Abstract
Metabotropic glutamate receptors (mGlus) are 7 Transmembrane Spanning Receptors (7TMs) that are differentially expressed throughout the brain and modulate synaptic transmission at both excitatory and inhibitory synapses. Recently, mGlus have been implicated as therapeutic targets for many disorders of the central nervous system, including Parkinson’s disease (PD). Previous studies have shown that nonselective agonists of group III mGlus have antiparkinsonian effects in several animal models of PD, suggesting that these receptors represent promising targets for treating the motor symptoms of PD. However, the relative contributions of different group III mGlu subtypes to these effects have not been fully elucidated. Here we report that intracerebroventricular (icv) administration of the mGlu8-selective agonist (S)-3,4-dicarboxyphenylglycine (DCPG [2.5, 10, or 30 nmol]) does not alleviate motor deficits caused by acute (two hour) treatment with haloperidol or reserpine. However, following prolonged pretreatment with haloperidol (three doses evenly spaced over 18–20 hours) or reserpine (18–20 hours), DCPG robustly reverses haloperidol-induced catalepsy and reserpine-induced akinesia. Furthermore, DCPG (10 nmol, icv) reverses the long-lasting catalepsy induced by 20 hour pretreatment with the decanoate salt of haloperidol. Finally, icv administration of DCPG ameliorates forelimb use asymmetry caused by unilateral 6-hydroxydopamine lesion of substantia nigra dopamine neurons. These findings suggest that mGlu8 may partially mediate the antiparkinsonian effects of group III mGlu agonists in animal models of PD in which dopamine depletion or blockade of D2-like dopamine receptors is prolonged and indicate that selective activation of mGlu8 may represent a novel therapeutic strategy for alleviating the motor symptoms of PD.
1. Introduction
Parkinson’s disease (PD) is a chronic neurodegenerative disorder characterized by primary motor symptoms such as resting tremor, rigidity, bradykinesia and postural instability. The major pathophysiological feature of PD is the progressive degeneration of dopaminergic neurons of the substantia nigra pars compacta (SNc). This loss of dopaminergic modulation of the striatum alters the control of motor activity by the basal ganglia-thalamo-cortical loop, resulting in the parkinsonian motor symptoms (DeLong and Wichmann, 2007). Current therapeutic strategies to manage and control motor symptoms in PD involve increasing dopaminergic neurotransmission, either by administration of the dopamine precursor L-DOPA or dopamine receptor agonists. Unfortunately, the efficacy of these therapeutic strategies decreases over time in many patients. In addition, long-term L-DOPA therapy is associated with a high incidence of disabling side effects such as dyskinesias and motor fluctuations (Prashanth et al., 2011). As a result, much attention has been focused on identifying novel therapeutic targets for PD and metabotropic glutamate receptors (mGlus) have now been implicated as promising new drug targets for alleviating motor symptoms (Conn et al., 2005).
mGlus are 7 Transmembrane Spanning Receptors (7TMRs) that are activated by glutamate, the major excitatory neurotransmitter of the central nervous system (CNS). Eight subtypes of mGlus have been identified to date, and are divided into three groups according to their sequence homology, ligand pharmacology, and downstream signaling pathways (Conn and Pin, 1997). Group I mGlus (mGlu1 and -5) signal through Gq/11 to increase phosphoinositide hydrolysis and mobilize intracellular calcium stores. Group II (mGlu2 and -3) and group III (mGlu4, -6, -7, and -8) mGlus couple to Gi/o G proteins and are often located presynaptically, where they modulate neurotransmitter release. The recent availability of selective pharmacological tools for mGlus has led to the identification of specific receptor subtypes as potential therapeutic targets for CNS disorders such as PD.
Several recent studies using group III mGlu-selective agonists, such as L-AP4 and ACPT-I, have demonstrated that these compounds have antiparkinsonian effects in rodent PD models, including reserpine-induced akinesia, haloperidol-induced catalepsy, and 6-hydroxydopamine lesion-induced motor deficits (Konieczny et al., 2007; Lopez et al., 2012; Lopez et al., 2007; Lopez et al., 2008; MacInnes et al., 2004; Sibille et al., 2007; Valenti et al., 2003). To determine the specific mGlu subtypes that mediate the antiparkinsonian effects of group III mGlu agonists, recent studies have taken advantage of subtype-selective agonists and positive allosteric modulators (PAMs) of mGlu4 (East et al., 2010; Goudet et al., 2012; Jones et al., 2011a; Jones et al., 2011b; Marino et al., 2003; Niswender et al., 2008b). In rats, these compounds reverse reserpine-induced akinesia, haloperidol-induced catalepsy, and motor deficits caused by unilateral 6-hydroxydopamine lesion, suggesting that mGlu4 activation is at least partially responsible for the antiparkinsonian effects of group III mGlu agonists in PD animal models. However, the potential contribution of other group III mGlu subtypes has not been fully elucidated. We now report that the mGlu8 agonist (S)-3,4-dicarboxyphenylglycine (DCPG) (Thomas et al., 2001) has behavioral effects predictive of antiparkinsonian actions in several rodent models of PD when administered via an intracerebroventricular route of administration. Interestingly, reversal of akinetic motor deficits by DCPG requires a prolonged state of dopamine depletion or dopamine receptor blockade, suggesting that mGlu8 function in the basal ganglia may differ in the intact versus dopamine-depleted states. These findings suggest that mGlu8 activation may partially mediate the anti-akinetic effects of group III mGlu agonists in prolonged dopamine depletion models.
2. Materials and Methods
2.1 Animals
Two hundred seventy-one third ventricle-cannulated (TVC) male Sprague-Dawley rats weighing 250 to 300 grams were purchased from Taconic Farms, Inc. (Hudson, NY). Cannula placement (AP= −0.8 mm, ML = 0.0 mm and DV= −8.0 mm, relative to bregma) allowed infusion of non-brain penetrant drugs into the third ventricle. Cannula placement was visually verified following sacrifice for all animals used in these studies. Animals that underwent forelimb asymmetry testing were lesioned by unilateral injection of 6-hydroxydopamine (6-OHDA) into the medial forebrain bundle prior to TVC surgery. 6-OHDA lesions were functionally verified using an apomorphine-induced rotation test (performed by Taconic Farms, Inc.). On day 21 post-lesion, apomorphine (0.05 mg/kg, sc) was administered and rotations contralateral to the lesion were measured in a rotometer for 6 consecutive 5 minute periods (30 minutes total). Only animals with greater than 180 rotations in 30 minutes or multiple 5 minute periods of more than 6 rotations per minute were used for the forelimb asymmetry study. For studies that did not require intracerebroventricular (icv) drug administration, thirty-eight male Sprague-Dawley rats (250 to 300 grams) that had not undergone TVC surgery were used (Harlan, Indianapolis, IN). Animals were maintained in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care under a 12 hour light/dark cycle (lights on 06:00 to 18:00) with free access to food and water. All experiments were performed during the light cycle, were approved by Vanderbilt University’s Institutional Animal Care and Use Committee, and conformed to guidelines established by the National Research Council Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and the number of animals used.
2.2 Drugs
(S)-3,4-DCPG and L-AP4 were purchased from Tocris Bioscience (Ellisville, MO). Haloperidol (free base) and reserpine were purchased from Sigma-Aldrich (St. Louis, MO). The decanoate salt of haloperidol was synthesized in-house. DCPG and L-AP4 were prepared in artificial cerebrospinal fluid (ACSF) and administered icv at a rate of 0.5 to 1 µl/min in the indicated volume. Injection cannulae were left in place for an additional five minutes after completion of infusion. Haloperidol (1.5 mg/kg) was dissolved in 0.2% lactic acid, and pH was adjusted to ~6.5 with 1N NaOH. Haloperidol was administered intraperitoneally (ip) in a volume of 1 ml/kg. Reserpine (5 mg/kg, dissolved in 1% acetic acid) was prepared as described previously (Valenti et al., 2003), and administered subcutaneously (sc) in a volume of 1 ml/kg under light isoflurane anesthesia. Haloperidol decanoate (50–200 mg/kg) was dissolved in sesame oil using a mortar and pestle, and was administered intramuscularly (im) in a volume of 2 ml/kg; half of the dose was given in each femoral muscle. All drugs were prepared fresh on the day of the experiment.
