Abstract
Brain tumors are devastating due to the high fatality rate and the devastating impact on life qualities of patients. Recent advancement of comparative transcriptome profiling tools and mouse genetic models has greatly deepened our understanding of the developmental origins of these tumors, which could lead to effective therapeutic strategies. We review recent progresses in three types of brain tumors: ependymoma, medulloblastoma, and malignant glioma. The conceptual framework established by these studies converged on three important aspects. First, subtypes in each tumor group originate from distinct cell types. Second, each cell-of-origin is uniquely sensitive to some but not other genetic mutations. Lastly, mutant stem cells may not transform until they differentiate into more restricted progenitor cell type. Overall, these findings indicate the existence of intricate interactions between gene mutations and developmental context for the formation of brain tumors.
Introduction
Brain cancers, such as glioblastoma, are often lethal because of their invasive nature and resistance to conventional surgical procedures, chemo- and radiation-therapies. The urgent need for novel therapies has led to great emphasis on the study of the cell of origin for these tumors, grounded within modern concepts on neuroglial lineage development. From the developmental perspective, the cell of origin is defined as a progenitor that gives rise to one or a few progeny cell types in a specific tissue or organ. As a deviation from normal development, the cell of origin for cancer can be viewed as a specific cell type that can become transformed to drive the formation of the tumor mass [1]. Brain tumors, including ependymoma, medulloblastoma and malignant glioma, are speculated to have distinct cellular origins, which can be discriminated on the basis of anatomical location, expression of cellular markers, and morphological resemblance to normal brain cells [2]. With the refinement of genomic analysis tools and genetic animal model systems, a surge of recent studies on brain tumors have revealed a new level of complexity and the dynamic nature of their origins. Here we provide an overview of this topic, focused on a few representative studies to illustrate how technical advancement enhanced our understanding of the developmental origins of brain tumors.
Developmental Origins of Ependymoma from Region-restricted Precursors
Ependymoma is a group of brain tumors localized on the ventricle wall along the cerebrospinal axis [2,3]. Despite histopathological resemblances, ependymomas at different regions, such as supratentorial, posterior fossa, and the spinal cord, have disparate prognoses [4,5]. Such a discrepancy could be caused either by different cellular origins or by distinct genetic mutations that led to these tumors. Using a powerful cross-species genomic comparison approach, Johnson et al [6] compared the transcriptome of human ependymoma samples with that of mouse NSCs isolated from discrete locations along the cerebrospinal axis, at both embryonic and adult stages. Remarkably, their molecular profiling data demonstrated that human supratentorial ependymomas with amplified EphB2 and deleted INK4a/ARF specifically matched the INK4a/ARF null mouse embryonic NSCs from the cerebral region but not those from other locations or at the adult age. Using a mouse model, they further showed that ependymomas resembling human supratentorial ependymoma could only be induced by over-expressing Ephb2 in embryonic cerebral NSCs. This study elegantly demonstrated that the heterogeneity of ependymomas is most likely rooted in the differences of their developmental origins. Importantly, this study also demonstrated a unique susceptibility of a subtype of NSCs to particular genetic lesions, suggesting a synergistic effect between mutations and signaling contexts in the cell of origin. Similar findings of regional expression differences have also been described in pilocytic astrocytoma [7], suggesting that the notion of region-restricted cellular origins is also supported by findings with other types of brain tumors.
