Abstract
Voltage-gated Ca2+ (Cav) channels control neuronal functions including neurotransmitter release and gene expression. The Cacna1a gene encodes the α1 subunit of the pore-forming Cav2.1 channel. Mice with mutations in this gene form useful tools for defining channel functions. The recessive ataxic tottering-6j strain that was generated in the Neuroscience Mutagenesis Facility at The Jackson Laboratory has a mutation in the Cacna1a gene. However, the effect of this mutation has not been investigated in detail. In this study, mutation analysis shows a base substitution (C-to-A) in the consensus splice acceptor sequence linked to exon 5, which results in the skipping of exon 5 and the splicing of exon 4 directly to exon 6. The effect of this mutation is expected to be severe as the expressed α1 subunit protein lacks a significant part of the S4–S5 linker, S5, and part of S5–S6 linker in domain I. Tottering-6j mice display motor dysfunctions in the footprint, rotating rod, and hind-limb extension tests. Although cytoarchitecture of the mutant brains appears normal, tyrosine hydroxylase was persistently expressed in cerebellar Purkinje cells in the adult mutant mice. These results indicate that tottering-6j is a useful model for functional studies of the Cav2.1 channel.
Introduction
Voltage-gated Ca2+ (Cav) channels play an important role in the regulation of diverse neuronal functions which are attributed to elevated intracellular Ca2+ concentrations [1], [2]. The pore-forming α1 subunit functions as a voltage sensor and is capable of generating channel activity [3]. The α1 subunit consists of four homologous transmembrane domains (I-IV), each containing six transmembrane spanning α-helices (S1–S6) [4], [5]. The four domains are connected through cytoplasmic linkers, and both the C- and N-termini are cytoplasmic and interact with regulatory proteins [6], [7].
Mutations within the α1 subunit (Cav2.1α1) gene from the Cav2.1 channel have been identified [8], [9]. In humans, these mutations cause several autosomal dominant neurological defects, including familial hemiplegic migraine (FHM), episodic ataxia type-2 (EA2), and spinocerebellar ataxia (SCA6) [10]. To examine the function and disease processes of the Cav2.1 channel, mouse genetic approaches can be useful. Mice with mutations in the Cav2.1α1 Cacna1a gene have been reported, and include the FHM1 model strains (R192Q and S218L knockin mice) [11], [12], a SCA6 model strain carrying additional CAG repeats in the Cacna1a locus of the knockin mice [13], and a knockout strain lacking Cav2.1 currents [14]. It has also been reported that in spontaneous or chemically-induced Cacna1a mutant strains, dominant mutations were detected in the tottering-5j and wobbly mice and recessive mutations were detected in the rocker, tottering, rolling Nagoya, tottering-4j, and leaner mice [15], [16], [17]. In contrast to the heterozygous tottering-5j and wobbly mice, which showed mild ataxia and had normal life spans, the homozygous tottering-5j and wobbly mice showed severe ataxia and died prematurely. All of the homozygous recessive mouse mutants developed ataxia and have normal life spans. The chemically induced ataxic groggy rat is a recessive Cacna1a mutant with a normal life span [18]. Cacna1a mutant strain serves as a motor neuron disease model. Cav2.1 channels express at the neuromuscular junction (NMJ) and regulate acetylcholine (ACh) release from motor neurons [19]; abnormal ACh release is the cause of NMJ dysfunction in tottering and rolling Nagoya [20], [21]. Mice with motor neuron disease displayed clasping behavior in the hind-limb extension test [22], similar to that shown by rolling Nagoya mice [23].
We describe here a novel Cacna1a gene mutant, the tottering-6j mouse, generated in the Neuroscience Mutagenesis Facility at The Jackson Laboratory (MN, USA). The tottering-6j mice are a chemically-induced mutant strain produced using ethylnitrosourea (ENU) and show a similar phenotype to the tottering mice in the Jackson Laboratory Database (http://jaxmice.jax.org/strain/008623.html). The database showed that the complementation test performed between tottering and tottering-6j mice indicated that the tottering-6j mice have a recessive mutation in the Cacna1a gene. However, the exact position of the mutation and the advanced motor behavior of this strain have not been examined. Motor behavior was studied using the footprint [24], traction [25], rotating rod [26], and hind-limb extension [23] tests, all of which are well characterized and reliable. Tyrosine hydroxylase is a key enzyme in the noradrenergic biosynthesis pathway. Its expression is normally transient in a subset of cerebellar Purkinje cells and is not present 40 days postnatally [27]. By contrast, this transient expression persists into adulthood in tottering mice [27], [28]. This expression pattern indicates that Ca2+ misregulation leading to the responsiveness of the tyrosine hydroxylase promoter and reflecting abnormal Ca2+ signaling causes motor dysfunction.
In this study, to characterize aberrant neuronal network in motor function of tottering-6j mice, we identified the causative mutation in the Cacna1a gene, and examined the poor motor coordination, and the altered tyrosine hydroxylase expression in the cerebellar Purkinje cells of the tottering-6j mice.
