Abstract
Binge alcohol drinking induces hepatic steatosis. Recent studies showed that chronic ethanol-induced fatty liver was, at least in part, CYP2E1 dependent. The mechanism of acute alcohol induced steatosis and whether CYP2E1 plays any role is still unclear. Increasing oxidative stress by alcohol can activate the JNK MAP kinase signaling pathway, suggesting that JNK might be a target for prevention of alcohol induced steatosis. We used CYP2E1 knockout (KO) mice, a JNK inhibitor, and JNK1 or JNK2 knockout mice to test the role of CYP2E1, JNK, and the individual role of JNK1 and JNK2 in acute alcohol-induced steatosis. In wild type (WT) mice, acute alcohol activates CYP2E1 and increases oxidative stress, which reciprocally increases activation of the JNK signaling pathway. Acute alcohol–induced fatty liver and oxidative stress was blunted in CYP2E1 KO mice and by the JNK inhibitor in WT mice. The antioxidant N-acetylcysteine decreased the acute alcohol induced oxidative stress, activation of JNK and the steatosis but not the activation of CYP2E1. Acute alcohol decreased autophagy and increased expression of SREBP, effects blocked by the JNK inhibitor. Acute alcohol–induced fatty liver was the same in either JNK1 or JNK2 KO mice as WT mice, thus either JNK1 or JNK2 per se is sufficient in induction of steatosis by acute alcohol.
Conclusion
acute alcohol elevation of CYP2E1, oxidative stress and activation of JNK interact to lower autophagy and increase lipogenic SREBP resulting in fatty liver.
Keywords: oxidative stress, CYP2E1, JNK, autophagy, liver steatosis
INTRODUCTION
Acute alcohol consumption induces steatosis in liver [1–2]. Steatosis is an early and reversible stage of alcohol induced liver injuries. However, steatosis can evolve towards steatohepatitis, which is characterized not only by lipid accumulation but also by necroinflammation and fibrosis. Previous work showed that chronic alcohol consumption induced fatty liver was, at least in part, CYP2E1 dependent [3–4]. Chlormethiazole, an inhibitor of CYP2E1, lowered chronic ethanol-induced fatty liver [4]. Chronic ethanol-induced fatty liver was significantly blunted in CYP2E1 knockout (KO) mice as compared to wild type (WT) mice. Restoring CYP2E1 to the KO mice restored the chronic alcohol-induced fatty liver [3]. CYP2E1 is also critical in alcohol induced steatosis in HepG2 cells [5]. However, whether CYP2E1 plays a role in acute alcohol induced fatty liver is unknown. One goal of the current study was to evaluate whether CYP2E1 plays a role in acute alcohol induced liver steatosis.
JNK signaling has been reported to be related to disease progressions, such as steatohepatitis, obesity, insulin resistance, non-alcoholic liver diseases etc [6–9]. Acute alcohol induced a moderate increase in the phosphorylation of JNK [10–11]. This may suggest that JNK plays a role in the progression of steatosis, and might be a target for the prevention of steatosis and further development of liver damage by alcohol. In most tissues especially liver, there are two forms of JNK, JNK1 and JNK2 [12]. Mice deficient in either JNK1 or JNK2 are viable but double knockouts are embryonic lethal. Recent studies showed either JNK-1 or JNK-2 played a role in chemical or drug induced fatty liver or liver toxicity [8, 13–17]. Recent reports showed that JNK-1 but not JNK2 played an important role in methionine choline deficient or high fat diet induced steatohepatitis [8, 14]. In this study, we evaluated whether JNK plays a critical role in acute alcohol induced liver steatosis and if it did, the individual role of JNK1 or JNK2 in this acute alcohol induced liver steatosis was studied.
The effect of autophagy on various biological effects to the liver [18–21] has been recently studied. Alcohol treatment to CYP2E1 expressing HepG2 cells decreased autophagy while inducing steatosis [5]. As a comparison, the change of autophagy was less in HepG2 cells without CYP2E1 expression [5]. This suggested that CYP2E1, autophagy and steatosis may correlate with each other. A recent report showed that autophagy was increased with in vivo alcohol treatment, or upon addition of ethanol to isolated hepatocytes [22]. Interestingly, induction of autophagy was found to be JNK dependent [23]. In the current study, using an acute alcohol model, the possible relationship between steatosis, CYP2E1 activation, JNK activation and autophagy was determined.
EXPERIMENTAL PROCEDURES
Experimental Models and Treatments
Animal experiments were performed with approval of the Mount Sinai Animal Care and Use Committee. SV/129-background CYP2E1 knockout mice were kindly provided by Dr. Frank J. Gonzalez (Laboratory of Metabolism, National Cancer Institute, Bethesda, MD) and breeding colonies established at Mount Sinai. Male SV/129 wild type mice, Male jnk1−/− (B6.129-Mapk8tm1Flv/J), jnk2−/− (B6.129-Mapk9tm1Flv/J), and wild type-C57BL/6J mice (JNK1 KO, JNK2 KO and WT), weighing 24–26g, at 8–10 weeks of age, were purchased from Jackson Laboratory (Bar Harbor, ME). JNK1 KO mice were backcrossed 7 generations to C57BL/6J mice while JNK2 KO mice were backcrossed 5 generations to C57BL/6J mice. Ethanol was given for 4 doses at 30 minutes interval between each dose. The first dose of ethanol was administered as IP injection at 0.93g/kg b.w‥ The other three doses were applied by gavage at 1.25g/kg b.w‥ Mice were fasted for 18 hrs before being sacrificed. JNK inhibitor XIII (EMD Chemicals, Inc., Gibbstown, USA) was dissolved in 30% ethanol and applied as IP injection at 5µg/kg b.w. (the dose of ethanol is equivalent to the first dose of ethanol treatment). The other three doses of ethanol were applied the same as in the ethanol treatment group. For N-acetylcysteine (NAC) treatment, two doses of NAC at 100mg/kg b.w. were injected IP 24 hrs and 1 hr respectively before the first dose of ethanol treatment. In the saline control group, the four doses of saline were applied at 30 min intervals, with the 1st dose as IP injection, and other three doses as gavage in the same volume of saline as the ethanol treatment group.
Sample Collection, Pathology Analysis, and Biochemical Assays
Liver tissue collection, Oil-red O staining and serum ALT and AST were assayed as previously described [3–5]. Activity of CYP2E1, TBARS assay, GSH and triglyceride levels were assayed as previously described [3–5]. GSSG was assayed by following the decrease in absorbance at 340 nm upon addition of NADPH plus glutathione reductase to sample liver extracts.
Western Blot Analysis
Levels of CYP2E1, pJNK, JNK, cJUN, pcJUN, LC3, P62, Atg7, Beclin, p-mTOR, pP70, P70, p4E1, 4E1, BIP, IRE1α, Bcl-2, and SREBP1α in 20–100µg of protein samples from freshly prepared liver homogenate fractions were determined using western blot analysis. Blots were scanned using an Odyssey Imaging System (LI-COR Biosciences, Lincoln, NE). All specific bands were quantified with the Automated Digitizing System (Image J programs, version 1.34S, National Institute of Health).
Immunohistochemistry staining
Paraffin slides were deparaffinized and blocked as previously reported [13]. Antibodies against 4-HNE, and pJNK were added at 1:200 dilution and incubated at 4°C overnight. Slides were washed with PBS and stained with the Histostain Plus Broad Spectrum (DAB) kit. Images were detected under the light microscope at 200× magnitude.
Statistical Analysis
Values reflect mean±SE. One-way ANOVA with subsequent posthoc comparisons by Tukey HSD were performed by SPSS analysis software (version 10.0). P values of less than 0.05 were considered statistically significant and results are from experiments using 3–6 mice of each genotype.
