On the basis of an RNA interference screen, we identify AKT1 and AKT2 as inhibitors of β1-integrin activity and invasion in prostate cancer. AKT1 siRNA induces β-integrin activity and up-regulation of RTKs known to function in cooperation with integrins. In contrast, AKT2 siRNA up-regulates microRNA-200, which increases integrin activity.
Abstract
AKT1 and AKT2 kinases have been shown to play opposite roles in breast cancer migration and invasion. In this study, an RNA interference screen for integrin activity inhibitors identified AKT1 as an inhibitor of β1-integrin activity in prostate cancer. Validation experiments investigating all three AKT isoforms demonstrated that, unlike in breast cancer, both AKT1 and AKT2 function as negative regulators of cell migration and invasion in PC3 prostate cancer cells. Down-regulation of AKT1 and AKT2, but not AKT3, induced activation of cell surface β1-integrins and enhanced adhesion, migration, and invasion. Silencing of AKT1 and AKT2 also resulted in increased focal adhesion size. Importantly, the mechanisms involved in integrin activity regulation were distinct for the two AKT isoforms. Silencing of AKT1 relieved feedback suppression of the expression and activity of several receptor tyrosine kinases, including EGFR and MET, with established cross-talk with β1-integrins. Silencing of AKT2, on the other hand, induced up-regulation of the microRNA-200 (miR-200) family, and overexpression of miR-200 was sufficient to induce integrin activity and cell migration in PC3 cells. Taken together, these data define an inhibitory role for both AKT1 and AKT2 in prostate cancer migration and invasion and highlight the cell type–specific actions of AKT kinases in the regulation of cell motility.
INTRODUCTION
Unlike early-stage, localized prostate cancer, castration-resistant metastatic prostate cancer is incurable. Pathways involved in the regulation of prostate cancer adhesion and migration are therefore central to prostate cancer mortality. Activation of the phosphatidylinositol 3′ kinase (PI3K) pathway, due to loss of the phosphatase and tensin homologue (PTEN) tumor suppressor gene, is one of the predominant genetic and cellular changes in human prostate cancer (Majumder and Sellers, 2005). Protein kinase B (PKB/AKT) is the primary downstream mediator of PI3K signaling, and it influences numerous cellular processes, including survival, proliferation, metabolism, and migration (Manning and Cantley, 2007). The AKT family of kinases includes three members—AKT1, AKT2 and AKT3—that share a high degree of homology. AKT1 and AKT2 are broadly expressed in most tissues, whereas AKT3 has a more limited expression pattern (Yang et al., 2003).
Several studies have investigated the specific roles of individual AKT family members in cell migration and invasion in breast and ovarian cancer, but such studies are lacking in prostate cancer. Overexpression of PI3K induces an invasive phenotype in breast and ovarian cancer cells, and similar effects are observed in cells overexpressing AKT2, but not AKT1 or AKT3, in vitro and in vivo (Arboleda et al., 2003). Subsequently, AKT1 has been found to function as an inhibitor of migration and invasion in breast (Irie et al., 2005; Yoeli-Lerner et al., 2005) and ovarian cancer (Meng et al., 2006). Silencing of AKT1 increases growth factor–induced migration of MCF10A breast epithelial cells dramatically, whereas inhibition of AKT2 has no effect (Irie et al., 2005). In line with these data, overexpression of AKT1 in breast cancer cell lines inhibits cell motility and invasion (Yoeli-Lerner et al., 2005). Thus the distinct roles for AKT1 as a migration inhibitor and AKT2 as a promigratory kinase are well defined in breast and ovarian cancer. These data have been validated in transgenic mouse models, in which AKT1 was shown to be critical for breast cancer induction and AKT2 for metastatic dissemination (Hutchinson et al., 2004; Maroulakou et al., 2007; Dillon et al., 2009).
Recently interesting molecular details have been identified regarding the opposing functions of the highly homologous AKT family members in cell migration. The relative expression levels of AKT1 and AKT2 correlate with the expression levels of microRNA miR-200 family members and epithelial–mesenchymal transition (EMT) of MCF10A cells (Iliopoulos et al., 2009). In addition, unexpected substrate specificity may explain the distinction between AKT1 and AKT2 in breast cancer. The actin-bundling protein palladin is a specific AKT1 substrate required for AKT1-mediated inhibition of breast cancer migration (Chin and Toker, 2010).
Integrins are a family of adhesion receptors implicated in many important cellular processes, including cancer progression and invasion. Integrins are abundantly expressed on the surface of all cell types, except erythrocytes. Their function in cells is regulated on the level of expression but also very importantly via modulation of integrin activity (i.e., affinity toward ligands; Gahmberg et al., 2009; Shattil et al., 2010; Kim et al., 2011; Regent et al., 2011). The most abundant matrix-binding integrins are heterodimers of a specific α subunit and the common β1 subunit. Changes in the levels or activity of the β1 subunit are therefore likely to reflect the overall adhesive status of cells (Regent et al., 2011). In breast and ovarian cancer cells, AKT2-induced migration and invasion is linked to the up-regulation of β1-integrin expression (Arboleda et al., 2003). Recently talin-induced increase of integrin activity was shown to stimulate focal adhesion kinase signaling and invasion in prostate cancer cells (Sakamoto et al., 2010). However, the contribution of AKT isoforms in the regulation of integrin activity has not been clearly elucidated.
In this study, we investigated the isoform-specific functions of AKT in prostate cancer and show that, unlike in breast cancer, both AKT1 and AKT2 function as negative regulators of cell migration and invasion in prostate cancer cells. We provide multiple lines of evidence to show that AKT1 and AKT2 both function as inhibitors of β1-integrin activity, migration, and invasion. Our results demonstrate a link between integrin activity and AKT and describe AKT1-mediated down-regulation of receptor tyrosine kinase (RTK) levels and AKT2-induced suppression of miR-200 family as pathways correlating with reduced integrin activity and the antimigratory effects of AKT1 and AKT2 in prostate cancer.
RESULTS
AKT inhibition augments β1-integrin activity in PC3 cells
Inhibitors of the PI3K pathway are undergoing clinical evaluation in prostate cancer (Amato et al., 2008). However, their efficacy in the clinical trials has been limited (Sawyers, 2003; Guertin and Sabatini, 2009). This is most likely due to the complex outcome of inhibition of this pathway. In breast cancer, AKT1 has a negative regulatory role on migration and AKT2 has a positive regulatory role on migration (Irie et al., 2005; Dillon and Muller, 2010). We recently reported a cell-spot microarray (CSMA) RNA interference (RNAi) screen for β1-integrin activity regulators in 12 cell lines by using Matrigel-spot–embedded small interfering RNA (siRNA) oligos to silence target genes (Pellinen et al., 2012). In this study, we focused our analysis specifically on the prostate cancer cell lines and noted that among the siRNAs that up-regulated β1-integrin activity (Supplemental Table S1), AKT1 was a strong hit. AKT1 silencing induced integrin activity in 7/8 of the cell lines studied (with either one or both of the reporter antibodies, z value > +1.0), such that only in the primary prostate stromal cells was β1-integrin activity not influenced by AKT1 siRNAs (Figure 1A; efficiency of the AKT1 siRNA used in the screen are shown in Supplemental Figure S1). This indicates that AKT1 functions as a negative regulator of β1-integrin activity in both androgen-sensitive (VCaP, MDAPCA2a, 22RV1, RWPE1) and androgen-insensitive (PC3, ALVA31) prostate cancer cell lines, as well as in primary prostate epithelial cells. This was also evident in the micrographs taken from PC3 cells growing on control or AKT1 siRNA-containing array spots (Figure 1B). This is interesting because AKT1 function has not been directly linked to regulation of integrin activity, and the possible role of AKT1 in prostate cancer cell migration remains poorly studied.
