Abstract
Heterogeneity is a hallmark of the adaptive immune system. This is most evident in the enormous diversity of B and T cell antigen receptors. There is also heterogeneity within antiviral T cell populations, and subsets of effector and memory T cells now permeate our thinking about specialization of T cell responses to pathogens. It has been less clear, however, how heterogeneity in developing virus-specific effector and memory T cells is related to cell-fate decisions in the immune response, such as the generation long-lived memory T cells. Here we discuss recent findings that might help redefine how heterogeneity in antiviral T cell populations gives rise to T cell subsets with short- and long-lived cell fates.
Cardinal Features of Memory T Cells
Immunological Memory (IM) is a defining characteristic of the adaptive immune system that, during primary infection, produces long-lived plasma cells and memory T and B cells. These memory B and T cells are endowed with unique properties that permit more vigorous and specific responses upon reinfection to protect against pathogens. These key memory B and T cell properties are central to our current understanding of IM, yet the pathways that give rise to optimal memory B and T cells remain poorly understood. This review will focus principally on the development of memory T cells during viral infection. The accompanying review by Dörner and Radbruch covers recent work on antiviral B cell responses (Dörner and Radbruch, 2007). Here we focus on recent advances in our understanding of antiviral memory CD8+ T cell differentiation and, although the majority of the discussion focuses on viral systems, selected data from nonviral experimental models are also discussed, where relevant to antiviral immunity.
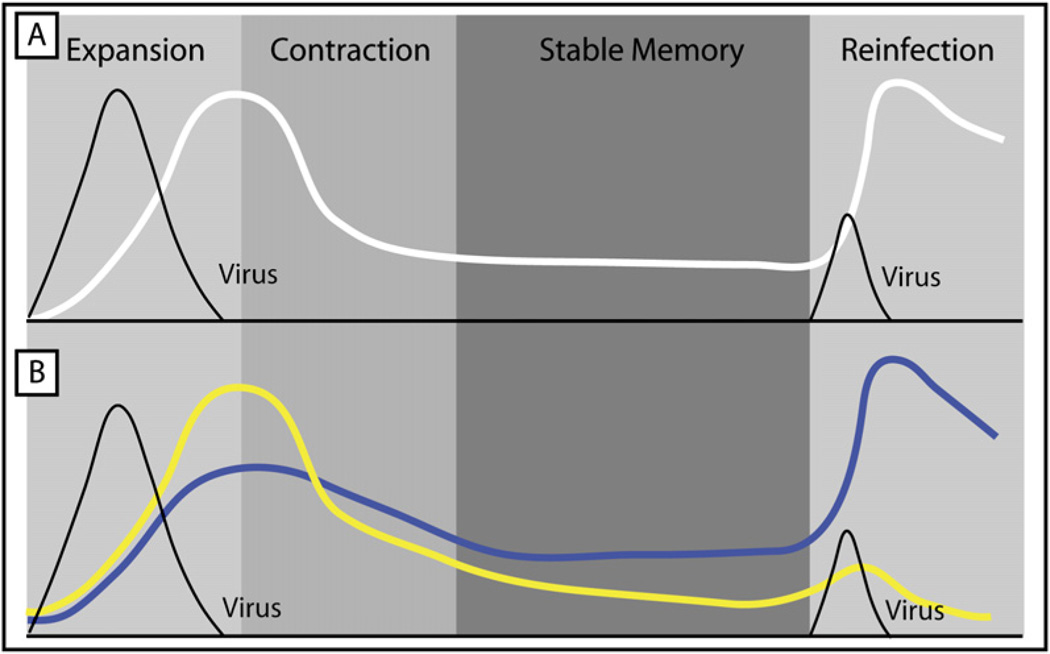
Over the last ten years, the innovation of major histocompatibility complex (MHC) class I and II tetramers, the use of T cell receptor (TCR) transgenic mice, and other techniques have led to the detailed quantitation, isolation, and characterization of virus-specific T cell populations in mice, nonhuman primates, and humans during infection. This work outlined the kinetics of virus-specific responses with great precision (Figure 1). A T cell response to a typical acute viral infection can be characterized by three distinct phases: expansion and effector T cell differentiation (profound clonal expansion and acquisition of effector functions), contraction (death of the majority of activated effectors T cells via apoptosis), and stable memory (formation of a numerically stable long-lived population of memory T cells) (Ahmed and Gray, 1996; Williams and Bevan, 2007; Zinkernagel et al., 1996). Virus-specific T cells can expand as much as 104-fold to 105-fold, in as little as 8 days, from as few as 100–200 naive precursors (Arstila et al., 1999; Blattman et al., 2002). This massive T cell proliferation is critical to long-term immunity because the magnitude of the initial clonal burst typically determines memory T cell numbers (Hou et al., 1994; Murali-Krishna et al., 1998). Moreover, extensive cellular differentiation occurs as these newly activated T cells become potent antiviral effector T cells and ultimately memory T cells. Effector T cells migrate to virtually all tissues and eliminate the pathogen by killing infected cells, producing cytokines, and recruiting other leukocytes via chemokine production. In general, effector CD8+ T cells control infection through cytotoxic activity (via perforin and granzymes) and the secretion of interferon (IFN)-γ and tumor necrosis factor (TNF)α (Kaech et al., 2002b; Wherry and Ahmed, 2004; Williams and Bevan, 2007). Effector CD4+ T cells take on a diverse set of roles and inhibit viral replication through the production of antiviral cytokines (and perhaps cytotoxicity), but they also activate dendritic cells (DCs) and provide help to B cells and CD8+ T cells (Seder and Ahmed, 2003).
Figure 1. A Homogenous and Heterogeneous View on Antiviral Memory T Cell Development.
(A) T cells clonally expand and homogeneously differentiate into effector T cells, of which most die, but some persist to become long-lived memory T cells. Upon reinfection, memory T cells re-expand and control infection faster than 1° infection.
(B) Two populations of effector T cells form with different memory T cell potential: short-lived effector T cells (SLECs; yellow line) that do not gain memory T cell potential, and memory precursor effector cells (MPECs; blue line) that do. Upon reinfection, it is primarily the descendents of MPECs that participate in secondary responses because of their enhanced proliferative capacity.
After the expansion and contraction phases, a fraction (i.e., typically 5%–20%) of virus-specific T cells survives, forming a pool of memory T cells. Unlike most somatic cells in which terminal differentiation results in a functional, but a nonmitotic cell, the major product of memory T cell differentiation is a population of T cells that retains stem cell-like qualities. That is, after acute viral infections, long-lived memory T cells are endowed with multipotency, a high proliferative potential, telomerase expression, and self renewal (Williams and Bevan, 2007). These memory T cells persist in an antigen-independent, but cytokine-dependent (namely interleukin-15 [IL-15] and IL-7), manner, and slowly divide (referred to here as homeostatic turnover) (Surh et al., 2006). But almost immediately upon reinfection, memory T cells begin to produce effector molecules, undergo dramatic clonal expansion, and differentiate into secondary effector T cells. This qualitatively and quantitatively enhanced memory T cell response results in faster control of infection compared to a primary response (Figure 1). Thus, the cardinal features of memory T cells are maintenance of (1) high proliferative potential, (2) a multipotent state, meaning that memory T cells can maintain memory T cell identity but also rapidly reactivate antiviral effector functions upon reinfection, and (3) long-term survival and self renewal in the absence of antigen via IL-7- and IL-15-driven homeostatic turnover.
This fairly customary description of effector and memory T cell differentiation during viral infection, however, does not incorporate the complexity of the multiple subpopulations of T cells that are now known to exist. These effector and memory T cell subsets differ not only in their effector functions, migratory properties, and proliferative potential, but also in their long-term persistence and ability to form protective memory T cells. How these distinct effector and memory T cell subsets form, function, and persist is a matter of great interest and will be the main topic of this review.