Developmental Origins of Medulloblastoma from Hindbrain Precursors
Medulloblastoma (MB) is a type of primitive neuroectodermal brain tumors that originates from the hindbrain/posterior fossa area and typically involves the cerebellum in the pediatric age range. Multiple MB subtypes have been described showing different genetic alterations, pathological features, and locations [8]. For example, the desmoplastic subtype tends to localize on the surface of the brain and to carry mutations in the Shh pathway, while the classic subtype tends to reside deep in the cerebellar region and to carry mutations in the Wnt pathway. The developmental origin of the desmoplastic subtype has been linked to granule neuron precursors (GNPs), based on multiple lines of evidences: the superficial tumor location that matches GNP location, augmented Shh signaling that is critical for GNP proliferation, and resemblance in transcriptome profiles to GNPs. This notion was further solidified by mouse models that recapitulated this MB subtype after introducing activating mutations in the Shh pathway with GNP-specific Atoh1-Cre (also known as Math1-Cre) [9,10]. However, the developmental origins of the classic subtype of MB remained unclear until recently. Gibson et al [11] suggests that MBs with aberrant activities of the Wnt pathway likely originate from Zic+ cells (the presumed precursor of mossy-fiber neurons) in the dorsal brain stem but not GNPs in the EGL. They demonstrated this convincingly with a mouse medulloblastoma model induced by overexpressing the activated version of Ctnnb1 (gene encoding β-catenin) in BLBP+ radial glia cells. The dorsal brainstem location, molecular and pathological features of induced tumors all matched well with human classic subtype but not the desmoplastic subtype of MB. These studies strongly suggest that these subtypes of MBs have distinct developmental origins, each of which is uniquely susceptible to corresponding mutations.
Developmental Origin of glioma: neural stem cells or restricted progenitors?
As the most common and fatal group of brain tumors, glioma poses significant challenges to both cancer biologists and clinicians. Traditionally, gliomas have been classified as astrocytoma and oligodendrocytoma based on morphological resemblance to normal glial cell types in the brain called astrocytes and oligodendrocytes, respectively. However, the existence of a subtype termed astro-oligodendroglioma manifesting mixed morphologies of both cells suggests that the boundary of astrocytoma and oligodendrocytoma may not be clear [2]. Taking advantage of the large genomic and transcriptomic dataset on human gliomas, a recent landmark paper [12] from the Cancer Genome Atlas (TCGA) consortium re-classified malignant gliomas into four distinct subtypes based on their different molecular signatures: neural, proneural, classic and mesenchymal. This new molecular classification should not only make a strong impact on molecule-based diagnoses of glioma subtypes, but also pave the way for the identification of the developmental origins for these subtypes using mouse cancer models.
During normal brain development, neural stem cells (NSCs) give rise to all the mature neurons, astrocytes, and oligodendrocytes via intermediate, more restricted progenitor cells [13–15]. Due to the transient nature of intermediate progenitors, most of them remain elusive despite many years of research, with oligodendrocytic progenitor cells (OPCs) as an exception [16]. Here we review recent papers that support the roles of NSCs and OPCs as the origin for gliomas.
Neural stem cells (NSCs) as the origin for gliomas
The NSC is characterized by its capacity to self-renew and its plasticity to differentiate into multiple cell types [15]. Observations of the cellular heterogeneity and renewability of glioma cells [17,18] led to the hypothesis that NSCs may be the origin for gliomas.
A few recent studies using genetic mouse models provided some supportive evidences for this hypothesis. For instance, the introduction of mutations in tumor suppressor genes p53 and NF1 [19], p53 and PTEN [20], or the overexpression of a mutant form of p53 [21], into NSCs with hGFAP- or Nestin-Cre efficiently induced mouse brain tumors that recapitulate human glioma pathology. Concordantly, introducing INK4a/Arf and EGFRVIII mutations into cultured neural spheres, which has been generally believed to enrich NSC population in vitro, afforded these cells capacities to initiate gliomas after intracranial grafting into immuno-compromised mice [22,23]. Lastly, Alcantara Llaguno et al [24] delivered Cre into specific anatomical structures in the brain via virus injection to inactivate tumor suppressor genes, and showed that subventricular zone (SVZ, where adult NSCs reside) but not other brain regions is particular susceptible to glioma formation. In summary, these collective evidences provided strong support for the hypothesis that NSC is the origin for gliomas.
However, the NSC hypothesis faces an intrinsic experimental quandary to unequivocally distinguish two scenarios: do NSCs serve as a direct origin, or do lineage-restricted progenitor cells that derive from NSCs function as the actual origin for gliomas. If the latter turns out to be true, what would be the identity of such progenitor cells?