Results
Transcript and Genomic Structure of the Cacna1a Gene in Tottering-6j Mutant Mice
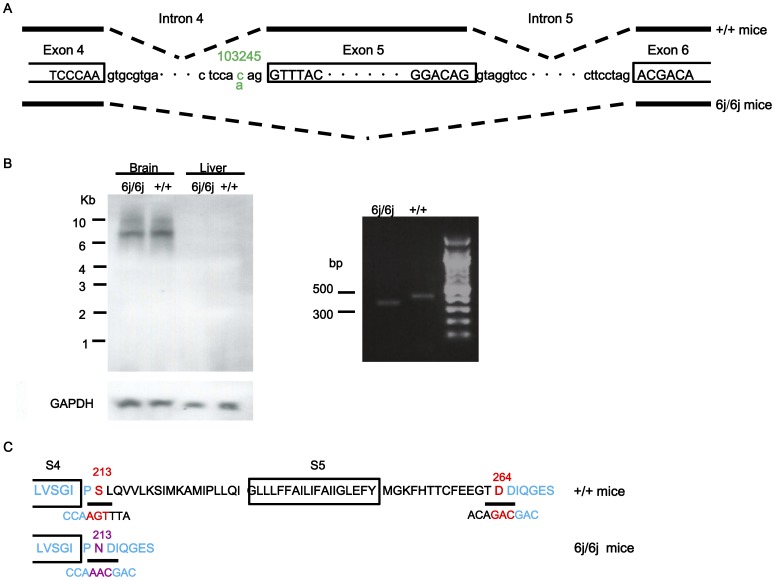
Sequencing of the Cacna1a genomic DNA from homozygous tottering-6j (6j/6j) mutants revealed a C-to-A transversion at nucleotide 103245 (Fig. 1A). The mutation is located in the consensus splice acceptor sequence linked to exon 5 and results in the skipping of exon 5, removing 153 bp and splicing directly to exon 6. These mutations were not found in the Cacna1a genomic and cDNA from wild-type (+/+) mice. Northern blot analyses did not detect any differences in the brain Cacna1a mRNA expression between 6j/6j and +/+ littermates (Fig. 1B). The RT-PCR fragment generated from the brain showed that a smaller size fragment was detected in 6j/6j mice (Fig. 1B). The expressed α1 subunit protein in the mutants is predicted to lack part of the S4–S5 linker, S5, and a part of S5–S6 in domain I consisting of amino acids at 213 (serine) to 264 (asparatic acid) and contain a new amino acid at 213 (asparagine) (Fig. 1C). Segregation analysis of the point mutation revealed coinheritance of the mutation and ataxic phenotype in all 42 6j/6j mice tested, while all 84 heterozygous tottering-6j (6j/+) and 42+/+ littermates were heterozygote and wild-type, respectively, at the tottering-6j locus (data not shown).
Figure 1. Structure and expression of the Cacna1a gene in tottering-6j mice.
Intron/exon and transcript structure in 6j/6j and +/+ strains is shown (A). Exon sequences are indicated by capital letters in boxes. Intron sequences indicate lowercase. The C-to-A substitution at the nucleotide residue 103245 is indicated in green. Transcripts are indicated below and above as genotypes; these are 6j/6j and +/+ strain, respectively. Splicing events are indicated by dashed lines. Analysis of Cacna1a gene expression in 6j/6j (n = 8) and +/+ (n = 8) mice by Western blot and RT-PCR (B). Representative protein expression pattern of the Cacna1a gene is shown. GAPDH was used to control for total RNA loading. No differences were observed between the 6j/6j and the +/+ mice. Representative RT-PCR results are shown. The fragment from the brain of the 6j/6j mice was smaller compared with that of the +/+ mice. A part of protein sequences of domain I in the Cav2.1α1 are shown (C). The protein sequence for the transmembrane segments of S4 and S5 is indicated by the boxes. The upper protein sequence is +/+ and the lower is 6j/6j, respectively. The nucleotide sequence encoding the amino acid is indicated under the protein sequence. The blue highlighting indicates the alternate exons. Red highlighting indicates amino acids encoded across a splice junction. The deleted amino acid region is 213–264. The altered amino acid is 213 is indicated in purple.
Normal Muscle Strength in Tottering-6j Mutant Mice
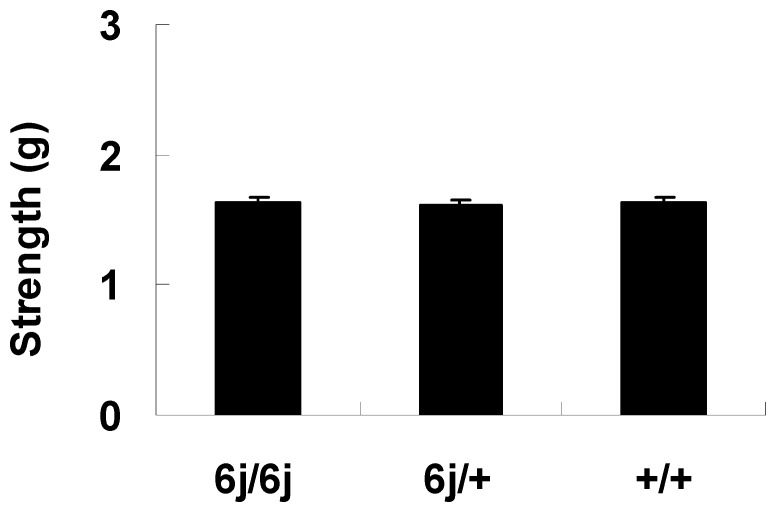
The results from the grip strength test showed the groups did not differ significantly in muscle strength (F(2, 27) = 0.045; P = 0.957) (Fig. 2).
