Abstract
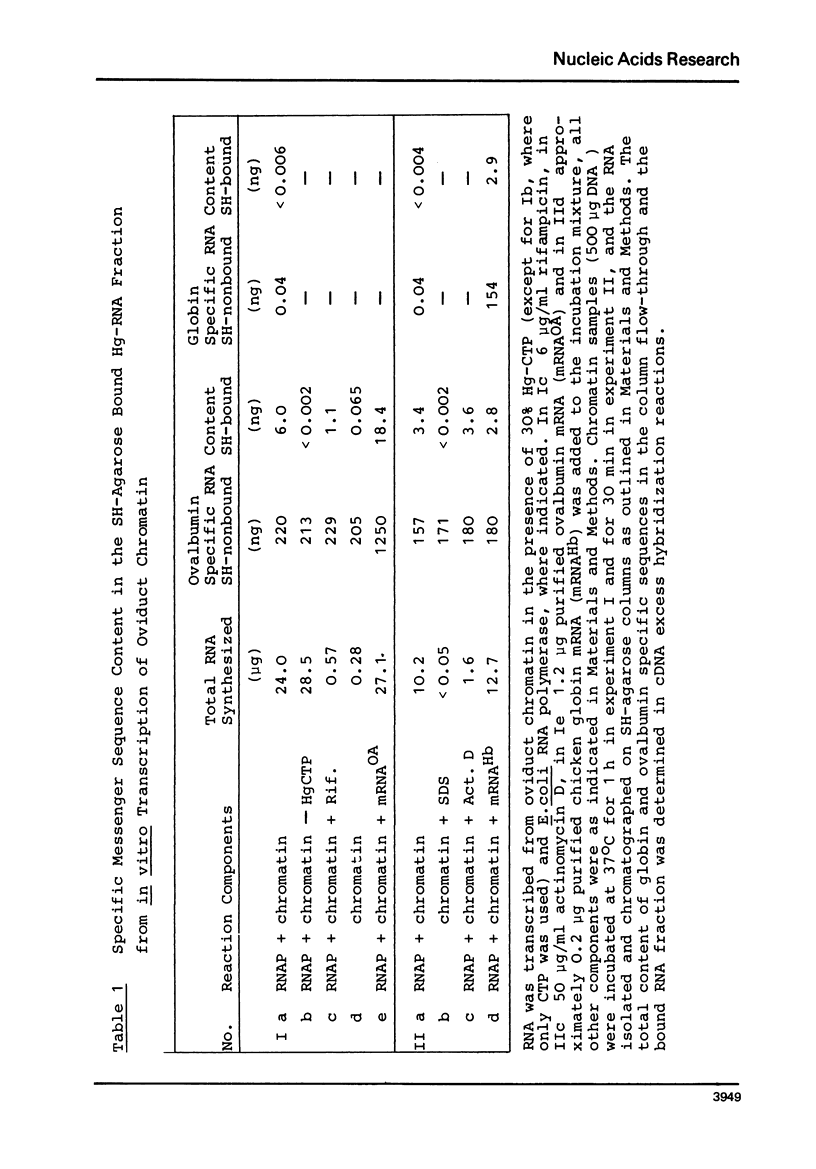
Mercurated nucleoside triphosphates have been used for transcription of chicken oviduct chromatin with E. coli RNA polymerase. The newly synthesized RNA was purified from preexisting RNA by SH-agarose chromatography and analyzed for the content of specific mRNA sequences. The apparent preferential production of ovalbumin mRNA sequences was not inhibited by actinomycin D, although total RNA synthesis was reduced by more than 90%. Furthermore, when globin mRNA alone, or added to oviduct chromatin, was incubated in the transcription assay, a significant fraction of this mRNA was retained on SH-agarose. The copurification of chromatin associated RNA with in vitro synthesized mercurated RNA was mainly due to a RNA-dependent synthesis of complementary sequences by the bacterial enzyme. Although denaturation of the transcripts prior to SH-agarose chromatography leads to a reduced contamination with endogenous ovalbumin specific RNA, we are unable to show that the messenger-specific RNA sequences purified with the newly mercurated RNA results from a DNA-dependent reaction.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrin S. M. In vitro transcription of simian virus 40 sequences in SV3T3 chromatin. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2304–2308. doi: 10.1073/pnas.70.8.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel R., Cedar H., Felsenfeld G. Synthesis of globin ribonucleic acid from duck-reticulocyte chromatin in vitro. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2029–2032. doi: 10.1073/pnas.70.7.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebee T. J., Butterworth P. H. The use of mercurated nucleoside triphosphate as a probe in transcription studies in vitro. Eur J Biochem. 1976 Jul 15;66(3):543–550. doi: 10.1111/j.1432-1033.1976.tb10580.x. [DOI] [PubMed] [Google Scholar]
- Biessmann H., Gjerset R. A., Levy B., McCarthy B. J. Fidelity of chromatin transcription in vitro. Biochemistry. 1976 Oct 5;15(20):4356–4363. doi: 10.1021/bi00665a002. [DOI] [PubMed] [Google Scholar]
- Crouse G. F., Fodor J. B., Doty P. In vitro transcription of chromatin in the presence of a mercurated nucleotide. Proc Natl Acad Sci U S A. 1976 May;73(5):1564–1567. doi: 10.1073/pnas.73.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Dale R. M., Martin E., Livingston D. C., Ward D. C. Direct covalent mercuration of nucleotides and polynucleotides. Biochemistry. 1975 Jun 3;14(11):2447–2457. doi: 10.1021/bi00682a027. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Ward D. C. Mercurated polynucleotides: new probes for hybridization and selective polymer fractionation. Biochemistry. 1975 Jun 3;14(11):2458–2469. doi: 10.1021/bi00682a028. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Ernest M. J., Schutz G., Feigelson P. RNA synthesis in isolated hen oviduct nuclei. Biochemistry. 1976 Feb 24;15(4):824–829. doi: 10.1021/bi00649a015. [DOI] [PubMed] [Google Scholar]
- FOX C. F., ROBINSON W. S., HASELKORN R., WEISS S. B. ENZYMATIC SYNTHESIS OF RIBONUCLEIC ACID. III. THE RIBONUCLEIC ACID-PRIMED SYNTHESIS OF RIBONUCLEIC ACID WITH MICROCOCCUS LYSODEIKTICUS RIBONUCLEIC ACID POLYMERASE. J Biol Chem. 1964 Jan;239:186–195. [PubMed] [Google Scholar]