Oligodendrocyte progenitor cells (OPCs) as the origin for gliomas
OPCs, reflected by their name, were originally believed to function solely as the precursor for mature oligodendrocytes. However, many studies have shown that OPCs persist into adulthood (4–5% of total adult brain cells) as the largest proliferating pool in both rodent and human brain parenchyma [16]. An initial clue that OPCs could be involved in glioma was the observation that Olig2, a bHLH transcription factor essential for OPC development, was not only universally expressed in human gliomas and also functionally required for gliomagenesis in a mouse model [22,25].
The possible role for OPCs as the glioma origin has been further supported by recent papers. Weiss and his colleagues [26,27] reported that human S100β promoter-directed expression of v-erbBan activated variant of EGFRcould induce glioma formation in a p53-null sensitized genetic background. S100β, though widely expressed in many mature cell types, does not turn on its expression in NSCs. Thus, this study provided strong evidence that cells other than NSCs can function as the glioma origin. In parallel, by injecting PDGF-BB expressing retrovirus into the white matter tract of mice, the Canoll group demonstrated that the well-known mitogen for developing OPCs could effectively initiate glioma formation in p53or PTEN or p53/PTEN double mutant mice [28]. Further analysis of tumors in both models revealed salient features of OPC, suggesting the critical role of OPC in gliomagenesis. More specifically, Lindberg et al [29] were able to generate gliomas by utilizing an RCAS/tv-a mouse model to over-express PDGF-BB in a CNPase-positive cell population that includes OPCs, although the penetrance was lower than what the Canoll group reported [28]. Lastly, the inactivation of p53 and NF1 by OPC-specific NG2-Cre transgene [30] led to the formation of malignant gliomas [31]. All these evidences clearly demonstrated that OPC could serve as the developmental origin for gliomas.
It is worth noting that cross-species transcriptome analysis revealed OPC-originated mouse gliomas matched well with human proneural subtype of GBM [12,28,31], suggesting OPCs as the developmental origin for this particular subtype of glioma. Importantly, in these animal studies the initial mutations used to drive transformation widely embodied those most frequently found in human GBMs, including p53PTENv-erbB, Nf1 and Ras [32], suggesting that OPCs could turn malignant with a wide range of mutations.
The Cell-of-origin and the cell-of-mutation are two distinct entities
Previously, the origin of cancer was defined by a “tried-and-true” methodology, whereby oncogenic mutations were introduced into populations of cells with particular developmental or regional characteristics to test their transforming potentials. However, the advent of new mouse genetic tools allowed us to dissect the tumor development process with unprecedented temporal and spatial resolution. It became clear that cells acquiring initial mutations (cell-of-mutation) might not directly transform; rather lineage-restricted progeny cells derived from the cell-of-mutation could inherit the mutations and subsequently transform into malignancy (see Figure 1B for an example). In the case of desmoplastic medulloblastoma model, although mutations that activate Shh signaling in both NSCs and GNPs could lead to tumor formation, the malignant growth is only seen in GNPs but barely in NSCs, suggesting that GNP is the cell-of-origin while both NSC and GNP could serve as the cell-of-mutation [9,10].
