Abstract
Background
Zinc (Zn) deficiency is one of the most widespread mineral nutritional problems that affect normal development in plants. Because Zn cannot passively diffuse across cell membranes, it must be transported into intracellular compartments for all biological processes where Zn is required. Several members of the Zinc-regulated transporters, Iron-regulated transporter-like Protein (ZIP) gene family have been characterized in plants, and have shown to be involved in metal uptake and transport. This study describes the first putative Zn transporter in grapevine. Unravelling its function may explain an important symptom of Zn deficiency in grapevines, which is the production of clusters with fewer and usually smaller berries than normal.
Results
We identified and characterized a putative Zn transporter from berries of Vitis vinifera L., named VvZIP3. Compared to other members of the ZIP family identified in the Vitis vinifera L. genome, VvZIP3 is mainly expressed in reproductive tissue - specifically in developing flowers - which correlates with the high Zn accumulation in these organs. Contrary to this, the low expression of VvZIP3 in parthenocarpic berries shows a relationship with the lower Zn accumulation in this tissue than in normal seeded berries where its expression is induced by Zn. The predicted protein sequence indicates strong similarity with several members of the ZIP family from Arabidopsis thaliana and other species. Moreover, VvZIP3 complemented the growth defect of a yeast Zn-uptake mutant, ZHY3, and is localized in the plasma membrane of plant cells, suggesting that VvZIP3 has the function of a Zn uptake transporter.
Conclusions
Our results suggest that VvZIP3 encodes a putative plasma membrane Zn transporter protein member of the ZIP gene family that might play a role in Zn uptake and distribution during the early reproductive development in Vitis vinifera L., indicating that the availability of this micronutrient may be relevant for reproductive development.
Background
Zinc is an essential micronutrient that plays many important roles in various physiological and metabolic processes in all living organisms. It functions as a cofactor for over 300 enzymes and proteins involved in cell division, nucleic acid metabolism and protein synthesis, and is critical in the control of gene transcription and the coordination of other biological processes regulated by proteins containing DNA-binding Zn-finger motifs, RING fingers and LIM domains [1-4].
It has been demonstrated that Zn deficiency is one of the most widespread mineral nutritional problems affecting normal development in plants [5-8]. This includes altered expression and/or function of proteins at the metabolic level that leads to different physiological symptoms characterized by root apex necrosis. At the same time, sub-lethal Zn deficiency induces spatial heterogeneous or interveinal chlorosis, development of reddish-brown or bronze tints and a range of auxin deficiency-like responses such as internode shortening, epinasty, inward curling of leaf lamina and reduction of leaf size [4-9]. In grapevines, deficit of Zn results in the development of leaves that are smaller than normal and/or mottled, and shortened internodes. It has been suggested that this reduction in shoot growth results from the fact that Zn is essential for the synthesis of tryptophan, a precursor of the phytohormone indoleacetic acid (IAA) [10]. Another important symptom of Zn deficiency in grapevines is the production of clusters with few berries that also vary in size from normal to very small [10-12]. In this way, vineyards commonly correct Zn deficiency with both soil and foliar application of fertilizers. Under conditions of Zn deficiency, application of foliar Zn fertilizer shortly before anthesis increases the number of flowers that set fruit [10-14].
Since Zn cannot passively diffuse across cell membranes, it must be transported into intracellular compartments for all biological processes where Zn is required. Several members of the 15 Zinc-regulated transporters, Iron-regulated transporter-like Protein (ZIP) gene family have been characterized in Arabidopsis thaliana[15], and their involvement in metal uptake and transport in plants has been demonstrated [16-18].
Arabidopsis ZIP1 and ZIP3 genes are expressed in roots in response to Zn deficiency, suggesting that they transport Zn from the soil to the plant. ZIP4 is expressed either in roots and shoots, showing a delicate regulation to control the homeostasis of Zn, thus avoiding potential toxic effects of this micronutrient [15,17]. Additionally, these three transporters restore Zn uptake in the yeast Zn-uptake mutant, Δzrt1/Δzrt2 (ZHY3 strain), confirming its implication in Zn homeostasis [15,19-22]. Moreover, ZIP2 and ZIP4 can rescue yeast mutants deficient in copper (Cu) transport, and ZIP4 is up-regulated in Cu-deficient roots [23]. Although several ZIP genes have been identified and functionally characterized at the molecular level [2,24], the complete gene family and their role in metal homeostasis is not fully understood. In this way, a considerably large family of ZIP genes has been currently characterized from various species, such as Thlaspi japonicum[25], Thlaspi caerulescens[17,26-28], soybean [29], Medicago truncatula[30,31] and rice [2,24,32,33]. The availability of the full-genome sequence of grapevine (http://www.genoscope.cns.fr/spip/Vitis-vinifera-e.html) provides the opportunity to investigate the Zn homeostasis and their importance for the reproductive development in this organism. 20 ZIP genes have been identified recently in Vitis vinifera[34], but none of them has been functionally characterized.
Here, we report the isolation of VvZIP3, a member of the ZIP gene family from Vitis vinifera L. cv. Carménère, which encodes a Zn uptake protein. This gene was isolated from an expression library of Carménère berries and is highly homologous to AtZIP1 gene from Arabidopsis thaliana. Expression of VvZIP3 in the yeast Zn-uptake deficient mutant ZHY3 complemented its growth defect, indicating that VvZIP3 has the function of a putative Zn transporter. Moreover, the expression of the VvZIP3-mGFP5 fusion protein in onion epidermal cells indicated that is located at the plasma membrane. Expression analysis revealed that VvZIP3 was transcribed principally in reproductive tissues - specifically in developing flowers -, organs that also present the highest accumulation of Zn. These results suggest that VvZIP3 participates in Zn uptake during flower development contributing to the normal development in Vitis vinifera L.
Results
VvZIP3 is a member of a Zn transporter encoding gene family
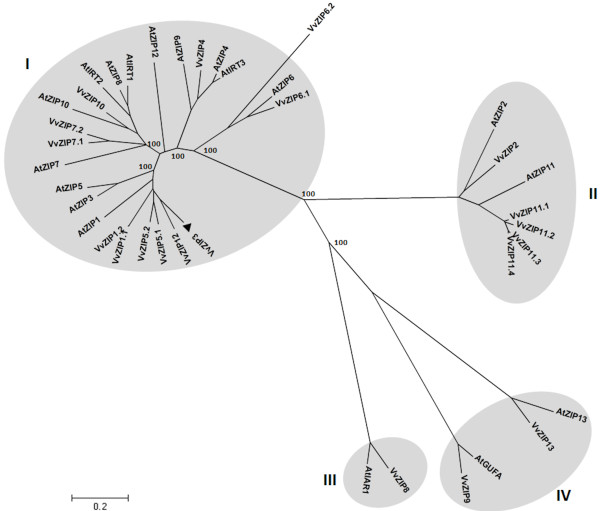
In a previous preliminary study, a macroarray containing approximately 4800 ESTs from grapevine reproductive tissue expression libraries was screened to compare the transcriptomic profiles of normal and seedless berries from Carménère cultivar [35]. A gene coding for a protein similar to a metal transporter from Medicago truncatula was found to be strongly repressed in seedless berries (see Additional file 1). The corresponding full length cDNA was isolated from an expression library of Carménère berries and its sequence was subjected to different in silico analyses for further characterization. When compared with the ZIP genes identified in the grapevine genome [34] the isolated gene was found to correspond to VvZIP3. Sequence analysis and comparison of the cDNA with genomic DNA shows that VvZIP3 is composed of three exons and two introns, being a single copy gene located in chromosome I of grapevine (data not shown). A phylogenetic tree was obtained compiling VvZIP3 protein with other sequences of known Arabidopsis thaliana and the recently identified Vitis vinifera L. ZIP members (Figure 1). This association revealed that VvZIP3 is closely related to AtZIP5, AtZIP3 and AtZIP1, three characterized Zn transporters in Arabidopsis[15].