FIGURE 1:
AKT1 is an inhibitor of β1-integrin activity in several different prostate cell lines. (A) The number of individual AKT1 siRNAs (y-axis) affecting β1-integrin activity in different prostate cell lines with z scores > +1 (the siRNA numbers with average siRNA z scores [n = 2] are indicated below the columns). (B) Representative images of AKT1- and control-silenced PC3 cells from array spots stained as indicated. Scale bar: 10 μM.
To investigate the role of AKT kinases in integrin regulation in more detail, we chose PC3 cells for further studies, as this cell line is highly migratory and invasive (Rantala et al., 2011; Pellinen et al., 2012). Western blot analysis revealed that PC3 cells express all three AKT isoforms (Figure 2A). First, we treated PC3 cells with a pan-AKT inhibitor, triciribine (AKTi), to achieve efficient inhibition of all AKT isoforms. Long-term treatment (20 h) with the inhibitor significantly reduced the levels of phosphorylated AKT (Thr-308 and Ser-473 sites) as well as all the AKT isoforms in the cells (Figure 2A). It was unlikely that this was due to an overall reduction in protein levels, as β1-integrin expression remained unaltered compared with untreated cells (Figure 2A). Furthermore, the number of live cells was not significantly reduced by the inhibitor (12% reduction; n.s.; Figure 2B).
FIGURE 2:
Inhibition of AKT kinases increases integrin activity and adhesion in PC3 cells. (A) Western blot analysis of lysates from AKTi-treated (10 μM for 20 h) PC3 cells with the indicated antibodies. Shown are representative blots of three independent experiments. Numbers below the bands indicate fold change of protein level normalized against tubulin and compared with DMSO control cells. (B) Proliferation of DMSO- or AKTi-treated PC3 cells was analyzed by using WST-1 reagent (mean ± SEM). The data are from a representative experiment of three. (C) FACS analysis of cell surface β1-integrin from DMSO- or AKTi-treated PC3 cells stained with 12G10 and total β1-integrin antibody (K20) (mean fluorescence intensity relative to DMSO cells ± SEM, n = 3; *, p < 0.05). (D) Adhesion (30 min) of DMSO- or AKTi-treated PC3 cells was analyzed in wells coated with different concentrations of collagen. Adherent cells were detected with crystal violet, and absorbance was measured at 620 nm (mean ± SEM, four technical replicates/experiment; *, p < 0.05, **, p < 0.005). The experiment was repeated twice with similar results.
Conformation-specific monoclonal antibodies and labeled ligands, such as fibronectin, are widely used as reporters for β1-integrin activity (Byron et al., 2009; Rantala et al., 2011). To investigate the effect of pan-AKT inhibition on β1-integrin activity in PC3 cells, we used 12G10, an antibody specifically recognizing active β1-integrin or the pan-β1 antibody K20. Staining of cell surface integrins followed by fluorescence-activated cell-sorting flow cytometry (FACS) demonstrated that AKTi-treated cells expressed significantly more active β1-integrin on the cell surface compared with untreated or dimethyl sulfoxide (DMSO)-treated cells (Figure 2C). This effect was specifically due to induction of the active conformation of the receptor, since total cell surface β1-integrin levels (K20 staining) remained unaltered (Figure 2C). In line with the increased integrin activity, AKTi-treated cells displayed significantly enhanced adhesion to collagen over a wide range of ligand concentrations (Figure 2D). Taken together, these data suggest that signaling mediated through AKT kinases negatively influences the activity and ligand binding of β1-integrins in PC3 cells.
AKT1 and AKT2 inhibit β1-integrin activity
We next investigated the relative contributions of the individual AKT isoforms on the regulation of integrin activity. siRNA-mediated silencing efficiently and specifically reduced the expression of each isoform (Figure 3A) without significant effects on PC3 cell viability (Figure 3B). Staining of cell surface β1-integrins in the silenced cells showed that AKT1 siRNA and AKT2 siRNA increased integrin activity by 1.4-fold and by 1.6-fold, respectively, whereas AKT3 siRNA had no significant effect on β1-integrin activity in the cells on plastic (Figure 3C). No difference was observed in the total cell surface β1-integrin expression (Figure 3C). These data were further validated by analyzing the binding of a labeled fibronectin fragment to the silenced cells using FACS (Figure S2A) and with additional siRNA oligos targeting AKT1 or AKT2 (Figure S2, B and C), demonstrating that the effect of AKT1 or AKT2 silencing on β1-integrin activity was specifically due to loss of expression of the kinase, rather than off-target effects.
FIGURE 3:
Silencing of AKT1 and AKT2 isoforms enhances cell surface and total levels of β1-integrin activity. (A) Western blot analysis of AKT-silenced PC3 cells. Cells were treated with the indicated siRNAs for 72 h and blotted with AKT isoform–specific antibodies or tubulin to control for equal loading. Representative blots from three independent experiments with similar results are shown. Numbers below the bands indicate fold change of protein level normalized against tubulin and compared with control siRNA–transfected cells. (B) Proliferation of AKT-silenced PC3 cells was analyzed using WST-1 reagent (mean ± SEM). The data are from a representative experiment of three. (C) FACS analysis of cell surface β1-integrin from control or AKT isoform–specific siRNA-treated PC3 cells stained with 12G10 and total β1-integrin antibody (K20) (mean fluorescence intensity relative to DMSO cells ± SEM; n = 3; *, p < 0.05, **, p < 0.005). Different numbers after the siRNAs indicate independent siRNA oligos. (D) Representative images from 12G10- and K20-stained, adherent, AKT-silenced cells stained as in (E). Scale bar: 10 μm. (E) ScanR microscopy analysis of levels of β1-integrin from adherent PC3 cells silenced as indicated. Adherent cells were fixed, permeabilized, and stained as indicated (>5000 cells/condition). Combined results from two individual experiments are shown (4 wells/experiment; the means, error bars, and p values are the averages of these eight replicas; mean fluorescence intensity relative to siRNA control cells: *, p < 0.05, **, p < 0.005).
Because PC3 cells have very rapid endosomal traffic of active β1-integrins from the cell surface (Arjonen et al., 2012), we tested the effects of AKT1 or AKT2 silencing to the total levels of active β1-integrin in cells. The analysis of β1epitope (12G10) staining from adherent siRNA-transfected cells following fixation and permeabilization also showed increased total levels of active β1-integrin in the cytoplasm (Figure 3D). ScanR automated microscope imaging and quantification of more than 5000 cells/transfection showed that AKT1 or AKT2 silencing also significantly increased the expression of 12G10 in adherent cells without influencing the total β1-integrin expression (K20; Figure 3E). Thus our data show that both AKT1 and AKT2 inhibit integrin activity in PC3 cells.
