Cyclin-dependent kinase 1 (Cdk1) is required for initiation and maintenance of polarized cell growth in budding yeast. Cdk1 activates Rho-family GTPases, which trigger polarization of the actin cytoskeleton for delivery of membrane to growth sites. It is found that Cdk1's function in polarized growth extends beyond that of actin organization.
Abstract
Cyclin-dependent kinase 1 (Cdk1) is required for initiation and maintenance of polarized cell growth in budding yeast. Cdk1 activates Rho-family GTPases, which polarize the actin cytoskeleton for delivery of membrane to growth sites via the secretory pathway. Here we investigate whether Cdk1 plays additional roles in the initiation and maintenance of polarized cell growth. We find that inhibition of Cdk1 causes a cell surface growth defect that is as severe as that caused by actin depolymerization. However, unlike actin depolymerization, Cdk1 inhibition does not result in a massive accumulation of intracellular secretory vesicles or their cargoes. Analysis of post-Golgi vesicle dynamics after Cdk1 inhibition demonstrates that exocytic vesicles are rapidly mistargeted away from the growing bud, possibly to the endomembrane/vacuolar system. Inhibition of Cdk1 also causes defects in the organization of endocytic and exocytic zones at the site of growth. Cdk1 thus modulates membrane-trafficking dynamics, which is likely to play an important role in coordinating cell surface growth with cell cycle progression.
INTRODUCTION
Oscillations in cyclin-dependent kinase (Cdk) activity drive the core cell cycle events of chromosome duplication and segregation (Nasmyth, 2001). These core events are coordinated with changes in cell polarity and cell growth as cells progress through the cell cycle (Moseley and Nurse, 2009). In budding yeast, a single cyclin-dependent kinase called Cdk1 controls chromosome duplication and segregation, as well as initiation of polarized cell growth that leads to formation of a daughter cell (Culotti and Hartwell, 1971; Lew and Reed, 1993; Moffat and Andrews, 2004). Cdk1 is thus the nexus at which cell growth and cell cycle progression are controlled.
Polarized cell growth in budding yeast requires coordination of the actin cytoskeleton with membrane-trafficking pathways. The Rho-family GTPases Rho1 and Cdc42 are activated in a Cdk1-dependent manner in a defined patch at the cortex, where they recruit formin proteins to initiate formation of actin cables (Evangelista et al., 1997; Nern and Arkowitz, 2000; Shimada et al., 2000). The actin cables extend radially into the cytoplasm, serving as tracks for myosin motors that deliver post-Golgi vesicles to the plasma membrane (Schott et al., 1999). Vesicles are tethered at the plasma membrane before fusion by the exocyst complex (TerBush and Novick, 1995). Cdc42 and Rho1 interact with specific exocyst subunits in a GTP-dependent manner, which, together with phosphatidylinositol 4,5-bisphosphate (PIP2) binding, is required for polarized growth (Guo et al., 2001; Zhang et al., 2008). These mechanisms direct secretory vesicles carrying components required for cell growth to a defined site of exocytosis on the cell surface.
Endocytosis-associated actin patches are also localized to the site of bud growth and form a ring that surrounds the presumptive bud site (Kilmartin and Adams, 1984; Amberg, 1998; Layton et al., 2011). Localized endocytosis in proximity to sites of exocytosis may optimize cell polarity by recycling polarity determinants that would otherwise diffuse as the membrane grows (Valdez-Taubas and Pelham, 2003; Marco et al., 2007). The mechanisms responsible for the organization of endocytic and exocytic zones at the site of cell growth are unknown.
Cdk1 is required for polarization of the actin cytoskeleton and phosphorylates key regulators of Rho-family GTPases that direct actin polarization (Knaus et al., 2007; McCusker et al., 2007; Sopko et al., 2007; Kono et al., 2008). Much attention has therefore focused on a role for Cdk1 in initiating cell growth via polarization of the actin cytoskeleton. However, Rho-family GTPases also control endocytic and exocytic trafficking events (Kroschewski et al., 1999; Adamo et al., 2001; Guo et al., 2001; Murray and Johnson, 2001; Wu et al., 2008). Moreover, a systematic mass spectrometry screen for targets of Cdk1 identified many endocytic and exocytic proteins (Holt et al., 2009). We therefore tested whether the role of Cdk1 in the control of polarized cell growth extends beyond its known role in controlling the actin cytoskeleton. To do so, we investigated the dynamics of membrane-trafficking pathways after Cdk1 inhibition.
RESULTS
Inhibition of Cdk1 attenuates cell surface growth to a similar extent as actin depolymerization
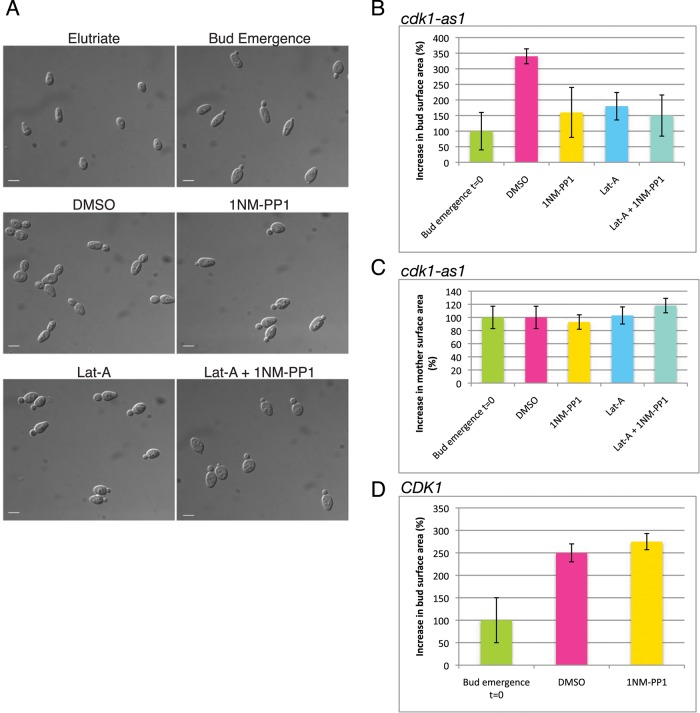
We previously discovered that acute Cdk1 inhibition caused rapid attenuation of secretory vesicle delivery to the growing bud, resulting in a block of polar growth (McCusker et al., 2007). These studies used an analogue-sensitive allele of Cdk1 (cdk1-as1), which enabled rapid and specific inhibition of Cdk1 activity with the adenine analogue 4-amino-1-tert-butyl-3-(1′-naphthylmethyl)pyrazolo[3,4-d]pyrimidine (1NM-PP1; Bishop et al., 2001). To investigate how Cdk1 controls polarized cell growth, we compared the effects of cdk1-as1 inhibition to the effects caused by latrunculin-A (Lat-A), an F-actin poison that causes rapid actin depolymerization and a block in delivery of vesicles to the growing bud (Ayscough et al., 1997). A synchronous population of unbudded cdk1-as1 cells was obtained by centrifugal elutriation and released into fresh media. On initiation of bud emergence, cells were treated for 1 h with 1NM-PP1, Lat-A, or both 1NM-PP1 and Lat-A (Figure 1A). The mean bud surface area was then calculated and compared with the mean at time zero, which was set to 100% (Figure 1B). In control cells treated with dimethyl sulfoxide (DMSO), buds increased in size by 340%. In contrast, buds in cells treated with 1NM-PP1 or Lat-A increased in size by only 160 and 180%, respectively (Figure 1B). Attenuation of bud growth by cdk1-as1 inhibition was not due simply to depolarization of growth, because mother cells did not grow after cdk1-as1 inhibition (Figure 1C). Treatment of wild-type CDK1 cells with 1NM-PP1 did not attenuate growth (Figure 1D). Inhibition of cdk1-as1 and F-actin simultaneously did not show strong additive effects. We conclude that the contribution of Cdk1 to polarized growth is comparable to that of F-actin, consistent with Cdk1 making a major contribution to polarized cell surface growth via actin-dependent processes.
FIGURE 1:
Inhibition of Cdk1 attenuates bud growth as severely as actin depolymerization. (A) Images showing representative samples of cells. Elutriate: cells immediately after elutriation; Bud Emergence: cells at the time when inhibitors were added. Cells were treated for 1 h with DMSO as a control or with the indicated inhibitors. Scale bar, 5 μm. (B) Quantitation of the surface area of buds in cdk1-as1 cells 1 h after treatment. The bar labeled t = 0 shows the size of buds at the time of inhibitor addition. Absolute bud size at t = 0 was 5 μm2. (C) Quantitation of mother cell surface area in cdk1-as1 cells treated with inhibitors. Absolute mother size at t = 0 was 29 μm2. (D) Quantitation of bud growth in wild-type cells treated with 1NM-PP1 for 1 h. Absolute bud size at t = 0 was 4 μm2. Error bars show mean ± SD, where n is at least 100 cells.
