Abstract
Methamphetamine damages monoamine-containing nerve terminals in the brains of both animals and human drug abusers, and the cellular mechanisms underlying this injury have been extensively studied. More recently, the growing evidence for methamphetamine’s influences on memory and executive function of human users has prompted studies of cognitive impairments in methamphetamine-exposed animals. After summarizing current knowledge about the cellular mechanisms of methamphetamine-induced brain injury, this review emphasizes research into the brain changes that underlie the cognitive deficits that accompany repeated methamphetamine exposure. Novel approaches to mitigating or reversing methamphetamine-induced brain and behavioral changes are described, and it is argued that the slow spontaneous reversibility of the injury produced by this drug may offer opportunities for novel treatment development.
Keywords: Addiction, Amphetamines, Recognition Memory, Striatum, Dopamine, Serotonin
Introduction
Methamphetamine (mAMPH) is a highly addictive, widely abused psychostimulant drug. First synthesized in 1893 [1], mAMPH has been used for the treatment of attentional deficit hyperactivity disorder, narcolepsy, asthma, and obesity [2–5]. Due to its ease of synthesis from commonly available precursors, mAMPH abuse in the U.S. has reached near-epidemic proportions. Acutely, mAMPH intake leads to euphoria, increased vigilance, hyperactivity, and cardiovascular changes -- actions that are similar to those of other psychostimulant drugs [6]. However, when taken repeatedly or in higher doses, mAMPH can result in adverse physiological and neurological consequences, including weight loss, muscular wasting, periodontal necrosis, brain injury, and impaired attention, memory and executive functions. After a single dose, amphetamines persist in the human body for nearly a day [7], and this long duration of action is thought to contribute to the brain injury and cognitive impairments seen in mAMPH abusers. Recently, much new information has been acquired about how mAMPH affects the brain and behavior of both human users and animals. This review will emphasize the evidence germane to three questions: (1) What are the brain changes seen in the mAMPH-using population, and do these changes represent enduring brain injury? (2) What can animal models reveal about the effects of mAMPH exposure on persisting neural and behavioral impairments? (3) Is brain injury needed for mAMPH-induced cognitive impairments to occur?
mAMPH-induced brain changes in animal models
When discussing any drug’s actions, it is important to distinguish acute vs. persistent effects. Like other amphetamines, mAMPH interacts with nerve terminals that use catecholamines (dopamine, DA and norepinephrine, NE) or the indoleamine, serotonin (5-HT), as neurotransmitters. Whether swallowed, snorted, smoked, or taken intravenously, mAMPH crosses the blood-brain barrier (BBB) and enters these terminals, inducing a non-exocytotic transmitter ‘release’. At least two general actions of mAMPH contribute to this monoamine transmitter release: (1) redistribution of amines from synaptic vesicle sites into the cytoplasm, and (2) reverse transport of the monoamine transmitters from cytoplasm to extracellular space, via their plasma membrane uptake carriers [8]. These acute drug actions, especially those occurring in the DA nerve terminals of the striatum (caudate nucleus, putamen, and nucleus accumbens (NAc) area of humans), account for the initial euphoric and other stimulatory actions of mAMPH [9].
In addition to these acute actions, mAMPH is capable of inducing long-lasting brain changes. Such long-term effects include: (1) the development of compulsive patterns of mAMPH use, (2) induction of brain injury, and (3) alterations in the cognitive functions of the mAMPH-using population. Research using animals (most often, rodents) and employing several different mAMPH dosing regimens (see Box 1), has been essential to characterize mechanisms underlying these enduring effects of mAMPH. These animal models include: (1) mAMPH self-administration to study drug-seeking, (2) escalating mAMPH dose regimens to examine the enhanced neurobehavioral influences of mAMPH with repeated exposure, (3) mAMPH conditioned place preference (CPP) to investigate the neural substrates of this drug’s rewarding effects, and (4) binge mAMPH administration models that readily induce neurotoxicity of brain DA and 5-HT systems. Animal research has played an especially important role in these investigations, because only in animals can the exposure to mAMPH be isolated from the other variables (pharmacologic, genetic, environmental) that affect brain and cognition.
BOX 1. Controversial aspects of mAMPH brain injury - Dose regimens.
Most of the evidence for mAMPH neurotoxicity comes from animals exposed to binge dosing regimens. Although human mAMPH abusers have been reported to take the drug in prolonged “runs” involving multiple sequential doses, binge administration is not the typical pattern of first or habitual mAMPH use. As is typical with many other drugs of abuse, human mAMPH users initially take low doses and progressively increase their drug intake. This type of escalating dose schedule has been studied in rodents and generally does not produce monoaminergic damage. In fact, escalating doses protect against damage arising from a subsequent single-day [22, 23], but not a multiple-day, mAMPH binge [25], suggesting that prior limited mAMPH exposure provides some, but not blanket, neuroprotection.