AKT1 and AKT2 regulate focal adhesions
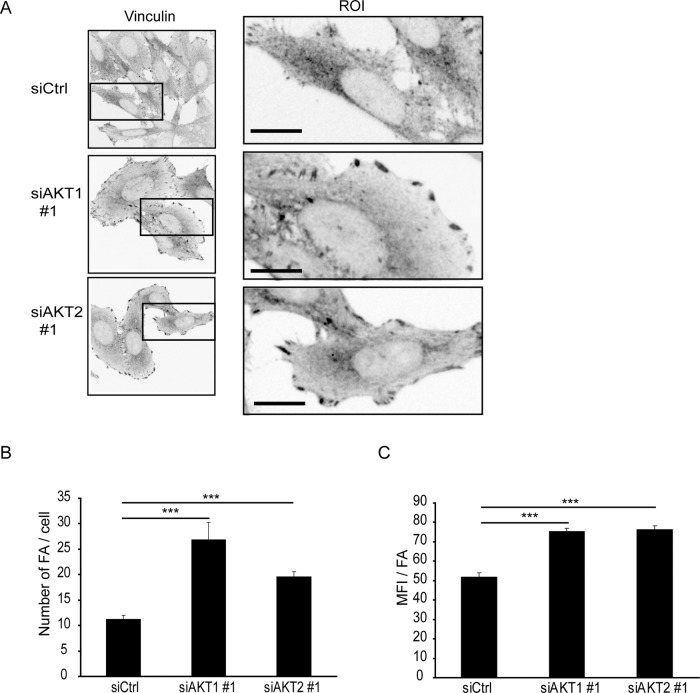
Integrin-mediated adhesion to matrix triggers the formation of focal adhesions. These are complex assemblies of adhesion receptors, signaling molecules, and scaffold proteins (e.g., vinculin) that link the extracellular matrix to the actin cytoskeleton (Zaidel-Bar et al., 2007; Kuo et al., 2011). The finding that AKT inhibition induced integrin-mediated adhesion in PC3 cells (Figure 2D), prompted us to investigate focal adhesions in AKT-silenced cells. In control siRNA–transfected PC3 cells, only a limited number of vinculin-positive clusters (clusters bigger than 50 pixel were scored as focal adhesions) were detected. However, silencing of AKT1 or AKT2 significantly increased the number of focal adhesions in PC3 cells. The AKT-silenced cells also appeared to spread more, and the focal adhesions were mainly detected at the cell periphery (Figure 4A). In addition to focal adhesion number (Figure 4B), also their size (based on vinculin fluorescence intensity) was significantly increased (Figure 4C).
FIGURE 4:
AKT1 and AKT2 regulate focal adhesions. (A) Confocal microscopy images from PC3 cells silenced with the indicated siRNAs. Cells were stained with vinculin antibody. Scale bar: 10 μm. (B) The number of vinculin-positive focal adhesions (FA) and (C) mean fluorescence intensity of vinculin per focal adhesion were analyzed with ImageJ (mean ± SEM; n = 3; 10 cells/transfection; ***, p < 0.001).
Thus AKT1 and AKT2 function as negative regulators of focal adhesions in PC3 cells.
AKT1 and AKT2 silencing induces migration and invasion
In PC3 cells, silencing of both AKT1 and AKT2 induced integrin activation. This encouraged us to investigate the effect of AKT silencing on cell migration in these cells using two different migration models. First, we performed time-lapse imaging of PC3 cells randomly migrating on plastic. Silencing of AKT1 induced a small but significant increase in the migration distance (path length), whereas the effect on persistence (distance to start and trajectories) was more evident (Figure 5, A–C). Silencing of AKT2 or AKT3 had no effect on migration distance on plastic, but interestingly, AKT3 silencing increased the persistence of PC3 cells on plastic (Figure S3, A and B). We then analyzed migration of the silenced cells on fibroblast-produced cell-derived matrix (CDM; Even-Ram and Yamada, 2005), in which the requirements for migration are more like those in a three-dimensional environment. Interestingly, the migration of PC3 cells along the matrix tracks (path length, distance to start, and trajectories) in these conditions was significantly induced by AKT2 silencing, whereas the increase in AKT1-silenced cells did not reach significance (p = 0.2), and AKT3 silencing had no significant effect (Figures 5, D–F, and S3, C and D).
FIGURE 5:
AKT kinases regulate prostate cancer cell motility. Migration of AKT-silenced PC3 cells on plastic or on CDM was followed by time-lapse imaging for 21 h at 20 min intervals. Quantification of path length (A and D) and distance to start (B and E) are shown (mean ± SEM; *, p < 0.05, **, p < 0.005; 46–62 cells were analyzed for (B) and 130 cells were analyzed for (E) from one representative experiment of three experiments). Representative cell tracks from cells silenced as indicated are shown (C and F).
Because increased migration on CDM often correlates with induced invasion (White et al., 2007; Caswell and Norman, 2008), we next evaluated the role of AKT1, AKT2, and AKT3 in a three-dimensional invasion assay. PC3 cells were transfected with the indicated siRNAs and plated on the bottom of Ibidi μ-slide wells (ibidi GmbH). Invasion through Matrigel toward increasing serum concentrations was monitored after 4 d. Confocal imaging of the invading cells revealed that silencing of AKT1 and AKT2 significantly induced PC3 cell invasion, but AKT3 silencing had no effect (Figure 6A). This was most likely due to the increased β1-integrin activity, since a function blocking anti–β1 antibody (Mab13) inhibited invasion of AKT1- and AKT2-silenced PC3 cells in Matrigel and reduced it to the levels of Mab13-treated control cells (Figure 6B). Therefore both AKT1 and AKT2 function as anti-invasive kinases in these prostate cancer cells.
FIGURE 6:
AKT kinases regulate prostate cancer cell invasion. (A) Invasion of AKT-silenced PC3 cells in Matrigel. siRNA-transfected cells were allowed to invade for 4 d and were then stained with Alexa Fluor 488 phalloidin. Cells were imaged with confocal microscopy. Side view (z-axis) of invading cells and x-y views of cells from the bottom (20 μm) and close to the top (invaded distance: 70 μm) of the Matrigel plug. Arrow indicates invasion direction. Analysis of invasion area was done with ImageJ (mean ± SEM; *, p < 0.05, **, p < 0.005; eight invasion areas were analyzed per experiment; n = 2). (B) Invasion of AKT-silenced and Mab13-treated PC3 cells (mean ± SEM; **, p < 0.005, ***, p < 0.0005; eight invasion areas were analyzed per experiment; n = 2).
