Abstract
Severe pre/eclampsia are associated with brain edema that forms preferentially in the posterior cerebral cortex possibly due to decreased sympathetic innervation of posterior cerebral arteries and less effective autoregulation during acute hypertension. In the present study, we examined the effect of pregnancy on the effectiveness of cerebral blood flow autoregulation using laser Doppler flowmetry and edema formation by wet:dry weight in acute hypertension induced by phenylephrine infusion in the anterior and posterior cerebrum from nonpregnant (n=8) and late-pregnant (n=6) Sprague Dawley rats. In addition, we compared the effect of pregnancy on sympathetic innervation by tyrosine hydroxylase staining of posterior and middle cerebral arteries (n=5–6/group) and endothelial and neuronal nitric oxide synthase expression using quantitative polymerase chain reaction (n=3/group). In nonpregnant animals, there was no difference in autoregulation between anterior and posterior cerebrum. However, in late-pregnant animals, the threshold of cerebral blood flow autoregulation was shifted to lower pressures in the posterior cerebrum, which was associated with increased neuronal nitric oxide synthase expression in the posterior cerebral cortex vs. anterior. Compared to the nonpregnant state, pregnancy increased the threshold of autoregulation in both brain regions that was related to decreased expression of endothelial nitric oxide synthase. Lastly, acute hypertension during pregnancy caused greater edema formation in both brain cortices that was not due to changes in sympathetic innervation. These findings suggest that although pregnancy shifted the cerebral blood flow autoregulatory curve to higher pressures in both the anterior and posterior cortices, it did not protect from edema during acute hypertension.
Keywords: Pregnancy, cerebral blood flow autoregulation, sympathetic innervation, brain edema
INTRODUCTION
Eclampsia is thought to be similar to hypertensive encephalopathy in which an acute and excessive elevation in blood pressure, secondary to the preeclamptic state, causes decreased cerebrovascular resistance (CVR), autoregulatory breakthrough, and a large increase in cerebral blood flow (CBF) in excess of metabolic demands.1,2 Breakthrough of autoregulation during acute hypertension can be damaging to the blood-brain barrier (BBB) and cause hydrostatic brain edema.3 Cerebral edema formation has been described in patients with severe preeclampsia and eclampsia and is thought to underlie the neurological symptoms associated with these conditions.1,2,4
The posterior cerebral cortex appears to be more susceptible to edema formation during acute hypertension associated with hypertensive encephalopathy and pre/eclampsia.1–4 The propensity for the edema to form in the posterior cerebrum has led to more recent terminology of posterior reversible encephalopathy syndrome (PRES) to better incorporate the posterior nature of the neurological symptoms that include uncontrolled vomiting, cortical blindness and severe and persistent headache.5 Although the underlying mechanism by which PRES arises is largely unknown, several have cited morphological studies showing a decrease in sympathetic innervation of the vertebro-basilar arteries vs. those of the internal carotid artery system as the cause.6 Cerebral arteries and arterioles on the brain surface (pial vessels) are innervated extrinsically by sympathetic neurons whose fibers originate in the superior cervical ganglia.7 This sympathetic innervation limits the pressure at which autoregulatory breakthrough occurs during acute hypertension.8 Thus, it has been speculated that decreased sympathetic innervation of posterior cerebral arteries (PCA) leads to a lower pressure of autoregulatory breakthrough and a propensity for hydrostatic edema to form in the posterior cerebrum during acute hypertension.6
Nitric oxide (NO) appears to be involved in CBF autoregulation and may have an effect during pregnancy. Pharmacological inhibition of NO synthase (NOS) shifts the pressure of CBF autoregulatory breakthrough to significantly higher pressures.9 Furthermore, a previous study by Talman and Dragon showed that selective inhibition of neuronal NOS (nNOS) attenuated autoregulatory breakthrough, suggesting that NO production may be affecting CBF autoregulation through nNOS, in addition to endothelial NOS (eNOS).10 Inhibition of eNOS and nNOS may reduce NO-dependent vasodilation in cerebral vasculature, thus providing protection against forced dilatation in acute hypertension.10
Eclampsia and PRES can occur at normal blood pressures, suggesting that autoregulation of CBF are shifted to a lower range of pressures during pregnancy.11–13 We previously measured CBF autoregulation in nonpregnant (NP) and late-pregnant (LP) rats and found that there was no difference in autoregulation or the pressure at which autoregulatory breakthrough occurred with pregnancy.9 However, our previous study measured CBF only in the anterior cerebral cortex during an acute infusion of phenylephrine to produce hypertension. Thus, the main goal of this study was to compare CBF autoregulation between anterior and posterior cerebral cortex in NP and LP rats to determine if autoregulation is less effective in the posterior cerebrum. In addition, brain water content was measured after acute hypertension to determine the influence of autoregulation and pregnancy on edema formation during increases in arterial pressure. In the current study, we used chloral hydrate as an anesthetic instead of pentobarbital that was used in our previous study since it may have relatively smaller effects on hemodynamic responses and less of an influence on CBF autoregulation.14,15 Sympathetic innervation of PCA and middle cerebral arteries (MCA) from NP and LP rats was measured to determine if there is a relationship between autoregulation of CBF and sympathetic nerve density of the posterior vs. anterior cerebrum during pregnancy as these are the major vessels that supply the posterior and anterior cerebral cortices, respectively.16 Finally, messenger RNA (mRNA) expression of eNOS and nNOS were compared between anterior and posterior brain cortices from NP and LP animals to investigate the role of NO in changes of CBF autoregulation in pregnancy.