Recently, it has been argued that rats that learn to self-administer mAMPH afford a more translationally relevant animal model for studying mAMPH-induced brain alterations [24, 104], because self-administering animals, like human abusers, control their own intake pattern and typically escalate their use over time. Since contingent (self-administered) doses of mAMPH have been shown to produce patterns of DA and GLU release in the NAc different from those produced by non-contingent (experimenter-administered) injections of equivalent doses [105], the type of mAMPH administration used could have important effects on the development of neurotoxicity. Self-administration studies in which access to mAMPH is limited to 1–2 hours/day showed no lasting changes in monoaminergic markers [24]. By contrast, experiments that allow rats to self-administer mAMPH for several hours per day report that one or two weeks of extended access mAMPH self-administration results in lasting decreases in DAT, but not in other markers associated with DA injury ([24, 104] but see [87]). This raises the possibility that self-administration of mAMPH may be less likely to induce neurotoxicity compared to experimenter-delivered doses. However, when allowed even longer daily access to mAMPH (15 hours/day), rats self-administer even more drug and, following 8d of access, they experience a long-lasting loss of dopaminergic markers (DA, DAT, and TH) and show glial activation similar to that seen in human mAMPH users and “binge” animal experiments [26]. Importantly, the daily intake (mg/kg) of mAMPH in the study using 15 hour drug access [26], but not 6 hour access [24], approximates the threshold daily dose for producing neurotoxicity in the “binge” paradigm [28, 106].
In view of the close relationship between the amount of mAMPH consumed by self-administering animals and consequent reductions in DA content as well as astroglial proliferation [26], it seems reasonable to conclude that rodent ‘models’ aimed at replicating the neurochemical deficits seen in mAMPH abusers need to employ dosing regimens that provide high enough sustained levels of mAMPH (whether experimenter- or self-administered) to achieve the same suite of brain changes as those documented in populations of mAMPH abusers.
Psychostimulant-induced neurotoxicity has most commonly been modeled in rodents by administration of moderate-to-high doses of mAMPH in a “binge” pattern, consisting of several doses given during a single day. Repeated mAMPH dosing in single day has also been shown effective in inducing neurotoxicity in non-human primates [10, 11]. Binge mAMPH dosing regimens produce long-lasting reductions in forebrain DA and 5-HT contents, accompanied by decreases in multiple additional enzymes and transporter molecules specific to DA and 5-HT nerve terminals (see Figure 1A–C; for recent reviews see [12–15]). Additional studies show that these reductions represent injury to monoamine nerve terminals, because they are accompanied by increases in histological markers for degenerating nerve terminals (i.e., silver staining [16] and Fluoro-Jade C fluorescence [17]) as well as astroglial and microglial activation in striatum [18–21]. Even when animals are exposed to mAMPH in dosing regimens that more carefully model features of human mAMPH use (i.e., self-administration, or experimenter-administered escalating doses followed by binge treatments), the mAMPH-induced injury to monoamine terminals, while blunted [22–24], still occurs [25, 26] (see Box 1). Additionally, non-human primates given mAMPH at doses that mimic recreational use in humans experience long-lasting damage to dopaminergic neurons [27].
Figure 1. Imaging studies show mAMPH-induced damage to dopaminergic terminals in both rats (A–C) and humans (D–G).
(A) A diagram of a coronal section of a rat brain showing the plane sampled and the location of the caudate-putamen (CP) and nucleus accumbens (NAc). (B) Binding of the DAT ligand [125I]RTI-55 in a control rat [97], as revealed by in vitro audtoradiography. (C) mAMPH-induced DAT losses in a rat treated with a binge regimen of mAMPH (4 doses of 4 mg/kg, with doses separated by 2 hr intervals) [97]. (D) A standard triaxial magnetic resonance image (MRI) of a human brain to show the plane sampled in images E–G and noting the locations of the caudate nucleus (Cd) and putamen (Put) [47]. (E) A representation of positron emission tomography (PET) images of the binding potential of the DAT ligand [11C]WIN 35,428 in control subjects [47]. (F) mAMPH-induced WIN binding decreases in a group of abusers who had used mAMPH an average of 21 times/month for 5.5 years, but had abstained from mAMPH use of an average of nearly 6.5 years before scanning [47]. (G) DAT depletions in patients diagnosed with Parkinson’s disease [47]. Figures are adapted, with permission, from [97] (panels A–C) and [47] (panels D–G).
How can mAMPH damage brain monoaminergic systems? Acutely, binge mAMPH dosing regimens cause extraordinary increases in extracellular levels of monoamines [28, 29] and other transmitters. Excess monoamine release activates an array of brain circuits whose effects are crucial to the development of mAMPH-induced terminal damage. Particularly important are the polysynaptic basal-ganglia-thalamo-cortical loops that, under the influence of mAMPH, increase glutamate (GLU) release from corticofugal fibers [30]. A second crucial effect of mAMPH is alteration of thermoregulation. Several lines of research have demonstrated that the combined actions of mAMPH increase (1) extracellular levels of monoamines, (2) extracellular GLU, and (3) body temperature, activating a range of downstream events that can compromise the integrity of monoamine terminals. Importantly, these downstream actions of mAMPH do not occur in isolation, but may interact to produce brain injury. Consequently, it is difficult to assign clear roles for each event in causing mAMPH neurotoxicity. As summarized diagrammatically in Figure 2, these downstream events triggered by mAMPH include:
Figure 2. Mechanisms of mAMPH neurotoxicity.