AKT2 silencing induces miR-200, and miR-200a expression results in increased integrin activity and migration
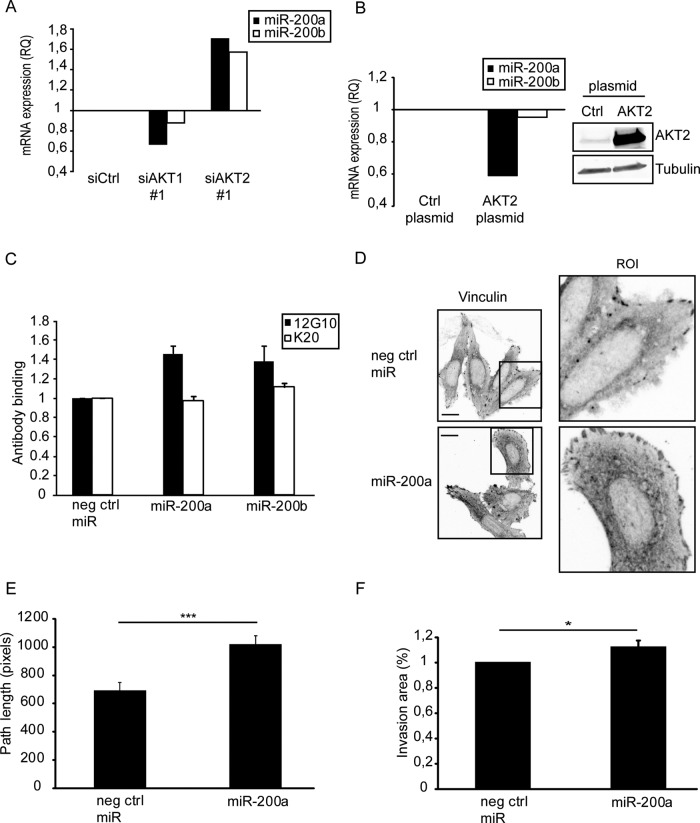
miRNAs are small (∼22 nucleotide) RNAs that regulate gene expression posttranscriptionally in a sequence-specific manner to influence cell differentiation, survival, and response to environmental cues (Bartel, 2004). Each miRNA may regulate the expression of many target genes. AKT isoforms were recently shown to differentially regulate the abundance of microRNA miR-200 family in breast epithelial cells, such that their levels are reduced in cells with activated AKT2 (Iliopoulos et al., 2009). This prompted us to analyze miR-200 family members 200a and 200b in prostate cancer cells. We investigated their levels by real-time reverse transcription PCR (qRT-PCR; Figure 7A). Silencing of AKT2 significantly induced both miR-200a and miR-200b microRNAs, whereas silencing of AKT1 resulted in modest reduction of miR-200 (Figure 7A). We further investigated the link between AKT2 and miR-200 by overexpressing AKT2 in PC3 cells. AKT2 reduced miR-200a levels but had no effect on miR-200b levels (Figure 7B).
FIGURE 7:
AKT1 and AKT2 kinases differ in their regulation of the levels of miR-200 family members. (A) qRT-PCR analysis of miR-200a and miR-200b levels from AKT1- and AKT2-silenced PC3 cells. RNA of siRNA-transfected PC3 cells was isolated and subjected to qRT-PCR analysis. (B) Western blot and qRT-PCR analysis of AKT2 protein levels and miR-200a and miR-200b levels from empty control plasmid or AKT2-transfected PC3 cells. (C) FACS analysis of cell surface β1-integrins from pre-miRNA–transfected PC3 cells stained with 12G10 and K20 (mean fluorescence intensity relative to control cells ± SEM; n = 2). (D) Confocal microscopy images from pre–miRNA-transfected PC3 cells. Cells were stained with vinculin antibody. Scale bar: 10 μm. (E) Migration of pre–miRNA-transfected PC3 cells was followed by time-lapse imaging for 21 h at 20 min intervals. Quantification of the path length (mean ± SEM; n = 65; ***, p < 0.001). (F) Invasion of pre–miRNA-transfected PC3 cells (mean ± SEM; *, p < 0.05; eight invasion areas were analyzed per experiment; n = 3).
While several studies have linked miR-200 to inhibition of EMT, in vivo breast cancer models have indicated that miR-200 enhances breast cancer cell metastasis (Dykxhoorn et al., 2009). In addition, invasive melanoma cells have been shown to increase levels of miR-200 (Elson-Schwab et al., 2010). Thus the effect of miR-200 on migration appears to be dependent on cell type and context. Because we found that AKT2 silencing increases integrin activity and migration, we wanted to test whether miR-200 expression in these cells would have the same effects. To test this, we transfected pre-miR200a or pre-miR200b to PC3 cells, and validated overexpression using qRT-PCR (unpublished data). FACS staining of cell surface integrins revealed up-regulation of active β1-integrin (but not total β1) in both miR-200a– and miR-200b–transfected cells (Figure 7C). In melanoma cells, miR-200 family member 200a results in an elongated and protrusive mode of invasion (Elson-Schwab et al., 2010), characteristic of integrin-dependent cell motility (Friedl and Wolf, 2003). In line with this, miRNA-200a induced the size of vinculin-positive focal adhesions (mean fluorescence intensity of vinculin per focal adhesion: 35%, p < 0.001, 10 cells) and there was a trend for increased the vinculin-positive focal adhesion number (mean fluorescence intensity of vinculin per focal adhesion: 28%, p = 0.06, 10 cells; Figure 7D), such that it reflected the effect of AKT2 silencing (Figure 4A). In line with these data, overexpression of miR-200a significantly increased PC3 cell migration as analyzed with time-lapse microscopy (Figure 7E) and also induced a modest but significant increase in invasion into Matrigel (Figure 7F). Thus, in PC3 cells, AKT2 silencing induces miR-200 family, and miR-200 overexpression increases integrin activity and migration.
AKT1 silencing up-regulates RTK expression and activity
Cross-talk with integrins and RTKs is well established. Several RTKs, including epidermal growth factor receptor (EGFR) and hepatocyle growth factor receptor (c-MET), are known to positively regulate integrins and, conversely, integrin-mediated adhesion has been linked to ligand-independent activation of RTKs (Moro et al., 1998; Mitra et al., 2010; Ivaska and Heino, 2011). Several oncogenes are known to trigger feedback-inhibitory loops in cells (Courtois-Cox et al., 2006), and AKT inhibition has been show to relieve feedback suppression of several RTKs in cancer cells (Chandarlapaty et al., 2011). Interestingly, we also observed that silencing of AKT1 induced an increase in the levels of some phosphorylated RTKs. We used an anti-phosphotyrosine receptor antibody array to compare levels of 42 active RTKs in AKT1- or AKT2-silenced and control-transfected PC3 cells. In PC3 cells, the most strongly phosphorylated RTKs were MET and EGFR (Figures 8A and S4A). On AKT1 silencing, the levels of both of these phosphorylated RTKs were increased by more than twofold compared with control siRNA–treated cells. In addition, up-regulation of several other less abundant phospho-RTKs was also observed in AKT1-silenced cells (Figure 8B). In contrast, AKT2 silencing did not induce up-regulation of these RTKs, apart from the strong up-regulation of platelet-derived growth factor α receptor (PDGFRα; Figure 8A). The up-regulation of EGFR and MET was confirmed with Western blotting of AKT1 siRNA and control siRNA–transfected PC3 cells (Figure S4B). Thus silencing of AKT1 in these PC3 cells correlates with up-regulation of a number of active RTKs to the increased integrin activity observed upon AKT1 silencing.