METHODS
Animal model of pregnancy
Female virgin NP and timed pregnant Sprague Dawley rats were used for all experiments. Pregnant animals were bought from Harlan (Dublin, VA) on day 15–16 of pregnancy and used on day 19–20. Changes in CBF autoregulation were measured in LP rats as this time during gestation is when eclampsia occurs most often.9 All procedures were approved by the institutional animal care and use committee at the University of Vermont, an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited institution. All protocols were in compliance with the National Institutes of Health guide for the care and use of animals.
Measurement of CBF autoregulation
Animals were anesthetized initially with isoflurane (3% in oxygen) and maintained at 1.5–2% for placement of arterial and venous catheters, laser Doppler probes and tracheostomy. Chloral hydrate was administered intravenously as bolus doses (a total of 200 mg/kg) while isoflurane was decreased stepwise until stopped. Two laser Doppler probes were placed above the MCA and PCA cerebral cortices after a burr hole was drilled. The laser Doppler probe for the MCA territory was placed 1 mm posterior from the coronal suture and 2 mm lateral from the sagittal suture, while the probe for PCA territory was placed 1 mm anterior from the lambdoid suture and 2 mm lateral from the sagittal suture. Animals were ventilated to maintain pH and blood gases within normal physiological ranges (see Table 1). CBF autoregulation was measured in NP (n=8) and LP (n=6) animals by continuously monitoring CBF in both the anterior and posterior cerebral cortex during an acute infusion of phenylephrine (Sigma, St. Louis, MO) at an increasing rate of 4–48 μg/min in lactated Ringer’s solution, to raise arterial blood pressure, as previously described.9 In addition to assessing CBF vs. pressure curves, the upper limit of CBF autoregulation was determined as the pressure at which CBF increased by 20% from baseline, as has been previously done.17, 18
Table 1.
Physiological parameters of nonpregnant (NP) and late-pregnant (LP) rats that underwent acute hypertension for measurement of CBF autoregulation.
| Physiological Parameters | NP (n=8) | LP (n=6) |
|---|---|---|
| Weight (g) | 351 ± 10 | 437 ± 7 |
| Arterial pH | 7.44 ± 0.01 | 7.41 ± 0.01 |
| Arterial pCO2 (mmHg) | 38.1 ± 1.3 | 40.7 ± 1.3 |
| Arterial pO2 (mmHg) | 121 ± 3 | 113 ± 8 |
Brain water content
After measurement of CBF, or sham operation for control (CTL, n=4), the animals were decapitated under anesthesia and the brain removed for measurement of water content, as previously described.9 Briefly, the cerebellum and brain stem were removed and the remaining cerebrum was sectioned in anterior and posterior regions that corresponded to the region for which CBF measurements were taken. The brain sections were weighed wet, dried overnight at 90°C and then weighed again dry. Percent water content was calculated from the wet:dry weights from the following equation: ([wet weight − dry weight]/wet weight) × 100%.
Perivascular sympathetic nerve density of MCA and PCA
Separate sets of NP (n=5–8) and LP (n=5–7) animals were used to determine sympathetic nerve density of the MCA and PCA, as previously described.19 Briefly, segments of MCA and PCA were carefully dissected and immunohistochemically stained for tyrosine hydroxylase (TH) or the pan neuronal marker protein gene product 9.5 (PGP 9.5) to determine sympathetic nerve density and total nerve density, respectively. Micrographs of three areas of each vessel were taken using an Olympus fluorescent microscope at 10X. Nerve density was determined from each image using morphometric analysis that consisted of a grid overlay and counting intersect points per vessel area. Averages of the three photomicrographs per vessel were used for comparison.
mRNA expression of eNOS and nNOS in anterior and posterior brain cortices
In a separate set of animals, anterior and posterior brain cortices from NP (n=3) and LP (n=3) rats were collected to determine expression levels of eNOS and nNOS using real-time quantitative polymerase chain reaction (qPCR) methods, as previously described.20 All collected samples were stored in RNase inhibitor (1 unit/μl, RiboLock, Fermentas, Glen Burnie, MD, USA) at −80°C. Standard techniques for qPCR were performed by the Vermont Cancer Center DNA Analysis Facility at the University of Vermont, as described previously.20 Samples were DNase treated. Primers of eNOS, nNOS and MAPK6 (housekeeping control) were purchased from Applied Biosystems (Foster City, CA). All Assay on Demand primers were validated by the manufacturer for efficiency and did not detect homologs. All samples were run in duplicates. Data were analyzed using the −2ΔΔCT method.21
Data analysis and statistics
CBF autoregulatory curves and brain water content were analyzed by one-way analysis of variance (ANOVA) to determine differences between NP vs. LP and anterior vs. posterior. Two-way ANOVA was used to determine the influence of pregnancy and brain region and their interaction on the upper limit of CBF autoregulation, perivascular nerve density and brain water content. T-test was used to compare mRNA expression levels between groups. Differences were considered significant when p<0.05.