- GOMATOS P. J., KRUG R. M., TAMM I. ENZYMIC SYNTHESIS OF RNA WITH REOVIRUS RNA AS TEMPLATE. I. CHARACTERISTICS OF THE REACTION CATALYZED BY THE RNA POLYMERASE FROM ESCHERICHIA COLI. J Mol Biol. 1964 Jul;9:193–207. doi: 10.1016/s0022-2836(64)80100-5. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour R. S., Paul J. Tissue-specific transcription of the globin gene in isolated chromatin. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3440–3442. doi: 10.1073/pnas.70.12.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M., Murphy R. F., Bonner J. Structure of transcriptionally active chromatin. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4404–4408. doi: 10.1073/pnas.72.11.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. E., Schwartz R. J., Tsai M. J., O'Malley B. W., Roy A. K. Effect of estrogen on gene expression in the chick oviduct. In vitro transcription of the ovalbumin gene in chromatin. J Biol Chem. 1976 Jan 25;251(2):524–529. [PubMed] [Google Scholar]
- Hynes N. E., Groner B., Sippel A. E., Nguyen-Huu M. C., Schütz G. mRNA complexity and egg white protein mRNA content in mature and hormone-withdrawn oviduct. Cell. 1977 Aug;11(4):923–932. doi: 10.1016/0092-8674(77)90303-8. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Groner Y., Monroy G., Hurwitz J. The in vitro synthesis of avian myeloblastosis viral RNA sequences. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3045–3049. doi: 10.1073/pnas.71.8.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAKOW J. S., OCHOA S. Ribonucleic acid polymerase of Azotobacter vinelandii. I. Priming by polyribonucleotides. Proc Natl Acad Sci U S A. 1963 Jan 15;49:88–94. doi: 10.1073/pnas.49.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel D. A., Ingram V. M. RNA aggregation during sulfhydryl-agarose chromatography of mercurated RNA. Nucleic Acids Res. 1977 Jun;4(6):1979–1988. doi: 10.1093/nar/4.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melli M., Pemberton R. E. New method of studying the precursor-product relationship between high molecular weight RNA and messenger RNA. Nat New Biol. 1972 Apr 12;236(67):172–174. doi: 10.1038/newbio236172a0. [DOI] [PubMed] [Google Scholar]
- NAKAMOTO T., WEISS S. B. The biosynthesis of RNA: printing by polyribonucleotides. Proc Natl Acad Sci U S A. 1962 May 15;48:880–887. doi: 10.1073/pnas.48.5.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pays E. Double-stranded RNA in chromatin transcripts formed by exogenous RNA polymerase. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1121–1125. doi: 10.1073/pnas.73.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder R. H. Transcription of chromatin by bacterial RNA polymerase. J Mol Biol. 1973 Oct 25;80(2):229–241. doi: 10.1016/0022-2836(73)90169-1. [DOI] [PubMed] [Google Scholar]
- STEVENS A., HENRY J. STUDIES ON THE RIBONUCLEIC ACID POLYMERASE FROM ESCHERICHIA COLI. I. PURIFICATION OF THE ENZYME AND STUDIES OF RIBONUCLEIC ACID FORMATION. J Biol Chem. 1964 Jan;239:196–203. [PubMed] [Google Scholar]
- Shih T. Y., Khoury G., Martin M. A. In vitro transcription of the viral-specific sequences present in the chromatin of cells transformed by simian virus 40. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3506–3510. doi: 10.1073/pnas.70.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Young H. A., Parks W. P., Scolnick E. M. In vitro transcription of Moloney leukemia virus genes in infected cell nuclei and chromatin: elongation of chromatin associated ribonucleic acid by Escherichia coli ribonucleic acid polymerase. Biochemistry. 1977 May 3;16(9):1795–1801. doi: 10.1021/bi00628a005. [DOI] [PubMed] [Google Scholar]
