The Rab GTPase Ypt7p and its effector complex HOPS participate in catalyzing the fusion of yeast vacuoles. The role of the vacuolar kinase Yck3p in this relation is examined. It is shown how the regulatory ability of the Rab GTPase cycle is enforced only by posttranslational modification of the effector complex HOPS.
Abstract
The homotypic fusion of yeast vacuoles requires the Rab-family GTPase Ypt7p and its effector complex, homotypic fusion and vacuole protein sorting complex (HOPS). Although the vacuolar kinase Yck3p is required for the sensitivity of vacuole fusion to proteins that regulate the Rab GTPase cycle—Gdi1p (GDP-dissociation inhibitor [GDI]) or Gyp1p/Gyp7p (GTPase-activating protein)—this kinase phosphorylates HOPS rather than Ypt7p. We addressed this puzzle in reconstituted proteoliposome fusion reactions with all-purified components. In the presence of HOPS and Sec17p/Sec18p, there is comparable fusion of 4-SNARE (soluble N-ethylmaleimide–sensitive factor attachment protein receptor) proteoliposomes when they have Ypt7p bearing either GDP or GTP, a striking exception to the rule that only GTP-bound forms of Ras-superfamily GTPases have active conformations. However, the phosphorylation of HOPS by recombinant Yck3p confers a strict requirement for GTP-bound Ypt7p for binding phosphorylated HOPS, for optimal membrane tethering, and for proteoliposome fusion. Added GTPase-activating protein promotes GTP hydrolysis by Ypt7p, and added GDI captures Ypt7p in its GDP-bound state during nucleotide cycling. In either case, the net conversion of Ypt7:GTP to Ypt7:GDP has no effect on HOPS binding or activity but blocks fusion mediated by phosphorylated HOPS. Thus guanine nucleotide specificity of the vacuolar fusion Rab Ypt7p is conferred through downstream posttranslational modification of its effector complex.
INTRODUCTION
Membrane fusion at each stage of exocytic and endocytic vesicular traffic is catalyzed by functionally conserved protein families (Wickner and Schekman, 2008). These include Rab GTPases, which have distinct conformations in their GTP-bound and GDP-bound states (Grosshans et al., 2006). GTP-bound Rab proteins bind “effectors,” which perform downstream functions leading to fusion. Among these effectors are large multisubunit tethering complexes (Yu and Hughson, 2010). Fusion also requires membrane-bound soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) proteins (Jahn and Scheller, 2006), each with at least one heptad-repeat SNARE domain that binds to (“snares”) the others in 4-helical bundles in-cis (when anchored to the same membrane) or in-trans (when anchored to apposed membranes). Tethering, the first step of docking, requires the Rab, and thus the Rab is the master regulator of fusion. Rab proteins have very low intrinsic rates of GTP hydrolysis unless they are activated by a GTPase-activating protein (GAP). Guanine nucleotide exchange factors (GEFs) trigger nucleotide exchange on the Rab, allowing them to recycle to their active, GTP-bound form. Rab proteins have been crystallized in their GTP- and GDP-bound forms (Constantinescu et al., 2002), have received extensive genetic study, and have been studied in vitro during the fusion of purified organelles (Hutagalung and Novick, 2011). Rabs are essential for the reconstitution of fusion with pure components, both for endosomes (Ohya et al., 2009) and vacuoles (Stroupe et al., 2009).
We study membrane fusion with yeast vacuoles (Wickner, 2010). Vacuoles undergo constant fission and fusion in the cell. Genetic disruption of fusion still allows fission to continue, resulting in a highly fragmented vacuole morphology (vam mutants), which gave an early definition of the genes needed for fusion (Wada et al., 1992). Purified vacuoles will fuse when incubated with ATP. A colorimetric assay of this fusion (Haas et al., 1994) allowed definition of the stages and catalysts of the fusion reaction (Wickner, 2010). On incubation with ATP, vacuoles disassemble their cis-SNARE complexes and synthesize phosphoinositides. They tether by means of the Ypt7p Rab-family GTPase and its hexameric effector complex, homotypic fusion and vacuole protein sorting complex (HOPS). HOPS has direct affinity for Ypt7p through its Vps39p and Vps41p subunits (Brett et al., 2008; Ostrowicz et al., 2010). HOPS also directly recognizes vacuolar lipids and SNAREs (Stroupe et al., 2006). Around each pair of apposed membranes, the proteins and lipids that are needed for fusion become enriched in a ring-shaped microdomain through a complex web of direct affinities: of Ypt7p for HOPS, of the SNAREs for each other, and of HOPS and one of the SNAREs (Vam7p) for phosphoinositides. Enrichment at the vertex ring of each lipid that is “regulatory” for fusion (diacylglycerol, ergosterol, and phosphoinositides) is regulated by the other regulatory lipids and by SNAREs (Fratti et al., 2004), although little is known of how this occurs. The SNAREs pair in-trans, and fusion occurs around the ring.
Rab GTPases are often thought of as master regulatory “switches” that are “on” when they bind GTP and “off” when GDP is bound. However, vacuole fusion is constitutive and may not require regulation during exponential growth in medium of a constant tonicity. In vitro vacuole fusion is blocked by the addition of a GAP, such as Gyp7p or Gyp1-46, which promotes GTP hydrolysis. This suggests that vacuole fusion, like many other Rab-regulated fusion events, is active only with the GTP-bound form of Ypt7p. Pioneering studies by LaGrassa and Ungermann (2005) and Cabrera et al. (2009) showed that the vacuolar kinase Yck3p, which regulates fusion in response to growth-medium osmolarity, is essential for inhibition of fusion by added GAP or Gdi1p. However, Yck3p phosphorylates the SNARE Vam3p and the Vps41p subunit of the HOPS complex but not Ypt7p. What, then, is the relationship of Yck3p action to the Gyp and Gdi1p sensitivity of fusion?
We now report that the HOPS complex works with either Ypt7:GTP or Ypt7:GDP to support fusion, whereas phosphorylated HOPS (P-HOPS), phosphorylated on its Vps41p subunit by the vacuolar kinase Yck3p (Cabrera et al., 2009), can support fusion only with Ypt7:GTP. Thus the guanine nucleotide specificity of Ypt7p is conferred through covalent modification of its downstream effector.