2.3 Induction and measurement of catalepsy
For acute catalepsy studies, haloperidol (1.5 mg/kg, ip) was administered two hours prior to baseline catalepsy measurement. For prolonged haloperidol-induced catalepsy studies, three doses of haloperidol (1.5 mg/kg, ip) were evenly spaced over 18–20 hours prior to baseline catalepsy measurement. For haloperidol decanoate-induced catalepsy studies, animals were pretreated for the indicated time prior to measurement of catalepsy. Animals were returned to their home cages during the haloperidol pretreatment period. Catalepsy was assessed by placing each rat’s forepaws on a horizontal bar positioned 6 cm above the testing surface and measuring the latency for the rat to remove one forepaw. Trials were ended after 60 seconds if no forepaw had been removed, and a score of 60 seconds was recorded for that trial. For reversal studies, rats were then given an icv infusion of either DCPG (2.5 or 10 nmol), L-AP4 (50 nmol or 100 nmol), or vehicle (ACSF). Rats were retested for catalepsy either five minutes after completion of drug infusion (haloperidol studies) or 10, 20, and 30 minutes after completion of infusion (haloperidol decanoate studies).
2.4 Induction and measurement of akinesia
Rats were treated with reserpine (5 mg/kg, sc) and returned to their home cages for either 2 hours (acute treatment) or 18–20 hours (prolonged treatment) prior to measurement of baseline akinesia. Locomotor activity was measured for 30 minutes by placing rats in photocell activity cages (Hamilton-Kinder, Poway, CA) equipped with 16×16 infrared beams. Akinetic rats then received an icv infusion of DCPG (2.5, 10, or 30 nmol), L-AP4 (50 nmol), or vehicle (ACSF), and activity was measured for an additional 30 minutes. For haloperidol decanoate characterization, locomotor activity was measured for 30 minutes at the indicated times after haloperidol decanoate administration following baseline catalepsy measurement as described above.
2.5 Measurement of striatal dopamine levels
Rats were treated with reserpine (5 mg/kg, sc) or vehicle for either 2 hours (acute treatment) or 18–20 hours (prolonged treatment) prior to sacrifice. Brains were removed rapidly and placed into ice-cold 0.9% NaCl solution. Tissue samples of caudate putamen (CPu) were taken bilaterally, immediately weighed, and stored on dry ice. Samples were analyzed for striatal monoamine content using high performance liquid chromatography (HPLC) with electrochemical detection (Hackler et al., 2006). Briefly, samples were homogenized in 0.1M trichloroacetic acid containing 10 mM sodium acetate, 100 µM EDTA, and 10.5% methanol (pH 3.8). Homogenized samples were centrifuged at 10,000 × g for 20 minutes, and the supernatant was removed and stored at −80°C prior to measurement of dopamine content. Total protein concentration was determined by assaying the pellet using the BCA Protein Assay Kit (Pierce Chemical Company, Rockford, IL).
2.6 Estimation of D2 receptor occupancy with PET imaging
D2 dopamine receptor occupancy following haloperidol administration was measured using positron emission tomography (PET) as described previously (Jones et al., 2008; Tantawy et al., 2009). One week prior to the first day of testing, rats underwent surgery to install catheters in the jugular vein for radiotracer administration. On the first testing day, vehicle (0.2% lactic acid, ip) was administered two hours prior to injection of ~13 MBq/0.2 mL [18F]fallypride [(S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3’-[18F]fluoropropyl)-2,3-dimethoxybenzamide]. Rats were then returned to their home cages with free access to food and water. Sixty minutes after [18F]fallypride injection, rats were anesthetized using isoflurane (~1.5%), placed in the microPET Focus 220 (Siemens, Knoxville, TN) and a 60 min dynamic acquisition was initiated. This delayed scan method was used in order to minimize the effects of anesthesia on binding potential estimates as previously described (Tantawy et al., 2011). One week later, the same rats were injected with haloperidol (free base, 1.5 mg/kg, ip) two hours prior to intravenous injection of [18F]fallypride. After 60 minutes, rats were anesthetized with ~1.5% isoflurane, and images were acquired using the same protocol as in the control session. Receptor occupancy for haloperidol decanoate was measured similarly, except that vehicle (sesame oil) or haloperidol decanoate (100 mg/kg, im) was administered 20 hours prior to radiotracer injection. Images were reconstructed after correcting for scatter and attenuation as described previously (Tantawy et al., 2009). Briefly, attenuation maps were created from a transmission image obtained using a 57Co source. Data were reconstructed on a 128 × 128 × 95 grid with a pixel size of 0.095 cm and a slice thickness of 0.080 cm. Dynamic images were reconstructed using an OSEM2D algorithm with a sequence of five 60s frames (5 × 60 s), 2 × 300 s, 2 × 600 s, 2 × 1200 s, 1 × 600 s, 6 × 300 s, 2 × 600 s, and 3 × 1200 s.
2.7 Measurement of 6-OHDA-induced forelimb use asymmetry
Rats with unilateral 6-OHDA lesion of the medial forebrain bundle, whose dopamine depletion status was functionally validated by the ability of apomorphine to induce contralateral rotation (see above) were used to assess the ability of DCPG and L-AP4 to reverse lesion-induced forelimb use deficits (Schallert et al., 2000; Valenti et al., 2003). To this end, 6-OHDA-lesioned rats with TVC surgery were used to allow for the icv administration of DCPG and L-AP4. Rats were handled daily prior to testing. On the test day, rats were placed in a transparent plastic cylinder (20 cm in diameter and 30 cm high). Animals were not habituated to the cylinder prior to the first test session. Baseline limb-use asymmetry was measured over a 10 minute period, after which rats were infused with either L-AP4 (100 nmol/2.5 µl) or DCPG (2.5 or 10 nmol/2.5 µl). Five minutes after the end of the infusion, limb-use behavior was measured for an additional 10 minute period. Experimental sessions were monitored with a camera located beneath the cylinder for off-line analysis of limb-use. Video analysis of forelimb use was performed by two raters who were blinded to the treatment groups. Forelimb use was scored as described previously and data were expressed as Forelimb Asymmetry (FLA) Index Score (Jones et al., 2011a; Lundblad et al., 2004). The FLA Index Score is a composite of the number of Wall contacts of the unaffected (Wunaffected) and affected (Wimpaired) forelimb and Landings of the unaffected (Lunaffected) and affected (Limpaired) forelimb (see section 2.8, Data analysis and statistics).
To verify the extent of 6-OHDA lesion-induced dopamine depletion a randomly selected cohort of rats (n = 24) was sacrificed and striatal tissue was dissected from the ipsilateral (lesioned) and contralateral (intact) dorsal striatum. Samples were frozen on dry ice and tissue concentrations of dopamine and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) were determined by HPLC with electrochemical detection as described above (2.5 Measurement of striatal dopamine levels). Correct placement of the guide cannula targeting the third ventricle was verified in all animals used for this study.
2.8 Data analysis and statistics
All calculations associated with data analysis were performed using Microsoft Excel. Statistical tests were performed using JMP IN 5.1 or StatView. Graphs were created using SigmaPlot 9.0. Catalepsy and akinesia results are summarized as percent change between baseline and post-drug measurements. For most behavioral experiments, data were analyzed for a main effect of drug treatment using whole model one-way analysis of variance (ANOVA). When a statistically significant effect was found, post hoc analysis was performed using a Dunnett’s test to compare drug treatment groups with the vehicle group. Data are expressed as mean ± SEM. For behavioral characterization of haloperidol decanoate effects, data were analyzed using repeated measures ANOVA followed by pairwise comparisons using a Bonferroni correction. Lesion-induced changes in transmitter concentrations and reversal of haloperidol effects by DCPG were analyzed using an unpaired Student’s t test. For D2 receptor occupancy studies, the microPET images were coregistered to a rat brain template (Rubins et al., 2003) using AMIDE (Loening and Gambhir, 2003) and volumetric regions of interest (ROIs) were drawn around the striata and the cerebellum generating time-activity curves (TACs) over the duration of the scans. Binding potentials (BPs) were estimated via a Logan plot (Logan, 2000). D2 receptor occupancy was calculated as [1 − (Drug BP/Vehicle BP)] × 100. Forelimb asymmetry data were analyzed as described previously (Valenti et al., 2003). To assess forelimb use in 6-OHDA-lesioned rats the FLA Index Score was calculated as follows (Jones et al., 2011a):
Using this method, rats showing preferential use of the unaffected forelimb (ipsilateral to the lesion) have negative FLA Index Scores, whereas rats that do not show a forelimb bias have FLA Index Scores that are close to zero. Statistical comparison of drug effects on forelimb asymmetry was performed using two-way ANOVA followed by Bonferroni comparisons.