mAMPH (mA) enters dopaminergic terminals, as shown in the right side of the diagram (1), causing efflux of DA from intraneuronal vesicles. This DA is broken down intracellularly, producing reactive species (2) such as hydrogen peroxide (H2O2) and superoxide (·O2−), and is transported to extracellular spaces (3) where it is also oxidized producing reactive oxygen species (ROS). High intracellular concentrations of DA and mAMPH can inhibit the electron transport chain (ETC) in mitochondria (4), causing leakage of high-energy electrons which trigger the formation of superoxide. mAMPH-induced increases in GLU release, seen on the left side of the diagram (5), stimulate NMDA receptors (NMDAR) on dopaminergic terminals causing increases in intracellular Ca++. These Ca++ increases stimulate nitric oxide synthase (NOS) activity, increasing the production of nitric oxide (NO), which can combine with superoxide to form highly-damaging peroxynitrite (·ONOO−)(6). High intracellular Ca++ also stimulates proteolytic enzymes such as calpain (7) which can break down structural proteins such as spectrin and tau, damaging terminal integrity. mAMPH also stimulates microglia to release ROS and cytokines (8) which further increase extracellular GLU levels. Finally, mAMPH causes leakage of the BBB, allowing plasma proteins to enter the brain (9), followed by water and ions, causing brain edema and further physiological disruption of neurotransmission.
1) Increased production of reactive oxygen and nitrogen species (ROS and RNS, respectively)
Both the DA and GLU released by mAMPH can result in the production of highly reactive oxygen and nitrogen species. Excess DA is enzymatically broken down, producing 3,4-dihydroxyphenylacetic acid DOPAC and the reactive oxygen compound hydrogen peroxide. DA can also be non-enzymatically autooxidized into cytotoxic DA-o-quinones or into DA semiquinones, with the production of highly reactive superoxide anions [31, 32]. The excessive production of ROS triggered by mAMPH can overwhelm the capacity of endogenous neuronal antioxidative systems, damaging cellular lipids, proteins, and nucleic acids [33].
The increased extracellular GLU occurring during binge mAMPH activates NMDA receptors, allowing intracellular Ca2+ influx. This increased intracellular Ca2+ activates enzymatic pathways that increase ROS and RNS production. For example, mAMPH activation of the protease calpain leads to increased uric acid formation (with concomitant free radical production [34]) and arachidonic acid breakdown (yielding oxygen radicals [35]). Increased Ca2+ also activates nitric oxide synthase, increasing nitric oxide (NO) levels, which can directly damage cells or react with superoxide forming highly reactive peroxynitrite [36]. It has been shown that mAMPH-induced RNS can damage the integrity of cellular proteins by covalently reacting with available tyrosine and cysteine residues [37].
2) Metabolic compromise
The electron transport chain in mitochondria passes high-energy electrons through a series of acceptor proteins to drive ATP formation, ultimately reducing oxygen to form water. Even under normal conditions, 1–3% of the high-energy electrons can “leak” from the chain, prematurely and incompletely reducing oxygen to form superoxide. Both mAMPH and DA inhibit electron transport chain complexes [38, 39], increasing superoxide production and depriving mAMPH-stressed neurons of the ATP they need for recovery/repair.
3) Induction of inflammatory responses
Microglia act as the resident macrophages in brain tissue and are the initial responders to tissue damage or foreign pathogens. mAMPH-induced increases in extracellular GLU and DA quinones activate microglia. These activated microglia release pro-inflammatory cytokines, which can both stimulate GLU release and inhibit GLU uptake [40–42], thereby increasing the activity of nitric oxide synthase and RNS production. In addition, activated microglia release a variety of cytotoxic compounds, including ROS, that are designed to attack foreign pathogens, but which can cause neuronal damage.
4) Alterations in cellular proteins
The mAMPH-induced activation of proteases such as calpain can cleave the cytoskeletal proteins spectrin and tau [43, 44]. In addition, recent studies have shown that binge mAMPH alters protein degradation by the ubiquitin proteasomal system, leading to dysregulation of protein turnover, accumulation of unwanted proteins and potential formation of protein aggregates [15].