Figure 2. Muscle strength in the grip strength test.
The 6j/6j (n = 10), 6j/+ (n = 10), and +/+ mice (n = 10) were examined in motor behavior tests.
Motor Dysfunctions in Tottering-6j Mutant Mice
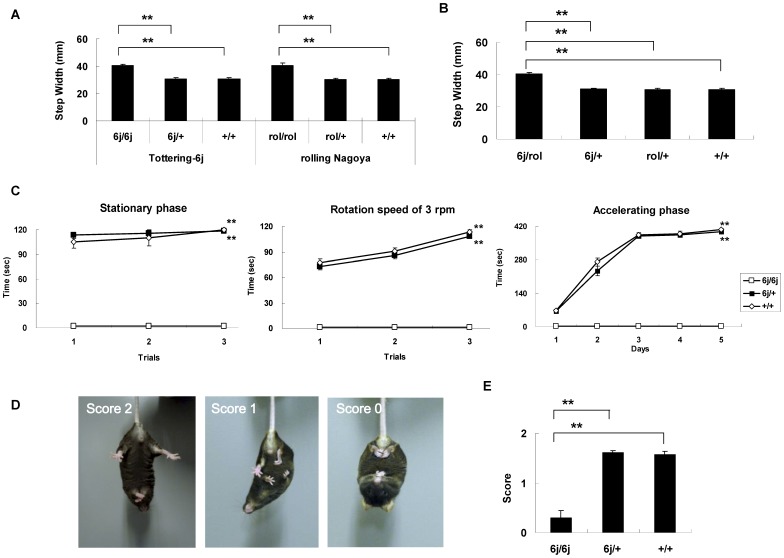
We examined the walking pattern using the footprint test to study ataxic phenotype and to perform the complementation test. There were no significant differences among 6j/6j, 6j/+ and +/+ mice comparing stride length (left; F(2, 42) = 0.014, P = 0.986, right; F(2, 42) = 0.069; P = 0.933) (data not shown). However, there was a significant difference when comparing step width (F(2, 42) = 26.802, P<0.001) (Fig. 3A). The 6j/6j mice had a larger step width than the 6j/+ mice (P<0.001) and the +/+ mice (P<0.001). There were no significant differences between the homozygous rolling Nagoya, (rol/rol), heterozygous rolling Nagoya, (rol/+) and the +/+ mice comparing the stride length (left; F(2, 42) = 0.008, P = 0.992, right; F(2, 42) = 0.013; P = 0.987) (data not shown). However, there were significant differences in step width (F(2, 42) = 21.845, P<0.001) (Fig. 3A). There was a significant difference between the rol/rol and rol/+ mice (P<0.001) and between the rol/rol and the +/+ mice (P<0.001). To test for complementarity to a known Cacna1a allele, such as the rolling Nagoya, 6j/+ mice were crossed with the rol/+ mice. The groups did not differ significantly between compound heterozygous (tottering-6j × rolling Nagoya, 6j/rol), 6j/+, rol/+, and +/+ mice when comparing stride length (left, F(3, 56) = 0.004; P = 0.999, right, F(3, 56) = 0.004; P = 0.991) (data not shown). There were significant differences between the strains when comparing step width (F(3, 56) = 31.180; P<0.001) (Fig. 3B). There was a significant difference between the 6j/rol and the 6j/+ mice (P<0.001), between the 6j/rol and the rol/+ mice (P<0.001), and between the 6j/rol and the +/+ mice (P<0.001).
Figure 3. Motor coordination was assessed using the footprint, rotating rod, and hind-limb extension tests.
The footprint test was used to examine the walking pattern (A, B). The tottering-6j strain including 6j/6j (n = 15), 6j/+ (n = 15), and +/+ mice (n = 15), and the rolling Nagoya strain including rol/rol (n = 15), rol/+ (n = 15), and +/+ mice (n = 15) were used (A). The heterozygous mice including 6j/rol (n = 15), 6j/+ (n = 15), rol/+ (n = 15), and +/+ (n = 15) mice were used (B). In the rotating rod test, retention time on the rotating rod was examined in 6j/6j (n = 10), 6j/+ (n = 10), and +/+ mice (n = 10) (C). In the hind-limb extension test, hind-limb posture was scored as 2, 1, or 0 (D), and the score for each strain is presented (E) for 6j/6j (n = 10), 6j/+ (n = 10), and +/+ mice (n = 10). **P<0.001 compared to the appropriate control (Tukey's test).
The 6j/6j mice were not able to maintain balance on a stationary rod (0 rpm) (F(2, 81) = 580.892; P<0.001) or during rotation speed of 3 rpm (F(2, 81) = 592.887; P<0.001) or in the accelerating phase (F(2, 435) = 871.034; P<0.001) (Fig. 3C). There were significant differences between the 6j/6j and the 6j/+ mice (0 rpm; P<0.001, 3 rpm; P<0.001, accelerating phase; P<0.001) and between the 6j/6j and the +/+ mice (0 rpm; P<0.001, 3 rpm; P<0.001, accelerating phase; P<0.001) (Fig. 3C).