3. Results
3.1 In contrast to the group III mGlu agonist L-AP4, the selective mGlu8 agonist DCPG does not reverse acute reserpine-induced akinesia and haloperidol-induced catalepsy
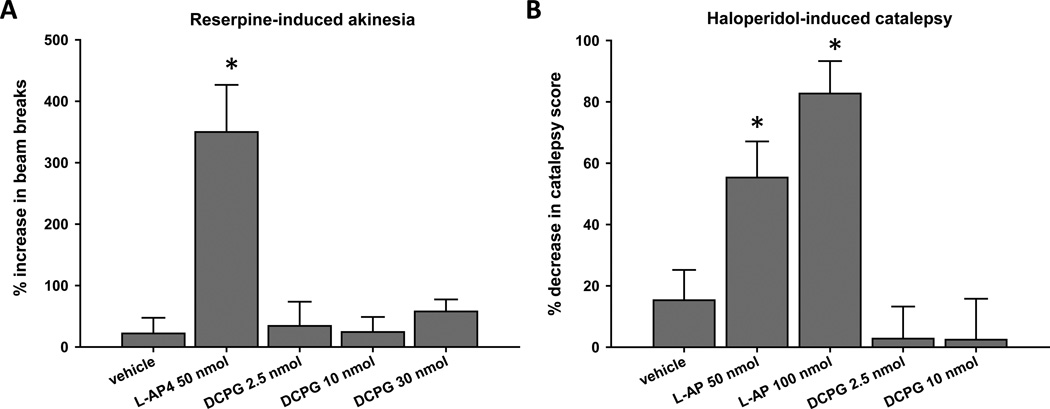
Reserpine-induced akinesia and haloperidol-induced catalepsy are preclinical models of PD that are commonly used in rodents. For example, antiparkinsonian drugs such as L-DOPA increase locomotor activity in reserpine-treated rats (Colpaert, 1987), indicating that reversal of akinesia by pharmacological agents is consistent with antiparkinsonian activity. The nonselective group III mGlu agonist L-AP4 (50 nmol, icv) and the mGlu8-selective agonist DCPG (2.5, 10, or 30 nmol, icv) were evaluated for their ability to reverse the akinesia induced by a two hour pretreatment with reserpine (5 mg/kg, sc; Fig. 1A). In agreement with previous findings (Valenti et al., 2003), L-AP4 significantly increased locomotor activity in rats acutely treated with reserpine (P < 0.05, Dunnett’s comparison with vehicle group; n = 10 animals per treatment group). Conversely, DCPG did not increase locomotor activity at any dose tested (Fig. 1A).
Figure 1. DCPG does not reverse acute reserpine-induced akinesia or haloperidolinduced catalepsy.
Animals were pretreated with reserpine (5 mg/kg, sc) for 2 hours prior to baseline locomotor activity measurement for 30 minutes (A), or with haloperidol (1.5 mg/kg, ip) for 2 hours prior to baseline catalepsy measurement (B). After baseline measurements, animals received an icv infusion of L-AP4 (50 or 100 nmol/2.5 µl), DCPG (2.5, 10, or 30 nmol/2.5 µl) or vehicle (ACSF). Five minutes after completion of infusion, locomotor activity (A) or catalepsy (B) was measured again, and the percent change from baseline was calculated. Data are shown as mean ± SEM. Results were obtained from ten animals per treatment group. *P < 0.05, Dunnett’s comparison with vehicle group.
In addition to studies in the reserpine model, we evaluated the ability of L-AP4 (50 or 100 nmol) and DCPG (2.5 or 10 nmol) to reverse the catalepsy induced by a two hour pretreatment with haloperidol (1.5 mg/kg, ip). Similar to previously reported results (Valenti et al., 2003), L-AP4 significantly reversed haloperidol-induced catalepsy at both doses (P < 0.05, Dunnett’s comparison with vehicle group; n = 10 animals per treatment group). In contrast, DCPG had no effect on catalepsy induced by a two hour pretreatment with haloperidol (Fig. 1B).
3.2 In contrast to acute models of dopamine depletion or dopamine receptor blockade, DCPG reverses effects of prolonged reserpine-induced akinesia and haloperidol-induced catalepsy
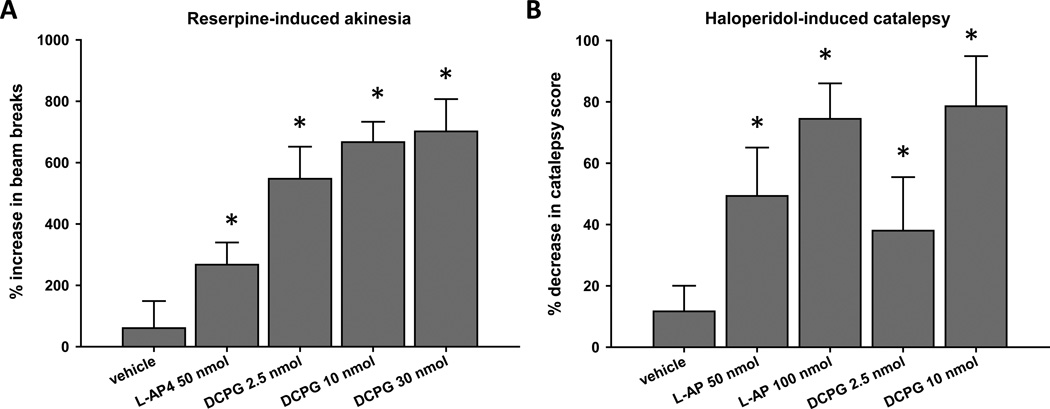
Because a more prolonged depletion of dopamine by reserpine or blockade of D2-like dopamine receptors by haloperidol has been shown to induce changes in mGlu function (Marino et al., 2002; Poisik et al., 2007), we also assessed the ability of L-AP4 (50 nmol, icv) and DCPG (2.5, 10, or 30 nmol, icv) to reverse the akinesia induced by an 18–20 hour pretreatment with reserpine (5 mg/kg, sc; Fig. 2A). In contrast to the effects of DCPG following acute reserpine treatment, all tested doses of DCPG robustly increased locomotor activity in rats after more prolonged reserpine treatment (P < 0.05, Dunnett’s comparison with vehicle group; n = 10 animals per treatment group). L-AP4 also significantly increased locomotor activity in rats pretreated with reserpine for 18–20 hours, and the magnitude of this effect was similar to that observed after acute reserpine treatment (Fig. 2A; compare to Fig. 1A).
Figure 2. DCPG reverses prolonged reserpine-induced akinesia and haloperidol-induced catalepsy.
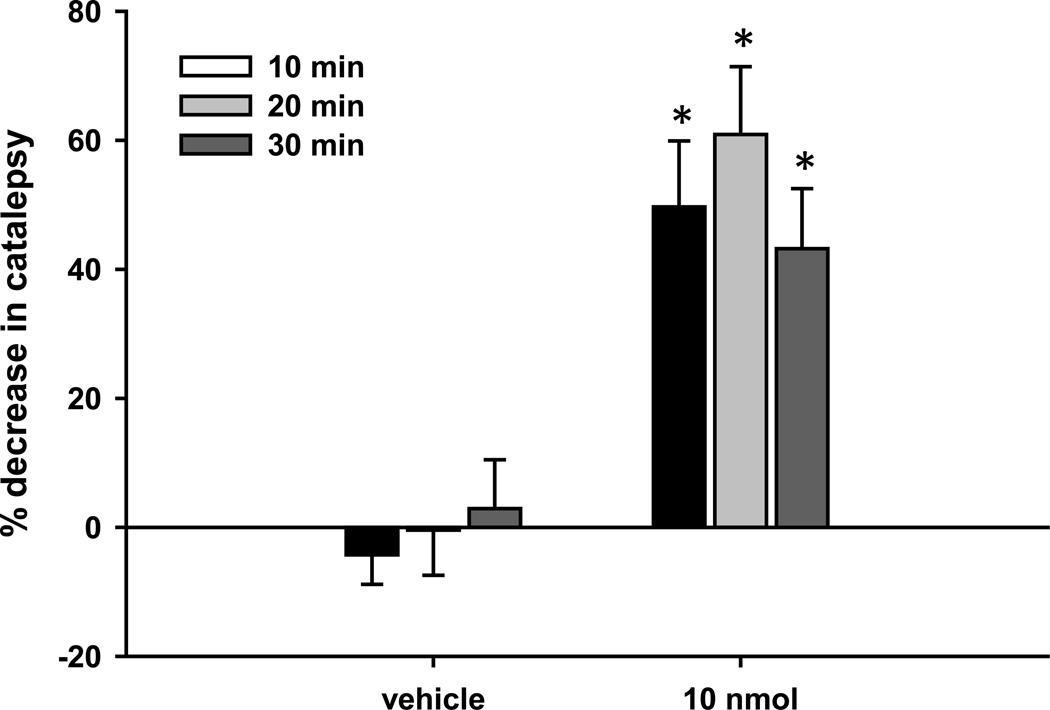
Animals were pretreated with reserpine (5 mg/kg, sc) for 18–20 hours prior to baseline locomotor activity measurement for 30 minutes (A), or with three injections of haloperidol (1.5 mg/kg, ip) evenly spaced over 18–20 hours prior to baseline catalepsy measurement (B). After baseline measurements, animals received an icv infusion of L-AP4 (50 or 100 nmol/2.5 µl), DCPG (2.5, 10, or 30 nmol/2.5 µl) or vehicle (ACSF, 2.5 µl). Five minutes after completion of infusion, locomotor activity (A) or catalepsy (B) was measured again, and the percent change from baseline measurement was calculated. Data are shown as mean ± SEM. Results were obtained from ten animals per treatment group. *P < 0.05, Dunnett’s comparison with vehicle group.