Effector and Memory T Cell Development and Fate Decisions
Models of Memory T Cell Development during Viral Infection
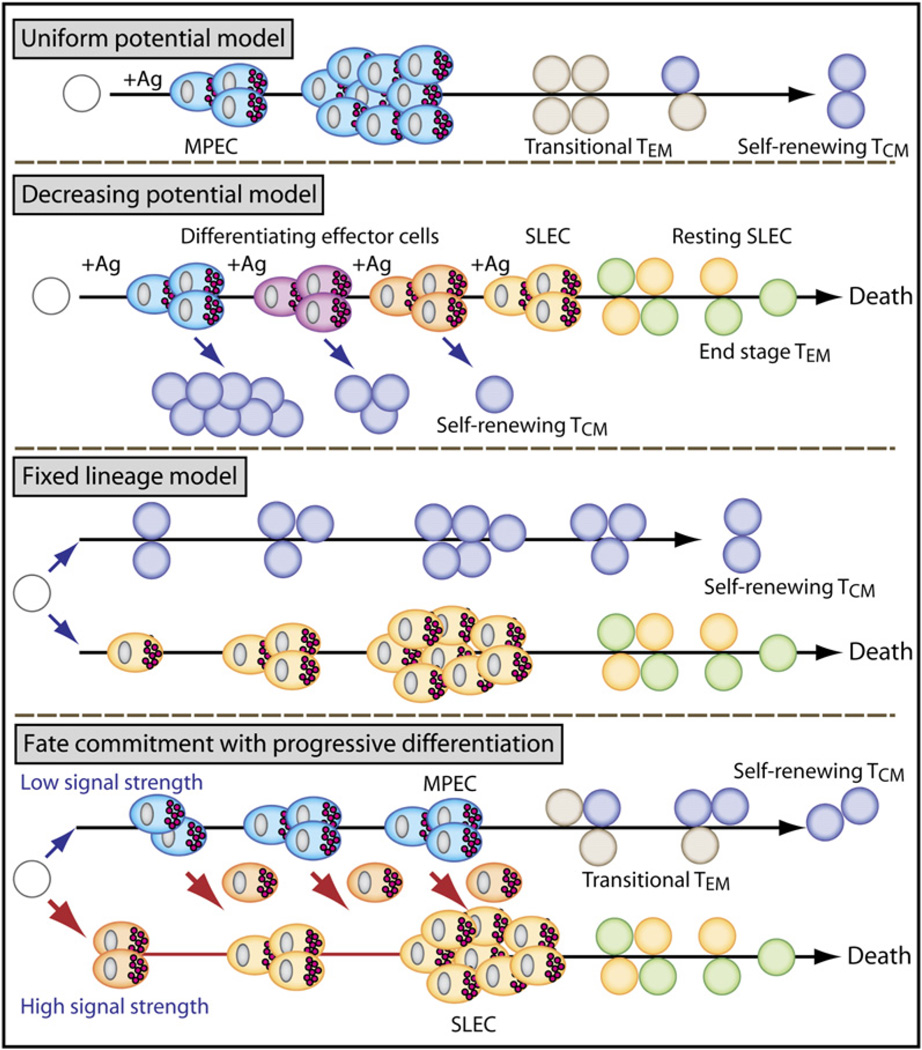
Despite tremendous advances in our characterization of memory T cells in the last decade, we still do not know when and how memory T cells actually form after infection. The answers might largely depend on how one defines a memory T cell. One of the oldest but still most widely used definitions is based simply on time after infection; once antigen-specific T cell numbers stabilized (several weeks to months after infection), these cells were typically deemed memory T cells. However, this definition does not take into account the more concrete functional aspects and subsets of memory T cells described above. When considering the development of T cell memory on the basis of measurable memory T cell properties (i.e., high proliferative potential, multipotency, rapid recall, and homeostatic turnover), at least four possible models can be envisioned (Figure 2).
Figure 2. Models of Effector and Memory Cell-Fate Decisions during Acute Viral Infection.
Model 1—uniform potential. All activated effector cells develop equal potential to become MPECs (aqua cells), but extrinsic factors limit the number of memory T cells generated. MPECs give rise to transitional TEM cells (gray) that convert into self-renewing TCM cells (dark blue).
Model 2—decreasing potential. Shorter durations of antigenic stimulation favor MPECs that give rise to TCM cells. Longer stimulation promotes terminal differentiation of SLECs (yellow) and end-stage TEM cells (green) that decline over time.
Model 3—fixed lineage. Upon activation, naive T cells develop into either SLECs or fully mature memory T cells. In this model, cells might bypass an effector stage and develop directly into self-renewing TCM.
Model 4—fate commitment with progressive differentiation. According to the strength of signal, either an MPEC or SLEC fate will be adopted early after activation. The MPECs acquire effector functions, but remain multipotent (e.g., can still become memory T cells or SLECs). Most SLECs die, but some persist with a limited lifespan as an end-stage TEM cell. MPECs survive, and give rise to transitional TEM cells that progressively mature into long-lived TCM cells.
Model 1 — Uniform Potential
The first model is rooted in an extrinsic viewpoint. Here, the effector T cell pool is relatively homogenous, with each cell having acquired effector functions and memory T cell developmental potential equivalently. Competition for, or withdrawal from, nutrients, cytokines, growth factors, antigen, or other environmental resources limits the number of T cells that can survive contraction and enter the memory T cell pool (Freitas and Rocha, 2000). However, this simple model of memory T cell formation is insufficient to explain the heterogeneity within effector and memory T cell populations and also does not help define when memory T cell properties are acquired.
Model 2—Decreasing Potential
In the second model, the early effector T cells also start off with relatively equal memory T cell developmental potential. However, the effector T cells progressively lose memory cell potential and are pushed toward terminal differentiation in a linear fashion as TCR stimulation is increased or prolonged (Ahmed and Gray, 1996). This second model provides a mechanism for creating a heterogeneous pool of effector T cells in various stages of differentiation according to their stimulation history during infection. This model might be particularly useful in explaining the properties of latecomers in the T cell response (Catron et al., 2006; D’Souza and Hedrick, 2006; Jelley-Gibbs et al., 2007) and T cell fates during chronic or latent infections or repetitive stimulation (Wherry and Ahmed, 2004). Although this model was originally based mostly on the effects of antigenic signaling, it does not rule out that exposure to other signals might be involved.
Model 3—Fixed Lineage
A third model posits that memory or effector T cell lineage commitment occurs very early after the initial T cell stimulation, such that fully mature memory T cells and effector T cells coexist within the effector T cell population (Farber, 1998; Sallusto and Lanzavecchia, 2001). According to this model, these preformed memory T cells might bypass the effector stage, possess all characteristic traits of memory T cells, and survive the contraction phase, whereas the effector T cells die. Some evidence for this model is that at the peak of clonal expansion, a minority of T cells bears phenotypic resemblance to memory T cells found several weeks later (Lefrancois and Marzo, 2006). Also, it is possible to isolate CD4+ T cells early in the immune response that do not have full effector function, but can persist long term (Wu et al., 2002). Furthermore, this model might more accurately portray the events that occur when naive T cells are primed under noninfectious conditions, such as with DC vaccines (Badovinac et al., 2005). In another recent study, asymmetric separation of daughter cells at the first T cell division was observed, and one daughter T cell adopted a memory cell fate and the other an effector T cell fate (Chang et al., 2007), suggesting that lineages might be fixed as early as the first cell division. It remains to be determined, though, how this 50:50 split in cell fates after division one leads to only 5%–20% of the clonal T cell burst entering the memory T cell pool.
Model 4—Fate Commitment with Progressive Differentiation
The fourth model builds on the first three, but differs in several important respects. One distinction is that it postulates the existence of memory precursor effector cells (MPECs). These MPECs are not fully mature memory T cells, as in the third model, but rather they possess effector properties (hence MPEC) and require further differentiation to gain quintessential memory T cell properties, such as a high proliferative potential and the ability to undergo homeostatic turnover. Also, the effector T cell pool is heterogeneous and contains many short-lived effector cells (SLECs) that will die after infection, and a smaller fraction of MPECs with the potential to become long-lived memory T cells. The SLECs are a terminally differentiated cell population, but, unlike in the third model, the MPEC fate is not fixed. That is, the MPECs retain plasticity to develop into SLECs if additional strong stimulatory signals are encountered (e.g., persisting antigen and/or inflammation). This model is also consistent with early fate commitment in the first T cell division (Chang et al., 2007) if the memory-fated daughter T cells require further differentiation events to mature into a long-lived memory T cell.
In this model, the primary basis for the cell-fate decision is the magnitude of the overall strength of signal, which includes the combined effects of antigen, costimulation, and inflammation (signals 1, 2, and 3). High or excessively strong signals drive greater clonal expansion but also promote terminal effector T cell differentiation. The fourth model differs from the decreasing-potential hypothesis in that different cell fates can be specified early according to the intensity of the signals received (Gett et al., 2003) but do not require multiple rounds of stimulation to create heterogeneous cell fates.