Figure 1.
Phylogenetic relationship between VvZIP3 and ZIP proteins from Vitis and Arabidopsis thaliana. Phylogenetic analysis was performed using the software MEGA 4.0 (http://www.megasoftware.net/mega.html). It is assumed that the length of the branches is proportional to phylogenetic distances. Position of VvZIP3 in the not rooted tree is marked by a black arrowhead. Grapevine protein sequences were deduced from the nucleotide sequence obtained in this work (VvZIP3) or from the GenBank accessions (see methods).
VvZIP3 is differentially expressed during grapevine development
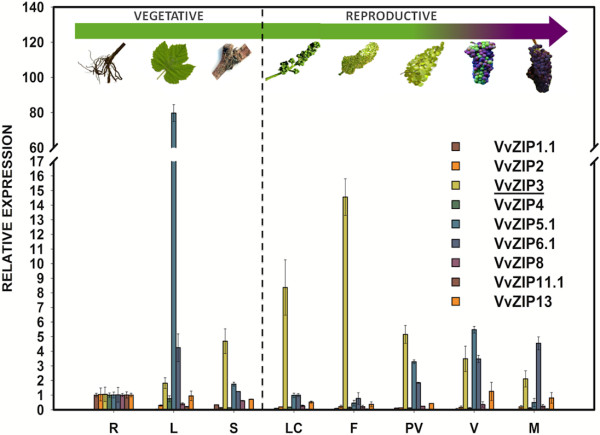
As a first approximation to determine the expression profile of VvZIP3 and its relevance during the development of Vitis vinifera L. cv. Carménère, relative expression level was measured by qPCR and compared with other representative ZIP genes identified in the grapevine genome [34]. Based on clusters determined in the phylogenetic comparison (Figure 1), VvZIP1.1VvZIP2VvZIP4VvZIP5.1VvZIP6.1VvZIP8VvZIP11.1 and VvZIP13 were selected for this analysis. Total RNA was isolated from roots, leaves, stems, little clusters, flowers, fruits and seeds at different developmental stages (from pre-veraison to mature grapes) during the S3 growing period, covering important events such as flower and berry development. This analysis reveal that VvZIP5.1 is mainly expressed in leaves during the vegetative development (about 80-fold compared to roots) but its expression decay during early stages of the reproductive development, being up-regulated again at pre-veraison (PV) and veraison (V) stages but to a lesser extent than in leaves (Figure 2). On the other hand, VvZIP3 presented a differential expression profile in both vegetative and reproductive tissues, characterized for a high expression during reproductive development. While in vegetative tissues the expression prevailed mainly in stems and at low levels in roots and leaves, in reproductive tissues VvZIP3 was mainly expressed in little cluster and flowers, with a significant up regulation (about fifteen-fold) in flowers. Contrary to this, reduction of the VvZIP3 transcripts was evident in berries as maturation stages progressed (Figure 2), suggesting that VvZIP3 could be important during the early stages of the reproductive development in Vitis vinifera L. Other members of VvZIP family analyzed shown a low expression level except for VvZIP6.1 that is induced as berry maturation stages progressed (Figure 2).
Figure 2.
Gene expression analysis of members ofVitis vinifera L. ZIPgene family. Expression profiles of representative members of Vitis vinifera ZIP gene family in R (roots); L (leafs); S (stems); LC (little clusters); F (flowers); PV (pre-veraison fruit); V (whole veraison fruit); M (whole mature fruit). Expression in root samples was adjusted to 1 relative unit. The end of vegetative development and the beginning of reproductive development is divided by a dotted line. The images are representations of each phenological stage. VvZIP3 is underlined. Data represent means ± SD (n = 3).
VvZIP3 protein has conserved motifs associated to Zn transporters
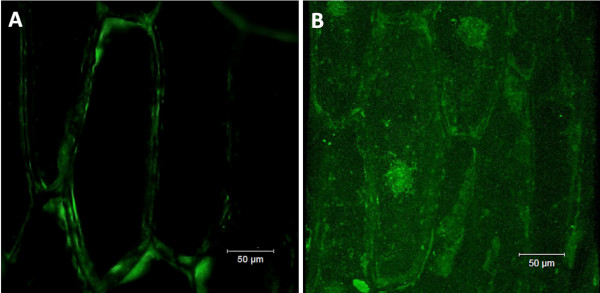
BLAST search on the translated protein sequence indicated strong homology with several members of the ZIP family from Arabidopsis thaliana as well as with other Zn and iron (Fe) transporters (Figures 1 and 3). Multiple alignments of the translated sequence with homologous proteins showed that VvZIP3 encodes a protein of 348 amino acid residues (of ca. 37.3 KDa). In agreement with the structure of other ZIP protein family members, principally AtZIP1 (accession AT3G12750), ZIP1 and ZIP2 from Fragaria X ananasa (AAX28838 and AAX28837, respectively), GmZIP1 (AAK37761), PtZIP12 (XP_002315075) and MtZIP1 (AAR08412), VvZIP3 was predicted to contain eight transmembrane (TM) domains, a very short C-terminal tail, and a hydrophilic region between TM domains III and IV, being this the most variable region in length and containing a potential metal-binding domain rich in histidine residues (Figure 3). This region was predicted to be directed toward the inside surface of the membrane. Further analyses with the Wolf PSORT-II software [36] (http://wolfpsort.org/) showed that VvZIP3 is predicted to be a plasma membrane protein with a potential signal peptide in the first 28 residues (Figure 3). The cellular localization assigned by in silico analysis was experimentally tested. VvZIP3 cDNA fused to the N-terminal coding part of the modified green fluorescent protein 5 (mGFP5) was transiently expressed under the control of the cauliflower mosaic virus (CaMV) 35 S promoter in onion epidermal cells. The fluorescence of the VvZIP3-mGFP5 fusion protein was observed at the plasma membrane (Figure 4A), while that of mGFP5 alone was localized to the cytoplasm and nucleus (Figure 4B), suggesting that VvZIP3 is a transporter protein located at the plasma membrane.
Figure 3.
Comparison of the VvZIP3 amino acid sequence with its homologues from other plant species. Alignment was performed with highest similar homologous sequences. Identical residues are blackened. The eight transmembrane domains are highlighted by black lines under the sequences and numbered from I to VIII, as predicted by TMHMM and TMpred (see methods). HMD, highlighted by light gray line, depicts the histidine-rich metal-binding domain. A potential metal binding motif highly conserved (HxGHVH) is marked between TM-III and TM-IV with asterisks under the sequences. The N-terminus line under the VvZIP3 sequence (grey box) highlights a possible signal peptide for plasma membrane localization obtained by WOLF-PSORT-II software (see methods).
Figure 4.
Cellular localization of VvZIP3-mGFP5 in planta. Transient expression of (A) VvZIP3-mGFP5 and (B) mGFP5 constructs in onion epidermal cells. Both genetic constructs were incorporated by particle bombardment and transformed bulb scale epidermal layers were incubated for 16 h at 25°C before visualization by confocal microscopy (Bars = 50 μm).