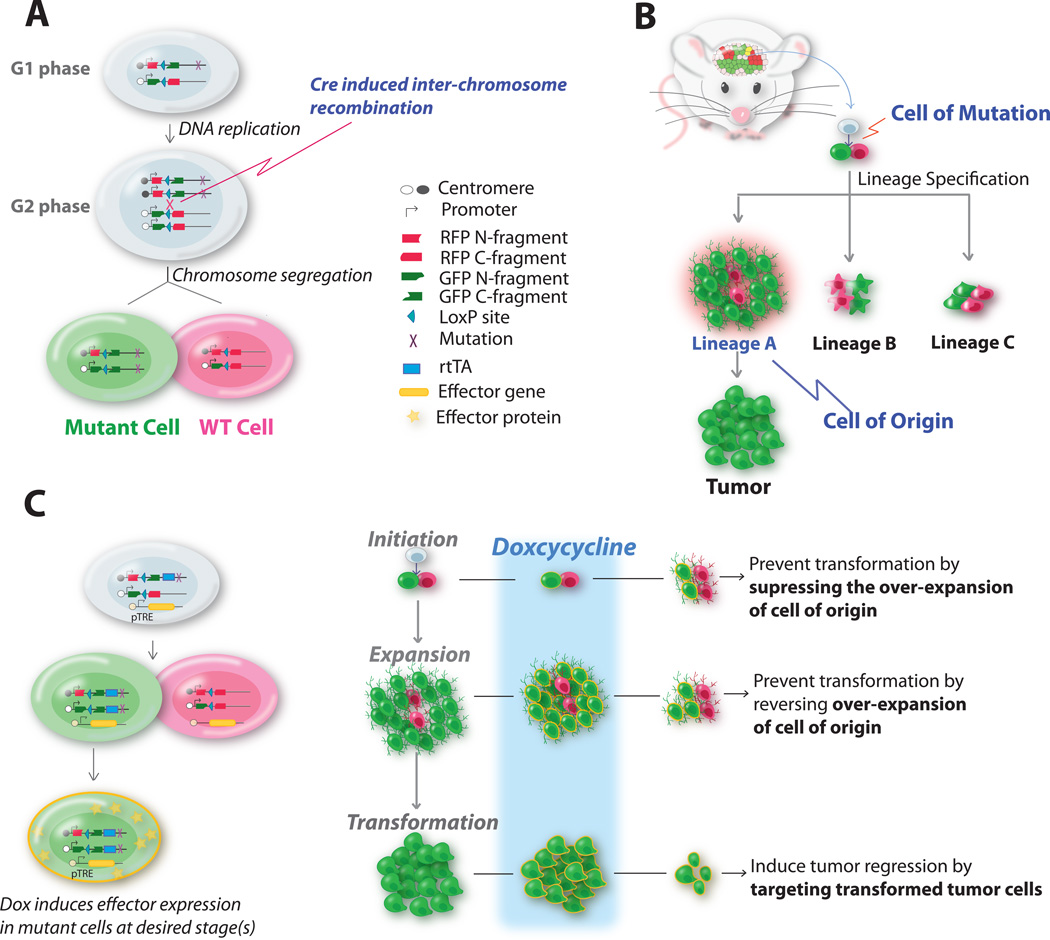
Figure 1. MADM, a mouse genetic mosaic model, can be used for identifying tumor cell of origin and evaluating novel targets against such cells for tumor prevention or treatment.
(A) Scheme that shows how MADM can concurrently mutate and label cells via a Cre/loxP-mediated inter-chromosomal mitotic recombination event. Green cells are homozygous null for the gene-of-interest that is distal to the MADM cassette on the same chromosome; red cells are WT cells. For details, see Zong et al 2005.
(B) Scheme that illustrates how to use MADM-based cancer model to identify cell of origin by analyzing the aberrant growth of all progeny lineages derived from mutant stem/progenitor cells in a defined organ. Although we use the brain as an example here, the same principle should be applicable to tumors in other organs. For details, see Liu et al 2011.
(C) The MADM system can be further modified by incorporating the Tet-ON system to investigate the effects of further genetic manipulation on the cell of origin. With the temporal control of the intervention, one could model the treatment effects of any gene on tumor initiation, progression, and malignancies.
The lesson learned from medulloblastoma studies now demands one to generate the same set of mutations in NSCs and each of progeny lineages to test their ability to form brain tumors before claiming the cell-of-origin. However, this is currently unfeasible for most brain tumors since some of the lineage-restricted progenitors have not been well defined and others have no specific genetic tools. An alternative solution to tease apart cell-of-origin and cell-of-mutation is to analyze individual cell lineages along the entire developmental process of tumorigenesis by using a mouse genetic mosaic model termed MADM (Mosaic Analysis with Double Markers) [31,33,34] (see also Figure 1A). Via Cre/loxP-mediated mitotic inter-chromosomal recombination, MADM generates a small number (0.1%–1% or much lower) of GFP-labeled homozygous mutant cells, thus mimicking the sporadic loss of heterozygosity (LOH) of TSGs in human cancers. MADM provides precise control for tumor initiation in vivo since the cell type specificity and the timing of mutation induction are completely determined by the activity of Cre transgenes. Since the efficiency of Cre/loxP-induced LOH is much higher than that of spontaneous LOH, the probability of tumor initiation from cells that are not labeled by GFP is very low. Moreover, MADM also labels sibling WT cells with red fluorescent protein such as DeRed or tdTomato, allowing the direct phenotypic comparison between mutant and WT sibling cells at a single-cell resolution in vivo, with the latter as an ideal internal control. Lastly, the permanent labeling of mutant cells with GFP allows one to trace the lineage of these cells, providing direct in vivo evidence of their aberrant capacity in proliferation, migration, differentiation, and even trans-differentiation into other cell types.