RESULTS
Acute alcohol treatment induces steatosis in liver, which involves activation of CYP2E1 and JNK
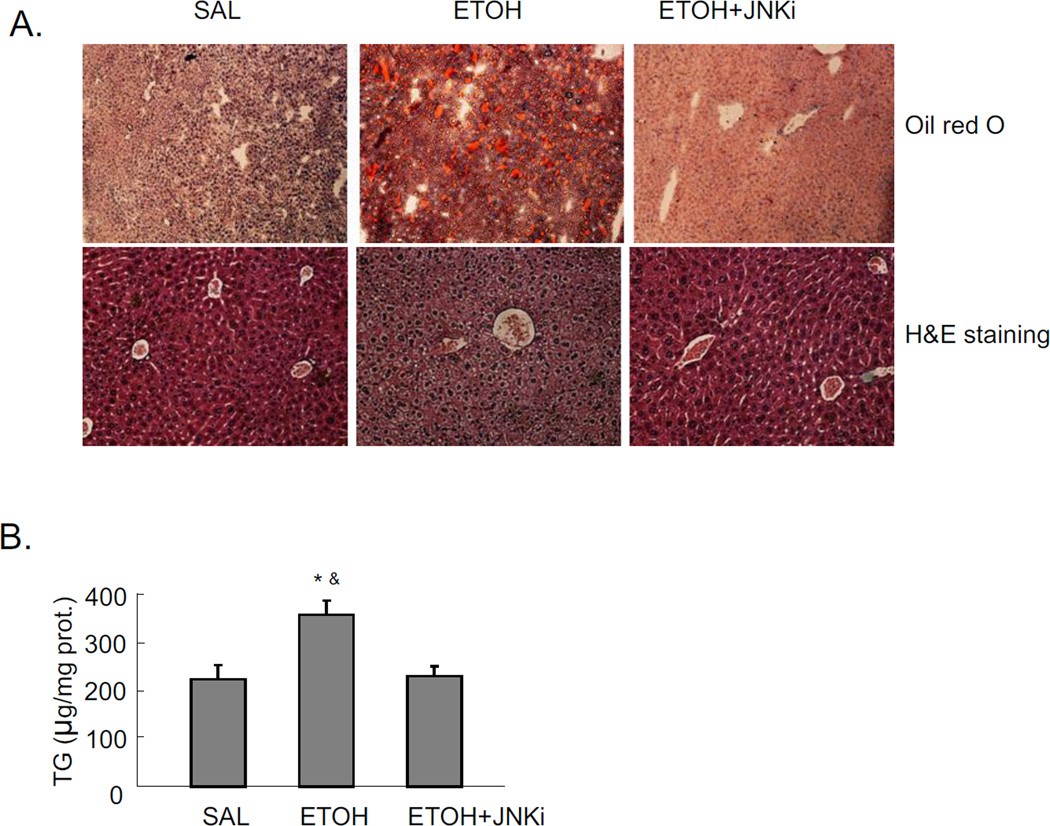
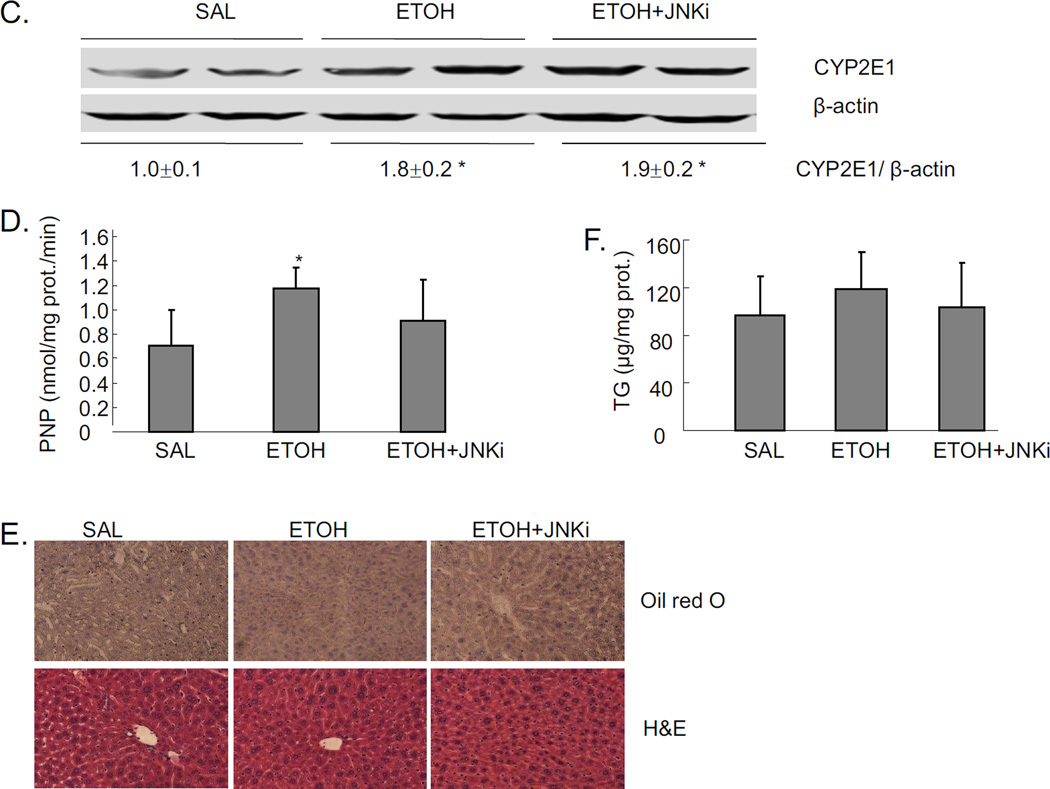
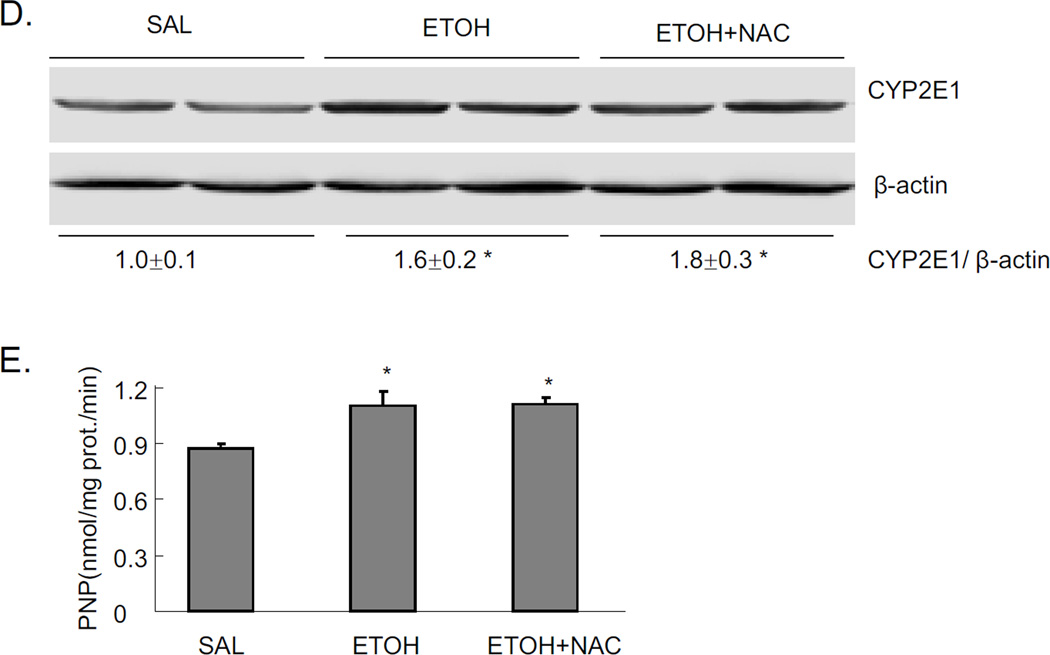
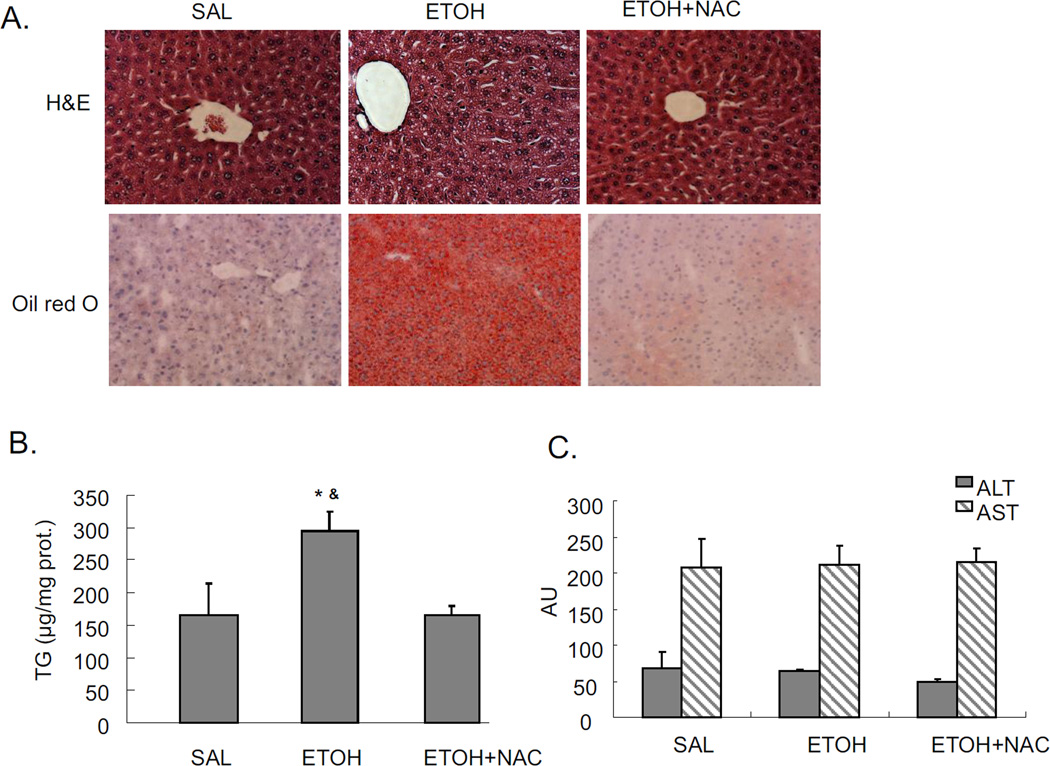
Mice were treated with a total of 4.68g/kg b.w. alcohol and sacrificed 18 hrs after the last dose of ethanol. Significant steatosis was produced in livers by acute alcohol treatment, as shown by H&E staining, Oil Red O staining, and TG levels in liver (Fig 1A and 1B). Acute alcohol treatment also increased CYP2E1 protein expression (Fig. 1C), and CYP2E1 activity (Fig. 1D). This suggests that CYP2E1 might play a role in the acute alcohol induced steatosis. This modest induction of CYP2E1 is due, in part, to the last dose of ethanol being administered 18 hrs prior to sacrifice, coupled to the rapid turnover of CYP2E1 [24–25]. Further evidence for a role of CYP2E1 was tested by treating CYP2E1 knockout mice with acute alcohol. No steatosis was induced by acute alcohol in CYP2E1 knockout mice (Fig. 1E and 1F), confirming CYP2E1 plays a role in formation of steatosis in this acute alcohol model.
Fig. 1.
Effect of CYP2E1 and JNK inhibitor XIII on acute ethanol-induced fatty liver. Mice were treated with a total dose of alcohol at 4.68g/kg b.w. with 4 applications at 30 mins interval. Mice were sacrificed 18 hrs after the last dose of ethanol. Results A–D are from wild type (WT) mice. A. Oil Red O and H&E staining. B. Liver TG level. C. Western blot of liver CYP2E1. D. CYP2E1 activity in livers. Panels E and F refer to CYP2E1 knockout mice. CYP2E1 knockout mice were fed a total amount of 4.68g/kg b.w. alcohol exactly as described for the WT mice. E. Oil Red O and H&E staining of liver. F. liver TG level. * p<0.05 comparing ethanol group and ethanol+JNK inhibitor group to saline control. & p<0.05 comparing ethanol group to JNK inhibitor group. n=6.
JNK inhibitor completely blocked the acute alcohol induced steatosis (Fig. 1A–B). We evaluated whether this blockage of steatosis could be due to inhibition of CYP2E1. The JNK inhibitor did not block either CYP2E1 protein expression or activity (Fig. 1C–D). This suggests that inhibition by the JNK inhibitor is not through direct inhibition of CYP2E1 but rather through inhibition of effects that are downstream of ethanol-CYP2E1 signaling as described below. Acute alcohol treatment per se or together with JNK inhibitor did not cause liver injury as reflected by ALT and AST assay (data not shown).