VvZIP3 restores Zn-limited growth in the yeast ZHY3 double mutant strain
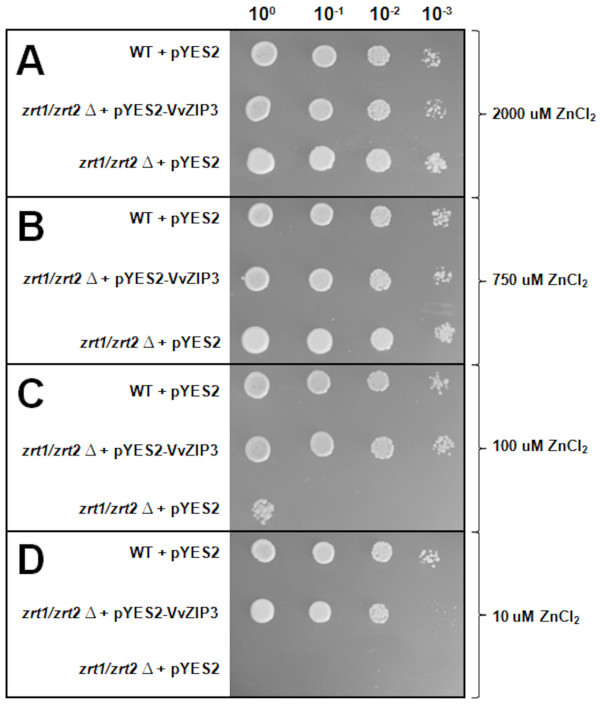
To support the role of VvZIP3 as a Zn uptake transporter, the yeast mutant ZHY3 [19,20] defective in Zn uptake, due to the inactivation of both its high (Δzrt1)- and low (Δzrt2)-affinity Zn transporters, was used in a complementation experiment. ZHY3 cells transformed with the empty pYES2 expression vector were able to grow on synthetically defined medium only when supplemented with Zn (750 μM to 2 mM) (Figure 5). However, ZHY3 transformed with the pYES2 expression vector containing the VvZIP3 cDNA (pYES2-VvZIP3) grew well on media supplemented with both low (10 μM and 100 μM) and high Zn (750 μM and 2 mM). The wild type parental yeast strain DY1457 also developed on media with both low and high Zn concentrations (Figure 5). These results indicate that VvZIP3 complemented the mutations of ZHY3, apparently by transporting Zn across the yeast plasma membrane.
Figure 5.
Complementation of the ZHY3 (zrt1zrt2Δ) yeast mutant with VvZIP3. The double mutant strain was transformed with either the empty vector pYES2 or with the pYES2-VvZIP3 construction. The wild type parental strain DY1457 (WT) was also transformed with pYES2 as a control. The transformed cells were adjusted to OD600 of 1 and 5 μl of serial dilutions (from left to right in each panel) and were spotted on SC-U plates supplemented with different concentrations of ZnCl2. Plates were incubated for 6 days at 30°C.
VvZIP3 is mainly expressed in the pericarp and skin of berries and is related to normal fruit development
A more detailed analysis of the transcriptional profile of VvZIP3 along fruit development showed that the expression level during berry growth was significant lower than that in early stages of reproductive development (inflorescences and flowers) and appears to be restricted to the pericarp and skin tissues since a very low transcriptional activity was detected in seeds (Figure 6A).
Figure 6.
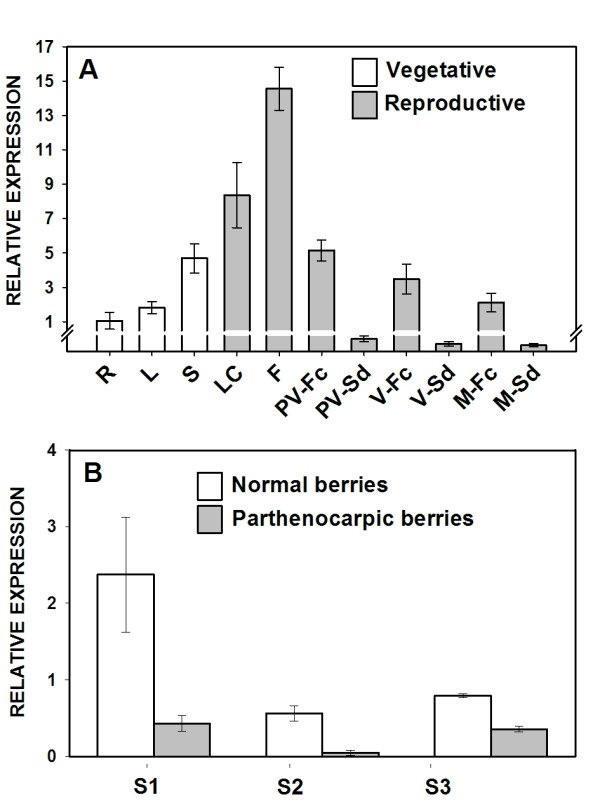
Gene expression analysis of VvZIP3 in grapevine tissues. (A) Expression analysis of VvZIP3 in R (roots); L (leafs); S (stems); LC (little clusters); F (flowers); PV-Fc (pre-veraison fruit complete); PV-Sd (pre-veraison seeds); V-Fc (whole veraison fruit); V-Sd (veraison seeds); M-Fc (whole mature fruit); M-Sd (mature seeds). Expression in root samples was adjusted to 1 relative unit. Relative expression values of PV-Sd, V-Sd and M-Sd are 0.039, 0.024 and 0.019, respectively. Data represent means ± SD (n = 3). (B) Expression analysis of VvZIP3 in normal and parthenocarpic berries at the pre-veraison stage during three growing seasons (S1, S2 and S3). qPCR analyses of VvZIP3 expression was normalized against the expression level of VvGAPDH. Data represent means ± SD (n = 3).
In field conditions, Zn deficiency is commonly associated to the production of clusters with few berries that vary in size from normal seeded to very small or parthenocarpic unseeded berries, a viticulture problem known as millerandage[10-12]. In order to test whether VvZIP3 expression is being affected in such phenotype, its transcriptional profile was compared in normal and parthenocarpic grapes in an early developmental stage (pre-veraison) from three different seasons (S1, S2 and S3). This analysis revealed that expression of VvZIP3 was consistently repressed in parthenocarpic green berries compared to normal berries in the three growing seasons analyzed (Figure 6B). Taken together, these results suggest that VvZIP3 is mainly expressed when the plant needs high requirement of Zn (flowering and fruit setting) and that its expression is associated with the physiological processes that affect the normal berry development in grapevine.
Zn accumulation in reproductive tissue of Vitis vinifera L. cv. Carménère is associated to VvZIP3 expression profiles
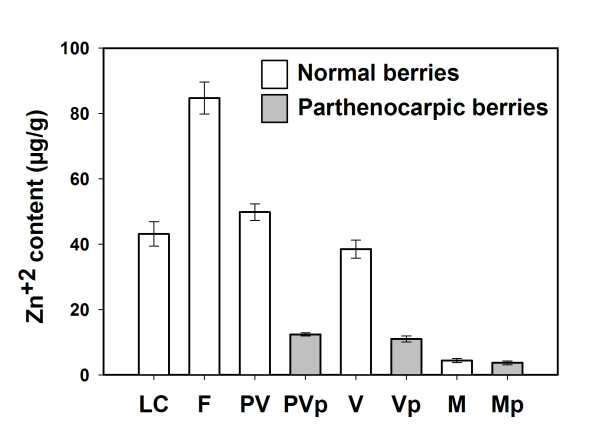
To examine whether the Zn content profiles correlate with the expression of VvZIP3 on the same season and to determine the profiles of accumulation of this metal during reproductive development, the concentration of Zn was measured in little clusters, flowers and fruits from season S3 (Figure 7). As expected, there was a significant increase of Zn levels in flowers, about two-fold compared to those in little clusters. This observation suggests that VvZIP3 plays a relevant role in Zn transport during flower development. After flowering stage, the reduction in Zn content was evident as maturation stages progressed. Additionally, parthenocarpic berries showed reduced Zn content when compared with normal berries before the maturation stage. These results seems to be consistent with the VvZIP3 expression profile (Figure 6), however, and since at least two other members of the grapevine ZIP gene family are also expressed during berry development (VvZIP5.1 and VvZIP6.1; Figure 2), the putative role of VvZIP3 in Zn-uptake in these tissues need to be further analyzed.