5) BBB leakage
Recent evidence indicates that binge mAMPH administration can disrupt the BBB [45, 46]. This disruption causes proteins and ions to leak into the brain causing water to osmotically follow, yielding brain edema. The mechanism by which mAMPH causes BBB disruption could involve mAMPH-induced ROS production and hyperthermia, both of which are known triggers of BBB breakdown. In addition, mAMPH has been shown to activate the metalloproteinases MMP-2 and MMP-9 which may alter the integrity of tight junctions between the vascular endothelial cells and cleave components of the extracellular matrix which provides vascular basement membrane support [12]. Two recent reviews [13, 15] provide a more detailed account of the cellular processes contributing to the mechanisms of mAMPH-induced neurotoxicity.
mAMPH-induced brain injury in humans
Consistent with the research findings in laboratory animals, positron emission tomography (PET) studies of abstinent human mAMPH abusers report significantly reduced striatal binding of radioligands to DA transporters (DAT), when compared to age- and sex-matched controls with no history of drug use/dependence (Figure 1E and F). These DAT binding reductions persist for at least 11 months after mAMPH was last used [47, 48] and therefore cannot represent short-term adaptations to mAMPH intake. Additional evidence for neurotoxicity in the brains of mAMPH users comes from post mortem analyses of striatal DA terminal markers in chronic mAMPH abusers and matched controls. These demonstrate lower levels of striatal DA, its synthetic enzyme tyrosine hydroxylase (TH), and DAT protein in the mAMPH users [49, 50]. Also consistent with the animal research, mAMPH users show reductions in forebrain 5-HT and 5-HT transporter (SERT) both in vivo by PET imaging and in post mortem tissue analysis [51, 52]. Additionally, PET imaging of a radiotracer for activated microglia reported dramatic (>250%) elevations in this tracer’s binding in brain regions with dopaminergic or serotonergic innervation in mAMPH abusers [53]. Because of the close relationship between activated microglia and brain injury [54–56], this finding provides further support for the conclusion that chronic mAMPH users suffer monoaminergic injury. In addition to demonstrating the translational value of the animal models for understanding brain changes of mAMPH abusers, these experiments point to a possible role for neural injury in the long-lasting motor (see Box 2) and cognitive changes documented in mAMPH users.
BOX 2. Controversial aspects of mAMPH brain injury - Motor symptoms.
Because repeated mAMPH exposure in humans and animals produces long-term damage to DA terminals, mAMPH abusers might be expected to show movement disorders associated with dopaminergic injury, e.g. Parkinson’s disease (PD). Although rats exposed to binge mAMPH are impaired in performing demanding motor tasks [107] and human mAMPH users with striatal DAT reductions have impairments in performing learned movements rapidly [48], the classical motor symptoms of PD (ie. tremor, bradykinesia, muscle rigidity, postural abnormalities) are rare.
Three possible explanations for the scarcity of PD symptoms in mAMPH users have been advanced. First, the symptoms of PD typically emerge only when the loss of dopaminergic innervation to the putamen (the sensorimotor striatal subdivision) reaches a critical threshold. mAMPH abusers may not exhibit parkinsonian symptoms because the their reductions in striatal dopaminergic terminal markers (such as DAT) is less (20–25%) than the 50–75% loss seen in symptomatic PD patients [47](Fig. 1F and G). Second, the types of dopaminergic damage seen in PD and induced by repeated mAMPH differ. PD is characterized by a progressive degeneration of dopaminergic neurons (cell bodies in the substantia nigra and terminals in striatum), whereas the injury induced by mAMPH exposure is limited to dopaminergic terminals and is slowly reversed during prolonged abstinence (12–17 months in humans [92] or 6–12 months in rodents [88, 89]). Third, the striatal pattern of DA loss in PD and mAMPH users differs. In PD patients, the dopaminergic damage is greater in the putamen than in the caudate nucleus, a relationship that is reversed in mAMPH abusers [50], suggesting that motor symptoms may not dominate the clinical symptomatology of mAMPH users.
However, recent evidence points to the possibility that people who abuse mAMPH face a greater probability of developing parkinsonian symptoms during their lifespan, compared with the general population. A recent epidemiological analysis of the risk of PD in a large population of individuals hospitalized with mAMPH use, compared to individuals with hospital records of cocaine abuse or appendicitis, showed that mAMPH users had a significantly elevated risk of subsequent admission with a PD diagnosis [108]. Although these findings require replication, this increased risk might occur because drug-induced injury to their striatal dopaminergic terminals renders mAMPH users more vulnerable to age-related reductions in striatal DA function.
Cognitive changes with mAMPH exposure
Human studies
It has been reported repeatedly that human mAMPH users show deficits in several cognitive domains, including memory, attention, and decision-making skills. An early survey of cognition in mAMPH users reported impairments in recognition/recall of words and pictures, information processing speed, selective attention, and abstract thinking [57]. Recent evidence confirms that tasks requiring either movement skills or perceptual speed are impaired [58]. In addition, deficiencies in the ability to flexibly shift attention from formerly relevant, but currently irrelevant, stimuli (i.e., attentional set-shifting) are commonly reported for both chronic amphetamine [59] and mAMPH users [60–62]. Other research utilizing the Stroop task, where words naming colors are printed in different colors of ink, and the subject is asked to respond with the color of the ink rather than with the word printed, supports the conclusion that mAMPH abusers have difficulties in tasks of selective attention [63–65]. Stimulant abusers also have impairments in ‘inhibitory control’ over choices, as evidenced by their tendency toward perseverative responding [66–68]. Finally, abstinent mAMPH abusers perform more poorly on a task of ‘prospective memory’ that requires remembering to perform intended acts [69]. It has been argued that, in aggregate, these cognitive impairments may seriously interfere with the mAMPH user’s ability to engage in and benefit from psychosocial therapy for their dependence [70].