Whereas a single dose of reserpine induces prolonged akinesia, the catalepsy produced by a single injection of haloperidol (1.5 mg/kg, ip) is no longer present 18–20 hours after administration. In order to produce a more prolonged dopamine receptor blockade, rats received three injections of haloperidol (1.5 mg/kg, ip) evenly spaced over 18–20 hours prior to baseline measurement of catalepsy. We then tested the ability of L-AP4 (50 or 100 nmol) and DCPG (2.5 or 10 nmol) to reverse the catalepsy induced by repeated haloperidol administration. In contrast to the lack of an effect of DCPG after acute haloperidol administration, catalepsy induced by repeated haloperidol administration was significantly reduced by 2.5 and 10 nmol DCPG (Fig. 2B; P < 0.05, Dunnett’s comparison with vehicle group; n = 10 animals per treatment group). In addition, both doses of L-AP4 significantly reduced the catalepsy induced by this haloperidol dosing schedule. Again, the reversal of catalepsy by L-AP4 was similar after both acute and prolonged haloperidol administration (Figs. 1B and 2B).
3.3 Striatal dopamine is reduced to a similar level after both acute and prolonged reserpine treatment
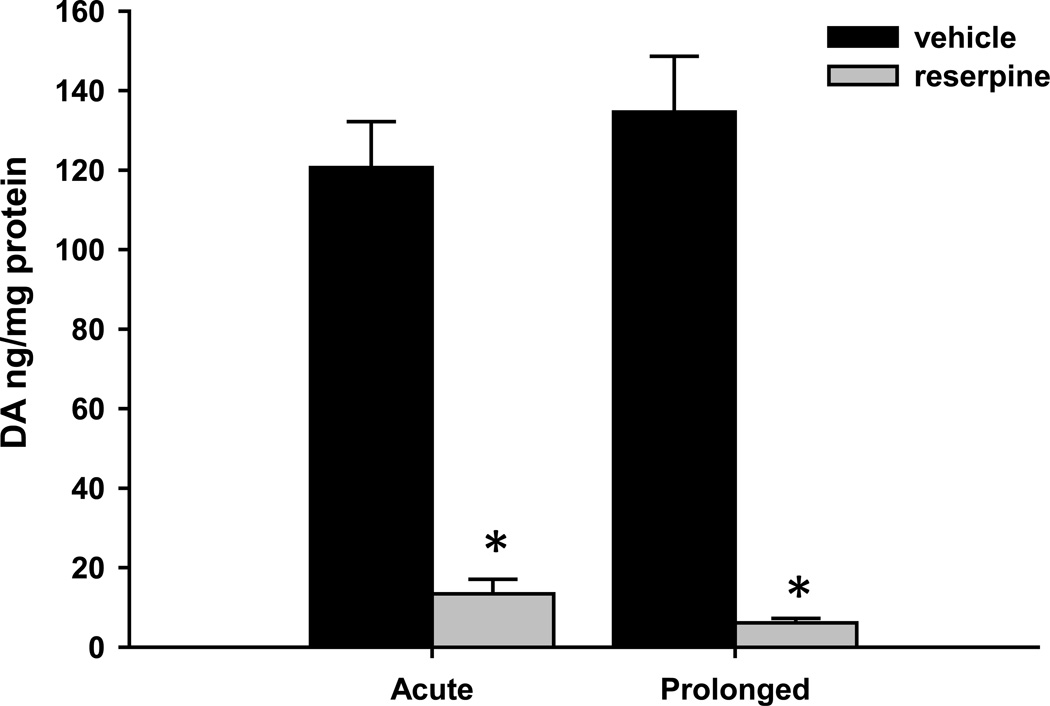
In order to ensure that our acute and prolonged reserpine treatments produced similar levels of dopamine depletion, we determined tissue dopamine levels in the striatum two hours and 18–20 hours after treatment with vehicle or reserpine (5 mg/kg, sc) using HPLC. Both acute and prolonged reserpine treatments caused a significant reduction in striatal dopamine levels (Fig. 3). Striatal dopamine content was reduced to 11.1% of control levels after two hour reserpine treatment (120 ± 11.6 ng/mg protein for vehicle-treated animals vs. 13.4 ± 3.7 ng/mg protein for reserpine-treated animals; mean ± SEM, n = 6 animals per treatment group; t10 = 8.829, P < 0.0001). After 18–20 hour reserpine treatment, striatal dopamine content was reduced to 4.5% of control levels (134.6 ± 13.1 ng/mg protein for vehicle-treated animals vs. 6.1 ± 1.1 ng/mg protein for reserpine-treated animals; mean ± SEM, n = 6 animals per treatment group; t10 = 9.113, P < 0.0001).
Figure 3. Striatal dopamine levels are significantly reduced by both acute and prolonged reserpine treatments.
Rats were treated with reserpine (5 mg/kg, sc) or vehicle (1% acetic acid) for 2 hours (acute) or 20 hours (prolonged) prior to sacrifice. Dopamine (DA) levels from micropunches of the CPu were measured by HPLC and normalized to the total amount of protein in each sample. Normalized DA levels were reduced to 11.1% of control levels after acute reserpine treatment and 4.5% of control levels after prolonged reserpine treatment. Data were obtained from six animals per treatment group, and are shown as mean ± SEM. *P < 0.0001, unpaired t test.
3.4 Haloperidol decanoate characterization
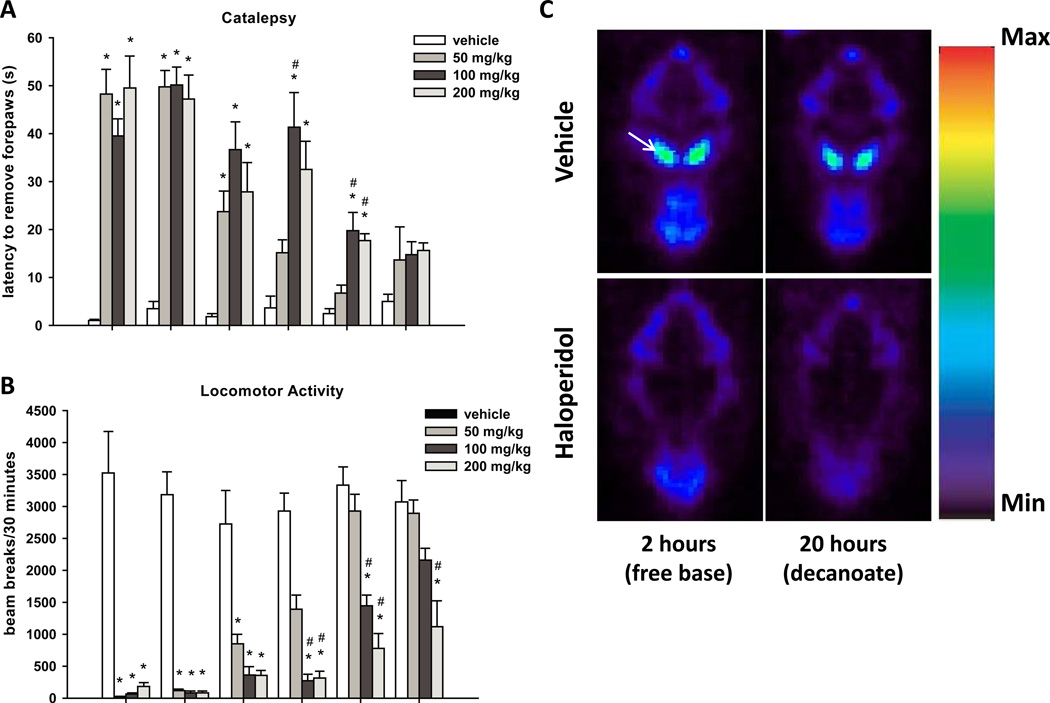
Because the cataleptic behavior induced by 1.5 mg/kg haloperidol administered intraperitoneally decreases after 6–12 hours (data not shown), several doses of haloperidol were required to maintain catalepsy 18–20 hours after the first dose. In order to achieve a more prolonged effect with a single dose of haloperidol, we administered the decanoate salt of haloperidol intramuscularly (50 to 200 mg/kg). This treatment paradigm induced prolonged catalepsy and akinesia (Figs. 4A and 4B, respectively). For catalepsy results (Fig. 4A), repeated measures ANOVA revealed a significant effect of drug treatment (F(3, 24) = 29.8, P < 0.0001; n = 6–8 animals per treatment group), a significant effect of time (F(5, 120) = 30.8, P < 0.0001) and a significant interaction between dose and time (F(15, 120) = 5.7, P < 0.0001). For haloperidol decanoate-induced akinesia results (Fig 4B), repeated measures ANOVA revealed a significant effect of drug treatment (F(3, 24) = 60.0, P < 0.0001), a significant effect of time (F(5, 120) = 37.7, P < 0.0001) and a significant interaction between drug treatment and time (F(15, 120) = 8.8, P < 0.0001).
