Abstract
Transcatheter aortic valve replacement (TAVR) was approved in the United States in late 2011, providing a critically needed alternative therapy for patients with severe aortic stenosis previously refused surgical aortic valve replacement (SAVR). Over 20,000 TAVR have been performed in patients worldwide since 2002 when Alain Cribier performed the first-in-man TAVR. This paper reviews the data from balloon expandable and self-expanding aortic stent valves as well as data comparing them with traditional surgical aortic valve replacement (SAVR). Complications using criteria established by the Valve Academic Research Consortium (VARC) are reviewed. Future challenges and possibilities are discussed and will make optimizing TAVR an important goal in the years to come.
Keywords: transcatheter aortic valve replacement, SAVR, TAVR, transcatheter, aortic stenosis, VARC, stent valve, complications, TAVI, valve academic research consortium, review, PARTNER, SAPIEN, CoreValve, JenaValve, Acurate
Introduction
Aortic stenosis (AS) is a degenerative valvular disease that worsens over time. The natural history of AS is well studied with worsening prognosis after the onset of angina, syncope, and dypsnea.1–3 In the past, the only effective treatment for AS has been surgical aortic valve replacement (SAVR) with guidelines being well established for when to refer for surgery.4 Pathophysiology of aortic valve stenosis is degenerative and calcific, and it may be exacerbated by the same cellular atherosclerotic processes which are involved with lipid accumulation and inflammation. Other diseases, such as end stage renal failure, can also accelerate the disease process. Patients often have a history of coronary artery disease, carotid artery disease, and peripheral vascular disease. Patients may require concomitant SAVR and coronary artery bypass grafting (CABG). In addition, surgeries may require several hours of cardioplegia. If the patient has comorbidities, such as renal failure, their peri-and post-operative mortality percentage increases. The United States Surgical Database, provided by the Society of Thoracic Surgery (STS), provides an approximate calculator for predicting mortality by factoring in variables such as risk factors, type of surgery, and comorbidities. This allows surgeons to better risk stratify patients.5 The European equivalent predictor of surgical outcome is the European EuroScore. Both databases take into account the patient’s comorbidities and assign a numerical value to them. The algorithm then generates an overall mortality score for the procedure. Surgeons may refuse to operate based on this operative mortality percentage.
In this setting, the development and Food and Drug Administration (FDA) approval of a percutaneous option was completed in 1992; Anderson described the first transcatheter aortic stent valve implanted in a pig.8 In 2000, Bonhoeffer implanted a transcatheter pulmonic valve in a human patient. In 2002, the first transcatheter aortic stent valve was implanted in humans by Dr Cribier.9 The approach used at the time was a transeptal one, where stents were implanted from femoral venous access given the bulkiness of the first device. Although this procedure was difficult, it was successful. Unfortunately the patient’s leg became ischemic post procedure, required an above the knee amputation, and died four months later.7 In 2005, with newer materials, the transfemoral position (common femoral artery) was developed in both Canada and Germany.8 Using this approach, placement of the valve became easier.
Stent Valves
Balloon expandable
Edwards SAPIEN
The first Cribier Edwards valve used equine pericardium to fashion the leaflets, and these were attached to a stainless steel stent. However, the equine material was soon replaced by bovine pericardium, and this formed the basis for the Edwards SAPIEN transcatheter aortic valve.8 The Edwards Life Sciences SAPIEN valve contains a bovine aortic trileaflet valve attached to a metallic scaffold. The stent is balloon expandable and is manually crimped onto the balloon immediately prior to deployment. For the 23- and 26-mm valve sizes, 22F and 24F sheaths are required.
Edwards SAPIEN XT
The Edwards SAPIEN XT is the next generation balloon expandable Edwards stent valve. Its sheath has since been downsized to 18F and 19F for the 23 mm and 26 mm stent valves, respectively. On computerized tomography (CT) scan, a minimum diameter of 6 and 6.5 mm was required at the level of the femoral artery for successful insertion of the 23 mm and 26 mm SAPIEN XT valves compared to 7 mm and 8 mm for the 23 mm and 26 mm SAPIEN valves, respectively. Recent analysis of 190 patients receiving either valve (71 SAPIEN vs. 112 SAPIEN XT) in the transfemoral approach, showed that the 30 day combined safety endpoint was similar (15.2% SAPIEN XT vs. 17.9% SAPIEN). Valve performance was also comparable at 30 days. In addition, transfemoral success was 91.1% using the SAPIEN XT vs. 61.4% using the SAPIEN.11
Self-expanding Medtronic CoreValve
CoreValve Inc. received the CE Mark in 2007 and was acquired by Medtronic in 2009.12 The Revalving stent valve itself has undergone several iterations: initially it began as a 24 Fr system, and it is currently an 18 Fr system. It contains porcine pericardium fashioned leaflets attached to a self-expanding nitinol cage. The stent is placed in the left ventricular outflow tract (LVOT) and extends into the aorta. Three distinct areas exist within the stent that have different radial and hoop strengths. The valve itself is self-centering and is partially retrievable.10 It rests in the supra-aortic position away from the coronary ostia.
Human Trials
Stent valve versus medical therapy/ balloon valvuloplasty
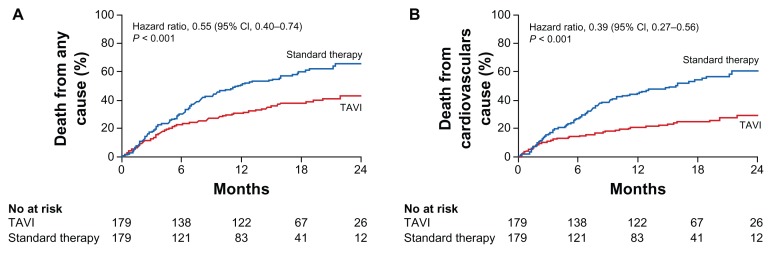
The PARTNER (Placement of AoRTic TraNscathetER) trial was the first randomized trial to evaluate the stent valve in humans across the United States.13 Prior data from tens of thousands of patients in Europe with the Edwards SAPIEN and Medtronic CoreValve showed that this modality might be an effective way to treat critical aortic stenosis.14 In the PARTNER B trial, 358 patients who were not considered suitable for surgery were randomized to either standard therapy or TAVR. Most strikingly, 1-year all-cause mortality was 50.7% for standard therapy vs. 30.7% for TAVR (95% confidence interval, 0.4 to 0.74, P < 0.001) (Fig. 4). However, complications including strokes were higher for TAVR (5.0% vs. 1.1%, P = 0.06).
Figure 4.
PARTNER trial data showing superior outcomes from TAVI vs. standard therapy for death at 1 and 2 years for: (A) death from any cause, and (B) death from a cardiovascular cause.
Reprinted with permission.13
Recent two-year outcomes were analyzed.15 Deaths at two years were 43.3% in the TAVR group and 68.0% in standard therapy group (P < 0.001), with cardiac related death associated with 31.0% in the TAVR group and 62.4% in the standard therapy group (P < 0.0019). The incidence of strokes was still higher (13.8% TAVR vs. 5.5% standard therapy, P = 0.01). Data further suggested that the mortality benefit after TAVR may be mainly limited to patients with fewer comorbidities.