Acute alcohol treatment induces activation of JNK MAP Kinase
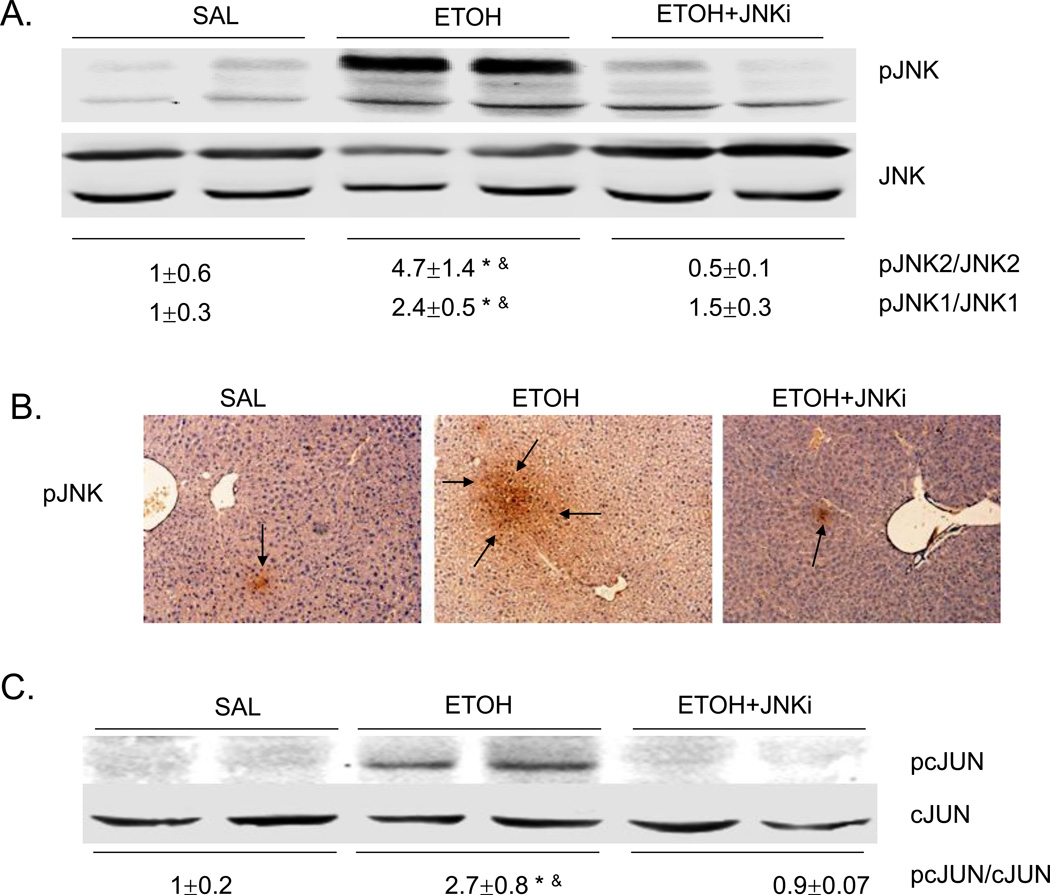
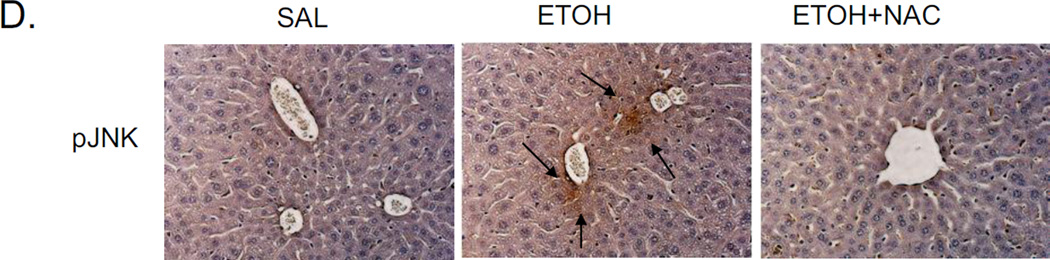
In chronic alcohol treatment, MAP kinase signaling pathways were involved in development of liver injuries [26–28]. Based upon the blunting of acute ethanol-induced steatosis by the JNK inhibitor, we evaluated whether JNK was indeed activated, and if so, which JNK isoform, JNK1 or JNK2 or both was activated. Both JNK1 and JNK2 were activated, by acute alcohol treatment with increases in the pJNK1/JNK1 and pJNK2/JNK2 ratio of 2.4 fold and 4.7 fold as compared to saline control, respectively (Fig. 2A). This activation was blocked by the JNK inhibitor. Immunohistochemistry staining also confirmed the activation of JNK by acute alcohol and the prevention of this activation by JNK inhibitor (Fig. 2B). Acute alcohol treatment also increased the activation of cJUN as shown by the elevated ratio of pcJUN/cJUN. JNK inhibitor blocked this cJUN activation (Fig. 2C).
Fig. 2.
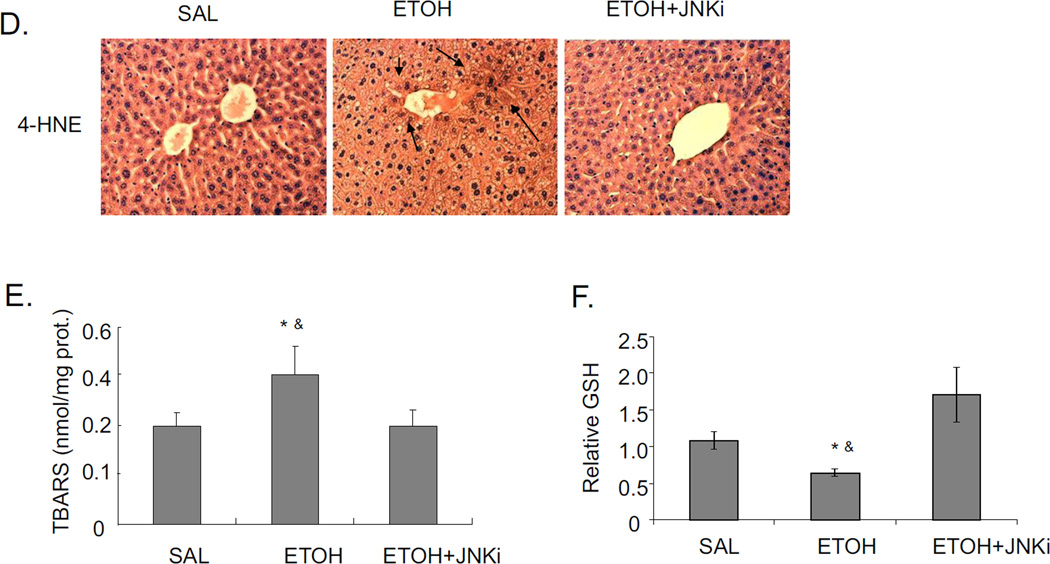
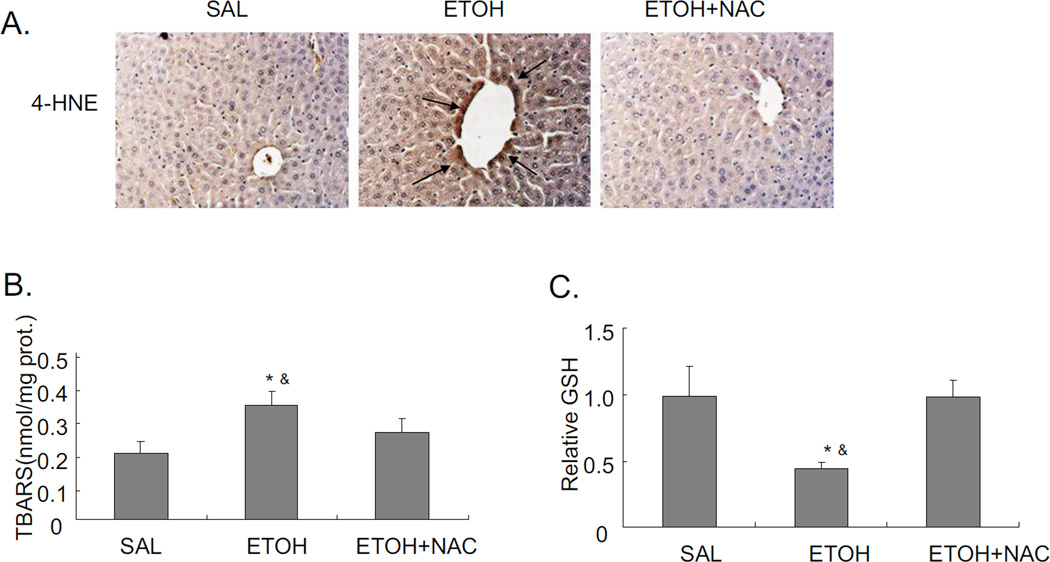
Acute alcohol induces the activation of JNK, downstream cJUN, and increases oxidative stress in liver. JNK inhibitor blocks these effects. A. Western blot of pJNK and JNK. B. Immunohistochemistry staining for pJNK. Arrows point to areas of positive staining. C. Western blot for pcJUN and cJUN. D. 4-HNE immunostaining. Arrows point to areas of positive staining. E. TBARS level. F. GSH level in livers. Values are the relative GSH level to saline control. * p<0.05 comparing ethanol group to saline control. & p<0.05 comparing ethanol group to ethanol+JNK inhibitor group. n=6.