RESULTS
We prepare proteoliposomes from a vacuolar-mimic lipid mixture with the four vacuolar SNAREs and Ypt7p (Zucchi and Zick, 2011). To determine whether a specific nucleotide state of Ypt7p is required for reconstituted proteoliposome fusion, we stripped bound guanine nucleotide from purified Ypt7p by incubation with EDTA, followed by reloading with either GTP or GDP before reconstituted proteoliposome (RPL) reconstitution by detergent dialysis. These proteoliposomes were prepared with lumenally entrapped Cy5-streptavidin (Sa-Cy5) or entrapped biotinylated R-phycoerythrin (PhycoE), as described (Zucchi and Zick, 2011). To assay their fusion, we mixed proteoliposomes with these two lumenal markers in the presence of ATP and a large molar excess of external biotin-dextran and added recombinant HOPS, Sec17p, and Sec18p to initiate fusion (at t = 0). The ensuing fusion allowed the lumenal probes to mix and bind through the high affinity of biotin for streptavidin, bringing the Cy5 and PhycoE fluorophores into close proximity, which was assayed as fluorescence resonance energy transfer (FRET). The large excess of external biotin dextran blocked the development of any FRET signal due to lysis (Zucchi and Zick, 2011). Fusion was completely dependent on Ypt7p and HOPS but was independent of whether the Ypt7p had been loaded with GTP or GDP (Figure 1). Because vacuole fusion is favored by the GTP-bound form of Ypt7p both in vivo and in vitro (Eitzen et al., 2000), a regulatory factor or condition was likely missing from our reconstitution.
FIGURE 1:
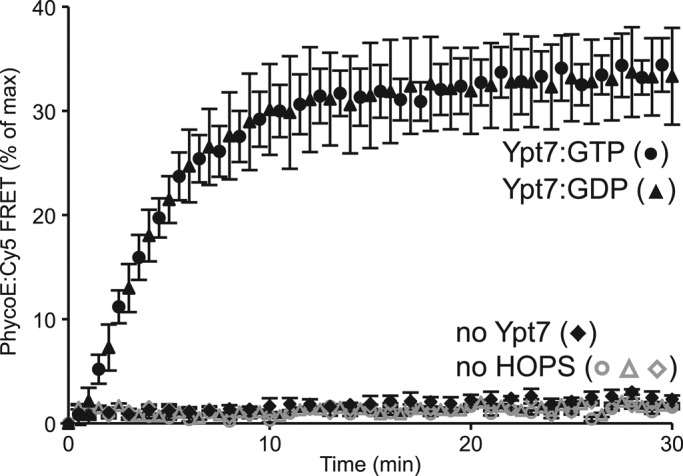
HOPS mediates the fusion of RPLs bearing Ypt7:GTP or Ypt7:GDP. Fusion was assayed as content mixing between proteoliposomes bearing either Cy5-labeled streptavidin (Sa-Cy5) or biotinylated R-phycoerythrin (PhycoE). All RPLs carried the four vacuolar SNAREs (Vam3p, Vti1p, Vam7p, Nyv1p; molar protein:lipid ratio, 1:1000) and the Rab GTPase Ypt7p (molar protein:lipid ratio, 1:2000) that had been preloaded with either GTP (circles) or GDP (triangles) before reconstitution. Reactions were incubated at 27°C with Sec17p, Sec18p, ATP, and HOPS, and the FRET signal between PhycoE and Sa-Cy5 was recorded over time (see Materials and Methods). Reactions without Ypt7p (diamonds) or HOPS (open symbols) served as controls. The increase of the FRET signal is presented as percentage of the maximal signal obtained by detergent lysis in samples that had not received biotin-dextran. Shown is the mean of three repeats; error bars represent standard deviations (SDs).
Yck3p, a vacuolar kinase that inhibits fusion, is required on the organelle for fusion to be sensitive to added GAPs such as Gyp1-46 or to Gdi1p (GDP dissociation inhibitor [GDI]; LaGrassa and Ungermann, 2005). Yck3p phosphorylates HOPS on its Vps41p subunit (Cabrera et al., 2009; Supplemental Figures S1 and S2) to yield P-HOPS, which has a lower affinity for vacuolar lipids and thus a stricter dependence on Ypt7p for its membrane association and the ensuing fusion (Hickey et al., 2009). A previous study (Cabrera et al., 2009) used a genetic approach to establish mutants that interfere with or mimic Vps41p phosphorylation, but the pattern of Vps41p residues that are phosphorylated on vacuoles by organelle-bound Yck3p in vivo is not known. Mass spectrometry analysis of our in vitro phosphorylation reactions, which employ a soluble, recombinant form of Yck3 lacking the C-terminal eight amino acids of the native protein required for palmitoylation in yeast, reveals that mainly three regions of Vps41p are modified under this conditions (Supplemental Figure S2B).
The addition of increasing levels of recombinant Yck3 inhibited the fusion of proteoliposomes bearing GDP-loaded Ypt7p to a far greater extent than for proteoliposomes with Ypt7:GTP (Figure 2A). Because Yck3p can phosphorylate other vacuolar proteins, including the SNARE Vam3p (Brett et al., 2008), we also used P-HOPS that had been reisolated after phosphorylation by Yck3p in vitro (Hickey et al., 2009). This P-HOPS, which is devoid of significant contamination with residual Yck3 (Supplemental Figure S3), has about one-half the fusion potency as HOPS with proteoliposomes bearing Ypt7:GTP (which may reflect its reduced affinity for vacuolar lipids; Hickey et al., 2009) but has entirely lost the capacity to support fusion when Ypt7p bears GDP (Figure 2B). Preincubation of the P-HOPS with lambda protein phosphatase completely relieved this inhibition and the induced nucleotide specificity. In light of the novelty of Rab functional guanine nucleotide specificity residing in the covalent modification of a downstream effector, we sought to determine which HOPS functions were being regulated.
FIGURE 2:
Phosphorylation of HOPS by Yck3p confers Ypt7p nucleotide specificity to the fusion reaction. Fusion reactions had 4-SNARE RPLs that either carried Ypt7:GTP or Ypt7:GDP. (A) The mix of Sec17p, Sec18p, HOPS, and Mg:ATP was incubated with increasing concentrations of Yck3p for 10 min at 27°C before being added to the RPL mix. The relative amount of content mixing after 30 min is compared with the control condition (RPLs with Ypt7:GTP, no Yck3p), which corresponded to 32.4% of the maximal signal obtained by detergent lysis. Shown is the mean of three experiments; error bars represent SDs. (B) Fusion reactions were performed with either purified, nonphosphorylated HOPS or phosphorylated HOPS (P-HOPS) isolated and characterized as described (Hickey et al., 2009) or P-HOPS that had been pretreated with lambda protein phosphatase (New England BioLabs, Ipswich, MA). The relative amount of content mixing after 30 min is compared with the control condition (RPLs with Ypt7:GTP incubated with nonphosphorylated HOPS), which corresponded to 24.3% of the maximal signal obtained by detergent lysis. Shown is the mean of three experiments; error bars represent SDs.
We reported (Price et al., 2000; Seals et al., 2000) that HOPS in a crude vacuolar extract binds directly to Ypt7p loaded with GTPγS, although neither the phosphorylation state of this HOPS nor its possible association with other proteins such as SNAREs (Collins et al., 2005) was known. By performing binding studies with purified Ypt7p that had been preloaded with either GTP or GDP and purified HOPS complex either in its nonphosphorylated or phosphorylated form, we found that HOPS binds efficiently to Ypt7p regardless of its nucleotide state, whereas P-HOPS shows a clear preference for Ypt7:GTP (Figure 3A).
