SUMMARY
Memory CD8+ T cells are the pivotal component of adaptive immunity, however the signal and genetic pathways that govern their formation remain poorly defined. This study shows that the IL-10-IL-21-STAT3 pathway is critical for memory CD8+ T cell development following acute LCMV infection. In the absence of either Interleukin-10 (IL-10) and IL-21 or STAT3, virus-specific CD8+ T cells retain terminal effector (TE) differentiation states and fail to mature into protective memory T cells that contain self-renewing central memory T (Tcm) cells. Expression of Eomes, BCL-6, Blimp-1 and SOCS3 was considerably reduced in STAT3-deficient memory CD8+ T cells, and BCL-6- or SOCS3-deficient CD8+ T cells also had perturbed memory cell development. Reduced SOCS3 expression rendered STAT3-deficient CD8+ T cells hyper-responsive to IL-12, suggesting that the STAT3-SOCS3 pathway helps to insulate memory precursor cells (MPCs) from inflammatory cytokines that drive TE differentiation. Thus, memory CD8+ T cell precursor maturation is an active process dependent on IL-10-IL-21-STAT3 signaling.
INTRODUCTION
Memory CD8+ T cells are an important component of long-term immunity against infectious disease. Their ability to protect dependents on their longevity and other cardinal features such as their robust capacity to proliferate and develop effector functions when antigen is reencountered (Cui and Kaech, 2010; Kaech and Wherry, 2007; Sallusto et al. 2011). How these memory T cell qualities are acquired and maintained by the pathogen-specific CD8+ T cells that form the memory T cell pool following infection is not well understood.
Together with antigen, cytokines and the Janus Kinase-Signal Transducer and Activator of Transcription (JAK-STAT) pathways they activate are essential mediators of T cell differentiation, function and survival during immune responses (O’Shea et al., 2011; Shuai and Liu, 2003). Prior work has shown that intense or prolonged exposure to antigen and inflammatory cytokines, such as IL-2, IL-12, IFN-γ, and type I IFNs, which signal through STAT-1, −2, −4 and −5, maximize effector CD8+ T cell proliferation and differentiation to aid pathogen clearance (Agarwal et al., 2009; Badovinac et al., 2005; Badovinac et al., 2004; Cui and Kaech, 2010; Joshi et al., 2007; Kalia et al.,2010; Kolumam et al., 2005; Nguyen et al., 2002). However, these signals also modulate memory cell fate because they drive formation of terminal effector (TE) CD8+ T cells, which despite their important role in pathogen clearance, are less fit to persist and populate the memory cell pool and acquire cardinal memory cell properties (Badovinac et al., 2005; Badovinac et al., 2004; Cui et al., 2009; Joshi et al., 2007; Kalia et al., 2010; Pipkin et al., 2010; Sarkar et al., 2008). These inflammatory cytokines enhance CD8+ TE cell formation via the transcription factors T-bet (Tbx21) and Blimp-1 (Prdm1), and in some cases, TE cells can be distinguished from memory precursor cells (MPCs) by increased KLRG1 and decreased IL-7Rα expression (Badovinac et al., 2005; Badovinac et al., 2004; Cui et al., 2009; Joshi et al., 2007; Kaech and Wherry, 2007; Kalia et al., 2010; Kallies et al., 2009; Pipkin et al., 2010; Rutishauser et al., 2009; Sarkar et al., 2008).
It is unclear how the MPC subset forms during infection and circumvents the signals that promote TE differentiation. One possibility is that, like TE fates, MPC fates are induced and sustained over time by particular cytokines produced during infection. However, such signaling pathways have not been formally identified. We postulated that STAT3 may be a critical factor in this process for a variety of reasons. STAT3-dependent cytokines, such as IL-6, IL-10 and IL-21, have been implicated in memory CD8+ T cell differentiation following vaccination and infection (Castellino and Germain, 2007; Foulds et al., 2006; Hinrichs et al., 2008; Yi et al. 2010). STAT3 has an important role in the differentiation other types of T cells including T helper 17 (Th17), Th2 and regulatory T (Treg) cells (Chaudhry et al., 2009; Stritesky et al., 2011; Yang et al., 2007). Lastly, low STAT3 activity is critical for embryonic stem cells to remain undifferentiated and self-renew (Matsuda et al., 1999) and it would be interesting if STAT3 functioned similarly in memory CD8+ T cells.
This study reveals that STAT3 was required for formation of mature, self-renewing and protective memory CD8+ T cells. Two upstream cytokines of STAT3, IL-10 and IL-21, acted together to promote memory CD8+ T cell differentiation and functional maturation during LCMV infection. In the absence of STAT3, KLRG1loIL-7Rhi MPCs formed, but these cells did not accumulate and populate the memory CD8+ T cell pool as normally observed. Rather the memory CD8+ T cell pool that formed was comprised mainly of TE-like cells that failed to undergo homeostatic proliferation or protect against secondary infection. During the effector-to-memory transition, the IL-10-IL-21-STAT3 signaling pathway sustained expression of the transcription factors BCL-6, Eomes and Blimp-1. In addition, expression of the cytokine signaling inhibitor SOCS3 was also reduced and, consequently, STAT3-deficient CD8+ T cells were hypersensitive to IL-12 signaling. Thus, we propose that STAT3 promotes and preserves memory cell potential in virus-specific CD8+ T cells by sustaining the expression of “pro-memory” transcription factors as well as proteins that shield T cells from responding to effector-inducing inflammatory cytokines in the environment.
RESULTS
STAT3 is intrinsically required for memory CD8+ T cell differentiation and maturation
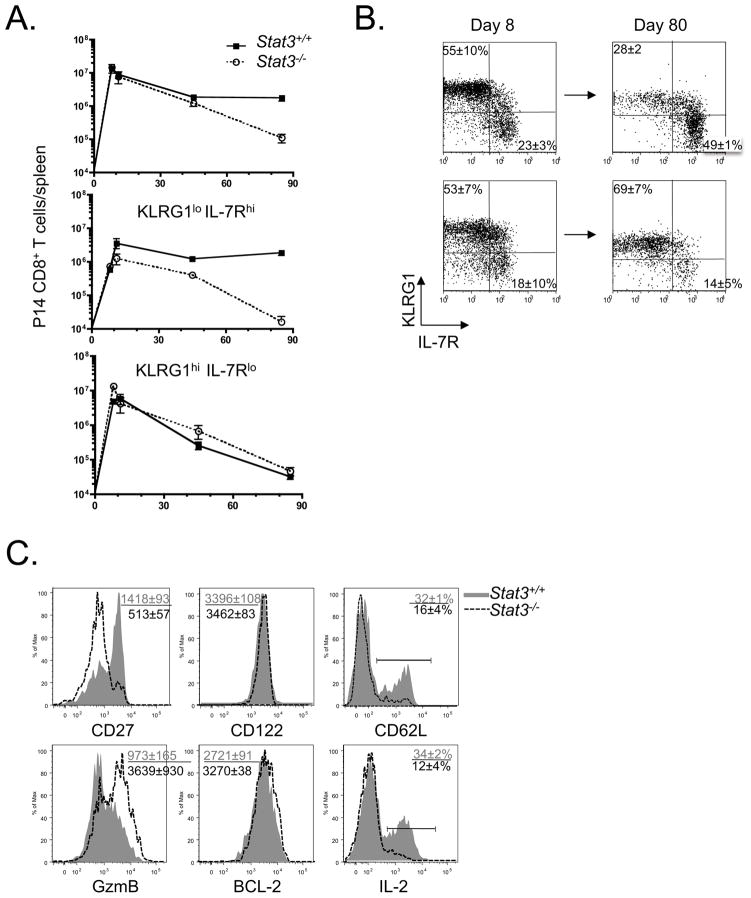
To examine the role of STAT3 in effector and memory CD8+ T cell development during viral infection, we generated mice in which the activated CD8+ T cells would be STAT3-deficient by crossing mice containing loxP-flanked Stat3 alleles (Stat3flox/flox) (Welte et al., 2003) to mice expressing the Cre recombinase under the control of the human Granzyme B promoter (GzB-cre) (Jacob and Baltimore, 1999). For simplicity, the GzB-cre+; Stat3flox/flox animals will be referred to as “Stat3−/−” and their littermate controls (GzB-cre+; Stat3+/+ or GzB-cre−; Stat3flox/flox) as “Stat3+/+” throughout the manuscript. Stat3−/− and Stat3+/+ mice were infected acutely with lymphocytic choriomeningitis virus (LCMV) and effector and memory CD8+ T cell development was analyzed. Proliferation of LCMV-specific effector CD8+ T cells (DbGP33–41 and DbNP396–404) in Stat3−/− mice was comparable to littermate control mice at day 8 post infection (p.i.) (Fig. 1A and Supplementary Fig. 1A). Additionally, clearance of LCMV at day 8 p.i. and the formation of CD44hi KLRG1hi CD27hi IL-7Rlo CD62Llo effector CD8+ T cells that produced IFN-γ, TNF-α and Granzyme B was also similar between the two groups of mice (Fig. 1B, C and Supplementary Fig. 1B, C). There was a slight reduction in the percentage of KLRG1lo IL-7Rhi effector CD8+ T cells that contain MPCs (Fig. 1C), but overall, effector CD8+ T cell expansion, differentiation and function at day 8 p.i was not substantially altered in the absence of STAT3.