TAVR versus SAVR
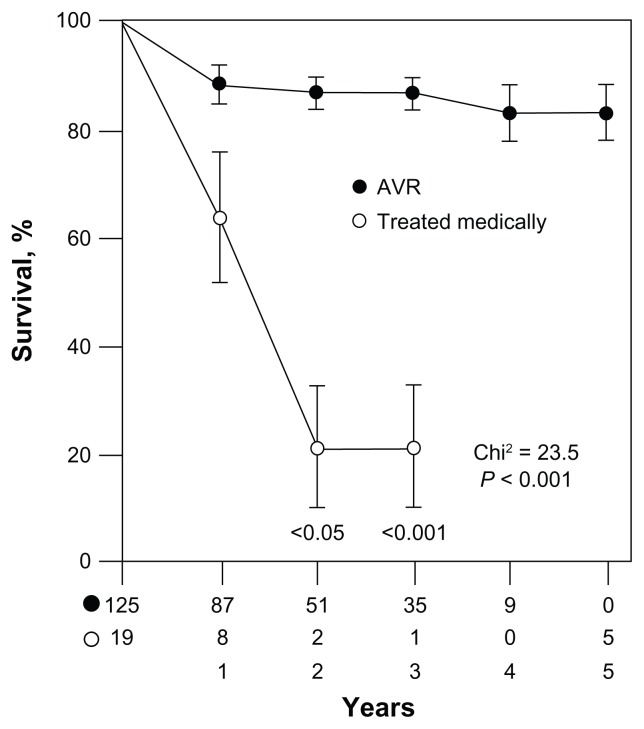
The PARTNER investigators also compared TAVR with SAVR (PARTNER A) among high-risk patients with STS 11.8%.16 Prior to TAVR, SAVR had been the only long-term effective therapeutic option for patients. If the patient did not qualify for SAVR due to high peri- and post-operative risk, prognosis was poor as shown in Figure 1. In this trial, patients screened for the PARTNER trial that qualified for both SAVR and TAVR were further randomized to either therapy. The goal was to determine if TAVR was more effective than SAVR. Results suggested that the overall 1 year mortality was similar post-procedure (24.2% TAVR vs. 26.8% SAVR, P = 0.44). Death rates from cardiovascular causes were equivalent at 1 year (14.3% TAVR vs. 13% SAVR, P = 0.63). However, major and minor stroke were more frequent in the TAVR arm (8.3% TAVR vs. 4.3% SAVR, P < 0.05). Vascular complications were also more frequent in the TAVR arm (18% TAVR vs. 4.8% SAVR, P < 0.001).
Figure 1.
Natural history of AS increases dramatically after onset of symptoms; without surgical intervention, mortality increases dramatically. Reprinted with permission.2
Notes: From the patient perspective, in the past patients had no options if the cardiothoracic surgeons refused to operate. Some would be given comfort care while others would have an aortic valvuloplasty (first described by Dr Alain Cribier in 1986) to temporize the AS.6 Data suggests that this has no significant effect on long-term survival.7
2 years was consistent with data collected after 1 year.17 Overall mortality rates were similar (33.9% TAVR vs. 35.0% SAVR, P = 0.78), as were the mortality rates associated with cardiovascular factors (21.4% TAVR vs. 20.5% SAVR, P = 0.8). The frequency of all neurologic events (major strokes and transient ischemic attacks) was higher at 2 years (11.2% TAVR vs. 6.5% SAVR, P = 0.05). Paravalvular leak was more common in the TAVR group (1 year: 7.0% TAVR vs. 1.9% SAVR, P < 0.001; 2 years: 6.9% TAVR vs. 0.9% SAVR, P < 0.001), and was associated with increased late mortality (hazard ratio, 2.11; 95% CI, 1.43 to 3.1; P < 0.001).
In a single center prospective Swiss registry,18 442 patients with severe aortic stenosis were assigned to medical treatment (MT, n = 78), TAVR (n = 257), or SAVR (n = 107). Mortality from all causes was higher in the MT arm (61.5% MT vs. 22.6% TAVR vs. 22.4% SAVR, P < 0.001). Patient operative mortality risk was calculated using both Euro-Score and STS (6.5 ± 4.1 MT vs. 6.4 ± 5.0 TAVR vs. 4.8 ± 5.3 SAVR, P = 0.009). The incidence of major stroke observed in this study was similar between both TAVR and SAVR (2.6% MT vs. 4.3% TAVR vs. 3.7% SAVR, P = 0.91). However, when compared to the PARTNER trial, these patients had overall lower STS scores (6.0 ± 5.0).
FDA approval
Given the results of the PARTNER trial, the FDA approved the Edward SAPIEN stent valve for use in the United States in late 2011 with The Centers for Medicare and Medicaid Services (CMS) proposing a payment plan in February of 2012 largely based on guidelines from the PARTNER trial. The final memo written in May of 201219 is shown in Table 1.
Table 1.
CMS guidelines for heart team and hospital requirements for TAVR.
| No TAVR experience | Prior TAVR experience |
|---|---|
| Hospital qualifications | |
| ≥50 total AVRs in the previous year prior to TAVR, including ≥10 high-risk patients | ≥20 AVRs per year or ≥40 AVRs every 2 years; and |
| ≥2 physicians with cardiac surgery privileges, and; | ≥2 physicians with cardiac surgery privileges; and |
| ≥1000 catheterizations per year, with ≥400 percutaneous coronary interventions (PCIs) per year. | ≥1000 catheterizations per year, including ≥400 percutaneous coronary interventions (PCIs) per year. |
| Heart team | |
| Cardiovascular surgeon | Cardiovascular surgeon and interventional cardiologist combined |
| ≥100 career AVRs including 10 high-risk patients; or | ≥20 TAVR procedures in the prior year; or |
| ≥25 AVRs in one year; or | ≥40 TAVR procedures in the prior 2 years. |
| ≥50 AVRs in 2 years; and which include at least 20 AVRs in the last year prior to TAVR initiation. | |
| Interventional cardiologist | |
| Professional experience with 100 structural heart disease procedures lifetime; or 30 left-sided structural procedures per year of which | |
| 60% should be balloon aortic valvuloplasty (BAV). | |
| Atrial septal defect and patent foramen ovale closure are not considered left-sided procedures. | |
Table adapted.19
TAVR Complications
Complications from these large caliber devices include stroke, myocardial infarction, bleeding, vascular injury such as perforation, dissection, trauma and arterial intussusception, device embolization, reverse placement of the stent valve, and geographic misplacement of the stent valve leading to the possible blocking of coronary ostia. Most of these complications can potentially be life threatening. Long-term complications include stroke, bleeding, paravalvular regurgitation, and endocarditis although there have been case reports of a broad spectrum of rare complications that can occur. Given the total number of TAVR performed worldwide, it was necessary to develop common criteria to describe complications related to the stent valve procedures. As with the Academic Research Consortium (ARC) criteria developed for stent thrombosis, a Valve Academic Research Consortium (VARC) was also created to help create a common language by which to quantify complications in a standardized and objective fashion. In the spirit of ARC, physicians from cardiology and cardiovascular surgical societies, industry representatives, and US FDA representatives met in San Francisco, California, USA, as well as in Amsterdam, the Netherlands, in 2009 to discuss TAVR and create VARC criteria.20
VARC criteria
VARC criteria separates stent valve placement into three important composite endpoints: (1) device success; (2) combined safety endpoint (30 days); and (3) combined efficacy endpoint (1 year) (Table 2). Device success entails: successful vascular access; delivery and deployment of the device; successful retrieval of the delivery system; correct positioning of the device; and the device performing to specification with only one stent valve implanted. Combined safety endpoints include: all-cause mortality, major stroke, life-threatening bleeding, acute kidney injury, peri-procedural myocardial infarction (MI), major vascular complications, and repeat valvular procedures. Of note, peri-procedural MI is defined as a CK-MB greater than 10x the upper limit of normal (in coronary databases, this is usually 3× the upper limit of normal as opposed to surgical databases, which is usually 5–10× the upper limit normal). Combined efficacy endpoint estimates longer outcomes (1 year or longer) including all-cause mortality, failure of current therapy for AS, and prosthetic heart valve dysfunction including worsening AS or AR. Each VARC complication was defined with previously published clinical-trial complication definitions in mind, but now specific to TAVR and SAVR.