Distinguishing which of the models of memory T cell development occurs during viral infections has been a difficult and controversial challenge. Indeed, these models are not mutually exclusive, and it is possible that multiple pathways exist for generating effector T cells and long-lived memory T cells depending on the T cell priming conditions. We will now discuss our current understanding of effector and memory T cell heterogeneity and the implications for T cell memory with these models as a guide.
Identification of Memory Precursors and Defining Their Progressive Differentiation
Initially, several reports suggested that the entire Naive → Effector → Memory (N → E → M) differentiation process could run on autopilot after a brief (~24 hr) stimulation with antigen (Williams and Bevan, 2007). Subsequent work has shown that CD4+ T cell help, IL-2, inflammation, and persisting antigen can greatly influence memory T cell differentiation. Moreover, functional, phenotypic and gene-expression profiling of antigen-specific T cell populations during the course of an antiviral T cell response has led to the notion that fully competent memory T cells develop gradually after the clearance of acute viral infection (as referred to in models 1 and 4) (Kaech et al., 2002a). After most acute viral infections, the memory T cell pool slowly converts from a population containing mostly effector memory T (TEM) cells (e.g., CD62LLo, CCR7Lo, IL-2Lo) to one containing central memory T (TCM) cells (e.g., CD62LHi, CCR7Hi, IL-2+) that exhibit a high proliferative potential and can homeostatically turn over (Wherry and Ahmed, 2004). A number of other important phenotypic changes also occur gradually in the memory pool, resulting in memory T cells with a more mature phenotype (IL-7RHi, CD27Hi, CD122Hi, Bcl-2Hi, KLRG1Lo, CXCR3Hi, and CD43Lo) that differs considerably from the starting effector T cell population (Figure 3 and Table 1) (Badovinac et al., 2007; Hikono et al., 2007; Kaech et al., 2003; Wherry et al., 2004; Wherry et al., 2003). Because this process is gradual, a snapshot taken at one or two time points early after infection might be insufficient to appreciate the full dynamics and timeframe of these changes. The phenotypic changes in the memory T cell population could result from selective survival of different subsets (model 3), actual cellular conversion (models 1 and 4), or both.
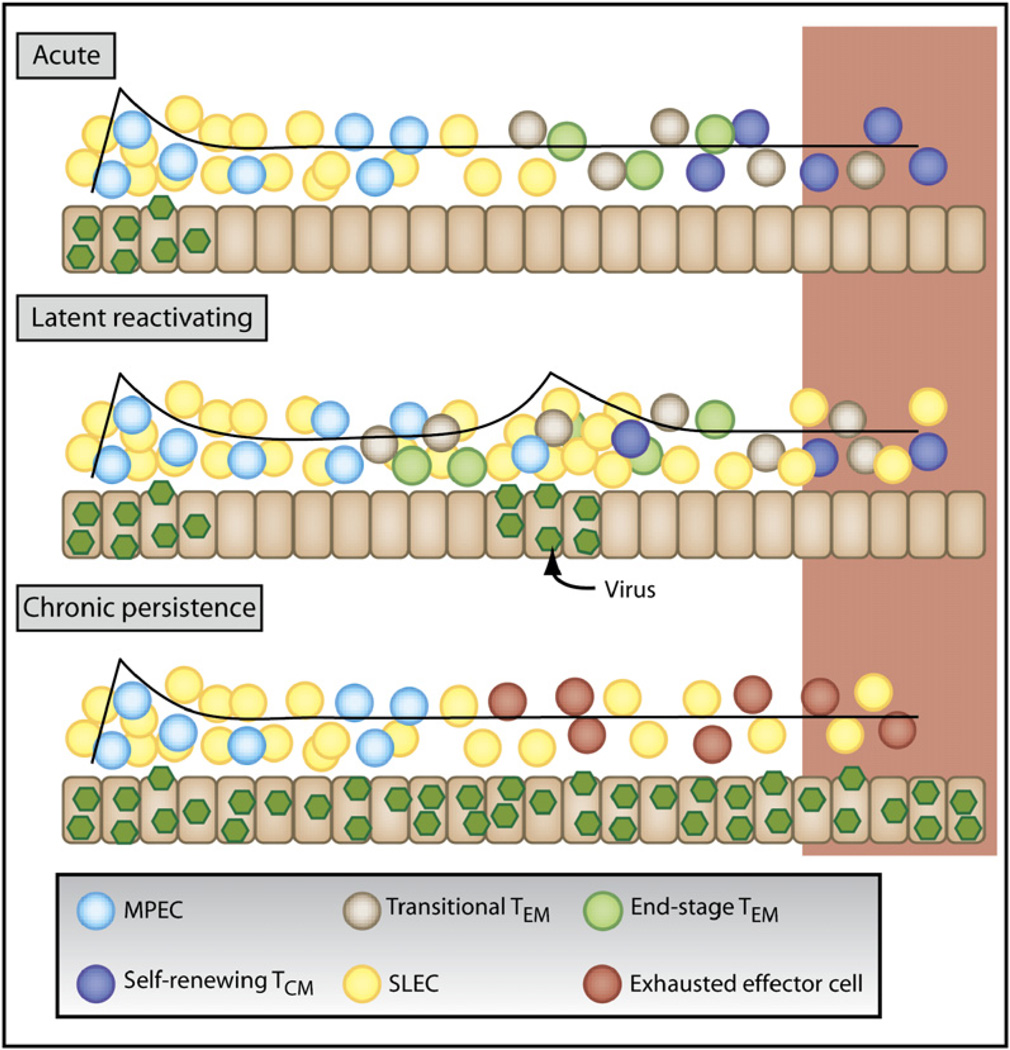
Figure 3. Differential Effects of Acute, Latent or Chronic Viral Infections on Memory T Cell Differentiation.
Viral infections fall into three categories based on the duration and pattern of viral infection—(1) acute, (2) latent with reactivation, and (3) chronic or persistent.
Primary acute infection generates SLECs (yellow) and MPECs (aqua). For a description of the cell types, see Figure 2. The majority of SLECs die, but some persist for finite intervals (TEM cells; green). In contrast, MPECs survive and progressively mature from transitional TEM cells (light blue) into protective TCM cells (dark blue).
During latent or reactivating viral infections, a mixed population with a variety of effector and memory differentiate states is formed. Typically, there will be more SLECs and TEM cells than after acute infection. The abundance of secondary MPECs, resting TCM cells, or transitional TEM cells will likely depend on the frequency and degree of viral reactivation.
During persistent or chronic viral infections, with high viremia, T cells can undergo altered differentiation and become exhausted (red). According to the type of infection, the snap-shot of effector and memory T cells present (outlined in red box) is likely to be different. See Table 1 for detailed descriptions of common attributes.
Table 1.
Characteristics of T Cells Responding to Different Types of Viral Infections
| Type of Infection Phenotype of T Cells Functional Properties | Examples of Infections | |
|---|---|---|
| Acute viral Infection (Memory Phase) | ||
| CD62LHi > CD62LLo | –High proliferative potential | LCMV (Acute Strains) |
| CD44Hi | –Potent effector functions (IFN-γ, TNF-α, IL-2, cytotoxicity) | VSV |
| CD27Int/Hi | –Potent homeostatic turnover | Vaccinia virus |
| CD11aHi | –Antigen-independent persistence | Influenza virus |
| CCR7Hi | RSV | |
| CD127Hi | Sendai Virus | |
| CXCR3Hi | ||
| KLRG1Lo | ||
| CD122Hi | ||
| CD43Lo | ||
| PD-1Lo | ||
| CD69Lo | ||
| CD57Lo | ||
| Latent, Reactivating Infection | ||
| CD62LLo > CD62LHi | –Intermediate proliferative potential | γHV |
| CD44Hi | –Weaker effector functions (lower IFN-γ, TNF-α, IL-2) | EBV |
| CD27Lo/Int | –Reduced homeostatic turnover | CMV |
| CD11aHi | HSV | |
| CCR7Lo/Hi | ||
| CD127Lo/Int | ||
| CD122Lo | ||
| KLRG1Hi/Lo | ||
| PD-1Int/Hi | ||
| CD69Lo | ||
| CD57Hi | ||
| Chronic, Persistent Infection | ||
| CD62LLo | –Low proliferative potential | LCMV (chronic strains) |
| CD27Lo/Int | –Poor effector functions (exhausted) | HCV |
| CD44Hi | –Little or no homeostatic turnover | HIV |
| CCR7Lo | –Antigen-dependent persistence | SIV |
| CD11aHi | ||
| CD122Hi/Lo | ||
| CD127Lo/int | ||
| PD-1Hi | ||
| KLRG1Hi/Lo | ||
| CD57Hi | ||
| CD69Hi | ||
The progressive changes in memory T cell function (e.g., high proliferative potential, homeostatic turnover) that accompany the E → M transition help to delineate when memory T cells form after acute infection. These data alone, however, do not distinguish whether the effector T cell population is homogenous or heterogeneous with regard to the potential to form memory T cells (model 1 versus models 2, 3, and 4). The examination of gene and surface marker expression has demonstrated that, like the memory T cell population, the effector T cell population is rich in cellular and functional heterogeneity (Wherry and Ahmed, 2004). In particular, a subset of effector CD8+ T cells can be identified that already expressed certain features of memory CD8+ T cells, such as increased IL-7R expression (Huster et al., 2004; Kaech et al., 2003). However, these IL-7RHi effector CD8+ T cells did not appear to be preformed memory T cells (model 3), but rather they were more like MPECs (model 4). The IL-7RHi T cells had full effector function and expressed cytotoxic molecules (e.g., granzyme B) and IFN-γ, but, similar to their IL-7RLo counterparts, the IL-7RHi effector T cells had a relatively low proliferative capacity compared to mature memory T cells (Huster et al., 2004; Kaech et al., 2003). The IL-7RHi effector T cells, however, had a substantially increased ability to become self-renewing memory T cells compared to the IL-7RLo effector T cells (Huster et al., 2004; Kaech et al., 2003). A number of subsequent studies in infectious models support the idea that the IL-7RHi fraction of effector CD8+ T cells contains MPECs, though in some cases (e.g., low inflammatory conditions), not all IL-7RHi effector T cells are destined to populate the memory pool (Castellino and Germain, 2007; Hand et al., 2007; Huster et al., 2004; Kaech et al., 2003; Lacombe et al., 2005). Taken together, it appears that by approximately 1 to 2 weeks after viral infection, effector CD8+ T cells have committed to short-lived or long-lived cell fates.