Normally, an extension reflex in the hind-limb is observed when a mouse is suspended in the air by its tail. However, in mice with motor neuron disease, hind-limb retraction is observed more commonly (Fig. 3D). The groups differed significantly in the score (F(2, 29) = 53.926; P<0.001) and a significant difference was observed between the 6j/6j and the 6j/+ mice (P<0.001) and between the 6j/6j and the +/+ mice (P<0.001) (Fig. 3E).
Brain Cytoarchitecture and Cerebellar Expression of Tyrosine Hydroxylase in Tottering-6j Mutant Mice
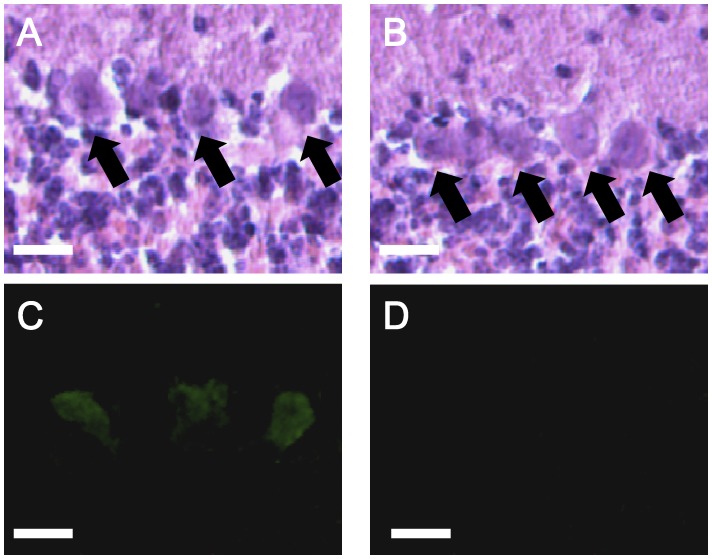
Our initial pathology studies involved gross histological examination with serial sections through the whole brain. The cytoarchitecture of the mutant brains appeared normal (data not shown). We investigated the cerebellum more closely to explain the ataxia observed in the mutant. We found a normal cerebellar morphology and cytoarchitecture in the 6j/6j mice compared with the +/+ mice. Tyrosine hydroxylase expression is detected in a subset of the cerebellar Purkinje cells of the 6j/6j mice but not in the +/+ mice at eight weeks of age (Fig. 4).
Figure 4. Representative histochemistry in the cerebellum of tottering-6j mice.
Hematoxylin eosin (HE) staining of 6j/6j (A) and +/+ (B) cerebella, and tyrosine hydroxylase (TH) staining of 6j/6j (C) and +/+ (D) cerebella are shown. TH was detected in the Purkinje cells of the 6j/6j mice (n = 6) but not in those of +/+ mice (n = 6). Arrows point to Purkinje cell somata. Scale bar, 20 µm.
Discussion
In this study, we identified the mouse mutant tottering-6j as a new allele of the Cav2.1 α1 subunit, Cacna1a gene using sequence analyses, behavior tests, and histological studies.
The Jackson Laboratory performed the complementation test between the tottering and the tottering-6j mice. The result indicated that the tottering-6j mice have a recessive mutation in the Cacna1a gene. To confirm this result, we used another Cacna1a mutant, the rolling Nagoya mice [23], [29]. Our complementation and segregation tests also showed that the tottering-6j mice have a recessive mutation in the Cacna1a allele. Most genes are divided into exons (coding region) and introns (intervening non-coding region). Transcription of genomic DNA creates large pre-RNAs from which the intervening non-coding regions must be precisely removed to create functional mRNAs. Splicing is performed by small nuclear RNAs (snRNAs) and proteins that recognize consensus sequences on the genomic DNA at or near the splice junctions [30]. We identified a CAG to AAG transverse at the 3′ splice acceptor sequence in intron 4, resulting in the skipping of exon 5 and the splicing of exon 4 directly with exon 6. Although the mechanism has remained unclear, the altered sequence is not recognized as a splice acceptor sequence. The expressed α1 subunit protein in the tottering-6j mice is predicted to lack part of the S4–S5 linker, S5, and a part of S5–S6 in domain I (Fig. 5). Because the S5 and S6 segments and the membrane-associated P-loop connecting them in the α1 subunit form the pore lining of the ion channel [31], at least, part of the pore lining of the Cav2.1 channel would be dysfunctional.
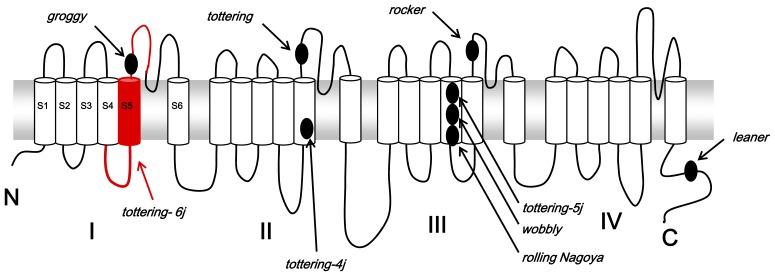
Figure 5. Proposed transmembrane topography of the Cav2.1α1 subunit and positions of known mutations identified in the Cacna1a mutant mice and rat.
The deletion region including part of the S4–S5 linker, S5, and a part of S5–S6 in domain I of Cav2.1α1 in the tottering-6j mice is shown by the red line.
