropene derivatives are capable of eliciting a strong fluorogenic response from quenched tetrazine fluorescent probes, and this feature can be used to perform live-cell imaging, which we demonstrated by labeling cyclopropene-modified phospholipids. We believe the use of methylcyclopropenes will extend the advantages of tetrazine cycloadditions to small-molecule tracking applications that require minimal reaction partners. We are currently pursuing applications that incorporate cyclopropenes into metabolic and enzyme activity reporters, such as lipid, monosaccharide, and amino acid analogues.
Supplementary Material
Footnotes
We greatly acknowledge Ralph Mazitschek, Michael Hardy, and Carlos Guerrero for helpful discussions. This work was funded in part by NIH grant K01EB010078, the University of California, San Diego, the NSF under CHE-0741968, and by NIH grant P30 NS047101 supported by Jennifer Santini.
Supporting information for this article (experimental details) is available on the WWW under http://dx.doi.org/10.1002/anie.201202122.
References
- 1.Blackman ML, Royzen M, Fox JM. J Am Chem Soc. 2008;130:13518– 13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]; Devaraj NK, Weissleder R, Hilder-brand SA. Bioconjugate Chem. 2008;19:2297– 2299. doi: 10.1021/bc8004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devaraj NK, Weissleder R. Acc Chem Res. 2011;44:816–827. doi: 10.1021/ar200037t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiessler M, Waldeck W, Kliem C, Pipkorn R, Braun K. Int J Med Sci. 2009;7:19– 28. doi: 10.7150/ijms.7.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devaraj NK, Hilderbrand S, Upadhyay R, Mazitschek R, Weissleder R. Angew Chem. 2010;122:2931– 2934. doi: 10.1002/anie.200906120. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:2869– 2872. doi: 10.1002/anie.200906120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu DS, Tangpeerachaikul A, Selvaraj R, Taylor MT, Fox JM, Ting AY. J Am Chem Soc. 2012;134:792– 795. doi: 10.1021/ja209325n. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lang K, Davis L, Torres-Kolbus J, Chou C, Deiters A, Chin JW. Nat Chem. 2012;4:298– 304. doi: 10.1038/nchem.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devaraj NK, Upadhyay R, Haun JB, Hilderbrand SA, Weissleder R. Angew Chem. 2009;121:7147– 7150. doi: 10.1002/anie.200903233. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:7013– 7016. doi: 10.1002/anie.200903233. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chen W, Wang D, Dai C, Hamelberg D, Wang B. Chem Commun. 2012;48:1736– 1738. doi: 10.1039/c2cc16716f. [DOI] [PubMed] [Google Scholar]; Plass T, Milles S, Koehler C, Szymański J, Mueller R, Wießler M, Schultz C, Lemke EA. Angew Chem. 2012;124:4242– 4246. doi: 10.1002/anie.201108231. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2012;51:4166– 4170. doi: 10.1002/anie.201108231. [DOI] [PubMed] [Google Scholar]; Kaya E, Vrabel M, Deiml C, Prill S, Fluxa VS, Carell T. Angew Chem. 2012;124:4542– 4545. doi: 10.1002/anie.201109252. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2012;51:4466– 4469. doi: 10.1002/anie.201109252. [DOI] [PubMed] [Google Scholar]