Pathways That Induce Effector and Memory T Cell Heterogeneity
What are the signals and genetic pathways that affect T cell longevity and specify different effector and memory T cell lineages? Although we are far from a complete understanding of these pathways, a substantial body of work has outlined some key factors, including (1) the role of CD4+ T cell help, (2) common gamma-chain cytokines, and (3) the strength of antigenic and inflammatory signals during T cell priming.
CD4+ T Cell Help and IL-2
CD4+ T cells, CD40-CD40L signals, and IL-2 help to maximize effector CD8+ T cell expansion, an effective E → M transition, and long-term maintenance after acute viral infections (Northrop and Shen, 2004; Rocha and Tanchot, 2004; Williams and Bevan, 2007). The result of defective CD4+ T cell help is the generation of a population of anti-viral CD8+ T cells that largely resembles TEM cells, responds poorly to rechallenge, and in some cases expresses the death receptor, Trail (Badovinac et al., 2006; Janssen et al., 2005). Interestingly, IL-2 acts early during infection to instill robust recall capacity in the memory CD8+ T cells that develop later (Williams et al., 2006). An early source of IL-2 during CD4+ T cell priming is also required for optimal memory CD4+ T cell differentiation (Dooms et al., 2007). One possible mechanism for early CD4+ T cell help for CD8+ T cells is by the enhancement of naive CD8+ T cell chemotaxis to helped DCs (Castellino et al., 2006). In addition, there is some evidence that the high-affinity antibodies produced by the CD4+ T:B cell collaboration may indirectly help memory CD8+ T cell development because passive immune therapy can improve memory CD8+ T cell function in CD4+-deficient animals (Bachmann et al., 2004). There might also be an interesting connection between elevated T-bet expression in unhelped CD8+ T cells and the enrichment of TEM-phenotype cells. (Intlekofer et al., 2007) Thus, CD4+ T cells appear to modulate the developing effector and memory T cell subpopulations through mechanisms that include APC activation, CD40:CD40L, IL-2, neutralizing antibody, Trail, and T-bet.
IL- 7 and IL-15
IL-7 and IL-15 are important for both antiviral memory CD8+ and CD4+ T cell generation and homeostasis (Purton et al., 2007; Surh et al., 2006). In vitro priming in the presence of IL-2 or IL-15 can lead to TEM cell-like or TCM cell-like populations of CD8+ T cells, respectively (Manjunath et al., 2001). IL-7 and IL-15, however, do not appear to play major instructive roles in the actual MPEC- versus SLEC-fate decision in vivo. Most data indicate that reducing or enhancing IL-7 or IL-15 signals during the N → E or E → M stages does not greatly alter the number IL-7RHi MPECs or memory CD8+ T cells that ultimately form (Hand et al., 2007; Klonowski et al., 2006; Sun et al., 2006; Tripathi et al., 2007) (S.M.K., unpublished data). Treatment with IL-2, IL-7, and IL-15 during the contraction phase will substantially protract effector T cell death, but it does not seem to affect the memory CD8+ T cell population quantitatively or qualitatively (Blattman et al., 2003; Hand et al., 2007; Klonowski et al., 2006; Melchionda et al., 2005; Sun et al., 2006; Tripathi et al., 2007) (S.M.K., unpublished data). Moreover, the forced expression of IL-7R on all effector CD8+ T cells does not save the terminally differentiated IL-7RLo SLECs from death (Hand et al., 2007). Altogether, these data suggest that IL-7R downregulation is symptomatic, not causal, to effector CD8+ T cell contraction after infection and that, although IL-7 and IL-15 can clearly provide survival signals, these important homeostatic cytokines are unlikely to provide differentiation signals for the generation of subsets of effector and memory T cells in vivo.
Strength of Signal: Antigen, Costimulation, and Inflammation
In general, there are three major classes of signals necessary for T cell activation and differentiation: signal 1 from antigen, signal 2 from costimulation, and signal 3 from inflammation. The strength (magnitude and/or duration) of signal 1 can influence the size of the virus-specific T cell response (Bullock et al., 2000; Wherry et al., 1998; Wherry et al., 1999). What has remained less clear is how the strength of TCR signaling during infection qualitatively influences memory T cell differentiation. Limiting the strength or duration of signals received by T cells during priming can alter both the types of effector T cell subsets produced and their rate of memory cell maturation. In general, blunting the infection, reducing the duration of stimulation, or increasing intraclonal T cell competition leads to enhanced formation of effector T cells expressing high amounts of IL-7R and CD62L and low amounts of KLRG1 (Badovinac et al., 2007; Badovinac et al., 2005; Joshi et al., 2007; Marzo et al., 2005; Williams and Bevan, 2004) (E.J.W., unpublished data). In some experiments, this effect was accompanied by more rapid, but not necessarily more abundant, memory T cell development (Badovinac and Harty, 2007; Badovinac et al., 2005). Together, these observations demonstrated that the strength or duration of infection is a critical factor regulating the quality and rate of memory CD8+ T cell differentiation, but it was unclear how the strength of antigenic, costimulatory, or inflammatory signals was individually involved.
Costimulatory signals (signal 2) from receptors such as CD28, CD40, 41BB, CD27, ICOS, and OX40 clearly impact the size of the antiviral effector and memory T cell populations, with some notable differences in their effects on CD4+ versus CD8+ T cells (Greenwald et al., 2005; Watts, 2005). Interestingly, the expression levels of some costimulatory receptors, including CD27, CD28, and others, are often used to distinguish subsets of antiviral T cells, especially in humans (van Lier et al., 2003). Costimulation certainly plays an important role in effector and memory T cell survival during infection, but what influence these signals have, if any, on instructing effector versus memory T cell-fate determination is not clear.
Although inflammation can augment the expression of costimulatory molecules, inflammatory cytokines can also directly impact effector T cell expansion and differentiation (Mescher et al., 2006). For example, type I IFN, IL-12, and IFN-γ signals can act directly on T cells for optimal effector T cell expansion and survival (Badovinac et al., 2000; Curtsinger et al., 2003; Haring et al., 2005; Kolumam et al., 2005; Mullen et al., 2001; Whitmire et al., 2005a; Whitmire et al., 2005b). An interesting point that emerged from these studies is that the nature of the pathogen can determine which cytokine will be most influential for developing effector T cells (Cousens et al., 1999). For instance, lymphocytic choriomeningitis virus (LCMV) specific CD8+ T cell responses are critically dependent on type I IFN, but do not require IL-12, whereas the opposite is true for CD8+ T cells responding to Listeria monocytogenes infection (Way et al., 2007).