Figure 7.
Zn accumulation patterns in grapevine. Zn concentration in developing organs of Vitis vinifera L. in season 3 (S3). LC (little clusters); F (flowers); PV (pre-veraison berries); PVp (pre-veraison parthenocarpic berries); V (veraison berries); Vp (veraison parthenocarpic berries); M (mature berries); Mp (mature parthenocarpic berries). Data represent means ± SD (n = 3).
The expression of VvZIP3 is induced by Zn in normal berries
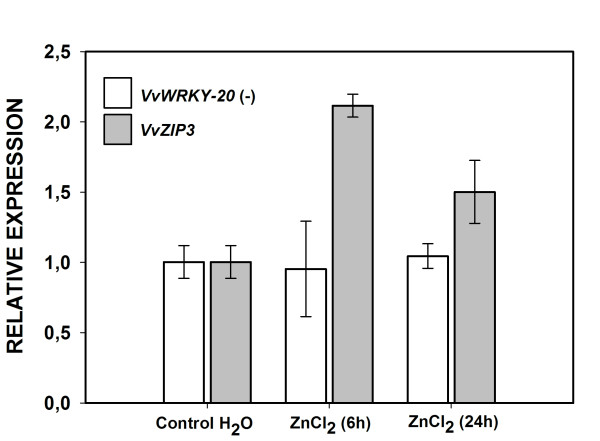
To test if the VvZIP3 expression is Zn-dependant in reproductive tissues, normal berries at pre-veraison stage were exposed to Zn treatment. To reproduce in planta situation, exogenous Zn2+ was added by generating a capillary ion flux to the sink tissues through the berry peduncle (see Methods). After 6 hours of treatment, expression of VvZIP3 was up-regulated about 2.1 fold compared to no-treated berries and this up-regulation was maintained until the end of the experiment (24 hours), while the negative control gene, VvWRKY-20, which encodes a putative zinc-finger transcription factor expressed in grapevine leaves (see Additional file 2), showed no alteration in its transcriptional level, along this treatment (Figure 8). This result suggests that the zinc flow to reproductive organs promotes its accumulation in these tissues by inducing VvZIP3 expression.
Figure 8.
VvZIP3response under zinc treatment. Expression analysis of VvZIP3 in pre-veraison berries under Zn treatment (2 mM ZnCl2) at 6 and 24 hours. Expression in non-treated (H2O) pre-veraison berries samples for VvZIP3 and VvWRKY-20 (negative control), was adjusted to 1 relative unit. qPCR analyses of VvZIP3 and VvWRKY-20 expression was normalized against the expression level of VvGAPDH. Data represent means ± SD (n = 3).
Discussion
Although several ZIP genes have been characterized in plants [15,23,37-40], to date, no ZIP gene has been isolated for Vitis species. The ZIP family of metal transporters shares several characteristics, including a molecular size between 36 and 39 kDa, 8 trans-membrane domains, a cytoplasmic ‘variable region’ localized between transmembrane domains 3 and 4 that provides a potential metal-binding domain and carboxy and amino termini located on the outer side of the targeted membrane [15,21,22]. Indeed, VvZIP3 displays all these structural characteristics allowing to be considered as a member of the ZIP family (Figure 3).
As shown in the phylogenetic tree (Figure 1), the predicted amino acid sequence of VvZIP3 was most closely related to AtZIP1, AtZIP3 and AtZIP5 (50–56% of identity) from A. thaliana[15,16,22] indicating that VvZIP3 and those proteins share, probably, a common evolutionary ancestor. All of these proteins are able to complement the growth of the yeast strain ZHY3. This yeast mutant type is very sensitive to Zn deprivation because of the mutation of their both high (ZRT1)- and low (ZRT2)-affinity Zn uptake systems [19,20]. Similar to the Arabidopsis ZIP proteins, the grape ZIP protein encoded by the cDNA of VvZIP3 complemented the growth of this yeast mutant (Figure 5). Moreover, VvZIP3 was localized to the plasma membrane, as shown by the WOLF-pSORT II prediction and transient expression of a VvZIP3-mGFP5 fusion protein in onion epidermal cells (Figure 4A and 4B). Hence, these findings demonstrate that VvZIP3 gene encodes a putative Zn transporter that may participate in the uptake of this element in Vitis vinifera L..
The tissue-specific expression determined for grapevine ZIP encoding genes reflects the complexity of this gene family and suggests a differential gene regulation associated to the nutritional requirements of grapevine. In this regard, several ZIP genes identified in other species display diverse transcriptional profiles regarding tissue specificity and response to Zn status. For example, in rice, OsZIP4 mRNA accumulates in the phloem cells of the stem as well as in the vascular bundles of the roots and leaves [2,32], OsZIP1 mRNA accumulates in Zn-deficient roots and shoots while OsZIP2 mRNA accumulates primarily in Zn-deficient roots [24]. In the model legume Medicago truncatulaMtZIP1 transcripts were only detected in Zn-deficient roots and leaves [30], while MtZIP2 gene was expressed in roots and stems, but not in leaves, and its transcriptional activity could be induced by Zn [31]. In addition, the VvZIP3 counterparts identified in Arabidopsis (principally AtZIP1AtZIP3 and AtZIP5) show a strong transcriptional activity in root tissue under Zn deficiency [15,22]. Compared to other VvZIP genes (i.e VvZIP5.1), VvZIP3 is mainly transcribed in grapevine reproductive tissues under field conditions, more specifically in developing flowers and in the pericarp and/or skin of berries at early growth stages (Figure 2 and 6A). In these tissues, VvZIP3 expression seems to be induced in response to Zn exposure as deduced from the experiments with normal berries at pre-veraison stage under exogenous Zn treatment (Figure 8).
In addition, and even when VvZIP3 is not expressed in seeds, its transcriptional activity was consistently repressed in parthenocarpic non-seeded berries (Figure 6B). This down-regulation is not due to a non-specific disruptive effect on gene expression caused by seedlessness. When non-seeded/seeded expression ratio was determined for several transporter encoding genes both, up-regulated and down-regulated genes were identified (See Additional file 1). Interestingly, such analysis revealed that a gene coding for a putative grapevine boron transporter also appears strongly repressed in non-seeded berries. Like Zn, boron is also an essential micronutrient required for normal reproductive development in plants, and B deficiency has been associated to parthenocarpic berry development in grapevines [10,41]. Similar to VvZIP3VvBOR1 is predominantly expressed in flowers at anthesis and normal berries at early growth stages, preceding a B accumulation in reproductive tissues [42]. This expression profile seems adequate to fulfill the B requirement for cross-linking of rhamnogalacturonane II, a polysaccharide essential for cell wall formation during pollen tube growth [43].
In a similar way, the VvZIP3 expression profile, its straight correlation with the Zn accumulation pattern during development of reproductive organs (Figure 7), and it’s up-regulation in response to an increase in vascular Zn content (Figure 8), suggests a participation of VvZIP3 in the Zn loading during early reproductive developmental stages. It has been reported that alteration of the expression of ZIP transporters affects Zn distribution. Recently, it has been demonstrated that constitutive over-expression of the OsZIP4 gene in transgenic rice plants confers disarrangement of Zn distribution in the transgenic plants [32]. In these regard, we can speculate that the alteration of VvZIP3 expression during flowering and fertilization can modify the distribution, remobilization and availability of Zn, and hence affects normal reproductive development. Considering that Zn is essential for the stabilization of many proteins involved in development such as proteins containing DNA-binding Zn-finger motifs, RING fingers and LIM domains [1,7,10] and that Zn-finger transcription factors have been involved in the development and function of floral tissues such as anthers, tapetum, pollen and pistil secretory tissues in several plant species [1,7,44,45], it is plausible to propose that VvZIP3 may play a key role in both flower and normal fruit development.