Figure 1. STAT3-deficient memory CD8+ T cells sustain a terminally differentiated effector phenotype.
(A) Stat3+/+ (filled square) and Stat3−/− (open circle) mice were infected with LCMV, and DbGP33–41-specific CD8+ T cells in spleen were enumerated at day 8, 40 and 80 p.i. (B) Bar graphs show viral titers in the serum at day 8 p.i. LOD denotes the level of detection. Serum from naïve mice infected with CL13 served as a positive control. (C) Dot plots show expression of KLRG1 and IL-7R in DbGP33–41 tetramer+ CD8+ T cells at day 8 and 80 p.i. (D) Numbers of KLRG1lo IL-7Rhi and KLRG1hi IL-7Rlo subsets from (A and B) were plotted in line graphs. (E) Histograms show the expression level of CD27, CD122, Granzyme B and BCL-2, and percentage of CD62L+ and IL-2+ in DbGP33–41 tetramer+ Stat3+/+ (shaded histogram) and Stat3−/− (dashed line histogram) CD8+ T cells at day 80 p.i. (F) Dot plots show the IFNγ and TNFα expression in GP33–41 peptide stimulated Stat3+/+ and Stat3−/− splenocytes at day 80 p.i. Data shown are representative of at least three independent experiments per time point. MFI or percentage of “positive” cells (mean±SEM) shown in the upper left or right corner throughout the whole manuscript.
Next, we followed the development of Stat3+/+ and Stat3−/− memory CD8+ T cells in the spleen and other tissues following LCMV infection, and although there was no significant alterations in the numbers or tissue distribution of GP33–41 and NP396–404-specific memory CD8+ T cells that formed up to ~80 days p.i. (Fig. 1A and Supplementary Fig. 1A, D), substantial differences in the types of memory CD8+ T cells that formed in the spleen were apparent. Whereas the Stat3+/+ memory cell pool contained mostly KLRG1lo IL-7Rhi CD27hi cells and had accumulated some CD62Lhi central memory T (Tcm) cells, the STAT3-deficient memory CD8+ T cells resembled TE cells and were predominantly KLRG1hiIL-7RloCD27loCD62Llo (Fig. 1C-E and Supplementary Fig. 1A, B). BCL-2 expression was not affected by STAT3-deficiency and increased in Stat3−/−- memory CD8 T cells as typically observed after viral clearance (compare Supplementary Fig. 1C and Fig. 1E and 2C) (Kaech et al., 2003). IL-15Rβ (CD122) expression was also unaffected (Fig. 1E).
Figure 2. STAT3 acts in a CD8+ T cell autonomous manner to generate a long-lived pool of mature memory CD8+ T cells.
(A) Mice that received a small number (5×105) of P14 TCR-tg Stat3+/+ (filled square) and Stat3−/− (open circle) cells were infected with LCMV. P14 CD8+ T cells in spleen were enumerated at day 8, 40 and 80 p.i. Numbers of total P14, KLRG1lo IL-7Rhi and KLRG1hi IL-7Rlo subsets were plotted in line graphs. (B) Dot plots show the expression of KLRG1 and IL-7R in P14 CD8+ T cells at day 8 and 80 p.i. (C) Histograms show the expression of CD27, CD122, Granzyme B and BCL-2, and percentage of CD62L+ and IL-2+ in P14 Stat3+/+ (shaded histogram) and Stat3−/− (dashed line histogram) CD8+ T cells at day 80 p.i. MFI or percentage of “positive” cells shown in the upper left corner.
STAT3-deficient memory CD8+ T cells were functional and produced IFN-γ and TNF-α when restimulated (Fig. 1F), but they were less “polyfunctional” than Stat3+/+ cells because very few made IL-2 (Fig. 1E). Splenic Stat3−/− memory CD8+ T cells also aberrantly sustained expression of GzmB following viral clearance (Fig. 1E). Moreover, the homing of STAT3-deficient P14 memory CD8+ T cells to all tissues was normal, except to the lymph nodes and this was likely due to the Tcm cell deficit (Supplementary Fig. 1E). Altogether, these results reveal that the formation of phenotypically and functionally mature memory CD8+ T cells, including Tcm cells, is considerably impaired in the absence of STAT3. Rather, the Stat3−/− virus-specific CD8+ T cells persist long-term in more of an effector-like state.
Because GzB-cre mediated Stat3 deletion is not restricted solely to CD8+ T cells, we verified that the above Stat3−/− phenotypes were CD8+ T cell autonomous by transferring small number of LCMV-specific P14 Stat3−/− CD8+ T cells (from P14 TCR-tg; GzB-cre+; Stat3flox/flox mice) into naïve P14neg littermates and examining their responses to LCMV infection. Indeed, the phenotypes of P14 Stat3−/− cells recapitulated all those observed in polyclonal Stat3−/− memory CD8+ T cells described above (Fig. 2), with one notable exception. That is, compared to the WT P14 CD8+ T cells, the STAT3-deficient P14 CD8+ T cells did not persist well over time despite normal BCL-2 expression (Fig. 2A, C). Thus, STAT3 acts in a CD8+ T cell autonomous manner to control memory CD8+ T cell differentiation, and in a competitive setting, Stat3−/− memory P14 CD8+ T cells are less fit and have a shortened life-span compared to WT memory T cells.