FIGURE 3:

Unlike HOPS, P-HOPS binds to Ypt7 in a nucleotide state–dependent manner. (A) HOPS or P-HOPS was incubated for 10 min at 27°C with amylose-resin-bound, MBP-tagged Ypt7 that had been preloaded with either GTP or GDP. Resin without any Ypt7p (X) served as a control for unspecific binding. After extensive washing, the amylose beads were incubated with 0.4% SDS at 95°C for 5 min, followed by SDS–PAGE and immunoblotting for Vps11p and Ypt7p. A representative immunoblot is shown with fractions (10%) of the input of HOPS and P-HOPS used in the assay as reference (top), as well as the quantification of three repeats in which the unspecific background binding to amylose without Ypt7p was subtracted (bottom). The quantification was performed with a Umax PowerLook 1100 scanner (Techville, Dallas, TX) and UN-SCAN-IT gel 5.3 software (Silk Scientific, Orem, UT). (B) Liposome-clustering assays of protein-free liposomes or proteoliposomes carrying Ypt7p in its GTP or GDP form were performed with HOPS, P-HOPS, or HOPS buffer. The core data of the liposome cluster size distribution after incubation for 20 min at 27°C is shown as a box plot. The box covers the interquartile range, that is, 50% of the data, with the median value displayed as a line. All data are shown as cumulative probability plots in Supplemental Figure S4.
To study the HOPS–Ypt7p interaction in the context of membranes, we prepared proteoliposomes of vacuolar lipids and bearing no Ypt7p, Ypt7:GDP, or Ypt7:GTP. These were incubated with HOPS, P-HOPS, or their buffer alone and subjected to a liposome-clustering assay (Hickey et al., 2009) that analyzes clustering by fluorescence microscopy, measuring the size distribution of all the clusters in several random fields. The core data of this analysis are presented as a box-plot (Frigge et al., 1989), which shows the median and 25th–75th percentile values (Figure 3B), and the complete particle size distribution is provided as a cumulative probability plot (Supplemental Figure S4). HOPS supports the clustering of protein-free vacuole lipid liposomes (Figure 3B, lane 1 vs. lane 3), as reported earlier (Hickey and Wickner, 2010), and there is comparable stimulation of this clustering by either Ypt7:GDP (lane 4) or Ypt7:GTP (lane 7). In contrast, P-HOPS promotes less clustering and only in the presence of Ypt7:GTP (lane 8). A lowered tethering efficiency might explain at least part of the reduced activity observed in fusion reactions of Ypt7:GTP with P-HOPS as compared with HOPS.
The effects of HOPS phosphorylation on the specificity of fusion for the Ypt7p-bound guanine nucleotide may also result from the diminished binding of P-HOPS to vacuolar lipids (Hickey et al., 2009) and its enhanced specificity for binding to the GTP-bound Rab. These binding interactions may also regulate ensuing Ypt7p and HOPS-dependent steps of the reaction, which are still ill defined.
Vacuoles require Yck3p for the sensitivity of fusion to GAP proteins. Do these pure components allow a reconstitution of this regulation? In the absence of Yck3p, proteoliposome fusion is independent of whether Ypt7p bears GDP, GTP, or nonhydrolyzable GTPγS (Figure 4A, lanes 1–3) and is unaffected by the presence of the GAP Gyp1-46p (lanes 4–12). In the presence of Yck3p (Figure 4B), Ypt7p loaded with GTP or GTPγS supports fusion (lanes 2 and 3), whereas GDP-loaded Ypt7p does not (lane 1). Addition of the GAP Gyp1-46 inhibits fusion supported by Ypt7:GTP (lanes 5, 8, and 11) but has no effect when Ypt7p bears the nonhydrolyzable GTPγS (lanes 6, 9, and 12). Although Ypt7:GDP cannot support fusion in the presence of Yck3p (Figure 4, B, lane 1, and C, lane 1), fusion is reactivated by the addition of the purified GEF Mon1p-Ccz1p in the presence of GTP (Figure 4C, lanes 5, 9, 13, 17, and 21) but not in the presence of CTP (Figure 4C, lanes 6, 10, 14, 18, and 22). Thus the requirement for Yck3p to allow fusion to be sensitive to an added GAP is the result of HOPS phosphorylation and the ensuing change in nucleotide specificity.
FIGURE 4:

Alteration of Ypt7p nucleotide state by GAP (Gyp1-46) and GEF (Mon1p-Ccz1p) activity modulates P-HOPS–catalyzed fusion reactions. (A, B) Fusion of 4-SNARE RPLs that either carried Ypt7:GDP, Ypt7:GTP, or Ypt7:GTPγS. The RPL mixes were incubated for 10 min at 27°C with increasing concentrations of Gyp1-46 before the addition of Sec17p/Sec18p/HOPS. The mix of Sec17p, Sec18p, HOPS, and Mg:ATP was preincubated without (A) or with Yck3p (B) for 10 min at 27°C to allow HOPS phosphorylation. The content mixing after 30 min is compared with the control condition (RPLs with Ypt7:GTP, no Gyp1-46), which corresponded to 29.6% of the maximal signal obtained by detergent lysis. Shown is the mean of three experiments; error bars represent SDs. (C) Fusion reactions of 4-SNARE RPLs that either carried Ypt7:GDP or Ypt7:GTP. The RPL mixes were incubated for 10 min at 27°C with increasing concentrations of Mon1p-Ccz1p and either GTP or CTP (0.5 mM) before the addition of Sec17p/Sec18p/HOPS. The mix of Sec17p, Sec18p, HOPS, and Yck3p was incubated for 10 min at 27°C before being added. The content mixing after 30 min is compared with the control condition (RPLs with Ypt7:GTP, no Mon1p-Ccz1p), which corresponded to 27.3% of the maximal signal obtained by detergent lysis. Shown is the mean of three experiments; error bars represent SDs.
Because Yck3p is also needed for vacuole fusion to be sensitive to added Gdi1p, we sought the mechanism of this inhibition. The fusion of proteoliposomes with Ypt7:GTP is unaffected by Gdi1p regardless of the presence or absence of Yck3p (Figure 5A). Proteoliposomes with Ypt7:GDP fuse only when Yck3p is absent, and this fusion is inhibited only by high levels of Gdi1p (Figure 5A, lanes, 3, 15, and 19) that begin to extract Ypt7:GDP from the membrane (Figure 5B). In the presence of Yck3p, the nucleotide exchange factor Mon1p-Ccz1p restores fusion of proteoliposomes bearing Ypt7:GDP (Figures 4C and 5C, lane 2 vs. lane 4) by converting Ypt7p to its GTP bound state. This restoration is blocked by 1–4 μM Gdi1p (Figure 5C, lanes 8 and 10), levels that show little extraction of Ypt7:GDP (Figure 5B) or inhibition of the fusion that is mediated by nonphosphorylated HOPS (Figure 5A, lanes 11 and 15). Thus Gdi1p can inhibit through both extracting Ypt7:GDP and blocking the GEF-mediated nucleotide exchange of Ypt7p, in accord with the pioneering studies of Garrett et al. (1994).