Conclusions
Considering that Zn deficiency produces several developmental problems in grapevines [10-12] and that no information is available regarding the specificity, regulation and function of any ZIP gene in Vitis vinifera L., this work provides relevant information about the functional characterization of a putative Zn transporter identified in this species. Using a functional molecular approach, our results suggest that VvZIP3 encodes a plasma membrane putative Zn transporter protein member of the ZIP gene family. VvZIP3 is principally expressed in reproductive tissues, being strongly repressed in parthenocarpic seedless berries that present lower zinc accumulation, suggesting that it may participate in Zn uploading for normal berry development and that changes in its expression could affect zinc availability during this process.
Methods
Plant material
Grapevine (Vitis vinifera L. var. Carménère) grown under field conditions in a commercial vineyard in the Maule Valley (Central Chile) during three growing seasons (S1, S2 and S3) were used in this study. Zn nutritional status in plant leaves was monitored and corrected by foliar spray applications to maintain a Zn-sufficiency condition equivalent to a foliar concentration of 45–55 ppm. Random sampling of different organs was performed starting at early flowering until mature fruit stage (from October to April) from plants grown in the same plot. Stages to be sampled were defined according to the Modified Eichhorn-Lorenz System [46]. Flowering stages collected were: EL19, inflorescences or little clusters (LC) and EL 23, flowers at full bloom (F). Fruit developmental stages were: EL31, berries at preveraison 7 mm in diameter (PV); EL35, berries at veraison (V) and EL38, berries at postveraison harvest-ripe (M). For sampling, phenological stages were determined for normal seeded berries, then clusters were collected and seeded and non-seeded berries from the some bunch were separated for further processing.
Identification and isolation of VvZIP3
From expression libraries made from RNA of normal and parthenocarpic Vitis vinifera L. cv. carménère berries (DEGECHIVID database; http://www.genomicafrutos.cl), an EST sequence highly similar to genes encoding Zn transporter proteins was obtained after comparison in the grapevine GENOSCOPE (http://www.genoscope.cns.fr/externe/GenomeBrowser/Vitis/) and NCBI (http://www.ncbi.nlm.nih.gov/genome/seq/BlastGen/BlastGen.cgi?taxid=29760) databases.
The sequence, named VvZIP3, was translated to obtain the open reading frame containing the initial methionine and the first stop codon using the OMIGA 2.0 software Vitis vinifera L.[47]. Identification of conserved domains in the predicted protein was carried out using InterProScan (Hunter, et al. 2009) and ScanProsite (de Castro, et al. 2006). Grand average hydropathy was obtained according to Kyte and Doolittle model [48]. Potential transmembrane domains in the predicted protein sequence were identified using TMPred [49], and TMHMM [50]. Signal peptides, as well as possible subcellular targeting sites were assessed using Wolf PSORT-II software [36]. Alignments were performed using ClustalW [51] and phylogenetic analyses were conducted with MEGA4 [52]. Phylogenetic trees were inferred using the Neighbor-Joining method [53]. The ZIP genes accession numbers used in tree construction are: Vitis vinifera (VvZIP1.1, GSVIVT00002087001; VvZIP1.2, GSVIVT00002088001; VvZIP2,GSVIVT00024285001; VvZIP3, GSVIVT00030117001; VvZIP4,GSVIVT00032208001; VvZIP5.1, GSVIVT00037538001; VvZIP5.2,GSVIVT00037540001; VvZIP6.1, GSVIVT00024060001; VvZIP6.2, GSVIVT00029326001; VvZIP7.1, GSVIVT00027686001; VvZIP7.2,GSVIVT00031911001; VvZIP8, GSVIVT00030650001; VvZIP9,GSVIVT00024638001; VvZIP10, GSVIVT00031915001; VvZIP11.1,GSVIVT00033348001; VvZIP11.2, GSVIVT00033353001; VvZIP11.3,GSVIVT00033352001; VvZIP11.4, GSVIVT00033350001; VvZIP12, GSVIVT00030116001; VvZIP13, GSVIVT00033649001) and Arabidopsis thaliana (AtZIP1, AT3G12750; AtZIP2, AT5G59520; AtZIP3, AT2G32270; AtZIP4, AT1G10970; AtZIP5, AT1G05300; AtZIP6, AT2G30080; AtZIP7, AT2G04032; AtZIP8, AT5G45105; AtZIP9, AT4G33020; AtZIP10, AT1G31260; AtZIP11, AT1G55910; AtZIP12, AT5G62160; AtZIP13, AT3G08650; AtIRT1, AT4G19690; AtIRT2, AT4G19680; AtIRT3, AT1G60960; AtIAR1, AT1G68100; AtGUFA, AT3G20870).
Plant RNA extraction
Total RNA was extracted from 2 to 3 g of frozen (−80°C) roots, leaves, stems, little clusters, flowers, fruits and seeds (from pre-veraison to mature grapes) using the modified CTAB method [54]. Total RNA integrity was corroborated by formaldehyde agarose gel electrophoresis and their purity by OD260/280 ratio >1.95. Following DNase (DNAse I, Ambion) treatment of total RNA, first-strand cDNA synthesis was carried out from 2 μg of total RNA for each sample using oligo (dT) according to the manufacturer’s instructions (Revertaid First Strand cDNA Synthesis K1622 Kit, Fermentas).
Gene expression analyses
Expression analysis was performed with three independent total RNA extractions (biological repeats). A standard curve was generated for each ZIP gene and VvGAPDH (as housekeeping gene) using a cDNA serial dilution. The resultant PCR efficiency calculations were imported into relative expression data analysis. PCR parameters used were: 94°C for 4 min; 94°C for 1 min, 60°C (annealing temperature) for 1 min, and 72°C for 1 min for 30 cycles; and a final step at 72°C for 7 min. The PCR products were visualized on agarose gels and isolated with the E.Z.N.A gel extraction kit (Omega Bio-Tek Inc.) to determine the primers efficiency. Gene transcript levels were measured by quantitative PCR (qPCR) using a DNA Engine Opticon 2 Cycler System (MJ Research). All reactions were performed using the Brilliant SYBR Green Master Mix (Stratagene) according to the procedure described by the manufacturer. For each sample, qPCR reactions were carried out in triplicate (technical repeats) using 10 μl Master Mix, 0.5 μl 250 nM each primer, 1 μl diluted cDNA and nuclease-free water in a final volume of 20 μl. Fluorescence was measured at the end of each amplification cycle. Amplification was followed by a melting curve analysis with continuous fluorescence data acquisition during the 65–95°C melt. The raw data were manually analyzed and expression was normalized to GAPDH gene (VvGADPH, NCBI/GenBank database accession number CN938023) Ubiquitin gene (VvUBQ, TIGR database accession number TC32075) to minimize variation in cDNA template levels. Primer sets used for qPCR were: VvZIP1.1rtFwd (5′-TGATATACATGGCGCTGG-3′) and VvZIP1.1rtRev (5′-CAGACACAAGAAAGAAAAGACG-3′) for VvZIP1.1; VvZIP2rtFwd (5′-CCATCAACCATCTCGTTGC-3′) and VvZIP2rtRev (5′-CAAACAAGGATCGTTTACAAGC-3′) for VvZIP2; VvZIP3rtFwd (5′-ACGACGAAAACAGCCCAAC-3′) and VvZIP3rtRev (5′-GGAGTCTCACATTGCTTTGC-3′) for VvZIP3; VvZIP4rtFwd (5′-CTGGTCATCGAAGGCATATTCG-3′) and VvZIP4rtRev (5′-AGGCCCCAAAACAAGAATTAGG-3′) for VvZIP4; VvZIP5.1rtFwd (5′-AAGTTGGAGAGCATGAAGG-3′) and VvZIP5.1rtRev (5′-ATTGGTGGAAAGTGAGAGC-3′) for VvZIP5.1; VvZIP6.1rtFwd (5′-GATGACAGTAGTGCGAATGC-3′) and VvZIP6.1rtRev (5′-TGGTCTCAACTCTCAACAAGC-3′) for VvZIP6.1; VvZIP8rtFwd (5′-AGGCCCTCTTCTTCAACTTCC-3′) and VvZIP8rtRev (5′-AGCCATGCCCAATATCAGC-3′) for VvZIP8; VvZIP11.1rtFwd (5′-CACCGGTATTGTCATAGATGC-3′) and VvZIP11.1rtRev (5′-GGAACACACTTCAAGATGAGC-3′) for VvZIP11.1; VvZIP13rtFwd (5′-GTCGACACATGGTCCTTCC-3′) and VvZIP13rtRev (5′-CCGCACTATTTTCCAAAAGC-3′) for VvZIP13; VvGAPDHFwd (5′-TTCCGTGTTCCTACTGTTG-3′) and VvGAPDHRev (5′-CCTCTGACTCCTCCTT GAT-3′) for VvGAPDH; VvUBQFwd (5′-GTGGTATTATTGAGCCATCCTT-3′) and VvUBQRev (5′-AACCTCCAATCCAGTCATCTAC-3′) for VvUBQ. For the Zn treatment assay in pre-veraison berries, a negative control gene, VvWRKY-20 (WRKY transcription factor 20-like, GSVIVT01030046001), whose expression is not altered during metal treatments (unpublished data) was included. The primers for VvWRKY-20 were: VvWRKY-20Fwd (5′-CAACAAACTCCAAGTGCAGAACC-3′) and VvWRKY-20Rev (5′-CACCCCCAAAAAATGAGAAGG-3′).