Cdk1 activity does not contribute to polarized cell growth solely via modulation of the actin cytoskeleton
Actin depolymerization results in accumulation of post-Golgi vesicles due to a failure in vesicle delivery to the growing bud (Novick and Botstein, 1985). Actin depolymerization also blocks endocytosis (Kubler and Riezman, 1993). If Cdk1 is primarily required for polarization of the actin cytoskeleton to deliver vesicles, inhibition of cdk1-as1 could result in post-Golgi vesicle accumulation and an endocytic block. To test this, we first visualized a green fluorescent protein (GFP)–tagged version of Sec4, which is a Rab-GTPase component of post-Golgi secretory vesicles that was previously used as a post-Golgi vesicle marker (Schott et al., 2002). Actin was visualized in the same cells by rhodamine–phalloidin staining, enabling imaging of endocytosis-associated actin patches and actin cables (Kilmartin and Adams, 1984). Deconvolved optical sections of asynchronously growing cells were projected to observe GFP-Sec4 particles and actin structures in the cell (Figure 2A and Supplemental Figures S1 and S2).
FIGURE 2:
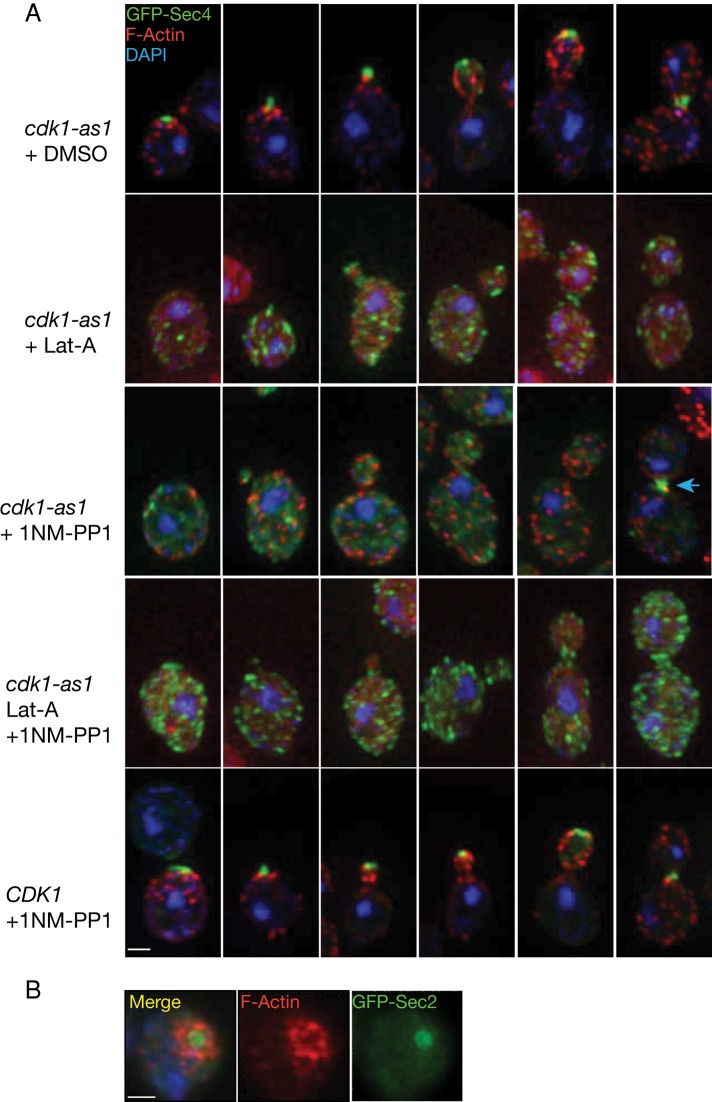
Behavior of GFP-Sec4 and actin after inhibition of Cdk1. (A) Asynchronous cells were treated for 1 h with DMSO or with the indicated inhibitors and were then fixed and stained. Images show maximum projections of 15–20 deconvolved z-sections through different cells at distinct cell cycle stages. GFP-Sec4 is shown in green, rhodamine–phalloidin in red, and DNA in blue. An arrow denotes the Cdk1-independent localization of GFP-Sec4 to the bud neck postanaphase. Scale bar, 2 μm. (B) Wide-field fluorescence images showing the top view of an unbudded cell initiating polarization of Sec2-GFP (green), F-actin (red), and DNA (blue). Scale bar, 2 μm.
In control cells, GFP-Sec4 localized to growth sites and to a few cytoplasmic particles, as previously reported (Figure 2A and Supplemental Figure S1; Schott et al., 2002). On Lat-A treatment, actin structures disappeared and many GFP-Sec4 particles accumulated in the cytoplasm, as expected for accumulation of secretory vesicles (Figure 2A and Supplemental Figures S1 and S2). In contrast, treatment of cdk1-as1 cells with 1NM-PP1 did not cause accumulation of GFP-Sec4 particles. Instead, GFP-Sec4 was observed as a diffuse cytoplasmic signal and in a few particles (Figure 2A and Supplemental Figure S1). Actin patches lost their polarized organization, and actin cables became disorganized (Figure 2A and Supplemental Figure S2; McCusker et al., 2007). Fewer cables were observed, and those that were visible were short and failed to orient along the mother-daughter axis (Supplemental Figure S2, arrows). Of importance, GFP-Sec4 localized normally to the bud neck in neighboring large-budded cells that had presumably undergone anaphase before inhibition of cdk1-as1 (Figure 2A, arrow). This demonstrated that inhibition of cdk1-as1 had specific effects on post-Golgi vesicles involved in polarized growth, since membrane addition at the site of cell division is Cdk1-independent in budding yeast and sea urchins (Shuster and Burgess, 2002; VerPlank and Li, 2005).
The failure to accumulate post-Golgi vesicles after inhibition of cdk1-as1 could be due to a failure to generate the vesicles. In this case, treatment of cdk1-as1 cells with 1NM-PP1 and Lat-A simultaneously should not lead to vesicle accumulation. However, we found that GFP-Sec4 particles accumulated to the same extent when cdk1-as1 cells were treated with Lat-A and 1NM-PP1 together as when cells were treated with Lat-A alone (Figure 2A and Supplemental Figure S1). We conclude that Cdk1 activity is not required for bulk post-Golgi vesicle production. Together these observations demonstrate that depolymerization of actin or inhibition of Cdk1 has fundamentally different effects on accumulation of post-Golgi vesicles, implying that Cdk1 does not act simply to generate and maintain a polarized actin cytoskeleton for delivery of vesicles to the growing bud.
Cdk1 is required for maintaining discrete zones of endocytosis and exocytosis at the site of polarized cell growth
Control cells showed a striking segregation of exocytic and endocytic compartments, as revealed by GFP-Sec4 or Sec2-GFP and actin staining (Figure 2, A, top, and B). Before bud emergence, a belt of endocytosis-associated actin patches surrounded a central zone of exocytosis, which was evident from views looking down at the polarized surface of the cell (Figure 2B). GFP-Sec4 remained polarized at the bud tip until after chromosome segregation, when it relocalized to the bud neck (Figure 2A). Previous work found that actin patches form a ring at the site of bud growth, and other studies found that exocytic proteins form a tightly focused spot (Kilmartin and Adams, 1984; Amberg, 1998; Finger et al., 1998; Layton et al., 2011). The merged image in Figure 2B shows the localization of endocytic and exocytic zones in the same cell, which emphasizes the interesting organization of these processes at the site of cell growth. Inhibition of cdk1-as1 eliminated the distinct zones of endocytosis and exocytosis (Figure 2A and Supplemental Figures S1 and S2).
Cdk1 is not required for endocytosis
The defects in post-Golgi vesicle accumulation and localization caused by cdk1-as1 inhibition could reflect defects in a membrane-trafficking pathway. We therefore tested whether cdk1-as1 activity is required for normal membrane-trafficking events in the endocytic and exocytic pathways. We first tested whether inhibition of cdk1-as1 caused defects in endocytosis. Wild-type CDK1 or cdk1-as cells were treated with 1NM-PP1 for 1 h. Cells were placed on ice to block endocytosis and then pulsed with the lipophilic dye FM4-64 (Vida and Emr, 1995). After washing away excess dye, we released cells at 25ºC to initiate endocytic uptake of the dye (Figure 3A). Imaging cells periodically during the pulse chase provided a means of assaying the function of the endocytic pathway after cdk1-as1 inhibition (Figure 3B). In wild-type CDK1 cells, FM4-64 was endocytosed and over time was chased through the endocytic system, where it terminally labeled the vacuole. After 12 min, FM4-64 could be visualized in punctate perivacuolar structures in both wild-type CDK1 and cdk1-as1 cells treated with 1NM-PP1. These structures most likely correspond to the prevacuolar compartment. By 16 min, FM4-64 had reached the limiting membrane of the vacuole in both CDK1 and cdk1-as1 cells treated with 1NM-PP1. To ensure that our pulse-chase assay was sufficiently sensitive to detect defects in endocytosis, we used the endocytosis-defective sla2Δ mutant as a control (Holtzman et al., 1993). In this mutant, persistent plasma membrane staining reflected defective internalization of the dye, and in many cells, bright fluorescent puncta formed as dye accumulated in an early endosomal compartment, as reported previously (Kim et al., 2006). Because the kinetics of FM4-64 internalization was not dramatically impaired after cdk1-as1 inhibition, we conclude that endocytosis and subsequent transport to the vacuole occur efficiently after cdk1-as1 inhibition, despite defects in polarized growth.