Cav2.1α1 is a molecular complex comprising several proteins [6], [7]. The intracellular N- and C-termini and the cytoplasmic loops connecting domains I–IV are important for interaction with other proteins including the β subunit of the channel that binds to the I–II loop, synaptic proteins that interact at the synaptic protein interaction (synprint) site found in the II-III loop, and G protein βγ heterodimers (Gβγ) that interact at three sites on the N-terminus, I–II loop and C-terminus. Because there was not a frame shift after the deletion in the tottering-6j mice, the binding site for these proteins would be intact. It has been reported that most Cav2.1 knockout mice do not survive past weaning [14]. However, most of the tottering-6j mice had normal life spans (data not shown). If there was a frame shift after the deletion site, the tottering-6j mice would show similar phenotypes to the knockout mice.
Given the pivotal role of the Cav2.1 channel in controlling neurotransmitter production and release, defects in the structure of the presynaptic Cav2.1 channel result in aberrant synaptic signaling leading to various patterns of neural network dysfunction and behavior disorders. In the behavior tests, tottering-6j mice clearly suffer from ataxia without the loss of muscle strength. All of the homozygous recessive Cacna1a mice developed ataxia, ranging from mild in the rocker, tottering, and rolling Nagoya mice to severe ataxia in the leaner mice [9]. The mutation identified in the tottering-6j mice is similar to the rocker, tottering, or groggy mutation in the structural location of the Cav2.1α1 subunit (Fig. 5). Particularly, the groggy rats have an amino acid substitution located in the S5–S6 linker in domain I [18]. The groggy rats show mild ataxia resembling the rocker or tottering mice rather than the rolling Nagoya mice. In our sturdy, the tottering-6j mice exhibited similar walking patterns to the rolling Nagoya mice. The rolling Nagoya mice have a mutation in the voltage-sensing S4 segment in the domain III [32], and leaner mice have a mutation in a splice donor consensus sequence, which results in an altered C-terminal sequence [27] (Fig. 5). The reduction in amplitude of the P-type Ca2+ current was greater in the Purkinje cells of the leaner mice (60%) [33] compared with the tottering and rolling Nagoya mice (40%) [32]. These results indicate that the threshold dose for ataxia differs for different mutants. The Purkinje cells of 8-week-old tottering-6j mice showed tyrosine hydroxylase expression. Although tyrosine hydroxylase expression was no longer detected in adult rocker mice [34], ectopic tyrosine hydroxylase expression occurred in the other recessive Cacna1a mouse mutants including tottering [27], rolling Nagoya [35], tottering-4j [17], and leaner [27] mice. Tyrosine hydroxylase is normally expressed only during development; thus, ectopic tyrosine hydroxylase expression in Cacna1a mutants may indicate delayed neuronal maturation. Because the Ca2+ concentration in Purkinje cells is an important determinant of tyrosine hydroxylase expression [36], [37], ectopic tyrosine hydroxylase expression is likely the direct result of Ca2+ dysregulation due to Cav2.1 dysfunction. The rocker mice exhibited the mildest ataxia of the homozygous recessive Cacna1a mutants [34]. It may be that the lower magnitude change in Ca2+ exhibited in the rocker mice results in the mild ataxia and inability to downregulate tyrosine hydroxylase. Electrophysiological examination of the Ca2+ flux in Purkinje cells may be helpful in evaluating the validity of this hypothesis. The cerebellum of Cacna1a mutants has been reported to show significant synaptic changes. An altered synaptic pattern between cerebellar parallel fibers and Purkinje cells has been reported in adult tottering and leaner mice [37]. Rolling Nagoya mice show abnormally shaped Purkinje cell dendritic spines and single parallel fiber varicosities making multiple synaptic contacts, which were not observed in the wild type [38], suggesting that aberrant branching may be present in the Purkinje cells of tottering-6j mice. The present study found that although the tottering-6j mutant mice showed normal muscle strength in the grip-strength test, they exhibited an abnormal hind-limb extension reflex. Mice with motor dysfunction displayed reduced hind-limb extension and abnormal ACh receptor expression at the NMJ [22]. These results indicate that the tottering-6j mice have a deficit in ACh release at the NMJ. We plan to conduct electorophysiological, ultrastructural, and morphological studies; however, a detailed comparison of allelic variants would be helpful in clarifying the relationships among the many different structural, physiological, and synaptic abnormalities, and the observed behavioral deficits and the understanding of the channel functions.
In summary, the recessive ataxic tottering-6j strain contains a mutation in the Cacna1a gene. The mutation analysis shows a base substitution (C-to-A) in the consensus splice acceptor sequence linked to exon 5, resulting in the skipping of exon 5 and the deletion of a part of the pore lining of the α1 subunit of Cav2.1 channel. Tottering-6j mice display motor dysfunctions in the footprint, rotating rod, and hind-limb extension tests. Although gross cytoarchitecture of the mutant brains appears normal, tyrosine hydroxylase was expressed in cerebellar Purkinje cells in the mice at eight weeks of age. These results indicate that the tottering-6j strain is useful model for functional studies of the Cav2.1 channel.
Materials and Methods
Ethics Statement
The research was conducted in accordance with the Declaration of the Helsinki and was approved by the Animal Experiments Committee of RIKEN Brain Science Institute. All animals were cared for and treated humanely in accordance with the Institutional Guideline for Experiments using Animals (Approved ID: No. H24-2-206).