The interpretation of deficient performance on cognitive tasks in the mAMPH-abusing population can be complex, since the differences may reflect either the influence of mAMPH or the users’ history of other-drug use, education, or genetic influences. Animal research provides an ideal approach to study the effects of mAMPH exposure separately from all other influences, thereby determining this drug’s lasting influence on domains of cognitive functioning similar to those affected in human mAMPH users. Strikingly, many of the same features, such as impairments in recognition memory, shifting attentional set, response learning, and reversals, are observed in mAMPH-exposed rodents. These research findings provide novel opportunities to (1) investigate how mAMPH exposure alters the brain to selectively influence cognitive function, and (2) develop novel treatments for these abnormalities.
Rodent studies
During the early 2000’s, the emerging research on the cognitive abnormalities of the mAMPH-using population prompted the identification of tasks that, in animals, were persistently affected by mAMPH exposure. Exposing rats to a binge mAMPH regimen that injured their DA and 5-HT systems produced lasting impairments in their novel object recognition (NOR) memory [71, 72, 23] as well as in their recognition of socially relevant odors [73] (see Figure 3A and B). Rats exposed to such neurotoxic mAMPH doses also had enduring impairments in motor learning. Specifically, mAMPH-exposed rats showed poor learning of sequences of turns in both a radial arm maze [74] and the Cincinnati water maze [75, 76]. Both of these tasks depend upon response learning rather than place learning, and this distinction highlights both the behavioral and neurobiological specificity of mAMPH-induced cognitive deficits. Maze navigation by response learning uses an internal reference point for remembered pathways, whereas place learning uses external reference points as directional cues. Not only are the strategies different in these two forms of navigation, the brain areas involved in learning and recalling each differ -- response learning tasks are dependent on striatal mechanisms [77], while place learning tasks are hippocampal-dependent [78]. Several studies have found that neurotoxic mAMPH dosing regimens that impair response learning or object recognition memory (which depends on the integrity of the perirhinal cortex, pRh [79, 80]) have little or no effect on rats’ place learning in tasks such as the Morris Water Maze [81, 72, 75].
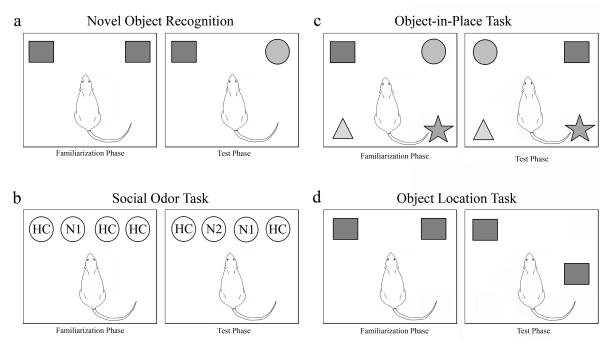
Figure 3. Recognition memory tasks have played a key role in animal research into the cognitive impairments associated with mAMPH exposure.
(a) Novel Object Recognition (NOR) Task assesses the ability of rats to remember the identity of objects in fixed positions in an arena. Initial presentation of objects occurs during a Familiarization phase, which is followed by a memory test (Test phase). Drug-naïve rodents spend more time exploring a novel object (here the circle) than a familiar object (the square) during Test phase, which can occur at intervals ranging from minutes to one day after the Familiarization phase. (b) Social Odor Recognition Task uses odors of conspecifics permeating wooden beads that are placed into rat’s home cage. Rats explore the odor of a conspecific (N1) more than odors from their own home cage (HC’s) during familiarization, but habituate to N1 during 3 successive familiarization trials (using short inter-trial intervals). During Test phase 24 h later, drug-naïve rats show higher exploration of the odor of a new conspecific (N2) than of the N1 or HC odors. (c) The Object-in-Place (OIP) Task requires memory of object identity and location. The same objects are used in the Familiarization and Test phases, but the location of a subset of the objects is exchanged. During test, drug-naïve rodents typically spend more time exploring the objects in changed locations (here, circle and square) than objects in their original locations. (d) Object Location Task requires only that the animal recognize that an object is in an unfamiliar location. During the Test phase, drug-naïve rodents spend more time investigating the square in the unfamiliar location. Comparison of the performance of mAMPH-treated rats in these four memory tasks can provide information about brain circuits affected by repeated stimulant use. Studies of excitotoxic injury to perirhinal cortex (pRh) show that damage to this area leads to memory deficits in each of these tasks except Object Location [102, 80]. By contrast, similar excitotoxic injury to hippocampus produces a different profile, with impairments in tasks requiring information about object location (i.e., OIR and Object Location tasks), but not in tasks requiring only memory of stimulus features (NOR, social odor recognition) [103, 80]. Studies on mAMPH-treated rats have reported impairments in each of these recognition tasks, with the exception of the Object Location task. Therefore, it is likely that mAMPH exposure--whether given in a single-day binge regimen or self-administered by rats for weeks--disrupts the operation of neural circuits that include the pRh while leaving hippocampal circuits less affected.