FIGURE 3:
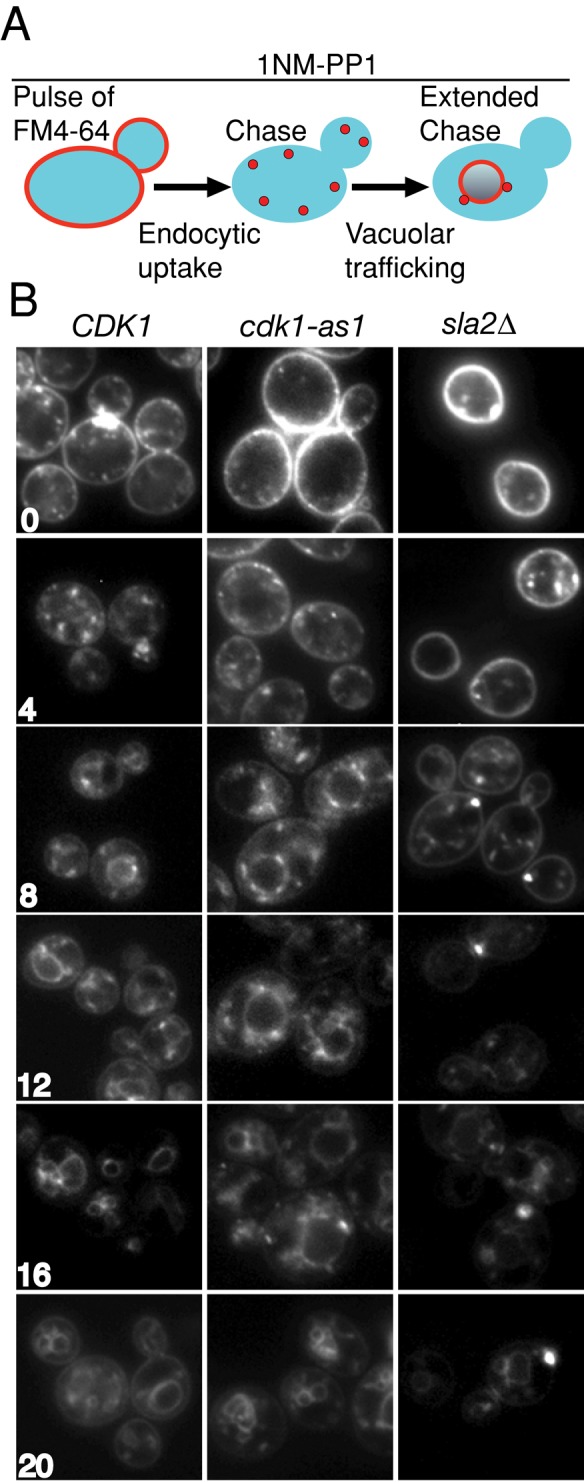
Cdk1 activity is not required for endocytosis. (A) Schematic representation of the experiment. CDK1 and cdk1-as1 cells were treated for 1 h with 1NM-PP1 and compared with an endocytosis-defective sla2Δ control in the following experiment: endocytosis was blocked by placing cells on ice, then a pulse of the lipophilic dye FM4-64 was provided, followed by a chase period in the absence of dye. During the chase, the dye is internalized by endocytosis, transits the endocytic pathway, and is delivered to the vacuole in the presence of 1NM-PP1. (B) Wide-field fluorescence images showing FM4-64 staining in live cells. Numbers indicate the time, in minutes, after initiation of the chase. In the endocytosis-defective sla2Δ mutant, FM4-64 accumulates in punctate intracellular structures, and the kinetics of its delivery to the vacuole is delayed.
Endocytosis is not required for secretion in budding yeast (Raths et al., 1993). Thus the accumulation of vesicles in cells treated with Lat-A, but not in cdk1-as1 cells treated with 1NM-PP1, cannot be explained by a model in which endocytic recycling of proteins needed for secretion continues after Cdk1 inhibition but not after Lat-A treatment.
Cdk1 is not required for transport from the endoplasmic reticulum to the vacuole
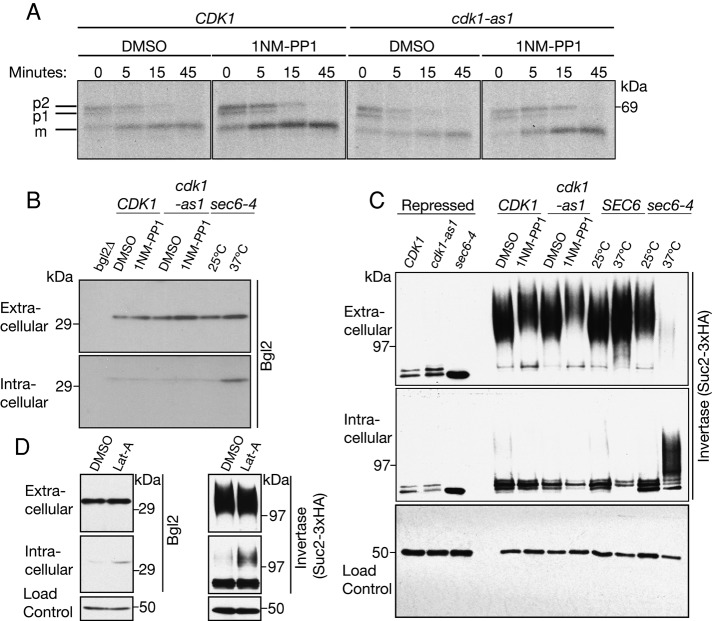
We next assayed the pathway that transports carboxypeptidase Y (CPY) from the endoplasmic reticulum (ER) to the vacuole via the Golgi (Hasilik and Tanner, 1978; Stevens et al., 1982). CPY is synthesized in the ER as a 67-kDa proenzyme (p1), which traffics to the Golgi, where it is glycosylated to yield a 69-kDa form (p2; Hasilik and Tanner, 1978). CPY is then routed to the vacuole, where it is cleaved to produce a mature (m) protease (Stevens et al., 1982). Wild-type CDK1 or cdk1-as1 cells were treated with DMSO or 1NM-PP1, and CPY transport kinetics were analyzed by pulse-chase and immunoprecipitation (Figure 4A). Inhibition of cdk1-as1 had no detectable effects on CPY processing. In an additional assay, we found that GFP-tagged carboxypeptidase S (GFP-CPS) was also efficiently targeted to the vacuole after cdk1-as1 inhibition (Supplemental Figure S3A). Immunoblotting revealed no difference in the levels of GFP-CPS after cdk1-as1 inhibition (Supplemental Figure S3B). Together these observations demonstrate that Cdk1 activity is not required for transport between the ER and Golgi, between the Golgi and the vacuole, or between endosomes and the vacuole. Of importance, these results also indicate that 1NM-PP1 did not cause nonspecific effects that completely disabled the secretory pathway.
FIGURE 4:
Cdk1 inhibition does not result in the intracellular accumulation of post-Golgi vesicle cargoes. (A) CDK1 and cdk1-as1 cells were treated with 1NM-PP1 or DMSO control. Cells were then pulsed with 35S-methionine for 10 min and chased for the times indicated. CPY was immunoprecipitated and analyzed by SDS–PAGE. (B) Immunoblot analysis of the extracellular and intracellular pools of the post-Golgi vesicle cargo protein Bgl2. Note that inactivation of sec6-4 caused an increase in intracellular levels of Bgl2. Nap1 was used as a loading control (not shown). (C) Immunoblot analysis of the extracellular and intracellular levels of Suc2-3xHA. Cells were grown in 2% glucose containing 1NM-PP1 or DMSO control for 1 h. Suc2-3xHA was then induced by shifting cells to 0.1% glucose in the presence of 1NM-PP1 or DMSO and harvesting cells after an additional hour. Note that inactivation of sec6-4 caused a complete block in secretion of Suc2-3xHA. Nap1 was used as a loading control. (D) Immunoblot analysis of the secreted and intracellular pools of Bgl2 and Suc2-3xHA levels after actin depolymerization for 1 h using latrunculin-A. Nap1 was used as a loading control. Note that the data presented in C and D were obtained in separate experiments.
Inhibition of Cdk1 does not result in accumulation of post-Golgi vesicle cargo proteins
We next tested whether Cdk1 inhibition caused defects in transport from the Golgi to the plasma membrane. In budding yeast, secreted proteins exit the Golgi in at least two classes of vesicles. The cell wall β-glucanase Bgl2 is transported directly from the Golgi to the plasma membrane, and another class of vesicles transports invertase, encoded by the SUC2 gene, to the plasma membrane via an endosomal compartment (Harsay and Schekman, 2002). We monitored trafficking of Bgl2 using an antibody against the protein and Suc2 using a hemagglutinin (HA)-tagged version of the protein that had been used previously (Wiederkehr et al., 2003; Gillingham et al., 2006). CDK1 or cdk1-as1 cells were treated with DMSO or 1NM-PP1 and processed to yield intracellular and extracellular fractions that were probed by Western blotting to assay levels of Bgl2 (Figure 4B) or Suc2 (Figure 4C). As a control, trafficking of Bgl2 and Suc2 were also assayed in cells carrying the sec6-4 temperature-sensitive mutant. Sec6 is a component of the exocyst complex, which is required for vesicle tethering and fusion at the plasma membrane (TerBush et al., 1996). Thus inactivation of Sec6 causes a rapid block of exocytosis and accumulation of post-Golgi secretory vesicles.