Inflammatory cytokines also appear to play a key role in some cell-fate decisions in the developing T cell response by altering the distribution of effector T cells that have memory T cell potential (i.e., MPECs) versus terminally differentiated cells (i.e., SLECs) (Badovinac et al., 2005; Joshi et al., 2007; Pearce and Shen, 2007). Under low inflammatory conditions, fewer IL-7RLo effector T cells are generated. As inflammation increases, the formation of more IL-7RLo effector T cells is favored. It appears that for a constant dose of antigen, increasing inflammatory signals can increase the SLEC to MPEC ratio. IL-12 and IFN-γ are two primary players in this process, but other signals are undoubtedly involved in this process because IL-12 and IFN-γ amounts can vary between different infections (Cousens et al., 1999), and likely candidates are type I IFNs (Curtsinger et al., 2005; Kolumam et al., 2005; Way et al., 2007), other IL-12 family members, or even Toll-like receptor ligands that act directly on T cells (Hervas-Stubbs et al., 2007). For the purposes of vaccination, one might predict that the type of inflammatory signal will be as important as the amount. Indeed, some recent evidence for this concept is emerging from vaccine studies (Wille-Reece et al., 2006), and this issue of the recognition of viral infection by the innate immune system and subsequent inflammatory response is covered in the accompanying review by Pichlmair and Reis e Sousa (2007).
Although a dose-dependent inflammation model suggests that for a given dose of antigen lower inflammatory conditions favor memory T cell development, this should not be incorrectly interpreted to mean that the absence of inflammation enhances memory T cell formation. The priming of T cells without any inflammation leads to tolerance and/or clonal deletion (Redmond and Sherman, 2005). Thus, some inflammation is clearly required for optimal T cell expansion, effector T cell development, and memory T cell generation. It is not clear, however, whether increased inflammation would induce greater numbers of SLECs at the expense of MPECs or whether MPEC numbers are determined by other factors. Moreover, if the SLEC versus MPEC decision is based on an overall strength of signal, then the effects of inflammation might vary according to the degree of TCR stimulation. This idea should be testable by varying inflammation at constant doses of antigen and vice versa, and then examining SLEC versus MPEC fates and memory T cell formation. In addition, it will be interesting to examine whether inflammation can act in a bystander manner with or without TCR signaling during memory T cell differentiation. To rationally apply these principles to vaccination, one might need to determine the optimal inflammatory signals for a given dose or regimen of antigen exposure.
Balancing Effector T Cell Differentiation and Memory T Cell Fates
Additional heterogeneity, other than simply short-term and long-term fates, exists in the functional specification of T cells during infection. This point is more obvious for CD4+ T cells, compared to CD8+ T cells, where a diverse array of effector cell types (Th1, Th2, Th17, TFH, Treg, etc.) exists. Thus, T cell fate decisions might exist in layers: Activated T cells commit to (1) which type of effector cell to become and (2) whether to be long lived or not. The specification of different effector CD4+ T cell populations is based on key innate immune cytokines that induce the appropriate effector T cell response for the given pathogen or adjuvant (IFN-γ and IL-12 → T-bet → Th1; IL-4 → GATA3 and c-maf → Th2; IL-6 and TGFβ → rorγ → TH17 and TGFγ → FoxP3 → Treg). It is unclear whether every effector T cell lineage produces long-lived, self-renewing memory T cells, and if so, whether common or separate pathways are used. For example, some evidence implies that IL-7R might also be a marker for Th1 CD4+ T cells (Dooms et al., 2007; Kondrack et al., 2003; Li et al., 2003; Purton et al., 2007), but it remains to be seen whether this will apply to all effector T cell lineages (e.g., Th2 or Th17).
Some data suggest that effector function specification and memory T cell potential are intertwined, but the precise mechanism is unclear (Intlekofer et al., 2005; Joshi et al., 2007; Takemoto et al., 2006; Wu et al., 2002). For example, Th1 cells with the potential to form memory T cells versus terminally differentiated effector Th1 could be distinguished based on low and high levels of IFN-γ production, respectively (Wu et al., 2002). It is worth pointing out that virus-specific IFN-γ-producing CD4+ T cells can efficiently populate the memory T cell pool (Whitmire et al., 2006), though the attrition of these cells has been documented in some infections (Homann et al., 2001). Another example is that recent work in CD8+ T cells suggests that memory T cell potential is inversely linked to T-bet expression. In LCMV infection, SLECs contain higher amounts of T-bet than do MPECs, and the overexpression of T-bet in these effector CD8+ T cells could induce an SLEC fate (Joshi et al., 2007). T-bet expression can be regulated by inflammatory cytokines in a dose-dependent manner, and hence these data provide a mechanism for how some inflammatory signals can influence the MPEC- versus SLEC-fate decision (Joshi et al., 2007; Takemoto et al., 2006). Also, it is not clear how T-bet and eomesodermin (Eomes), another T-box transcription factor involved in effector CD8+ T cell differentiation, cooperate to regulate effector CD8+ T cell fates (Takemoto et al., 2006).
The control of innate cytokines on T cell expression of lineage-determining transcription factors, such as T-bet, that can specify both effector functions and memory fates creates a sensible way for the innate immune system to influence T cell homeostasis long term. It will be interesting to examine whether this model applies to other key transcription factors involved in lymphocyte differentiation.
Memory T Cell Subsets
T cell heterogeneity also has considerable implications for the protective immunity afforded by memory T cells. Indeed, it was the seminal work of a few groups in the late 1990s that first outlined the existence of specific memory T cell subsets (coined TEM and TCM cells) and alerted us to the importance of heterogeneity within antigen-specific T cell populations (Hamann et al., 1997; Sallusto et al., 1999). However, it is quite evident today that memory T cell heterogeneity is more complex than simply TCM and TEM cells. Nonetheless, the concept of TEM and TCM cells remains an important paradigm, though a number of important questions remain unresolved with regard to these memory T cell subsets. These include understanding how TEM and TCM cells contribute to protective immunity to different pathogens, the precise lineage relationships between these cell types, and how the heterogeneity in the memory T cell pool is related to the early effector T cell heterogeneity discussed above.
TEM and TCM cells were first distinguished in humans based on lymph node (LN) homing receptor CD62L and CCR7 expression, effector functions, and proliferative capacity (Sallusto et al., 1999). These studies gave rise to the original views of TEM and TCM cells in which TEM cells exist at the portals of pathogen entry and possess immediate effector functions and a reduced proliferative capacity, and TCM cells exist mainly in lymph nodes, express effector function upon restimulation, and mount robust secondary responses. Although these largely accepted definitions persist, it is worth noting that one important caveat of using strictly CD62LLo and CCR7Lo to define TEM cells is that recently activated effector T cells share this phenotype, thus blurring the line between true effector T cells and resting TEM cells. In general, TEM cells are not in LNs, but they can be found in spleen, blood, and a variety of nonlymphoid tissues, whereas TCM cells are found in LN, spleen, and blood, and they also enter nonlymphoid tissues, albeit less efficiently than do TEM cells (Marshall et al., 2001; Masopust et al., 2001; Reinhardt et al., 2001; Wherry et al., 2003). In addition, some of the functional differences first noted in CD4+ TEM and TCM cells do not hold up as well in memory CD8+ T cell subsets because CD8+ TEM and TCM cells are nearly equivalent in their production of effector cytokines and cytotoxicity (Barber et al., 2003; Ravkov et al., 2003; Unsoeld et al., 2002; Wherry et al., 2003). However, the augmented capacity of TCM cells to proliferate to antigen and homeostatic signals and to produce IL-2, compared to TEM cells has held true in most analyses (Wherry and Ahmed, 2004). The ability of TCM cells to undergo homeostatic turnover in response to IL-7 and IL-15 is a key factor in the longevity of these memory T cells.