Animals
The tottering-6j mouse strain with the C57BL/6J and BALB/cByJ mixed genetic background was provided by the Jackson Laboratory. Tottering-6j mice backcrossed to C57BL/6J mice for 10 generations produced tottering-6j mice with a C57BL/6J genetic background. We used the rolling Nagoya mouse strain [23] with a C57BL/6J genetic background (backcross generations; N = 10) for complementation tests. The mice were allowed ad libitum access to water and food pellets (CRF-1; Oriental Yeast, Tokyo, Japan) and kept at room temperature (23±1°C) and 55±5% humidity under a 12∶12-h light-dark cycle (light from 8∶00 am to 8∶00 pm).
Mutation Analysis of the Transcript and Genomic Structure of the Cacna1a Gene
The mice were anesthetized with isofluorane and killed by decapitation, and whole brains were dissected. Total RNA was isolated from brains of 24 eight-week-old homozygous tottering-6j (6j/6j) and 24 wild-type (+/+) mice using TRIzol reagent, according to the manufacture’s protocol (Invitrogen, ON, Canada). The first-strand complementary DNA (cDNA) was synthesized by oligo(dT) priming (SuperScript First-Strand Synthesis System; Invitrogen, ON, Canada). Reverse transcriptase-polymerase chain reaction (RT-PCR) primers were designed to create fourteen 400- to 800-bp fragments covering the entire 7929-bp messenger RNA (mRNA) sequence of Cacna1a. The RT-PCR products were sequenced using an automated sequencer (ABI Prism 3730; Applied Biosystems, CA, USA). Alternation in the transcript structure was discovered in Cav2.1α1 using the C57BL/6J, and BALB/cByJ, and database sequence of Cacna1a cDNA (GenBank ID: NM_007578). The following PCR primers (Forward: 5′-CCTCTCTGTGGGTACACATAT-3′, reverse: 5′-GGGAATACTGAATTCAGGATT-3′) were used to confirm the mutation position in the fragment consisting of the nucleotides 99881 (in intron 3) - 108044 (in intron 6) of the mouse genomic Cacna1a DNA (GenBank ID: NC_000074) from spleens of 24 eight-week-old 6j/6j and 24+/+ mice. Northern blotting was used to examine the transcript levels, where 10 µg of the total RNA isolated from the brain and the liver of eight-week-old 6j/6j and +/+ mice was used for blot hybridization with a DIG labeled probe consisting of nucleotides 3864–4664 of the mouse Cacna1a cDNA (GenBank ID: NM_007578). For RT-PCR analysis, the following PCR primers (Forward: 5′-TCCTACCTGAGGAATGGCTGGAAC-3′, reverse 5′-CAGCCTTCCATGGTGATGCACTCC-3′) were used to identify the genotypes with the wild-type 473-bp or mutant-type 320-bp fragments consisting of nucleotides 493 (in exon 4) - 965 (in exon 6) of the mouse Cacna1a cDNA (GenBank ID: NM_007578) from brains.
Motor Behavior Tests
The mice, including eight-week-old male 6j/6j, heterozygous tottering-6j (6j/+), homozygous rolling Nagoya, (rol/rol), heterozygous rolling Nagoya, (rol/+), compound heterozygous (tottering-6j × rolling Nagoya, 6j/rol), and +/+ mice were subjected to motor behavior studies. In the footprint test, black ink was applied to the hind paws of each mouse and they were then placed in a narrow alley (9 × 25 × 10 cm) on white paper. Stride length and step width were measured. The black ink used for the footprint analysis was non-toxic. The footprint test was conducted between 10∶00 am and 12∶00 pm. In the traction test, the grip strength of each mouse was measured using a traction apparatus (Ohara & Co., Ltd., Tokyo, Japan). Each mouse was made to grasp the attached bar (1 mm diameter) with the forepaws and was slowly pulled back by its tail. The maximum tension (in g) before release was recorded and normalized to body weight. The traction test was conducted between 11∶00 am and 11∶30 pm. In the rotating rod test, motor coordination was assessed with a rotating rod apparatus (Ugo Basile RotaRod Treadmills, Model 7650; Ugo Basile S.R.L., Comerio, Italy). The mice were first placed on the stationary rod (0 rpm) for three trials, followed by three trials at a rotation speed of 3 rpm. Latency until a fall occurred was monitored for 120 s and the intra-trial intervals for each animal were greater than 20 min. The rotation of the rotarod was accelerated from 3 to 30 rpm over 300 s at a constant rate, and the rotation speed of 30 rpm was maintained for 120 s. Mice were trained for five days and received three trials per day, with an interval of 1 h between trials. The time taken for each mouse to maintain balance on the rotarod was measured. The rotating rod test was conducted between 1∶00 am and 4∶00 pm. In the hind-limb extension test, the mice were suspended by the tail and the extent of hind-limb extension was observed during 10 s. A score of 2 corresponded to a normal extension reflex in both hind-limbs, with splaying of toes. A score of 1 corresponded to an extension reflex in only one hind-limb or extension of both hind-limbs, without splayed toes. A score of 0 corresponded to clasping behavior with both hind-limbs. The hind-limb extension test was conducted between 1∶00 am and 2∶00 pm. All behavioral analyses were conducted by a well-trained experimenter who was blinded to the mouse genotypes. The mice were moved into the behavioral testing room at least 1 h before testing. The data are presented as the mean ± standard error of the mean (SEM). Statistical analyses were conducted using Excel Statistics 2006 (SSRI, Tokyo, Japan). The data were analyzed using an analysis of variance (ANOVA). Tukey's post hoc test between groups was performed when appropriate. The results were considered significant at a 5% or lower probability of error.