Table 2.
VARC definition of composite endpoints.
| Device success | Combined safety (30 d) | Combined efficacy (1+ yr) |
|---|---|---|
| Vascular access | All cause mortality | All cause mortality (>30 d) |
| Delivery and deployment | Major stroke | Hospitalization for AS/CHF |
| Retrieval | Life-threatening bleeding | Worsening valve performance |
| Correct positioning | Acute kidney injury stage 3 | |
| Optimal valve performance | Peri-procedure MI | |
| One stent valve only | Major vascular complication | |
| Repeat procedure for valve dysfunction |
Table adapted.20
VARC meta-analysis
Consortium authors screened a total of 482 articles written about TAVR in 2011 and came up with 16 unique articles that used VARC criteria meta-analysis of 3519 patients.21 Stent valves used were both the Medtronic CoreValve and the Edwards SAPIEN. The 30-day STS score associated with TAVR was 8.7% (95% CI: 7.0% to 10.3%). All-cause 30-day mortality was 7.8% (95% CI: 5.5% to 11.1%). The 1-year mortality rate was 22.1% (95% CI: 17.9% to 26.9%) with 1 year cardiovascular mortality at 14.4% (95% CI: 10.6% to 19.5%, P = 0.0002). The prevalence of major stroke was 3.2% (95% CI: 2.1% to 4.8%, P < 0.0001). Moderate to severe residual aortic regurgitation was 7.4% (95% CI: 4.6% to 10.2%). Myocardial infarction was 1.1% (95% CI: 0.2% to 2%, P < 0.0001). Life-threatening bleeding was noted in 15.6% (95% CI: 11.7% to 20.7%). Major vascular complications were noted in 11.9% (95% CI: 8.6% to 16.4%). Medtronic CoreValve use resulted in a higher rate of permanent pacemaker implantation when compared to the Edwards SAPIEN (28.9% vs. 4.9%, P < 0.0001). Composite endpoints of safety at 30 days were 32.7% (95% CI: 27.5% to 38.8%, P < 0.0001) and efficacy at 1 year 71.1% (95% CI: 65.6% to 76.0%, P = 0.58).
Although this analysis was a random sampling of patients undergoing TAVR in 2011, whose authors used the newly defined VARC criteria to categorize complications without strict 3rd party/ unbiased adjudication, this initial meta-analysis still provides a better understanding of the degree to which complications can occur. Furthermore, this can be compared in detail with the PARTNER trial to better understand how real world patients perform compared to patients selected for clinical trials with strict exclusion criteria.
Stroke
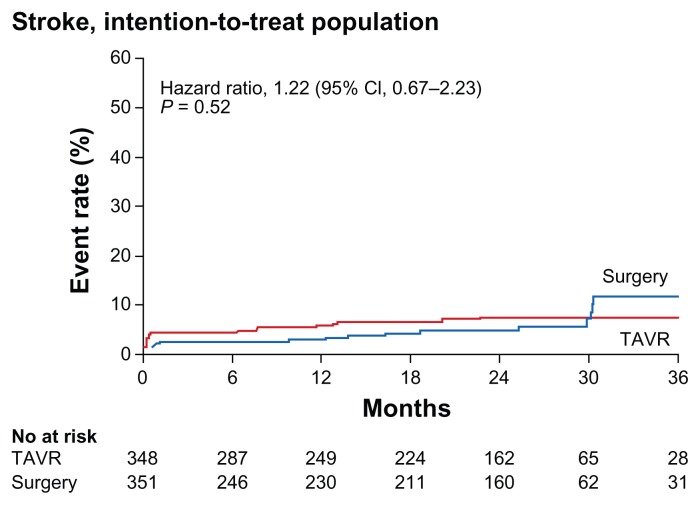
The incidence of both major and minor stroke has been discussed extensively as it relates to TAVR given the relatively high rates of stroke peri- and post-procedure. In the PARTNER B trial, TAVR was randomized against standard therapy.13 The 30-day major stroke rate was 5.0% in the TAVR group vs. 1.1% in the standard therapy group (P = 0.06). The 1-year major stroke incidence was 7.8% in the TAVR group vs. 3.9% in the standard therapy group (P < 0.18). Given that standard therapy in the PARTNER B trial included balloon valvuloplasty (82.3%), it is possible that with medical therapy alone (without balloon valvuloplasty), the stroke rate would have been even lower. In the PARTNER A study,16 stroke increased the hazard of death (hazard ratio, 2.47; 95% CI: 1.42 to 4.3, P < 0.001). Using VARC criteria for stroke shown in Table 3, in the 16 study meta-analysis,21 patients had an average STS of 8.7% and major stroke at 30 days was 3.2% versus the PARTNER B trial where patients had an average STS of 11.2%, and major stroke at 30 days was 5.0%. Similarly, in the PARTNER A trial, it was noted that the incidence of a stroke within 30 days was 3.8% for those in the TAVR arm vs. 2.1% among those in the SAVR arm. Interestingly, it was found that the overall number of strokes within 3 years in both arms did not differ significantly until the 30-month mark, where more strokes occurred in SAVR arm; the significance of this result is unknown (see Figure 5).17
Table 3.
VARC criteria for stroke.
Rapid onset of focal/global deficit with one of the following:
|
Duration of focal/global deficit ≥24 h; Only <24 h if:
|
Confirmation by one of the following:
|
Notes: Non-neurologic causes of stroke need to be ruled out prior to application of these criteria (eg, brain tumor, trauma, infection, hypoglycemia, peripheral lesion, pharmacologic agents).
Table adapted.20
Figure 5.
Time-to-event curve for stroke.
Reprinted with permission.17
The high incidence of stroke, in particular, may be prohibitive in making TAVR first line treatment when compared to SAVR, especially in low to moderate risk patients. Because the native calcific, degenerative aortic valve is not removed surgically, but rather compressed against the aortic annulus by the stent balloon and stent upon inflation, the compressed native valve material may not be stable. Possible emboli from ejection of this material likely increases stroke risk until it is stabilized. In addition, scraping of the aorta during the procedure may also dislodge a stroke-causing atheroma. Thus the increased risk of stroke may be prohibit the performance of a TAVR, and benefits and risks must be weighed cautiously. Stroke risk has been reviewed in depth and remains a concern.22 Imaging studies by transcranial Doppler during TAVR showed embolic events occurring during the procedure itself, including balloon valvuloplasty, catheter manipulation, and stent valve deployment.23 Diffusion weighted magnetic resonance imaging (DW-MRI) studies showed abnormalities in 68% to 84% of patients post TAVR, although these did not translate into clinical stroke most of the time.24–27 Risk factors for stroke in TAVR are not clear, but established risk factors are age and left ventricular dysfunction.22 Major stroke also increases the 1-year mortality rate significantly as seen in the PARTNER trial (66.7% with stroke vs. 27.7% without stroke, P < 0.0001).