Lineage Development of Memory T Cell Subsets and Relationship to Early Effector Heterogeneity
There is ongoing debate about the development and lineage relationship of memory T cell subsets. Some studies suggest that TCM and TEM cells are fixed populations that do not convert over time (Manjunath et al., 2001; Marzo et al., 2005; Sallusto and Lanzavecchia, 2001) (model 3), whereas others propose that TEM cells can convert to TCM cells in the absence of antigen (Badovinac et al., 2007; Bouneaud et al., 2005; Wherry et al., 2004; Wherry et al., 2003; Zaph et al., 2006) (model 4). On the basis of recent work, we propose that after acute viral infection, there are at least three distinct T cell populations in the early memory T cell pool. First, there exist TEM cells derived from IL-7RHi MPECs that represent a transitional or convertible T cell population, which can further differentiate and transform slowly into TCM cells (Badovinac et al., 2007; Marzo et al., 2005; Wherry et al., 2004; Wherry et al., 2003) (E.J.W., unpublished data). Second, there is a population of TCM cells primarily derived from these convertible TEM cells. These memory T cells have acquired the ability to efficiently self renew via homeostatic turnover (Wherry et al., 2004; Wherry et al., 2003) (E.J.W., unpublished data). Some TCM cells might descend directly from CD62LHi MPECs present at the peak of clonal expansion. Whether TCM cells that arise as a result of conversion from TEM cells are functionally equivalent to TCM cells derived from CD62LHi MPEC is unclear. Third, there remain some terminally differentiated IL-7RLo KLRG1Hi CD62LLo SLECs that can enter the memory phase and downregulate markers of effector activation (e.g., granzyme B). These resting SLECs in the memory pool, however, do not efficiently persist long term, self renew, or undergo conversion to TCM cells (Joshi et al., 2007) (E.J.W., unpublished data). These resting SLECs seem to represent an end-stage TEM cell population.
The segregation of the memory T cells into self-renewing TCM cells, transitional TEM cells, and end-stage TEM cells fits with a number of experimental observations and might help to explain some apparently disparate results in the field. For instance, such a model helps to account for the differences in phenotypic heterogeneity and protective immunity in TEM cell subsets across different studies (Bachmann et al., 2005a; Bachmann et al., 2005b; Huster et al., 2006; Roberts et al., 2005; Roberts and Woodland, 2004; Wherry et al., 2003). These results might reflect differing proportions of transitional, end-stage TEM cells, depending on the infection and time of analysis. Lastly, reducing the overall strength of T cell stimulation by increasing naive CD8+ T cell precursor frequency and T cell competition appears to enrich the development of transitional TEM cells (Badovinac et al., 2007) (E.J.W., unpublished data), increasing the evidence that these T cells occupy a particular stage of memory T cell differentiation.
Protective Immunity
One of the major implications of memory T cell subsets with different functional properties or anatomical localization is for protective immunity. Relatively few studies, however, have directly compared the protective capacity of TCM and TEM cells. Mouse studies demonstrated that CD8+ TCM cells conferred greater protective immunity compared to TEM cells after systemic, local, and respiratory challenge infections, and this was associated with a greater proliferative burst of TCM cell-derived secondary effector T cells (Wherry et al., 2003). Evidence is accumulating that TCM cells also correlate with more favorable responses against simian immunodeficiency virus (SIV) and human immunodeficiency virus (HIV) (Seaman et al., 2004; Vaccari et al., 2005) and protect better against tumors (Klebanoff et al., 2005). A recent study delineated multiple memory CD8+ T cell subsets to Sendai virus that do not conform to the strict TEM and TCM cell definitions. With additional phenotypic markers, including CXCR3, CD27, CD43 (high MW form), and KLRG1, a hierarchical series of subsets was described with varying degrees of recall potency (Hikono et al., 2007). However, other studies using respiratory viruses found interesting kinetics in which TEM cells responded better than did TCM cells at early time points after infection, but in which at later time points, TCM cells mounted the more vigorous secondary responses (Roberts et al., 2005; Roberts and Woodland, 2004), perhaps consistent with the gradual change in the composition of transitional versus end-stage TEM cells over time.
There might be an advantage to maintaining end-stage TEM cells for finite lengths of time after viral clearance because this is the most likely period for reinfection to occur. Indeed, immunity to respiratory Sendai virus or influenza virus infection correlates best with the persistence of TEM cells in the lungs (Hogan et al., 2001; Liang et al., 1994). However, if the end-stage TEM cells gradually disappear, the host could still be protected for longer periods by transitional TEM cells that have become TCM cells. It is interesting to consider whether functional, but relatively senescent, TEM cells are not preferentially maintained in the lung and intestinal mucosa to optimally balance immunological protection and immunopathological damage to delicate mucosal tissues.
Altered Memory T Cell Differentiation during Repetitive or Chronic Infections
Although the above discussion of memory T cell development pertains mostly to events occurring during primary infection with viruses that are completely cleared from the host, there are several situations in which memory T cell differentiation might differ. First, recent work indicates that secondary (2°) and tertiary (3°) memory CD8+ T cells are distinct from primary (1°) memory T cells. More 2° and 3° memory CD8+ T cells persist in a CD62LLo state, with heightened levels of granzyme B and cytotoxicity, for longer periods of time compared to the 1° memory T cells (Jabbari and Harty, 2006; Masopust et al., 2006). In addition, although a substantially larger number of virus-specific T cells can be generated with repeated infection, the E → M transition appears extended, and longer time might be needed to reach stable memory T cell numbers (Grayson et al., 2002; Masopust et al., 2006). These observations point out that memory T cell phenotype, function, and maintenance can be influenced by stimulation history, and special consideration should be given to this issue when developing prime-boost regimens for vaccination.
A second situation that can dramatically alter memory CD8+ T cell differentiation is persisting infection (Klenerman and Hill, 2005; Wherry and Ahmed, 2004) (Figure 3 and Table 1). A substantial body of literature exists on the defects in T cell effector functions that can occur during chronic infections with hierarchical loss in proliferative capacity, cytotoxicity, and the ability to produce cytokines (Lieberman et al., 2001; Shin and Wherry, 2007) and the topic of chronic HIV infection is covered in the accompanying review by Deeks and Walker (2007). Virus-specific CD8+ T cells that persist during chronic infection are quite different from memory T cells found after acute infection. For example, during chronic viral infection, virus-specific T cells remain CD62LLo CCR7Lo, and TCM cells rarely develop (Wherry et al., 2004). These T cells also have a low proliferative capacity, express low amounts of IL-7 and IL-15 receptors, and fail to respond efficiently to these cytokines (Shin and Wherry, 2007). Rather, long-term maintenance of virus-specific CD8+ T cells during chronic infection depends on periodic bursts of cell division due to persisting antigen (Shin et al., 2007) and, in some cases, the priming of new thymic emigrants (Vezys et al., 2006). The precise details of impaired memory T cell development could differ depending on the whether T cells are subjected to repetitive stimulation, for example by herpes virus infections, or protracted, continuous stimulation (e.g., LCMV, HIV, hepatitis C virus [HCV]).
In addition to the changes in memory T cell differentiation and homeostasis, virus-specific CD8+ T cells can become exhausted and lose effector functions during chronic infection (Shin and Wherry, 2007). The inhibitory receptor PD-1 is highly expressed during many chronic infections and blockade of the PD-1-PD-L1 pathway can restore function in these exhausted T cells (Barber et al., 2006; Day et al., 2006; Petrovas et al., 2006; Radziewicz et al., 2006; Trautmann et al., 2006; Zhang et al., 2007). The extent of PD-1 expression and functional exhaustion in a virus-specific T cells likely depends on the degree of ongoing viral replication, antigen abundance, and inflammation associated with, as well as the anatomical location or cellular tropism of, the persisting virus. Moreover, T cell populations from persistent or latent infections will consist of a mixture of various phenotypes and functional states (i.e., activated versus resting) depending on their recent or history of exposure to antigen, inflammation, and other factors (Figure 3 and Table 1).
Memory T Cell Differentiation in Humans
The above issues are particularly relevant for studying virus-specific T cell populations in humans, and T cell phenotype and function often varies for different pathogens (van Lier et al., 2003). For example, influenza virus (flu)-, respiratory syncytial virus (RSV)-, and vaccinia virus (VV)-specific memory CD8+ T cells, all infections that do not persist, are usually IL-7RHi, CD62LHi, and CD27Hi (van Leeuwen et al., 2005). In contrast, a greater proportion of IL-7RLo, CD62LLo, and CD27Hi/Int cells are found in Epstein-Barr virus (EBV)- and cytomegalovirus (CMV)-specific CD8+ T cell populations (Boutboul et al., 2005; van Leeuwen et al., 2005; Wherry et al., 2006). Further, HIV-specific CD8+ T cells are mainly IL-7RLo CD62LLo, but they express high amounts of CD27 (Appay et al., 2002; Boutboul et al., 2005; Champagne et al., 2001; Paiardini et al., 2005; Sabbaj et al., 2007; van Leeuwen et al., 2005; Wherry et al., 2006). Overall, the parallels between humans and mice are striking. For example, human memory T cells from acute infections (e.g., flu and RSV) respond substantially better to IL-7 and IL-15 compared to those from latent infections (e.g., EBV and CMV) (van Leeuwen et al., 2005). Thus, pathogen persistence for chronic, highly replicating, as well as latent, reactivating viruses, can substantially impact human memory T cell differentiation. It is important to keep in mind the effects of persistent and latent viral infections on the phenotypic heterogeneity of antiviral T cells in humans, especially when performing cross-sectional rather than longitudinal types of studies (Figure 3 and Table 1).