STAT3 is required for memory CD8+ T cell self-renewal and protective immunity
Next, we examined whether Stat3−/− memory CD8+ T cells possess other cardinal memory properties such as the ability to self-renew (i.e., homeostatically proliferate) and protect against secondary infection. First, we infected Stat3+/+ and Stat3−/− mice with LCMV and fed them 5-bromodeoxyuridine (BrdU) in their drinking water from days 30 to 40 p.i. The Stat3−/− memory CD8+ T cells had markedly reduced rates of BrdU incorporation, despite their normal expression of IL-15Rβ and responsiveness to IL-15 (Fig. 3A and data not shown). Second, we adoptively transferred equal numbers of LCMV-specific memory CD8+ T cells from Stat3+/+ and Stat3−/− mice into naïve recipients that were then infected with the more virulent LCMV Clone 13 strain. This showed that the secondary expansion of Stat3−/− memory CD8+ T cells was reduced 90% relative to WT controls and this correlated with poor viral control (Fig. 3B,C). Similar, if not more profound, defects in memory CD8+ T cell-mediated protection were also observed in the P14 Stat3−/− CD8+ T cells when rechallenged with either LCMV Clone 13 or recombinant Listeria monocytogenes that express LCMV GP-33 epitope (LM-33) (Supplementary Fig. 2 and data not shown). Thus, in the absence of STAT3, the virus-specific CD8+ T cells retain a TE-like phenotype and fail to form memory T cells that persist well (in a competitive setting), self-renew and provide protective immunity to a secondary infection.
Figure 3. STAT3 is required for memory CD8+ T cell self-renewal and protective immunity.
(A) LCMV infected mice were given BrdU in drinking water from days 30 to 40 p.i., BrdU incorporation (±SEM) in Stat3+/+ (solid line) and Stat3−/− (dashed line) memory CD8+ T cell and isotype control (shaded) is shown in histograms. (B and C) Equal numbers of Stat3+/+ and Stat3−/− LCMV-specific memory CD8+ T cells were adoptively transferred into naïve hosts that were subsequently infected with LCMV Cl13. A control group of “Naive” mice that did not receive donor memory CD8+ T cells are also shown. Bar graphs show the numbers of secondary effector CD8+ T cells (B) and serum viral titers (C) in the recipient mice at day 6 p.i. Data shown are representative of three independent experiments (** denotes p<0.01).
IL-10 and IL-21 cooperatively promote memory CD8+ T cell differentiation
To determine which extracellular signals might govern memory CD8+ T cell development in a STAT3-dependent manner, we examined the ability of LCMV-specific CD8+ T cells to respond to several STAT3-dependent cytokines directly ex vivo by measuring STAT3727 and STAT3705 phosphorylation via flow cytometry. This analysis identified IL-10 and IL-21 as candidate cytokines because both LCMV-specific effector and memory CD8+ T cells responded to these cytokines (Supplementary Fig. 3A and data not shown). To examine the roles of IL-10 and IL-21 on effector and memory CD8+ T cell differentiation during LCMV infection, we deprived cells of these cytokines using a combination of genetic and mAb blockade approaches. WT or Il21−/− mice were infected with LCMV and either left alone or treated with IL-10 mAb from days 1–25 p.i. The virus-specific CD8+ T cell responses were analyzed at days 8 and 25 p.i. and consistent with previous reports in acute LCMV infection (Brooks et al., 2010, Yi et al., 2010; Maris et al., 2007), mice lacking either IL-10 or IL-21 alone showed modest effects of these cytokines on effector and memory CD8 T cell differentiation, function and viral clearance. Likewise, when both IL-10 and IL-21 were blocked simultaneously, the ability to clear LCMV, produce IFNγ and the numbers of virus-specific CD8 T cells recovered from at day 25 p.i. were unaffected (Fig. 4 and Supplementary Fig. 3B-D). However, in this latter group we observed that the formation and persistence of KLRG1lo IL-7Rhi MPCs was significantly impaired (Fig. 4). This result suggests that IL-10 and IL-21 cooperate to promote memory CD8+ T cell maturation, and deprivation of both cytokines recapitulates the phenotype found in Stat3−/− CD8+ T cells.
Figure 4. Both IL-10 and IL-21 promote memory CD8+ T cell differentiation.
(A) WT and Il21−/− mice were infected with LCMV and either treated with αIL-10 mAb or mock injected with PBS for 25 days. (A) KLRG1 and IL-7R expression on DbGP33–41 tetramer+ CD8+ T cells was examined at days 25 p.i. (B) The numbers of total DbGP33–41 tetramer+ (open bars) and KLRG1lo IL-7Rhi (black bars) CD8+ T cells at day 25 p.i. are shown in the stacked bar graphs. Data shown are representative of two independent experiments (Statistical analyses show the comparison between KLRG1lo IL-7Rhi groups, * denotes p<0.05 and n.s. denotes “not significant”).
STAT3-deficient CD8+ T cells have reduced Eomes, BCL-6 and Blimp-1 expression
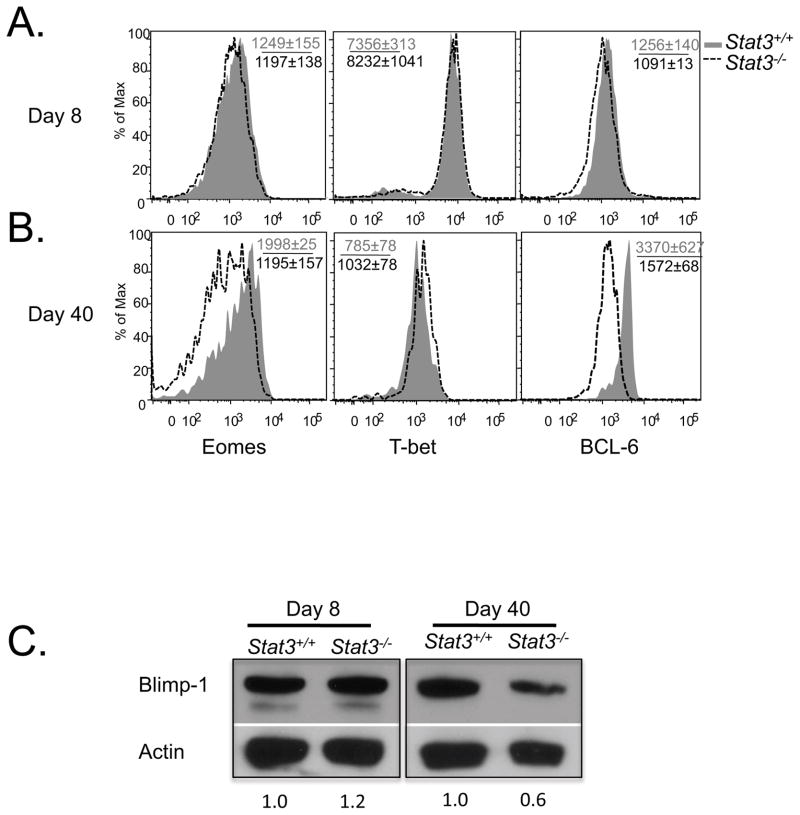
Being a transcription factor, there are likely multiple downstream gene targets of STAT3 involved in memory CD8+ T cell development. We first examined how STAT3-deficiency affects the expression of other genes associated with effector and memory CD8+ T cell fate decisions. Transcription factors such as BCL-6, Eomes, and TCF-1 are involved in Tcm cell differentiation and longevity, and others such as T-bet (Tbx21) and Blimp-1 (Prdm1) promote TE cell differentiation (Banerjee et al., 2010; Ichii et al., 2007; Ichii et al., 2004; Intlekofer et al., 2005; Joshi et al., 2007; Kallies et al., 2009; Rutishauser et al., 2009; Zhou et al. 2010). Correlating with these roles, KLRG1hi IL-7Rlo TE cells express greater amounts of Tbx21 and Prdm1, and conversely, KLRG1lo IL-7Rhi MPCs express greater amounts of Bcl6, Eomes and Tcf1 and these are important for Tcm cell formation (Ichii et al., 2004; Ichii et al., 2007; Intlekofer et al., 2007; Joshi et al., 2007; Rutishauser et al., 2009; Zhou et al. 2010). We examined the expression of these transcription factors and found that at day 8 p.i. the Stat3−/− CD8+ T cells contained comparable amounts of Eomes, T-bet, BCL-6 and Blimp-1 protein to the Stat3+/+ cells, indicating that STAT3 signaling was not critical for normal expression these proteins during effector CD8+ T cell differentiation (Fig. 5). However, the memory CD8+ T cells that formed in the absence of STAT3 had normal amounts of T-bet, but reduced amounts of Eomes, BCL-6 and Blimp-1 compared to the Stat3+/+ cells (Fig. 5). In agreement with prior studies in B cells (Ozaki et al., 2004; Spolski and Leonard, 2008b), STAT3 may act directly on BCL-6 in CD8+ T cells because this protein was elevated in effector CD8+ T cells after treatment with IL-10 + IL-21, but the same was not true for Eomes and T-bet (Supplementary Fig. 4; note, Blimp-1 was not examined in these experiments). Together, these data show that IL-10/IL-21/STAT3 signaling is necessary to sustain expression of particular transcription factors in virus-specific CD8+ T cells during the effector to memory transition.