Using MADM, the developmental origin of gliomas induced by p53/NF1 mutations was examined by first introducing the mutations into NSCs and then analyzing the overexpansion in each progeny lineage. Surprisingly, detailed analysis of lineage-specific expansions prior to full malignancy revealed subtle or no over-proliferation in NSCs, neurons and astrocytes, but dramatic aberrant growth in OPCs. Along with histological, transcriptomic profiling, and genetic evidences, it became clear that OPCs function as the cell-of-origin in this model, while NSCs act as the cell-of-mutation [31]. This study not only helped reconcile one of the debates in the glioma field, but also provided experimental principles that could be used to identify the origin of other tumor types (illustrated in Figure 1B). To model treatment effects of a gene of interest at distinct tumor developmental stages, one can incorporate the Tet-ON/OFF binary system into MADM by fusing one chimeric MADM cassette with Dox-inducible transcription factor rtTA or tTA [35]. The modified MADM system would allow one to turn on/off the expression of TRE-driven effector gene at desired time, including but not limited to tumor suppressor gene, oncogene, or shRNAs against such genes (Figure 1C).
The distinction between cell-of-mutation and cell-of-origin carries great significance for both medical and basic research. For clinicians, the cell-of-origin is the transforming step of tumors thus would be an ideal therapeutic target. For basic researchers, the selectivity of transforming potentials in particular cell types clearly demonstrates signaling synergies between gene mutations and signaling context in the cell-of-origin. Further studies of such synergies will not only lead to novel ideas for clinical interventions, but also provide deep insights of the robustness of normal developmental programs.
Conclusions
Recent advancement in bioinformatics analysis and mouse genetic tools has greatly enhanced our understanding for the developmental origins of brain tumors. Large-scale genomic comparison between tumors and normal cell types, sometimes cross-species, has been highly successful in many studies. However, due to the highly plastic nature of end-stage cancer cells, their “birthmark” could be tampered during tumor progression. For example, the entire transcriptome profile of GBM could change dramatically by disturbing a single gene [36,37]. Therefore, it is imperative to complement bioinformatics analyses with mouse genetic studies to consolidate the claim of the developmental origin of brain tumors.
Finally, it is important to note that recent findings brought about even more new questions. Each type of brain tumor now can be divided into subgroups based on their unique molecular signatures in addition to their pathological distinctions. This implies that distinct cell types could serve as the cell of origin for each of these subtypes, a notion best supported by studies of medulloblastoma. In the case of gliomas, it is highly possible that multiple cell types, such as NSCs, glial progenitors, and even mature astrocytes, could serve as the developmental origins for non-proneural subtypes. Although these questions can only be resolved with further studies, the breathtaking progresses in recent years gave us the confidence that these goals should be within our reach.
Highlights.
Distinct developmental origins and susceptibility to mutations contribute to subtypes of brain tumors
Gene expression profiling, especially cross-species analysis, is a powerful tool to reveal the heterogeneity of tumor origin
Mouse cancer models with lineage tracing capacity provide concrete evidences of brain tumor origins
Cell-of-mutation and cell-of-origin are distinct concepts: former refers to cells with initial mutations, while the latter refers to cells that transform into tumors.