Neurotoxic doses of mAMPH also impair inhibitory control, as evidenced by animals’ performance on tasks involving reversal learning. In rats taught a touchscreen-based visual discrimination task, subsequent mAMPH binge dosing had no effect on their memory for the discrimination, but impaired their ability to reverse their response by choosing the previously unreinforced stimulus [82]. A more recent study suggests that mAMPH-exposed rats also have impairments in an additional facet of cognition termed effortful decision-making, in which reward choices are balanced against the effort required to obtain rewards of differing magnitudes [83].
Although most of the research on the enduring cognitive changes of mAMPH-exposed animals has relied on the use of binge dosing regimens that damage DA and 5-HT nerve terminals, evidence increasingly points to the conclusion that obvious neurotoxicity to these terminals is not necessary for mAMPH-induced cognitive deficits to occur. Significant impairments in NOR were observed in rats given a sensitizing mAMPH dose regimen (3 mg/kg every other day for 20 d) that failed to alter striatal DAT or forebrain SERT [84]. Further, rats trained to self-administer mAMPH for approximately 3 weeks, including a period of long access (6 hr/day) mAMPH self-administration, had impairments in a NOR task [85, 86] as well as in an ‘object in place’ (OIP) task, which requires memory for both objects’ identities and locations in an arena [87] (Figure 3C). This self-administration regimen appears not to result in striatal dopaminergic terminal loss [24, 86, 87], but it does reduce levels of SERT and NE transporter (NET) as well as the metabotropic GLU receptor mGluR5 in cerebral cortex [86, 87]. Importantly, the reductions in SERT and mGluR5 occurred in the pRh, a cortical area critical for recognition memory.
The finding of diminished mGluR5 protein in the pRh accompanying NOR deficits of mAMPH-experienced rats prompted an investigation of the potential restorative influences of the mGluR5 receptor positive allosteric modulator, 3-cyano-N-1,3-diphenyl-1H-pyrazol-5-yl (CDPPB) on NOR in these animals [86]. When administered prior to a short-term object recognition memory test, 30 mg/kg CDPBB restored the recognition memory of mAMPH-exposed rats to a level similar to that of mAMPH-naïve control animals. Although questions remain about how mGluR5 allosteric modulation exerts its beneficial effects on NOR memory, these findings demonstrate the potential clinical benefits of research into the mAMPH-induced behavioral and neurochemical deficits in animals.
Recovery
Although incompletely characterized, recovery from mAMPH-induced injury has been repeatedly observed in both human abusers and rodents, suggesting that therapeutic interventions could be developed to hasten reversal of mAMPH-induced neurochemical and cognitive deficits. In contrast to the destruction of dopaminergic cell bodies seen after treatment with neurotoxins such as 6-hydroxydopamine or 1- methyl -4- phenyl -1,2,3,6-tetrahydro pyridine (MPTP), the damage due to binge regimens of mAMPH in rodents is almost exclusively confined to nerve terminals of monoaminergic neurons. The mAMPH-induced decreases in dopaminergic [88] and serotonergic [89] terminal markers recover to control levels within 6–12 months after mAMPH administration. Similar recovery of mAMPH-induced decreases in DAT and striatal DA content have been seen in vervet monkeys over the course of 1.5 years [90, 91].
Studies of human mAMPH abusers also suggest that drug-induced changes may be reversed with increasing abstinence. Striatal DAT levels, as measured by PET imaging, were nearly 20% greater in mAMPH abusers who had been abstinent for 12–17 months when compared to DAT values from those same users when less than 6 months abstinent [92]. Similarly, long-abstinence mAMPH users showed greater thalamic glucose utilization [93] and lower levels of microglial activation [53] than short-abstinence users. Also, global cerebral glucose utilization during a vigilance task increased in a group of mAMPH abusers between tests at 5–9 days of abstinence and a test 4 weeks later [94]. However, it is unclear whether the improvements in neurochemical parameters that can occur with increased abstinence translate into better cognitive performance. Higher levels of DAT binding in mAMPH abusers have been found unrelated to improvements in memory deficits [92, 95], whereas increased glucose utilization was positively correlated with improved performance on motor and verbal memory tasks [93] and improved reaction times in a vigilance task [94].