Blocking post-Golgi secretion with the sec6-4 mutant led to an intracellular accumulation of both Bgl2 and Suc2, as expected (Figure 4, B and C; Adamo et al., 2001). In contrast, intracellular levels of Bgl2 or Suc2 did not increase after cdk1-as1 inhibition, and instead Suc2 levels appeared to drop slightly (Figure 4, B and C). The failure to accumulate intracellular post-Golgi vesicle cargoes after cdk1-as1 inhibition contrasted with the effect of actin depolymerization, which caused a moderate accumulation (Figure 4D). This is concordant with earlier work demonstrating that although the actin cytoskeleton is required for polarized growth, it is only partially required for secretion of Suc2 (Novick and Botstein, 1985). Cell viability was not reduced after 1 h of cdk1-as1 inhibition, making it unlikely that cell death accounted for the lack of cargo accumulation (Supplemental Figure S4). These observations further support the conclusion that inhibition of 1NM-PP1 does not lead to an accumulation of post-Golgi secretory vesicles.
Cdk1 activity is required for normal trafficking of post-Golgi secretory vesicles
To learn more about the fate of post-Golgi vesicles after cdk1-as1 inhibition, we studied the dynamics of post-Golgi vesicle markers in live cells. CDK1 and cdk1-as1 cells were pulse chased with FM4-64 to label the vacuole and treated with 1NM-PP1 or DMSO. Post-Golgi vesicle dynamics were then monitored 1 h after treatment by imaging GFP-Sec4. In cdk1-as1 cells treated with DMSO and in wild-type CDK1 cells treated with 1NM-PP1, GFP-Sec4 particles were rapidly transported to the bud. Kymographs were generated to analyze GFP-Sec4 dynamics, revealing that under these imaging conditions, particles were transported at a rate of around 1.7 μm/s (Figure 5A and Supplemental Movies S1–S4). GFP-Sec4 particles often dwelt at the bud neck, presumably reflecting either a time lag before association of the vesicle with an actin cable or the presence of a septin-dependent barrier (Takizawa et al., 2000; Supplemental Movies S1 and S3). A subset of GFP-Sec4 particles cycled between the plasma membrane and the cytoplasm at the end of the mother cell opposite the bud. To our knowledge, the existence of this trafficking behavior has not previously been reported.
FIGURE 5:
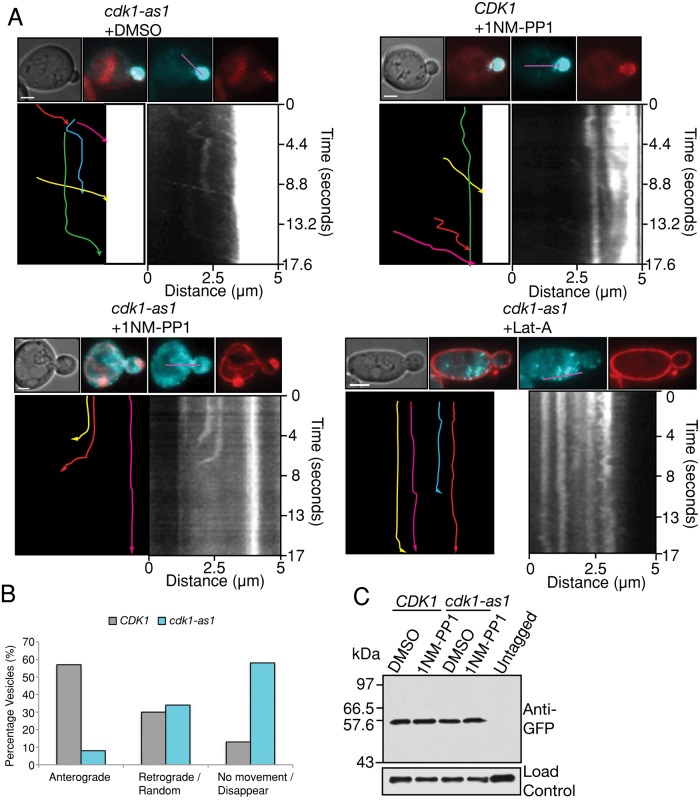
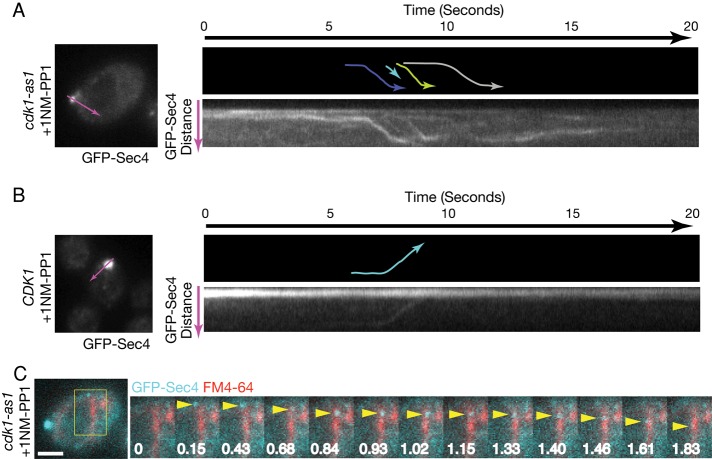
Cdk1 inhibition results in aberrant targeting of post-Golgi vesicles. (A) Cells of the indicated genotypes were treated with 1NM-PP1 or Lat-A for 1 h and pulse chased with FM4-64 to label the vacuole. From 80 to 100 images were then acquired at 220-ms intervals and stacked vertically to generate kymographs. In each set, shown at the top are a differential interference contrast image, merged images of GFP-Sec4 (cyan) and FM4-64 (red), and the individual images used to generate the merge. The longitudinal line used to generate kymographs is shown in purple. Lower right, the resulting kymograph; lower left, a tracing of the kymograph in which each colored line represents the trajectory of a different GFP-Sec4 particle, with the arrowhead showing the direction of movement. Scale bar, 2 μm. (B) Quantitation of vesicle behavior. n = 282 vesicles for CDK1 and n = 671 vesicles for cdk1-as1. (C) Levels of GFP-Sec4 after 1 h of treatment with 1NM-PP1 or DMSO. Nap1 was used as a loading control.
Inhibition of cdk1-as1 severely disrupted the dynamics of GFP-Sec4 trafficking. Few GFP-Sec4 particles were visible, and those that were visible either made short, retrograde movements away from the bud before disappearing or completely failed to move and then often disappeared (Figure 5A and Supplemental Movie S4). GFP-Sec4 disappearance coincided with endomembrane/vacuole localization, suggesting that some vesicles may be consumed in the endomembrane/vacuole (Figure 5A and Supplemental Movie S4). Quantitation of 200–600 particle movements revealed that >55% of GFP-Sec4 particles showed anterograde movements in wild-type CDK1 cells treated with 1NM-PP1. In contrast, <10% of GFP-Sec4 particles were transported in this manner in cdk1-as1 cells treated with 1NM-PP1, and many particles disappeared during analysis, which may be due to their targeting to the vacuole (Figure 5B). Western blotting revealed that GFP-Sec4 levels were unchanged after cdk1-as1 inhibition (Figure 5C). Unlike cdk1-as1 inhibition, treatment with Lat-A led to the accumulation of post-Golgi particles in the cytoplasm that did not disappear during analysis (Figure 5A and Supplemental Movie S2). These results indicate that Cdk1 activity is critical for the polarized anterograde trafficking of post-Golgi vesicles and that the effects of inhibition of Cdk1 are distinct from those of actin depolymerization.
The technical challenge of following particles that move at high speeds in an x, y, z-axis is formidable, and it is not possible to image all particles at all times as one would ideally wish. Therefore, we also observed the fate of post-Golgi particles in cells with small buds, which minimized the concern that vesicles were moving out of the focal plane, since the z axis is greatly reduced. These experiments were carried out using a total internal reflection (TIRF) imaging system equipped with a sensitive electron-multiplying charge-coupled device (EM-CCD) camera, which enabled image acquisition at speeds of up to 30 ms. The improved sensitivity of this system facilitated longer observations of GFP-Sec4 dynamics after inhibition of cdk1-as1. Consistent with the results presented in Figure 5, kymographs of GFP-Sec4 dynamics 20 min after cdk1-as1 inhibition revealed a stream-like flow of GFP-Sec4 particles out of the bud and into the mother cell (Figure 6A). Some of these particles disappeared upon reaching the vacuole membrane (Figure 6C). These observations suggest that all vesicles are not fusing with the plasma membrane in or near the bud after cdk1-as1 inhibition.
FIGURE 6:
Retrograde streaming of post-Golgi particles from sites of polarized growth into the mother cell after cdk1-as1 inhibition. (A) cdk1-as1 cells or (B) CDK1 cells were treated with 1NM-PP1 for 20 min and imaged. Approximately 650 images were acquired at 30-ms intervals and stacked vertically to generate kymographs. In each set of images, shown at the top is a schematic representation of the kymograph in which the colored lines indicate individual particle trajectories and the arrow indicates the direction of movement. The longitudinal line used to generate kymographs is shown in purple on an image of the cell used for imaging. (C) A series of images from the cdk1-as1 cell shown in A, where a GFP-Sec4 particle (indicated by a yellow arrowhead) moves to the vacuolar membrane (shown in red after staining with FM4-64) and then disappears. Time is shown in seconds. Scale bar, 2 μm.