- 7.Saxon E, Bertozzi CR. Science. 2000;287:2007– 2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 8.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Proc Natl Acad Sci USA. 2007;104:16793– 16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sauer J, Heinrich G. Tetrahedron Lett. 1966;7:4979– 4984. [Google Scholar]; Maier G. Angew Chem. 1967;79:446– 458. [Google Scholar]; Angew Chem Int Ed Engl. 1967;6:402– 413. [Google Scholar]; Sauer J, Bauerlein P, Ebenbeck W, Dyllick-Brenzinger R, Gousetis C, Sichert H, Troll T, Wall-fahrer U. Eur J Org Chem. 2001:2639–2657. [Google Scholar]; Sauer J, Bauerlein P, Ebenbeck W, Gousetis C, Sichert H, Troll T, Utz F, Wallfahrer U. Eur J Org Chem. 2001:2629– 2638. [Google Scholar]
- 10.Thalhammer F, Wallfahrer U, Sauer J. Tetrahedron Lett. 1990;31:6851– 6854. [Google Scholar]
- 11.Carter FL, Frampton VL. Chem Rev. 1964;64:497– 525. [Google Scholar]
- 12.Dowd P, Gold A. Tetrahedron Lett. 1969;10:85– 86. [Google Scholar]
- 13.von W, Doering E, Mole T. Tetrahedron. 1960;16:65– 70. [Google Scholar]
- 14.Closs GL, Boll WA, Closs LE. J Am Chem Soc. 1963;85:3796– 3800. [Google Scholar]
- 15.Yan N, Liu XZ, Pallerla MK, Fox JM. J Org Chem. 2008;73:4283– 4286. doi: 10.1021/jo800042w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petiniot N, Anciaux AJ, Noels AF, Hubert AJ, Teyssie P. Tetrahedron Lett. 1978;19:1239– 1242. [Google Scholar]
- 17.Yang J, Karver MR, Li W, Sahu S, Devaraj NK. Angew Chem. 2012;124:5312– 5315. doi: 10.1002/anie.201201117. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2012;51:5222– 5225. doi: 10.1002/anie.201201117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Royzen M, Yap GPA, Fox JM. J Am Chem Soc. 2008;130:3760– 3761. doi: 10.1021/ja8001919. [DOI] [PubMed] [Google Scholar]
- 19.Matsuura M, Saikawa Y, Inui K, Nakae K, Igarashi M, Hashimoto K, Nakata M. Nat Chem Biol. 2009;5:465– 467. doi: 10.1038/nchembio.179. [DOI] [PubMed] [Google Scholar]
- 20.Jewett JC, Bertozzi CR. Org Lett. 2011;13:5937– 5939. doi: 10.1021/ol2025026. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hangauer MJ, Bertozzi CR. Angew Chem. 2008;120:2428– 2431. doi: 10.1002/anie.200704847. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:2394– 2397. doi: 10.1002/anie.200704847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dumas-Verdes C, Miomandre F, Lepicier E, Galangau O, Vu TT, Clavier G, Meallet-Renault R, Audebert P. Eur J Org Chem. 2010:2525– 2535. [Google Scholar]
- 22.Sletten EM, Bertozzi CR. Angew Chem. 2009;121:7108–7133. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hang HC, Linder ME. Chem Rev. 2011;111:6341– 6358. doi: 10.1021/cr2001977. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hang HC, Wilson JP, Charron G. Acc Chem Res. 2011;44:699– 708. doi: 10.1021/ar200063v. [DOI] [PMC free article] [PubMed] [Google Scholar]; Best MD, Rowland MM, Bostic HE. Acc Chem Res. 2011;44:686– 698. doi: 10.1021/ar200060y. [DOI] [PubMed] [Google Scholar]; Jao CY, Roth M, Welti R, Salic A. Proc Natl Acad Sci USA. 2009;106:15332– 15337. doi: 10.1073/pnas.0907864106. [DOI] [PMC free article] [PubMed] [Google Scholar]; Neef AB, Schultz C. Angew Chem. 2009;121:1526– 1529. doi: 10.1002/anie.200805507. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:1498– 1500. doi: 10.1002/anie.200805507. [DOI] [PubMed] [Google Scholar]
- 24.Chiou SH. J Biochem. 1983;94:1259– 1267. doi: 10.1093/oxfordjournals.jbchem.a134471. [DOI] [PubMed] [Google Scholar]; Kennedy DC, McKay CS, Legault MCB, Danielson DC, Blake JA, Pegoraro AF, Stolow A, Mester Z, Pezacki JP. J Am Chem Soc. 2011;133:17993– 18001. doi: 10.1021/ja2083027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.