Aknowledgements
We thank David Rowitch for helpful comments and discussions, and Jesse Goldfarb for proofreading. Grant support to HZ: NIH R01-CA136495, Department of Defense (DoD) Peer Reviewed Cancer Research Program (PRCRP) CA100469, and the W. M. Keck Foundation. HZ is Pew Scholar in Biomedical Sciences, supported by The Pew Charitable Trusts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of special interest
** of outstanding interest
- 1. Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781.. ** This review not only summarized the most recent findings of cell-of-origin in a few highly studied tumor types, but also emphasized the distinction between cell-of-origin and cancer stem cell concepts and pointed out that clonal level lineage tracing is the "gold standard" for identifying cell-of-origin.
- 2.Fuller GN. The WHO Classification of Tumours of the Central Nervous System, 4th edition. Arch Pathol Lab Med. 2008;132:906. doi: 10.5858/2008-132-906-TWCOTO. [DOI] [PubMed] [Google Scholar]
- 3.Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. discussion 226-219. [DOI] [PubMed] [Google Scholar]
- 4.Modena P, Lualdi E, Facchinetti F, Veltman J, Reid JF, Minardi S, Janssen I, Giangaspero F, Forni M, Finocchiaro G, et al. Identification of tumor-specific molecular signatures in intracranial ependymoma and association with clinical characteristics. J Clin Oncol. 2006;24:5223–5233. doi: 10.1200/JCO.2006.06.3701. [DOI] [PubMed] [Google Scholar]
- 5.Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 6. Johnson RA, Wright KD, Poppleton H, Mohankumar KM, Finkelstein D, Pounds SB, Rand V, Leary SE, White E, Eden C, et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature. 2010;466:632–636. doi: 10.1038/nature09173.. ** By comparing transcriptomic profiles between human ependymoma samples and mouse NSCs along the cerebrospinal axis, heterogeneity of NSCs was identified and accounted for prognostic variations among human ependymoma patients. Mouse models were further used to confirm their findings.
- 7.Sharma MK, Mansur DB, Reifenberger G, Perry A, Leonard JR, Aldape KD, Albin MG, Emnett RJ, Loeser S, Watson MA, et al. Distinct genetic signatures among pilocytic astrocytomas relate to their brain region origin. Cancer Res. 2007;67:890–900. doi: 10.1158/0008-5472.CAN-06-0973. [DOI] [PubMed] [Google Scholar]
- 8.Gilbertson RJ, Ellison DW. The origins of medulloblastoma subtypes. Annu Rev Pathol. 2008;3:341–365. doi: 10.1146/annurev.pathmechdis.3.121806.151518. [DOI] [PubMed] [Google Scholar]
- 9.Schuller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, Huillard E, Sun T, Ligon AH, Qian Y, et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14:123–134. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang ZJ, Ellis T, Markant SL, Read TA, Kessler JD, Bourboulas M, Schuller U, Machold R, Fishell G, Rowitch DH, et al. Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell. 2008;14:135–145. doi: 10.1016/j.ccr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, Kranenburg TA, Hogg T, Poppleton H, Martin J, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468:1095–1099. doi: 10.1038/nature09587.. ** This study presented the first non-desmoplastic medulloblast model in mice, and illustrated that distinct subtypes of medulloblastoma oirginate from different cell types that are susceptible to unique genetic mutations.
- 12. Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020.. ** This study re-classified glioma subtypes according to their molecular profiling rather than histo-pathology, providing important framework for the identification of developmental origins of each subtype of gliomas.
- 13.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 14. Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001.. * The most recent and comprehensive review of adult neural stem cells.
- 15.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 16.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 17.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 18.Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, Messing A, Parada LF. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8:119–130. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455:1129–1133. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Yang J, Zheng H, Tomasek GJ, Zhang P, McKeever PE, Lee EY, Zhu Y. Expression of mutant p53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model. Cancer Cell. 2009;15:514–526. doi: 10.1016/j.ccr.2009.04.001.. ** By tracking mutatant p53 expression, this studies demonstrated that early transformation occurs in type C* cells around subventricular zone.
- 22.Ligon KL, Huillard E, Mehta S, Kesari S, Liu H, Alberta JA, Bachoo RM, Kane M, Louis DN, Depinho RA, et al. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007;53:503–517. doi: 10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 24. Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, Alvarez-Buylla A, Parada LF. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15:45–56. doi: 10.1016/j.ccr.2008.12.006.. ** Using both genetic and viral injection into restricted brain regions, this study showed that adult NSCs are sufficient in generating gliomas upon genetic mutations.