Conclusions and Implications for Vaccines and Long-Term Antiviral Immunity
Understanding the pathways that lead to optimal generation and maintenance of memory T cells is of considerable importance for both prophylactic and therapeutic vaccines. One would like to define the subsets of T cells that will provide the most robust protective immunity for different types of pathogens and also those capable of persisting independent of repetitive or continual boosting. Defining how the diversity in phenotype and function of effector and memory T cells arises during primary immune responses will likely lead to the discovery of multiple signals and pathways that control the differentiation of T cells with different functions, protective capacities, and long-term fates. Decoding the pathways and molecules that can be used to therapeutically enhance long-term T cell immunity is a major future goal. Applying emerging new observations such as possible fate-determining signals imprinted by early inflammatory cytokines or the asymmetric separation of daughter T cells (Chang et al., 2007) and other signals should empower and enhance vaccine design. Moreover, the improved ability to implement functional genomic approaches and forward and reverse genetics to study memory T cell differentiation should provide rich opportunities to take our understanding of effector and memory T cell differentiation to a new level of practical application.
ACKNOWLEDGMENTS
We thank Kaech and Wherry lab members and Rafi Ahmed for insightful discussion. S.M.K. is supported by the Burroughs-Wellcome Fund (1004313), the Edward Mallinckrodt, Jr. Foundation, the Cancer Research Institute, and the National Institutes of health (NIH) (AI066232 and AI077075). E.J.W. is supported by the Ellison Medical Foundation, the Commonwealth Universal Research Enhancement Program, the Pennsylvania Department of Health, and the NIH (AI071309 and HHSN26620050030C).
REFERENCES
- Ahmed R, Gray D. Immunological memory and protective immunity: Understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286:958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Hunziker L, Zinkernagel RM, Storni T, Kopf M. Maintenance of memory CTL responses by T helper cells and CD40–CD40 ligand: Antibodies provide the key. Eur. J. Immunol. 2004;34:317–326. doi: 10.1002/eji.200324717. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J. Immunol. 2005a;175:4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Wolint P, Schwarz K, Oxenius A. Recall proliferation potential of memory CD8+ T cells and antiviral protection. J. Immunol. 2005b;175:4677–4685. doi: 10.4049/jimmunol.175.7.4677. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Harty JT. Manipulating the Rate of Memory CD8+ T Cell Generation after Acute Infection. J. Immunol. 2007;179:53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat. Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Messingham KA, Griffith TS, Harty JT. TRAIL deficiency delays, but does not prevent, erosion in the quality of “helpless” memory CD8 T cells. J. Immunol. 2006;177:999–1006. doi: 10.4049/jimmunol.177.2.999. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Ahmed R. Cutting edge: Rapid in vivo killing by memory CD8 T cells. J. Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Blattman JN, Antia R, Soudive DJD, Wang X, Kaech SM, Murali-Krishan K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J. Exp. Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- Bouneaud C, Garcia Z, Kourilsky P, Pannetier C. Lineage relationships, homeostasis, and recall capacities of central- and effector-memory CD8 T cells in vivo. J. Exp. Med. 2005;201:579–590. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutboul F, Puthier D, Appay V, Pelle O, Ait-Mohand H, Combadiere B, Carcelain G, Katlama C, Rowland-Jones SL, Debre P, et al. Modulation of interleukin-7 receptor expression characterizes differentiation of CD8 T cells specific for HIV, EBV and CMV. AIDS. 2005;19:1981–1986. doi: 10.1097/01.aids.0000191919.24185.46. [DOI] [PubMed] [Google Scholar]
- Bullock TN, Colella TA, Engelhard VH. The density of peptides displayed by dendritic cells affects immune responses to human tyrosinase and gp100 in HLA-A2 transgenic mice. J. Immunol. 2000;164:2354–2361. doi: 10.4049/jimmunol.164.5.2354. [DOI] [PubMed] [Google Scholar]
- Castellino F, Germain RN. Chemokine-guided CD4+ T cell help enhances generation of IL-6RalphahighIL-7Ralpha high pre-memory CD8+ T cells. J. Immunol. 2007;178:778–787. doi: 10.4049/jimmunol.178.2.778. [DOI] [PubMed] [Google Scholar]
- Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- Catron DM, Rusch LK, Hataye J, Itano AA, Jenkins MK. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. J. Exp. Med. 2006;203:1045–1054. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Long-worth SA, Vinup KE, Mrass P, Oliaro J, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- Cousens LP, Peterson R, Hsu S, Dorner A, Altman JD, Ahmed R, Biron CA. Two roads diverged: interferon alpha/beta-and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J. Exp. Med. 1999;189:1315–1328. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J. Immunol. 2003;171:5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- D’Souza WN, Hedrick SM. Cutting edge: Latecomer CD8 T cells are imprinted with a unique differentiation program. J. Immunol. 2006;177:777–781. doi: 10.4049/jimmunol.177.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, Depierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Deeks SG, Walker BD. Human immunodeficiency virus controllers: Mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. this issue. [DOI] [PubMed] [Google Scholar]
- Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R alpha-expressing cells. J. Exp. Med. 2007;204:547–557. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörner T, Radbruch A. Antibodies and B cell memory in viral immunity. Immunity. 2007;27:384–392. doi: 10.1016/j.immuni.2007.09.002. this issue. [DOI] [PubMed] [Google Scholar]
- Farber D. Differential TCR signaling and the generation of memory T cells. J. Immunol. 1998;160:535–539. [PubMed] [Google Scholar]
- Freitas AA, Rocha B. Population biology of lymphocytes: The flight for survival. Annu. Rev. Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]
- Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat. Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8(+) T cells to apoptosis in vivo. J. Immunol. 2002;169:3760–3770. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Hamann D, Baars P, Rep M, Hooibrink B, Kerkhof-Garde S, Klein M, van Lier R. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 1997;9:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor {alpha} is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc. Natl. Acad. Sci. USA. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring JS, Corbin GA, Harty JT. Dynamic regulation of IFN-gamma signaling in antigen-specific CD8+ T cells responding to infection. J. Immunol. 2005;174:6791–6802. doi: 10.4049/jimmunol.174.11.6791. [DOI] [PubMed] [Google Scholar]
- Hervas-Stubbs S, Olivier A, Boisgerault F, Thieblemont N, Leclerc C. TLR3 ligand stimulates fully functional memory CD8+ T cells in the absence of CD4+ T-cell help. Blood. 2007;109:5318–5326. doi: 10.1182/blood-2006-10-053256. [DOI] [PubMed] [Google Scholar]
- Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J. Exp. Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, Woodland DL. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol. 2001;166:1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- Hou S, Hyland L, Ryan K, Portner A, Doherty P. Virus-specific CD 8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. USA. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster KM, Koffler M, Stemberger C, Schiemann M, Wagner H, Busch DH. Unidirectional development of CD8+ central memory T cells into protective Listeria-specific effector memory T cells. Eur. J. Immunol. 2006;36:1453–1464. doi: 10.1002/eji.200635874. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J. Exp. Med. . 2007 doi: 10.1084/jem.20070841. Published online August 13, 2007. 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J. Exp. Med. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- Jelley-Gibbs DM, Dibble JP, Brown DM, Strutt TM, McKinstry KK, Swain SL. Persistent depots of influenza antigen fail to induce a cytotoxic CD8 T cell response. J. Immunol. 2007;178:7563–7570. doi: 10.4049/jimmunol.178.12.7563. [DOI] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs a gradient of T-bet expression that specifies memory precursor and short-lived effector CD8 T cell fates. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002a;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2002b;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerman P, Hill A. T cells and viral persistence: Lessons from diverse infections. Nat. Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- Klonowski KD, Williams KJ, Marzo AL, Lefrancois L. Cutting edge: IL-7-independent regulation of IL-7 receptor alpha expression and memory CD8 T cell development. J. Immunol. 2006;177:4247–4251. doi: 10.4049/jimmunol.177.7.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J. Exp. Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe MH, Hardy MP, Rooney J, Labrecque N. IL-7 receptor expression levels do not identify CD8+ memory T lymphocyte precursors following peptide immunization. J. Immunol. 2005;175:4400–4407. doi: 10.4049/jimmunol.175.7.4400. [DOI] [PubMed] [Google Scholar]