Zinc Treatment
In order to determine whether VvZIP3 expression is affected by Zn, Vitis vinifera L. cv. Carménère grape clusters were harvested from field-grown vines in a commercial vineyard in the Maule Valley (Central Chile) during the season S3 at the preveraison stage (EL31, PV) [46]. Uniform berries with their respective peduncles were excised under water, and were positioned on perforated plastic trays (Kim trak 25 × 14 cm) so that the cut pedicels protruded through the holes into a dish containing the proper solution [55]. The experimental conditions were: temperature at 25°C and light at 156 W cm-2. 2 mM of ZnCl2 was applied as a dip solution throughout the experiment and distilled water as a control. Eight random berries from each tray were collected at 6 and 24 hours, pooled together and processed for RNA extraction. The experiment included three trays per treatment (Zn and water) and was repeated twice.
Determination of Zn content
Total Zn content was determined in little clusters, flowers and fruits at different developmental stages (pre-veraison, veraison and mature grapes) according to Karla [56]. The reagents used were of high purity (Suprapur, Merck, Darmstadt). The cleaning of the material was fundamental to guarantee the optimum result in analysis. Tissues were washed with deionized water and oven-dried (80°C) to constant weight. The samples were subsequently homogenized and kept in plastic containers for later analysis. Dried tissues were ground into powder, then ashed at 500°C and dissolved in 2 M HCl [56]. The resulting solution was filtered and washed with bidistilled water to a final volume of 50 mL in a pre-treated volumetric flask. The analyses were done in duplicate. The measurements were done by flame atomic absorption spectroscopy (air/acetylene), using a Unicam 969 spectrophotometer with deuterium background corrector. The method of analysis was validated using the SRM-1570 certified reference material (spinach), supplied by the National Institute of Standards and Technology (USA). Replications of the reference material showed good exactness with relative errors varying between 2.2 – 3.4%.
Yeast complementation assay
The following strains of the yeast Saccharomyces cerevisiae were used in this study: wild type parent strain DY1457 (MATa, ade6, can1, ura3, leu2, his3, trp1) and the Zn2+ uptake defective double mutant ZHY3 Δzrt1/Δzrt2 (MATa, his3, leu2, met1, lys2, ura3, zrt1, zrt2) [19,20]. Growth occurred in yeast potato dextrose or in synthetic defined (SD) medium with 2% (w/v) glucose or galactose, supplemented as necessary [57]. For metal complementation assay, yeast was grown in liquid SD medium (with 2% [w/v] galactose) until OD = 1, and drop assays were performed on SD plates containing different concentrations of Zn (10 μM, 100 μM, 750 μM and 2 mM). Yeast cells were transformed using the S.c. EasyComp Transformation Kit (Invitrogen). The VvZIP3 cDNA was subcloned from the TOPO vector clone (TOPO TA Cloning Vector System, Invitrogen) into the pYES2 plasmid (Invitrogen) using the primers VvZIP3-fwd (5-CATCTGGATCCATGAGCAAGCTTCAGTTCTATCCAT-3) and VvZIP3-rev (5-CCATCTCTCGAGGATTCCACCCCATTTGGCCAGA-3) to introduce the BamHI and XhoI sites (underlined).
Transient expression assay in onion epidermal cells
The intracellular localization of VvZIP3 was determined by monitoring the transient expression of a VvZIP3-mGFP5 translational fusion product in onion epidermal cells after DNA particle bombardment. The coding region of mGFP5, a green fluorescent protein modified for plants [58] was fused to the XbaI site of the pART7 vector [59]. The VvZIP3 cDNA was subcloned from pYES2-VvZIP3 to the XhoI-KpnI–digested pART7 between the 35 S cauliflower mosaic virus promoter and the octopine synthase terminator and in frame with mGFP5, using the VvZIP3XhoI-fwd (5-CCATCTCTCGAGATGAGCAAGCTTCAGTTCTATC-3) and VvZIP3KpnI-rev (5-CCCCGGTACCCGATTCCACCCCATTTGGCCAGA-3) primers (sites underlined). Gold particles (0.6 μm) coated with the resulting construct was delivered into onion bulb scale epidermal cell layers with a PDS-1000/He Particle Delivery System (Bio-Rad). The bombardment parameters were as follows: discharge pressure of 1100 p.s.i. with a 900 p.s.i. rupture disk, and distance to target tissue of 6 cm. Onion epidermal layers were placed onto MS agar plates before bombardment and incubated at 22°C for 24 h after particle delivery. Viewing of bombarded samples was done with a confocal microscope (Zeiss LSM-510 system).
Authors’ contributions
FGC participated in the design of the study, carried out the experiments, interpreted the data, and drafted the manuscript. RP carried out part of the qPCR experiment. RPC carried out part of the sequence analyses and gave extensive advices on qPCR data analysis. JT carried out the metal determination experiments. HPC directed the overall project and helped to draft the manuscript. JC participated in the design of the study and helped to draft the manuscript. SG carried out Zn treatment and gene expression analysis. SRL and EG conceived the research, participated in the design of the study and played a major part in results interpretation. All authors read and approved the final manuscript.
Supplementary Material
NS/S Expression ratio of different ESTs associated to transport. Putative genes associated to transport that showing a significant expression variation (p-value < 0.05) from expression libraries from parthenocarpic (NS) and normal (S) Vitis vinifera L. cv. carménère berries (DEGECHIVID database; http://www.genomicafrutos.cl).
A) Comparison of the VvWRKY-20 amino acid sequence with its putative homologue AtZAP1. Alignment was performed with the highest homologous sequence from Vitis vinifera L. Genome to AtZAP1 (AT2G04880), named VvWRKY-20 (GSVIVT01030046001, http://www.genoscope.cns.fr). Alignments were performed using ClustalW. The WRKY motifs are highlight in yellow boxes and a blue underline indicate the DNA-binding motifs. Identical residues are blackened; similar residues are highlight in grey. B) Gene expression analysis of VvWRKY-20 in grapevine tissues. Expression profiles of VvWRKY-20 in R (roots); L (leafs); S (stems); LC (little clusters); F (flowers); PV (pre-veraison fruit); V (whole veraison fruit); M (whole mature fruit). Expression in root samples was adjusted to 1 relative unit. Data represent means ± SD (n = 3).