JNK inhibitor blocks oxidative stress induced by acute alcohol treatment
Chronic alcohol treatment of mice increases oxidative stress in the liver [3, 29]. There are also reports on increased oxidative stress in acute alcohol treatment [30–32]. In the current binge alcohol model, oxidative stress was increased, as shown by elevated 4-HNE staining, increased TBARS levels, and decreased reduced GSH levels compared to saline controls (Fig. 2D–F). Levels of GSSG were elevated two-fold by the acute ethanol treatment (2 nmol/mg protein for saline controls and 3.8 nmol/mg protein for the ethanol-treated) (see time course below). JNK inhibitor blocked this acute alcohol induced oxidative stress decreasing levels of 4-HNE and TBARS, and increasing levels of GSH in liver (Fig. 2D–F). To further evaluate the role of oxidative stress in steatosis formation in this acute alcohol model, mice were pretreated with the antioxidant NAC prior to acute alcohol treatment. NAC pretreatment blocked oxidative stress induced by acute alcohol treatment decreasing 4-HNE protein adduct formation and TBARS levels and increasing reduced GSH levels (Fig. 3A–C). The NAC treatment did not block the acute alcohol induction of CYP2E1 (Fig 3D and 3E), indicating that the blockage of oxidative stress by NAC is not due to the inhibition of CYP2E1. NAC blocked acute alcohol induced steatosis as shown by reduced H&E staining, Oil Red O staining and liver triglyceride levels (Fig. 4A–B). This suggests that oxidative stress indeed plays an important role in the acute alcohol induced steatosis. As mentioned above (Fig. 2), inhibition of JNK decreased the acute alcohol-induced oxidative stress. Results in Fig 4D showed that inhibition of oxidative stress with NAC also blocked JNK activation. This suggests there is a reciprocal relationship between the increase of oxidative stress and activation of JNK which may play important roles in the acute alcohol-induced steatosis. No liver toxicity was found with acute alcohol only or pretreatment with NAC (Fig. 4C).
Fig. 3.
Effect of the antioxidant NAC on acute alcohol induced oxidative stress and CYP2E1. A. 4-HNE staining in liver. Arrows point to areas of positive staining. B. TBARS level. C. GSH level in liver. Values are the relative GSH level to saline control. D. Western blot of CYP2E1. E. CYP2E1 activity. * p<0.05 comparing ethanol group or ethanol+NAC to saline control. & p<0.05 comparing ethanol group to ethanol+NAC group. n=3–4.
Fig. 4.
Effect of NAC on acute alcohol induced liver steatosis and activation of JNK. A. H&E staining and Oil Red O staining. B. TG level in liver. * p<0.05 comparing ethanol group to saline control. & p<0.05 comparing ethanol group to ethanol+NAC group. n=3–4. C. ALT and AST level in serum. D. Immunohistochemistry staining of pJNK. Arrows point to areas of positive staining.
Effect of acute alcohol and JNK inhibition on autophagy signaling pathways
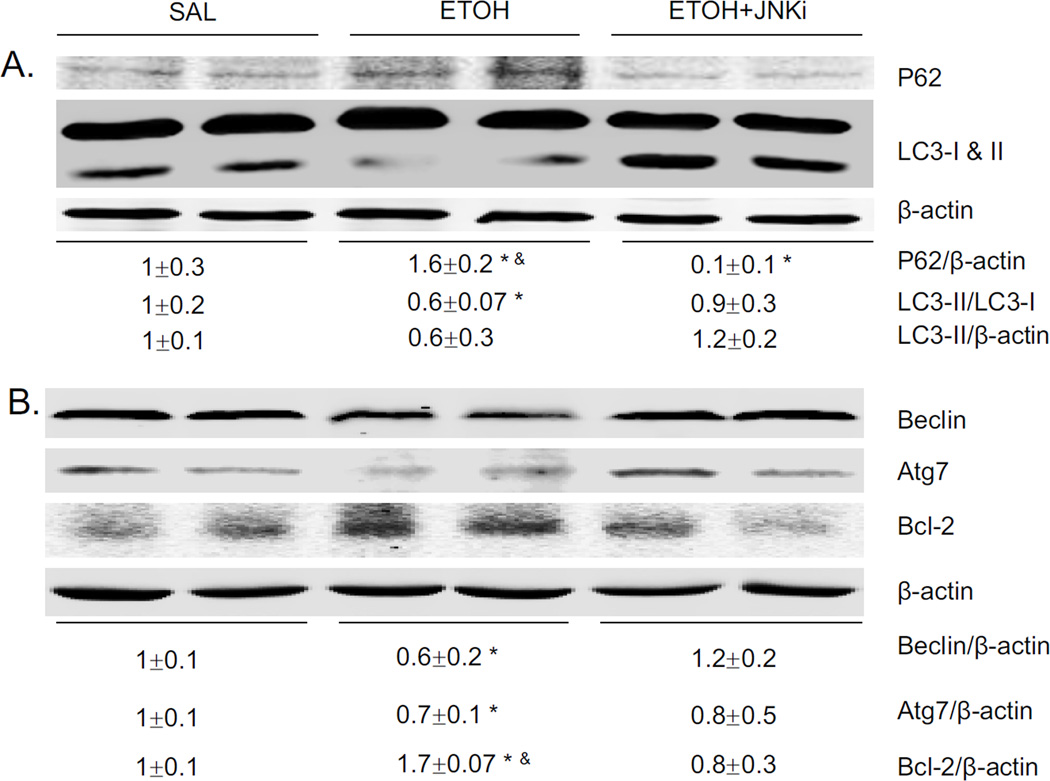
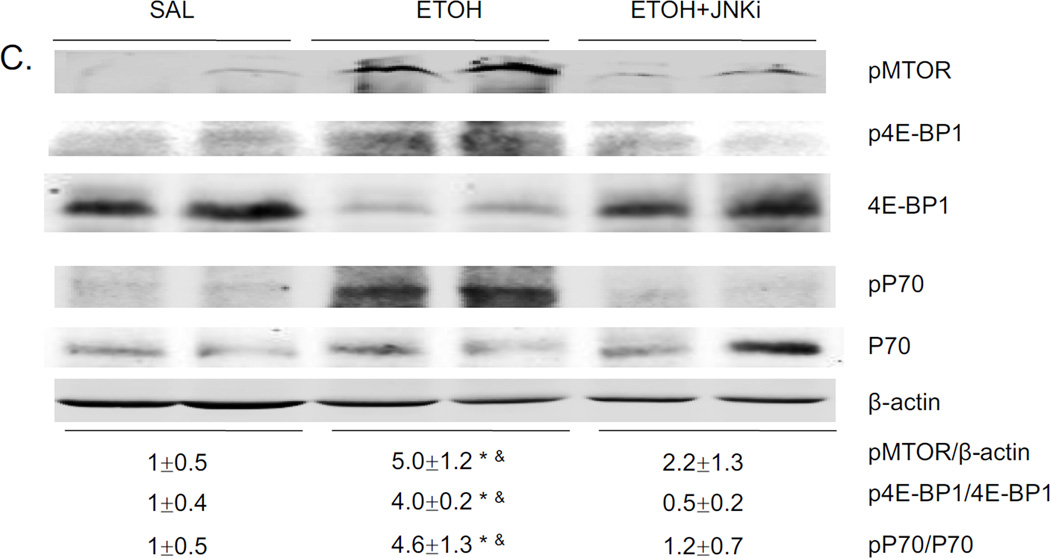
Autophagy could either be increased or decreased by alcohol depending on the model, tissue, experimental conditions [5, 22, 33, 34]. Acute alcohol decreased autophagy, as shown by decreased LC3-II/LC3-I ratio, decreased LC3-II/β-actin ratio and increased P62 levels (Fig. 5A). This decrease in autophagy was prevented by the JNK inhibitor. Beclin-1 is an important component in inducing the formation of autophagosomes in mammalian systems, and Bcl-2 can bind to Beclin-1 and inhibit autophagy [35]. In addition, Atg 7 is critical in the transition of LC3-I to LC3-II [36]. Acute alcohol caused decreased expression of Beclin, Atg7, and increased expression of Bcl-2 (Fig. 5B). The JNK inhibitor reversed these effects (Fig 5B). Ethanol induced oxidative stress can promote the activation of mTOR, which inhibits autophagy [47]. mTOR promotes protein synthesis and cell growth by phosphorylating p70 ribosomal S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E-binding protein-1 (4EBP1) [37]. Acute alcohol treatment increased mTOR phosphorylation, 4E1-BP and P70 phosphorylation (Fig. 5C), downstream targets of mTOR activation. The JNK inhibitor blocked these phosphorylations. These data show that acute alcohol treatment increased activation of mTOR and its downstream factors and decreased autophagy. JNK inhibitor blocked the effect of acute alcohol on autophagy.
Fig. 5.
Effect of acute ethanol treatment and JNK inhibitor on liver autophagy. Western blot of P62 and LC3 (A), Beclin, Atg7, Bcl2 (B), pmTOR, p4E1 and 4E1, pP70 and P70 (C). * p<0.05 comparing ethanol group or ethanol+JNK inhibitor group to saline control. & p<0.05 comparing ethanol group to ethanol+JNK inhibitor group. n=6.
