Abstract
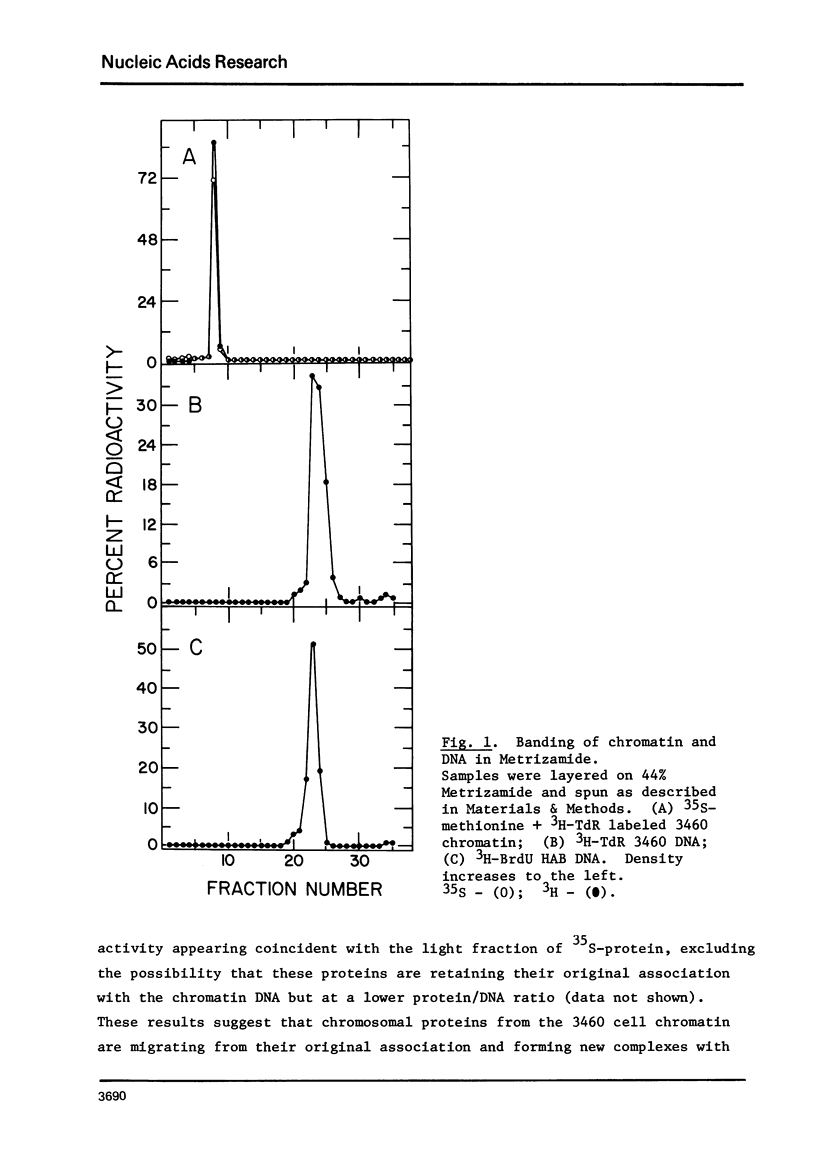
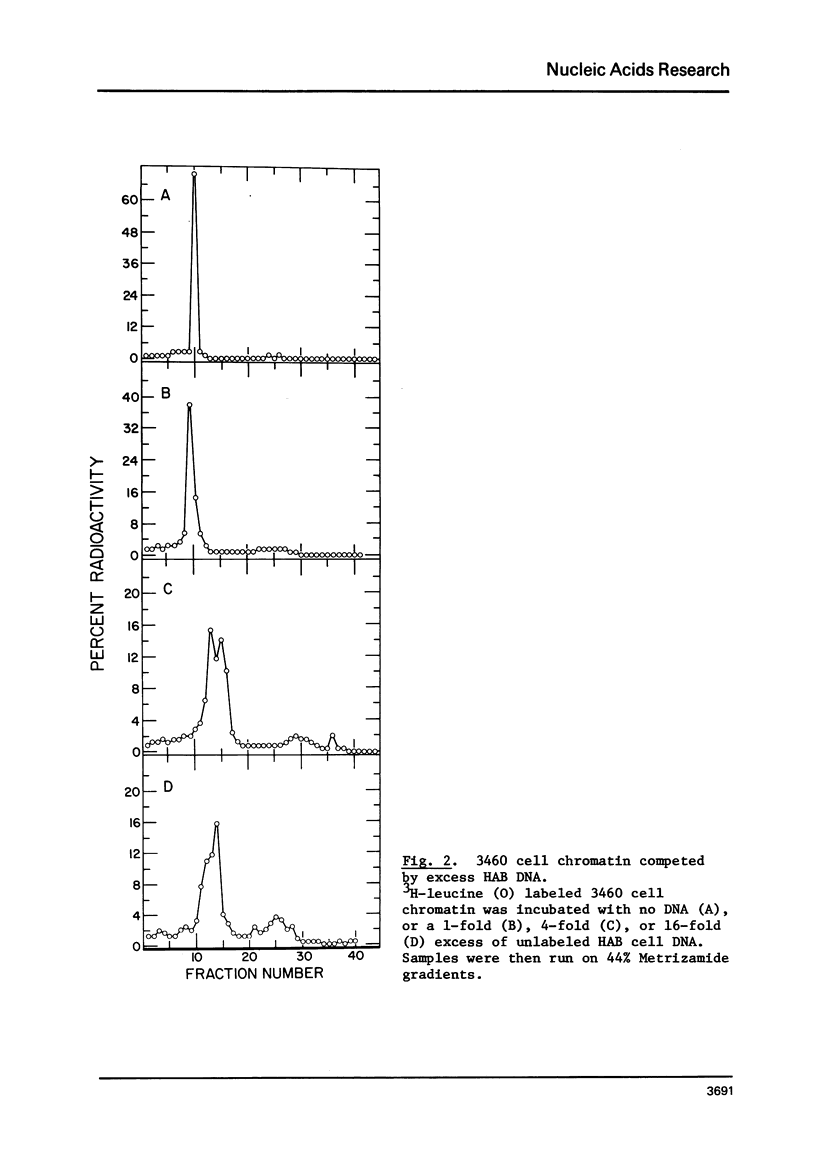
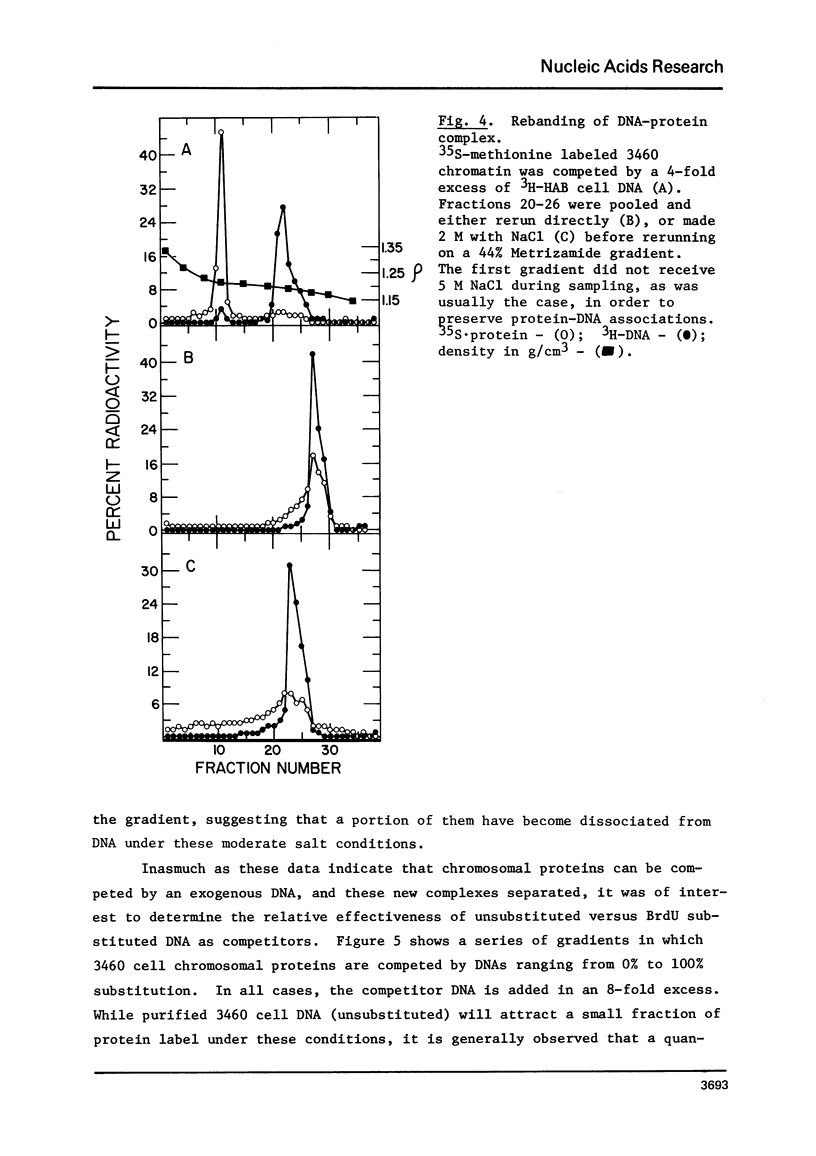
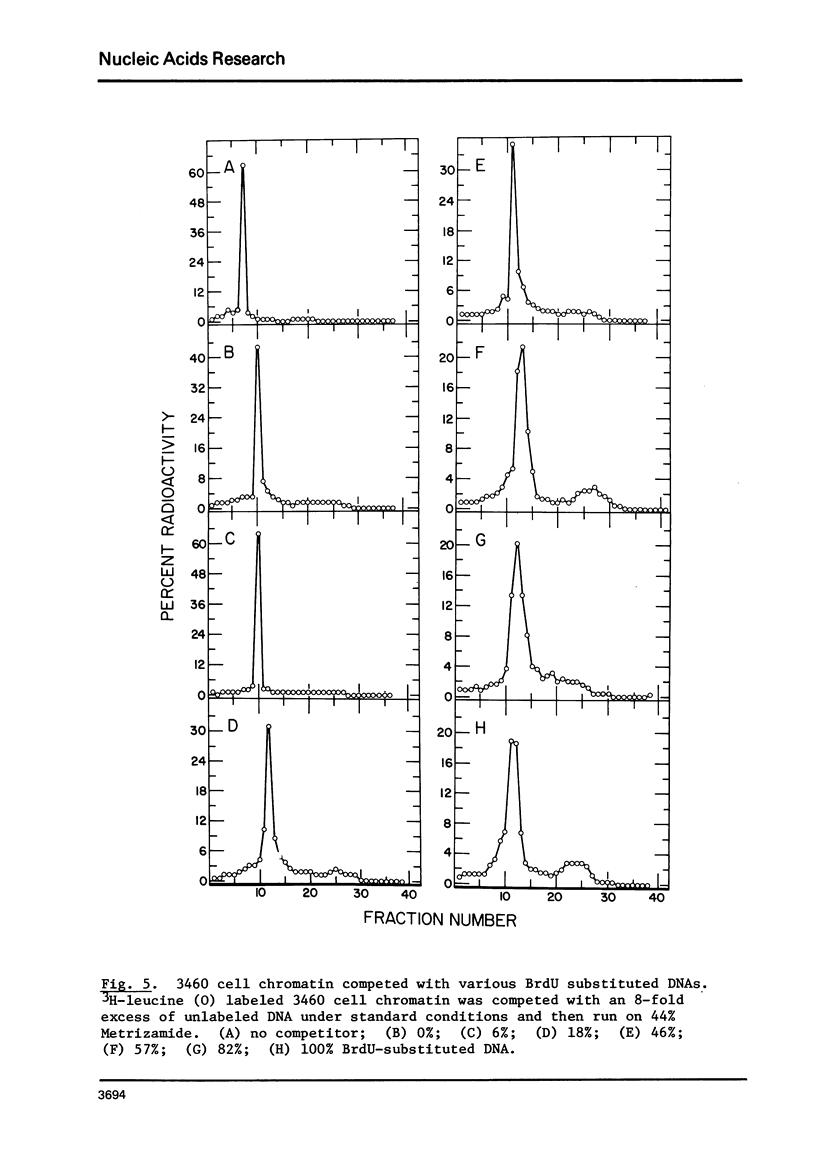
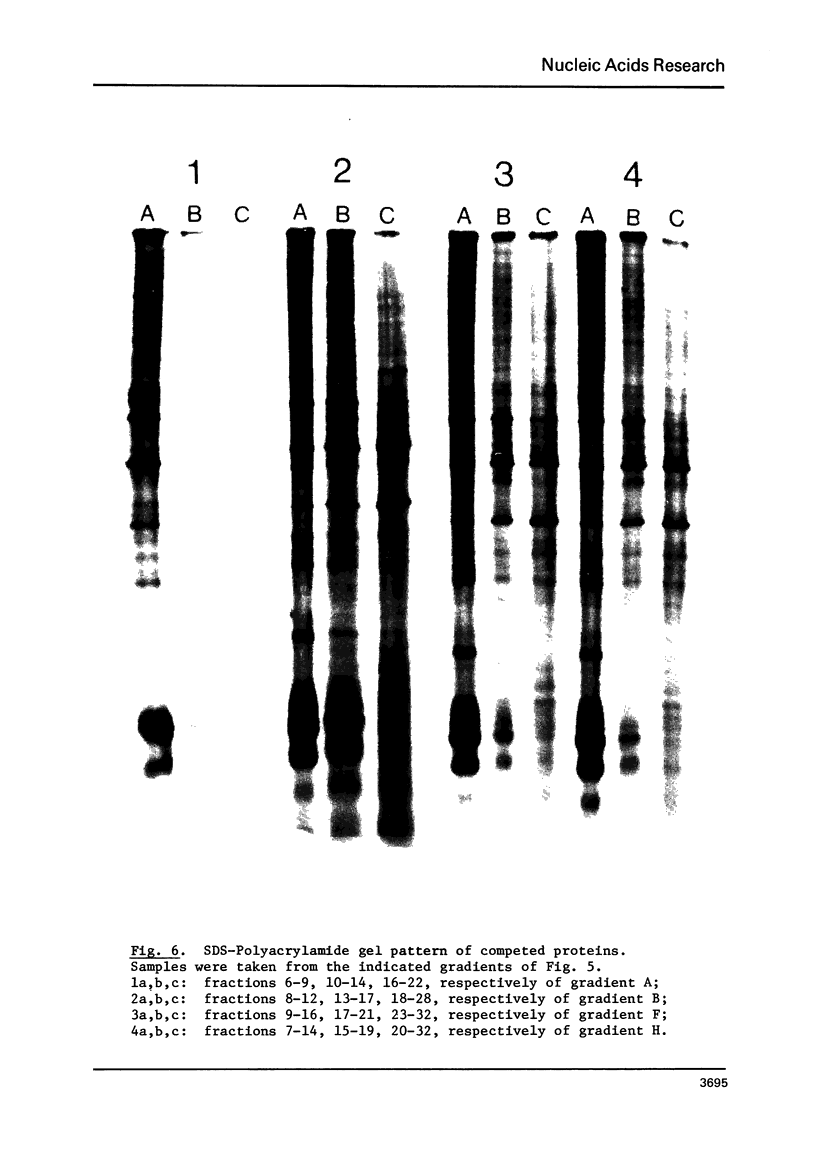
Chromatin-DNA competition has been utilized to examine the general nature of chromosomal proteins interacting more strongly with BrdU substituted DNA. When chromatin is incubated with an excess of purified DNA, a portion of the chromosomal proteins will exchange to the purified DNA. These two complexes can then be separated on Metrizamide gradients due to their differing protein/DNA ratios. Using this technique we observe that most nonhistone chromosomal proteins will exchange to a competitor DNA, the extent of exchange being directly dependent upon the competitor DNA being present