Figure 4. A single dose of haloperidol decanoate induces prolonged catalepsy, akinesia, and D2 dopamine receptor occupancy.
Animals were treated with haloperidol decanoate (50, 100, or 200 mg/kg, im) or vehicle (sesame oil), and catalepsy (A) and locomotor activity (B) were measured at the indicated times. For both catalepsy and locomotor activity, repeated measures ANOVA revealed a significant effect of dose and time, as well as a significant interaction between dose and time. Catalepsy and locomotor activity measurements were obtained from six to eight rats per treatment group. For haloperidol decanoate-induced akinesia results (Fig 4B), repeated measures ANOVA revealed a significant effect of drug treatment (F(3, 24) = 60.0), a significant effect of time (F(5, 120) = 37.7) and a significant interaction between drug treatment and time (F(15, 120) = 8.8). Data are shown as mean ± SEM. *P < 0.0083 vs. vehicle group, pairwise comparison using Bonferroni correction. #P < 0.0083 vs. 50 mg/kg group, pairwise comparison using Bonferroni correction. Images showing [18F]fallypride binding in the striatum were obtained using microPET (C). The striatum is indicated by an arrow in the top left panel. Images were captured following two hour treatment with 0.2% lactic acid (vehicle, top left panel) or 1.5 mg/kg haloperidol (free base, ip, bottom left panel), or 20 hour pretreatment with sesame oil (vehicle, top right panel) or 100 mg/kg haloperidol decanoate (im, bottom right panel). Images represent single horizontal slices (0.08 cm thick) from a representative animal summed over the duration of the sixty minute scan. Scans were performed on four rats for each treatment group.
Previous studies in rats have demonstrated that greater than 80% of D2 receptors must be occupied by haloperidol to produce robust catalepsy (Natesan et al., 2006; Wadenberg et al., 2001). In our studies, rats were cataleptic for up to five days after haloperidol decanoate administration, suggesting high levels of D2 receptor occupancy. In order to confirm this, we determined the in vivo D2 dopamine receptor occupancy 20 hours after 100 mg/kg haloperidol decanoate administration using microPET. The high affinity D2 receptor antagonist [18F]fallypride was used as a PET tracer to evaluate D2 receptor occupancy by haloperidol. In agreement with our prediction, 20 hour haloperidol decanoate treatment resulted in 97.9% D2 receptor occupancy (Fig. 4C, right panels, and Table 1; n = 4). This level of D2 receptor occupancy was comparable to that observed after 2 hour pretreatment with 1.5 mg/kg haloperidol (free base, 97.3%), which produced a level of catalepsy 2–4 hours post-administration similar to that observed 20 hours after haloperidol decanoate administration (Fig. 4C, left panels, and Table 1; n = 4).
Table 1.
D2 receptor occupancy following two hour haloperidol or twenty hour haloperidol decanoate treatment.
| Drug treatment | Vehicle BP1 | Drug BP | D2 receptor occupancy2 |
|---|---|---|---|
| Haloperidol (free base) | 18.4 ± 1.8 | 0.5 ± 0.2 | 97.3% |
| Haloperidol decanoate | 19.1 ± 0.9 | 0.4 ± 0.2 | 97.9% |
Binding potential (BP) was calculated as Logan DVR – 1.
D2 dopamine receptor occupancy was calculated as [1 − (Drug BP/Vehicle BP)] × 100.
3.5 DCPG reverses haloperidol decanoate-induced catalepsy
After characterization of time course of behavioral effects induced by haloperidol decanoate, we assessed the ability of DCPG (10 nmol, icv) to reverse the catalepsy induced by 20 hour treatment with haloperidol decanoate (100 mg/kg, im). DCPG significantly reversed catalepsy when measured ten minutes after completion of infusion (Fig. 5; t16 = 5.157, P < 0.05, unpaired t test; n = 10 animals for vehicle group and 8 animals for DCPG group). Significant reversal of catalepsy was also observed 20 and 30 minutes after completion of DCPG infusion.
Figure 5. DCPG reverses prolonged haloperidol decanoate-induced catalepsy.
Animals were pretreated with haloperidol decanoate (100 mg/kg, im) 20 hours prior to baseline catalepsy measurement. After baseline measurements, animals received an icv infusion of DCPG (10 nmol/5 µl) or vehicle (ACSF, 5 µl). Ten, twenty, and thirty minutes after completion of infusion, catalepsy was measured again, and the percent change from baseline measurement was calculated. Data are shown as mean ± SEM. Results were obtained from eight to ten animals per treatment group. *P < 0.05, unpaired t test.
3.6 L-AP4 and DCPG decrease forelimb use asymmetry following 6-OHDA lesion
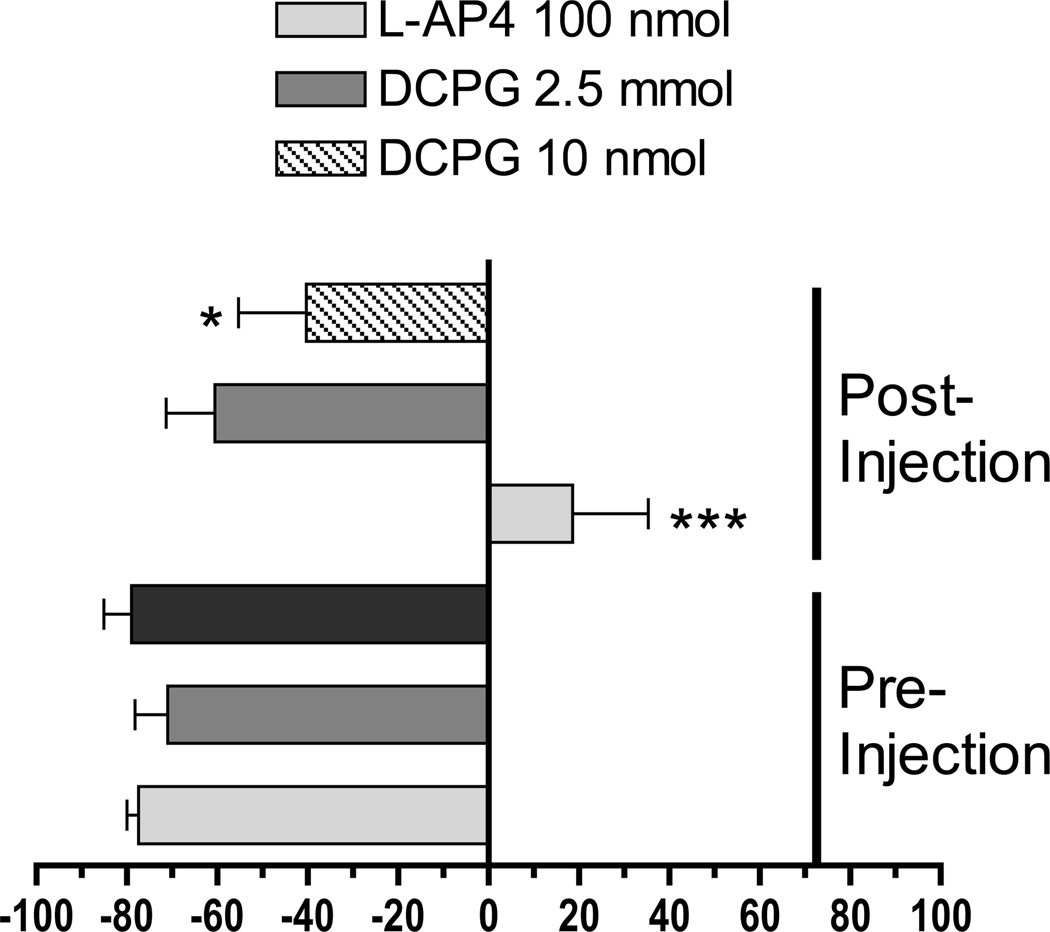
Unilateral 6-OHDA lesion represents an additional rat model of prolonged dopamine depletion. This model differs from reserpine-induced akinesia and haloperidol-induced catalepsy because it involves degeneration of the dopaminergic neurons of the SNc rather than pharmacological manipulation of intact dopamine systems. Rats with an extensive unilateral lesion of the SNc favor use of the forepaw that is ipsilateral to the site of the lesion when supporting their weight during a wall contact following a rearing event or after landing from a rearing event. Drugs that effectively alleviate PD symptoms in humans, such as L-DOPA and dopamine receptor agonists, reduce forelimb-use asymmetry caused by unilateral 6-OHDA lesion (Lundblad et al., 2002). We therefore tested the ability of L-AP4 (100 nmol, icv) and DCPG (2.5 or 10 nmol, icv) to reduce forelimb use asymmetry in unilaterally 6-OHDA-lesioned animals. Two-way ANOVA revealed significant effects of Treatment (pre vs. post injection [F1,62 = 29.78]), Drug (F2,62 = 6.43) and a significant Treatment X Drug interaction (F2,62 = 8.07). As shown in Figure 6, L-AP4 [t = 5.90, p < 0.001, n = 10] and the high dose of DCPG (10 nmol [t = 2.60, p < 0.05, n = 12]), but not the low dose of DCPG (2.5 nmol [t = 0.70, p > 0.05, n = 12] caused a significant reversal of the FLA Index Score. Tissue neurochemistry revealed that, compared to the unaffected hemisphere, 6-OHDA-lesioned rats exhibited a greater than 95% loss of striatal dopamine and the dopamine metabolites DOPAC and HVA in the lesioned hemisphere (Table 2).