Causes of stroke may include manipulation across the degenerated aortic valve, and atheroma along the aortic arch. To try to prevent this, distal protection devices have been proposed in the greater vessels, and recent studies using this technique have indicated some, but not complete, success.28,29 More data will be forthcoming about the feasibility of these filters. In addition, in the future, standardized neurologic stroke scales (eg, National Institutes of Health Stroke Scale NIHSS/Rankin) will likely be used in conjunction with neurology specialist consultants to further ascertain stroke status in patients pre- and post-procedure until the incidence of stroke decreases.
Paravalvular leak
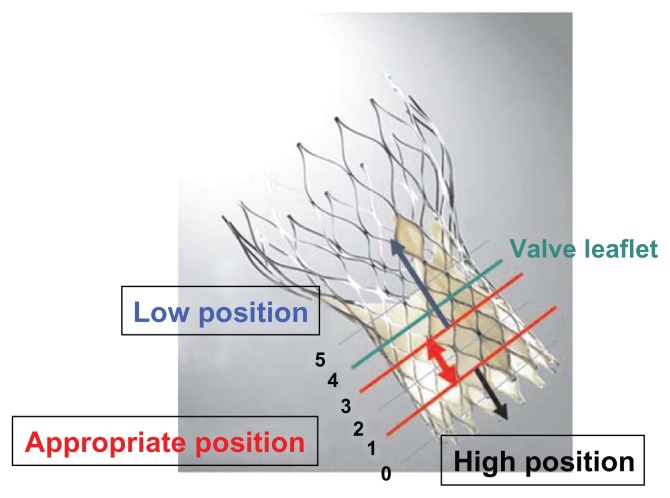
Paravalvular leak is a result of poor apposition of the stent valve with the aortic annulus. This is significantly higher in patients with TAVR vs. SAVR (6.8% vs. 1.9%, PARTNERS A)16 Criteria for assessment vary, but center on Doppler parameters of the jet30 as well as on diastolic flow reversal in the descending aorta. The significance of paravalvular regurgitation on mortality is unclear. It seems that patients with even mild paravalvular regurgitation may have a higher mortality rate.17 Further analysis suggests positioning of the CoreValve is a key factor in determining post-procedure paravalvular aortic regurgitation (see Figure 6), as well as annulus size to prosthetic ratio.31 Progression from moderate to severe paravalvular regurgitation does not occur often.17,32 Given this data, it seems clear that full apposition, positioning, and correct sizing of the stent valve are important to prevent paravalvular leak. To this end, different imaging methods for accurately sizing the aortic annulus are being investigated, including both CT and MRI.33,34
Figure 6.
Position for deployment is an important factor in determining paravalvular regurgitation with the Medtronic CoreValve. Reprinted with permission.31
Vascular complications
Vascular complications occurred in 32.4% of patients with TAVR in the PARTNER B trial, with major vascular complications found in 16.8% of patients at one year.13 In the PARTNER A trial, vascular complications occurred in 18% of patients with TAVR, with 11.3% patients experiencing major complications at one year.16 Vascular complications in the PARTNER A trial were a predictor of mortality (hazard ratio 1.71; 95% CI: 1.07 to 2.73, P = 0.02). A VARC meta-analysis of 16 recent studies showed a pooled vascular complication rate of 18.8% in patients with TAVR, and 11.9% of patients experienced major cardiovascular events,21 with the criteria shown in Table 4. When assessing data from the UK TAVI Registry using the VARC criteria, it was found that major complications occurred in 6.3% of patients (55/869 patients); specifically, rates for major complications were 6.2% among patients with the CoreValve (28/451 patients) and 6.3% among patients with the Edwards SAPIEN (26/410 patients).35
Table 4.
VARC definition of vascular complications.
| Major | Minor |
|---|---|
| Thoracic aortic dissection | Failure of percutaneous access site closure resulting in intervention/surgical correction |
| Access site/related vascular injury leading to death, blood transfusion ≥4U, surgical intervention, irreversible end-organ damage | Access site/related vascular injury requiring compression or thrombin injections therapy, or hematoma requiring transfusion of ≥2 but <4U, not requiring unplanned intervention/surgery |
| Distal embolization (non-cerebral) requiring surgery or causing irreversible end-organ damage | Distal embolization treated with embolectomy and/or thrombectomy with no amputation or irreversible end-organ damage |
Table adapted.20
As the valve prostheses become smaller, and more centers move to a complete percutaneous approach, major vascular complications may decrease further. New technologies have been developed to help decrease the sheath size for the femoral artery in patients. The Edwards eSheath allows for transient sheath expansion during delivery of the stent valve. The sheath expands with the passage of the prosthesis and returns to its lower profile diameter after passage (eg, 20 F to 27 F, outer diameter).36 In addition to the transapical approach used with the Edward SAPIEN, JenaValve, and Acurate TA valves, other approaches are also being explored, including subclavian and direct aortic insertion.
Bleeding
In the PARTNER B trial, major bleeding occurred in 22.3% of patients with TAVR at 1 year.13 In the PARTNER A trial, major bleeding occurred in 14.7% of patients with TAVR.16 Predictors of mortality in the PARTNER A trial included major bleeding (TAVR: hazard ratio, 2.11; 95% CI: 1.41 to 3.17, P < 0.001). VARC criteria further categorized bleeding into life-threatening bleeding, major bleeding, and minor bleeding (Table 5). In the recent VARC meta-analysis of 16 studies, life-threatening bleeding occurred in 15.6% (95% CI: 11.7% to 20.7%) of patients, and major bleeding occurred in 22.3% (95% CI: 17.8% to 28.3%) of patients.21
Table 5.
VARC criteria for bleeding.
| Life-threatening/disabling bleeding | Major bleeding | Minor bleeding |
|---|---|---|
| Fatal | No criteria for life-threatening/disabling | Any bleeding worthy of clinical mention, but not life-threatening-or major |
| Hgb decrease ≥5 g/dL, or whole blood/pRBC transfusion ≥4U | Hgb decrease ≥3 g/dL, or whole blood/pRBC transfusion 2–3U | |
| Causing hypovolemic shock/severe hypotension requiring vasopressors/surgery | ||
| Critical area/organ eg, Intracranial, intraspinal, intraocular, pericardial requiring pericardiocentesis, intramuscular with compartment syndrome |
Notes: Either of the conditions can be satisfied in column one, “Life-threatening/disabling bleeding.” Major bleeding, both criteria have to be fulfilled.
Table adapted.20
When comparing 30-day major bleeding in TAVR to 1-year major bleeding in the PARTNER A trial, bleeding increased from 9.3% to 14.7%. Given that this bleeding risk is post-procedural, antiplatelet and anticoagulation therapy may have to be examined in detail to determine the optimal length of time for treatment (ie, dual antiplatelet therapy) to prevent valve thrombosis, while minimizing the risk of bleeding.
