Abstract
The basal ganglia network serves to integrate information about context, actions, and outcomes to shape an animal’s behavior based on its past experience. Clinically, the basal ganglia receive the most attention for their role in movement disorders. Recent advances in technology have opened new avenues of research into the structure and function of basal ganglia circuits. One emerging theme is the importance of GABAergic interneurons in coordinating and regulating network function. Here, we discuss evidence that changes in striatal GABAergic microcircuits contribute to basal ganglia dysfunction in a number of movement disorders. Because interneurons are genetically and neurochemically unique from striatal projection neurons, they may provide promising therapeutic targets for treating a variety of striatal-based disorders.
Keywords: interneuron, basal ganglia, Parkinson’s disease, Huntington’s disease, dystonia, Tourette syndrome
Introduction
The 1980s were a golden era for basal ganglia research, culminating in circuit models that continue to guide hypothesis-based studies of basal ganglia function in clinical and experimental contexts 1–4. Within the basal ganglia, the striatum is the most prominent nucleus, serving as a major site of input and integration for cortical, thalamic, and midbrain afferents. The striatum is functionally divided along a dorsolateral/ventromedial axis, where the dorsolateral portion subserves sensorimotor functions and the ventromedial portion is more involved in cognitive and limbic functions 5. Because the focus of this review is neural circuits involved in movement disorders, much of our discussion is concentrated on neural circuits in the dorsolateral striatum.
The projection neurons of the striatum, called spiny projection neurons (SPNs), integrate glutamatergic inputs from the cortex and thalamus and send GABAergic projections to neurons in downstream basal ganglia nuclei. Based on anatomical projection patterns and biochemical differences, SPNs are divided into two classes. D1-type dopamine receptor–expressing neurons project directly to basal ganglia output nuclei and are called “direct-pathway” SPNs (dSPNs), while D2-type dopamine receptor–expressing neurons, known as “indirect-pathway” SPNs (iSPNs), project indirectly to basal ganglia output nuclei via the globus pallidus external segment (GPe) and the subthalamic nucleus (STN). These pathways are well segregated in the dorsolateral striatum, with fewer than 5% of SPNs expressing both classes of dopamine receptors 6. Activity of dSPNs leads to the disinhibition of motor circuits to facilitate movement. Overactivity of the direct pathway has been proposed to cause hyperkinetic movement disorders such as Huntington’s disease (HD), dystonia, and Tourette’s syndrome. In contrast, iSPN activity inhibits motor circuits to suppress movement. Overactivity of the indirect pathway is thought to underlie hypokinetic motor symptoms in disorders such as Parkinson’s disease (PD).
Approximately 80–90% of striatal SPNs in the dorsolateral striatum fall into the direct/indirect pathway classification system. The remaining 10–20% are found in neurochemically distinct patches throughout the striatum, called striosomes or patches 4. SPNs in striosomes typically express D1 receptors and directly project to a subset of dopaminergic neurons in the substantia nigra compacta (SNc). Their direct projections to a subset of dopamine neurons suggest that striosomal SPNs are particularly important for regulating dopamine signaling, but their immediate effects on movement are not clear.
These classic models of basal ganglia function illustrate the importance of understanding how and when specific classes of SPNs are activated. Historically, the cellular and synaptic mechanisms controlling dSPN vs. iSPN activation were hard to elucidate, because SPN subtypes could only be differentiated using manually intensive anatomical methods or antidromic stimulation in vivo. This has rapidly changed thanks to the development of transgenic mouse lines that fluorescently label dSPNs, iSPNs, and local interneurons within the striatum 7–11.
Interneurons tune and regulate dynamical properties of neural circuits in many brain regions. Interneurons comprise only ~5% of all striatal neurons, but they critically regulate striatal output. Compared to the broad diversity of interneuron subtypes in the hippocampus 12 and cortex 13, interneurons in the striatum are considerably less heterogeneous (Figure 1). Electrophysiologically, most striatal GABAergic interneurons fall into two categories (1) fast-spiking interneurons (FSIs) and (2) persistent and low-threshold spike interneurons (PLTSs) 14. Neurochemically, FSIs may be distinguished by their expression of the calcium binding protein parvalbumin (PV), while PLTS interneurons express neuropeptides such as somatostatin (SOM), neuropeptide Y (NPY), and the enzyme nitric oxide synthase (NOS). Neurons broadly classified physiologically as PLTSs might also include several subtypes of GABAergic interneurons, including those that express tyrosine hydroxylase (TH) 9. In addition, about 20% of NPY-expressing interneurons have the electrophysiological properties of neurogliaform cells (NGF) 10. The striatum also contains calretinin-expressing interneurons, but these cells are much sparser in rodents compared to primates 15 and their electrophysiological properties are not well characterized.
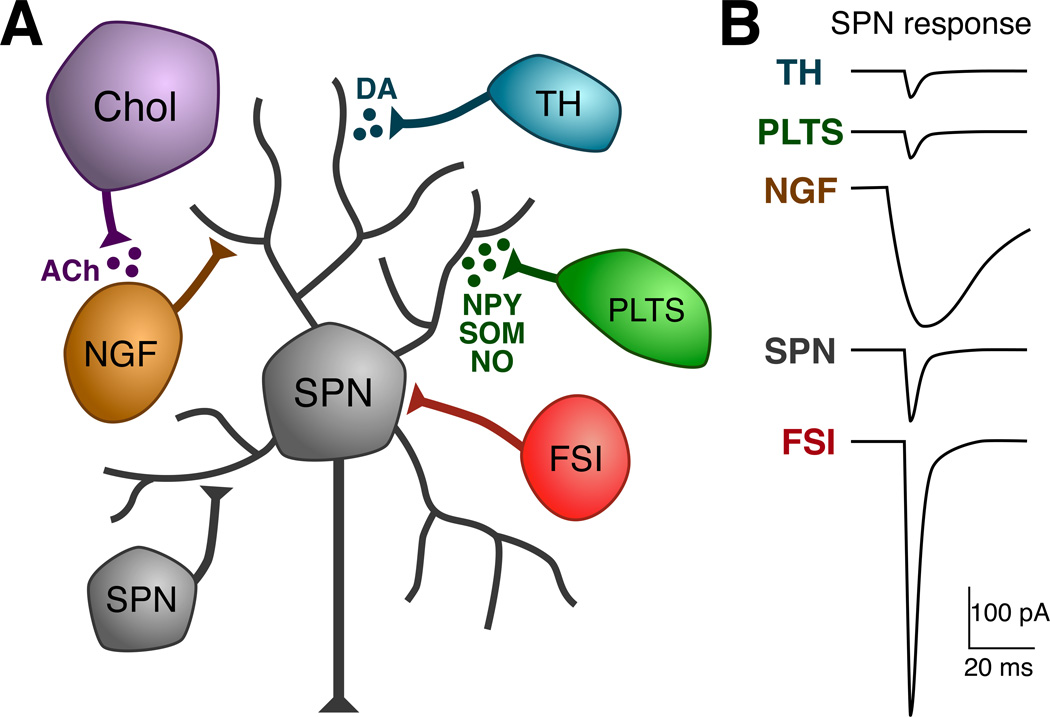
Figure 1.