in excess. While essentially the same proteins will migrate to either unsubstituted or BrdU substituted DNA, the substituted DNA is found to be a quantitatively better competitor and its effectiveness as a competitor is directly related to the level of BrdU substitution.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick M. D., Davidson R. L. Total substitution of bromodeoxyuridine for thymidine in the DNA of a bromodeoxyuridine-dependent cell line. Proc Natl Acad Sci U S A. 1974 May;71(5):2082–2086. doi: 10.1073/pnas.71.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnie G. D., Rickwood D., Hell A. Buoyant densities and hydration of nucleic acids, proteins and nucleoprotein complexes in metrizamide. Biochim Biophys Acta. 1973 Dec 7;331(2):283–294. doi: 10.1016/0005-2787(73)90441-3. [DOI] [PubMed] [Google Scholar]
- Bischoff R., Holtzer H. Inhibition of myoblast fusion after one round of DNA synthesis in 5-bromodeoxyuridine. J Cell Biol. 1970 Jan;44(1):134–150. doi: 10.1083/jcb.44.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., Bick M. D. Bromodeoxyuridine dependence--a new mutation in mammalian cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):138–142. doi: 10.1073/pnas.70.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levitt D., Dorfman A. The irreversible inhibition of differentiation of limb-bud mesenchyme by bromodeoxyuridine. Proc Natl Acad Sci U S A. 1972 May;69(5):1253–1257. doi: 10.1073/pnas.69.5.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac operator analogues: bromodeoxyuridine substitution in the lac operator affects the rate of dissociation of the lac repressor. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2574–2576. doi: 10.1073/pnas.69.9.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. The binding of lac repressor and the catabolite gene activator protein to halogen-substituted analogues of poly[d(A-T)]. Biochim Biophys Acta. 1976 May 3;432(2):185–191. doi: 10.1016/0005-2787(76)90160-x. [DOI] [PubMed] [Google Scholar]
- Lin S., Lin D., Riggs A. D. Histones bind more tightly to bromodeoxyuridine-substituted DNA than to normal DNA. Nucleic Acids Res. 1976 Sep;3(9):2183–2191. doi: 10.1093/nar/3.9.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk D. C., Bick M. D. Determination of 5'-bromodeoxyuridine in DNA by buoyant density. Anal Biochem. 1977 Feb;77(2):346–349. doi: 10.1016/0003-2697(77)90247-0. [DOI] [PubMed] [Google Scholar]
- Rickwood D., Hell A., Malcolm S., Birnie G. D., MacGillivray A. J., Paul J. Fractionation of unfixed chromatin by buoyant-density centrifugation. Biochim Biophys Acta. 1974 Jul 11;353(3):353–361. doi: 10.1016/0005-2787(74)90029-x. [DOI] [PubMed] [Google Scholar]
- Scher W., Preisler H. D., Friend C. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro. 3. Effects of 5-bromo-2'-deoxyuridine, dimethylformamide and dimethylsulfoxide. J Cell Physiol. 1973 Feb;81(1):63–70. doi: 10.1002/jcp.1040810108. [DOI] [PubMed] [Google Scholar]
- Silagi S., Bruce S. A. Suppression of malignancy and differentiation in melanotic melanoma cells. Proc Natl Acad Sci U S A. 1970 May;66(1):72–78. doi: 10.1073/pnas.66.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]