Time course for the effects of acute alcohol treatment
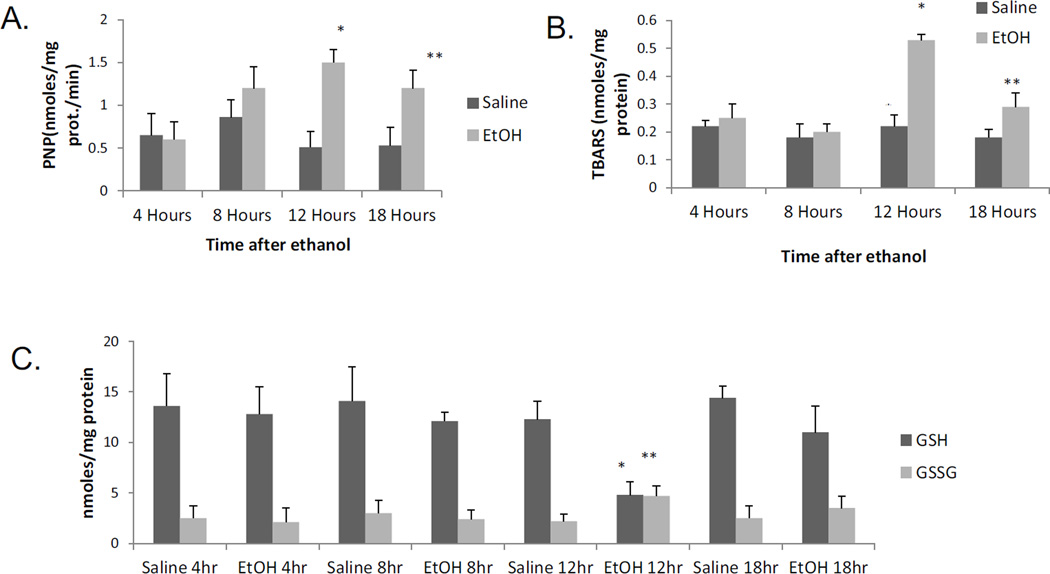
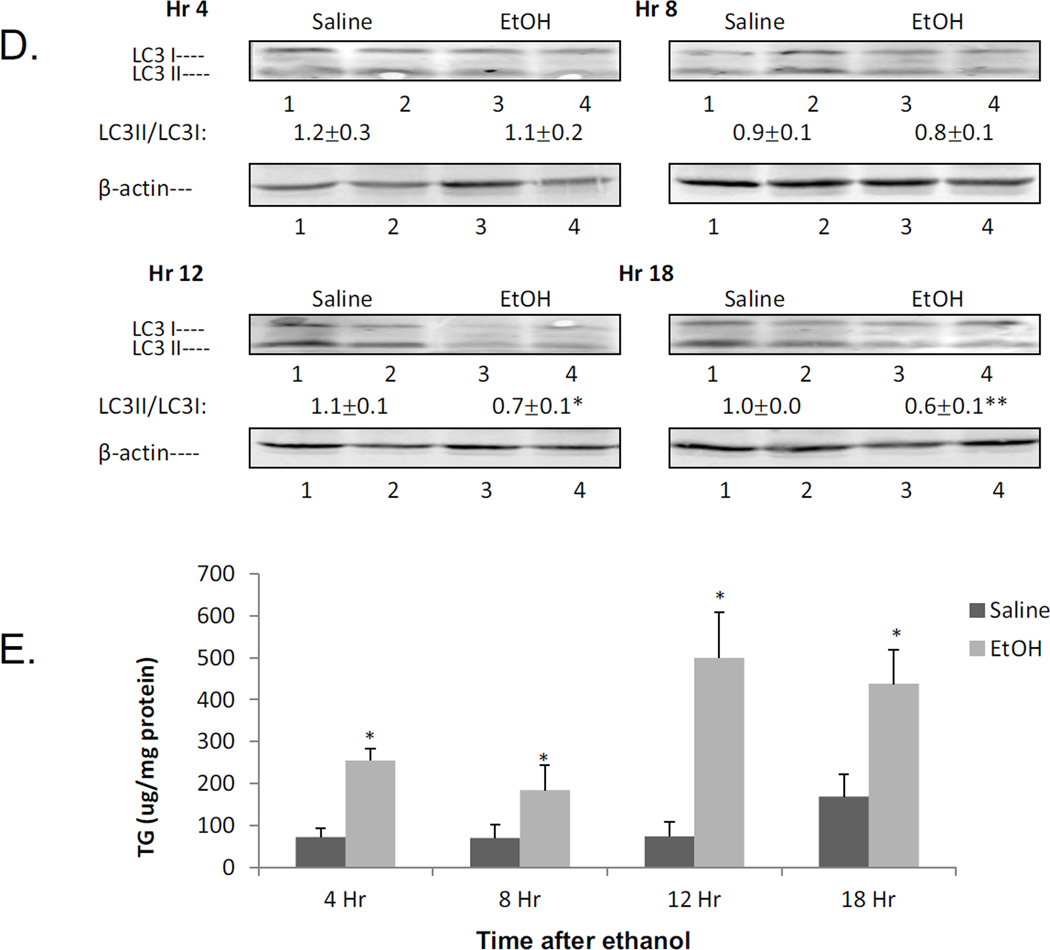
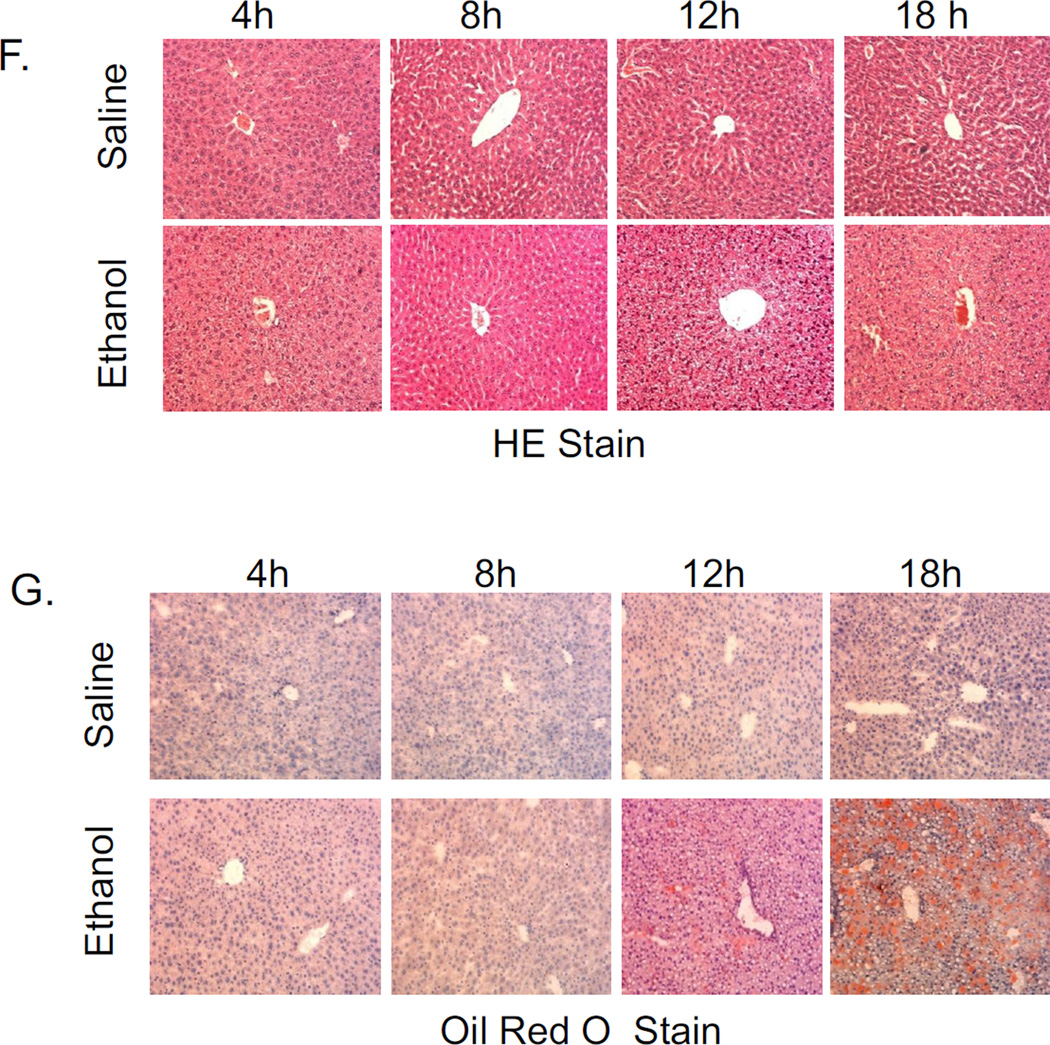
To evaluate the kinetics of some of the changes produced by the acute alcohol treatment, mice were treated with a total of 4.68g/ kg b.w. alcohol and sacrificed 4,8, 12 and 18 hrs after the last dose of ethanol. The catalytic activity of CYP2E1 began to rise 8 hr and was significantly elevated at 12 and 18 hr after ethanol as compared to saline controls (Fig.6A). Oxidant stress was elevated at 12 hr, with smaller increases at 18 hr after the last dose of alcohol as reflected by increases in levels of TBARS and GSSG coupled to decreases in GSH (Fig 6B,6C). The LC3-II/ LC3-I ratio was lowered at 12 and 18 hrs after the last dose of ethanol (Fig 6D). These changes in oxidant stress and autophagy (12 hr after ethanol) are associated with corresponding changes in CYP2E1 (8–12 hr after ethanol). Results on triglyceride accumulation showed a biphasic response to ethanol(Fig 6E). There was an early increase in TGs at 4 and 8 hrs after ethanol. This was followed by a more striking second phase of TG accumulation at 12 and 18 hrs after ethanol (Fig 6E). This secondary burst of TG formation is associated with lipid droplet formation and steatosis becoming elevated at 12 and 18 hrs after ethanol (Fig. 6F,6G). Both the steatosis score and Oil Red O staining were −, +/−, +/++ and ++ at 4,8, 12 and 18 hrs after the last dose of ethanol, respectively (saline values were all −).
Fig. 6.
Time course for the effects of acute alcohol treatment on liver. Mice were gavaged with a total of 4.68 g/kg b.w. of alcohol and sacrificed at 4, 8, 12, and 18 hrs after the last dose of alcohol. A:CYP2E1 catalytic activity (oxidation of PNP). B, C:parameters of oxidative stress (TBARS, GSH,GSSG). D: the LC3-II/LC3-I ratio. E:TG accumulation. F: H&E staining: G: Oil Red O staining. The various measures were assayed as described in the above Figure legends. Results are from 4 saline-treated and 4 alcohol-treated mice at each time point. *, ** p< 0.05 comparing ethanol group to the saline control for that time point.
Effect of acute alcohol and JNK inhibitor on SREBP and ER stress
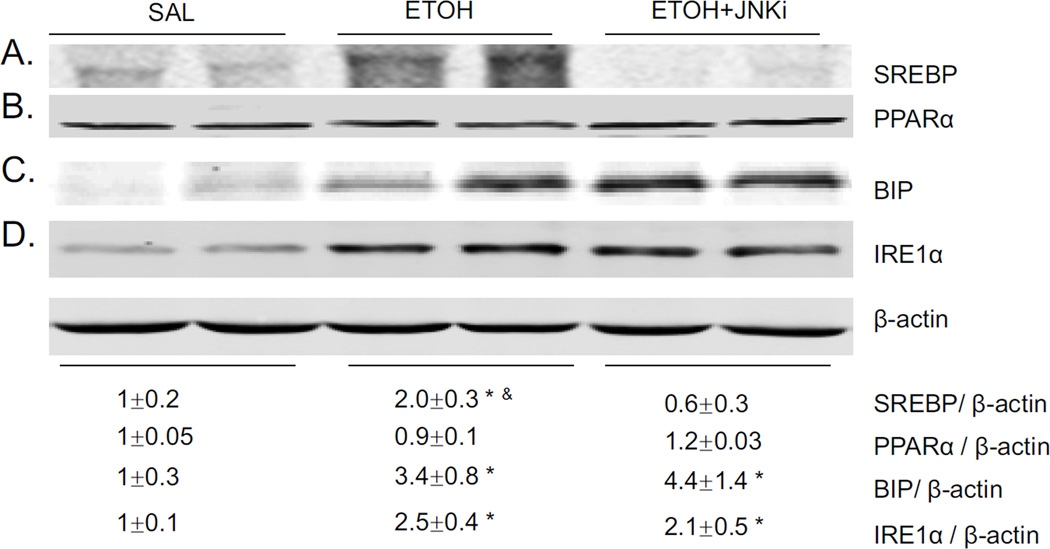
SREBP is an important transcription factor controlling lipid homeostasis [38]. Acute alcohol increased SREBP expression in liver 2 fold. JNK inhibitor blocked this increase (Fig. 7A). No change of PPARα expression was found either by acute alcohol or by acute alcohol and JNK inhibitor together (Fig. 7B). Whether ER stress could be related to the acute alcohol induced steatosis was evaluated. Acute alcohol increased expression of BIP and IRE1α (Fig. 7C–D). However, JNK inhibitor did not block the increased expression of these two ER stress markers, suggesting that inhibition of steatosis by JNK inhibitor is not ER stress related. No significant change of CHOP was found either after acute alcohol treatment or alcohol plus JNK inhibitor pretreatment (data not shown).