When cdk1-as1 was inhibited, those particles that moved did so in a vectoral manner at a similar velocity, consistent with motor-mediated transport of post-Golgi vesicles along actin cables. The velocities of vesicle movement in the four vesicle tracks shown in Figure 6A were calculated from the kymographs and found to be 2.6, 2.7, 2.7, and 3.5 μm/s, which is within the range of velocity previously reported for myosin motor–mediated transport (Schott et al., 2002). The speed is slightly faster than that of the vesicles shown in Figure 5. This may reflect the fact that the cells in Figure 5 were treated with inhibitor for 1 h before imaging, whereas the cells shown in Figure 6 were treated for 20 min. The movement of vesicles out of the bud after cdk1-as1 inhibition contrasted with control CDK1 cells treated with 1NM-PP1, where GFP-Sec4 particles moved from the mother to the bud, as expected (Figure 6B).
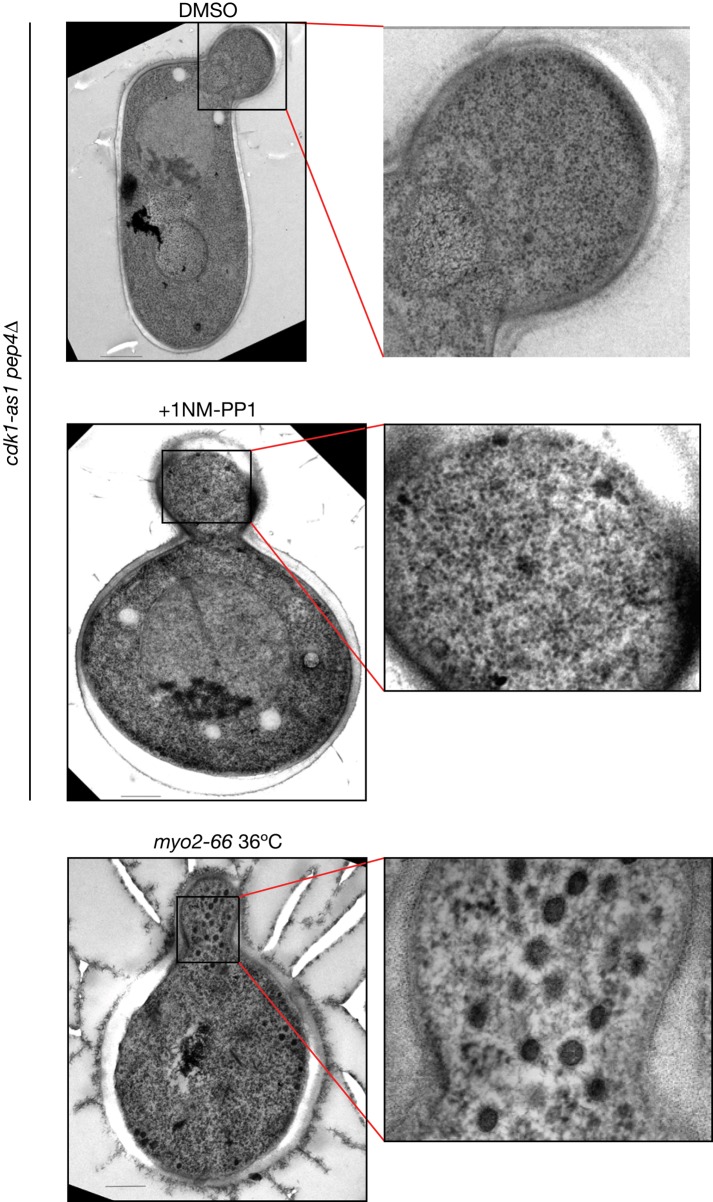
Previous work found that the myo2-66 temperature-sensitive allele caused accumulation of vesicles without causing accumulation of secretory cargoes (Govindan et al., 1995). MYO2 encodes the myosin that moves vesicles along actin tracks to the bud (Johnston et al., 1991). The failure to accumulate secretory cargoes in myo2-66 cells is similar to the effects of inhibiting cdk1-as1. We therefore considered the possibility that inhibition of cdk1-as1 blocks Myo2 function and causes accumulation of a class of vesicles that cannot be detected with GFP-Sec4. In previous work, we did not observe accumulation of vesicles by electron microscopy after inhibition of cdk1-as1 (McCusker et al., 2007). To ensure that our imaging methods can detect the class of vesicles that accumulate in myo2-66 cells, we repeated the electron microscopy analysis and included a myo2-66 control. We found that myo2-66 cells accumulated vesicles, whereas cdk1-as1 cells treated with 1NM-PP1 did not, which argues against the possibility that inhibition of cdk1-as1 blocks Myo2 function (Figure 7). In this experiment, we also tested whether pep4∆, which inactivates a major vacuolar degradation pathway, allowed accumulation of vesicles in cdk1-as1 cells. We reasoned that pep4∆ could block consumption of vesicles by the vacuole; however, we observed that the effects of inhibiting cdk1-as1 in pep4∆ cells were no different from the effects that we previously observed of inhibiting cdk1-as1 in an otherwise wild-type background (Figure 7; McCusker et al., 2007).
FIGURE 7:
Accumulation of cytoplasmic vesicles after inactivation of myo2-66 but not after cdk1-as1 inhibition. Cells were grown to early-log phase and treated for 1 h with DMSO as a control, with 1NM-PP1 to inactivate cdk1-as1, or shifted to 36ºC for 1 h to inactivate myo2-66. After rapid freezing, cells were freeze substituted and processed for transmission electron microscopy. Scale bar, 200 nm.
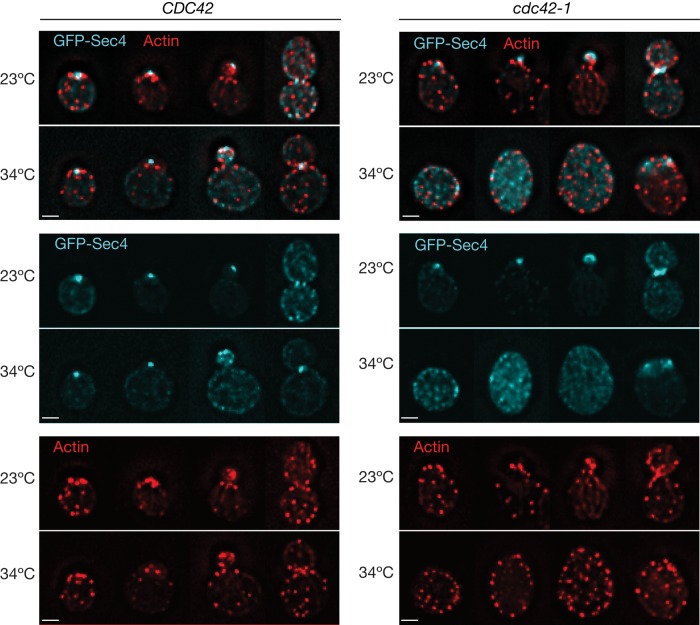
We also considered the possibility that the effects of inactivating cdk1-as1 are due solely to depolarization of the actin cytoskeleton. In this case, the effects of cdk1-as1 inhibition ought to resemble the effects of a cdc42 mutant, in which actin filaments are depolarized and disorganized (Adams et al., 1990). To test this, we monitored GFP-Sec4 and endocytosis-associated actin patch localization in wild-type and cdc42-1 cells. In the cdc42-1 cells at the restrictive temperature, cells arrested with no buds and depolarized actin patches, as previously reported, and GFP-Sec4 particles accumulated (Figure 8). These results are consistent with previous work on Igq1, which interacts with Cdc42 and is required for normal polarization of the actin cytoskeleton. Like cdc42 mutants, deletion of the IQG1 gene causes a loss of polarity phenotype, as well as accumulation of vesicles that can be seen by electron microscopy (Osman and Cerione, 1998). Actin mutants that cause disorganization of the actin cytoskeleton also cause accumulation of vesicles (Novick and Botstein, 1985). Together these results indicate that cdk1-as1 inhibition results in a defect that is distinct from that produced by actin depolymerization or actin depolarization.
FIGURE 8:
Accumulation of cytoplasmic GFP-Sec4 in cdc42-1 mutant cells. Cells expressing GFP-Sec4 (cyan) were shifted to the temperature indicated and fixed after 3 h. The actin cytoskeleton (red) was stained with Alexa 546–phalloidin. Images show maximum projections of 15–20 deconvolved z-sections through different cells at distinct cell cycle stages. Shown are individual GFP-Sec4 and actin channels and the merge of the two signals. Scale bar, 2 μm.
DISCUSSION
We propose that Cdk1 controls membrane trafficking events that are required for proper delivery of vesicles to the cell surface during polarized growth. A recent proteomics study identified many components of the secretory pathway as potential direct targets of Cdk1, consistent with a role for Cdk1 in controlling membrane-trafficking events (Holt et al., 2009).