- 25.Ligon KL, Alberta JA, Kho AT, Weiss J, Kwaan MR, Nutt CL, Louis DN, Stiles CD, Rowitch DH. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol. 2004;63:499–509. doi: 10.1093/jnen/63.5.499. [DOI] [PubMed] [Google Scholar]
- 26.Weiss WA, Burns MJ, Hackett C, Aldape K, Hill JR, Kuriyama H, Kuriyama N, Milshteyn N, Roberts T, Wendland MF, et al. Genetic determinants of malignancy in a mouse model for oligodendroglioma. Cancer Res. 2003;63:1589–1595. [PubMed] [Google Scholar]
- 27. Persson AI, Petritsch C, Swartling FJ, Itsara M, Sim FJ, Auvergne R, Goldenberg DD, Vandenberg SR, Nguyen KN, Yakovenko S, et al. Non-stem cell origin for oligodendroglioma. Cancer Cell. 2010;18:669–682. doi: 10.1016/j.ccr.2010.10.033.. ** This study showed that gliomas could be induced from cell types other than NSCs.
- 28. Lei L, Sonabend AM, Guarnieri P, Soderquist C, Ludwig T, Rosenfeld S, Bruce JN, Canoll P. Glioblastoma models reveal the connection between adult glial progenitors and the proneural phenotype. PLoS One. 2011;6:e20041. doi: 10.1371/journal.pone.0020041.. ** By injecting PDGF-coding retrovirus into the white matter of adult mutant mice, this study demonstrated the sufficiency of OPC in gliomagenesis.
- 29.Lindberg N, Kastemar M, Olofsson T, Smits A, Uhrbom L. Oligodendrocyte progenitor cells can act as cell of origin for experimental glioma. Oncogene. 2009;28:2266–2275. doi: 10.1038/onc.2009.76. [DOI] [PubMed] [Google Scholar]
- 30.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 31. Liu C, Sage JC, Miller MR, Verhaak RG, Hippenmeyer S, Vogel H, Foreman O, Bronson RT, Nishiyama A, Luo L, et al. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146:209–221. doi: 10.1016/j.cell.2011.06.014.. ** This study used MADM, a genetic mosaic mouse model, to demonstrated that while NSCs serve as "cell-of-mutation", OPCs are the actual "cell-of-origin" for gliomagenesis. It reconciles the debate between NSC and OPC hypotheses in the field.
- 32.Barrett LE, Granot Z, Coker C, Iavarone A, Hambardzumyan D, Holland EC, Nam HS, Benezra R. Self-renewal does not predict tumor growth potential in mouse models of high-grade glioma. Cancer Cell. 2012;21:11–24. doi: 10.1016/j.ccr.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 33.Zong H, Espinosa JS, Su HH, Muzumdar MD, Luo L. Mosaic analysis with double markers in mice. Cell. 2005;121:479–492. doi: 10.1016/j.cell.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Muzumdar M, Luo L, Zong H. Modeling sporadic loss of heterozygosity in mice by using mosaic analysis with double markers (MADM) Proc Natl Acad Sci USA. 2007;104:4495–4500. doi: 10.1073/pnas.0606491104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tasic B, Miyamichi K, Hippenmeyer S, Dani VS, Zeng H, Joo W, Zong H, Chen-Tsai Y, Luo L. Extensions of MADM (Mosaic Analysis with Double Markers) in Mice. PLoS One. 2012;7:e33332. doi: 10.1371/journal.pone.0033332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, Sulman EP, Anne SL, Doetsch F, Colman H, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463:318–325. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhat KP, Salazar KL, Balasubramaniyan V, Wani K, Heathcock L, Hollingsworth F, James JD, Gumin J, Diefes KL, Kim SH, et al. The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes Dev. 2011;25:2594–2609. doi: 10.1101/gad.176800.111. [DOI] [PMC free article] [PubMed] [Google Scholar]