GABAergic microcircuits in the striatum. A. Schematic showing different classes of striatal neurons that contribute to local inhibitory networks. B. Illustration comparing the typical time course and amplitudes of unitary IPSCs (uIPSCs) observed in SPNs from each class of inhibitory neuron. FSIs typically synapse onto the somatic compartment of SPNs 112 and produce large amplitude, fast kinetic responses in SPNs 8, 22–24. PLTS interneurons are sparsely connected to SPNs and their synapses are found on the dendrites of SPNs 112. uIPSCs from PLTS interneurons are very small compared to those from FSIs, typically < 100 pA 8. A more important role for PLTS interneurons in regulating SPN function may be the release of neuromodulators such as NPY, SOM, and NO. TH-positive interneurons are similar electrophysiologically to PLTS interneurons. They also make inhibitory connections onto the distal dendrites of SPNs and produce small amplitude uIPSCs 9. The local release of dopamine by these neurons may be particularly important in diseases like PD, where the normally massive dopaminergic innervation of the striatum from the SNc is lost. NPY-NGF interneurons likely target the distal dendrites of SPNs 10, 113. Although NPY-NGF interneurons receive some excitatory inputs (presumably from both cortex and thalamus), they are also well activated by acetylcholine (ACh) release from striatal cholinergic interneurons 19. The uIPSCs from NPY-NGF interneurons have distinctive slow kinetics compared to uIPSCs from all other cell types 10, 19. Finally, SPNs also make lateral inhibitory connections with each other and these collateral synapses also target the dendritic compartments of SPNs 28, 114. Although the probability of finding a connection between any two SPNs is small and uIPSCs are weak, due to the large number of SPNs in the striatum relative to all other cell types (95% of striatal neurons are SPNs), this inhibitory collateral network may be a major source of local inhibition 29. Abbreviations: Chol, cholinergic interneuron; DA, dopamine.
Although this review will focus on inhibitory microcircuits within the striatum, it is important to note the presence of an additional type of interneuron in the striatum that releases the neurotransmitter acetylcholine. Cholinergic interneurons play an important role in regulating striatal output 16, 17, possibly through the modulation of local inhibitory circuits 18, 19.
GABAergic Microcircuits in the Striatum
FSIs give rise to one of the best-characterized inhibitory microcircuits in the striatum. They are thought to mediate feedforward inhibition because they are activated earlier and at lower thresholds than SPNs 20, 21. FSIs make strong, dense projections onto SPNs within a 300 µm radius and inhibit SPN firing 8, 21–23. A single FSI inhibits an estimated total of 135–541 SPNs 24 of both the direct- and indirect-pathway subtypes 8, 23.
NGF cells that express NPY represent a second major class of densely-projecting interneuron 10, 19. Whereas FSIs are connected to SPNs with connection probabilities ranging from 25% 24 to 75% 23, NPY-NGF neurons are connected to SPNs with >86% connection probability 10. However, the comparatively low number of NPY-NGF interneurons in the striatum relative to FSIs might explain why the influence of NPY-NGF interneurons on striatal function has not been more apparent. Inhibitory postsynaptic currents (IPSCs) from NPY-NGF neurons give rise to distinct, long-lasting IPSCs in SPNs that have nearly 10-fold longer decay kinetics than those from FSIs (123 ms vs. 8 ms) 10, 25. Interestingly, NPY-NGF interneurons are activated by acetylcholine released from synchronously firing cholinergic interneurons, suggesting that NPY-NGF interneurons may be an integral part of cholinergic-mediated control of striatal output 19.
SPNs make collateral projections onto other SPNs, creating a lateral inhibitory network whose strength and function has been the topic of much theoretical work and some controversy 26. Unitary connections between SPNs are individually weak and connectivity is sparse 22, 27, 28, but in aggregate, lateral inhibition from thousands of SPNs can generate substantial inhibition 29 that might be important for creating distinct cell assemblies within the striatal circuit 30–32.
SPNs also receive inhibitory inputs from TH-positive interneurons and PLTS interneurons. Inhibitory inputs from these neurons are not readily detected with somatic whole-cell recordings and connectivity is sparse 8–10, so the extent to which inhibitory synaptic transmission from these interneurons contributes to striatal function is unclear. A more intuitive role for TH-positive and PLTS interneurons would be as sources of neuropeptides and neuromodulators such as dopamine, SOM, NPY, and NO, although further investigation will be required to clarify their functions.
GABAergic microcircuits are emerging as important coordinators of activity across distinct information processing streams within the striatum including the direct- and indirect-pathways and the patch/matrix compartments. FSIs, for example, are synaptically connected to both dSPNs and iSPNs 23, 25, and their dendrites cross the boundaries between the patch and matrix compartments, unlike the dendrites of SPNs which are restricted to their respective compartments 33. The dendrites of PLTS-like interneurons also cross the boundaries between patch and matrix compartments 33 and their release of neuromodulators and neuropeptides may enable regulation of large areas of the striatum by volume transmission. In this review, we discuss evidence that dysfunction of striatal GABAergic microcircuits leads to imbalances in striatal output pathways in a number of movement disorders. We hope that a better understanding of microcircuit dysfunction may open the door for new therapeutic strategies for the treatment of striatal-based movement disorders
Parkinson’s Disease
Hypokinetic motor impairments in PD patients are thought to arise from the loss of dopamine neurons in the substantia nigra that densely innervate the striatum. In rate-based models of striatal function, dopamine depletion leads to increased firing rates of iSPNs and excessive activity of neurons in basal ganglia output nuclei 1, 3, 34. In support of these rate-based models, a recent study demonstrated that increased firing of iSPNs was sufficient to decrease movement, and motor impairments in dopamine-depleted mice were diminished by increasing the firing rates of dSPNs 35.