The events controlled by Cdk1 that are required for normal membrane trafficking are unknown. One possibility is that Cdk1 directs post-Golgi vesicles to the site of polarized growth. It could do this by initiating events that mark vesicles with a targeting signal or by activating a docking site at the site of cell growth. In the absence of the targeting signals, vesicles could be redirected to the endomembrane system or vacuole via a default trafficking pathway. Previous studies found that the vacuole may be the default trafficking destination in the yeast secretory pathway and that additional targeting or retention signals exist for sorting to other destinations (Roberts et al., 1992; Wilcox et al., 1992). Alternatively, Cdk1 could be required to suppress signals that target post-Golgi vesicles to the endomembrane system or vacuole. Additional models are possible, and further work will be necessary to fully understand the molecular mechanisms underlying the effects of Cdk1 on membrane traffic during growth of the daughter cell membrane.
Disappearance of vesicles after Cdk1 inhibition was dependent upon actin filaments. Previous work found that Cdc42 stimulates actin polymerization at the vacuole, and Cdc42 and Rho1 localize to the vacuole membrane, where they regulate actin-dependent membrane fusion events (Eitzen et al., 2001; Isgandarova et al., 2007). Thus actin filaments associated with the vacuole or endomembrane system could be responsible for movement of vesicles toward these compartments, where they could be consumed via a Rho-GTPase–dependent mechanism. This model would be consistent with our observation that disappearance of vesicles in vivo appears to occur near the endomembrane system.
Although our results suggest that vesicles are redirected to the endomembrane system or vacuole, we cannot rule out other fates. For example it is possible that vesicles are redirected to the Golgi or that the vesicles are dismantled via a novel mechanism.
Several observations suggest that the effects of cdk1-as1 inhibition are not due to depolarization of the actin cytoskeleton. First, the effects of cdk1-as1 inhibition are different from the effects of inactivating Cdc42, which is required for actin polarization. Inhibition of cdk1-as1 with the concentrations of 1NM-PP1 used here results in rapid cessation of bud growth, and the mother cell does not increase in volume (Bishop et al., 2000; McCusker et al., 2007). In contrast, when cdc42-1 is inactivated, cells undergo a uniform arrest as very large unbudded cells (Adams et al., 1990). This indicates that buds and unbudded cells continue to grow when cdc42-1 is inactivated. These observations suggest that slow depolarized growth takes place when the actin cytoskeleton is depolarized, in contrast to the effects of inactivating cdk1-as1. Second, if inhibition of cdk1-as1 exerted its effects via depolarization of the actin cytoskeleton, one should be able to detect significant numbers of vesicles in cells after inhibition of cdk1-as1. However, we detected few GFP-Sec4 marked vesicles when cdk1-as was inhibited, whereas vesicles were detected in cdc42-1 cells.
It may seem surprising that inhibition of cdk1-as1 causes severe defects in trafficking of post-Golgi vesicles but does not cause severe defects in trafficking of several secretory cargoes. However, previous work showed that even quite strong blocks in exocytic trafficking such as those seen in exo84-112, sec6-4, cdc42-6, or ypt31/32Δ can leave other trafficking pathways such as the CPY route intact and operational with normal kinetics (Jedd et al., 1997; Adamo et al., 2001; Zhang et al., 2005). Conversely, endocytic mutants such as sla2Δ and end4Δ are normal in CPY trafficking and invertase secretion (Raths et al., 1993; Mulholland et al., 1997). Similarly, invertase secretion has been reported to be normal in the endosome-to–plasma membrane recycling mutant rcy1Δ (Wiederkehr et al., 2000).
We observed that sites of actin-dependent endocytosis form a ring that surrounds a central axis of exocytosis at the site of polarized growth, as suggested by previous studies that separately observed sites of endocytosis or exocytosis (Kilmartin and Adams, 1984; Amberg, 1998; Finger et al., 1998; Layton et al., 2011). Endocytic and exocytic compartments are also closely apposed in Aspergillus nidulans (Taheri-Talesh et al., 2008). Studies suggest that close coordination of endocytic uptake and recycling helps to optimize cell polarity (Valdez-Taubas and Pelham, 2003; Marco et al., 2007). The molecular mechanisms underlying the relative organization of endocytic and exocytic sites are poorly understood, but our observations here suggest that Cdk1 plays a role.
In summary, these observations suggest that Cdk1 plays a role in regulating membrane dynamics to coordinate membrane growth with cell cycle progression. Yeast cells are able to maintain the same basic size and shape despite widely varying growth rates caused by changes in nutrient availability or other external stresses. Direct control of membrane dynamics by Cdk1 would offer an attractive model for linking membrane growth to cell cycle progression. Identification of the molecular mechanisms targeted by Cdk1 to control membrane dynamics will be an important focus for future work.
MATERIALS AND METHODS
Yeast strains and procedures
A list of the yeast strains used in this study is provided in Table 1. Tagging of open reading frames at the C-terminus was achieved by PCR-based homologous recombination at the endogenous gene locus. GFP-Sec4 was expressed from a CEN vector (pRS315) under the control of the Sec4 promoter (Schott et al., 2002). Unless stated, cells were grown in yeast extract/peptone/dextrose (ThermoFisher Scientific, Waltham, MA) or selective media supplemented with 50 μg/ml adenine sulfate (ICN Biomedicals, Irvine, CA) at 23ºC.
TABLE 1:
Strains used in this work.
| Name | Genotype | Reference |
|---|---|---|
| DK186 | MATa bar1 his3-11,15 leu2-3112 trp1-1 ura3-1 ade2-1 can1-100 | |
| DMY496 | MATa bar1 cdc28-as1a | Bishop et al. (2001) |
| DMY401 | MATa bar1 cdc28-as1 sla2Δ::kanMX6 | This study |
| DMY543 | MATa bar1 cdc28-as1 SEC2-GFP::HIS3MX6 | This study |
| DMY891 | MATa bar1 BGL2-6XHA::HIS3MX6 | This study |
| DMY894 | MATa bar1 cdc28-as1 BGL2-6XHA::HIS3MX6 | This study |
| DMY870 | MATa bar1 SUC2-3XHA::HIS3MX6 | This study |
| DMY872 | MATa bar1 cdc28-as1 SUC2-3XHA::HIS3MX6 | This study |
| DMY890 | MATa sec6-4 SUC2-3XHA::HIS3MX6 | This study |
aNote that the budding yeast CDC28 gene encodes Cdk1. We refer to cyclin-dependent kinase 1 as Cdk1 throughout this article.
Treatment of cells with inhibitors
In all experiments involving addition of inhibitors, cells were treated with 25 μM 1NM-PP1 (Calbiochem, La Jolla, CA) to inhibit cdk1-as1 activity or 100 μM latrunculin-A to inhibit F-actin.
Centrifugal elutriation
A 1.3-l culture of cells was grown overnight to OD600 1.4. A synchronous population of unbudded cells was obtained by centrifugal elutriation. Cells were centrifuged at 2700 rpm and elutriated at 31 ml/min. Cells were released into fresh media and monitored for the synchronous formation of small buds, an indicator of the initiation of polarized growth. At bud formation, cells were treated with DMSO (Sigma-Aldrich, St. Louis, MO) or with inhibitors. Treated cells were incubated at 23ºC for 1 h and then fixed in 3.7% formaldehyde (ThermoFisher Scientific) for 1 h.
Microscopy
Cells were grown to early logarithmic phase in selective media, treated with 1NM-PP1 for the times indicated, and attached to coverslips that had been coated with 1 mg/ml concanavalin-A (Sigma-Aldrich). Coverslips were then mounted onto glass slides using two pieces of double-sided tape. For single-section wide-field images (Figures 1, 2B, 3, and 5), cells were visualized using a Zeiss Axioskop II microscope with a Zeiss AxioCam HRm camera (Carl Zeiss, Jena, Germany). Photobleaching was minimized using a Uniblitz VMMD1 external shutter driver (Uniblitz, Rochester, NY). A 100× (numerical aperture [NA] 1.3) Plan-Neofluar (Carl Zeiss) oil objective was used to visualize cells. Image acquisition was performed using Zeiss AxioVision software. Kymographs were generated using MetaMorph software (Molecular Devices, Sunnyvale, CA). Multiple z-sections shown in Figure 2A were acquired on a DMI600B inverted Leica fluorescent microscope (Leica, Wetzlar, Germany) equipped with a Hamamatsu ORCA C9100 EM-CCD camera (Hamamatsu Photonics, Hamamatsu, Japan) and a 100× (NA 1.4) Plan-Apochromat oil objective. From 15 to 20 0.2-μm steps were acquired using Leica LASAF6000 software. Ten iterations of a blind deconvolution algorithm were run, using a refractive index of 1.5.
High-speed near-TIRFM was used to monitor GFP-Sec4 puncta in Figure 6. In near-TIRFM, the incidence angle of the laser with respect to the sample is reduced, generating a deeper evanescent field. This configuration was used to resolve puncta moving beneath the cell wall in budding yeast. The TIRFM system comprised an Axiovert 200M (Carl Zeiss) microscope chassis with a 100× (NA 1.46) Plan Apochromat oil objective with an additional 1.6 Optovar lens, an Evolve EM-CCD camera (Photometrics, Tucson, AZ) and a Dual View DV2 beam-splitter (Photometrics). Samples were illuminated using acousto-optic tunable filter–controlled 488- and 561-nm laser lines coupled to a motorized TIRF slider (Carl Zeiss) via a single-mode fiber. The system was controlled by MetaMorph software. The data presented in Figure 6 were generated by acquiring images at 30-ms intervals and generating kymographs using MetaMorph.