Endocarditis
Endocarditis is an important issue that needs to be addressed. Whereas bioprosthetic or mechanical infected valves may need to be removed and replaced, it is unclear what options TAVR patients will have if vegetations develop on their stent valve. A recent case report and review of the literature suggests that the mortality rate is 33% if a patient with TAVR develops endocarditis.37 If surgery is indicated, the STS score will increase given the active infection. Thus, if the patient initially had TAVR because of high surgical risk, then surgery may still be refused. In a published case report,37 a review of the literature showed that only one-third of patients with medically treated endocarditis survived. Furthermore, in the elderly, the onset may be insidious. Vigilance must be exercised to prevent endocarditis during the stent valve procedure, and when seeing the patient on follow up visits.
Antiplatelet/anticoagulation therapy
Unlike TAVR, guidelines for percutaneous coronary intervention (PCI) are well established, with the placement of bare metal stents requiring dual antiplatelet therapy (DAPT) for 1 month. For drug-eluting stents, US guidelines suggest that DAPT be used for 1 year, with aspirin (ASA) taken lifelong thereafter. Guidelines for stent valve antiplatelet and anticoagulation have yet to be created. The incidence of prosthetic valve thrombosis post procedure seems to be minimal, whereas life-threatening bleeding has been a significant risk and a predictor of mortality. In the PARTNER trials, DAPT was given for 6 months post procedure; the range for most trials is between 3 to 6 months. In a small pilot study, Ussia et al compared the use of 100 mg of aspirin daily alone with 100 mg of aspirin and 75 mg of clopidogrel taken daily for 3 months post procedure.38 Mortality and Major Adverse Cardiovascular and Cerebral Events (MACCE – in this paper inclusive of death MI, life-threatening bleed, major stroke, urgent/conversion to SAVR) were not significantly different at 30 days (ASA: 15% vs. DAPT: 13%, P = 0.71) and 6 months (ASA: 15% vs. DAPT: 18%, P = 0.85). Larger studies will need to be conducted to determine appropriate antiplatelet and anticoagulation therapies to minimize MACCE. If decreasing antiplatelet duration will lead to less MACCE, then patients requiring PCI pre-TAVR may need to have their DAPT regimen reassessed.
US consensus document on management
A recent expert consensus document has been published with expert recommended guidelines for TAVR mainly based on PARTNER data.39 The summary table is shown below (Table 6).
Table 6.
Consensus guidelines to SAVR, TAVR, or standard therapy.
| Treatment | Indication | Major complications |
|---|---|---|
| Surgical aortic valve replacement |
|
|
| Transcatheter aortic valve replacement |
|
|
| Balloon aortic valvuloplasty |
|
|
| Medical therapy |
|
|
Reprinted with permission.39
Emerging Technologies: New Stent Valves
Multiple new stent valves are being developed as TAVR has gained approval in the United States and Europe. In addition to the Edwards SAPIEN and Medtronic CoreValve, several valves are in development. Even in 2008, first in-man results were published for the Sadra-Lotus Valve™ (Boston Scientific, MN, USA), Direct Flow™ Medical Valve (Direct Flow Medical Inc., CA, USA), the Paniagua Heart Valve (Colibri Heart Valve, LLC, CO, USA), in addition to human implants of the JenaValve (JenaValve Technologies Gmbh, Munich, Germany) and the AorTx valve (Hansen Medical Inc, CA, USA).14
Many of the larger device companies have acquired different valve technologies, which are in various stages of development. The new valves try to improve the deliverability, positioning, sealing, and repositioning/ removal when compared to prior Edward SAPIEN XT and Medtronic CoreValve,36 as well as have the same or smaller caliber transfemoral catheter sizes of 18 Fr or less. Some valves currently being evaluated include the LotusTM (Boston Scientific, MN, USA), Direct FlowTM (Direct Flow Medical Inc, CA, USA), CENTERA™ valve (Edward Lifesciences, Inc, CA USA), PorticoTM (St Jude Medical, Inc, MN, USA), AcurateTM (Symetic, Ecublens, VD, Switzerland), EngagerTM (Medtronic Inc, MN, USA), and the JenaValve (JenaValve Technologies GmbH, Munich, Germany).
Even with 50,000 stent valves deployed, both Edwards Lifesciences and Medtronic have multiple new and improved products in their pipeline. The Jena Valve is a second generation transapical stent valve currently being developed in Germany. It has a CE Mark of approval that was granted in late 2011. It has a porcine pericardial valve attached to a self-expanding nitinol stent that is placed from the transapical approach using a 32F sheathless catheter. The stent valve has three “feelers” that fit into the native sinuses. As with the Medtronic CoreValve, there is no need for rapid pacing prior to valve deployment. Safety of the valve was evaluated in a prospective, multicenter single arm study at seven German sites.40 A total of 73 patients were enrolled. Valve sizes used were 23 mm, 25 mm, and 27 mm. Primary endpoint was 30-day all-cause mortality (7.6%). Procedural success was 89.6%. Crossover to SAVR was 6% (n = 4) and valve-in-valve was performed in 3% (n = 2). Stroke occurred in 3% of patients. Given the “feeler” technology of the JenaValve, some manipulation in the aortic root and the ascending aorta is required. Further data acquisition is needed to determine whether this may contribute to more strokes. It should be noted that this effect did not occur in this study clinically, although transcranial Doppler and DW-MRI imaging were not performed to look for emboli. Long-term composite efficacy endpoints will be forthcoming.
The first in-man study of the Acurate TA transapical valve was recently completed.41 Forty patients with STS score of 9.0% ± 4.7% had TAVR with the Acurate system. By VARC criteria, device success was 92.5%, with a 30-day safety profile of 25%. Mean aortic gradient improved from 51 mmHg to 11.9 mm Hg at 6 months. Thus, in addition to the Edwards SAPIEN valve, both the JenaValve and the Acurate TA will provide more transapical options for TAVR.
A Possible Expanded Role for Balloon Aortic Valvuloplasty
Although the PARTNER B trial compared TAVR to standard therapy, standard therapy mainly consisted of balloon valvuloplasty. The rationale for this was that balloon valvuloplasty was not considered superior to medical therapy alone, and at most could only extend life a few more months. Furthermore, given the potential complications associated with standard therapy (including aortic regurgitation and stroke), balloon valvuloplasty is no longer used at many medical centers. However, it appears that balloon valvuloplasty may actually provide a bridge or a precious window of additional time for significantly symptomatic AS patients who are waiting to obtain TAVR but cannot yet receive it for various reasons.42 The risk of stroke in these patients is also not trivial. In the PARTNER B trial, the rate of stroke within 30 days for those treated with standard therapy was 1.1%, and the rate of stroke within 1 year was 3.9%. When taking into account the CMS criteria for TAVR, it should be noted that of the 30 structural heart procedures that need to be performed by the interventionalist each year, at least 60% of these procedures are required to be balloon valvuloplasty. As interventional cardiologists resurrect an old treatment that was once contraindicated to bridge critically ill patients for future TAVR or SAVR, the caveat and caution is that more strokes will probably be seen in patients with critical AS.