A great deal of work has been devoted to unraveling the cellular and synaptic mechanisms underlying the imbalanced firing rates of dSPNs and iSPNs following dopamine depletion. However, a purely rate-based model of basal ganglia dysfunction in PD remains controversial. Under conditions of low dopamine levels, neurons in the basal ganglia and motor areas of the cortex show profound changes in firing pattern. Neurons in these regions fire in bursts 36–38, and become highly synchronous, oscillating in the 5–30 Hz frequency range 36–43. These dramatic alterations in firing pattern are thought to disrupt information processing throughout the basal ganglia-thalamo-cortical circuit 44–47.
In response to decreased dopaminergic signaling, SPNs in the striatum fire more synchronously and there is an increase in local field potential (LFP) power in the 10–30 Hz frequency range 38, 41–43, 48, 49. In part, the emergence of aberrant synchrony and oscillations in the striatum could reflect increased cortical influence over striatal activity that develops under conditions of low dopamine levels 41, 50, 51. Aberrant synchrony and oscillations could also reflect changes in inhibitory microcircuits. Acutely, dopamine signaling has been proposed to increase FSI excitability 52 through the activation of D5-type dopamine receptors 53, 54 and acute depletion of dopamine with α-methyl-paratyrosine in brain slices reduces FSI excitability 55. During more prolonged dopamine depletion, however, changes in FSI excitability are no longer observed 8, 51, suggesting that excitability may be homeostatically regulated.
FSI microcircuits are dramatically altered by dopamine depletion. Under normal conditions, FSIs make synaptic contacts onto both dSPNs and iSPNs, but preferentially target dSPNs 8 (Fig. 2A). Dopamine depletion induces a rapid, pathway-selective plasticity whereby FSIs nearly double their innervation of iSPNs (Fig. 2B). This inverts the normal pathway preference expressed by FSIs and is sufficient to induce aberrant synchrony across the population of iSPNs in a computer model of the striatal circuit 25. This suggests a mechanism through which the plasticity of striatal microcircuits could contribute directly to the amplification or propagation of pathological oscillations and synchrony in PD.
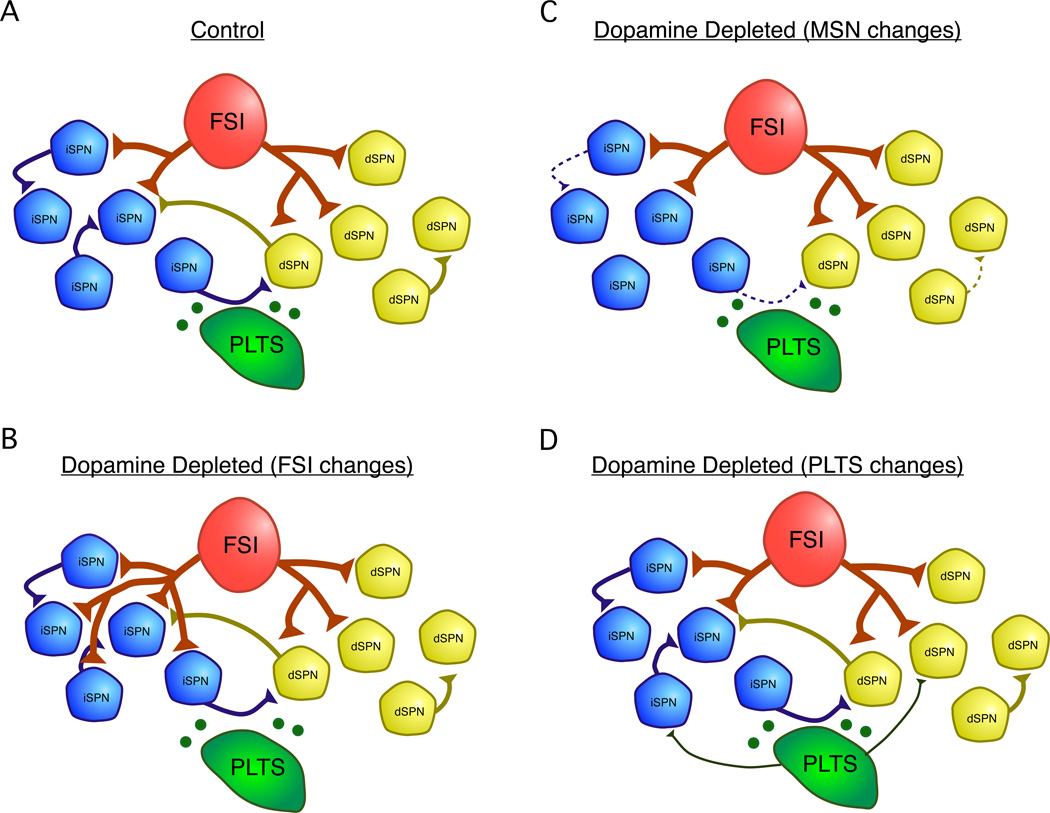
Figure 2.
Summary of changes in GABAergic microcircuits following dopamine depletion. A. Under normal conditions, SPNs laterally inhibit each other and inhibition is observed both across and within dSPN and iSPN subtypes 28. FSIs inhibit both dSPNs and iSPNs, but preferentially target dSPNs 8. PLTS interneurons release neuropeptides such as NPY and SOM and the neuromodulator NO which can modulate SPN activity 14. Under control conditions, inhibitory synapses from PLTS interneurons onto SPNs are hard to detect 8. The following changes have been observed to GABAergic microcircuits following pharmacological dopamine depletion in mice: B. Sprouting of FSI axons and the formation of new axons specifically onto iSPNs. This causes an inversion of the normal pathway-selectivity of FSIs such that after dopamine depletion, FSIs are more likely to target iSPNs than dSPNs 25. C. Reduction in connectivity and unitary strength of lateral inhibition between SPNs. Connections between dSPNs were sparse under control conditions and were no longer detected in dopamine depleted mice 28. D. An increase in the strength or connectivity of inhibitory inputs from PLTS interneurons onto SPNs may occur. This finding is based on increases in the frequency of large amplitude inhibitory postsynaptic currents (IPSCs) observed in SPNs after dopamine depletion 59, presumably arising from spontaneously active PLTS interneurons in the slice.