FIGURE 8:
Silencing of AKT1 increases activation and total levels of EGFR and c-Met. (A) Control and AKT1- and AKT2-silenced PC3 cell lysates were applied to phospho-RTK arrays. (B) Changes in RTK levels between control siRNA and siAKT1- and siAKT2-transfected cells were quantified and are indicated in the table. (C and D) AKT1, but not AKT2, expression anticorrelates with MET expression in prostate carcinoma samples (n = 208 patients) as analyzed by cDNA microarray expression analysis (Kilpinen et al., 2008). In contrast, both AKT1 and AKT2 expression highly correlate with MET expression in skin cancers (n = 147 patients). (E) Model for AKT-dependent regulation of β1-integrin activity in prostate cancer cells. Integrins and RTKs trigger signaling pathways that include activation of AKTs. Activated AKT1 signals via a negative feedback loop to suppress expression of RTKs (Chandarlapaty et al., 2011), as well as cell surface levels of active RTKs (Chandarlapaty et al., 2011) and β1-integrins (shown here). AKT2 signals to down-regulate miR-200a, which contributes to increased β1-integrin activity in PC3 cells via an unknown mechanism. Silencing of AKT1 or AKT2 (red crosses) induces integrin activity via these two pathways (red text) and contributes to increased migration and invasion of prostate cancer cells.
The negative correlation between AKT1 and MET was particularly intriguing and prompted us to investigate whether these in vitro findings correlate with the in vivo situation in clinical samples. In silico meta-analysis of 208 prostate cancer samples and 147 skin tumors (Kilpinen et al., 2008) revealed that AKT1 mRNA levels showed a strong anticorrelation with MET mRNA levels specifically in prostate, but not in skin cancers (Figure 8C). In contrast, AKT2 levels correlated to some extent with MET levels in both cancer types (Figure 8D), indicating that the expression of antimigratory kinase AKT1 in vivo also correlates with the reduced expression of a well-established promigratory RTK, namely MET.
DISCUSSION
Activation of the PI3K pathway is implicated in many cancer types, and PI3K or its downstream components, including AKTs, are considered attractive targets for inhibitors. However, several studies have highlighted the complexity of biological outcomes obtained upon AKT inhibition (Irie et al., 2005; Dillon and Muller, 2010; Chandarlapaty et al., 2011), including the potential cell type–specific effects of AKT isoforms on cell migration and invasion (Elson-Schwab et al., 2010). We used our recent high-throughput RNAi screen to study prostate cancer cells (Pellinen et al., 2012) as a basis for this study and identified AKT1 as an inhibitor of β1-integrin activity. On detailed investigation of the specific roles of the different AKT isoforms, we found that down-regulation of AKT1 and AKT2, but not AKT3, induced activity of cell surface β1-integrins and enhanced adhesion, migration, and invasion (Figure 8E).
To the best of our knowledge, AKT1 and AKT2 have not been directly implicated in the regulation of β1-integrin conformation on the cell surface. However, several excellent studies in breast and ovarian carcinoma both in vitro and in vivo have demonstrated that in these cancer types, AKT1 functions as an inhibitor of invasion, whereas AKT2 activity has the opposite effect on motility and cancer dissemination (Arboleda et al., 2003; Irie et al., 2005; Meng et al., 2006). These functions appear rather cell type–specific and context-specific, since AKT1 is promigratory in fibroblasts (Zhou et al., 2006), and an RNAi screen in MCF10A cells identified both AKT1 and AKT2 as promigratory (Simpson et al., 2008). The role of AKTs in the regulation of focal adhesions is also context dependent. AKT activity has been shown to function as a positive regulator of focal adhesion, but AKT2 has been shown to decrease focal adhesions (Winograd-Katz et al., 2009). However, details regarding the mechanistic differences underlying isoform specificity downstream of AKTs remain incompletely understood. In this study, we show that the pathways correlating with integrin activity inhibition in prostate cancer cells are distinct for AKT1 and AKT2. Silencing of AKT1 relieves a feedback suppression of expression and activity of RTKs such as EGFR and MET. This might be linked to the established positive cross-talk between β1-integrins and these RTKs (Ivaska and Heino, 2011). Furthermore, our analysis of gene expression in clinical tumor samples showed that AKT1 mRNA levels anti-correlate with MET mRNA levels in prostate cancer. Silencing of AKT2, on the other hand, induced up-regulation of miR-200 family microRNAs, and overexpression of miR-200a and miR-200b is sufficient to induce integrin activity in PC3 cells. Thus our data define an inhibitory role for both AKT1 and AKT2 in prostate cancer migration and highlight two distinct signaling pathways triggered by AKT1 or AKT2 silencing that correlate with alterations in integrin activity (Figure 8E).
Studies of the AKT isoforms in clinical prostate cancer samples have shown that more than 60% of cancerous tissues overexpressed all three AKT isoforms. Interestingly, in this study, expression of a given AKT isoform correlated with rather different clinically significant prognostic parameters (Le Page et al., 2006), suggesting a distinct role for the isoforms in vivo in cancer. In our recent high-throughput RNAi screen for integrin activity regulators (Pellinen et al., 2012) in VCAP, an androgen-dependent prostate cancer cell line AKT3 was identified as a positive regulator for integrin activity (Pellinen et al., 2012). In the subsequent secondary screens with four additional siRNA oligos and 12 cell lines from different cancer types, AKT3 silencing inhibited β1-integrin activity in five out of 12 cell lines and significantly increased β1 activity in one of the cell lines (Pellinen et al., 2012). In light of the growing amount of data on AKT isoforms in cancer and the data presented here (with no significant β1 inhibition by AKT3 observed in PC3 cells on plastic), the highly context-dependent nature of AKT-mediated control on cell motility is becoming increasingly obvious.
Several studies have demonstrated functional cross-talk between integrins and RTKs in different cell types. We show here that loss of AKT1 in PC3 cells induces up-regulation of the levels of several active RTKs, which is in line with a recent publication investigating the biological outcome of long-term pharmacological AKT inhibition (Chandarlapaty et al., 2011). Furthermore, we observed a significant anti-correlation between AKT1 and c-MET mRNA expression, specifically in clinical prostate tumor samples. This prompted us to investigate whether up-regulation of the two most abundant RTKs, MET and EGFR, was sufficient to duplicate AKT1 silencing–induced β1-integrin activation. However, mere overexpression or silencing of either kinase alone or the two in conjunction was not sufficient to influence β1-integrin activity in these cells (unpublished data), suggesting that the AKT1-specific inhibitory effect is likely to be influenced by several of the seven up-regulated kinases and their downstream signaling to activate, for example, extracellular signal–regulated kinases (ERK). Recently AXL was found to be necessary for vascular endothelial growth factor (VEGF)-induced signaling downstream of VEGFR2 in endothelial cells (Ruan and Kazlauskas, 2012). Interestingly, silencing of AKT1 in PC3 cells also results in strong up-regulation of VEGFR2. This raises the possibility that Axl and VEGFR2 function together to regulate integrins in these cells. However, this remains to be investigated. Another possibility would be that AKT1-induced inhibition of the expression of kinases such as AXL in these cells would suppress an EMT phenotype (Gjerdrum et al., 2010; Vuoriluoto et al., 2010) and thus negatively influence migration.