Further research into neurobiological factors tending to a normalize brain chemistry and cognition of mAMPH users could hold great benefit (See Box 3). Because the mAMPH-induced damage to brain DA and 5-HT projections are spontaneously reversed over a period of several months to years, investigations into the processes underlying this reversal hold promise for new treatment approaches to accelerate recovery. The single published study investigating the mechanisms underlying recovery from mAMPH-induced impaired dopaminergic transmission [96] reported that unilateral intrastriatal infusions of glial-derived neurotrophic factor (GDNF) into rats one week after a neurotoxic dose of mAMPH significantly accelerated normalization of evoked DA release. This finding suggests that GDNF therapy can accelerate recovery from mAMPH-induced striatal dopaminergic damage, raising the possibility that other interventions applied systemically might have similar beneficial influences. Recently, it was reported [97] that voluntary exercise in running wheels ameliorated the dopaminergic and serotonergic damage resulting from a neurotoxic mAMPH binge. In these experiments, rats were allowed constant access to running wheels, or kept in non-wheel cages, for 3 weeks before and after a binge dosing regimen of mAMPH or saline. Correlation analyses showed that the amount of post-mAMPH running (but not pre-mAMPH running) predicted the extent of individual animals’ amelioration of damage, suggesting that exercise may have accelerated recovery from mAMPH-induced forebrain monoaminergic injury.
BOX 3. Outstanding Questions and Future Directions.
Mechanisms of Methamphetamine-induced Neurotoxicity. Further investigation is needed into the mechanisms by which mAMPH injures neurons. A coherent hypothesis that involves mAMPH-induced DA release and the subsequent release of GLU, resulting in increased production of damaging free radicals, has gained wide acceptance as the primary mechanism of mAMPH-induced neurotoxicity. However, questions remain about how other mAMPH-induced changes such as hyperthermia, stress, metabolic compromise, opening of the BBB, and changes in proteasomal activity might interact with elements in this hypothesized system to produce enduring injury to monoaminergic nerve terminals.
Brain Changes beyond Neurotoxicity. Much work has been done to characterize changes in monoamine nerve terminals associated with mAMPH’s neurotoxic effects in humans and animals. However, mAMPH-induced cellular and molecular changes occurring downstream from the monoamine neurons may contribute to the functional impairments. Two examples of such changes are (i) the reductions in striatal DA D2 receptors that occur in human mAMPH users [109], and (ii) the decrease in mGluR5 seen in rats that have undergone long access mAMPH self-administration [84]. These alterations have been hypothesized to contribute, respectively, to the compulsive pattern of mAMPH abuse and to the impairments in recognition memory of mAMPH-exposed animals. It is likely that additional investigations will reveal other brain molecules of functional significance to the treatment of mAMPH abuse.
Cognitive Impairments: Furthering the Handshake between Human and Animal Research. Research on mAMPH neurotoxicity in animals helped inform research on loss of DAT in human mAMPH abuse. In turn, the study of cognitive changes in mAMPH abusers helped drive the search for animal models of these cognitive impairments. The utility of studying the cognitive deficits of mAMPH-exposed animals derives from (i) the ability of the experimenter to control drug exposure, (ii) the range of potential therapeutic interventions that can be tried, and (iii) the extent of current knowledge about the involvement of particular neural systems rodent memory tasks. From the perspective of neural systems, much remains to be learned about how mAMPH exposure affects the functions of brain regions that are involved in mAMPH-induced cognitive impairments.
Facilitating Recovery: Although the gradual recovery of monoamine neurotransmitters observed after mAMPH exposure has been described in both humans and rodents, the mechanism(s) for this recovery are largely unknown. Does restoration of monoamines represent a sprouting of axon collateral branches or a phenotypic upregulation that occurs without adding membrane to damaged neurons? How do neurotrophins and growth factors contribute? What cellular signaling pathways stimulate this recovery? Apart from these questions about the mechanism, can the rate of recovery from either the mAMPH-induced neurochemical or cognitive deficits be accelerated by either pharmacological or non-pharmacological therapies?
Although the mechanism(s) by which exercise counteracts mAMPH-induced damage are unknown, exercise has been shown to upregulate several growth factors in the nervous system [98, 99]. One promising avenue of investigation is based on reports of exercise-induced increases in vascular endothelial growth factor (VEGF) in the CNS. Exercise-induced VEGF production has been implicated in recovery from cerebral ischemia (stroke) [100, 101] and may play a similar role in accelerating recovery from mAMPH-induced monoaminergic damage.
Conclusions
Humans who use mAMPH, or animals that are exposed to this drug by experimenter delivery or self-administration, can suffer long-lasting brain injury. The damage to monoamine pathways has been extensively characterized in rodents, confirmed in non-human primates, and seen in both PET and post mortem studies of the brains of mAMPH abusers. Studies of the cellular mechanisms contributing to this mAMPH-induced injury point to a series of events that arise from mAMPH-induced DA and GLU efflux, including the production of ROS and RNS, inflammatory responses, opening of the BBB, and alterations in cellular proteins and energy metabolism.
The cognitive functioning of human mAMPH abusers has been found to be below that of control subjects on a range of tasks that assess memory, attention, and executive functions. Recently, animal research has succeeded in providing model systems for these behavioral effects of mAMPH exposure. Tasks of recognition memory have been especially useful, as the effect of mAMPH exposure on this class of memory is robust across several tasks that depend upon perirhinal cortex. Using recognition memory as an assessment tool, the development of treatments to reverse the cognitive impairments of mAMPH-exposed animals has met with some initial success. Further advancements will be based on our still-evolving understanding of mAMPH’s persistent influences on multiple neurotransmitter receptor systems.