Electron microscopy
Cells were pelleted, and the pellets were placed on the surface of a copper electron microscopy grid (400 mesh) that had been coated with Formvar. Each loop was quickly immersed in liquid propane precooled and maintained at −180°C using liquid nitrogen. The loops were then transferred to a precooled solution of 4% osmium tetroxide in dry acetone in a 1.8-ml polypropylene vial at −82°C for 72 h (substitution) and warmed gradually to room temperature, followed by three washes in dry acetone. Specimens were stained in the dark for 1 h in 1% uranyl acetate in acetone at 4°C. After an additional rinse in dry acetone, the samples were infiltrated progressively with araldite (epoxy resin; Fluka, Sigma-Aldrich). Ultrathin sections were contrasted with lead citrate and observed with a Hitachi 7650 electron microscope (Hitachi, Tokyo, Japan; Bordeaux Imaging Center, Electronic Microscopy Pole of the University of Bordeaux 2).
Assays for secretion and endocytosis
Invertase (Suc2) secretion was monitored using a 3xHA-tagged version of the protein, as used previously (Gillingham et al., 2006). Cells were grown to early logarithmic phase in yeast extract/peptone/dextrose (YPD) and then shifted to yeast extract/peptone (YEP) media containing 0.1% dextrose and 1NM-PP1 or DMSO (control) for 1 h to induce invertase secretion. sec6-4 cells were resuspended in YEP + 0.1% dextrose and then immediately shifted to 34ºC for 1 h. At the end of the experiment, NaN3 and NaF were added to 10 mM, and cells were incubated on ice for 10 min. Cells were then rinsed in wash buffer (20 mM Tris-HCl, pH 7.5, 10 mM NaN3, 10 mM NaF). We removed 1.5 OD600 of cells and resuspended them in 150 μl of spheroplast buffer (50 mM Tris-HCl, pH 7.5, 1.4 M sorbitol, 10 mM NaN3, 10 mM NaF, 30 mM 2-mercaptoethanol, 0.2 mg ml−1 Zymolyase 100T; Seikagaku, Tokyo, Japan) and incubated at 37ºC for 20 min to digest the cell wall. All subsequent steps were carried out at 4ºC. Spheroplasts were pelleted by centrifugation at 2000 × g for 3 min. A 100-μl supernatant (external secreted fraction) was removed and boiled in 4× SDS–PAGE sample buffer. The spheroplast pellet was washed twice in spheroplast wash buffer (50 mM Tris-HCl, pH 7.5, 1.4 M sorbitol, 10 mM NaF, 10 mM NaN3). Spheroplasts were resuspended in 120 μl of lysis buffer (20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 2 mM MgCl2, 0.5% Triton-X100, 2 mM phenylmethylsulfonyl fluoride [PMSF], 1 μg/ml leupeptin, 1 μg/ml pepstatin-A, 1 μg/ml chymostatin), vortexed for 30 s, and then centrifuged at 82 × g for 5 min. A 100-μl supernatant (internal fraction) was boiled in 1× SDS–PAGE sample buffer. Samples were resolved using 7.5% SDS–PAGE, blotted, and probed with anti-HA polyclonal antisera. Internal and external Bgl2 levels were measured in the same manner, except that cells were maintained in YPD media, and Bgl2 was resolved using 12.5% SDS–PAGE. The cytoplasmic protein Nap1 was not detected in the extracellular fraction, indicating that cells were not overspheroplasted (unpublished data).
Endocytosis was assayed by pulse chasing the lipophilic dye FM4-64 (Invitrogen, Carlsbad, CA) through the endocytic pathway. Early-logarithmic-phase cells were treated with 25 μM 1NM-PP1 for 1 h at 23ºC and placed on ice for 5 min to block endocytosis, and FM4-64 was then added to 16 μM for 15 min (on ice). Cells were then washed into fresh YPD media at 23ºC containing 25 μM 1NM-PP1 to initiate the endocytic chase and imaged at the indicated times.
CPY transport assays
An overnight culture of logarithmic-phase cells was resuspended in minimal media lacking methionine and containing 2 mg/ml bovine serum albumin (Sigma-Aldrich). After 1 h of treatment with 1NM-PP1, or DMSO as a control, cells were adjusted to a concentration of 1 OD600/ml. Cells were incubated with 25 μCi 35S-methionine (Amersham-Pharmacia Biotech, GE Healthcare Bio-Sciences, Piscataway, NJ) per OD600 for 10 min (pulse), and the chase was then initiated by the addition of 10× chase mix (20% glucose, 50 mM methionine) to a final concentration of 1×. At the times indicated, 1 OD600 of cells was centrifuged and rapidly frozen in liquid nitrogen.
To immunoprecipitate CPY, metabolically labeled cells were resuspended in 75 μl of lysis buffer (1% SDS, 8 M urea, 2 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml pepstatin-A, 1 μg/ml chymostatin) and lysed by glass bead agitation for 1 min, 30 s in a cell homogenizer (BioSpec, Bartlesville, OK). Fresh buffer was added to give a final lysis buffer composition (10 mM Tris-HCl, pH 8.0, 0.1% Tween-20, 2 mM EDTA, 0.05% SDS, 40 mM urea, 100 mM NaCl). Insoluble debris was removed after centrifugation at 16,000 × g for 15 min. A 2-μl amount of anti-CPY was added to each tube, and immunoprecipitations were carried out for 1 h at room temperature. We added 20 μl of protein-A agarose (Bio-Rad, Hercules, CA) to each tube for 1 h and then washed the samples four times in 1.5 ml of wash buffer (10 mM Tris-HCl pH 8.0, 0.1% SDS, 0.1% Tween-20, 2 mM EDTA). Immunoprecipitates were resolved by SDS–PAGE (7.5% acrylamide) and analyzed using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Supplementary Material
Acknowledgments
We gratefully acknowledge the expertise of Benedicte Salin in conducting the electron microscopy experiments presented in Figure 7. We also thank Robert Arkowitz, Patrick Brennwald, Wei Guo, David Drubin, Ruth Collins, Marcus Babst, Randy Schekman, and Phil Crews for reagents and/or advice. Finally, we thank members of the Kellogg lab for helpful discussions. This work was funded by National Institutes of Health Grant GM053959-10 to D.K. Work in D.M.'s lab is funded by FP7 Marie Curie Grant IRG249298/Growth and Division, Agence Nationale de la Recherche Grant 2010 JCJC 1210 01, Fondation pour la Recherche Medicale Grant (INE20100518678), the Centre National de la Recherche Scientifique, Université de Bordeaux 2, and Conseil Régional d'Aquitaine Volet Recherche 20091301015.
Abbreviations used:
- 1NM-PP1
4-amino-1-tert-butyl-3-(1′-naphthylmethyl)pyrazolo[3,4-d]pyrimidine
- Cdk
cyclin-dependent kinase
- GFP
green fluorescent protein
- Lat-A
latrunculin-A
- PIP2
phosphatidyl inositol 4,5-bisphosphate
- PMSF
phenylmethylsulfonyl fluoride
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-10-0834) on July 5, 2012.
*These authors contributed equally to this work.