Figure 5. Stat3−/− CD8+ T cells have reduced expression of Eomes and BCL-6.
(A and B) Histogram plots showing the expression of Eomes, T-bet and BCL-6 in DbGP33–41 tetramer+ Stat3+/+ (shaded) and Stat3−/− (dashed line) CD8+ T cells at days 8 (A) and 40 (B) post infection. (C) Western blot shows the amount of Blimp-1 and Actin (loading control) in P14 CD8+ T cells isolated at days 8 and 40 p.i. by FACS. Numbers below the graph indicate the normalized abundance of Blimp-1 measured by densitometry.
BCL-6 promotes development of KLRG1lo IL-7Rhi memory CD8+ T cells
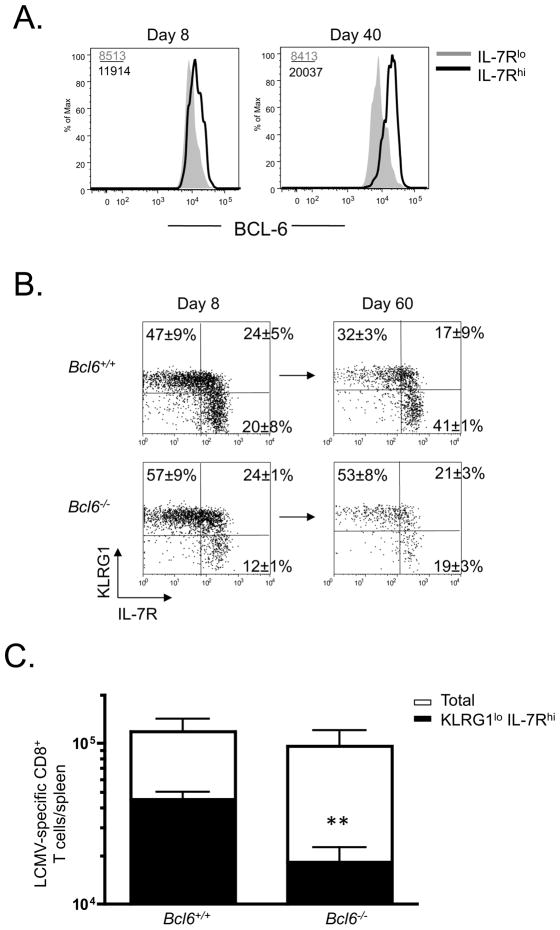
Prior work has shown that Bcl6 mRNA expression is elevated in IL-7Rhi effector cells relative to IL-7Rlo effector cells (Rutishauser et al., 2009) and that Bcl6 is involved in the generation of CD8+ Tcm cells (Ichii et al., 2007; Ichii et al., 2004), but it has not been examined if Bcl6 is required intrinsically in CD8+ cells or whether it is involved in the formation and persistence of KLRG1lo IL-7Rhi MPCs. Because of the reduced BCL-6 expression in Stat3−/− memory CD8+ T cells shown above, we wanted to investigate these questions in greater detail. First, we measured the amount of BCL-6 protein using flow cytometry and found that, indeed, the IL-7Rhi effector and memory CD8+ T cells express greater amounts of BCL-6 relative to their IL-7Rlo counterparts. Interestingly, BCL-6 expression progressively increased within the IL-7Rhi cells during the effector-to-memory transition whereas the expression in the IL-7Rlo cells remained stable (Fig. 6A). To examine the intrinsic role of Bcl6 in memory CD8+ T cells we made mixed bone marrow chimeras (BMCs) by combining either Bcl6−/− or Bcl6+/+ bone marrow with Cd8a−/− bone marrow to generate mice containing Bcl6-deficient or Bcl6-sufficient CD8+ T cells. Following reconstitution, the two groups of mice were infected with LCMV and effector and memory CD8+ T cell development was monitored at days 8 and ~60 p.i. At day 8, the Bcl6−/− effector CD8+ T cell population contained fewer KLRG1lo IL-7Rhi MPCs on average, but this was not statistically significant (Fig. 6B). However, during the effector to memory transition, the phenotypic differences between the two groups grew more apparent and the Bcl6−/− memory CD8+ T cell population contained significantly fewer KLRG1lo IL-7Rhi cells and more KLRG1hi IL-7Rlo cells (Fig. 6B, C). Thus, BCL-6 is also important for the persistence and accumulation of KLRG1lo IL-7Rhi in the memory CD8+ T cell population and the similarity in phenotypes between Bcl6−/− and Stat3−/− CD8+ T cells suggests that reduced BCL-6 expression in the Stat3−/− CD8+ T cells contributes to their impaired memory CD8+ T cell development.
Figure 6. BCL-6 is important for maintaining KLRG1lo IL-7Rhi memory CD8+ T cells following infection.
(A) Histograms show the expression of BCL-6 in LCMV-specific IL-7Rhi (black line) and IL-7Rlo (shaded) subsets at day 8 and 40 p.i. based on intracellular staining and flow cytometry. (B) Cd8α −/− bone marrow was mixed with either Bcl6−/− or Bcl6+/+ bone marrow (at a 90:10 ratio) and used to reconstitute irradiated Cd8+α −/− mice that were then infected with LCMV two months later. DbNP396–404 tetramer+ CD8+ T cells were examined for expression of KLRG1 and IL-7R at days 8 and 60 p.i. (C) Numbers of total (open) or KLRG1lo IL-7Rhi (solid) DbNP396–404+ memory CD8+ in spleen at day 60 were plotted in the bar graphs. Data shown are representative of two independent experiments (** denotes to p<0.01).