FIGURE 5:

GDI inhibits P-HOPS–catalyzed fusion reactions by arresting Ypt7p in its GDP state. (A) Fusion reactions contained 4-SNARE RPLs with either Ypt7:GDP or Ypt7:GTP. The RPL mixes were incubated for 10 min at 27°C with increasing concentrations of Gdi1p before the addition of Sec17p/Sec18p/HOPS. The mix of Sec17p, Sec18p, HOPS, and Mg:ATP was preincubated without or with Yck3p, as indicated, for 10 min at 27°C to allow HOPS phosphorylation. The content mixing after 30 min is compared with the control conditions (RPLs with Ypt7:GTP, no Gdi1p; without or with Yck3p), which corresponded to 30.7 and 14.5% of the maximal signal obtained by detergent lysis. Shown is the mean of three experiments; error bars represent SDs. (B) RPLs (0.5 mM lipid) carrying Ypt7:GDP were incubated in 20 μl of RB150/Mg2+ with increasing concentrations of Gdi1p for 10 min at 27°C. The reactions were then diluted fivefold with RB150/Mg2+ and centrifuged for 30 min at 50,000 rpm in a TLA-100 rotor (Beckman Coulter, Brea, CA). Supernatants (95 μl) were removed and the pellets resuspended in 90 μl RB150/Mg2+ containing 0.1% Thesit (wt/vol). Equal fractions of pellet and supernatant were spotted on nitrocellulose membranes and immunostained for Ypt7p. These dot blots were quantified with an Umax PowerLook 1100 scanner and UN-SCAN-IT gel 5.3 software. The average distribution of membrane-bound (pellet) and extracted (supernatant) Ypt7p for three experiments is shown; error bars represent SDs. (C) Fusion of 4-SNARE RPLs that carried either Ypt7:GDP or Ypt7:GTP. The RPL mixes were incubated for 10 min at 27°C with Mon1p-Ccz1p, GTP, and increasing concentrations of Gdi1p before the addition of Sec17p/Sec18p/HOPS. The mix of Sec17p, Sec18p, HOPS, and Yck3p was incubated for 10 min at 27°C before being added. The relative amount of content mixing after 30 min is compared with the control conditions (RPLs with Ypt7:GTP, no Mon1p-Ccz1p, no Gdi1p), which corresponded to 25.7% of the maximal signal obtained by detergent lysis. Shown is the mean of three experiments; error bars represent SDs.
Under standard conditions for in vitro vacuole fusion, which include ATP, Ypt7p is needed on both vacuole fusion partners (Haas et al., 1995; Eitzen et al., 2000; Figure 6A, lanes 1–6). ATP supports not only cis-SNARE complex disassembly by Sec17p/Sec18p (Ungermann et al., 1998) and phosphoinositide synthesis (Mayer et al., 2000), but also the phosphorylation of HOPS by Yck3p (LaGrassa and Ungermann, 2005). However, fusion can also occur in the absence of ATP when the soluble SNARE Vam7p is added (Thorngren et al., 2004; Figure 6A, lane 8). Under this condition, where there is no phosphorylation of HOPS, Ypt7p on one vacuole fusion partner suffices (Figure 6A, lane 12). This is also seen with reconstituted proteoliposomes, where the fusion that is supported by HOPS in the absence of Yck3p only requires Ypt7p on one of the fusion partners (Figure 6B, odd lanes), whereas in the presence of Yck3p, which converts HOPS to P-HOPS, Ypt7:GTP is needed on both fusion partners (even lanes).
FIGURE 6:

Fusion reactions catalyzed by nonphosphorylated HOPS complex require Ypt7 on one membrane only. (A) Fusion of isolated vacuoles. Vacuoles isolated from yeast strains DKY6281 (∆pho8 PEP4) and BJ3505 (PHO8 ∆pep4), which had been deleted for YPT7 (∆) or not (+), were incubated in pairs for 90 min at 27°C under fusion conditions (see Materials and Methods) either in the presence of ATP or after depleting ATP through hexokinase/glucose (HK/Gluc). To measure fusion, alkaline phosphatase activity, which results from Pep4p-mediated proteolytic activation of the precursor form of Pho8p after lumenal content mixing, was assayed and is displayed as percentage relative to the control condition (YPT7 vacuoles incubated in the presence of ATP). The mean of three experiments is shown; error bars represent SDs. (B) Fusion reactions of heterotypic pairs of 4-SNARE RPLs that carried Ypt7:GTP, Ypt7:GDP, or no Ypt7. The mix of Sec17p, Sec18p, and HOPS was preincubated without or with Yck3p, as indicated, for 10 min at 27°C before being added. The content mixing after 30 min is compared with the control condition (all RPLs with Ypt7:GTP, no Yck3) as the mean of three experiments; error bars represent SDs.
DISCUSSION
Both Ypt7p and HOPS participate in cycles that modify their functional interactions (Figure 7). HOPS is phosphorylated by Yck3p (LaGrassa and Ungermann, 2005) on its Vps41p subunit (Cabrera et al., 2009) and dephosphorylated by as-yet-undefined phosphatases. Both HOPS and P-HOPS are found in fresh cell extracts (LaGrassa and Ungermann, 2005), but their ratio in the cell is unknown. Yck3p phosphorylates other proteins, including the SNARE Vam3p, which participates in complexes with HOPS, but Yck3p does not modify Ypt7p. Why, then, are ATP and Yck3p needed for vacuole fusion to be sensitive to a GAP or to Gdi1p, which directly target the Rab, but not the SNAREs or HOPS? It has been suggested that Ypt7p might both activate HOPS and inhibit phosphorylation by Yck3p (Brett et al., 2008), although inhibition has not been seen in a reconstituted reaction (Hickey and Wickner, 2010), which is now seen to faithfully reproduce the need for Yck3p for fusion to be blocked by Gyp1-46 or Gdi1p. Instead, we find that HOPS supports membrane fusion whether Ypt7p bears GTP or GDP, whereas P-HOPS functions only with Ypt7:GTP (Figure 2).
FIGURE 7:
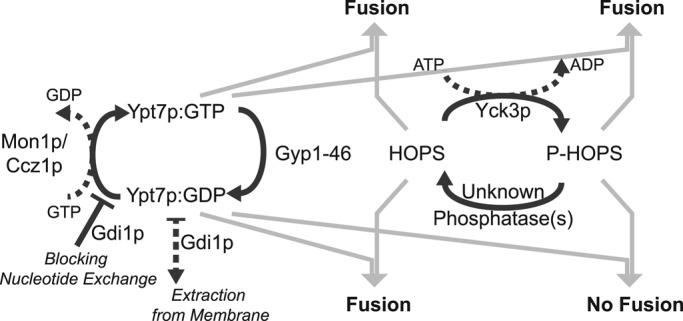
Scheme of the regulatory cycles of yeast vacuole fusion. Two functionally interconnected regulatory cycles have been reconstituted and analyzed for their potential to modulate vacuolar fusion activity. The Rab GTPase Ypt7p cycles between GTP- and GDP-bound states, catalyzed by a GTPase-activating protein (Gyp1-46) and the guanine nucleotide exchange factor Mon1p-Ccz1p. The GDI Gdi1p can arrest this cycle by extracting Ypt7:GDP from the membrane and by blocking the conversion of Ypt7:GDP to Ypt7:GTP. The HOPS complex cycles between a phosphorylated and nonphosphorylated state, catalyzed by the vacuolar kinase Yck3p and thus-far-unidentified phosphatase. Whereas HOPS is active with either Ypt7:GTP or Ypt7:GDP, phosphorylated HOPS mediates fusion only with Ypt7p in its GTP-bound form and is inactive with Ypt7p in its GDP-bound state.