Relevant Topics that Need to be Addressed in the Future
Measurement of left ventricular end diastolic pressure (LVEDP) and aortic valve gradient
In the catheterization laboratory, the standard method for measuring aortic valve gradients is by crossing the aortic valve and measuring pressures in both the left ventricle (LV) and the aorta. In the setting of a stent valve, it may be difficult and dangerous to cross the valve with a pigtail catheter. One option that has been used for mechanical prostheses43 that may be used here is the pressure wire, which will avoiding damaging the stent valve, yet transduce a high-fidelity pressure waveform to measure the LVEDP as well as the aortic valve gradient. One setup might be to attach the catheter to a transducer and measure that versus the pressure wire to determine the gradient. Pullback of the pressure wire could confirm the gradient.
CABG/SAVR vs. PCI/TAVR vs. hybrid
It is estimated that 75% of patients requiring aortic valve replacement (AVR) have coronary artery disease (CAD) (Serruys and PARTNER A).16,35 If coronary revascularization is needed along with AVR, that increases the STS morbidity and mortality score further.5 The approach to TAVR in a patient with CAD is still unclear. Two percutaneous strategies have evolved: (1) stage with PCI first, then TAVR; or (12) concomitant. In the setting of left main stenosis and chronic total occlusions, the best course of action it is unclear since these patients will undoubtedly have moderate to high SYNTAX scores, suggesting better surgical rather than percutaneous long-term outcomes. It is possible, as has been stressed in recent statements, that a “Heart Team” approach will be more effective. Perhaps in one such scenario, a hybrid procedure involving grafting of the left internal mammary artery (LIMA) to the left anterior descending (LAD) artery by cardiothoracic surgery, PCI to the obtuse marginal artery (OM) and the right coronary artery (RCA) by interventional cardiology, and TAVR by the cardiothoracic and interventional teams together would be ideal. Certainly, endovascular aneurysm repair (EVAR) has been performed in conjunction with TAVR as well.
AVR risk score (TRS)—patient selection
A TRS has been proposed to determine if a patient is an appropriate candidate for TAVR.36 Analogous to the coronary SYNTAX score, a TRS would help determine who should get TAVR, SAVR, or medical therapy. In Europe, TAVR is not recommended in patients with an expected lifespan of less than 1 year. The reason for this is likely economical as well as clinically driven. Even with TAVR, the 1-year mortality rate is about 22.5% to 26%. The 2-year mortality rate is even higher (35% in the PARTNER A study;16 30% noted in the CoreValve Italian Registry;32 and 26.3% noted in the UK TAVI Registry35). Therefore, even though the stent valve does save lives, a large percentage of patients will still die within 2 years. Although it is clear that the device has saved a good percentage of the 50,000 patients implanted with it, a high percentage (35%, PARTNER A) will die within two years. Unlike PCI where the mortality rate is 0.1%, the TAVR mortality rate is difficult to discuss with a patient and his or her family. Having a TRS would help stratify the patients according to risk so that the ones that are ideal candidates are appropriately screened based on age, aortic valve annulus anatomy, vascular health, STS score/EuroScore, and other comorbidities.
The most eye opening statistic of the TAVR/ SAVR trials is perhaps the mortality rate associated with SAVR. As seen in Figure 1, after treatment with SAVR, the implication is that patients do well for a long period of time as their lifespan is extended considerably. However, this is likely only true of a certain percentage of patients with few comorbidities. In a retrospective, single center study evaluating 1061 patients with SAVR, the 10-year life expectancy based on age for 70–74 year-olds was 54%; for 75–79 year-olds was 43%; and for 80 years old and greater was 17%.44 In the PARTNER A trial, death at 2 years in SAVR was 35%. Extrapolating to 3 years, the mortality rate appears to be approximately 45%–50% for patients with TAVR and for patients with SAVR. Therefore, although the lifespan of high-risk patients is extended, is not necessarily extended indefinitely after either SAVR or TAVR.
SAVR vs. TAVR
In the United States, current outcomes for cardiothoracic surgeons are closely scrutinized and available to the public; for instance, the California CABG Outcomes Reporting (CCORP) is mandatory because of US Senate bill 680.45 Surgeons also voluntarily report their data to the STS. The rating for complicated operations with high operative mortality rates (ie, a high STS score) may not be weighted substantially differently (eg, compassionate surgery) in calculating a particular surgeon’s operative mortality. Therefore, any peri- or post-surgical deaths count against the surgeon and go towards their annual mortality count. Thus, surgeons may be penalized for taking difficult cases with likely poor outcomes. Each surgeon is allowed to refuse to operate independently of an absolute STS score. Yet there are still surgeons out there who do not refuse any aortic valve surgery, but these cases are rare and these surgeons are likely not subject to current outcomes scrutiny.
PARTNER B trial showed that TAVR was better than standard therapy for a patient if cardiothoracic surgeons refused to operate due to high peri- and postoperative mortality. However, it is difficult to state how much better TAVR was compared to standard therapy. Two-year follow up suggests mortality is still high at 43.3% of those receiving TAVR vs. 68% for standard therapy. Furthermore, there was an increased risk of stroke with TAVR (13.8%) vs. standard therapy (5.5%). Importantly, TAVR did not show that it was superior to surgery. Surgeons refused to perform the operation given the high operative and post-operative mortality. An important question to ask is that since mortality rates are going to be 30.7% at 1 year and 43.3% at 2 years (even with TAVR), would most surgeons still operate? Chances are that surgeons would most likely still operate, since surgical outcomes (had there been a surgery arm in the PARTNER B cohort study) would have been similar to those noted with TAVR. Therefore, an even better study would be to compare patients at high risk for TAVR vs. patients receiving SAVR in inoperable aortic stenosis. Certainly extrapolating from the data in the PARTNER A study (and in most of the other studies reviewed here) comparing TAVR to SAVR suggest that surgical outcomes would be similar.
Currently, interventional cardiologists are not scrutinized closely because the operative and postoperative mortality of PCI is very low. However, with the advent of TAVR, closer scrutiny of mortality rates of interventional cardiologists with the Heart Team is inevitable. Ultimately, interventional cardiologists may also begin to refuse cases. From a physician-patient perspective, it is certainly difficult to tell a patient and their family that there is a 50% chance of survival after 2 years, even with TAVR.
Longevity
In the excitement of pushing forward with TAVR, one key question remains: how long will these valves last? SAVR porcine valve bioprostheses degrade over time, and the current estimate is that 50%–60% of them will not last more than 10 years.46 It is unlikely that the Edward SAPIEN or the Medtronic CoreValve leaflets will outperform current bioprosthetic valves used for SAVR. If TAVR is used on low to moderate risk patients between the ages of 60 and 70 years old, then what happens when the valve degenerates significantly in another 10 years? Although over 100 successful valve-in-valve (mostly TAVR in SAVR) procedures have been reported,47 will a valve-in-valve (TAVR in TAVR) even be feasible, or would it be dangerous to the patient given that it could create another comorbidity? Perhaps these cases will require a SAVR from a TAVR, although explanting a TAVR 10 years post-procedure may be an extremely difficult surgical procedure, and it may be associated with a high mortality rate.
Bioprosthetic vs. mechanical
In the United States, current real world decisions for most patients with severe aortic stenosis do not revolve around TAVR, but rather SAVR. SAVR is still the gold standard for aortic valve replacement. The most important question for current SAVR use is whether the patient will receive a bioprosthetic (eg, porcine) or a mechanical (eg, Carbomedics ATS) valve. In the past, mechanical valves were always thought to be superior to bioprosthetic valves; now that TAVR is a possible rescue option (valve in valve, TAVR in SAVR) for bioprosthetic valves, guidelines are no longer clear. Prior placement of a mechanical valve would likely be an absolute contraindication for a patient being assessed for a TAVR.