Figure 6. DCPG reduces forelimb use asymmetry in rats with unilateral 6-OHDA lesion.
Animals were evaluated for forelimb use asymmetry for 10 minutes prior to icv infusion of L-AP4 (100 nmol) or DCPG (2.5 or 10 nmol). Five minutes after completion of drug administration, forelimb use asymmetry was evaluated for an additional 10 minute period. The FLA Index Scores were calculated as described in the Methods. A negative FLA Index Score signifies preferential use of the unaffected forelimb, whereas FLA Index Scores close to zero indicate the absence of a forelimb use preference. Drug treatment resulted in significant reversal of forelimb asymmetry in rats treated with L-AP4 or 10 nmol DCPG. * P < 0.05, *** P < 0.001 vs. pretreatment (two-way ANOVA followed by Bonferroni comparison).
Table 2.
Concentrations of dopamine and its metabolites in the dorsal striatum of unilaterally 6-OHDA-lesioned rats.
| Treatment | Dopamine | DOPAC | HVA |
|---|---|---|---|
| (ng/mg protein) | |||
| 6-OHDA (n = 24) | |||
| contralateral | 66.31 ± 13.10 | 18.10 ± 1.28 | 13.59 ± 1.51 |
| ipsilateral | 0.37 ± 0.061*** | 0.27 ± 0.04*** | 0.54 ± 0.08*** |
| % remaining | 0.56 | 1.49 | 3.97 |
P < 0.001 vs. contralateral concentrations (Student’s t test).
4. Discussion
Metabotropic glutamate receptors have recently been identified as promising new therapeutic targets for alleviating the primary motor symptoms of PD (Johnson et al., 2009). Recent studies have demonstrated that central or systemic administration of group III mGlu-selective agonists elicits behavioral effects consistent with antiparkinsonian actions in both acute and prolonged animal models of PD. For example, icv administration of L-AP4 reverses reserpine-induced akinesia and haloperidol-induced catalepsy after a short (1.5–2 hour) pretreatment with reserpine or haloperidol (Valenti et al., 2003). Similarly, icv administration of L-AP4 or the group III mGlu agonist L-SOP has been shown to alleviate akinesia following a longer (18 hour) pretreatment with reserpine (MacInnes et al., 2004), demonstrating that group III mGlu activation remains efficacious after a more prolonged state of dopamine depletion. In addition, icv or systemic administration of L-AP4 and ACPT-I, respectively, can reverse motor deficits caused by 6-OHDA lesion, demonstrating that group III mGlu activation is also efficacious in this model of chronic dopamine depletion (Lopez et al., 2012; Lopez et al., 2007; Lopez et al., 2008; Valenti et al., 2003). Previous efforts to identify the group III mGlu subtypes that mediate the antiparkinsonian actions of group III mGlu-selective agonists have demonstrated that mGlu4 activation is likely to play a major role (Beurrier et al., 2009; Broadstock et al., 2011; East et al., 2010; Goudet et al., 2012; Jones et al., 2011a; Jones et al., 2011b; Marino et al., 2003; Niswender et al., 2008b). However, expression of mGlu7 and mGlu8 has also been detected in the basal ganglia (Bradley et al., 1999; Messenger et al., 2002), indicating that these receptor subtypes may also contribute to the antiparkinsonian actions of group III mGlu agonists.
In the present study, we used the mGlu8-selective agonist (S)-3,4-DCPG to explore the role of mGlu8 activation in mediating the antiparkinsonian effects of group III mGlu agonists in both acute and prolonged models of dopamine depletion. The only previous study to explore potential antiparkinsonian effects of mGlu8 activation in an acute model of PD evaluated the effects of direct infusion of DCPG into the globus pallidus (GP) and substantia nigra pars reticulata (SNr) of rats that had been acutely treated with haloperidol (1.5 hour pretreatment) (Lopez et al., 2007). This study demonstrated that DCPG did not reverse haloperidol-induced catalepsy when infused into either structure, a finding consistent with our observation that DCPG does not reverse the motor deficits induced by acute haloperidol or reserpine administration. Here we report that after prolonged (18–20 hour) treatment with haloperidol or reserpine, icv DCPG robustly reverses PD-like akinetic motor deficits. The differential ability of DCPG to exert antiparkinsonian effects following acute versus prolonged reserpine treatment is unlikely to be due to differences in the extent of dopamine depletion, because both treatments produce a similar reduction of striatal dopamine levels. Similarly, the level of dopamine receptor occupancy produced by 2 hour haloperidol (free base) treatment versus 20 hour haloperidol decanoate treatment is virtually identical, indicating that differences in the extent of dopamine receptor antagonism between these two models cannot account for the differential ability of DCPG to reverse catalepsy. Finally, DCPG alleviates forelimb-use asymmetry in the unilateral 6-OHDA rat model of PD. Taken together, these results indicate that the antiparkinsonian actions of DCPG are only present after prolonged dopamine hypofunction.
Studies in heterologous expression systems have shown that DCPG has 50 to 100-fold selectivity for mGlu8 over mGlu4, and has little or no activity at other mGlu subtypes (Niswender et al., 2008a; Thomas et al., 2001). The ability of DCPG to activate mGlu4 raises the possibility that the antiparkinsonian effects observed in our studies are due to agonist activity at mGlu4 rather than mGlu8. However, selective positive allosteric modulators of mGlu4 and mGlu4-preferring agonists reverse akinetic deficits induced by acute (one to two hour) pretreatment with haloperidol or reserpine, suggesting that after a short period of dopamine receptor blockade or dopamine depletion, enhanced mGlu4 activation can reverse catalepsy and akinesia (Beurrier et al., 2009; Goudet et al., 2012; Marino et al., 2003; Niswender et al., 2008b). Based on these findings, if the doses of DCPG used in our studies were sufficient to activate mGlu4, we would predict that DCPG would also reverse catalepsy and akinesia induced by two hour haloperidol or reserpine treatment. In contrast, we have found that the doses of DCPG used in our study did not produce any reversal of akinetic behaviors in those acute experimental paradigms, suggesting that the doses of DCPG that used here are not sufficient to activate mGlu4. While we cannot exclude the possibility that other receptors may be involved in mediating the effects of DCPG, the results presented here suggest that DCPG likely mediates antiparkinsonian effects in catalepsy, akinesia, and forelimb use asymmetry models that are due to selective activation of mGlu8.