Yeast vacuole fusion offers a novel paradigm in which guanine nucleotide specificity is imposed by regulated, covalent modification of the downstream effector. There are other reports of effectors binding to the GDP-bound Rab, such as coronin 3 in association with Rab27a (Kimura et al., 2008) and Legionella pneumophila SidM binding to Rab1 (Machner and Isberg, 2006). However, to our knowledge, this is the first report of guanine nucleotide specificity being induced by modification of the effector. Two HOPS subunits have direct affinity for Ypt7p, Vps39p, and Vps41p (Brett et al., 2008; Cabrera et al., 2009); substantial work will be needed to determine the affinity of each—as part of the HOPS complex—for Ypt7:GTP and Ypt7:GDP and any alteration in affinities when the other Rab-binding site is occupied.
Our findings provide a molecular explanation for the observation (LaGrassa and Ungermann, 2005) that in vitro vacuole fusion is sensitive only to added Gyp1-46/Gyp7-47 or to Gdi1p when Yck3p is present, even though Yck3p does not phosphorylate Ypt7p, the vacuolar target of GAP and GDI action. This explanation begins with two important aspects of vacuole fusion. Most of the Ypt7p on purified vacuoles must have bound GTP, since fusion occurs despite Yck3p converting HOPS to P-HOPS, and ∆yck3 vacuoles fuse at comparable rates to wild type (LaGrassa and Ungermann, 2005). Second, since vacuole fusion is fully sensitive to added Gdi1p even though most of the Ypt7p is bound with GTP at steady state, vacuole-bound GAPs such as Gyp7p and the exchange factor Mon1p-Ccz1p must be catalyzing frequent Ypt7p passage through its guanine nucleotide association cycle (Figure 7). Because, as expected, Gdi1p does not inhibit proteoliposome fusion when added to incubations with HOPS or P-HOPS and RPLs bearing Ypt7:GTP (Figure 5), it is presumably acting on Ypt7:GDP. To form Ypt7:GDP, the Ypt7p must hydrolyze its bound GTP under the influence of a vacuolar GAP such as Gyp7p. To complete the cycle, Ypt7:GDP would be triggered to release its bound GDP by Mon1p-Ccz1p (Nordmann et al., 2010). Nucleoside diphosphokinases would then convert this GDP to GTP, which could rebind to the Ypt7p. Added Gdi1p would strongly inhibit guanine nucleotide exchange (Garrett et al., 1994), shifting the balance to Ypt7p with bound GDP. In the presence of Yck3p, P-HOPS is formed that cannot function with Ypt7:GDP, but vacuoles from ∆yck3 strains will not form P-HOPS, and the HOPS will then support fusion with Ypt7:GDP. This model is supported by our data and by the observation that “bypass” vacuole fusion, performed in the absence of ATP and hence without P-HOPS (Thorngren et al., 2004; Brett et al., 2008), is strikingly less sensitive to Gyp or to Gdi1p, as seen for ∆yck3 vacuole fusion reactions (LaGrassa and Ungermann, 2005; Brett et al., 2008). Furthermore, the observation that Gyp7 overexpression leads to a greater fragmentation of vacuoles in wild-type cells than in ∆yck3 cells (Brett et al., 2008) or Vps41p phosphorylation-deficient cells (Cabrera et al., 2009) suggests that Yck3p also enforces such a Ypt7:GTP requirement in vivo. It has been shown (LaGrassa and Ungermann, 2005) that HOPS release from vacuoles during fusion assay incubations required Gdi1p, ATP, and Yck3p; this can now be seen to reflect the fact that HOPS can remain bound to vacuoles by its affinities for vacuolar lipids and for the GDP- or GTP-bound form of Ypt7p, whereas P-HOPS has lost this affinity for vacuolar lipids and relies on its affinity for Ypt7:GTP. It is therefore released only as Gdi1p extracts Ypt7p-GDP or blocks Ypt7p recycling to its GTP-bound form.
To gain a better mechanistic understanding of how the HOPS complex mediates vacuole fusion, future studies will have to decipher the complex network of its multiple interactions. A recent study of HOPS structure (Brocker et al., 2012) is an important first step. It will be important to understand whether Ypt7p activates HOPS beyond its mere recruitment to the membrane (Hickey et al., 2009; Stroupe, 2012) and how the multiple affinities of HOPS are orchestrated to support any function beyond tethering (Xu et al., 2010; Kramer and Ungermann, 2011).
Which role does, for example, the SM protein Vps33p, a subunit of the HOPS complex, play in facilitating fusion (Pieren et al., 2010)? HOPS can bind to either Ypt7p or to the SNARE complex but has not yet been observed to bind to both at the same time (unpublished data). Could that mean that HOPS binds to Ypt7p only during tethering and is then transferred to the SNARE complex for further posttethering activities? The ability to bypass the need for HOPS as the tethering agent and to prevent its activity in this capacity will be essential for study of its functions in downstream reactions.
MATERIALS AND METHODS
Proteins and reagents
The SNARE proteins GST-Vam3p, Vti1p, Vam7p, and Nvy1p were isolated as previously described (Mima et al., 2008). Vti1p and Nyv1p were gel filtered into RB150/β-OG (20 mM HEPES-NaOH, pH 7.4, 150 mM NaCl, 10% glycerol [vol/vol], 1% [wt/vol] β-octyl glucoside) with Sephacryl S-200 HR (GE Healthcare Biosciences, Pittsburgh, PA). A complete detergent exchange was confirmed by determining the CHAPS concentrations in eluate fractions (Urbani and Warne, 2005). GST-Ypt7p (Hickey et al., 2009), His6-Yck3(2–516) (Hickey et al., 2009), His6-Gyp1-46 (Wang et al., 2003), Gdi1p (Garrett and Novick, 1995), Sec17p (Schwartz and Merz, 2009), His6-Sec18p (Haas and Wickner, 1996), and HOPS (Hickey and Wickner, 2010) were isolated as described. MBP (maltose-binding protein)-tagged Ypt7p was a kind gift of Christopher Stroupe. HOPS that had been incubated with Yck3p prior to reisolation and the mock treated control (Hickey et al., 2009) were a kind gift of Christopher Hickey. Purified Mon1p-Ccz1p was a generous gift from Mirjana Nordmann and Christian Ungermann.