Under normal conditions, dSPNs and iSPNs extend collaterals to SPNs in both pathways, with the strongest connections between iSPNs→iSPNs and the weakest between dSPNs→ iSPNs 28. Dopamine depletion weakens collaterals between all SPNs 28 (Fig. 2C), and the loss of SPN-SPN collaterals provides a mechanism through which SPN cell assemblies can become pathologically large and synchronous when dopamine levels are low 32, 56.
Although the effects of dopamine depletion on NPY-NGF microcircuits have not been studied, the cholinergic interneurons that drive NPY-NGF signaling show increased acetylcholine release due to reduced autoreceptor function after dopamine depletion 57 and fire in a pathologically synchronous manner 58. Synchronous firing of cholinergic cells effectively drives NPY-NGF interneurons 19, so inhibitory signaling from NPY-NGF interneurons might be upregulated under conditions of low dopamine expression.
Finally, a fourth inhibitory microcircuit that might be affected by dopamine depletion arises from PLTS interneurons. Under normal conditions, PLTS interneurons make sparse, weak inhibitory projections onto SPNs 8, 10, but in dopamine-depleted mice, large, rhythmic inhibitory inputs develop onto SPNs that are thought to arise from PLTS interneurons 59 (Fig. 2D). Another possibility is that the large IPSCs observed after dopamine depletion arise from TH-positive interneurons 9, which increase in number after dopamine depletion 60.
Huntington’s Disease
A pathological hallmark of HD is the progressive loss of striatal SPNs. Indirect-pathway SPNs are more susceptible at early stages of the disease, whereas iSPNs, dSPNs, and cortical neurons die at later stages 61. Animal models of HD have revealed that dysfunction of neural circuits in the striatum and other brain regions can cause motor impairments even without cell death 62.
According to the classic model of basal ganglia function, reduced activity of iSPNs could underlie the hyperkinetic symptoms of HD 1, 2. In support of the classic model, disrupting indirect pathway activity in mice has been shown to increase locomotion 63–65. In a recent study, diphtheria toxin was injected into mice genetically engineered to express diptheria toxin receptors selectively in iSPNs, resulting in a ~90% reduction of these cells within 5 days. Loss of iSPNs specifically in the dorsomedial striatum increased locomotion and impaired learning of an accelerating rotarod task. Taking a different approach, a second group genetically deleted dopamine- and cAMP-regulated phosphoprotein-32 (DARPP-32)—a phosphatase that critically regulates dopamine receptor signal transduction—selectively in iSPNs to show that indirect-pathway disruption blocked long-term potentiation (LTP) and increased locomotor activity in an open field behavioral assay.
Reduced activity of iSPNs in HD could have dramatic effects on the structure and function of local cell assemblies in the striatum 30, 66, 67. Indirect-pathway SPNs make stronger collateral projections onto other SPNs than do dSPNs 28. Therefore, the loss of iSPNs could be particularly disruptive to lateral inhibitory circuits within the striatum.
The contribution of striatal GABAergic interneurons to circuit dysfunction in HD has not been well characterized. At early stages of motor impairments in two mouse models of HD, R6/2 and R6/1, IPSC frequency onto SPNs was nearly doubled, and SPNs showed increased expression of GABAA receptors 68. Although this study demonstrated changes in striatal inhibitory circuits early in the progression of HD, it is not clear whether increased inhibitory signaling arises from SPNs, FSIs, or another population of local GABAergic interneurons.
There is some evidence to suggest striatal FSIs are susceptible to HD-mediated cell death. In the R6/2 mouse model of HD, the numbers of FSIs were reduced by ~50% 69. In contrast, both calretinin-positive and NPY/SOM/NOS-positive interneurons were spared by the disease 70–72. The extent to which changes in the proportions of interneurons contribute to striatal pathophysiology in HD remains an open question. The preferential death of SPNs and FSIs in HD results in an increased relative proportion of PLTS and CR interneurons in the striatum. Under normal conditions, PLTS interneurons do not produce strong IPSCs in SPNs 8, 10, although their inhibitory projections might be increased under some pathological conditions 59. NO released from PLTS interneurons might increase the sensitivity of SPNs to their excitatory inputs 73, which could exacerbate glutamate excitotoxicity and contribute to cell death 74. However, NO has also been shown to enhance long-term depression of excitatory inputs onto SPNs 75. Thus, the overall effect of increased NO on striatal output remains controversial.
Dystonia
Dystonia is a clinical disorder in which involuntary and often painful muscle contractions generate twisting and repetitive movements. Although the pathophysiology of dystonia is still poorly understood, symptoms often correlate with increased striatal metabolic activity 76 and reduced GABAergic signaling 77, suggesting dysfunction of inhibitory circuits within the striatum.
A series of experiments characterizing striatal dysfunction in dystonia have been carried out in the dtsz hamster model of dystonia 78, 79. In dtsz hamsters, the firing rates of SPNs are abnormally high at 32–43 days of age, when dystonic attacks are at their peak, but drop back to normal levels at 96–100 days of age, when attacks no longer occur79. One mechanism that could account for the abnormally high SPN firing rates is reduced inhibition from local inhibitory interneurons, and indeed, fewer numbers of PV-positive interneurons are found in the striatum of dtsz hamsters relative to controls80.
In a recent study, directly reducing the activity of PV-positive FSIs in the dorsolateral striatum was sufficient to induce dystonia-like dyskinesias in mice81. When FSI firing was transiently suppressed by the infusion of IEM-1460—an antagonist selective for GluA2-lacking AMPA receptors—into the dorsolateral striatum, the mice exhibited action-induced dyskinesias that were characterized by prolonged, twisted postures and jerky, repetitive movements, similar to symptoms of dystonia in human patients. Although SPN firing rates tended to be higher when FSIs were inhibited, the difference was not significant, suggesting that dystonia is not caused by a simple increase or decrease in overall SPN firing rates but perhaps by a more complex change in their activation pattern. This concept would be consistent with changes in firing patterns observed in downstream basal ganglia nuclei in patients with dystonia 34, 82, 83.
At least two forms of dystonia are associated with the dysfunction of striosomal SPNs: dopa-responsive dystonia (DYT5) 84 and X-linked recessive dystonia parkinsonism (XDP; DYT3)85. In DYT3 dystonia, SPNs in striosomes degenerate, while DYT5 dystonia results from the selective reduction of dopaminergic innervation of striosomal SPNs, as evidenced by immunostaining 84 and reduced dopamine receptor binding by D3-type receptors that are enriched in striosomal compartments 86, 87. Disruption of striosomal SPNs presumably alters dopaminergic signaling, which could alter striatal output through direct effects on SPNs or modulation of corticostriatal plasticity 88.