Future
In this era of both SAVR and TAVR, one thing is clear: the treatment for aortic stenosis has changed. Cardiologists now have more options for their patients. The prevalence of the disease is highlighted by the sheer volume of patients that have had TAVR since 2002, despite regulatory hurdles. Outcomes associated with the implantation of either device appear to be equivalent to surgery, although mortality and major complications including stroke, vascular injury, bleeding, and paravalvular leak will need to be further reduced. The VARC criteria provide a good standardized framework by which to categorize these complications among a wide range of operators. New and next generation TAVR bioprostheses are being developed that may eventually make TAVR first line treatment. A TAVR risk score may help determine which patients will derive the most benefit from TAVR given the potential complications associated with the procedure. Future issues will need to be addressed as stated above, including assessing the efficacy of hybrid procedures such as CABG with TAVR, and determining whether an ideal patient with few comorbidities (but with critical AS) should receive a SAVR with mechanical prosthesis versus bioprosthesis in the event that the patient could benefit from a TAVR after his surgical valve deteriorates.
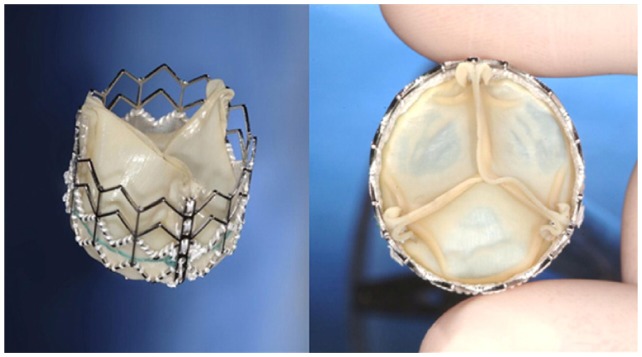
Figure 2.
Edwards SAPIEN Valve.
Reprinted with permission.10
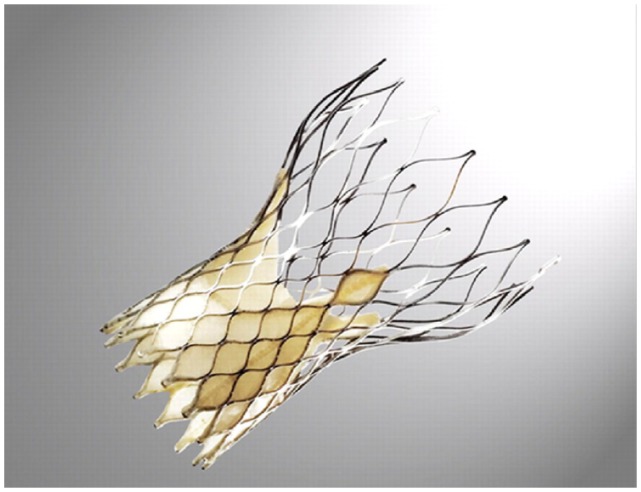
Figure 3.
Medtronic CoreValve.
Reprinted with permission.10
Footnotes
Author Contributions
Analysed the data: PPH. Wrote the first draft of the manuscript: PPH. Contributed to the writing of the manuscript: PPH. Agree with manuscript results and conclusions: PPH. Made critical revisions and approved final version: PPH. The author reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest. Provenance: the authors were invited to submit this paper.
Funding
Funding was provided in part by the International Cardiovascular Institute. Copyright permissions were graciously paid for by the International Cardiovascular Institute and Libertas Academica.
References
- 1.Carabello BA, Paulus WJ. Aortic stenosis. Lancet. 2009;373(9667):956–66. doi: 10.1016/S0140-6736(09)60211-7. [DOI] [PubMed] [Google Scholar]
- 2.Schwarz F, Banmann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation. 1982;66(5):1105–10. doi: 10.1161/01.cir.66.5.1105. [DOI] [PubMed] [Google Scholar]
- 3.Ross J, Jr, Braunwald E. Aortic stenosis. Circulation. 1968;38(Suppl 1):61–7. doi: 10.1161/01.cir.38.1s5.v-61. [DOI] [PubMed] [Google Scholar]
- 4.Bonow RO, Carabello BA, Chatterjee K, et al. for American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52(13):e1–142. doi: 10.1016/j.jacc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 5.The Society of Thoracic Surgeons. The Society of Thoracic Surgeons [web-page] Chicago: The Society of Thoracic Surgeons; 2012. Available from: http://www.sts.org. [Google Scholar]
- 6.Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet. 1986;1(8472):63–7. doi: 10.1016/s0140-6736(86)90716-6. [DOI] [PubMed] [Google Scholar]
- 7.Cribier A. Development of transcatheter aortic valve implantation (TAVI): a 20-year odyssey. Arch Cardiovasc Dis. 2012;105(3):146–52. doi: 10.1016/j.acvd.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Webb JG, Binder RK. Transcathter aortic valve implantation: the evolution of prostheses, delivery systems and approaches. Arch Cardiovasc Dis. 2012;105(3):153–9. doi: 10.1016/j.acvd.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Leon MB. Transcatheter aortic valve replacement: a breakthrough medical therapy. The 20-year odyssey, and now, a 10-year anniversary. Arch Cardiovasc Dis. 2012;105(3):145–52. doi: 10.1016/j.acvd.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Zajarias A, Cribier AG. Outcomes and safety of percutaneous aortic valve replacement. J Am Coll Cardiol. 2009;53(20):1829–36. doi: 10.1016/j.jacc.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 11.Eltchaninoff H, Durand E, Borz B, et al. Prospective analysis of 30-day safety and performance of transfemoral transcatheter aortic valve implantation with Edwards SAPIEN XT versus SAPIEN prostheses. Arch Cardiovasc Dis. 2012;105(3):132–40. doi: 10.1016/j.acvd.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Dvir D, Barbash IM, Ben-Dor I, et al. The development of transcatheter aortic valve replacement in the USA. Arch Cardiovasc Dis. 2012;105(3):160–4. doi: 10.1016/j.acvd.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Leon MB, Smith CR, Mack M, et al. for PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(1):1597–607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 14.Del Valle-Fernandez R, Ruiz CE. Transcatheter heart valves for the treatment of aortic stenosis: state-of-the-art. Minerva Cardioangiol. 2008;56(5):543–6. [PubMed] [Google Scholar]
- 15.Makkar RR, Fontana GP, Jilaihawi H, et al. for PARTNER Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366(18):1696–704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 16.Smith CR, Leon MB, Mack MJ, et al. for PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 17.Kodali SK, Williams MR, Smith CR, et al. for PARTNER Trial Investigators. Two-year outcomes after transcatheter or surgical aortic valve replacement. N Engl J Med. 2012;366:1686–95. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 18.Wenaweser P, Pilgrim T, Kadner A, et al. Clinical outcomes of patients with severe aortic stenosis at increased surgical risk according to treatment modality. J Am Coll Cardiol. 2011;58(21):2151–62. doi: 10.1016/j.jacc.2011.05.063. [DOI] [PubMed] [Google Scholar]
- 19.CMS Decision Memo for TAVR. 2012. http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=257&ver=4&NcaName=Transcatheter+Aortic+Valve+Replacement+%28TAVR%29&bc=ACAAAAAAIAAA&.