Our data suggest that one target of the inhibitory activity of AKT2 in prostate cancer is miR-200 family expression. While the exact mechanistic link between AKT2 silencing and induction of miR-200 remains to be investigated, we show that increased miR-200 levels correlate with increased integrin activity, focal adhesion formation, and migration. These data are consistent with the observation that up-regulation of miR-200 correlates with increased cancer dissemination in breast cancer in mice and invasion in melanoma cells (Dykxhoorn et al., 2009; Elson-Schwab et al., 2010) but is in obvious conflict with the published role of miR-200 as an EMT suppressor in breast cancer cells (Iliopoulos et al., 2009). The relationship between miR-200 family members and migration or invasion in prostate cancer is likely to be complex. A recent study showed that PDGF-D–induced EMT and invasion in PC3 cells was inhibited by miR-200b. However, our studies highlight the possibility that the function of cancer-linked miRNAs can also be context dependent and may vary between different cancer types.
Taken together, our data highlight an important and previously unknown difference between breast and prostate cancer with respect to the distinct roles for the AKT isoforms in the regulation of cell motility and invasion. The picture emerging from this work and studies by others is a complex one and warrants careful assessment of cancer type–specific biological outcomes if oncogenic PI3K signaling is targeted in the clinical setting.
MATERIALS AND METHODS
CSMA screening
CSMA screening is described in Pellinen et al. (2012).
Cell lines, inhibitors, and transfections
PC3 human prostate cancer cell line was grown in RPMI 1640 medium supplemented with 1% l-glutamine, 10% fetal bovine serum, and 1% penicillin–streptomycin. The PAN-AKT inhibitor AKTi (10 μg/ml; www.proteinkinase.de) and DMSO as a negative control were used for 20 h. siRNA-mediated silencing and pre-miRNA transfections were done using HiPerFect transfection reagent (Qiagen, Valencia, CA) according to the manufacturer´s protocol, and the cells were cultured for 2–3 d. Annealed siRNAs against AKT1 (four: Hs_AKT1_5 Flexitube siRNA, Hs_AKT1_8 Flexitube siRNA, Hs_AKT1_10 Flexitube siRNA, Hs_AKT1_11 Flexitube siRNA), AKT2 (two: Hs_AKT2_5 Flexitube siRNA, Hs_AKT2_6 Flexitube siRNA), AKT3 (Hs_AKT3_2 HP siRNA), and GAPDH (Hs_GAPDH_3 HP validated siRNA) were used as negative controls at 60 nM final concentrations (all were from Qiagen). Human pre-miRNA precursors for miR-200a and miR-200b and pre-miR negative control were used at 20 nM final concentrations (Ambion, Austin, TX). Plasmid transfections were done using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA) according to the manufacturer´s protocol, and the cells were cultured for 24 h. Both plasmids, pcDNA3.1 as a negative control and pcDNA3_Hygro_HA_AKT2 (plasmid 16000 from Morris Birnbaum, University of Pennsylvania, Philadelphia, PA), were from Addgene.
Antibodies
The following antibodies were used in this study: 12G10 (active β1-integrin [ Byron et al., 2009]; FACS 1:100, immunofluorescence 1:100; Abcam, Cambridge, MA), K20 (total β1-integrin [ Byron et al., 2009]; FACS 1:100; Immunotech, Marseille, France), and Mab13 as a β1-integrin function–blocking antibody (inactive β1-integrin [ Byron et al., 2009]; invasion assay 5 μg/ml; BD PharMingen, San Diego, CA). For Western blotting, the following antibodies were used: AKT1 (1:1000; Cell Signaling Technology, Danvers, MA), AKT2 (1:1000; Cell Signaling Technology), AKT3 (1:1000; Cell Signaling Technology), PAN-AKT (1:1000; Cell Signaling Technology), pAKT Ser473 (1:1000; Cell Signaling Technology), pAKT Thr308 (1:1000; Cell Signaling Technology), pGSK 3β Ser9 (1:1000; Cell Signaling Technology), β1-integrin (MAB2252; 1:1000; BD Transduction Laboratories, Franklin Lakes, NJ), p-Met Tyr1234/1235 (1:1000; Cell Signaling Technology), Met (L41G3) (1:1000; Cell Signaling Technology), p-EGFR Tyr1068 (1:500; Cell Signaling Technology), EGF receptor (1:1000; Cell Signaling Technology), and α-tubulin 12G10 (1:1000; Hybridoma Bank, University of Iowa, Iowa city, IA). Vinculin was used to stain focal adhesions (1:10000; Sigma-Aldrich, St. Louis, MO) and Alexa Fluor 488/647–conjugated phalloidin (1:50; Molecular Probes, Eugene, OR) was used to stain filamentous actin. Secondary antibodies conjugated with Alexa Fluor 488 or 647 were used in immunofluorescence, FACS, and ScanR (1:400; Invitrogen).
Western blot assay
Western blot assay was performed as described in Mattila et al. (2008).
Flow cytometry and ScanR
The FACS staining was performed as described earlier (Brandt et al., 2009). Briefly, fixed cells were washed with Tyrodes buffer (10 mM HEPES-NaOH at pH 7.5, 137 mM NaCl, 2.68 mM KCl, 1.7 mM MgCl2, 11.9 mM NaHCO3, 5 mM glucose, 0.1% bovine serum albumin [BSA]) and stained with primary antibodies against active β1-integrins (12G10, 1:100), total β1-integrin (K20, 1:50) or with secondary antibody only in control cells for 1 h. Cells were then washed with Tyrodes buffer and stained with Alexa Fluor 488–conjugated secondary antibody (1:400). After being washed, cells were suspended in Tyrodes buffer, and fluorescence was analyzed with flow cytometry (FACScalibur; BD Biosciences, Franklin Lakes, NJ). For analyzing the binding of labeled fibronectin repeat 7–10 (Moser et al., 2008), cells in Tyrodes buffer were incubated with the ligand (250 μg/ml) for 30 min at room temperature. After being washed, cells were fixed and measured with flow cytometry. ScanR analysis was done as described in Rantala et al. (2011), except Hoechst 33342 was used to stain DNA.
Proliferation and adhesion assays
Inhibitor or siRNA-treated cells ([3–5] × 103) in 100 μl medium were plated on Costar 96-well plates with clear bottoms (Corning, Corning, NY). After 24 h for measurement of proliferation, 10 μl of WST-1 reagent was added and incubated for 45 min at 37ºC. Absorbance was measured at 450 nm with Envision multilabel plate reader (Perkin Elmer-Cetus, Waltham, MA). For adhesion assays, 96-well plates were coated with different concentrations of collagen I (Calf skin Collagen I; Sigma-Aldrich) in phosphate-buffered saline (PBS) overnight at 4ºC. Wells were washed once with PBS and blocked with 0.5% BSA in PBS for 1 h at 37ºC. Inhibitor-treated cells (10 × 103) in serum-free medium were allowed to adhere for 20 min at 37ºC. Wells were washed with cold PBS, fixed in cold 4% paraformaldehyde, and stained with 0.05% crystal violet for 10 min, which was followed by washing with Milli-Q water (MQ) and drying. Then 100 μl of 10% acetic acid was added, and absorbance was measured at 620 nm with a Victor2V multilabel reader (Perkin Elmer-Cetus).