Tourette Syndrome
Tourette syndrome is a movement disorder that first presents during childhood and typically declines in adulthood 89. Patients with Tourette syndrome exhibit highly stereotyped movements called tics. It has been proposed that the stereotyped motor patterns of tics are driven by some of the same motor circuits as those involved in habit learning 90 and highly repetitive behaviors or compulsions 89, 91.
A circuit-level model of Tourette’s syndrome, put forward by Mink and colleagues, posits that tics arise from the aberrant activation of small “pockets” of SPNs that correspond to specific motor commands 89, 92. Experimental support for these movement-related pockets of SPN activity comes from striatal recordings of monkeys performing a saccade task 93. Just before saccade onset, small pockets of focal activity within the striatum “pop-out” from the global LFP, presumably reflecting activity of a small group of SPNs. Furthermore, microstimulation of small regions of the striatum elicit movements in individual body parts 94.
Disruption of local inhibitory circuits within the striatum could be a main contributor to the aberrant activation of small groups of SPNs. Small, local infusions of the GABAA blocker bicuculline into the striatum elicits tics 95–97. An important outstanding question is whether bicuculline produces tics because of a disruption of local SPN-SPN collaterals, blockade of inhibition from FSIs, or blockade of inhibition from another source. Some support for a deficiency in FSI signaling comes from the observation that the brains of Tourette’s patients have fewer striatal FSIs 98.
Drug-induced Motor Impairments
Many drugs that alter dopamine signaling can impact movement. Cocaine and amphetamine increase locomotion acutely and their chronic use can produce repetitive behaviors called motor stereotypies, which include head bobbing and lip smacking. Psychostimulant-induced hyperlocomotion may arise from increased dopamine levels caused by these drugs, but due to the complex and widespread actions of dopamine on most synapses and cell types in the striatum 99, the specific neural circuits involved remain unknown. In the doroslateral striatum, amphetamine has been shown to increase the firing rates of striatal FSIs 100, potentially through the activation of D5 receptors 52. Increases in FSI firing rates, but not SPN firing rates, were correlated with increased locomotor activity 100.
In rats chronically treated with cocaine or amphetamine, the severity of motor stereotypies correlated with a unique pattern of neuronal activation in the striatum 101, where rats with the most severe stereotypies showed higher ratios of patch:matrix neuron activation than those with weaker stereotypies. Similar results were observed in monkeys 102. Chronic cocaine and amphetamine treatments also resulted in increased c-fos expression in NOS-positive striatal interneurons, suggesting a role for these neurons in the manifestation of motor stereotypies.
Another common motor disorder that results from prolonged treatment with dopamine-enhancing drugs is L-DOPA-induced dyskinesia. The incidence of developing L-DOPA-induced dyskinesias is as high as 50% in patients with early-onset PD (i.e., between 40–59 years of age). These dyskinesias typically first present on the side most affected by PD, and are typically observed in the legs before the arms 103. Although the cellular mechanisms of L-DOPA-induced dyskinesias remain unknown, a great deal of research has focused on altered glutamatergic plasticity onto SPNs 104. However, there is also evidence to suggest that GABAergic signaling is altered in the striatum and thus could be a contributing factor. For example, treatment with L-DOPA in rats has been shown to increase glutamic acid decarboxylase (GAD) mRNA levels in the striatum, suggesting an increase in local GABAergic signaling 105.
Increases in striatal GABA could reflect increased GABA release from SPN-SPN collaterals or increased FSI activity. Neither of these possibilities has been explicitly tested, but a recent study provides indirect evidence supporting the involvement of overactivity of striatal FSIs 106. In this study, mice with L-DOPA-induced dyskinesias were given systemic injections of IEM-1460, a selective antagonist of GluA2-lacking, Ca2+-permeable AMPA receptors. Co-administration of IEM-1460 with L-DOPA significantly reduced the development of L-DOPA-induced dyskinesias 106. Although Ca2+-permeable AMPA receptors are found throughout the brain, the only GABAergic neurons in the striatum that express these receptors are FSIs 81, suggesting that overactivity of striatal FSIs could contribute to the development of L-DOPA-induced dyskinesias. Future experiments are needed to determine whether there are structural or functional changes in FSIs that are consistent with this hypothesis.
Future research directions
A growing body of evidence points to dysfunction of striatal microcircuits as a common theme in a variety of movement disorders. Changes in FSIs, for example, are observed in both hypokinetic and hyperkinetic movement disorders. These observations are reconciled by the fact that FSIs target SPNs in a pathway-selective manner that is regulated by plasticity 8, 25. The selective targeting of subsets of principal neurons by interneurons is an emerging theme in various neural circuits 107. Identifying the molecular mechanisms that control interneuron target specificity holds great promise for developing new techniques that could modulate neural circuits in highly selective ways. It will also be important to gain a better understanding of the extent to which changes in excitatory and inhibitory connections onto interneurons and the connections between them are involved in circuit dysfunction in movement disorders.
Many interneurons express unique receptors or ion channels compared to projection neurons. Thus, specific subsets of interneurons might represent pharmacologically tractable drug targets for movement disorders that would have fewer side effects than current drug treatments. Specific promoters also open the possibility for the selective targeting of interneuron subtypes with optogenetic or pharmacogenetic constructs such as opsins or engineered G-protein coupled receptors108–111. A number of questions still remain about the role of specific striatal microcircuits in regulating striatal output in health and disease. However, recent advances in our abilities to target and manipulate specific microcircuits in the striatum suggest that important new discoveries are just on the horizon.