Contributor Information
Felipe Gainza-Cortés, Email: fgainza@ceaf.cl.
Ricardo Pérez-Dïaz, Email: josperez@utalca.cl.
Ramón Pérez-Castro, Email: rperez@ucm.cl.
Jaime Tapia, Email: jtapia@utalca.cl.
José A Casaretto, Email: jcasaretto@utalca.cl.
Sebastián González, Email: sgonzalez@utalca.cl.
Hugo Peña-Cortés, Email: hugo.pena@usm.cl.
Simón Ruiz-Lara, Email: sruiz@utalca.cl.
Enrique González, Email: egonzale@utalca.cl.
Acknowledgements
We thank Dr. Damien Blaudez and Dr. Michel Chalot (Unité Mixte de Recherche INRA/Université Henri Poincaré “Tree–microbe Interactions”, Faculty of Sciences and Technology, Nancy University, France) and Dr. Sebastien Duplessis (INRA-Nancy, France) for providing the yeast strains and for collaboration in this work. This work was supported by FONDEF G07I1003. FGC, RPC, SG and RP were supported by Universidad de Talca doctoral fellowships.
References
- Kobayashi A, Sakamoto A, Kubo K, Rybka Z, Kanno Y, Takatsuji H. Seven zinc-finger transcription factors are expressed sequentially during the development of anthers in petunia. Plant J. 1998;13:571–576. doi: 10.1046/j.1365-313X.1998.00043.x. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Suzuki M, Kobayashi T, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. OsZIP4, a novel zinc-regulated zinc transporter in rice. J Exp Bot. 2005;56(422):3207–3214. doi: 10.1093/jxb/eri317. [DOI] [PubMed] [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. II. Academic, Neustadt; 1998. [Google Scholar]
- Broadley MR, Philip JW, John PH, Ivan Z, Alexander L. Zinc in plants. New Phytologist. 2007;173(4):677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- Pinton R, Cakmak I, Marschner H. Effect of zinc deficiency on proton fluxes in plasma membrane-enriched vesicles isolated from bean roots. J Exp Bot. 1993;44(3):623–630. doi: 10.1093/jxb/44.3.623. [DOI] [Google Scholar]
- Chandler WH. Zinc as a nutrient for plants. Botanical Gazette. 1937;98(4):625–646. doi: 10.1086/334670. [DOI] [Google Scholar]
- Sharma PN, Chatterjee C, Sharma CP, Agarwala SC. Zinc deficiency and anther development in maize. Plant Cell Physiol. 1987;28(1):11–18. [Google Scholar]
- Brune A, Urbach W, Dietz K-J. Zinc stress induces changes in apoplasmic protein content and polypeptide composition of barley primary leaves. J Exp Bot. 1994;45(9):1189–1196. doi: 10.1093/jxb/45.9.1189. [DOI] [Google Scholar]
- Volschenk CG, Hunter JJ, Watts JE. The effect of different zinc levels on the growth of grapevines. J Plant Nut. 1996;19(6):827–837. doi: 10.1080/01904169609365165. [DOI] [Google Scholar]
- Mullins MG, Bouquet A, Williams LE. Biology of the Grapevine. Cambridge University Press, Cambridge; 1992. [Google Scholar]
- Christensen P, Jensen F. Grapevine response to concentrate and to dilute application of Two zinc compounds. Am J Enol Vitic. 1978;29(3):213–216. [Google Scholar]
- Vasconcelos MC, Greven M, Winefield CS, Trought MCT, Raw V. The flowering process of vitis vinifera: a review. Am J Enol Vitic. 2009;60(4):411–434. [Google Scholar]
- Christensen P. Timing of zinc foliar sprays. I. Effects of application intervals preceding and during the bloom and fruit-set stages. II. Effects of day vs. night application. Am J Enol Vitic. 1980;31(53):9. [Google Scholar]
- Srinivasan C, Mullins MG. Physiology of flowering in the grapevine - a review. Am J Enol Vitic. 1981;32(1):47–63. [Google Scholar]
- Guerinot ML. The ZIP family of metal transporters. Biochimica et Biophysica Acta (BBA) Biomembranes. 2000;1465(1–2):190–198. doi: 10.1016/s0005-2736(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Eng BH, Guerinot ML, Eide D, Saier JMH. Sequence analyses and phylogenetic characterization of the ZIP family of metal Ion transport proteins. Journal of Membrane Biology. 1998;166(1):1–7. doi: 10.1007/s002329900442. [DOI] [PubMed] [Google Scholar]
- Grotz N, Guerinot ML. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochimica et Biophysica Acta (BBA) Mol Cell Res. 2006;1763(7):595–608. doi: 10.1016/j.bbamcr.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Puig S, Peñarrubia L. Placing metal micronutrients in context: transport and distribution in plants. Curr Opin Plant Biol. 2009;12(3):299–306. doi: 10.1016/j.pbi.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Zhao H, Eide D. The ZRT2 Gene Encodes the Low Affinity Zinc Transporter in Saccharomyces cerevisiae. J Biol Chem. 1996;271(38):23203–23210. doi: 10.1074/jbc.271.38.23203. [DOI] [PubMed] [Google Scholar]
- Zhao H, Eide D. The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc Nat Acad Sci. 1996;93(6):2454–2458. doi: 10.1073/pnas.93.6.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot ML, Eide D. Zeroing in on zinc uptake in yeast and plants. Current Opinion in Plant Biology. 1999;2(3):244–249. doi: 10.1016/S1369-5266(99)80042-9. [DOI] [PubMed] [Google Scholar]
- Grotz N, Fox T, Connolly E, Park W, Guerinot ML, Eide D. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc Nat Acad Sci. 1998;95(12):7220–7224. doi: 10.1073/pnas.95.12.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintz H, Fox T, Wu Y-Y, Feng V, Chen W, Chang H-S, Zhu T, Vulpe C. Expression Profiles of Arabidopsis thaliana in Mineral Deficiencies Reveal Novel Transporters Involved in Metal Homeostasis. J Biol Chem. 2003;278(48):47644–47653. doi: 10.1074/jbc.M309338200. [DOI] [PubMed] [Google Scholar]
- Ramesh SA, Shin R, Eide DJ, Schachtman DP. Differential metal selectivity and gene expression of Two zinc transporters from rice. Plant Physiol. 2003;133(1):126–134. doi: 10.1104/pp.103.026815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Usui K, Horie K, Nosaka S, Mizuno N, Obata H. Cloning of three ZIP/Nramp transporter genes from a Ni hyperaccumulator plant Thlaspi japonicum and their Ni2+-transport abilities. Plant Physiol Biochem. 2005;43(8):793–801. doi: 10.1016/j.plaphy.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Pence NS, Larsen PB, Ebbs SD, Letham DLD, Lasat MM, Garvin DF, Eide D, Kochian LV. The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens. Proc Nat Acad Sci. 2000;97(9):4956–4960. doi: 10.1073/pnas.97.9.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assunção AGL, Martins PDC, Folter SD, Vooijs R, Schat H, Aarts MGM. Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens. Plant Cell Env. 2001;24(2):217–226. doi: 10.1111/j.1365-3040.2001.00666.x. [DOI] [Google Scholar]
- Plaza S, Tearall KL, Zhao F-J, Buchner P, McGrath SP, Hawkesford MJ. Expression and functional analysis of metal transporter genes in two contrasting ecotypes of the hyperaccumulator Thlaspi caerulescens. J Exp Bot. 2007;58(7):1717–1728. doi: 10.1093/jxb/erm025. [DOI] [PubMed] [Google Scholar]
- Moreau S, Thomson RM, Kaiser BN, Trevaskis B, Guerinot ML, Udvardi MK, Puppo A, Day DA. GmZIP1 Encodes a symbiosis-specific zinc transporter in soybean. J Biol Chem. 2002;277(7):4738–4746. doi: 10.1074/jbc.M106754200. [DOI] [PubMed] [Google Scholar]