- 20.Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for Transcatheter Aortic Valve Implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57(3):253–69. doi: 10.1016/j.jacc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Genereux P, Head SJ, Van Mieghem NM, et al. Clinical outcomes after transcatheter aortic valve replacement using Valve Academic Research Consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol. 2012;59(23):2317–26. doi: 10.1016/j.jacc.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Deneault B, Kirtane AJ, Kodali SK, et al. Stroke associated with surgical and transcatheter treatment of aortic stenosis. J Am Coll Cardiol. 2011;58(21):2143–50. doi: 10.1016/j.jacc.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Drews T, Pasic M, Buz S, et al. Transcranial Doppler sound detection of cerebral microembolism during aortic valve implantation. Thorac Cardiovasc Surg. 2011;59(4):237–42. doi: 10.1055/s-0030-1250495. [DOI] [PubMed] [Google Scholar]
- 24.Ghanem A, Muller A, Nahle CP, et al. Risk and fate of cerebral embolism after transfemoral aortic valve implantation: a prospective pilot study with diffusion-weighted magnetic resonance imaging. J Am Coll Cardiol. 2010;55(14):1427–32. doi: 10.1016/j.jacc.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 25.Rodes-Cabau J, Dumont E, Boone RH, et al. Cerebral embolism following transcatheter aortic valve implantation: comparison of transfemoral and transapical approaches. J Am Coll Cardiol. 2011;57(1):18–28. doi: 10.1016/j.jacc.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 26.Arnold M, Schulz-Heise S, Achenbach S, et al. Embolic cerebral insults after transapical aortic valve implantation detected by magnetic resonance imaging. JACC Cardiovasc Interv. 2010;3(11):1126–32. doi: 10.1016/j.jcin.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Kahlert P, Knipp SC, Schlamann M, et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation. 2010;121(7):870–8. doi: 10.1161/CIRCULATIONAHA.109.855866. [DOI] [PubMed] [Google Scholar]
- 28.Nietlispach F, Wijesinghe N, Gurvitch R, et al. An embolic deflection device for aortic valve interventions. JACC Cardiovasc Interv. 2010;3(11):1133–8. doi: 10.1016/j.jcin.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Onsea K, Agostoni P, Samim M, et al. First-in-man experience with a new embolic deflection device in transcatheter aortic valve interventions. Euro Intervention. 2012;8(1):51–6. doi: 10.4244/EIJV8I1A9. [DOI] [PubMed] [Google Scholar]
- 30.Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009;22(9):975–1014. doi: 10.1016/j.echo.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Takagi K, Latib A, Al-Lamee R, et al. Predictors of moderate-to-severe paravalvular aortic regurgitation immediately after CoreValve implantation and the impact of postdilatation. Catheter Cardiovasc Interv. 2011;78(3):432–43. doi: 10.1002/ccd.23003. [DOI] [PubMed] [Google Scholar]
- 32.Ussia GP, Barbanti M, Petronio AS, et al. for CoreValve Italian Registry Investigators. Transcatheter aortic valve implantation: 3-year outcomes of self-expanding CoreValve prosthesis. Eur Heart J. 2012;33(8):969–76. doi: 10.1093/eurheartj/ehr491. [DOI] [PubMed] [Google Scholar]
- 33.Jilaihawi H, Kashif M, Fontana G, et al. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol. 2012;59(14):1275–86. doi: 10.1016/j.jacc.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 34.Jabbour A, Ismail TF, Moat N, et al. Multimodality imaging in transcatheter aortic valve implantation and post-procedural aortic regurgitation: comparison among cardiovascular magnetic resonance, cardiac computed tomography, and echocardiography. J Am Coll Cardiol. 2011;58(1):2165–73. doi: 10.1016/j.jacc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Moat NE, Ludman P, de Belder MA, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the UK TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol. 2011;58(20):2130–8. doi: 10.1016/j.jacc.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 36.Piazza N, Lange R, Martucci G, Serruys PW. Patient selection for transcatheter aortic valve implantation: patient risk profile and anatomical selection criteria. Arch Cardiovasc Dis. 2012;105(3):165–73. doi: 10.1016/j.acvd.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Loh PH, Bundgaard H, Søndergaard L. Infective endocarditis following transcatheter aortic valve replacement: diagnostic and management challenges. Catheter Cardiovasc Interv. 2012 doi: 10.1002/ccd.24368. 1002/ccd.24368. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 38.Ussia GP, Scarabelli M, Mule M, et al. Dual antiplatelet therapy versus aspirin alone in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2011;108(12):1772–6. doi: 10.1016/j.amjcard.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 39.Holmes DR, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59(13):1200–54. doi: 10.1016/j.jacc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Treede H, Mohr FW, Baldus S, et al. Transapical transcathetr aortic valve implantation using the JenaValve system: acute and 30-day results of the multicenter CE-mark study. Eur J Cardio-Thoracic Sur. 2012:1–8. doi: 10.1093/ejcts/ezs129. [DOI] [PubMed] [Google Scholar]
- 41.Kempfert J, Treede H, Rastan AJ, et al. Transapical aortic valve implantation using a new self-expandable bioprosthesis (ACURATE TA™): 6-month outcomes. Eur J Cardiothorac Surg. 2012 doi: 10.1093/ejcts/ezs139. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 42.Malkin CJ, Judd J, Chew DP, Sinhal A. Balloon aortic valvuloplasty to bridge and triage patients in the era of trans-catheter aortic valve implantation. Catheter Cardiovasc Interv. 2012 doi: 10.:1002/ccd.24325. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 43.Parham W, El Shafei A, Rajjoub H, Ziaee A, Kern MJ. Retrograde left ventricular hemodynamic assessment across bileaflet prosthetic aortic valves: the use of a high-fidelity pressure sensor angioplasty guidewire. Catheter Cardiovasc Interv. 2003;59(4):509–13. doi: 10.1002/ccd.10596. [DOI] [PubMed] [Google Scholar]
- 44.Yamashita MH, Ye J, Jamieson WR, Cheung A, Lichtenstein SV. Conventional aortic valve replacement remains a safe option in patients aged ≥> 70 years: a 20-year experience. J Heart Valve Dis. 2012;21(2):148–55. [PubMed] [Google Scholar]
- 45.California Coronary Artery Bypass Graft Outcomes Reporting Program (CCORP) Senate Bill 680 [website] California: State of California Office of Statewide Health Planning and Development; 2012. Available from: http://www.oshpd.ca.gov. [Google Scholar]
- 46.Kalejs M, Stradins P, Lacis R, Ozolanta I, Pavars J, Kasyanov V. St Jude Epic heart valve bioprostheses versus native human and porcine aortic valves—comparison of mechanical properties. Interact Cardiovasc Thorac Surg. 2009;8(5):553–6. doi: 10.1510/icvts.2008.196220. [DOI] [PubMed] [Google Scholar]
- 47.Piazza N, Bleiziffer S, Brockmann G, et al. Transcatheter aortic valve implantation for failing surgical aortic bioprosthetic valve: from concept to clinical application and evaluation (part 2) J Am Coll Cardiol Intv. 2011;4:733–742. doi: 10.1016/j.jcin.2011.05.007. [DOI] [PubMed] [Google Scholar]