Acknowledgements
The authors would like to thank Robyn Javier for assistance with figure design. Work in the Kreitzer lab is supported by National Institutes of Health grants R01NS064984 and R01NS078435 (to A.C.K.), K99 NS076524 (to A.H.G.), and the McKnight Endowment for Neuroscience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albin RL, et al. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 2.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 3.Gerfen CR, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science (New York, N.Y. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 4.Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 5.Voorn P, et al. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Matamales M, et al. Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PloS one. 2009;4:e4770. doi: 10.1371/journal.pone.0004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valjent E, et al. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci. 2009;32:538–547. doi: 10.1016/j.tins.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Gittis AH, et al. Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J Neurosci. 2010;30:2223–2234. doi: 10.1523/JNEUROSCI.4870-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibanez-Sandoval O, et al. Electrophysiological and morphological characteristics and synaptic connectivity of tyrosine hydroxylase-expressing neurons in adult mouse striatum. J Neurosci. 2010;30:6999–7016. doi: 10.1523/JNEUROSCI.5996-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibanez-Sandoval O, et al. A novel functionally distinct subtype of striatal neuropeptide Y interneuron. J Neurosci. 2011;31:16757–16769. doi: 10.1523/JNEUROSCI.2628-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Partridge JG, et al. Excitatory and inhibitory synapses in neuropeptide Y-expressing striatal interneurons. Journal of neurophysiology. 2009;102:3038–3045. doi: 10.1152/jn.00272.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maccaferri G, Lacaille JC. Interneuron Diversity series: Hippocampal interneuron classifications--making things as simple as possible, not simpler. Trends Neurosci. 2003;26:564–571. doi: 10.1016/j.tins.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Parent A. Striatal interneurons expressing calretinin, parvalbumin or NADPH-diaphorase: a comparative study in the rat, monkey and human. Brain research. 2000;863:182–191. doi: 10.1016/s0006-8993(00)02135-1. [DOI] [PubMed] [Google Scholar]
- 16.Pisani A, et al. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30:545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Oldenburg IA, Ding JB. Cholinergic modulation of synaptic integration and dendritic excitability in the striatum. Current opinion in neurobiology. 2011;21:425–432. doi: 10.1016/j.conb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witten IB, et al. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science (New York, N.Y. 2011;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.English DF, et al. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nature neuroscience. 2011;15:123–130. doi: 10.1038/nn.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parthasarathy HB, Graybiel AM. Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. J Neurosci. 1997;17:2477–2491. doi: 10.1523/JNEUROSCI.17-07-02477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallet N, et al. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci. 2005;25:3857–3869. doi: 10.1523/JNEUROSCI.5027-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koos T, et al. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J Neurosci. 2004;24:7916–7922. doi: 10.1523/JNEUROSCI.2163-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Planert H, et al. Dynamics of synaptic transmission between fast-spiking interneurons and striatal projection neurons of the direct and indirect pathways. J Neurosci. 2010;30:3499–3507. doi: 10.1523/JNEUROSCI.5139-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nature neuroscience. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- 25.Gittis AH, et al. Rapid target-specific remodeling of fast-spiking inhibitory circuits after loss of dopamine. Neuron. 2011;71:858–868. doi: 10.1016/j.neuron.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plenz D. When inhibition goes incognito: feedback interaction between spiny projection neurons in striatal function. Trends Neurosci. 2003;26:436–443. doi: 10.1016/S0166-2236(03)00196-6. [DOI] [PubMed] [Google Scholar]
- 27.Jaeger D, et al. Surround inhibition among projection neurons is weak or nonexistent in the rat neostriatum. Journal of neurophysiology. 1994;72:2555–2558. doi: 10.1152/jn.1994.72.5.2555. [DOI] [PubMed] [Google Scholar]
- 28.Taverna S, et al. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson's disease. J Neurosci. 2008;28:5504–5512. doi: 10.1523/JNEUROSCI.5493-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chuhma N, et al. Functional connectome of the striatal medium spiny neuron. J Neurosci. 2011;31:1183–1192. doi: 10.1523/JNEUROSCI.3833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponzi A, Wickens J. Sequentially switching cell assemblies in random inhibitory networks of spiking neurons in the striatum. J Neurosci. 2010;30:5894–5911. doi: 10.1523/JNEUROSCI.5540-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrillo-Reid L, et al. Encoding network states by striatal cell assemblies. Journal of neurophysiology. 2008;99:1435–1450. doi: 10.1152/jn.01131.2007. [DOI] [PubMed] [Google Scholar]
- 32.Humphries MD, et al. Dopamine-modulated dynamic cell assemblies generated by the GABAergic striatal microcircuit. Neural Netw. 2009;22:1174–1188. doi: 10.1016/j.neunet.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 33.Crittenden JR, Graybiel AM. Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Frontiers in neuroanatomy. 2011;5:59. doi: 10.3389/fnana.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeLong M, Wichmann T. Update on models of basal ganglia function and dysfunction. Parkinsonism & related disorders. 2009;15(Suppl 3):S237–S240. doi: 10.1016/S1353-8020(09)70822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kravitz AV, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mallet N, et al. Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J Neurosci. 2008;28:14245–14258. doi: 10.1523/JNEUROSCI.4199-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mallet N, et al. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 2008;28:4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldberg JA, et al. Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson's disease. J Neurosci. 2002;22:4639–4653. doi: 10.1523/JNEUROSCI.22-11-04639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raz A, et al. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci. 2000;20:8559–8571. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nini A, et al. Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of parkinsonism. Journal of neurophysiology. 1995;74:1800–1805. doi: 10.1152/jn.1995.74.4.1800. [DOI] [PubMed] [Google Scholar]
- 41.Costa RM, et al. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron. 2006;52:359–369. doi: 10.1016/j.neuron.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 42.Burkhardt JM, et al. Synchronous oscillations and phase reorganization in the basal ganglia during akinesia induced by high-dose haloperidol. The European journal of neuroscience. 2007;26:1912–1924. doi: 10.1111/j.1460-9568.2007.05813.x. [DOI] [PubMed] [Google Scholar]