Histochemistry
At 8 weeks of age, animals were anesthetized using sodium pentobarbitone and perfused transcardially with 4% paraformaldehyde in phosphate buffered saline (PBS). The brains were immediately removed from the cranium and fixed for an additional 4 hr at 4°C. The brains were then cryoprotected by submersion in 18% (w/v) sucrose in PBS at 4°C overnight. The samples were embedded in OCT, frozen in powdered dry ice for 5 min, and then allowed to equilibrate to the cutting temperature (−20°C) of the cryostat (Microm Cryo-Star HM 560 Cryostat, Thermo Fisher Scientific, Inc., MA, USA). Frozen serial sections were sliced at 15 µm and allowed to air dry on gelatin-coated slides for hematoxylin and eosin staining and immunocytochemistry. For immunocytochemistry a primary antibody to tyrosine hydroxylase (Chemicon International Inc., CA, USA) was used at a dilution of 1∶500, and the secondary antibody (Alexa Fluor 568-conjugated goat anti-mouse IgG antibody; Invitrogen, ON, Canada) was used at a dilution of 1∶500.
Funding Statement
This work was supported by Grants-in-Aid for Scientific Research KAKENHI (22500396) to ET and China 973 Program (2010CB529604) to WL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21. [DOI] [PubMed] [Google Scholar]
- 2. Liu L, Zwingman TA, Fletcher CF (2003) In vivo analysis of voltage-dependent calcium channels. J Bioenerg Biomembr 35: 671–685. [DOI] [PubMed] [Google Scholar]
- 3. Mori Y, Friedrich T, Kim MS, Mikami A, Nakai J, et al. (1991) Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature 350: 398–402. [DOI] [PubMed] [Google Scholar]
- 4. Catterall WA (1998) Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium 24: 307–323. [DOI] [PubMed] [Google Scholar]
- 5. Catterall WA (1999) Interactions of presynaptic Ca2+ channels and snare proteins in neurotransmitter release. Ann NY Acad Sci 868: 144–159. [DOI] [PubMed] [Google Scholar]
- 6. Tedford HW, Zamponi GW (2006) Direct G protein modulation of Cav2 calcium channels. Pharmacol Rev 58: 837–862. [DOI] [PubMed] [Google Scholar]
- 7. Buraei Z, Yang J (2010) The ß subunit of voltage-gated Calcium channels. Physiol Rev 90: 1461–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pietrobon D (2010) Cav2.1 channelopathies. Pflugers Arch 460: 375–393. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi E (2012) Cav2.1 channelopathies and mouse genetic approaches for investigating Cav2.1 channel function and dysfunction. In: Yamaguchi M, editor. Calcium Signaling. New York: Nova Science Publishers Inc. 149–158.
- 10. Rajakulendran S, Kaski D, Hanna MG (2012) Neuronal P/Q-type calcium channel dysfunction in inherited disorders of the CNS. Nat Rev Neurol 8: 86–96. [DOI] [PubMed] [Google Scholar]
- 11. van den Maagdenberg AM, Pietrobon D, Pizzorusso T, Kaja S, Broos LA, et al. (2004) A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron 41: 701–710. [DOI] [PubMed] [Google Scholar]
- 12. van den Maagdenberg AM, Pizzorusso T, Kaja S, Terpolilli N, Shapovalova M, et al. (2010) High CSD susceptibility and migraine-associated symptoms in Cav2.1 S218L mice. Ann Neurol 67: 85–98. [DOI] [PubMed] [Google Scholar]
- 13. Watase K, Barrett CF, Miyazaki T, Ishiguro T, Ishikawa K, et al. (2008) Spinocerebellar ataxia type 6 knockin mice develop a progressive neuronal dysfunction with age-dependent accumulation of mutant Cav2.1 channels. Proc Natl Acad Sci U S A 105: 11987–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fletcher CF, Tottene A, Lennon VA, Wilson SM, Dubel SJ, et al. (2001) Dystonia and cerebellar atrophy in Cacna1a null mice lacking P/Q calcium channel activity. Faseb J 15: 1288–1290. [DOI] [PubMed] [Google Scholar]
- 15. Pietrobon D (2005) Function and dysfunction of synaptic calcium channels: insights from mouse models. Curr Opin Neurobiol 15: 257–265. [DOI] [PubMed] [Google Scholar]
- 16. Xie G, Clapcote SJ, Nieman BJ, Tallerico T, Huang Y, et al. (2007) Forward genetic screen of mouse reveals dominant missense mutation in the P/Q-type voltage-dependent calcium channel, CACNA1A. Genes Brain Behav 6: 717–727. [DOI] [PubMed] [Google Scholar]
- 17. Miki T, Zwingman TA, Wakamori M, Lutz CM, Cook SA, et al. (2008) Two novel alleles of tottering with distinct Cav2.1 calcium channel neuropathologies. Neuroscience 155: 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tokuda S, Kuramoto T, Tanaka K, Kaneko S, Takeuchi IK, et al. (2007) The ataxic groggy rat has a missense mutation in the P/Q-type voltage-gated Ca2+ channel alpha1A subunit gene and exhibits absence seizures. Brain Res 1133: 168–177. [DOI] [PubMed] [Google Scholar]