For stripping Ypt7p of its bound nucleotide and reloading prior to reconstitution, purified Ypt7p was incubated at 4°C for 2 h in the presence of 1 mM of the desired nucleotide and 5 mM EDTA, then for another 2 h at 4°C after the addition of MgCl2 to a final concentration of 10 mM. GDP, GTPγS (Sigma-Aldrich, St. Louis, MO), GTP, and CTP (Roche Diagnostics, Indianapolis, IN) were prepared as 50 mM stock solutions and stored at -80°C.
Liposome preparation
Proteoliposomes for content-mixing assays were prepared by detergent dialysis in RB150/Mg2+ (20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES]-NaOH, pH 7.4, 150 mM NaCl, 1 mM MgCl2, 10% glycerol [vol/vol]) as described (Zucchi and Zick, 2011) from lipid mixes mimicking the vacuolar composition (43.6 mol% [donor] or 46.6 mol% [acceptor] 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine, 18 mol% 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine, 18 mol% soy l-α-phosphatidylinositol, 4.4 mol% 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine, 2 mol% 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphate, and 1 mol% 1,2-dipalmitoyl-sn-glycerol, all from Avanti Polar Lipids [Alabaster, AL]; 8 mol% ergosterol [Sigma-Aldrich]; 1 mol% each of di-C16 phosphatidylinositol 3-phosphate and phospatidylinositol 4,5-bisphosphate [Echelon Biosciences, Salt Lake City, UT]; and only for donor liposomes 1.5 mol% each of Marina-Blue–1,2-dipalmitoyl-sn-glycero-3-phosphatidylethanolamine [DPPE] and 7-nitrobenz-2-oxa-1,3-diazole)-DPPE [Life Technologies, Carlsbad, CA]), four vacuolar SNAREs, and Ypt7p, entrapping Cy5-labeled streptavidin or biotinylated R-phycoerythrin. Molar protein:lipid ratios were 1:1000 for SNAREs and 1:2000 for Ypt7p. Isolation after reconstitution was achieved by floatation on a four-step Histodenz gradient (35, 30, 25% Histodenz [wt/vol; Sigma-Aldrich] and RB150/Mg2+). Histodenz solutions were prepared as 70% stock solution in modified RB150/Mg2+ with a reduced concentration (2% [vol/vol]) of glycerol to compensate for the osmotic activity of the density medium; lower-concentration solutions were obtained by dilution with RB150/Mg2+. The RPLs harvested at the topmost interface were then dialyzed 1:2000 (vol/vol) against RB150/Mg2+ for 3 h at 4°C. Liposomes for clustering assays were prepared the same way with Ypt7:GDP, Ypt7:GTP (molar protein:lipid ratio, 1:2000), or no Ypt7 (buffer was added instead) but without addition of SNARE proteins or content markers and with 1.5 mol% rhodamine-DPPE (Life Technologies) as fluorescent lipid marker.
RPL fusion assays
Proteoliposome fusion was measured by the resulting lumenal content mixing. Fusion assays were prepared from three premixes: a mix of RPLs, a mix of soluble SNARE chaperones, and bovine serum albumin (BSA). Donor and acceptor RPLs were mixed for each reaction condition in RB150 with ATP, Mg2+, and dextran-biotin, molecular weight 70,000. Gyp1-46, Gdi1p, Mon1p-Ccz1p, and additional nucleotides were added to the RPL mix, where indicated. In experiments that contained Mon1p-Ccz1p, the final concentration of Mg2+ was increased by 1 mM, as a spontaneous nucleotide exchange was observed with substoichiometric amounts of Mg2+ (i.e., total nucleotide concentrations exceeding the Mg2+ concentration). Sec17p, Sec18p, and HOPS were mixed in RB150 for each reaction condition. Yck3p was included in the mix of Sec17p/Sec18p/HOPS where indicated; the presence of Mg:ATP (∼0.5 mM) allowed Yck3p to phosphorylate HOPS during the 10-min preincubation at 27°C. The RPL mix (10 μl) was added to wells of a 384-well plate that contained 3 μl of 10% (wt/vol) defatted BSA in RB150; the Sec17p/Sec18p/HOPS mix was added to adjacent wells. After a 10-min preincubation at 27°C, fusion reactions were started by adding 7 μl of the Sec17p/Sec18p/HOPS mix to the adjacent wells with RPL mix with a multichannel pipette; reactions were mixed by gentle pipetting up and down two to three times. Fusion reactions (20 μl) contained 4-SNARE donor and acceptor RPLs (250 μM lipid each), BSA (1.5% [wt/vol]), Sec17p (32.5 nM), Sec18p (0.9 μM), HOPS (100 nM), ATP (1.2 mM), Mg2+ (1.2–2.2 mM), biotin-dextran, molecular weight 70,000 (1 μM biotin), and, where indicated, Yck3p (3 μM, unless otherwise specified), Gyp1-46 (0–2 μM), Mon1p-Ccz1p (0–100 nM), Mg:GTP or Mg:CTP (0.5 mM), and Gdi1p (0–16 μM); the average final salt (NaCl/KCl) concentration was 165 mM resulting from the addition of the various protein buffers, The plates were further incubated at 27°C in a fluorescence plate reader for 30–60 min, and FRET between PhycoE and Cy5 (excitation, 565 nm; emission, 670 nm; cutoff, 630 nm) was continuously measured at intervals of 15–60 s. Maximal values were determined after addition of 0.1% (wt/vol) Thesit to samples that had not received any dextran-biotin.
HOPS-binding assays
To assess the binding of HOPS and P-HOPS to Ypt7p in its GTP or GDP form, we stripped MBP-tagged Ypt7p and reloaded it with either nucleotide (as described). These samples were diluted in RB150/Mg2+, and 20 μM Ypt7p was mixed with an equal volume of amylose beads (New England BioLabs, Ipswich, MA) that had been washed with RB150/Mg2+ (three times centrifuged at 14,000 × g and 4°C for 5 min with subsequent resuspension in 10 bed volumes). These suspensions of amylose beads with MBP-Ypt7p were incubated for 30 min at 4°C. After three 1:10 (vol/vol) washes with RB150/Mg2+, halves of each sample were incubated with HOPS or P-HOPS (75 nM final) for 10 min at 27°C in the presence of 2% (wt/vol) defatted BSA (Sigma-Aldrich). The amylose beads were washed three times 1:10 (vol/vol) with RB150/Mg2+ before they were mixed 1:1 with 2× SDS sample buffer and incubated at 95°C for 5 min. Samples were then subjected to SDS–PAGE and immunoblotting with antibodies against Ypt7p and Vps11p, a subunit of the HOPS complex.