RESULTS
Effect of brain region on CBF autoregulation in NP and LP rats
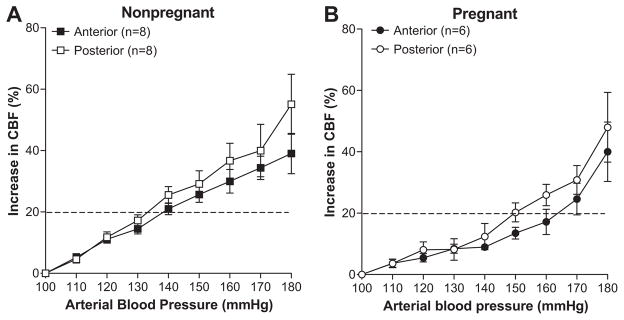
Figure 1 shows the change in CBF vs. arterial blood pressure in the anterior and posterior cerebral cortices in NP (Figure 1A) and LP (Figure 1B) rats. In NP rats, CBF rose relatively linearly in both brain regions, and there was no difference in autoregulation between anterior and posterior cortex. The pressure at which CBF increased 20 % from baseline in NP rats was 133±4 mmHg in posterior cortex and 141±4 mmHg in anterior cortex (not significant). In LP rats, there was a difference in autoregulation between the two brain regions. The pressure at which CBF increased 20% from baseline was 149±2 mmHg in the posterior cortex, but was increased to 165±3 mmHg in the anterior cortex (p<0.05), demonstrating less effective autoregulation of CBF in the posterior brain region during pregnancy with elevated arterial pressure.
Figure 1. Graph showing autoregulation of cerebral blood flow (CBF) of nonpregnant (A) and late-pregnant (B) animals in anterior vs. posterior cerebral cortex.
The dashed line represents the threshold at which CBF increases 20% from baseline and was considered the threshold for CBF autoregulation. In nonpregnant animals, there was no difference in autoregulation of CBF in the different brain regions. However, in late-pregnant animals the CBF autoregulation curve was shifted to the right in the anterior vs. posterior cerebral cortex.
Effect of pregnancy on CBF autoregulation in the anterior vs. posterior cerebral cortex
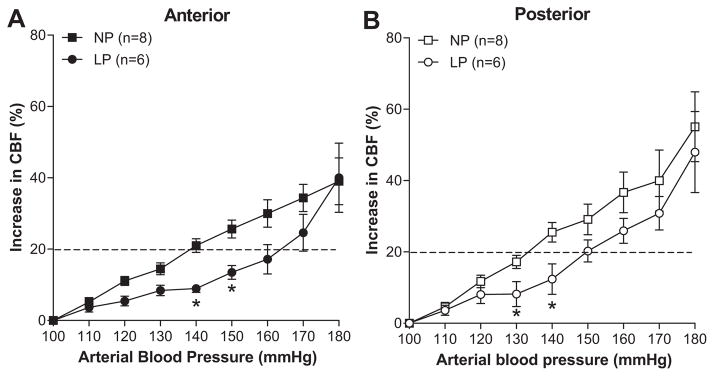
Because pre/eclampsia are pregnancy specific disorders, it is possible that pregnancy affects autoregulation differently in the posterior vs. anterior cerebrum. Thus, we also compared the effect of pregnancy on autoregulation of CBF in each brain region. Figure 2 shows the change in CBF vs. arterial blood pressure in the anterior (Figure 2A) and posterior (Figure 2B) cerebral cortex for NP and LP rats. In both brain regions, autoregulation of CBF was shifted to higher pressures during pregnancy. Thus, although there was less effective autoregulation of CBF in the posterior cerebral cortex with acute hypertension during pregnancy, when compared to the NP state, pregnancy had more effective autoregulation in both brain regions.
Figure 2. Effect of pregnancy on autoregulation of cerebral blood flow (CBF) in anterior (A) and posterior (B) cerebral cortex.
In both brain regions, pregnancy shifted the CBF autoregulation curve to the right, suggesting improved autoregulation of CBF during increases in arterial pressure. * p<0.05 vs. NP by one-way ANOVA.