Fig. 7.
Effect of acute alcohol and JNK inhibitor on levels of SREBP, PPARα, and ER stress markers. Western blot of SREBP, PPARα, BIP, and IRE1α(A–D). * p<0.05 comparing ethanol group or ethanol+JNK inhibitor group to saline control. & p<0.05 comparing ethanol group to ethanol+JNK inhibitor group. n=6.
Either JNK1 or JNK2 is sufficient for acute ethanol induced steatosis
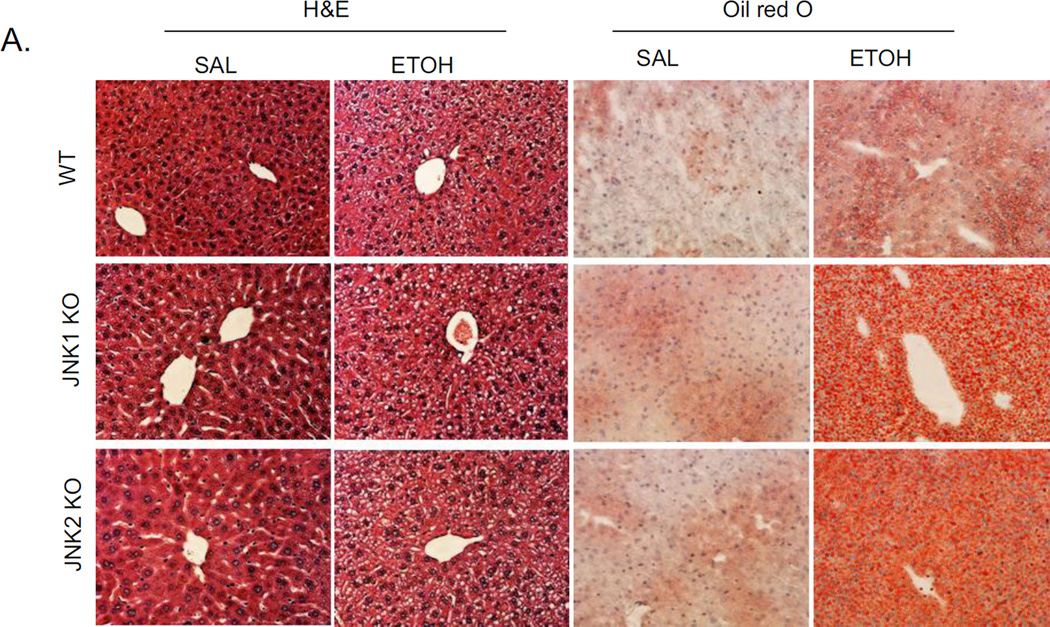
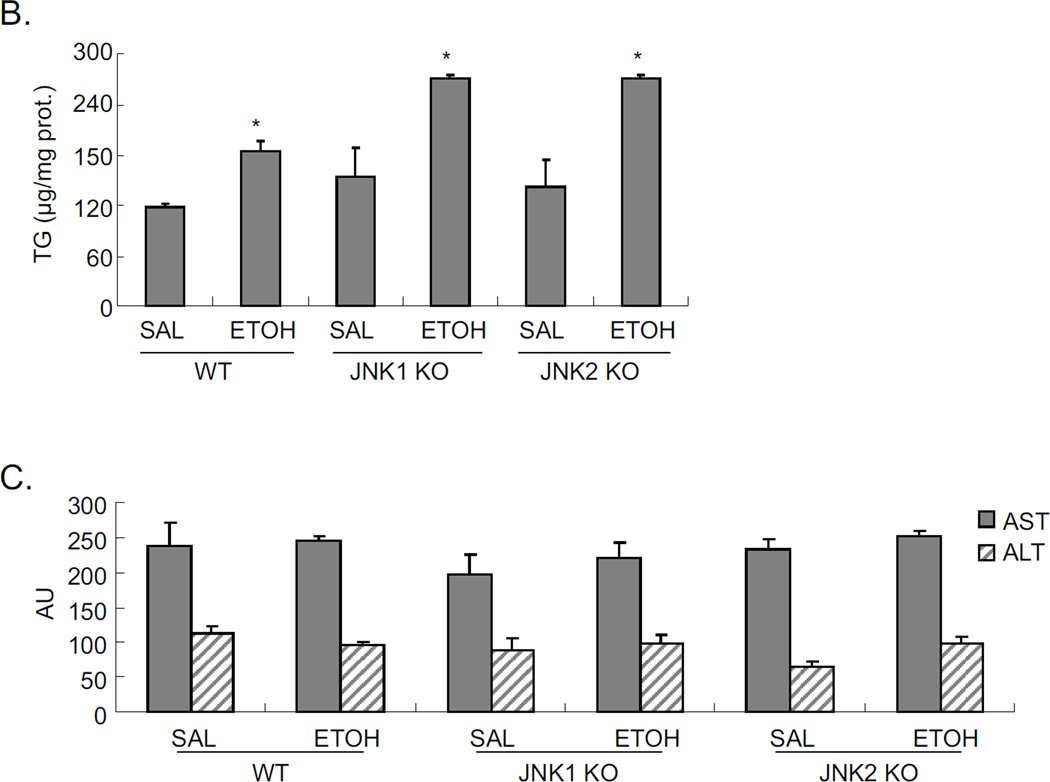
Since JNK inhibitor strongly blocked the steatosis induced by acute alcohol, and both JNK1 and JNK2 were each activated by alcohol (Fig 2A), we evaluated whether JNK1 or JNK2 plays a major role in this alcohol increase in steatosis. Either JNK1 knockout mice or JNK2 knockout mice or wild type mice were treated with acute alcohol. Acute alcohol induced steatosis, as shown by H&E and Oil Red O staining (Fig. 8A), and hepatic TG level (Fig. 8B), is the same in both JNK knockouts as in wild type mice treated acutely with alcohol. This suggests that either JNK1 or JNK2 is sufficient for acute alcohol induced steatosis. No liver toxicity was found in WT, JNK1 KO, and JNK2 KO with acute alcohol treatment (Fig. 8C).
Fig. 8.
Comparison of acute alcohol induced steatosis in WT, JNK1 KO, and JNK2 KO mice. JNK1 KO, JNK2 KO and wild type C57BL/6J mice were gavaged with ethanol as indicated in ‘Material and Methods’. A. H&E staining and Oil Red O staining. B. Liver TG level. * p<0.05 comparing ethanol group to saline control for each genotype. n=3–4. C. Serum ALT and AST levels.
DISCUSSION
Role of CYP2E1 in acute alcohol induced liver steatosis
We have previously shown that CYP2E1 plays a major role in chronic ethanol induced fatty liver and oxidative stress [29]. After feeding CYP2E1 knockout mice, CYP2E1 knockin mice and their wild type control with chronic alcohol, there was a significant decrease of oxidative stress and steatosis in CYP2E1 knockout mice compared to wild type control, and restoration of oxidant stress and steatosis in CYP2E1 knockin mice [3]. Steatosis was found after addition of ethanol in vitro in HepG2 cells expressing CYP2E1, but not in HepG2 cells not expressing CYP2E1 [5]. In the current study, acute alcohol administration, produced an activation of CYP2E1 (Fig 1C,1D), an increase in oxidative stress (Fig 2D,E,F) and activation of JNK (Fig 2A,2B) in WT mice. CYP2E1 knockout mice treated with acute alcohol did not develop steatosis (Fig 1E,1F), indicating that CYP2E1 plays an important role in steatosis formation in this acute alcohol model.
Time course experiments showed that increases in oxidant stress and decline in autophagy (12 hrs after ethanol) occur in association with elevation of CYP2E1 activity (8–12 hrs after ethanol). However, the acute alcohol treatment elevated TG levels at time points (4 hr) when CYP2E1 or oxidant stress was not yet altered by ethanol. The mechanism for this early increase in TG levels , which appears to be independent of CYP2E1 and ROS is not known and will require further study. The second burst in TG accumulation which occurs at 12 hr after the last dose of ethanol, a time point where CYP2E1 and ROS are elevated, and autophagy declines is associated with elevated lipid droplet formation and Oil Red O staining. This suggests the latter elevation in TGs is likely due to CYP2E1-mediated induction of ROS. e.g NAC blunts the elevation in TGs , the steatosis and the Oil Red O staining at 18 hrs (Fig 4).