- 43.Burkhardt JM, et al. Dissociable effects of dopamine on neuronal firing rate and synchrony in the dorsal striatum. Frontiers in integrative neuroscience. 2009;3:28. doi: 10.3389/neuro.07.028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bevan MD, et al. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci. 2002;25:525–531. doi: 10.1016/s0166-2236(02)02235-x. [DOI] [PubMed] [Google Scholar]
- 45.Hutchison WD, et al. Neuronal oscillations in the basal ganglia and movement disorders: evidence from whole animal and human recordings. J Neurosci. 2004;24:9240–9243. doi: 10.1523/JNEUROSCI.3366-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammond C, et al. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson's disease. Mov Disord. 2003;18:357–363. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- 48.Walters JR, et al. Phase relationships support a role for coordinated activity in the indirect pathway in organizing slow oscillations in basal ganglia output after loss of dopamine. Neuroscience. 2007;144:762–776. doi: 10.1016/j.neuroscience.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moran RJ, et al. Alterations in brain connectivity underlying beta oscillations in Parkinsonism. PLoS computational biology. 2011;7:e1002124. doi: 10.1371/journal.pcbi.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tseng KY, et al. Cortical slow oscillatory activity is reflected in the membrane potential and spike trains of striatal neurons in rats with chronic nigrostriatal lesions. J Neurosci. 2001;21:6430–6439. doi: 10.1523/JNEUROSCI.21-16-06430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mallet N, et al. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci. 2006;26:3875–3884. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bracci E, et al. Dopamine excites fast-spiking interneurons in the striatum. Journal of neurophysiology. 2002;87:2190–2194. doi: 10.1152/jn.00754.2001. [DOI] [PubMed] [Google Scholar]
- 53.Centonze D, et al. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J Neurosci. 2003;23:6245–6254. doi: 10.1523/JNEUROSCI.23-15-06245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rivera A, et al. Molecular phenotype of rat striatal neurons expressing the dopamine D5 receptor subtype. The European journal of neuroscience. 2002;16:2049–2058. doi: 10.1046/j.1460-9568.2002.02280.x. [DOI] [PubMed] [Google Scholar]
- 55.Fino E, et al. Effects of acute dopamine depletion on the electrophysiological properties of striatal neurons. Neuroscience research. 2007;58:305–316. doi: 10.1016/j.neures.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Jaidar O, et al. Dynamics of the parkinsonian striatal microcircuit: entrainment into a dominant network state. J Neurosci. 2010;30:11326–11336. doi: 10.1523/JNEUROSCI.1380-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding J, et al. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nature neuroscience. 2006;9:832–842. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- 58.Raz A, et al. Activity of pallidal and striatal tonically active neurons is correlated in mptp-treated monkeys but not in normal monkeys. J Neurosci. 2001;21:RC128. doi: 10.1523/JNEUROSCI.21-03-j0006.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dehorter N, et al. Dopamine-deprived striatal GABAergic interneurons burst and generate repetitive gigantic IPSCs in medium spiny neurons. J Neurosci. 2009;29:7776–7787. doi: 10.1523/JNEUROSCI.1527-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huot P, Parent A. Dopaminergic neurons intrinsic to the striatum. Journal of neurochemistry. 2007;101:1441–1447. doi: 10.1111/j.1471-4159.2006.04430.x. [DOI] [PubMed] [Google Scholar]
- 61.Reiner A, et al. Differential loss of striatal projection neurons in Huntington disease. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5733–5737. doi: 10.1073/pnas.85.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raymond LA, et al. Pathophysiology of Huntington's disease: time-dependent alterations in synaptic and receptor function. Neuroscience. 2011;198:252–273. doi: 10.1016/j.neuroscience.2011.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bateup HS, et al. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14845–14850. doi: 10.1073/pnas.1009874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Durieux PF, et al. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nature neuroscience. 2009;12:393–395. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- 65.Durieux PF, et al. Differential regulation of motor control and response to dopaminergic drugs by D1R and D2R neurons in distinct dorsal striatum subregions. The EMBO journal. 2012;31:640–653. doi: 10.1038/emboj.2011.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller BR, et al. Dysregulated information processing by medium spiny neurons in striatum of freely behaving mouse models of Huntington's disease. Journal of neurophysiology. 2008;100:2205–2216. doi: 10.1152/jn.90606.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cayzac S, et al. Changes in striatal procedural memory coding correlate with learning deficits in a mouse model of Huntington disease. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9280–9285. doi: 10.1073/pnas.1016190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cepeda C, et al. Increased GABAergic function in mouse models of Huntington's disease: reversal by BDNF. Journal of neuroscience research. 2004;78:855–867. doi: 10.1002/jnr.20344. [DOI] [PubMed] [Google Scholar]
- 69.Giampa C, et al. Phosphodiesterase type IV inhibition prevents sequestration of CREB binding protein, protects striatal parvalbumin interneurons and rescues motor deficits in the R6/2 mouse model of Huntington's disease. The European journal of neuroscience. 2009;29:902–910. doi: 10.1111/j.1460-9568.2009.06649.x. [DOI] [PubMed] [Google Scholar]
- 70.Cicchetti F, et al. Sparing of striatal neurons coexpressing calretinin and substance P (NK1) receptor in Huntington's disease. Brain research. 1996;730:232–237. doi: 10.1016/0006-8993(96)00307-1. [DOI] [PubMed] [Google Scholar]
- 71.Beal MF, et al. Somatostatin and neuropeptide Y concentrations in pathologically graded cases of Huntington's disease. Annals of neurology. 1988;23:562–569. doi: 10.1002/ana.410230606. [DOI] [PubMed] [Google Scholar]
- 72.Dawbarn D, et al. Survival of basal ganglia neuropeptide Y-somatostatin neurones in Huntington's disease. Brain research. 1985;340:251–260. doi: 10.1016/0006-8993(85)90921-7. [DOI] [PubMed] [Google Scholar]
- 73.West AR, Grace AA. The nitric oxide-guanylyl cyclase signaling pathway modulates membrane activity States and electrophysiological properties of striatal medium spiny neurons recorded in vivo. J Neurosci. 2004;24:1924–1935. doi: 10.1523/JNEUROSCI.4470-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fan MM, Raymond LA. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington's disease. Progress in neurobiology. 2007;81:272–293. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 75.Calabresi P, et al. A critical role of the nitric oxide/cGMP pathway in corticostriatal long-term depression. J Neurosci. 1999;19:2489–2499. doi: 10.1523/JNEUROSCI.19-07-02489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Playford ED, et al. Increased activation of frontal areas during arm movement in idiopathic torsion dystonia. Mov Disord. 1998;13:309–318. doi: 10.1002/mds.870130218. [DOI] [PubMed] [Google Scholar]
- 77.Levy LM, Hallett M. Impaired brain GABA in focal dystonia. Annals of neurology. 2002;51:93–101. [PubMed] [Google Scholar]