Immunofluorescence
siRNA-treated cells were plated on acid-washed coverslips and allowed to adhere and spread. The following day, cells were washed with cold PBS, fixed in cold 4% paraformaldehyde, washed with PBS, permeabilized with 0.1% Triton X-100 in 2% BSA/PBS for 15 min at room temperature, washed with PBS, and blocked with 1 mM MgCl2, 1 mM ethylene glycol tetraacetic acid in 2% BSA/PBS for 1 h at room temperature. Indicated primary antibodies diluted in blocking buffer were then added and the cells were incubated overnight. After cells were washed three times with PBS, Alexa-conjugated secondary antibodies were added for 1 h at room temperature. Coverslips were washed with PBS and MQ and mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) containing 4′6-diamidino-2-phenylindole (DAPI) to counterstain nuclei. Confocal three-dimensional images were taken with a Zeiss Axiovert 200 M with a spinning-disk confocal unit (CSU22; Yokogawa, Japan) and a Zeiss Plan-Neofluar 63× oil immersion lens/1.4 numerical aperture (NA) objective (Carl Zeiss Microscopy, Thornwood, NY) and analyzed with ImageJ (http://rsb.info.nih.gov/ij/download.html).
Migration and invasion assays
For live-cell imaging, CDM was generated by using NIH 3T3 fibroblasts as described previously in Caswell et al. (2007). AKT or control silenced cells were seeded sparsely on plastic or on CDM and allowed to adhere for ∼5 h before imaging was started. Phase-contrast images were taken every 18 or 20 min for 20–22 h with a Zeiss inverted wide-field microscope with an El Plan-Neofluar 10×/0.5 NA objective and equipped with a heated chamber (37ºC) and CO2 controller (4.8%). Cell movement (the path length and distance to start) was measured by tracking the cell body, defined as a nucleus, during the imaged time. The ImageJ MTrackJ (Meijering et al., 2012) was used for the analysis. The invasion assay was done as described in Hognas et al. (2012), except siRNA mix (70 nM siRNA and HiPerFect transfection reagent, used according to the manufacturer's protocol) was included in the Matrigel when the Matrigel was added. Mab13 antibody was used to block the function of β1-integrin. Cells were preincubated with 5 μg/ml Mab13 antibody, and Matrigel was also supplemented with this antibody.
qRT-PCR
Cells were detached with trypsin/EDTA solution, and cellular RNA was isolated with Trizol reagent (Invitrogen). RNA (20 ng) was used as a template for the RT reaction, and 1/10 of the synthesized cDNA was used for qRT-PCR. Primers and probes for miR-200a, miR-200b, and endogenous control (U6B) were included in Taqman MicroRNA Assay kits (Applied Biosystems, Bedford, MA). qRT PCR was performed according to manufacturer´s instructions (Applied Biosystems). The expression of miR-200a and miR-200b was determined by the quantitation method using U6B as a control.
RTK arrays
pRTK array was utilized according to manufacturer´s instructions (R&D Systems, Minneapolis, MN). Briefly, array membranes were blocked and lysates of AKT-silenced PC3 cells were incubated with membranes overnight. After the cells were washed, anti–phospho-tyrosine-horseradish peroxidase detection antibody was added, and the cells were incubated for 2 h at room temperature; this was followed by washing. Membranes were exposed to chemiluminescent reagent and exposed to x-ray film. Quantification of pixel intensities was done with ImageJ.
Statistical analysis
All statistical analyses were done using Student´s t test.
Supplementary Material
Acknowledgments
We thank L. Lahtinen and J. Siivonen for excellent technical assistance. M. Birnbaum and R. Fässler are acknowledged for the plasmids provided for the study. This study was supported by the Academy of Finland, a European Research Council Starting Grant, the Sigrid Juselius Foundation, and Finnish Cancer Organizations. R.V. was supported by the Turku Doctoral Program of Biomedical Sciences.
Abbreviations used:
- 12G10
β1-integrin epitope
- AKTi
triciribine
- BSA
bovine serum albumin
- CDM
cell-derived matrix
- CSMA
cell-spot microarray
- DAPI
4′6-diamidino-2-phenylindole
- DMSO
dimethyl sulfoxide
- EMT
epithelial–mesenchymal transition
- FACS
fluorescence-activated cell-sorting flow cytometry
- miR
microRNA
- NA
numerical aperture
- PBS
phosphate-buffered saline
- PI3K
phosphatidylinositol 3′ kinase
- PKB/AKT
protein kinase B
- PTEN
phosphatase and tensin homologue
- RNAi
RNA interference
- RTK
receptor tyrosine kinase
- siRNA
small interfering RNA
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E12-03-0213) on July 18, 2012.
The authors declare no conflict of interest.
Present addresses: *FIMM Finnish Institute for Molecular Medicine, Helsinki FIN 00014, Finland
†Knight Cancer Institute, Oregon Health and Science University, Portland, OR 97239.