Effect of pregnancy and brain region on water content after acute hypertension
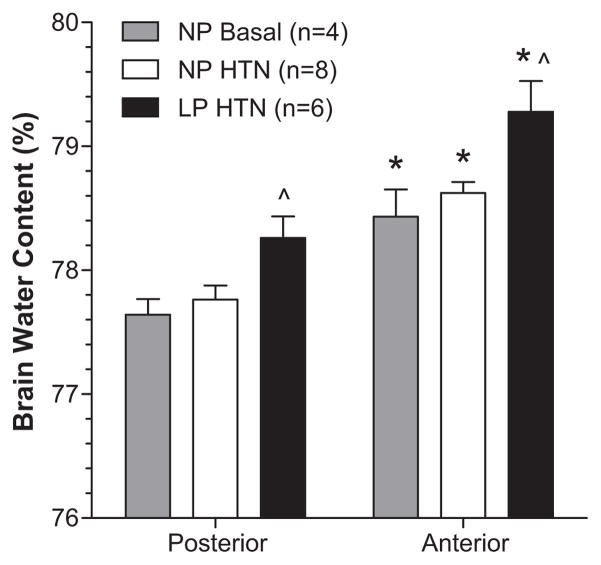
Brain water content was compared as a measure of edema formation in the anterior vs. posterior cerebrum in the same NP and LP animals for which autoregulation of CBF was determined. Figure 3 shows that the anterior cerebrum had significantly greater water content vs. posterior cerebrum in both NP and LP rats. However, LP animals had significantly greater edema in both brain regions vs. NP despite having more effective autoregulation of CBF during acute hypertension. To determine if the increase in water content in the anterior cerebrum was due to an effect of acute hypertension or to an overall increase in that region at baseline, water content was compared in sham-operated NP animals (NP Basal). We found that water content was significantly increased in the anterior cortex vs. posterior at baseline without hypertension, as shown in Figure 3.
Figure 3. Brain water content of anterior and posterior cerebral cortices from nonpregnant control (NP Basal), NP hypertensive (NP HTN) and late-pregnant (LP HTN) hypertensive rats.
Brain water content was significantly greater in the anterior vs. posterior cerebral cortex in all groups studied, regardless of hypertension. However, pregnancy caused an increase in edema in both brain regions compared to the NP state. *p<0.05 vs. posterior cerebral cortex; ^p<0.05 vs. NP HTN and NP Basal.
Sympathetic innervation of MCA vs. PCA from NP and LP rats
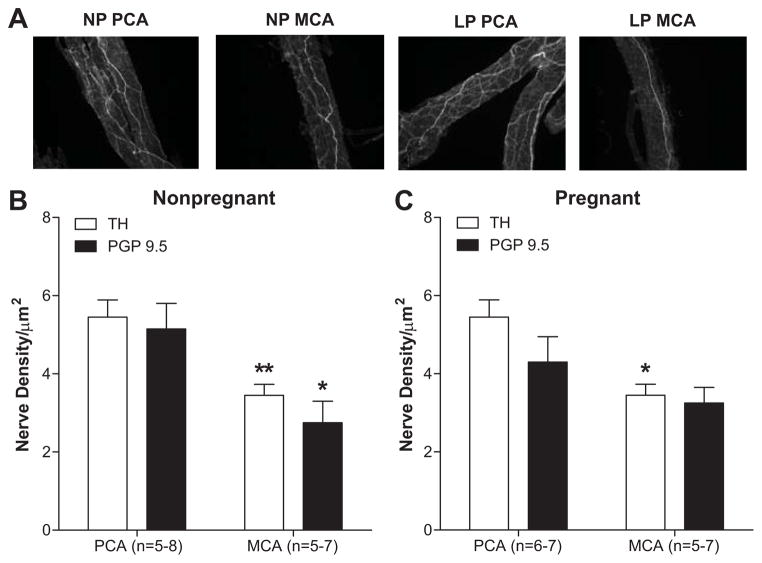
To assess the relationship between sympathetic innervation and autoregulation of CBF in the anterior vs. posterior cerebral cortex, we measured nerve density of TH- and PGP 9.5-stained PCA and MCA from NP and LP rats. Figure 4 shows that PCA had significantly increased sympathetic innervation vs. MCA in both NP (Figure 4B) and LP (Figure 4C) animals. In addition, there was no effect of pregnancy on sympathetic innervation of PCA or MCA. Comparison of TH and PGP 9.5 innervation showed no difference between the two, suggesting that sympathetic nerve fibers comprise a major proportion of perivascular innervation of cerebral arteries.
Figure 4. Perivascular sympathetic innervation of posterior (PCA) and middle (MCA) cerebral arteries from nonpregnant and late-pregnant rats.
Nerve density of tyrosine hydroxylase-stained arteries (A) was significantly greater in PCA vs. MCA from both nonpregnant and late-pregnant rats. (B) and (C) show nerve density of tyrosine hydroxylase-stained arteries compared to the pan neuronal stain protein gene product 9.5 (PGP 9.5) from nonpregnant and late-pregnant animals, respectively. There was no difference in innervation with pregnancy. *p<0.05 and **p<0.01 vs. PCA.