REFERENCES
- Adamo JE, Moskow JJ, Gladfelter AS, Viterbo D, Lew DJ, Brennwald PJ. Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud. J Cell Biol. 2001;155:581–592. doi: 10.1083/jcb.200106065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams AE, Johnson DI, Longnecker RM, Sloat BF, Pringle JR. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J Cell Biol. 1990;111:131–142. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg DC. Three-dimensional imaging of the yeast actin cytoskeleton through the budding cell cycle. Mol Biol Cell. 1998;9:3259–3262. doi: 10.1091/mbc.9.12.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin DG. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AC, Buzko O, Shokat KM. Magic bullets for protein kinases. Trends Cell Biol. 2001;11:167–172. doi: 10.1016/s0962-8924(01)01928-6. [DOI] [PubMed] [Google Scholar]
- Bishop AC, et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- Culotti J, Hartwell LH. Genetic control of the cell division cycle in yeast. 3. Seven genes controlling nuclear division. Exp Cell Res. 1971;67:389–401. doi: 10.1016/0014-4827(71)90424-1. [DOI] [PubMed] [Google Scholar]
- Eitzen G, Thorngren N, Wickner W. Rho1p and Cdc42p act after Ypt7p to regulate vacuole docking. EMBO J. 2001;20:5650–5656. doi: 10.1093/emboj/20.20.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista M, Blundell K, Longtine MS, Chow CJ, Adames N, Pringle JR, Peter M, Boone C. Bni1p, a yeast formin linking cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 1997;276:118–122. doi: 10.1126/science.276.5309.118. [DOI] [PubMed] [Google Scholar]
- Finger FP, Hughes TE, Novick P. Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell. 1998;92:559–571. doi: 10.1016/s0092-8674(00)80948-4. [DOI] [PubMed] [Google Scholar]
- Gillingham AK, Whyte JR, Panic B, Munro S. Mon2, a relative of large Arf exchange factors, recruits Dop1 to the Golgi apparatus. J Biol Chem. 2006;281:2273–2280. doi: 10.1074/jbc.M510176200. [DOI] [PubMed] [Google Scholar]
- Govindan B, Bowser R, Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J Cell Biol. 1995;128:1055–1068. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Tamanoi F, Novick P. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat Cell Biol. 2001;3:353–360. doi: 10.1038/35070029. [DOI] [PubMed] [Google Scholar]
- Harsay E, Schekman R. A subset of yeast vacuolar protein sorting mutants is blocked in one branch of the exocytic pathway. J Cell Biol. 2002;156:271–285. doi: 10.1083/jcb.200109077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A, Tanner W. Biosynthesis of the vacuolar yeast glycoprotein carboxypeptidase Y. Conversion of precursor into the enzyme. Eur J Biochem. 1978;85:599–608. doi: 10.1111/j.1432-1033.1978.tb12275.x. [DOI] [PubMed] [Google Scholar]
- Holt LJ, Tuch BB, Villen J, Johnson AD, Gygi SP, Morgan DO. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science. 2009;325:1682–1686. doi: 10.1126/science.1172867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DA, Yang S, Drubin DG. Synthetic-lethal interactions identify two novel genes, SLA1 and SLA2, that control membrane cytoskeleton assembly in Saccharomyces cerevisiae. J Cell Biol. 1993;122:635–644. doi: 10.1083/jcb.122.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgandarova S, Jones L, Forsberg D, Loncar A, Dawson J, Tedrick K, Eitzen G. Stimulation of actin polymerization by vacuoles via Cdc42p-dependent signaling. J Biol Chem. 2007;282:30466–30475. doi: 10.1074/jbc.M704117200. [DOI] [PubMed] [Google Scholar]
- Jedd G, Mulholland J, Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J Cell Biol. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GC, Prendergast JA, Singer RA. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol. 1991;113:539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J, Adams AEM. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984;98:922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Galletta BJ, Schmidt KO, Chang FS, Blumer KJ, Cooper JA. Actin-based motility during endocytosis in budding yeast. Mol Biol Cell. 2006;17:1354–1363. doi: 10.1091/mbc.E05-10-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus M, Pelli-Gulli MP, van Drogen F, Springer S, Jaquenoud M, Peter M. Phosphorylation of Bem2p and Bem3p may contribute to local activation of Cdc42p at bud emergence. EMBO J. 2007;26:4501–4513. doi: 10.1038/sj.emboj.7601873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono K, Nogami S, Abe M, Nishizawa M, Morishita S, Pellman D, Ohya Y. G1/S cyclin-dependent kinase regulates small GTPase Rho1p through phosphorylation of RhoGEF Tus1p in Saccharomyces cerevisiae. Mol Biol Cell. 2008;19:1763–1771. doi: 10.1091/mbc.E07-09-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschewski R, Hall A, Mellman I. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat Cell Biol. 1999;1:8–13. doi: 10.1038/8977. [DOI] [PubMed] [Google Scholar]
- Kubler E, Riezman H. Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J. 1993;12:2855–2862. doi: 10.1002/j.1460-2075.1993.tb05947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton AT, Savage NS, Howell AS, Carroll SY, Drubin DG, Lew DJ. Modeling vesicle traffic reveals unexpected consequences for Cdc42p-mediated polarity establishment. Curr Biol. 2011;21:184–194. doi: 10.1016/j.cub.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew DJ, Reed SI. Morphogenesis in the yeast cell cycle: regulation by Cdc28 and cyclins. J Cell Biol. 1993;120:1305–1320. doi: 10.1083/jcb.120.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco E, Wedlich-Soldner R, Li R, Altschuler SJ, Wu LF. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell. 2007;129:411–422. doi: 10.1016/j.cell.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker D, Denison C, Anderson S, Egelhofer TA, Yates JR, 3rd, Gygi SP, Kellogg DR. Cdk1 coordinates cell-surface growth with the cell cycle. Nat Cell Biol. 2007;9:506–515. doi: 10.1038/ncb1568. [DOI] [PubMed] [Google Scholar]
- Moffat J, Andrews B. Late-G1 cyclin-CDK activity is essential for control of cell morphogenesis in budding yeast. Nat Cell Biol. 2004;6:59–66. doi: 10.1038/ncb1078. [DOI] [PubMed] [Google Scholar]
- Moseley JB, Nurse P. Cdk1 and cell morphology: connections and directions. Curr Opin Cell Biol. 2009;21:82–88. doi: 10.1016/j.ceb.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Mulholland J, Wesp A, Riezman H, Botstein D. Yeast actin cytoskeleton mutants accumulate a new class of Golgi-derived secretary vesicle. Mol Biol Cell. 1997;8:1481–1499. doi: 10.1091/mbc.8.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JM, Johnson DI. The Cdc42p GTPase and its regulators Nrf1p and Scd1p are involved in endocytic trafficking in the fission yeast Schizosaccharomyces pombe. J Biol Chem. 2001;276:3004–3009. doi: 10.1074/jbc.M007389200. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. A prize for proliferation. Cell. 2001;107:689–701. doi: 10.1016/s0092-8674(01)00604-3. [DOI] [PubMed] [Google Scholar]
- Nern A, Arkowitz RA. Nucleocytoplasmic shuttling of the Cdc42p exchange factor Cdc24p. J Cell Biol. 2000;148:1115–1122. doi: 10.1083/jcb.148.6.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985;40:405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Osman MA, Cerione RA. Iqg1p, a yeast homologue of the mammalian IQGAPs, mediates cdc42p effects on the actin cytoskeleton. J Cell Biol. 1998;142:443–455. doi: 10.1083/jcb.142.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raths S, Rohrer J, Crausaz F, Riezman H. end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J Cell Biol. 1993;120:55–65. doi: 10.1083/jcb.120.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CJ, Nothwehr SF, Stevens TH. Membrane protein sorting in the yeast secretory pathway: evidence that the vacuole may be the default compartment. J Cell Biol. 1992;119:69–83. doi: 10.1083/jcb.119.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott D, Ho J, Pruyne D, Bretscher A. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J Cell Biol. 1999;147:791–808. doi: 10.1083/jcb.147.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott DH, Collins RN, Bretscher A. Secretory vesicle transport velocity in living cells depends on the myosin-V lever arm length. J Cell Biol. 2002;156:35–39. doi: 10.1083/jcb.200110086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y, Gulli MP, Peter M. Nuclear sequestration of the exchange factor Cdc24 by Far1 regulates cell polarity during yeast mating. Nat Cell Biol. 2000;2:117–124. doi: 10.1038/35000073. [DOI] [PubMed] [Google Scholar]
- Shuster CB, Burgess DR. Targeted new membrane addition in the cleavage furrow is a late, separate event in cytokinesis. Proc Natl Acad Sci USA. 2002;99:3633–3638. doi: 10.1073/pnas.052342699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopko R, Huang D, Smith JC, Figeys D, Andrews BJ. Activation of the Cdc42p GTPase by cyclin-dependent protein kinases in budding yeast. EMBO J. 2007;26:4487–4500. doi: 10.1038/sj.emboj.7601847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T, Esmon B, Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982;30:439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Taheri-Talesh N, Horio T, Araujo-Bazan L, Dou X, Espeso EA, Penalva MA, Osmani SA, Oakley BR. The tip growth apparatus of Aspergillus nidulans. Mol Biol Cell. 2008;19:1439–1449. doi: 10.1091/mbc.E07-05-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa PA, DeRisi JL, Wilhelm JE, Vale RD. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science. 2000;290:341–344. doi: 10.1126/science.290.5490.341. [DOI] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Novick P. Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J Cell Biol. 1995;130:299–312. doi: 10.1083/jcb.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez-Taubas J, Pelham HR. Slow diffusion of proteins in the yeast plasma membrane allows polarity to be maintained by endocytic cycling. Curr Biol. 2003;13:1636–1640. doi: 10.1016/j.cub.2003.09.001. [DOI] [PubMed] [Google Scholar]
- VerPlank L, Li R. Cell cycle-regulated trafficking of Chs2 controls actomyosin ring stability during cytokinesis. Mol Biol Cell. 2005;16:2529–2543. doi: 10.1091/mbc.E04-12-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr A, Avaro S, Prescianotto-Baschong C, Haguenauer-Tsapis R, Riezman H. The F-box protein Rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in Saccharomyces cerevisiae. J Cell Biol. 2000;149:397–410. doi: 10.1083/jcb.149.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr A, Du Y, Pypaert M, Ferro-Novick S, Novick P. Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum. Mol Biol Cell. 2003;14:4770–4782. doi: 10.1091/mbc.E03-04-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CA, Redding K, Wright R, Fuller RS. Mutation of a tyrosine localization signal in the cytosolic tail of yeast Kex2 protease disrupts Golgi retention and results in default transport to the vacuole. Mol Biol Cell. 1992;3:1353–1371. doi: 10.1091/mbc.3.12.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Rossi G, Brennwald P. The ghost in the machine: small GTPases as spatial regulators of exocytosis. Trends Cell Biol. 2008;18:397–404. doi: 10.1016/j.tcb.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Orlando K, He B, Xi F, Zhang J, Zajac A, Guo W. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J Cell Biol. 2008;180:145–158. doi: 10.1083/jcb.200704128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zajac A, Zhang J, Wang P, Li M, Murray J, TerBush D, Guo W. The critical role of Exo84p in the organization and polarized localization of the exocyst complex. J Biol Chem. 2005;280:20356–20364. doi: 10.1074/jbc.M500511200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.