The differential ability of DCPG to produce antiparkinsonian effects after acute vs. prolonged dopamine depletion or dopamine receptor blockade suggests that prolonged dopamine depletion leads to a change in the function or expression of mGluf8. In situ hybridization studies have detected mGlu8 mRNA in all basal ganglia structures (Messenger et al., 2002). Interestingly, chronic administration of amphetamine increases mGlu8 mRNA levels in the rat striatum (Parelkar and Wang, 2008), suggesting that dysregulation of dopaminergic signaling in the basal ganglia can alter the expression of mGlu8. However, no changes in mGlu8 mRNA levels have been observed following 6-OHDA lesion (Messenger et al., 2002), suggesting that changes in mGlu8 transcription may not underlie the differential effects of DCPG after acute vs. prolonged dopamine depletion. Alternatively, changes in the functional expression of mGlu8 at the protein level, such as increased translation, trafficking to the plasma membrane, or posttranslational modifications, represent possible explanations for this phenomenon.
Although there is a lack of anatomical evidence regarding changes in mGlu8 expression following dopamine depletion, previous studies using direct-site infusions of group III mGlu agonists in prolonged models of dopamine depletion suggest that several basal ganglia structures may be candidates for mediating the antiparkinsonian effects of DCPG. Following 18 hour reserpine treatment, direct infusion of the group III mGlu agonist L-SOP into the rat GP or SNr reverses akinesia (MacInnes et al., 2004), suggesting that the GP and the SNr are possible sites of action for an mGlu8-mediated antiparkinsonian effect. The ability of intrapallidal infusion of group III mGlu agonists to reverse akinetic deficits caused by bilateral 6-OHDA lesion also points to the GP as a possible site of action of DCPG (Lopez et al., 2007). However, other results suggest that these structures are unlikely to be the sites of action for the antiparkinsonian effects of DCPG. For example, a reduction of inhibitory striatopallidal transmission is thought to underlie the antiparkinsonian effects of group III mGlu activation in the GP (Lopez et al., 2007; Valenti et al., 2003), and electrophysiological studies have shown that DCPG does not reduce inhibitory striatopallidal transmission in brain slices obtained from normal or reserpinized rats (Valenti et al., 2003). In agreement with this finding, intrapallidal DCPG administration fails to reverse akinetic deficits following bilateral 6-OHDA (Beurrier et al., 2009). These results suggest that activation of mGlu8 in the GP is unlikely to mediate the antiparkinsonian effects of DCPG. In the SNr, direct infusion of group III mGlu agonists worsens the impaired motor performance of rats with bilateral 6-OHDA lesion (Lopez et al., 2007), so it is not likely that the improvement in motor performance observed after icv administration of DCPG is mediated by activation of mGlu8 receptors in the SNr. Interestingly, a recent study showed that direct infusion of very high doses of DCPG into the SNr reverses motor deficits following overnight reserpine treatment (Broadstock et al., 2011). However, this effect was not readily blocked by pretreatment with a group III mGlu antagonist, supporting the idea that activation of mGlu8 in the SNr may not be responsible for the alleviation of PD-like motor deficits observed following icv administration of DCPG. Alternatively, other basal ganglia structures, such as the striatum, as well as CNS structures outside of the basal ganglia, represent possible sites of action for the antiparkinsonian effects of DCPG.
In conclusion, we have found that the mGlu8 agonist DCPG produces anti-akinetic effects consistent with antiparkinsonian-like actions in prolonged but not acute models of PD. These findings suggest that mGlu8 activation may partially mediate the alleviation of motor deficits by group III mGlu-selective agonists in prolonged models of dopamine depletion and indicate that selective activation of mGlu8, or simultaneous activation of multiple group III mGlus, may represent a novel therapeutic strategy for the treatment of PD. Further studies will be necessary to elucidate the mechanisms underlying the antiparkinsonian effects of DCPG.
Highlights.
The mGlu8 agonist DCPG fails to reverse motor deficits in acute DA depletion models
DCPG reverses akinesia induced by overnight (18–20 hour) reserpine treatment
DCPG also reverses catalepsy induced by overnight haloperidol treatment
Haloperidol decanoate induces prolonged catalepsy and D2 receptor occupancy
DCPG reverses forelimb use asymmetry caused by 6-hydroxydopamine lesion of the SNc
Acknowledgements
This work was supported by NIH grant NS048334 and the Michael J. Fox Foundation. The authors would like to thank Zou Yue and Jordan Fritz for technical assistance, and Dr. Craig Lindsley for providing haloperidol decanoate. Parts of this work have been presented in abstract form (Johnson et al., 2008).
Nonstandard abbreviations
- PD
Parkinson’s disease
- DA
dopamine
- SNc
substantia nigra pars compacta
- mGlu
metabotropic glutamate receptor
- 7TM
seven transmembrane spanning receptor
- CNS
central nervous system
- PAM
positive allosteric modulator
- DCPG
(S)-3,4-dicarboxyphenylglycine
- TVC
third ventricle cannulation
- 6-OHDA
6-hydroxydopamine
- icv
intracerebroventricular
- ACSF
artificial cerebrospinal fluid
- ip
intraperitoneal
- sc
subcutaneous
- im
intramuscular
- CPu
caudate-putamen
- HPLC
high performance liquid chromatography
- PET
positron emission tomography
- FLA
forelimb asymmetry
- DOPAC
3,4-dihydroxyphenylacetic acid
- HVA
homovanillic acid
- SNr
substantia nigra pars reticulata
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beurrier C, Lopez S, Revy D, Selvam C, Goudet C, Lherondel M, Gubellini P, Kerkerian-LeGoff L, Acher F, Pin JP, Amalric M. Electrophysiological and behavioral evidence that modulation of metabotropic glutamate receptor 4 with a new agonist reverses experimental parkinsonism. Faseb J. 2009;23:3619–3628. doi: 10.1096/fj.09-131789. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Standaert DG, Rhodes KJ, Rees HD, Testa CM, Levey AI, Conn PJ. Immunohistochemical localization of subtype 4a metabotropic glutamate receptors in the rat and mouse basal ganglia. J Comp Neurol. 1999;407:33–46. [PubMed] [Google Scholar]
- Broadstock M, Austin P, Betts M, Duty S. Antiparkinsonian potential of targeting group III metabotropic glutamate receptor subtypes in the rodent substantia nigra pars reticulata. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpaert FC. Pharmacological characteristics of tremor, rigidity and hypokinesia induced by reserpine in rat. Neuropharmacology. 1987;26:1431–1440. doi: 10.1016/0028-3908(87)90110-9. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci. 2005;6:787–798. doi: 10.1038/nrn1763. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- East SP, Bamford S, Dietz MG, Eickmeier C, Flegg A, Ferger B, Gemkow MJ, Heilker R, Hengerer B, Kotey A, Loke P, Schanzle G, Schubert HD, Scott J, Whittaker M, Williams M, Zawadzki P, Gerlach K. An orally bioavailable positive allosteric modulator of the mGlu4 receptor with efficacy in an animal model of motor dysfunction. Bioorg Med Chem Lett. 2010;20:4901–4905. doi: 10.1016/j.bmcl.2010.06.078. [DOI] [PubMed] [Google Scholar]
- Goudet C, Vilar B, Courtiol T, Deltheil T, Bessiron T, Brabet I, Oueslati N, Rigault D, Bertrand HO, McLean H, Daniel H, Amalric M, Acher F, Pin JP. A novel selective metabotropic glutamate receptor 4 agonist reveals new possibilities for developing subtype selective ligands with therapeutic potential. Faseb J. 2012 doi: 10.1096/fj.11-195941. [DOI] [PubMed] [Google Scholar]
- Hackler EA, Airey DC, Shannon CC, Sodhi MS, Sanders-Bush E. 5-HT(2C) receptor RNA editing in the amygdala of C57BL/6J, DBA/2J, and BALB/cJ mice. Neurosci Res. 2006;55:96–104. doi: 10.1016/j.neures.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Conn PJ, Niswender CM. Glutamate receptors as therapeutic targets for Parkinson's disease. CNS Neurol Disord Drug Targets. 2009;8:475–491. doi: 10.2174/187152709789824606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Jones CK, Marvanova M, Thompson AD, Tantawy MN, Baldwin RM, Conn PJ. (S)-3,4-DCPG has antiparkinsonian effects in chronic models of Parkinson's disease. Neuropharmacology. 2008;55:603. [Google Scholar]