- López-Millán A-F, Ellis D, Grusak M. Identification and characterization of several New members of the ZIP family of metal Ion transporters in medicago truncatula. Plant Mol Biol. 2004;54(4):583–596. doi: 10.1023/B:PLAN.0000038271.96019.aa. [DOI] [PubMed] [Google Scholar]
- Burleigh SH, Kristensen BK, Bechmann IE. A plasma membrane zinc transporter from Medicago truncatula is up-regulated in roots by Zn fertilization, yet down-regulated by arbuscular mycorrhizal colonization. Plant Mol Biol. 2003;52(5):1077–1088. doi: 10.1023/A:1025479701246. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Masuda H, Suzuki M, Bashir K, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. Overexpression of the OsZIP4 zinc transporter confers disarrangement of zinc distribution in rice plants. J Exp Bot. 2007;58(11):2909–2915. doi: 10.1093/jxb/erm147. [DOI] [PubMed] [Google Scholar]
- Chen W, Feng Y, Chao Y. Genomic analysis and expression pattern of OsZIP1, OsZIP3 , and OsZIP4 in two rice ( Oryza sativa L.) genotypes with different zinc efficiency. Russian J Plant Physiol. 2008;55(3):400–409. doi: 10.1134/S1021443708030175. [DOI] [Google Scholar]
- Migeon A, Blaudez D, Wilkins O, Montanini B, Campbell M, Richaud P, Thomine S, Chalot M. Genome-wide analysis of plant metal transporters, with an emphasis on poplar. Cell Mol Life Sci. 2010;67(22):3763–3784. doi: 10.1007/s00018-010-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Cortes H, Cuadros A, Fichet T, Pinto M, Ramirez I, Valdés J, Riquelme A, González D, González E, Hinrischsen P. et al. Chilean effort for improving fruit quality in grapevine. A genomic approach to understanding seed formation, fruit ripening and phatogen response. Acta Hort. 2005;689:505–512. [Google Scholar]
- Horton P, Park K-J, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35(suppl_2):W585–587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Huang J, Jiang Y, Zhang H-S. Cloning and functional identification of two members of the ZIP (Zrt, Irt-like protein) gene family in rice (Oryza sativa L.) Mol Biol Rep. 2009;36(2):281–287. doi: 10.1007/s11033-007-9177-0. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Hirano K, Kato S, Obata H. Cloning of ZIP family metal transporter genes from the manganese hyperaccumulator plant Chengiopanax sciadophylloides, and its metal transport and resistance abilities in yeast. Soil Sci Plant Nut. 2008;54(1):86–94. doi: 10.1111/j.1747-0765.2007.00206.x. [DOI] [Google Scholar]
- Lee S, Kim S, Lee J, Guerinot M, An G. Zinc deficiency-inducible OsZIP8 encodes a plasma membrane-localized zinc transporter in rice. Mol Cells. 2010;29(6):551–558. doi: 10.1007/s10059-010-0069-0. [DOI] [PubMed] [Google Scholar]
- Lee S, Jeong H, Kim S, Lee J, Guerinot M, An G. OsZIP5 is a plasma membrane zinc transporter in rice. Plant Mol Biol. 2010;73(4-5):507–517. doi: 10.1007/s11103-010-9637-0. [DOI] [PubMed] [Google Scholar]
- Keller M. Deficit Irrigation and Vine Mineral Nutrition. Am J Enology Viticulture. 2005;56(3):267–283. [Google Scholar]
- Pérez-Castro R, Kasai K, Gainza-Cortés F, Ruiz-Lara S, Casaretto JA, Peña-Cortés H, Tapia J, Fujiwara T, González E. VvBOR1, the Grapevine Ortholog of AtBOR1, Encodes an Efflux Boron transporter that is differentially expressed throughout reproductive development of Vitis vinifera L. Plant Cell Physiol. 2012;53(2):485–494. doi: 10.1093/pcp/pcs001. [DOI] [PubMed] [Google Scholar]
- Dell B, Huang L. Physiological response of plants to low boron. Plant Soil. 1997;193(1):103–120. [Google Scholar]
- Kapoor S, Kobayashi A, Takatsuji H. Silencing of the Tapetum-Specific Zinc Finger Gene TAZ1 Causes Premature Degeneration of Tapetum and Pollen Abortion in Petunia. Plant Cell Online. 2002;14(10):2353–2367. doi: 10.1105/tpc.003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo K-, Kanno Y, Nishino T, Takatsuji H. Zinc-finger genes that specifically express in pistil secretory tissues of Petunia. Plant Cell Physiol. 2000;41(3):377–382. doi: 10.1093/pcp/41.3.377. [DOI] [PubMed] [Google Scholar]
- Coombe BG. Growth stages of the grapevine: adoption of a system for identifying grapevine growth stages. Aust J Grape Wine Res. 1995;1(2):104–110. doi: 10.1111/j.1755-0238.1995.tb00086.x. [DOI] [Google Scholar]
- Charles S. Omiga 2.0. Biotech Softw Int Rep. 2000;1(5):198–207. doi: 10.1089/152791600750034721. [DOI] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Hofmann K, Stoffel W. TMbase - A database of membrane spanning proteins segments. Biol Chem Hoppe-Seyler. 1993;374(374):166. [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden markov model: application to complete genomes. J Mol Biol. 2001;305(3):567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Mol Biol Evol. 2007;24(8):1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Reid K, Olsson N, Schlosser J, Peng F, Lund S. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol. 2006;6(1):27. doi: 10.1186/1471-2229-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubelakis-Angelakis KA, Kliewer WM. Effects of Exogenous Factors on Phenylalanine Ammonia-Lyase Activity and Accumulation of Anthocyanins and Total Phenolics in Grape Berries. Am J Enology Viticulture. 1986;37(4):275–280. [Google Scholar]
- Karla Y. Handbook of reference methods for plant analysis, I edn. Florida. 1998. Inc. CRC Press, USA; 1998. [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;28(28):3–20. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Haseloff J, Siemering KR, Prasher DC, Hodge S. Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Nat Acad Sci. 1997;94(6):2122–2127. doi: 10.1073/pnas.94.6.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleave AP. A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol. 1992;20(6):1203–1207. doi: 10.1007/BF00028910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NS/S Expression ratio of different ESTs associated to transport. Putative genes associated to transport that showing a significant expression variation (p-value < 0.05) from expression libraries from parthenocarpic (NS) and normal (S) Vitis vinifera L. cv. carménère berries (DEGECHIVID database; http://www.genomicafrutos.cl).
A) Comparison of the VvWRKY-20 amino acid sequence with its putative homologue AtZAP1. Alignment was performed with the highest homologous sequence from Vitis vinifera L. Genome to AtZAP1 (AT2G04880), named VvWRKY-20 (GSVIVT01030046001, http://www.genoscope.cns.fr). Alignments were performed using ClustalW. The WRKY motifs are highlight in yellow boxes and a blue underline indicate the DNA-binding motifs. Identical residues are blackened; similar residues are highlight in grey. B) Gene expression analysis of VvWRKY-20 in grapevine tissues. Expression profiles of VvWRKY-20 in R (roots); L (leafs); S (stems); LC (little clusters); F (flowers); PV (pre-veraison fruit); V (whole veraison fruit); M (whole mature fruit). Expression in root samples was adjusted to 1 relative unit. Data represent means ± SD (n = 3).