Effect of pregnancy on expression of eNOS and nNOS in brain cortices
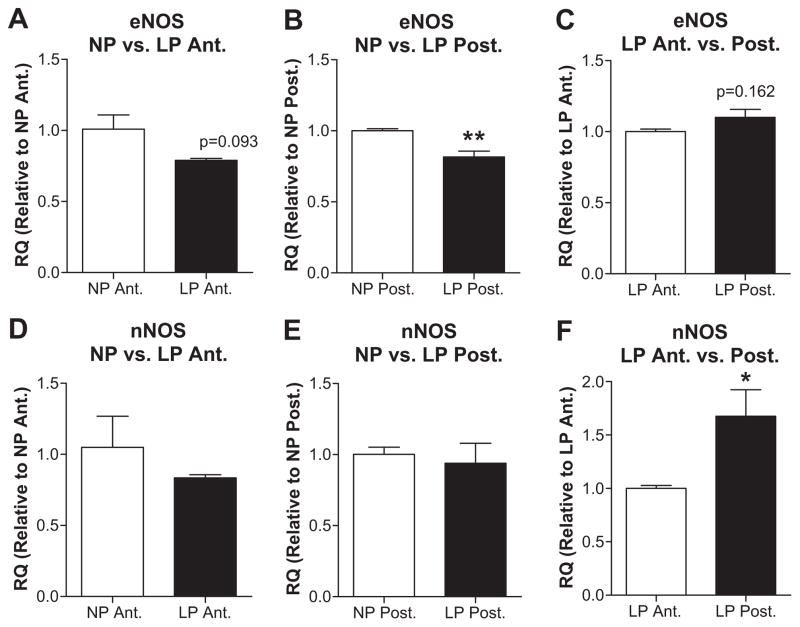
To investigate the potential role of NOS as an underlying mechanism by which pregnancy affected CBF autoregulation, mRNA expression levels of eNOS and nNOS were compared in NP and LP animals in anterior and posterior cerebral cortices (Figure 5A, B, D, E). Expression of eNOS was decreased in anterior (p=0.093) and posterior (p<0.01) cortices from LP vs. NP animals. However, nNOS was unaffected by pregnancy in either brain region. Because the posterior cerebral cortex was significantly less effective than the anterior at autoregulation of CBF during pregnancy, eNOS and nNOS expression was compared between brain regions in LP animals (Figure 5C and F). Expression of nNOS was significantly decreased in the anterior cortex vs. posterior whereas eNOS expression was decreased but not significantly.
Figure 5. Messenger RNA expression of eNOS and nNOS in anterior and posterior brain cortices from nonpregnant (NP) and late-pregnant (LP) rats.
eNOS (A, B, C) and nNOS (D, E, F) expression in NP vs. LP animals in anterior (A, D) and posterior (B, E) cortex. eNOS expression was decreased in both the anterior and posterior cerebral cortices with pregnancy. Regional differences in expressions in LP rats are presented in (C) and (F) for eNOS and nNOS, respectively. nNOS expression was increased in posterior brain cortex from LP rats compared to that of anterior. *p<0.05; **p<0.01 vs. corresponding controls.
DISCUSSION
In the present study, we compared autoregulation of CBF in the anterior and posterior cerebral cortex from NP and LP rats and the relationship between effectiveness of autoregulation and edema formation during acute hypertension. We also investigated how pregnancy affected sympathetic innervation of cerebral arteries and eNOS and nNOS expression as these factors may underlie changes in CBF autoregulation. We found that in NP animals, there was no difference in CBF autoregulation between brain regions, however, in LP animals, autoregulation was shifted to lower pressures in the posterior vs. anterior cortex. Interestingly, pregnancy shifted the autoregulatory curves in both brain regions to higher pressures compared to the NP state. The increased effectiveness of CBF autoregulation during pregnancy was not associated with an increase in perivascular sympathetic innervation, but was related to decreased eNOS expression in both brain regions compared to NP. In addition, there was a regional difference in nNOS expression in LP animals such that the posterior cerebral cortex, that had less effective CBF autoregulation, had increased nNOS expression. Lastly, despite more effective autoregulation of CBF during pregnancy, LP animals had greater edema formation during acute hypertension in both brain regions compared to NP, a result that is similar to what we have previously shown.9
In contrast to our previous study that measured CBF autoregulation in the anterior cerebral cortex only and found no difference with pregnancy,9 the current study found that both anterior and posterior cerebral cortices had CBF autoregulatory curves that were shifted to higher pressures compared to NP (Figure 2). The major difference in the present study was the use of chloral hydrate as an anesthetic vs. pentobarbital. Although all anesthetics affect CBF autoregulation, chloral hydrate has been shown to have less effect on the cardiovascular system and thus is used often for in vivo studies.14,15 Indeed, compared to pentobarbital, chloral hydrate anesthesia limited the percent change in CBF during acute hypertension to only ~50% compared to ~250% in the previous study at similar arterial pressures. However, chloral hydrate has been shown to uncouple CBF from metabolism to a greater extent than pentobarbital.23 Thus, the difference in the shape of the CBF autoregulation curves from our previous study that used pentobarbital and the current study that used chloral hydrate, could reflect differences in CVR and/or metabolism induced by the different anesthetics.