- Lefrancois L, Marzo AL. The descent of memory T-cell subsets. Nat. Rev. Immunol. 2006;6:618–623. doi: 10.1038/nri1866. [DOI] [PubMed] [Google Scholar]
- Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J. Exp. Med. 2003;198:1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J. Immunol. 1994;152:1653–1661. [PubMed] [Google Scholar]
- Lieberman J, Shankar P, Manjunath N, Andersson J. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood. 2001;98:1667–1677. doi: 10.1182/blood.v98.6.1667. [DOI] [PubMed] [Google Scholar]
- Manjunath N, Shankar P, Wan J, Weninger W, Crowley MA, Hieshima K, Springer TA, Fan X, Shen H, Lieberman J, von Andrian UH. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J. Clin. Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall DR, Turner SJ, Belz GT, Wingo S, Andreansky S, Sangster MY, Riberdy JM, Liu T, Tan M, Doherty PC. Measuring the diaspora for virus-specific CD8+ T cells. Proc. Natl. Acad. Sci. USA. 2001;98:6313–6318. doi: 10.1073/pnas.101132698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat. Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J. Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J. Clin. Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K, Altman J, Suresh M, Sourdive D, Zajac A, Miller J, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: A reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Northrop JK, Shen H. CD8+ T-cell memory: Only the good ones last. Curr. Opin. Immunol. 2004;16:451–455. doi: 10.1016/j.coi.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Paiardini M, Cervasi B, Albrecht H, Muthukumar A, Dunham R, Gordon S, Radziewicz H, Piedimonte G, Magnani M, Montroni M, et al. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J. Immunol. 2005;174:2900–2909. doi: 10.4049/jimmunol.174.5.2900. [DOI] [PubMed] [Google Scholar]
- Pearce EJ, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J. Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. this issue. [DOI] [PubMed] [Google Scholar]
- Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J. Exp. Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J. Virol. 2006;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravkov EV, Myrick CM, Altman JD. Immediate early effector functions of virus-specific CD8+CCR7+ memory cells in humans defined by HLA and CC chemokine ligand 19 tetramers. J. Immunol. 2003;170:2461–2468. doi: 10.4049/jimmunol.170.5.2461. [DOI] [PubMed] [Google Scholar]
- Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22:275–284. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lung. J. Immunol. 2004;172:6533–6537. doi: 10.4049/jimmunol.172.11.6533. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J. Exp. Med. 2005;202:123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B, Tanchot C. Towards a cellular definition of CD8+ T-cell memory: The role of CD4+ T-cell help in CD8+ T-cell responses. Curr. Opin. Immunol. 2004;16:259–263. doi: 10.1016/j.coi.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Sabbaj S, Heath SL, Bansal A, Vohra S, Kilby JM, Zajac AJ, Goepfert PA. Functionally competent antigen-specific CD127(hi) memory CD8+ T cells are preserved only in HIV-infected individuals receiving early treatment. J. Infect. Dis. 2007;195:108–117. doi: 10.1086/509510. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Exploring pathways for memory T cell generation. J. Clin. Invest. 2001;108:805–806. doi: 10.1172/JCI14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Seaman MS, Peyerl FW, Jackson SS, Lifton MA, Gorgone DA, Schmitz JE, Letvin NL. Subsets of memory cytotoxic T lymphocytes elicited by vaccination influence the efficiency of secondary expansion in vivo. J. Virol. 2004;78:206–215. doi: 10.1128/JVI.78.1.206-215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr. Opin. Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Lehar SM, Bevan MJ. Augmented IL-7 signaling during viral infection drives greater expansion of effector T cells but does not enhance memory. J. Immunol. 2006;177:4458–4463. doi: 10.4049/jimmunol.177.7.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol. Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J. Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- Tripathi P, Mitchell TC, Finkelman F, Hildeman DA. Cutting edge: Limiting amounts of IL-7 do not control contraction of CD4+ T cell responses. J. Immunol. 2007;178:4027–4031. doi: 10.4049/jimmunol.178.7.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsoeld H, Krautwald S, Voehringer D, Kunzendorf U, Pircher H. Cutting Edge: CCR7(+) and CCR7(−) memory T cells do not differ in immediate effector cell function. J. Immunol. 2002;169:638–641. doi: 10.4049/jimmunol.169.2.638. [DOI] [PubMed] [Google Scholar]
- Vaccari M, Trindade CJ, Venzon D, Zanetti M, Franchini G. Vaccine-induced CD8+ central memory T cells in protection from simian AIDS. J. Immunol. 2005;175:3502–3507. doi: 10.4049/jimmunol.175.6.3502. [DOI] [PubMed] [Google Scholar]
- van Leeuwen EM, de Bree GJ, Remmerswaal EB, Yong SL, Tesselaar K, ten Berge IJ, van Lier RA. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood. 2005;106:2091–2098. doi: 10.1182/blood-2005-02-0449. [DOI] [PubMed] [Google Scholar]
- van Lier RA, ten Berge IJ, Gamadia LE. Human CD8(+) T-cell differentiation in response to viruses. Nat. Rev. Immunol. 2003;3:931–939. doi: 10.1038/nri1254. [DOI] [PubMed] [Google Scholar]
- Vezys V, Masopust D, Kemball C, Barber DL, O’Mara LA, Larsen CP, Pearson TC, Ahmed R, Lukacher AE. Continuous recruitment of naïve T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J. Exp. Med. 2006;203:2263–2269. doi: 10.1084/jem.20060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- Way SS, Havenar-Daughton C, Kolumam GA, Orgun NN, Murali-Krishna K. IL-12 and type-I IFN synergize for IFN-gamma production by CD4 T cells, whereas neither are required for IFN-gamma production by CD8 T cells after Listeria monocytogenes infection. J. Immunol. 2007;178:4498–4505. doi: 10.4049/jimmunol.178.7.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J. Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Puorro KA, Porgador A, Eisenlohr LC. Increasing MHC class I-restricted epitope density elicits progressively larger CTL populations at all but the highest epitope expression levels. Proceedings of the 10th International Congress of Immunology; 1–6 November 1998; New Delhi, India. 1998. pp. 393–399. [Google Scholar]
- Wherry EJ, Puorro KA, Porgador A, Eisenlohr LC. The induction of virus-specific CTL as a function of increasing epitope expression: responses rise steadily until excessively high levels of epitope are attained. J. Immunol. 1999;163:3735–3745. [PubMed] [Google Scholar]
- Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Day CL, Draenert R, Miller JD, Kiepiela P, Woodberry T, Brander C, Addo M, Klenerman P, Ahmed R, Walker BD. HIV-specific CD8 T cells express low levels of IL-7Ralpha: Implications for HIV-specific T cell memory. Virology. 2006;353:366–373. doi: 10.1016/j.virol.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmire JK, Benning N, Whitton JL. Cutting edge: Early IFN-gamma signaling directly enhances primary antiviral CD4+ T cell responses. J. Immunol. 2005a;175:5624–5628. doi: 10.4049/jimmunol.175.9.5624. [DOI] [PubMed] [Google Scholar]
- Whitmire JK, Tan JT, Whitton JL. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. J. Exp. Med. 2005b;201:1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmire JK, Benning N, Whitton JL. Precursor frequency, nonlinear proliferation, and functional maturation of virus-specific CD4+ T cells. J. Immunol. 2006;176:3028–3036. doi: 10.4049/jimmunol.176.5.3028. [DOI] [PubMed] [Google Scholar]
- Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J. Exp. Med. 2006;203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Bevan MJ. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J. Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu. Rev. Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CY, Kirman JR, Rotte MJ, Davey DF, Perfetto SP, Rhee EG, Freidag BL, Hill BJ, Douek DC, Seder RA. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat. Immunol. 2002;3:852–858. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- Zaph C, Rook KA, Goldschmidt M, Mohrs M, Scott P, Artis D. Persistence and function of central and effector memory CD4+ T cells following infection with a gastrointestinal helminth. J. Immunol. 2006;177:511–518. doi: 10.4049/jimmunol.177.1.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Zhang Z, Wang X, Fu JL, Yao J, Jiao Y, Chen L, Zhang H, Wei J, Jin L, et al. PD-1 upregulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors, but not in long-term non-progressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R, Bachmann M, Kundig T, Oehen S, Pirchet H, Hengartner H. On Immunological Memory. Annu. Rev. Immunol. 1996;14:333–367. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]