- 19. Day NC, Wood SJ, Ince PG, Volsen SG, Smith W, et al. (1997) Differential localization of voltage-dependent calcium channel alpha1 subunits at the human and rat neuromuscular junction. J Neurosci. 17: 6226–6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Plomp JJ, Vergouwe MN, Van Den Maagdenberg AM, Ferrari MD, Frants RR, et al. (2000) Abnormal transmitter release at neuromuscular junctions of mice carrying the tottering alpha(1A) Calcium channel mutation. Brain 123: 463–471. [DOI] [PubMed] [Google Scholar]
- 21. Kaja S, Van De Ven RC, Van Dijk JG, Verschuuren JJ, Arahata K, et al. (2007) Severely impaired neuromuscular synaptic transmission causes muscle weakness in the Cacna1a-mutant mouse rolling Nagoya. Eur J Neurosci. 25: 2009–2020. [DOI] [PubMed] [Google Scholar]
- 22. Jaworski DM, Soloway P, Caterina J, Falls WA (2006) Tissue inhibitor of metalloproteinase-2 (TIMP-2)-deficient mice display motor deficits. J Neurobiol. 66: 82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takahashi E, Niimi K, Itakura C (2009) Motor coordination impairment in aged heterozygous rolling Nagoya, Cav2.1 mutant mice. Brain Res 1279: 50–57. [DOI] [PubMed] [Google Scholar]
- 24. Ogura H, Matsumoto M, Mikoshiba K (2001) Motor discoordination in mutant mice heterozygous for the type 1 inositol 1,4,5-trisphosphate receptor. Behav Brain Res 122: 215–219. [DOI] [PubMed] [Google Scholar]
- 25. Van Damme P, Leyssen M, Callewaert G, Robberecht W, Van Den Bosch L (2003) The AMPA receptor antagonist NBQX prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis. Neurosci Lett 343: 81–84. [DOI] [PubMed] [Google Scholar]
- 26. Niimi K, Takahashi E, Itakura C (2009) Analysis of motor function and dopamine systems of SAMP6 mouse. Physiol Behav 96: 464–469. [DOI] [PubMed] [Google Scholar]
- 27. Fletcher CF, Lutz CM, O'Sullivan TN, Shaughnessy JD Jr, Hawkes R, et al. (1996) Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell 87: 607–617. [DOI] [PubMed] [Google Scholar]
- 28. Austin MC, Schultzberg M, Abbott LC, Montpied P, Evers JR, et al. (1992) Expression of tyrosine hydroxylase in cerebellar Purkinje neurons of the mutant tottering and leaner mouse. Brain Res Mol Brain Res 15: 227–240. [DOI] [PubMed] [Google Scholar]
- 29. Oda S (1973) The observation of rolling mouse Nagoya (rol), a new neurological mutant, and its maintenance. Jikken Dobutsu 22: 281–288. [DOI] [PubMed] [Google Scholar]
- 30. Fu XD (1995) The superfamily of arginine/serine rich splicing factor. RNA 1: 663–680. [PMC free article] [PubMed] [Google Scholar]
- 31. Catterall WA (2000) Structure and regulation of voltage-gated calcium channels. Annu Rev Cell Dev Biol 16: 521–555. [DOI] [PubMed] [Google Scholar]
- 32. Mori Y, Wakamori M, Oda S, Fletcher CF, Sekiguchi N, et al. (2000) Reduced voltage sensitivity of activation of P/Q-type calcium channels is associated with the ataxic mouse mutation rolling Nagoya (tg(rol)), J Neurosci. 20: 5654–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lorenzon NM, Lutz CM, Frankel WN (1998) BeamKG (1998) Altered calcium channel currents in Purkinje cells of the neurological mutant mouse leaner. J Neurosci 18: 4482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zwingman TA, Neumann PE, Noebels JL, Herrup K (2001) Rocker is a new variant of the voltage-dependent calcium channel gene Cacna1a. J Neurosci 21: 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muramoto O, Kanazawa I, Ando K (1981) Neurotransmitter abnormality in Rolling mouse Nagoya, an ataxic mutant mouse. Brain Res 215: 295–304. [DOI] [PubMed] [Google Scholar]
- 36. Brosenitsch TA, Katz DM (2001) Physiological patterns of electrical stimulation can induce neuronal gene expression by activating N-type calcium channels. J Neurosci 21: 2571–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rhyu IJ, Abbott LC, Walker DB, Sotelo C (1992) An ultrastructural study of granule cell/Purkinje cell synapses in tottering (tg/tg), leaner (tg(la)/tg(la)) and compound heterozygous tottering/leaner (tg/tg(la)) mice. Neuroscience. 90: 717–728. [DOI] [PubMed] [Google Scholar]
- 38. Rhyu IJ, Oda S, Uhm CS, Kim H, Suh YS, Abbott LC (1999) Morphologic investigation of rolling mouse Nagoya (tg(rol)/tg(rol)) cerebellar Purkinje cells: an ataxic mutant, revisited. Neurosci Lett. 266: 49–52. [DOI] [PubMed] [Google Scholar]