Interestingly, the use of chloral hydrate unmasked a difference in CBF autoregulation with pregnancy. This rightward shift in the CBF autoregulatory curve during pregnancy was not due to increased perivascular sympathetic innervation, but was related to decreased eNOS expression. Inhibition of NOS has been shown to shift the CBF autoregulatory curve to higher pressures9 and although we did not measure eNOS activity or NO production itself, it is possible that decreased eNOS in the brain during pregnancy was at least partially responsible for the shift in CBF autoregulation to higher pressures during pregnancy.
The shift in the CBF autoregulatory curve to higher pressures during pregnancy was greater in the anterior compared posterior cerebral cortex. Again, this regional difference in CBF autoregulation in the pregnant brain was related to increased nNOS in the posterior vs. anterior cerebral cortex (Figure 5C and F). Selective inhibition of nNOS was also shown to shift the CBF autoregulatory curve to higher pressures.10 Thus, it is possible that decreased eNOS expression increases CVR overall during pregnancy is responsible for the shift in the CBF autoregulatory curve whereas decreased nNOS in the anterior cortex during pregnancy results in more effective autoregulation of CBF in that brain region. Although studies have shown increased eNOS expression in pregnancy in other vascular beds22, it has not been measured previously in the brain. Similar to the present study, a previous study found that nNOS expression and activity was decreased in the periventricular nuclei of the brain during late-pregnancy.24
Despite more effective autoregulation of CBF during pregnancy, only pregnant animals developed edema formation during acute hypertension. This result is similar to our previous study.9 Unlike CBF autoregulation, no regional difference in edema was observed. Although all animals had more water content in the anterior cortex, the magnitude of the increase in water with acute hypertension in pregnant animals was similar in the anterior and posterior cerebrum. It is likely that changes in BBB permeability and vascular volume are responsible for edema formation during pregnancy. For example, we previously showed that LP animals had significantly greater BBB permeability in response to acute hypertension compared to NP.25 The increased BBB permeability in vivo during pregnancy was shown to be due to outward remodeling of brain arterioles that increased vascular volume and decreased CVR during acute hypertension. Pregnancy also increased BBB permeability of isolated arterioles in response to an acute elevation in intravascular pressure, but only at pressures >180 mmHg.25 Another study showed that pregnancy did not increase hydraulic conductivity of the BBB26, further suggesting that hemodynamic changes during pregnancy, including decreased CVR in response to acute hypertension that increases BBB permeability, are responsible for the increase in edema formation.
There are several limitations of our study that are important to note. First, we did not find that the posterior brain region was more susceptible to edema formation as has been shown in humans. 1,2,4 This may be due to limitations of the model of acute hypertension used that includes the use of anesthesia. In addition, this model of acute hypertension does not allow for sustained blood pressure elevation beyond ~30 minutes due to systemic effects that precipitously drops arterial pressure. This short duration of hypertension may limit edema formation especially in the NP animals. Second, previous studies in humans found less perivascular sympathetic innervation of PCA vs. MCA6, a result we did not find. In fact, we found that the PCA was more innervated with TH-stained nerves than the MCA. The discrepancy may be due to species differences (rat vs. human) or staining methods. Both TH and dopamine β-hydroxylase are markers for sympathetic innervation.27 Depending on the activation state of the sympathetic nerves, the concentration of these two enzymes varies which may be causing the difference between the two studies. Lastly, vasoconstriction elicited by release of norepinephrine from sympathetic nerves is thought to be protective against breakthrough of CBF autoregulation in acute hypertension.28 However, we did not find any association of perivascular sympathetic innervation and CBF autoregulation.
It is worth noting that we used a model of normal pregnancy to measure CBF autoregulation and edema formation in response to acute hypertension. An understanding of CBF autoregulation changes during normal pregnancy is important because women who develop eclampsia exhibit a wide spectrum of signs and symptoms ranging from severe hypertension and proteinuria to mild or absent hypertension with no proteinuria.11,12 However, changes in CBF autoregulation may be different during preeclampsia in which there is endothelial dysfunction. One study that used transcranial Doppler to measure changes in CBF velocity in response to increases in blood pressure induced by a postural change found that preeclamptic women had a more pronounced decrease in mean flow velocity, suggesting a stronger autoregulatory response.29 However, intact autoregulation may not be the only factor important for neurological complications associated with pre/eclampsia. Circulating cytokines and growth factors that increase BBB permeability during preeclampsia have been shown to cause neuronal hyperexcitability and seizure activity.30,31
Perspectives
Despite improved autoregulation of CBF during pregnancy, brain edema was pronounced in pregnant animals after acute hypertension. This is likely due to enhanced BBB permeability and increased vascular volume that occurs during pregnancy. 25 Thus, a focus on changes in BBB and edema formation during normal pregnancy and pre/eclampsia may be more important than hemodynamics for the development of neurologic complications during these conditions.