SOCS3 suppresses IL-12 signaling in virus-specific CD8+ T cells
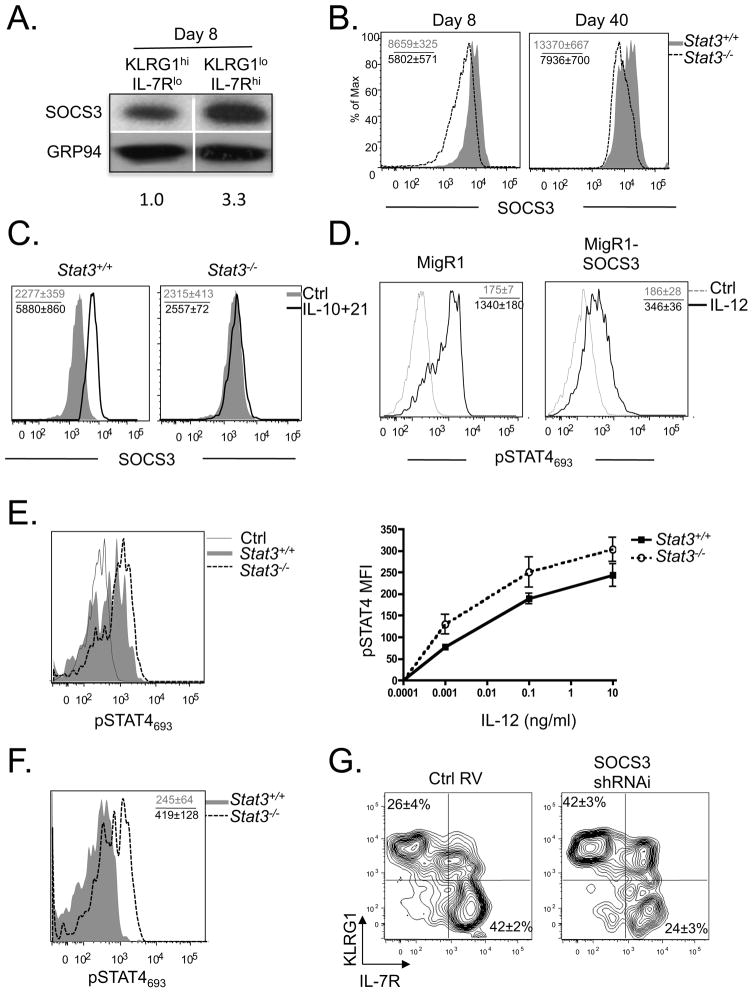
In addition to the reduced expression of pro-memory genes Eomes and Bcl6, it is also possible that the Stat3−/− virus-specific CD8+ T cells persist in TE or effector memory T (Tem) cell states because that Stat3−/− CD8+ T cells are more responsive to pro-inflammatory cytokine signals, such as IL-12, that drive effector cell differentiation. This idea stemmed from the observation that suppressor of cytokine signaling 3 (SOCS3), another well-characterized STAT3 target gene that degrades Jak2, IL-6Ra and other proteins to inhibit cytokine signaling (Egwuagu et al., 2002; Yoshimura et al., 2007), was expressed to a higher degree in IL-7Rhi MPCs compared to IL-7Rlo TE cells following LCMV infection (Fig. 7A). Additionally, Stat3−/− effector and memory CD8+ T cells expressed considerably less SOCS3 than their WT counterparts direct ex vivo (Fig. 7B, Supplementary Fig. 5A), and SOCS3 could be induced or maintained in LCMV-specific CD8+ T cells in a STAT3-dependent manner when cultured with IL-10 and IL-21 in vitro (Fig. 7C). We tested whether elevated SOCS3 could dampen IL-12-dependent phosphorylation of STAT4693 signaling by retroviral (RV) over-expression of SOCS3 in activated CD8+ T cells and found that this was sufficient to suppress IL-12 induced STAT4693 phosphorylation (Fig. 7D). Furthermore, activated CD8+ T cells pretreated with IL-10 and IL-21 in vitro for 48 hours prior to IL-12 stimulation, contained lower amounts of phosphorylated STAT4 than control cells that were not exposed to IL-10 and IL-21 (Supplementary Fig. 5B). More importantly, we observed that compared to Stat3+/+ effector and memory CD8+ T cells, Stat3−/− cells were hyper-responsive to IL-12 signaling and STAT4693 phosphorylation in vitro (Fig. 7E) or directly ex vivo one day after Listeria monocytogenes infection, which potently induces IL-12 production (Fig. 7F). Type I IFNs also activate STAT4 in effector CD8+ T cells (Nguyen et al., 2002). Therefore, we examined STAT4 phosphorylation in Stat3−/− effector CD8+ T cells following in vitro IFNβ stimulation and found comparable amounts of pSTAT4693 between Stat3+/+ and Stat3−/− effector CD8+ T cells (data not shown). These results show that STAT3-deficient cells are particularly more responsive to IL-12 signaling and suggest that increased basal STAT4 activity by bystander inflammatory cytokines sustains effector states in the Stat3−/− CD8+ T cells.
Figure 7. STAT3-dependent SOCS3 expression suppresses IL-12 signaling and enhances memory CD8+ T cell differentiation.
(A) Western blot shows the amount of SOCS3 and GRP94 (loading control) in KLRG1hi IL-7Rlo (TE) or KLRG1lo IL-7Rhi (MPC) P14 CD8+ T cells isolated by FACS 8 days p.i. Numbers below the graph indicate the normalized amount of SOCS3 measured by densitometry. (B) Histogram plots showing the expression of SOCS3 in DbGP33–41 tetramer+ Stat3+/+ (shaded) and Stat3−/− (dashed line) CD8+ T cells at day 8 and 40 p.i. (C) SOCS3 expression in P14 Stat3+/+ or Stat3−/− LCMV-specific day 6 effector CD8+ T cells treated in vitro with IL-10 and IL-21 for 8 hrs (black line) or left untreated (shaded). (D) Activated P14 CD8+ T cells were transduced with retroviruses (RV) containing MigR1 empty vector or MigR1-SOCS3, stimulated with IL-12 (black line) or left untreated (dashed line), and then the amount of pSTAT4693 was measured by flow cytometry. (E) Histogram plot (left) shows the levels of pSTAT4693 in day 7 P14 Stat3+/+ (shaded) and Stat3−/− (dashed line) effector CD8+ T cells after IL-12 (10ng ml−1) stimulation. Grey line shows untreated control cells. Line graph (right) shows MFI of pSTAT4693 (normalized to unstimulated controls) in day 7 P14 Stat3+/+ (squares) and Stat3−/− (open circles) CD8+ T cells stimulated over a range of IL-12 concentrations. (F) Plots show the amount of pSTAT4693 directly ex vivo in P14 Stat3+/+ (shaded) and Stat3−/− (dashed line) LCMV-specific memory CD8+ T cells one day after infection with Listeria monocytogenes. (G) Activated P14 CD8+ T cells were transduced with control RV (MigR1) or a SOCS3 shRNAi RV and adoptively transferred into C57BL/6 mice that were subsequently infected with LCMV. Contour plots show expression of KLRG1 and IL-7R in the RV transduced cells at day 30 p.i. Data shown are representative of at least three independent experiments.
To further evaluate the effects of SOCS3 in preserving memory CD8+ T cell fates, we knocked down (KD) SOCS3 in LCMV-specific CD8+ T cells using shRNAi RV transduction of P14 CD8+ T cells (Supplementary Fig. 5C). Although SOCS3 KD had little impact on effector CD8+ T cell differentiation and function (Supplementary Fig. 5D-F), it impaired the maintenance of KLRG1lo IL-7Rhi memory CD8+ T cells (Fig. 7G). However, the SOCS3 KD effect was not as robust as STAT3-deficiency suggesting that other genes downstream of STAT3 are involved in this process. Taken together, these data support a model that STAT3 serves two major roles in virus-specific CD8+ T cells. One is to sustain expression of transcription factors that enhance formation of long-lived, self-renewing memory CD8+ T cells (such as BCL-6 and Eomes) and the other is to “insulate” virus-specific CD8+ T cells, via SOCS3, from inflammatory signals (e.g. IL-12) that drive TE differentiation, thereby, preserving memory cell potential in a proportion of the surviving cells.