JNK activation and steatosis
In response to acute and chronic ethanol exposure, mitogen-activated protein kinase family members including JNKs are activated [27]. These responses to ethanol translate into activation of nuclear transcription factors and altered gene expression within the liver, leading to the development of steatosis and the early stages of alcohol-induced liver injury [39]. JNK is encoded for by three genes, each of which is alternatively spliced to yield a 54- and 46-kDa protein. In hepatocytes, only two of the genes, JNK1 and JNK2, are expressed [12]. JNK has been implicated in hepatic injury produced by alcohols, acetaminophen, TNFα, ischemia-reperfusion and hepatitis virus [8, 13–17, 40]. Although it has been suggested that JNK1 may promote cell death and JNK2 may promote proliferation and survival, it appears that depending on the toxin, tissue and cell type, either JNK1 or JNK2 or both play the major role in cell injury. Liver specific JNK1 knockout mice treated with a high fat diet developed an increase of steatosis in liver, while treating adipose tissue specific JNK1 knockout mice with high fat diet caused an inhibition of steatosis in liver [7, 41]. This suggests that JNK1 in liver and adipose tissue play opposite roles in steatosis formation. There appears to be no data which shows a tissue specific role of JNK in response to alcohol treatment. In addition to tissue specificity, the isoform of JNK may also decide its function. JNK1 appears to play a more important role in liver toxicity or steatosis by different stimuli as compared to JNK2 [8, 13, 14]. In fibroblasts, JNK1, but not JNK2, appears to be essential for TNFα-induced apoptosis [42]. JNK2 was found to be predominant in acetaminophen toxicity, and 6-hydroxydopamine-induced apoptosis in PC12 cells [16, 17]. Liver injury produced by either LPS/D-galactosamine or TNFα/D-galactosamine was lower in JNK2 KO mice, compared to JNK1 KO mice [16]. Systemic inhibition of JNK by JNK inhibitors can protect against the effects of feeding a high fat diet [43].
To clarify a possible role of JNK on acute alcohol induced steatosis, a systemic JNK inhibitor (JNK inhibitor XIII) was used in the current study to suppress JNK before acute alcohol treatment (Fig 2A,2B). It was reported that a JNK inhibitor blunted liver toxicity by acetaminophen partly by reducing oxidative stress in mitochondria [44]. One possible mechanism for the JNK inhibitor’s blunting of steatosis by acute alcohol may be by reducing oxidative stress. Indeed, the acute alcohol elevation of oxidant stress was blocked by the JNK inhibitor (Fig 2D,E,F). The role of oxidative stress in acute alcohol induced steatosis was confirmed by the fact that NAC which blocked the elevation in oxidative stress by acute alcohol (Fig 3A,B,C), also blocked the acute alcohol induced steatosis (Fig 4A, 4B).
Both JNK1 and JNK2 were activated by the acute alcohol treatment and the JNK inhibitor blocked the activation of either JNK (Fig 2A). To clarify the individual role of JNK1 or JNK2 on acute alcohol induced steatosis, either JNK1 knockout or JNK2 knockout mice were treated with acute alcohol. A comparable level of steatosis was found in JNK1 knockout mice and JNK2 knockout mice as in the WT mice (Fig 8). Either JNK1 or JNK2 is sufficient in inducing steatosis in this acute alcohol model.
Autophagy and steatosis
Autophagy is a lysosomal degradative pathway critical for the removal and breakdown of cellular components such as organelles and proteins. The regulation and function of autophagy and lipid metabolism were reported to be reciprocally related to each other [45]. Studies in hepatocytes and liver have demonstrated that macroautophagy mediates the breakdown of lipids stored in lipid droplets and that an inhibition of autophagy leads to the development of fatty liver [45]. Lipid droplets are substrates for macroautophagy. Besides the classic pathway of lipid metabolism by cytosolic lipases, lipid droplets are sequestered in autophagosomes and then broken down by lysosomal enzymes [45].
The effects of alcohol on autophagy are not clear. A recent study showed that autophagy protected against liver injury and fatty liver after acute alcohol administration to mice and that acute alcohol increased autophagosome formation [22]. Chronic ethanol treatment disrupts lysosomes and inhibits lysosomal hydrolytic activity suggesting that ethanol might inhibit autophagic degradation [46]. Addition of ethanol to VL-17A HepG2 cells which express alcohol dehydrogenase and CYP2E1 increases autophagosome formation but blocks autolysosome formation [34]. Ethanol in vitro increased total LC3-II levels in precision- cut rat liver slices [34]. Recent studies using hepatocytes, MCF-7 cells and HepG2 cells showed decreased autophagy after alcohol treatment in vitro [5, 47, 48] e.g. ethanol downregulated autophagy-related proteins such as Beclin-1 and LC3-II in immune cells [48].
In the current study, there was a decline in autophagy as reflected by decreased LC3 flux and decreased Beclin and Atg 7 levels coupled to increased expression of P62 and Bcl-2 (Fig 5A,5B) after acute alcohol administration. mTOR regulates protein synthesis, autophagy, and lipogenesis [49]. The acute alcohol administration increased phosphorylation of mTOR and activation of mTOR downstream targets 4E-BP1 and P70 (Fig 5C). This suggests that acute alcohol activated mTOR, which then decreases autophagy, resulting in steatosis. The JNK inhibitor prevented the effect on mTOR, autophagy markers and steatosis by acute alcohol. Importantly, the decrease in the LC3-II/LC3-I ratio and Beclin-1 and the increase in P62, Bcl-2, pmTOR and mTOR targets were all blocked by the JNK inhibitor (Fig 5) which likely contributes to the prevention of steatosis by the JNK inhibitor (Fig 1A, 1B).
Accumulation of lipids in liver may occur either via an increase of lipid synthesis, or decrease of lipid degradation or both. SREBP is an important transcription factor regulating lipid synthesis. Ethanol added in vitro to liver cell lines or chronic ethanol feeding elevated levels of SREBP [38]. Acute alcohol treatment increased SREBP expression in liver (Fig 7A). The JNK inhibitor prevented this increase by acute alcohol which suggests that the steatosis induced by acute alcohol may occur via the activation of JNK, followed by SREBP upregulation. Inhibition of this upregulation by the JNK inhibitor in addition to the inhibition of autophagy likely contributes to the inhibition of the steatosis.
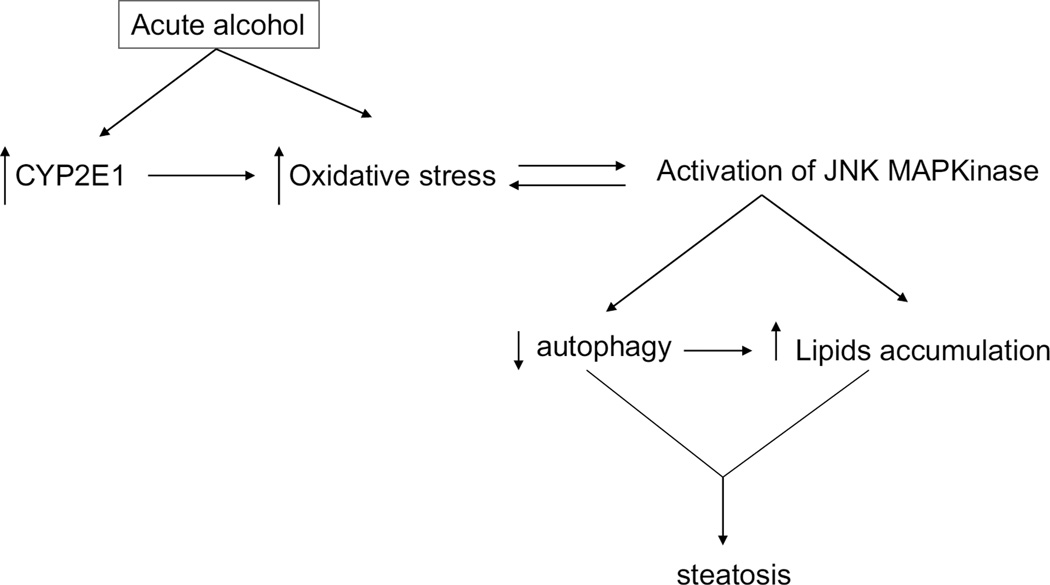
In conclusion, as depicted in the model (Fig. 9), acute alcohol treatment initially induces CYP2E1 activation followed by increases in oxidative stress in the liver. Increased oxidative stress activates JNK, while activated JNK further promotes oxidative stress. We propose the increased oxidative stress and activation of JNK results in a decrease of autophagy. The decrease in autophagy may also result from the increase in lipid accumulation. Interactions between CYP2E1, oxidative stress, JNK activation and decrease of autophagy play a role in the mechanism by which acute alcohol treatment promotes lipid accumulation in the liver. Inhibition of CYP2E1 or of JNK or antioxidant treatment or stimulation of autophagy may prove useful in preventing acute alcohol –induced fatty liver.
Fig. 9.
Scheme of acute alcohol induced steatosis. Acute alcohol treatment increases oxidative stress in liver. The increased oxidative stress activates JNK, which in turn reciprocally increases oxidative stress. Activation of JNK decreases autophagy and increase expression of SREBP-activated lipid synthesis enzymes, resulting in steatosis.
HIGHLIGHTS.
-
.
Acute alcohol induces hepatic lipid accumulation via a CYP2E1-dependent reaction.
-
.
Activation of JNK is necessary for the acute alcohol-induced steatosis.
-
.
CYP2E1-derived oxidant stress plays key role in the lipid accumulation and the activation of JNK.
-
.
A decrease in autophagy is associated with the alcohol-induced steatosis.
Acknowledgement
We appreciate greatly the help of Dr. Yongke Lu in genotyping of CYP2E1 KO mice.
Financial Support:
These studies were supported by USPHS Grants AA017425 and AA 018790 from The National Institute on Alcohol Abuse and Alcoholism.