- Sippel A. E., Hynes N., Groner B., Schütz G. Frequency distribution of messenger sequences within polysomal mRNA and nuclear RNA from rat liver. Eur J Biochem. 1977 Jul 1;77(1):141–151. doi: 10.1111/j.1432-1033.1977.tb11652.x. [DOI] [PubMed] [Google Scholar]
- Smith M. M., Huang R. C. Transcription in vitro of immunoglobulin kappa light chain genes in isolated mouse myeloma nuclei and chromatin. Proc Natl Acad Sci U S A. 1976 Mar;73(3):775–779. doi: 10.1073/pnas.73.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelsberg T. C., Cox R. F. Effects of estrogen and progesterone on transcription, chromatin and ovalbumin gene expression in the chick oviduct. Biochim Biophys Acta. 1976 Jul 16;435(4):376–390. doi: 10.1016/0005-2787(76)90203-3. [DOI] [PubMed] [Google Scholar]
- Steggles A. W., Wilson G. N., Kantor J. A., Picciano D. J., Falvey A. K., Anderson W. F. Cell-free transcription of mammalian chromatin: transcription of globin messenger RNA sequences from bone-marrow chromatin with mammalian RNA polymerase. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1219–1223. doi: 10.1073/pnas.71.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G., Park W., Thrall C., Mans R., Stein J. Regulation of cell cycle stage-specific transcription of histone genes from chromatin by non-histone chromosomal proteins. Nature. 1975 Oct 30;257(5529):764–767. doi: 10.1038/257764a0. [DOI] [PubMed] [Google Scholar]
- Sternbach H., Engelhardt R., Lezius A. G. Rapid isolation of highly active RNA polymerase from Escherichia coli and its subunits by matrix-bound heparin. Eur J Biochem. 1975 Dec 1;60(1):51–55. doi: 10.1111/j.1432-1033.1975.tb20974.x. [DOI] [PubMed] [Google Scholar]
- Sugiura M., Miura K. Transcription of double-stranded RNA by Escherichia coli DNA-dependent RNA polymerase. Eur J Biochem. 1977 Feb 15;73(1):179–184. doi: 10.1111/j.1432-1033.1977.tb11305.x. [DOI] [PubMed] [Google Scholar]
- Terao T., Dahlberg J. E., Khorana H. G. Studies on polynucleotides. CXX. On the transcription of a synthetic 29-unit long deoxyribopolynucleotide. J Biol Chem. 1972 Oct 10;247(19):6157–6166. [PubMed] [Google Scholar]
- Towle H. C., Tsai M. J., Tsai S. Y., O'Malley B. W. Effect of estrogen on gene expression in the chick oviduct. J Biol Chem. 1977 Apr 10;252(7):2396–2404. [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Young B. D., Harrison P. R., Gilmour R. S., Birnie G. D., Hell A., Humphries S., Paul J. Kinetic studies of gene frequency. II. Complexity of globin complementary DNA and its hybridization characteristics. J Mol Biol. 1974 Apr 25;84(4):555–568. doi: 10.1016/0022-2836(74)90116-8. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Felsenfeld G. Use of mercury-substituted ribonucleoside triphosphates can lead to artefacts in the analysis of in vitro chromatin transcrits. Biochem Biophys Res Commun. 1977 Apr 11;75(3):598–603. doi: 10.1016/0006-291x(77)91514-5. [DOI] [PubMed] [Google Scholar]
- de Pomerai D. I., Chesterton C. J., Butterworth P. H. Preparation of chromatin. Variation in the template properties of chromatin dependent on the method of perparation. Eur J Biochem. 1974 Aug 1;46(3):461–471. doi: 10.1111/j.1432-1033.1974.tb03639.x. [DOI] [PubMed] [Google Scholar]