DISCUSSION
Following acute viral or bacterial infection, a subset of memory precursor cells arise from a heterogeneous effector cell pool, and these cells progressively differentiate into functionally mature memory T cells that are long-lived and possess increased capacity to proliferate to antigen and homeostatic cytokines (e.g., IL-15) (Cui and Kaech, 2010; Kaech et al., 2002; Kaech and Wherry, 2007). Heightened or prolonged antigenic stimulation, inflammation, IL-2 signaling and mTOR activity promote terminal differentiation of effector CD8+ T cells (Agarwal et al., 2009; Araki et al., 2009; Badovinac et al., 2005; Badovinac et al., 2004; Cui and Kaech, 2010; Joshi et al., 2007; Kalia et al., 2010; Kolumam et al., 2005; Nguyen et al., 2002; Pearce et al., 2009; Sarkar et al., 2008), whereas truncation of these signals at priming and effector phase of the immune response accelerates memory development (Badovinac et al., 2005; Badovinac et al., 2004; Cui et al., 2009; Kalia et al., 2010; Sarkar et al., 2008). Recently, increasing evidence also suggests that further signals are required during effector→ memory transition stage for memory cells to gradually mature (Kaech et al., 2002; Sun et al., 2004). In this study, we demonstrated that, together, IL-10 and IL-21 are critical cytokines for promoting and maintaining memory cell formation and maturation and that STAT3 is an integral mediator of those cytokines. In the absence of STAT3, KLRG1lo IL-7Rhi MPCs formed (albeit at a slightly reduced frequency), but this population did not preferentially populate the memory pool as normally observed. STAT3 enhanced survival of memory CD8+ T cells in a competitive setting and was necessary to sustain expression of transcriptional regulators, such as BCL-6 and Eomes, which helped MPCs persist and mature into self-renewing Tcm cells. We propose that the STAT3-dependent expression of SOCS3 acts as an “insulator” of memory cell potential to prevent virus-specific CD8+ T cells from responding to inflammatory cytokines (such as IL-12) present during infection or basally that induce TE differentiation. Together, these data create a model wherein the induction and maintenance of memory CD8+ T cell fates is an active, rather than default, process that is dependent on cytokines that sustain expression of “pro-memory” transcription factors as well as proteins that shield T cells from responding to effector-inducing inflammatory cytokines in the environment.
Consistent with our data, human studies have found that memory T cells from patients with autosomal dominant hyper IgE syndrome (AD-HIES), whom carry dominant negative STAT3 alleles, share several of the phenotypic abnormalities described here in murine memory CD8+ T cells. AD-HIES patients have reduced numbers of memory T cells, especially Tcm cells, and T cells from AD-HIES patients are impaired from adopting Tcm cell traits when stimulated in vitro. The memory CD8+ T cells also show reduced BCL6, PRDM1 and SOCS3 expression. Most importantly, many AD-HIES patients are unable to control latent Epstein-Barr Virus (EBV) and Varicella Zoster Virus (VZV) infection and have considerably elevated EBV viral titers compared to healthy subjects (Siegel et al., co-submitted manuscript). Thus, STAT3 is an important regulator of memory T cells in both mouse and man.
Interestingly, although STAT3-deficient virus-specific CD8+ T cells fail to form functional memory cells, these KLRG1hi IL-7Rlo TE-like cells could persist for several months. This was unexpected given that cells with this phenotype tend to be shorter-lived (Joshi et al., 2007). However, our data show that one feature of Stat3−/− memory cells that differs from early effector TE cells is BCL-2 expression; BCL-2 accumulated in Stat3−/− memory cells normally following viral clearance (Kaech et al., 2003; Tripathi et al. 2010). KLRG1hi IL-7Rlo TE cells depend on IL-15 and there was also no defect in IL-2/15 receptor β chain expression in Stat3−/− memory cells. Lastly, gene expression profiling revealed that Stat3−/− CD8+ T cells expressed higher than normal amounts of Spi2A, another prosurvival factor for memory CD8+ T cells (Liu et al., 2004). Thus, it is possible that these factors combined account for the persistence of Stat3−/− memory CD8+ T cells.
An important finding in this study is that IL-10 and IL-21 cooperate to induce memory CD8+ T cell fates and promote their maturation during LCMV infection. Prior work has implicated IL-10 and IL-21 in memory CD8+ T cell differentiation and in agreement with recent reports, we found Il21−/− mice had slightly reduced polyfunctional Tcm cell formation during LCMV infection (Yi et al. 2010). However, IL-21 may have more profound roles in the expansion and survival of effector CD8+ T cells and persistence of memory cells in other types of infections such as vaccinia virus and adenovirus (Barker et al., 2010; Novy et al. 2011). Although it remains to be investigated more rigorously, CD4+ T cells are likely the physiological cell source for IL-21 (Spolski and Leonard, 2008a); thus, IL-21 production represents a key aspect of CD4+ T cell help provided to memory CD8+ T cells during infection. However, it will be important to determine if CD4+ T cells secrete IL-21 in a trophic manner to “help” CD8+ T cells or if it is delivered to CD8+ T cells by particular types of CD4+ T cells during immune responses.
IL-10 is traditionally thought of as an “anti-inflammatory” cytokine and therefore its role in promoting memory CD8+ T cell development may seem counterintuitive. Although IL-10-deficiency alone does not strongly perturb LCMV-specific CD8 T cell responses during acute infection (Brooks et al., 2010; Maris et al., 2007), IL-10 does support memory CD8+ T cell development during Listeria infection because fewer antigen-specific memory CD8+ T cells formed in Il10−/− mice compared to wild type controls (Foulds et al., 2006). However, another study analyzed the responses of Il10rb−/− CD8+ T cells during Listeria infection and their results suggested that IL-10 acts directly to suppress antigen-specific CD8+ T cell clonal expansion (Biswas et al., 2007). In the absence of IL-10R signaling greater numbers of memory CD8+ T cells formed as a result of the increased expansion (Biswas et al., 2007). Synthesizing all the data, we postulate that IL-10 simultaneously acts in both a direct and indirect manner to promote memory CD8+ T cell development. On the one hand IL-10 may act directly on CD8+ T cells to repress proliferation and induce SOCS3 and other STAT3-dependent genes that “insulate” and promote development of memory CD8+ T cells and their precursors. On the other hand, IL-10 may also act indirectly as an anti-inflammatory cytokine to antagonize production of IL-12, Type I IFNs and other inflammatory cytokines that drive TE differentiation during infection. Thus, IL-10 likely serves a compound role to promote and preserve MPC fates, and an IL-10-deficiency may have a larger effect on memory CD8+ T cell development than specific deletion of IL-10R signaling in CD8+ T cells. Given that many cells can produce IL-10, it will be interesting to determine the pertinent cell types involved in this process. In addition, although it remains to be determined more precisely how the STAT3 transcriptional activity is differentially regulated in MPC vs. TE or TCM vs. TEM cells, it does not likely stem from asymmetric expression of STAT3 or IL-10 and IL-21 receptors because all the subsets phosphorylated STAT3 fairly equivalently following stimulation with these two cytokines (data not shown). Lastly, it remains unclear if IL-10 and IL-21 function individually at different phases of the immune response or in an overlapping fashion to preserve memory potential and promote memory maturation. It will be important to further dissect if IL-10/IL-21/STAT3 signaling cascade is required to induce or maintain MPC fates, or both.