List of Abbreviations
- CYP2E1
cytochrome P4502E1
- WT
wild type
- KO
knockout
- NAC
N-acetylcysteine
- TBARS
Thiobarbituric Acid Reactive Substances
- GSH
glutathione
- 4EBP1
4E-binding protein-1
- SREBP
Sterol Regulatory Element-Binding Proteins
- PPAR
peroxisome proliferator-activated receptors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Korkusuz H, Keese D, Raschidi BA, Hubner F, Namgaladze D, Hintereder G, Korkusuz Y, Monch C, Vogl TJ. Detection of a Fatty Liver After Binge Drinking Correlation of MR-spectroscopy, DECT, Biochemistry and Histology in a Rat Model. Acad Radiol. 2011;18:1349–1357. doi: 10.1016/j.acra.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Passeri MJ, Cinaroglu A, Gao C, Sadler KC. Hepatic steatosis in response to acute alcohol exposure in zebrafish requires sterol regulatory element binding protein activation. Hepatology. 2009;49:443–452. doi: 10.1002/hep.22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu Y, Wu D, Wang X, Ward SC, Cederbaum AI. Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice. Free Rad. Biol. Med. 2010;49:1406–1416. doi: 10.1016/j.freeradbiomed.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- 5.Wu D, Wang X, Zhou R, Cederbaum A. CYP2E1 enhances ethanol-induced lipid accumulation but impairs autophagy in HepG2 E47 cells. Biochem Biophys Res Commun. 2011;402:116–122. doi: 10.1016/j.bbrc.2010.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kodama Y, Kisseleva T, Iwaisako K, Miura K, Taura K, De Minicis S, Osterreicher CH, Schnabl B, Seki E, Brenner DA. c-Jun N-terminal kinase-1 from hematopoietic cells mediates progression from hepatic steatosis to steatohepatitis and fibrosis in mice. Gastroenterology. 2009;137:1467–1477. e5. doi: 10.1053/j.gastro.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Xu A, Chung SK, Cresser JH, Sweeney G, Wong RL, Lin A, Lam KS. Selective inactivation of c-Jun NH2-terminal kinase in adipose tissue protects against diet-induced obesity and improves insulin sensitivity in both liver and skeletal muscle in mice. Diabetes. 2011;60:486–495. doi: 10.2337/db10-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–172. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 9.Czaja MJ. JNK regulation of hepatic manifestations of the metabolic syndrome. Trends Endocrinol Metab. 2010;21:707–713. doi: 10.1016/j.tem.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aroor AR, James TT, Jackson DE, Shukla SD. Differential changes in MAP kinases, histone modifications, and liver injury in rats acutely treated with ethanol. Alcohol Clin Exp Res. 2010;34:1543–1551. doi: 10.1111/j.1530-0277.2010.01239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishitani Y, Matsumoto H. Ethanol rapidly causes activation of JNK associated with ER stress under inhibition of ADH. FEBS Lett. 2006;580:9–14. doi: 10.1016/j.febslet.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 12.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Wu D, Yang L, Cederbaum AI. Hepatotoxicity mediated by pyrazole (CYP2E1) Plus TNF-alpha treatment occurs in jnk2(−/−) but not in jnk1(−/−) Mice. Hepatology. 2011;54(5):1753–1766. doi: 10.1002/hep.24540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49:87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Minemoto Y, Lin A. C-Jun N-terminal kinase1 (JNK1) but not JNK2 is essential for TNFα-induced c-Jun kinase activation and apoptosis. Mol Cell Biol. 2004;24:10844–10856. doi: 10.1128/MCB.24.24.10844-10856.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Singh R, Leflkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. Tumor necrosis factor induced liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem. 2006;281:15258–15267. doi: 10.1074/jbc.M512953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eminel S, Klettner K, Roemer L, Herdegan T, Waerzig B. JNK2 translocates to the mitochondria and mediates cytochrome release in PC12 cells in response to 6-hydroxydopamine. J Biol Chem. 2004;279:55385–55392. doi: 10.1074/jbc.M405858200. [DOI] [PubMed] [Google Scholar]
- 18.Amir M, Czaja MJ. Autophagy in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol. 2011;5:159–166. doi: 10.1586/egh.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caviglia JM, Gayet C, Ota T, Hernandez-Ono A, Conlon DM, Jiang H, Fisher EA, Ginsberg HN. Different fatty acids inhibit apoB100 secretion by different pathways: unique roles for ER stress, ceramide, and autophagy. J Lipid Res. 2011;52:1636–1651. doi: 10.1194/jlr.M016931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Degli Esposti D, Sebagh M, Pham P, Reffas M, Pous C, Brenner C, Azoulay D, Lemoine A. Ischemic preconditioning induces autophagy and limits necrosis in human recipients of fatty liver grafts, decreasing the incidence of rejection episodes. Cell Death Dis. 2011;2:e111. doi: 10.1038/cddis.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishina S, Korenaga M, Hidaka I, Shinozaki A, Sakai A, Gondo T, Tabuchi M, Kishi F, Hino K. Hepatitis C virus protein and iron overload induces hepatic steatosis through the unfolded protein response in mice. Liver Int. 2010;30:683–692. doi: 10.1111/j.1478-3231.2010.02210.x. [DOI] [PubMed] [Google Scholar]
- 22.Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, Lu B, Stolz DB, Clemens DL, Yin XM. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–1752. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komiya K, Uchida T, Ueno T, Koike M, Abe H, Hirose T, Kawamori R, Uchiyama Y, Kominami E, Fujitani Y, Watada H. Free fatty acids stimulate autophagy in pancreatic beta-cells via JNK pathway. Biochem Biophys Res Commun. 2010;401:561–567. doi: 10.1016/j.bbrc.2010.09.101. [DOI] [PubMed] [Google Scholar]
- 24.Song BJ, Gelboin HV, Park SS, Yang CS, Gonzalez FJ. Complimentary DNA and protein sequences of ethanol-inducible rat and human cytochrome P450s: transcriptional and posttranscriptional regulation of the rat enzyme. J Biol Chem. 1986;261:16689–16697. [PubMed] [Google Scholar]
- 25.Roberts BJ, Shoaf SE, Song BJ. Rapid changes in cytochrome P4502E1(CYP2E1) activity and other P40 isozymes following ethanol withdrawal in rat. Biochem Pharmacol. 1995a;49:1665–1673. doi: 10.1016/0006-2952(95)00098-k. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Lu Y, Xie B, Cederbaum AI. Chronic ethanol feeding potentiates Fas Jo2-induced hepatotoxicity: role of CYP2E1 and TNF-alpha and activation of JNK and P38 MAP kinase. Free Radic Biol Med. 2009;47:518–528. doi: 10.1016/j.freeradbiomed.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aroor AR, Shukla SD. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004;74:2339–2364. doi: 10.1016/j.lfs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Weng YI, Shukla SD. Effects of chronic ethanol treatment on the angiotensin II-mediated p42/p44 mitogen-activated protein kinase and phosphorylase a activation in rat hepatocytes. Alcohol. 2003;29:83–90. doi: 10.1016/s0741-8329(02)00325-7. [DOI] [PubMed] [Google Scholar]
- 29.Cederbaum AI. Role of CYP2E1 in ethanol-induced oxidant stress, fatty liver and hepatotoxicity. Dig Dis. 2010;28:802–811. doi: 10.1159/000324289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma A, Saurabh K, Yadav S, Jain SK, Parmar D. Ethanol induced induction of cytochrome P450 2E1 and activation of mitogen activated protein kinases in peripheral blood lymphocytes. Xenobiotica. 2011 doi: 10.3109/00498254.2011.624648. [DOI] [PubMed] [Google Scholar]
- 31.Mansouri A, Tarhuni A, Larosche I, Reyl-Desmars F, Demeilliers C, Degoul F, Nahon P, Sutton A, Moreau R, Fromenty B, Pessayre D. MnSOD overexpression prevents liver mitochondrial DNA depletion after an alcohol binge but worsens this effect after prolonged alcohol consumption in mice. Dig Dis. 2010;28:756–775. doi: 10.1159/000324284. [DOI] [PubMed] [Google Scholar]
- 32.Conde de la Rosa L, Moshage H, Nieto N. Hepatocyte oxidant stress and alcoholic liver disease. Rev Esp Enferm Dig. 2008;100:156–163. doi: 10.4321/s1130-01082008000300006. [DOI] [PubMed] [Google Scholar]
- 33.Yin XM, Ding WX, Gao W. Autophagy in the liver. Hepatology. 2008;47:1773–1785. doi: 10.1002/hep.22146. [DOI] [PubMed] [Google Scholar]
- 34.Osna NA, Thomes PG, Jr, TM Involvement of autophagy in alcoholic liver injury and hepatitis C pathogenesis. World J Gastroenterol. 2011;17:2507–2514. doi: 10.3748/wjg.v17.i20.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008;90:313–323. doi: 10.1016/j.biochi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Letters. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.You M, Fisher M, Deeg MA, Grabb DW. Ethanol induces fatty acid synthesis pathways by activation of SREBP. J. Biol Chem. 2002;277:29342–29347. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 39.Nagy LE. Molecular aspects of alcohol metabolism: transcription factors involved in early ethanol-induced liver injury. Annu Rev Nutr. 2004;24:55–78. doi: 10.1146/annurev.nutr.24.012003.132258. [DOI] [PubMed] [Google Scholar]
- 40.Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 41.Sabio G, Cavanagh-Kyros J, Ko HJ, Jung DY, Gray S, Jun JY, Barrett T, Mora A, Kim JK, Davis RJ. Prevention of steatosis by hepatic JNK1. Cell Metab. 2009;10:491–498. doi: 10.1016/j.cmet.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Minemoto Y, Lin A. c-Jun N-terminal protein kinase 1 (JNK1), but not JNK2, is essential for tumor necrosis factor alpha-induced c-Jun kinase activation and apoptosis. Mol Cell Biol. 2004;24:10844–10856. doi: 10.1128/MCB.24.24.10844-10856.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaneto H, Nakatani Y, Miyatsuka T, Kawamori D, Matsuoka TA, Matsuhisa M, Kajimoto Y, Ichijo H, Yamasaki Y, Hori M. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med. 2004;10:1128–1132. doi: 10.1038/nm1111. [DOI] [PubMed] [Google Scholar]
- 44.Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Czaja MJ. Autophagy in health and disease. 2. Regulation of lipid metabolism and storage by autophagy: pathophysiological implications. Am J Physiol Cell Physiol. 2010;298:C973–C978. doi: 10.1152/ajpcell.00527.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donohue TM., Jr Autophagy and ethanol-induced liver injury. World J Gastroent. 2009;15:1178–1185. doi: 10.3748/wjg.15.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noh BK, Lee JK, Jun HJ, Lee JH, Jia Y, Hoang MH, Hoang MH, Kim JW, Park KH, Lee SJ. Restoration of autophagy by puerarin in ethanol-treated hepatocytes via the activation of AMP-activated protein kinase. Biochem Biophys Res Commun. 2011;414:361–366. doi: 10.1016/j.bbrc.2011.09.077. [DOI] [PubMed] [Google Scholar]
- 48.von Haefen C, Sifringer M, Menk M, Spies CD. Ethanol enhances susceptibility to apoptotic cell death via down-regulation of autophagy-related proteins. Alcohol Clin Exp Res. 2011;35:1381–1391. doi: 10.1111/j.1530-0277.2011.01473.x. [DOI] [PubMed] [Google Scholar]
- 49.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]