Liposome-clustering assays
To test the tethering activity of different combinations of Ypt7:GTP or Ypt7:GDP with HOPS or P-HOPS, we performed a clustering assay (Hickey and Wickner, 2010) with minor modifications. RPLs (0.5 mM lipid) were incubated at 27°C in 20 μl of RB150/Mg2+ containing defatted BSA (2% wt/vol) and HOPS or P-HOPS (100 nM). After 25 min, each reaction was moved to ice and diluted sixfold with ice-cold RB150/Mg2+. Aliquots (4 μl) were placed on a microscope slide (Gold Seal no. 3051; Thermo Scientific, Portsmouth, NH) and covered with a 22-mm coverslip (2810-22; Corning Life Sciences, Lowell, MA). Images were collected using a DeltaVision Imaging System (Applied Precision, Issaquah, WA), comprising a customized Olympus (Center Valley, PA) IX-71 inverted wide-field microscope, a Photometrics (Tucson, AZ) CoolSNAP HQ2 camera, and an InsightSSI (Applied Precision) solid-state illumination unit. Random fields (>20) were obtained for each condition with a 60× objective. Occasional images were removed from analyses due to the obvious presence of large, highly fluorescent foreign material. Otherwise, successive images were used for analyses (≥10 for each condition). Cluster sizes were measured using ImageJ, version 10.2 (National Institutes of Health, Bethesda, MD), after setting the lower threshold level to 50 and the upper threshold level to 255. Each pixel equals 0.0116 μm2. Statistical analysis was performed with KaleidaGraph, version 3.6 (Synergy Software, Reading, PA).
Vacuole isolation and fusion assays
Vacuoles were purified from Saccharomyces cerevisiae strains BJ3505, DKY6281, and BJ3505 ∆ypt7 and their fusion assayed as described (Haas et al., 1994; Cabrera and Ungermann, 2008). All fusion reactions (30 μl) contained 20 mM 1,4-piperazinediethanesulfonic acid–KOH (pH 6.8), 200 mM sorbitol, 125 mM KCl, 6 mM MgCl2, 10 μM CoA, 10 μg/ml Pbi2p (IB2), 10 mg/ml defatted BSA, 0.3 μM Vam7, 60 nM HOPS, 3 μg ∆pep4 vacuoles (from BJ3505 strains), and 3 μg ∆pho8 vacuoles (from DKY6281). The “+ATP” incubations also contained 1 mM ATP and an ATP-regenerating system (1 mg/ml creatine kinase and 29 mM creatine phosphate), whereas the “–ATP” conditions had 33 U/ml hexokinase and 20 mM glucose. After incubation at 27°C for 90 min, reactions were added to 270 μl of developer solution (250 mM Tris-Cl, pH 8.5, 10 mM MgCl2, 0.4% [vol/vol] Triton X-100, 2 mM 4-nitrophenol phosphate) in a 96-well plate, mixed, and incubated at 30°C, and alkaline phosphatase activity was assayed by continuously measuring absorbance at 400 nm, reflecting 4-nitrophenol production, in a VersaMax plate reader (Molecular Devices, Sunnyvale, CA) for 20 min.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Heath Grant R01 GM23377-36. We thank Amy Orr, Holly Jakubowski, and Deborah Douville (Geisel School of Medicine at Dartmouth) for expert assistance, Devin Schweppe and Scott Gerber (Geisel School of Medicine at Dartmouth) for performing mass spectrometry analyses, Mirjana Nordmann and Christian Ungermann (University of Osnabrück, Osnabrück, Germany) for a very generous gift of purified Mon1p-Ccz1p, Christopher Stroupe (now at the University of Virginia, Charlottesville, VA) and Christopher Hickey (now at Yale University, New Haven, CT) for purified proteins, and James Moseley (Geisel School of Medicine at Dartmouth) for access to his microscope. Michael Zick was supported by a research fellowship (ZI 1339/1-1) from the German Research Foundation.
Abbreviations used:
- FRET
fluorescence resonance energy transfer
- GAP
GTPase-activating protein
- GDI
GDP-dissociation inhibitor
- GEF
guanine nucleotide exchange factor
- HOPS
homotypic fusion and vacuole protein sorting complex
- P-HOPS
phosphorylated HOPS
- PhycoE
R-phycoerythrin
- RPL
reconstituted proteoliposome
- Sa-Cy5
Cy5-labeled streptavidin
- SNARE
soluble N-ethylmaleimide–sensitive factor attachment protein receptor
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E12-04-0279) on July 11, 2012.
REFERENCES
- Brett CL, Plemel RL, Lobingier BT, Vignali M, Fields S, Merz AJ. Efficient termination of vacuolar Rab GTPase signaling requires coordinated action by a GAP and a protein kinase. J Cell Biol. 2008;182:1141–1151. doi: 10.1083/jcb.200801001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocker C, Kuhlee A, Gatsogiannis C, Balderhaar HJ, Honscher C, Engelbrecht-Vandre S, Ungermann C, Raunser S. Molecular architecture of the multisubunit homotypic fusion and vacuole protein sorting (HOPS) tethering complex. Proc Natl Acad Sci USA. 2012;109:1991–1996. doi: 10.1073/pnas.1117797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera M, Ostrowicz CW, Mari M, LaGrassa TJ, Reggiori F, Ungermann C. Vps41 phosphorylation and the Rab Ypt7 control the targeting of the HOPS complex to endosome-vacuole fusion sites. Mol Biol Cell. 2009;20:1937–1948. doi: 10.1091/mbc.E08-09-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera M, Ungermann C. Purification and in vitro analysis of yeast vacuoles. Methods Enzymol. 2008;451:177–196. doi: 10.1016/S0076-6879(08)03213-8. [DOI] [PubMed] [Google Scholar]
- Collins KM, Thorngren NL, Fratti RA, Wickner WT. Sec17p and HOPS, in distinct SNARE complexes, mediate SNARE complex disruption or assembly for fusion. EMBO J. 2005;24:1775–1786. doi: 10.1038/sj.emboj.7600658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu AT, Rak A, Alexandrov K, Esters H, Goody RS, Scheidig AJ. Rab-subfamily-specific regions of Ypt7p are structurally different from other RabGTPases. Structure. 2002;10:569–579. doi: 10.1016/s0969-2126(02)00737-2. [DOI] [PubMed] [Google Scholar]
- Eitzen G, Will E, Gallwitz D, Haas A, Wickner W. Sequential action of two GTPases to promote vacuole docking and fusion. EMBO J. 2000;19:6713–6720. doi: 10.1093/emboj/19.24.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratti RA, Jun Y, Merz AJ, Margolis N, Wickner W. Interdependent assembly of specific regulatory lipids and membrane fusion proteins into the vertex ring domain of docked vacuoles. J Cell Biol. 2004;167:1087–1098. doi: 10.1083/jcb.200409068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigge M, Hoaglin DC, Iglewicz B. Some implementations of the boxplot. Am Statistician. 1989;43:50–54. [Google Scholar]
- Garrett MD, Novick PJ. Expression, purification, and assays of Gdi1p from recombinant Escherichia coli. Methods Enzymol. 1995;257:232–240. doi: 10.1016/s0076-6879(95)57028-4. [DOI] [PubMed] [Google Scholar]