Novelty and Significance.
1) What is new?
During pregnancy, the posterior cerebrum was less effective at autoregulation of CBF during acute hypertension compared to the anterior cerebrum that was related to increased nNOS expression.
Compared to the nonpregnant state, pregnancy improved autoregulation in both the anterior and posterior cerebrum that was not related to perivascular sympathetic innervation but was related to decreased eNOS expression.
2) What’s relevant?
Despite improved autoregulation of CBF during pregnancy, acute hypertension caused a significant increase in brain edema compared to NP animals in both the anterior and posterior cerebrum, suggesting that factors such as increased BBB permeability during pregnancy are responsible for edema formation and not changes in hemodynamics.
Acknowledgments
We thank Mr. Timothy Hunter and Ms. Mary Lou Shane and the Vermont Cancer Center DNA Analysis Facility at the University of Vermont for their technical expertise in performing the PCR measurements.
Funding Sources
This study was supported by National Institute of Neurologic Disorders and Stroke (NINDS) grants NS045940, ARRA supplement NS045940-05S1 and the Neural Environment Cluster Supplement NS045940-06S1.
Footnotes
Conflict of Interest. None.
References
- 1.Easton JD. Severe preeclampsia/eclampsia: Hypertensive encephalopathy of pregnancy? Cerebrovasc Dis. 1998;8:53–58. doi: 10.1159/000015818. [DOI] [PubMed] [Google Scholar]
- 2.Servillo G, Striano P, Striano S, Tortora F, Boccella P, De Robertis E, Rossano F, Briganti F, Tufano R. Posterior reversible encephalopathy syndrome (PRES) in critically ill obstetric patients. Intensive Care Med. 2003;29:2323–2326. doi: 10.1007/s00134-003-1901-1. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RB, Feske SK, Polak JF, DeGirolami U, Iaia A, Beckner KM, Bravo SM, Klufas RA, Chai RY, Repke JT. Preeclampsia-eclampsia: Clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology. 2000;217:371–376. doi: 10.1148/radiology.217.2.r00nv44371. [DOI] [PubMed] [Google Scholar]
- 4.Engelter ST, Provenzale JM, Petrella JR. Assessment of vasogenic edema in eclampsia using diffusion imaging. Neuroradiology. 2000;42:818–820. doi: 10.1007/s002340000439. [DOI] [PubMed] [Google Scholar]
- 5.Mirza A. Posterior reversible encephalopathy syndrome: A variant of hypertensive encephalopathy. J Clin Neurosci. 2006;13:590–595. doi: 10.1016/j.jocn.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 6.Lincoln J. Innervation of cerebral arteries by nerves containing 5-hydroxytryptamine and noradrenaline. Pharmacol Ther. 1995;68:473–501. doi: 10.1016/0163-7258(95)02017-9. [DOI] [PubMed] [Google Scholar]
- 7.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 8.Sadoshima S, Fujii K, Yao H, Kusuda K, Ibayashi S, Fujishima M. Regional cerebral blood flow autoregulation in normotensive and spontaneously hypertensive rats--effects of sympathetic denervation. Stroke. 1986;17:981–984. doi: 10.1161/01.str.17.5.981. [DOI] [PubMed] [Google Scholar]
- 9.Euser AG, Cipolla MJ. Cerebral blood flow autoregulation and edema formation during pregnancy in anesthetized rats. Hypertension. 2007;49:334–340. doi: 10.1161/01.HYP.0000255791.54655.29. [DOI] [PubMed] [Google Scholar]
- 10.Talman WT, Nitschke Dragon D. Neuronal nitric oxide mediates cerebral vasodilatation during acute hypertension. Brain Res. 2007;1139:126–132. doi: 10.1016/j.brainres.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douglas KA, Redman CW. Eclampsia in the United Kingdom. BMJ. 1994;309:1395–1400. doi: 10.1136/bmj.309.6966.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sibai BM. Eclampsia. Vi. Maternal-perinatal outcome in 254 consecutive cases. Am J Obstet Gynecol. 1990;163:1049–1054. doi: 10.1016/0002-9378(90)91123-t. [DOI] [PubMed] [Google Scholar]
- 13.Katz VL, Farmer R, Kuller JA. Preeclampsia into eclampsia: Toward a new paradigm. Am J Obstet Gynecol. 2000;182:1389–1396. doi: 10.1067/mob.2000.106178. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Schuler B, Vogel O, Arras M, Vogel J. What is the optimal anesthetic protocol for measurements of cerebral autoregulation in spontaneously breathing mice? Exp Brain Res. 2010;207:249–258. doi: 10.1007/s00221-010-2447-4. [DOI] [PubMed] [Google Scholar]