Our data show that STAT3 is a key integrator of multiple signal inputs and outputs to coordinate effector and memory differentiation. Two important signal outputs of STAT3 in CD8+ T cells are Eomes and BCL-6 that, interestingly, both function to enhance survival of IL-7Rhi cells and development of TCM cells (Banerjee et al., 2010; Ichii et al., 2007; Ichii et al., 2004). Recent data showed that Eomes could be regulated by TCF-1 and Wnt signals (Zhou et al. 2010), and it remains to be determined if the reduction of Eomes expression observed in Stat3−/− CD8+ T cells similarly stems from decreased Tcf1 expression or if the Wnt-TCF-1 and IL-21-IL-10-STAT3 function as parallel pathways for regulating Eomes expression. Although the T-box factors, Eomes and T-bet coordinate to regulate CTL differentiation (Intlekofer et al., 2008; Pipkin et al. 2010), they are also expressed in a reciprocal manner to a certain degree with KLRG1lo IL-7Rhi effector cells and Tcm cell expressing more Eomes than KLRG1hi IL-7Rlo and Tem cells (Banerjee et al., 2010; Intlekofer et al., 2007). Thus, the ratio of these transcription factors in CD8+ T cells appears to be critical determinants of their long-term fates. Similarly, in extension to our prior study (Rutishauser et al., 2009), this report shows that BCL-6 and Blimp-1, two additional STAT3 target genes (Calame, 2008; Diehl et al., 2008; Ozaki et al., 2004; Spolski and Leonard, 2008b), function in a reciprocal manner in CD8+ T cells; Blimp-1 promotes TE differentiation and BCL-6 promotes MPC and TCM formation. We also show that BCL-6 expression increases overtime as memory CD8+ T cells mature and TCM cells accumulate, and we postulate that this relies on sustained IL-21-STAT3 signaling after viral infection. Sustained Blimp-1 expression in memory CD8+ T cells was also dependent on Stat3 and this was somewhat surprising given that Stat3−/− CD8+ T cells maintained several TE-like attributes. Several possible explanations may account for this result, for instance, it is possible that Blimp-1 predominantly acts during effector CD8+ T cell differentiation to induce TE-fates, but is dispensable at later times. Alternatively, despite the reduced expression of Blimp-1 and BCL-6 in Stat3−/− cells, possibly, the overall ratio of the two factors remains unaffected such that TE-fates dominate over time. It will be interesting to determine if the STAT3-dependent cytokines that function during the effector-to-memory transition to sustain Blimp-1 and BCL-6 expression in virus-specific CD8+ T cells are one in the same or different. IL-21 is a likely candidate since it induces the expression of both transcription factors in B cells (Calame, 2008; Diehl et al., 2008; Ozaki et al., 2004; Spolski and Leonard, 2008b), and if true, this would suggest that the same cytokine could simultaneously promote divergent cell fates in a “context-dependent manner” that would be determined by additional STAT3 co-factors in the MPC and TE subsets.
SOCS3 is an important target of STAT3 in CD8+ T cells and we propose that SOCS3 acts as an insulator to shield MPCs from cytokine signals that drive TE-fates. Interestingly, like SOCS3, expression of SOCS1 is also increased in MPCs relative to TE cells (data not shown). Possibly, additional suppressors of cytokine signaling are involved in memory cell fate specification and maintenance. IL-21-STAT3 signaling pathway can also induce SOCS1 expression in T cells (Palmer and Restifo, 2009), and this may explain the partial effect of SOCS3 KD on KLRG1lo IL-7Rhi TCM differentiation. Altogether, the identification of the IL-10-IL-21-STAT3 pathway as a pivotal regulator of memory CD8+ T cell development provides important mechanistic insight that could lead to novel immunotherapies and enhance vaccine development.
EXPERIMENTAL PROCEDURES
Mice
Stat3flox/flox mice were originally generated by Dr. Xin-Yuan Fu (Welte et al., 2003) (Indiana University School of Medicine, Indianapolis, IN), and obtained from Dr. Patty Lee (Yale University School of Medicine, New Haven, CT). Granzyme B-Cre (Gzm-Cre+) mice were kindly provided by Dr. Joshy Jacobs (Emory University, Atlanta, GA) via Dr. Richard Flavell’s lab (Yale University School of Medicine, New Haven, CT), and were crossed to Stat3flox/flox mice to generate GzB-cre+; Stat3flox/flox (Stat3−/−) mice and GzB-cre+; Stat3+/+ or GzB-cre−; Stat3flox/flox (Stat3+/+) mice. Stat3−/− and Stat3+/+ mice were further crossed to P14 TCR transgenic (tg) mice to create P14 Stat3−/− and P14 Stat3+/+ mice. Cd8a−/− and Il21−/− mice mice were purchased from Jackson Laboratories (Bar Harbor, ME) and Taconic (Hudson, NY) respectively. Bcl6−/− mice were kindly provided by Dr. Alexander Dent (Indiana University School of Medicine, Indianapolis, IN). All animal experiments were done with approved Institutional Animal Care and Use Committee protocols.
Infections and Treatments
For infections of mice, 2 × 105 PFU of LCMV Armstrong strain were administered intraperitoneally (i.p.). For recall experiments, mice were infected with 2 × 106 PFU of LCMV clone 13 strain intravenously (i.v.). For BrdU labeling, BrdU (1mg mL−1) was administered in the drinking water daily for at least 10 days. For IL-10 blockade, αIL-10 mAb antibody (JES5-2A5 clone, kindly provided by Dr. Joke M. M. den Haan, UV University Medical Center, Amsterdam, Netherland) was administered 0.5 mg ml−1 i.p. every other day for indicated period of time according to the experiments.
Bcl6−/− mixed bone marrow chimeras
To generate mixed bone marrow chimeras, bone marrow from Cd8+α −/− animals was mixed in a 90:10 ratio with bone marrow from either Bcl6+/+ or Bcl6−/− and used to reconstitute lethally irradiated C57/B6 recipient mice. Two months later, these mice were infected with LCMV, and lymphocytes were isolated from tissues as described previously (Rutishauser et al., 2009).
Retroviral Transduction
P14 Stat3−/− mice were directly infected with 2×106 PFU LCMV-Armstrong i.v. and one day later, P14 Stat3−/− splenocytes were spin-transduced for 90 min at 37°C with fresh viral supernatants from 293T cells (transfected with Eco-helper and either MigR1 control, MigR1-SOCS3 or MSCV-SOCS3-shRNAi plasmids in the presence of 8 μg mL−1 polybrene and 10 ng mL−1 IL-2) and then 0.5×106 splenocytes were transferred i.v. to recipient mice that were subsequently infected with LCMV.
Surface and intracellular staining and antibodies
All antibodies were purchased from ebiosciences (San Diego, CA) and BD Biosciences (San Jose, CA), except anti-Granzyme B PE (Caltag, Burlingame, CA), anti-BCL-6 PE (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-KLRG1 (2F1, was kindly provided by Dr. David Raulet, University of California, Berkeley, CA). Class I MHC tetramers were generated as described previously (Kaech et al., 2003). All flow cytometry was analyzed on a FACSCalibur or LSRII (BD) with FlowJo software (Treestar, San Carlos, CA).
pSTAT4693 Staining
For in vitro assays, P14 effector CD8+ T cells were harvested and incubated with IL-12 in vitro with culture media for 30 minutes. Cells were fixed with cytofix and permeabilized with 90% methanol, then stained intracellularly with pSTAT4693 antibody. For in vivo assays, we first infected mice containing 5×104 naïve P14 Stat3+/+ and Stat3−/− CD8+ T cells with LCMV and waited 40 days until memory P14 cells were formed. We then infected these immune mice with 2 × 104 CFU Listeria monocytogenes i.v. One day later, splenocytes were harvested and made cell suspension directly in cytofix, then stained with pSTAT4693 antibody as described above.