- 78.Fredow G, Loscher W. Effects of pharmacological manipulation of GABAergic neurotransmission in a new mutant hamster model of paroxysmal dystonia. European journal of pharmacology. 1991;192:207–219. doi: 10.1016/0014-2999(91)90045-r. [DOI] [PubMed] [Google Scholar]
- 79.Richter A, Loscher W. Alterations in pharmacological sensitivity of GABAergic but not dopaminergic and glutamatergic systems during ontogenesis in dystonic mutant hamsters. European journal of pharmacology. 1993;231:111–119. doi: 10.1016/0014-2999(93)90691-a. [DOI] [PubMed] [Google Scholar]
- 80.Gernert M, et al. Deficit of striatal parvalbumin-reactive GABAergic interneurons and decreased basal ganglia output in a genetic rodent model of idiopathic paroxysmal dystonia. J Neurosci. 2000;20:7052–7058. doi: 10.1523/JNEUROSCI.20-18-07052.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gittis AH, et al. Selective inhibition of striatal fast-spiking interneurons causes dyskinesias. J Neurosci. 2011;31:15727–15731. doi: 10.1523/JNEUROSCI.3875-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vitek JL, et al. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Annals of neurology. 1999;46:22–35. doi: 10.1002/1531-8249(199907)46:1<22::aid-ana6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 83.Starr PA, et al. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson's disease and normal macaque. Journal of neurophysiology. 2005;93:3165–3176. doi: 10.1152/jn.00971.2004. [DOI] [PubMed] [Google Scholar]
- 84.Sato K, et al. Differential involvement of striosome and matrix dopamine systems in a transgenic model of dopa-responsive dystonia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12551–12556. doi: 10.1073/pnas.0806065105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goto S, et al. Functional anatomy of the basal ganglia in X-linked recessive dystonia-parkinsonism. Annals of neurology. 2005;58:7–17. doi: 10.1002/ana.20513. [DOI] [PubMed] [Google Scholar]
- 86.Murray AM, et al. Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:11271–11275. doi: 10.1073/pnas.91.23.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karimi M, et al. Decreased striatal dopamine receptor binding in primary focal dystonia: a D2 or D3 defect? Mov Disord. 2011;26:100–106. doi: 10.1002/mds.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peterson DA, et al. Convergent evidence for abnormal striatal synaptic plasticity in dystonia. Neurobiology of disease. 2010;37:558–573. doi: 10.1016/j.nbd.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Albin RL, Mink JW. Recent advances in Tourette syndrome research. Trends Neurosci. 2006;29:175–182. doi: 10.1016/j.tins.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 90.Marsh R, et al. Habit learning in Tourette syndrome: a translational neuroscience approach to a developmental psychopathology. Archives of general psychiatry. 2004;61:1259–1268. doi: 10.1001/archpsyc.61.12.1259. [DOI] [PubMed] [Google Scholar]
- 91.Berridge KC, et al. Sequential super-stereotypy of an instinctive fixed action pattern in hyper-dopaminergic mutant mice: a model of obsessive compulsive disorder and Tourette's. BMC biology. 2005;3:4. doi: 10.1186/1741-7007-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Archives of neurology. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- 93.Courtemanche R, et al. Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J Neurosci. 2003;23:11741–11752. doi: 10.1523/JNEUROSCI.23-37-11741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alexander GE, DeLong MR. Microstimulation of the primate neostriatum. I. Physiological properties of striatal microexcitable zones. Journal of neurophysiology. 1985;53:1401–1416. doi: 10.1152/jn.1985.53.6.1401. [DOI] [PubMed] [Google Scholar]
- 95.McCairn KW, et al. The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain. 2009;132:2125–2138. doi: 10.1093/brain/awp142. [DOI] [PubMed] [Google Scholar]
- 96.Worbe Y, et al. Behavioral and movement disorders induced by local inhibitory dysfunction in primate striatum. Cereb Cortex. 2009;19:1844–1856. doi: 10.1093/cercor/bhn214. [DOI] [PubMed] [Google Scholar]
- 97.Bronfeld M, et al. Spatial and temporal properties of tic-related neuronal activity in the cortico-basal ganglia loop. J Neurosci. 2011;31:8713–8721. doi: 10.1523/JNEUROSCI.0195-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kalanithi PS, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annual review of neuroscience. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wiltschko AB, et al. Opposite effects of stimulant and antipsychotic drugs on striatal fast-spiking interneurons. Neuropsychopharmacology. 2010;35:1261–1270. doi: 10.1038/npp.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nature neuroscience. 2000;3:377–383. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- 102.Saka E, et al. Repetitive behaviors in monkeys are linked to specific striatal activation patterns. J Neurosci. 2004;24:7557–7565. doi: 10.1523/JNEUROSCI.1072-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thanvi B, et al. Levodopa-induced dyskinesia in Parkinson's disease: clinical features, pathogenesis, prevention and treatment. Postgraduate medical journal. 2007;83:384–388. doi: 10.1136/pgmj.2006.054759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Calabresi P, et al. Levodopa-induced dyskinesias in patients with Parkinson's disease: filling the bench-to-bedside gap. Lancet neurology. 2011;9:1106–1117. doi: 10.1016/S1474-4422(10)70218-0. [DOI] [PubMed] [Google Scholar]
- 105.Katz J, et al. Comparative effects of acute or chronic administration of levodopa to 6-hydroxydopamine-lesioned rats on the expression of glutamic acid decarboxylase in the neostriatum and GABAA receptors subunits in the substantia nigra, pars reticulata. Neuroscience. 2005;132:833–842. doi: 10.1016/j.neuroscience.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 106.Kobylecki C, et al. Calcium-permeable AMPA receptors are involved in the induction and expression of l-DOPA-induced dyskinesia in Parkinson's disease. Journal of neurochemistry. 2010;114:499–511. doi: 10.1111/j.1471-4159.2010.06776.x. [DOI] [PubMed] [Google Scholar]
- 107.Krook-Magnuson E, et al. New dimensions of interneuronal specialization unmasked by principal cell heterogeneity. Trends Neurosci. 2012;35:175–184. doi: 10.1016/j.tins.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kokaia M, Sorensen AT. The treatment of neurological diseases under a new light: the importance of optogenetics. Drugs Today (Barc) 2011;47:53–62. doi: 10.1358/dot.2011.47.1.1543306. [DOI] [PubMed] [Google Scholar]
- 109.Fenno L, et al. The development and application of optogenetics. Annual review of neuroscience. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nature reviews. 2012;13:251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Conklin BR, et al. Engineering GPCR signaling pathways with RASSLs. Nature methods. 2008;5:673–678. doi: 10.1038/nmeth.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kubota Y, Kawaguchi Y. Dependence of GABAergic synaptic areas on the interneuron type and target size. J Neurosci. 2000;20:375–386. doi: 10.1523/JNEUROSCI.20-01-00375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tamas G, et al. Identified sources and targets of slow inhibition in the neocortex. Science (New York, N.Y. 2003;299:1902–1905. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- 114.Wilson CJ. GABAergic inhibition in the neostriatum. Progress in brain research. 2007;160:91–110. doi: 10.1016/S0079-6123(06)60006-X. [DOI] [PubMed] [Google Scholar]