- Jones CK, Brady AE, Davis AA, Xiang Z, Bubser M, Tantawy MN, Kane AS, Bridges TM, Kennedy JP, Bradley SR, Peterson TE, Ansari MS, Baldwin RM, Kessler RM, Deutch AY, Lah JJ, Levey AI, Lindsley CW, Conn PJ. Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci. 2008;28:10422–10433. doi: 10.1523/JNEUROSCI.1850-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, Bubser M, Thompson AD, Dickerson JW, Turle-Lorenzo N, Amalric M, Blobaum AL, Bridges TM, Morrison RD, Jadhav S, Engers DW, Italiano K, Bode J, Daniels JS, Lindsley CW, Hopkins CR, Conn PJ, Niswender CM. The mGlu4 positive allosteric modulator VU0364770 produces efficacy alone and in combination with L-DOPA or an adenosine A2A antagonist in preclinical rodent models of Parkinson's disease. J Pharmacol Exp Ther. 2011a doi: 10.1124/jpet.111.187443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, Engers DW, Thompson AD, Field JR, Blobaum AL, Lindsley SR, Zhou Y, Gogliotti RD, Jadhav S, Zamorano R, Bogenpohl J, Smith Y, Morrison R, Daniels JS, Weaver CD, Conn PJ, Lindsley CW, Niswender CM, Hopkins CR. Discovery, synthesis, and structure-activity relationship development of a series of N-4-(2,5-dioxopyrrolidin-1-yl)phenylpicolinamides (VU0400195, ML182): characterization of a novel positive allosteric modulator of the metabotropic glutamate receptor 4 (mGlu(4)) with oral efficacy in an antiparkinsonian animal model. J Med Chem. 2011b;54:7639–7647. doi: 10.1021/jm200956q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny J, Wardas J, Kuter K, Pilc A, Ossowska K. The influence of group III metabotropic glutamate receptor stimulation by (1S,3R,4S)-1-aminocyclo-pentane-1,3,4-tricarboxylic acid on the parkinsonian-like akinesia and striatal proenkephalin and prodynorphin mRNA expression in rats. Neuroscience. 2007;145:611–620. doi: 10.1016/j.neuroscience.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging. 2003;2:131–137. doi: 10.1162/15353500200303133. [DOI] [PubMed] [Google Scholar]
- Logan J. Graphical analysis of PET data applied to reversible and irreversible tracers. Nucl Med Biol. 2000;27:661–670. doi: 10.1016/s0969-8051(00)00137-2. [DOI] [PubMed] [Google Scholar]
- Lopez S, Jouve L, Turle Lorenzo N, Kerkerian-Legoff L, Salin P, Amalric M. Antiparkinsonian action of a selective group III mGlu receptor agonist is associated with reversal of subthalamonigral overactivity. Neurobiol Dis. 2012 doi: 10.1016/j.nbd.2011.12.045. [DOI] [PubMed] [Google Scholar]
- Lopez S, Turle-Lorenzo N, Acher F, De Leonibus E, Mele A, Amalric M. Targeting group III metabotropic glutamate receptors produces complex behavioral effects in rodent models of Parkinson's disease. J Neurosci. 2007;27:6701–6711. doi: 10.1523/JNEUROSCI.0299-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez S, Turle-Lorenzo N, Johnston TH, Brotchie JM, Schann S, Neuville P, Amalric M. Functional interaction between adenosine A2A and group III metabotropic glutamate receptors to reduce parkinsonian symptoms in rats. Neuropharmacology. 2008;55:483–490. doi: 10.1016/j.neuropharm.2008.06.038. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson's disease. Eur J Neurosci. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Picconi B, Lindgren H, Cenci MA. A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2004;16:110–123. doi: 10.1016/j.nbd.2004.01.007. [DOI] [PubMed] [Google Scholar]
- MacInnes N, Messenger MJ, Duty S. Activation of group III metabotropic glutamate receptors in selected regions of the basal ganglia alleviates akinesia in the reserpine-treated rat. Br J Pharmacol. 2004;141:15–22. doi: 10.1038/sj.bjp.0705566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MJ, Awad-Granko H, Ciombor KJ, Conn PJ. Haloperidol-induced alteration in the physiological actions of group I mGlus in the subthalamic nucleus and the substantia nigra pars reticulata. Neuropharmacology. 2002;43:147–159. doi: 10.1016/s0028-3908(02)00097-7. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Williams DL, Jr, O'Brien JA, Valenti O, McDonald TP, Clements MK, Wang R, DiLella AG, Hess JF, Kinney GG, Conn PJ. Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson's disease treatment. Proc Natl Acad Sci U S A. 2003;100:13668–13673. doi: 10.1073/pnas.1835724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger MJ, Dawson LG, Duty S. Changes in metabotropic glutamate receptor 1–8 gene expression in the rodent basal ganglia motor loop following lesion of the nigrostriatal tract. Neuropharmacology. 2002;43:261–271. doi: 10.1016/s0028-3908(02)00090-4. [DOI] [PubMed] [Google Scholar]
- Natesan S, Reckless GE, Nobrega JN, Fletcher PJ, Kapur S. Dissociation between in vivo occupancy and functional antagonism of dopamine D2 receptors: comparing aripiprazole to other antipsychotics in animal models. Neuropsychopharmacology. 2006;31:1854–1863. doi: 10.1038/sj.npp.1300983. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Johnson KA, Luo Q, Ayala JE, Kim C, Conn PJ, Weaver CD. A novel assay of Gi/o-linked G protein-coupled receptor coupling to potassium channels provides new insights into the pharmacology of the group III metabotropic glutamate receptors. Mol Pharmacol. 2008a;73:1213–1224. doi: 10.1124/mol.107.041053. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Johnson KA, Weaver CD, Jones CK, Xiang Z, Luo Q, Rodriguez AL, Marlo JE, de Paulis T, Thompson AD, Days EL, Nalywajko T, Austin CA, Williams MB, Ayala JE, Williams R, Lindsley CW, Conn PJ. Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Mol Pharmacol. 2008b;74:1345–1358. doi: 10.1124/mol.108.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parelkar NK, Wang JQ. Upregulation of metabotropic glutamate receptor 8 mRNA expression in the rat forebrain after repeated amphetamine administration. Neurosci Lett. 2008;433:250–254. doi: 10.1016/j.neulet.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisik OV, Smith Y, Conn PJ. D1- and D2-like dopamine receptors regulate signaling properties of group I metabotropic glutamate receptors in the rat globus pallidus. Eur J Neurosci. 2007;26:852–862. doi: 10.1111/j.1460-9568.2007.05710.x. [DOI] [PubMed] [Google Scholar]
- Prashanth LK, Fox S, Meissner WG. l-Dopa-induced dyskinesia-clinical presentation, genetics, and treatment. Int Rev Neurobiol. 2011;98:31–54. doi: 10.1016/B978-0-12-381328-2.00002-X. [DOI] [PubMed] [Google Scholar]
- Rubins DJ, Melega WP, Lacan G, Way B, Plenevaux A, Luxen A, Cherry SR. Development and evaluation of an automated atlas-based image analysis method for microPET studies of the rat brain. Neuroimage. 2003;20:2100–2118. doi: 10.1016/j.neuroimage.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Sibille P, Lopez S, Brabet I, Valenti O, Oueslati N, Gaven F, Goudet C, Bertrand HO, Neyton J, Marino MJ, Amalric M, Pin JP, Acher FC. Synthesis and biological evaluation of 1-amino-2-phosphonomethylcyclopropanecarboxylic acids, new group III metabotropic glutamate receptor agonists. J Med Chem. 2007;50:3585–3595. doi: 10.1021/jm070262c. [DOI] [PubMed] [Google Scholar]
- Tantawy MN, Jones CK, Baldwin RM, Ansari MS, Conn PJ, Kessler RM, Peterson TE. [(18)F]Fallypride dopamine D2 receptor studies using delayed microPET scans and a modified Logan plot. Nucl Med Biol. 2009;36:931–940. doi: 10.1016/j.nucmedbio.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantawy MN, Peterson TE, Jones CK, Johnson K, Rook JM, Conn PJ, Baldwin RM, Ansari MS, Kessler RM. Impact of isoflurane anesthesia on D2 receptor occupancy by [18F]fallypride measured by microPET with a modified Logan plot. Synapse. 2011;65:1173–1180. doi: 10.1002/syn.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NK, Wright RA, Howson PA, Kingston AE, Schoepp DD, Jane DE. (S)-3,4-DCPG, a potent and selective mGlu8a receptor agonist, activates metabotropic glutamate receptors on primary afferent terminals in the neonatal rat spinal cord. Neuropharmacology. 2001;40:311–318. doi: 10.1016/s0028-3908(00)00169-6. [DOI] [PubMed] [Google Scholar]
- Valenti O, Marino MJ, Wittmann M, Lis E, DiLella AG, Kinney GG, Conn PJ. Group III metabotropic glutamate receptor-mediated modulation of the striatopallidal synapse. J Neurosci. 2003;23:7218–7226. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadenberg ML, Soliman A, VanderSpek SC, Kapur S. Dopamine D(2) receptor occupancy is a common mechanism underlying animal models of antipsychotics and their clinical effects. Neuropsychopharmacology. 2001;25:633–641. doi: 10.1016/S0893-133X(01)00261-5. [DOI] [PubMed] [Google Scholar]