REFERENCES
- Amato RJ, Jac J, Mohammad T, Saxena S. Pilot study of rapamycin in patients with hormone-refractory prostate cancer. Clin Genitourin Cancer. 2008;6:97–102. doi: 10.3816/CGC.2008.n.015. [DOI] [PubMed] [Google Scholar]
- Arboleda MJ, Lyons JF, Kabbinavar FF, Bray MR, Snow BE, Ayala R, Danino M, Karlan BY, Slamon DJ. Overexpression of AKT2/protein kinase Bβ leads to up-regulation of β1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003;63:196–206. [PubMed] [Google Scholar]
- Arjonen A, Alanko J, Veltel S, Ivaska J. Distinct recycling of active and inactive β1 integrins. Traffic. 2012;13:610–625. doi: 10.1111/j.1600-0854.2012.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Brandt DT, Baarlink C, Kitzing TM, Kremmer E, Ivaska J, Nollau P, Grosse R. SCAI acts as a suppressor of cancer cell invasion through the transcriptional control of β1-integrin. Nat Cell Biol. 2009;11:557–568. doi: 10.1038/ncb1862. [DOI] [PubMed] [Google Scholar]
- Byron A, Humphries JD, Askari JA, Craig SE, Mould AP, Humphries MJ. Anti-integrin monoclonal antibodies. J Cell Sci. 2009;122:4009–4011. doi: 10.1242/jcs.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell P, Norman J. Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol. 2008;18:257–263. doi: 10.1016/j.tcb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Caswell PT, et al. Rab25 associates with α5β1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin YR, Toker A. The actin-bundling protein palladin is an Akt1-specific substrate that regulates breast cancer cell migration. Mol Cell. 2010;38:333–344. doi: 10.1016/j.molcel.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois-Cox S, Genther Williams SM, Reczek EE, Johnson BW, McGillicuddy LT, Johannessen CM, Hollstein PE, MacCollin M, Cichowski K. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10:459–472. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon RL, Marcotte R, Hennessy BT, Woodgett JR, Mills GB, Muller WJ. Akt1 and akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer Res. 2009;69:5057–5064. doi: 10.1158/0008-5472.CAN-08-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon RL, Muller WJ. Distinct biological roles for the akt family in mammary tumor progression. Cancer Res. 2010;70:4260–4264. doi: 10.1158/0008-5472.CAN-10-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykxhoorn DM, Wu Y, Xie H, Yu F, Lal A, Petrocca F, Martinvalet D, Song E, Lim B, Lieberman J. miR-200 enhances mouse breast cancer cell colonization to form distant metastases. PLoS One. 2009;4:e7181. doi: 10.1371/journal.pone.0007181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson-Schwab I, Lorentzen A, Marshall CJ. MicroRNA-200 family members differentially regulate morphological plasticity and mode of melanoma cell invasion. PLoS One. 2010;5:e13176. doi: 10.1371/journal.pone.0013176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17:524–532. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- Gahmberg CG, Fagerholm SC, Nurmi SM, Chavakis T, Marchesan S, Gronholm M. Regulation of integrin activity and signalling. Biochim Biophys Acta. 2009;1790:431–444. doi: 10.1016/j.bbagen.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerdrum C, et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci USA. 2010;107:1124–1129. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- Hognas G, Tuomi S, Veltel S, Mattila E, Murumagi A, Edgren H, Kallioniemi O, Ivaska J. Cytokinesis failure due to derailed integrin traffic induces aneuploidy and oncogenic transformation in vitro and in vivo. Oncogene. 2012;31:3597–3606. doi: 10.1038/onc.2011.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JN, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt-1 (PKB-α) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer Res. 2004;64:3171–3178. doi: 10.1158/0008-5472.can-03-3465. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Polytarchou C, Hatziapostolou M, Kottakis F, Maroulakou IG, Struhl K, Tsichlis PN. MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci Signal. 2009;2:ra62. doi: 10.1126/scisignal.2000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska J, Heino J. Cooperation between integrins and growth factor receptors in signaling and endocytosis. Annu Rev Cell Dev Biol. 2011;27:291–320. doi: 10.1146/annurev-cellbio-092910-154017. [DOI] [PubMed] [Google Scholar]
- Kilpinen S, et al. Systematic bioinformatic analysis of expression levels of 17,330 human genes across 9,783 samples from 175 types of healthy and pathological tissues. Genome Biol. 2008;9:R139. doi: 10.1186/gb-2008-9-9-r139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Ye F, Ginsberg MH. Regulation of integrin activation. Annu Rev Cell Dev Biol. 2011;27:321–345. doi: 10.1146/annurev-cellbio-100109-104104. [DOI] [PubMed] [Google Scholar]
- Kuo JC, Han X, Hsiao CT, Yates JR, III, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol. 2011;13:383–393. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Page C, Koumakpayi IH, Alam-Fahmy M, Mes-Masson AM, Saad F. Expression and localisation of Akt-1, Akt-2 and Akt-3 correlate with clinical outcome of prostate cancer patients. Br J Cancer. 2006;94:1906–1912. doi: 10.1038/sj.bjc.6603184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24:7465–7474. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 2007;67:167–177. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- Mattila E, Auvinen K, Salmi M, Ivaska J. The protein tyrosine phosphatase TCPTP controls VEGFR2 signalling. J Cell Sci. 2008;121:3570–3580. doi: 10.1242/jcs.031898. [DOI] [PubMed] [Google Scholar]
- Meijering E, Dzyubachyk O, Smal I. Methods for cell and particle tracking. Methods Enzymol. 2012;504:183–200. doi: 10.1016/B978-0-12-391857-4.00009-4. [DOI] [PubMed] [Google Scholar]
- Meng Q, Xia C, Fang J, Rojanasakul Y, Jiang BH. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal. 2006;18:2262–2271. doi: 10.1016/j.cellsig.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Mitra AK, Sawada K, Tiwari P, Mui K, Gwin K, Lengyel E. Ligand-independent activation of c-Met by fibronectin and α(5)β(1)-integrin regulates ovarian cancer invasion and metastasis. Oncogene. 2010;30:1566–1576. doi: 10.1038/onc.2010.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro L, Venturino M, Bozzo C, Silengo L, Altruda F, Beguinot L, Tarone G, Defilippi P. Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 1998;17:6622–6632. doi: 10.1093/emboj/17.22.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14:325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- Pellinen T, Rantala JK, Arjonen A, Mpindi JP, Kallioniemi O, Ivaska J. A functional genetic screen reveals new regulators of β1-integrin activity. J Cell Sci. 2012;125:649–661. doi: 10.1242/jcs.090704. [DOI] [PubMed] [Google Scholar]
- Rantala JK, et al. SHARPIN is an endogenous inhibitor of β1-integrin integrin activation. Nat Cell Biol. 2011;11:1315–1324. doi: 10.1038/ncb2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regent M, Planus E, Bouin AP, Bouvard D, Brunner M, Faurobert E, Millon-Fremillon A, Block MR, Albiges-Rizo C. Specificities of β1 integrin signaling in the control of cell adhesion and adhesive strength. Eur J Cell Biol. 2011;90:261–269. doi: 10.1016/j.ejcb.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Ruan GX, Kazlauskas A. Axl is essential for VEGF-A-dependent activation of PI3K/Akt. EMBO J. 2012;31:1692–1703. doi: 10.1038/emboj.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto S, McCann RO, Dhir R, Kyprianou N. Talin1 promotes tumor invasion and metastasis via focal adhesion signaling and anoikis resistance. Cancer Res. 2010;70:1885–1895. doi: 10.1158/0008-5472.CAN-09-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyers CL. Will mTOR inhibitors make it as cancer drugs? Cancer Cell. 2003;4:343–348. doi: 10.1016/s1535-6108(03)00275-7. [DOI] [PubMed] [Google Scholar]
- Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson KJ, Selfors LM, Bui J, Reynolds A, Leake D, Khvorova A, Brugge JS. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol. 2008;10:1027–1038. doi: 10.1038/ncb1762. [DOI] [PubMed] [Google Scholar]
- Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C, Tiron C, Lorens JB, Ivaska J. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene. 2010;30:1436–1448. doi: 10.1038/onc.2010.509. [DOI] [PubMed] [Google Scholar]
- White DP, Caswell PT, Norman JC. αvβ3 and α5β1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J Cell Biol. 2007;177:515–525. doi: 10.1083/jcb.200609004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winograd-Katz SE, Itzkovitz S, Kam Z, Geiger B. Multiparametric analysis of focal adhesion formation by RNAi-mediated gene knockdown. J Cell Biol. 2009;186:423–436. doi: 10.1083/jcb.200901105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings BA. Protein kinase Bα/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell. 2005;20:539–550. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou GL, Tucker DF, Bae SS, Bhatheja K, Birnbaum MJ, Field J. Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration. J Biol Chem. 2006;281:36443–36453. doi: 10.1074/jbc.M600788200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.