- Garrett MD, Zahner JE, Cheney CM, Novick PJ. GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J. 1994;13:1718–1728. doi: 10.1002/j.1460-2075.1994.tb06436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A, Conradt B, Wickner W. G-protein ligands inhibit in vitro reactions of vacuole inheritance. J Cell Biol. 1994;126:87–97. doi: 10.1083/jcb.126.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A, Scheglmann D, Lazar T, Gallwitz D, Wickner W. The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO J. 1995;14:5258–5270. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A, Wickner W. Homotypic vacuole fusion requires Sec17p (yeast alpha-SNAP) and Sec18p (yeast NSF) EMBO J. 1996;15:3296–3305. [PMC free article] [PubMed] [Google Scholar]
- Hickey CM, Stroupe C, Wickner W. The major role of the Rab Ypt7p in vacuole fusion is supporting HOPS membrane association. J Biol Chem. 2009;284:16118–16125. doi: 10.1074/jbc.M109.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey CM, Wickner W. HOPS initiates vacuole docking by tethering membranes before trans-SNARE complex assembly. Mol Biol Cell. 2010;21:2297–2305. doi: 10.1091/mbc.E10-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Kimura T, Kaneko Y, Yamada S, Ishihara H, Senda T, Iwamatsu A, Niki I. The GDP-dependent Rab27a effector coronin 3 controls endocytosis of secretory membrane in insulin-secreting cell lines. J Cell Sci. 2008;121:3092–3098. doi: 10.1242/jcs.030544. [DOI] [PubMed] [Google Scholar]
- Kramer L, Ungermann C. HOPS drives vacuole fusion by binding the vacuolar SNARE complex and the Vam7 PX domain via two distinct sites. Mol Biol Cell. 2011;22:2601–2611. doi: 10.1091/mbc.E11-02-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGrassa TJ, Ungermann C. The vacuolar kinase Yck3 maintains organelle fragmentation by regulating the HOPS tethering complex. J Cell Biol. 2005;168:401–414. doi: 10.1083/jcb.200407141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machner MP, Isberg RR. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell. 2006;11:47–56. doi: 10.1016/j.devcel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Mayer A, Scheglmann D, Dove S, Glatz A, Wickner W, Haas A. Phosphatidylinositol 4,5-bisphosphate regulates two steps of homotypic vacuole fusion. Mol Biol Cell. 2000;11:807–817. doi: 10.1091/mbc.11.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima J, Hickey CM, Xu H, Jun Y, Wickner W. Reconstituted membrane fusion requires regulatory lipids, SNAREs and synergistic SNARE chaperones. EMBO J. 2008;27:2031–2042. doi: 10.1038/emboj.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann M, Cabrera M, Perz A, Brocker C, Ostrowicz C, Engelbrecht-Vandre S, Ungermann C. The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr Biol. 2010;20:1654–1659. doi: 10.1016/j.cub.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Ohya T, Miaczynska M, Coskun U, Lommer B, Runge A, Drechsel D, Kalaidzidis Y, Zerial M. Reconstitution of Rab- and SNARE-dependent membrane fusion by synthetic endosomes. Nature. 2009;459:1091–1097. doi: 10.1038/nature08107. [DOI] [PubMed] [Google Scholar]
- Ostrowicz CW, Brocker C, Ahnert F, Nordmann M, Lachmann J, Peplowska K, Perz A, Auffarth K, Engelbrecht-Vandre S, Ungermann C. Defined subunit arrangement and rab interactions are required for functionality of the HOPS tethering complex. Traffic. 2010;11:1334–1346. doi: 10.1111/j.1600-0854.2010.01097.x. [DOI] [PubMed] [Google Scholar]
- Pieren M, Schmidt A, Mayer A. The SM protein Vps33 and the t-SNARE H(abc) domain promote fusion pore opening. Nat Struct Mol Biol. 2010;17:710–717. doi: 10.1038/nsmb.1809. [DOI] [PubMed] [Google Scholar]
- Price A, Seals D, Wickner W, Ungermann C. The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J Cell Biol. 2000;148:1231–1238. doi: 10.1083/jcb.148.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ML, Merz AJ. Capture and release of partially zipped trans-SNARE complexes on intact organelles. J Cell Biol. 2009;185:535–549. doi: 10.1083/jcb.200811082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DF, Eitzen G, Margolis N, Wickner WT, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA. 2000;97:9402–9407. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroupe C. The yeast vacuolar Rab GTPase Ypt7p has an activity beyond membrane recruitment of the homotypic fusion and protein sorting-Class C Vps complex. Biochem J. 2012;443:205–211. doi: 10.1042/BJ20110687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroupe C, Collins KM, Fratti RA, Wickner W. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 2006;25:1579–1589. doi: 10.1038/sj.emboj.7601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroupe C, Hickey CM, Mima J, Burfeind AS, Wickner W. Minimal membrane docking requirements revealed by reconstitution of Rab GTPase-dependent membrane fusion from purified components. Proc Natl Acad Sci USA. 2009;106:17626–17633. doi: 10.1073/pnas.0903801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorngren N, Collins KM, Fratti RA, Wickner W, Merz AJ. A soluble SNARE drives rapid docking, bypassing ATP and Sec17/18p for vacuole fusion. EMBO J. 2004;23:2765–2776. doi: 10.1038/sj.emboj.7600286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Nichols BJ, Pelham HR, Wickner W. A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J Cell Biol. 1998;140:61–69. doi: 10.1083/jcb.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani A, Warne T. A colorimetric determination for glycosidic and bile salt-based detergents: applications in membrane protein research. Anal Biochem. 2005;336:117–124. doi: 10.1016/j.ab.2004.09.040. [DOI] [PubMed] [Google Scholar]
- Wada Y, Ohsumi Y, Anraku Y. Genes for directing vacuolar morphogenesis in Saccharomyces cerevisiae. I. Isolation and characterization of two classes of vam mutants. J Biol Chem. 1992;267:18665–18670. [PubMed] [Google Scholar]
- Wang L, Merz AJ, Collins KM, Wickner W. Hierarchy of protein assembly at the vertex ring domain for yeast vacuole docking and fusion. J Cell Biol. 2003;160:365–374. doi: 10.1083/jcb.200209095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles. Annu Rev Cell Dev Biol. 2010;26:115–136. doi: 10.1146/annurev-cellbio-100109-104131. [DOI] [PubMed] [Google Scholar]
- Wickner W, Schekman R. Membrane fusion. Nat Struct Mol Biol. 2008;15:658–664. doi: 10.1038/nsmb.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Jun Y, Thompson J, Yates J, Wickner W. HOPS prevents the disassembly of trans-SNARE complexes by Sec17p/Sec18p during membrane fusion. EMBO J. 2010;29:1948–1960. doi: 10.1038/emboj.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu IM, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol. 2010;26:137–156. doi: 10.1146/annurev.cellbio.042308.113327. [DOI] [PubMed] [Google Scholar]
- Zucchi PC, Zick M. Membrane fusion catalyzed by a Rab, SNAREs, and SNARE chaperones is accompanied by enhanced permeability to small molecules and by lysis. Mol Biol Cell. 2011;22:4635–4646. doi: 10.1091/mbc.E11-08-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