- 15.Nakao Y, Itoh Y, Kuang TY, Cook M, Jehle J, Sokoloff L. Effects of anesthesia on functional activation of cerebral blood flow and metabolism. Proc Natl Acad Sci U S A. 2001;98:7593–7598. doi: 10.1073/pnas.121179898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berman SA, Hayman LA, Hinck VC. Correlation of ct cerebral vascular territories with function: 3. Middle cerebral artery. AJR Am J Roentgenol. 1984;142:1035–1040. doi: 10.2214/ajr.142.5.1035. [DOI] [PubMed] [Google Scholar]
- 17.Banaji M, Tachtsidis I, Delpy D, Baigent S. A physiological model of cerebral blood flow control. Math Biosci. 2005;194:125–173. doi: 10.1016/j.mbs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Takada J, Ibayashi S, Ooboshi H, Ago T, Ishikawa E, Kamouchi M, Kitazono T, Iida M. Valsartan improves the lower limit of cerebral autoregulation in rats. Hypertens Res. 2006;29:621–626. doi: 10.1291/hypres.29.621. [DOI] [PubMed] [Google Scholar]
- 19.Aukes AM, Bishop N, Godfrey J, Cipolla MJ. The influence of pregnancy and gender on perivascular innervation of rat posterior cerebral arteries. Reprod Sci. 2008;15:411–419. doi: 10.1177/1933719107314067. [DOI] [PubMed] [Google Scholar]
- 20.Chan SL, Cipolla MJ. Relaxin causes selective outward remodeling of brain parenchymal arterioles via activation of peroxisome proliferator-activated receptor-gamma. FASEB J. 2011;25:3229–3239. doi: 10.1096/fj.10-175471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Uematsu M, Takasawa M, Hosoi R, Inoue O. Uncoupling of flow and metabolism by chloral hydrate: a rat in-vivo autoradiographic study. Neuroreport. 2009;20:219–222. doi: 10.1097/wnr.0b013e328302ee46. [DOI] [PubMed] [Google Scholar]
- 23.Boeldt DS, Yi FX, Bird IM. eNOS activation and no function: Pregnancy adaptive programming of capacitative entry responses alters nitric oxide (NO) output in vascular endothelium--new insights into eNOS regulation through adaptive cell signaling. J Endocrinol. 2011;210:243–258. doi: 10.1530/JOE-11-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heesch CM, Zheng H, Foley CM, Mueller PJ, Hasser EM, Patel KP. Nitric oxide synthase activity and expression are decreased in the paraventricular nucleus of pregnant rats. Brain Res. 2009;1251:140–150. doi: 10.1016/j.brainres.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cipolla MJ, Sweet JG, Chan SL. Cerebral vascular adaptation to pregnancy and its role in the neurological complications of eclampsia. J Appl Physiol. 2011;110:329–339. doi: 10.1152/japplphysiol.01159.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreurs MP, Houston EM, May V, Cipolla MJ. The adaptation of the blood-brain barrier to vascular endothelial growth factor and placental growth factor during pregnancy. FASEB J. 2012;26:355–362. doi: 10.1096/fj.11-191916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otten U, Schwab M, Gagnon C, Thoenen H. Selective induction of tyrosine hydroxylase and dopamine beta-hydroxylase by nerve growth factor: Comparison between adrenal medulla and sympathetic ganglia of adult and newborn rats. Brain Res. 1977;133:291–303. doi: 10.1016/0006-8993(77)90765-x. [DOI] [PubMed] [Google Scholar]
- 28.Faraci FM, Mayhan WG, Werber AH, Heistad DD. Cerebral circulation: Effects of sympathetic nerves and protective mechanisms during hypertension. Circ Res. 1987;61:II102–106. [PubMed] [Google Scholar]
- 29.Zatik J, Major T, Aranyosi J, Molnar C, Limburg M, Fulesdi B. Assessment of cerebral hemodynamics during roll over test in healthy pregnant women and those with pre-eclampsia. BJOG. 2001;108:353–358. doi: 10.1111/j.1471-0528.2001.00095.x. [DOI] [PubMed] [Google Scholar]
- 30.Amburgey OA, Chapman AC, May V, Bernstein IM, Cipolla MJ. Plasma from preeclamptic women increases blood-brain barrier permeability: Role of vascular endothelial growth factor signaling. Hypertension. 2010;56:1003–1008. doi: 10.1161/HYPERTENSIONAHA.110.158931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cipolla MJ, Pusic AD, Grinberg YY, Chapman AC, Poynter ME, Kraig RP. Pregnant serum induces neuroinflammation and seizure activity via TNFalpha. Exp Neurol. 2012;234:398–404. doi: 10.1016/j.expneurol.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]