Western blot analysis
Protein lysates from 1 × 106 day 8 LCMV-specific total (CD8+CD44+ CD62L−), KLRG1hi IL-7Rlo or KLRG1lo IL-7Rhi sorted effector CD8+ T cells were lysed and resolved by SDS-PAGE. Blimp-1 (Santa Cruz Biotechnology, Santa Cruz, CA), SOCS3 (Cell Signaling Technology, Danvers, MA), Actin (Sigma-Aldrich, St. Louis, MO) and GRP94 (Enzo Life Sciences, Plymouth Meeting, PA) were detected by Western Blotting. The abundance of each protein expression was measured by densitometry using ImageJ 1.43u software (NIH).
Statistical Analyses
Where indicated, p values were determined by a two-tailed unpaired Student’s t test. p values < 0.05 were considered significant. All graphs show averages of the mean ± SEM.
Supplementary Material
Acknowledgments
We thank the members of the Kaech laboratory, Drs. Nikhil Joshi and Timothy Hand for helpful comments and suggestions. We also thank Drs. Xin-Yuan Fu and Alexander Dent for providing us with the Stat3f/f and Bcl6−/− mice, respectively, and Dr. Joke M. M. den Haan the kind gift of αIL-10 mAb. This work was supported by grants from the NIH (RO1AI066232 (SMK), R21AI081150 (SMK), R01AR40072 (JC), AR44076 (JC), AI075157 (JC)), the Arthritis Foundation (JSW) and the Howard Hughes Medical Institute (SMK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFENRENCES
- Agarwal P, Raghavan A, Nandiwada SL, Curtsinger JM, Bohjanen PR, Mueller DL, Mescher MF. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J Immunol. 2009;183:1695–1704. doi: 10.4049/jimmunol.0900592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nature medicine. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nature immunology. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol. 2010;185:4988–4992. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker BR, Gladstone MN, Gillard GO, Panas MW, Letvin NL. Critical role for IL-21 in both primary and memory anti-viral CD8+ T-cell responses. European journal of immunology. 2010;40:3085–3096. doi: 10.1002/eji.200939939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas PS, Pedicord V, Ploss A, Menet E, Leiner I, Pamer EG. Pathogen-specific CD8 T cell responses are directly inhibited by IL-10. J Immunol. 2007;179:4520–4528. doi: 10.4049/jimmunol.179.7.4520. [DOI] [PubMed] [Google Scholar]
- Brooks DG, Walsh KB, Elsaesser H, Oldstone MB. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3018–3023. doi: 10.1073/pnas.0914500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calame K. Activation-dependent induction of Blimp-1. Current opinion in immunology. 2008;20:259–264. doi: 10.1016/j.coi.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Castellino F, Germain RN. Chemokine-guided CD4+ T cell help enhances generation of IL-6RalphahighIL-7Ralpha high prememory CD8+ T cells. J Immunol. 2007;178:778–787. doi: 10.4049/jimmunol.178.2.778. [DOI] [PubMed] [Google Scholar]
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science (New York, NY) 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Joshi NS, Jiang A, Kaech SM. Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177–2187. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Kaech SM. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunological reviews. 2010;236:151–166. doi: 10.1111/j.1600-065X.2010.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl SA, Schmidlin H, Nagasawa M, van Haren SD, Kwakkenbos MJ, Yasuda E, Beaumont T, Scheeren FA, Spits H. STAT3-mediated up-regulation of BLIMP1 Is coordinated with BCL6 down-regulation to control human plasma cell differentiation. J Immunol. 2008;180:4805–4815. doi: 10.4049/jimmunol.180.7.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egwuagu CE, Yu CR, Zhang M, Mahdi RM, Kim SJ, Gery I. Suppressors of cytokine signaling proteins are differentially expressed in Th1 and Th2 cells: implications for Th cell lineage commitment and maintenance. J Immunol. 2002;168:3181–3187. doi: 10.4049/jimmunol.168.7.3181. [DOI] [PubMed] [Google Scholar]
- Foulds KE, Rotte MJ, Seder RA. IL-10 is required for optimal CD8 T cell memory following Listeria monocytogenes infection. J Immunol. 2006;177:2565–2574. doi: 10.4049/jimmunol.177.4.2565. [DOI] [PubMed] [Google Scholar]
- Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichii H, Sakamoto A, Arima M, Hatano M, Kuroda Y, Tokuhisa T. Bcl6 is essential for the generation of long-term memory CD4+ T cells. International immunology. 2007;19:427–433. doi: 10.1093/intimm/dxm007. [DOI] [PubMed] [Google Scholar]
- Ichii H, Sakamoto A, Kuroda Y, Tokuhisa T. Bcl6 acts as an amplifier for the generation and proliferative capacity of central memory CD8+ T cells. J Immunol. 2004;173:883–891. doi: 10.4049/jimmunol.173.2.883. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science (New York, NY) 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. The Journal of experimental medicine. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nature immunology. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Jacob J, Baltimore D. Modelling T-cell memory by genetic marking of memory T cells in vivo. Nature. 1999;399:593–597. doi: 10.1038/21208. [DOI] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature immunology. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. The Journal of experimental medicine. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Phillips T, Zhang M, Wang Y, Opferman JT, Shah R, Ashton-Rickardt PG. Serine protease inhibitor 2A is a protective factor for memory T cell development. Nature immunology. 2004;5:919–926. doi: 10.1038/ni1107. [DOI] [PubMed] [Google Scholar]
- Maris CH, Chappell CP, Jacob J. Interleukin-10 plays an early role in generating virus-specific T cell anergy. BMC immunology. 2007;8:8. doi: 10.1186/1471-2172-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, Yokota T. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. The EMBO journal. 1999;18:4261–4269. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KB, Watford WT, Salomon R, Hofmann SR, Pien GC, Morinobu A, Gadina M, O’Shea JJ, Biron CA. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science (New York, NY) 2002;297:2063–2066. doi: 10.1126/science.1074900. [DOI] [PubMed] [Google Scholar]
- Novy P, Huang X, Leonard WJ, Yang Y. Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection. J Immunol. 2011;186:2729–2738. doi: 10.4049/jimmunol.1003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea JJ, Lahesmaa R, Vahedi G, Laurence A, Kanno Y. Genomic views of STAT function in CD4(+) T helper cell differentiation. Nature reviews. 2011;11:239–250. doi: 10.1038/nri2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- Palmer DC, Restifo NP. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends in immunology. 2009;30:592–602. doi: 10.1016/j.it.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2011;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. The Journal of experimental medicine. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nature reviews. 2003;3:900–911. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annual review of immunology. 2008a;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- Spolski R, Leonard WJ. The Yin and Yang of interleukin-21 in allergy, autoimmunity and cancer. Current opinion in immunology. 2008b;20:295–301. doi: 10.1016/j.coi.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stritesky GL, Muthukrishnan R, Sehra S, Goswami R, Pham D, Travers J, Nguyen ET, Levy DE, Kaplan MH. The transcription factor STAT3 is required for T helper 2 cell development. Immunity. 2011;34:39–49. doi: 10.1016/j.immuni.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nature immunology. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi P, Kurtulus S, Wojciechowski S, Sholl A, Hoebe K, Morris SC, Finkelman FD, Grimes HL, Hildeman DA. STAT5 is critical to maintain effector CD8+ T cell responses. J Immunol. 2010;185:2116–2124. doi: 10.4049/jimmunol.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte T, Zhang SS, Wang T, Zhang Z, Hesslein DG, Yin Z, Kano A, Iwamoto Y, Li E, Craft JE, et al. STAT3 deletion during hematopoiesis causes Crohn’s disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1879–1884. doi: 10.1073/pnas.0237137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. The Journal of biological chemistry. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- Yi JS, Ingram JT, Zajac AJ. IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection. J Immunol. 2010;185:4835–4845. doi: 10.4049/jimmunol.1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nature reviews. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.