Finally, evidence for the reversibility of the neural damage in mAMPH users as well as in mAMPH-exposed rodents and primates suggests a novel approach to improving outcome of mAMPH users by facilitating the brain’s own repair mechanisms to enhance recovery. The research on mAMPH’s influences on brain and behavior has been characterized by insights from the clinic informing preclinical research, and vice-versa. This pattern of cooperative interaction between human and animal research provides a strong foundation for future insights into treatment strategies for .mAMPH dependence and its cognitive consequences.
Acknowledgments
We acknowledge the National Institute on Drug Abuse for funding our research on methamphetamine (grant 5RO1DA012204).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Suwaki H, et al. Methamphetamine abuse in Japan: its 45 year history and the current situation. In: Klee H, editor. Amphetamine Misuse: International Perspectives on Current Trends. Harwood; 1997. pp. 199–214. [Google Scholar]
- 2.Prinzmetal M, Bloomberg W. Use of benzidrine for the treatment of narcolepsy. J Amer Med Assoc. 1935;105:2051–2054. [Google Scholar]
- 3.Penick SB. Amphetamines on obesity. Seminars in Psychiatry. 1969;1:144–162. [Google Scholar]
- 4.Lambert NM, et al. Hyperactive children and the efficacy of psychoactive drugs as a treatment intervention. Am J Orthopsychiatry. 1976;46:335–352. doi: 10.1111/j.1939-0025.1976.tb00933.x. [DOI] [PubMed] [Google Scholar]
- 5.Feldman RS, et al. Principles of Neuropsychopharmacology. Sinauer Associates; 1997. [Google Scholar]
- 6.Koob GF, Le Moal M. Neurobiology of Addiction. Elsevier; 2006. [Google Scholar]
- 7.Cook CE, et al. Pharmacokinetics of methamphetamine self-administered to human subjects by smoking S-(+)-methamphetamine hydrochloride. Drug Metab Dispos. 1993;21:717–723. [PubMed] [Google Scholar]
- 8.Sulzer D, et al. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Vollm BA, et al. Methamphetamine activates reward circuitry in drug naive human subjects. Neuropsychopharmacology. 2004;29:1715–1722. doi: 10.1038/sj.npp.1300481. [DOI] [PubMed] [Google Scholar]
- 10.Melega WP, et al. Dizocilpine and reduced body temperature do not prevent methamphetamine-induced neurotoxicity in the vervet monkey: [11C]WIN 35,428 - positron emission tomography studies. Neurosci Lett. 1998;258:17–20. doi: 10.1016/s0304-3940(98)00845-3. [DOI] [PubMed] [Google Scholar]
- 11.Villemagne V, et al. Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neurosci. 1998;18:419–427. doi: 10.1523/JNEUROSCI.18-01-00419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto BK, Raudensky J. The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J Neuroimmune Pharmacol. 2008;3:203–217. doi: 10.1007/s11481-008-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cadet JL, Krasnova IN. Molecular bases of methamphetamine-induced neurodegeneration. Int Rev Neurobiol. 2009;88:101–119. doi: 10.1016/S0074-7742(09)88005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleckenstein AE, et al. Psychostimulant-induced alterations in vesicular monoamine transporter-2 function: neurotoxic and therapeutic implications. Neuropharmacology. 2009;56(Suppl 1):133–138. doi: 10.1016/j.neuropharm.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto BK, et al. Amphetamine toxicities: classical and emerging mechanisms. Ann N Y Acad Sci. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricaurte GA, et al. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- 17.Bowyer JF, Schmued LC. Fluoro-Ruby labeling prior to an amphetamine neurotoxic insult shows a definitive massive loss of dopaminergic terminals and axons in the caudate-putamen. Brain Res. 2006;1075:236–239. doi: 10.1016/j.brainres.2005.12.062. [DOI] [PubMed] [Google Scholar]
- 18.Pu C, Vorhees CV. Protective effects of MK-801 on methamphetamine-induced depletion of dopaminergic and serotonergic terminals and striatal astrocytic response: an immunohistochemical study. Synapse. 1995;19:97–104. doi: 10.1002/syn.890190205. [DOI] [PubMed] [Google Scholar]
- 19.Miller DB, O’Callaghan JP. Elevated environmental temperature and methamphetamine neurotoxicity. Environ Res. 2003;92:48–53. doi: 10.1016/s0013-9351(02)00051-8. [DOI] [PubMed] [Google Scholar]
- 20.LaVoie MJ, et al. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Thomas DM, et al. Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci Lett. 2004;367:349–354. doi: 10.1016/j.neulet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 22.Segal DS, et al. Escalating dose methamphetamine pretreatment alters the behavioral and neurochemical profiles associated with exposure to a high-dose methamphetamine binge. Neuropsychopharmacology. 2003;28:1730–1740. doi: 10.1038/sj.npp.1300247. [DOI] [PubMed] [Google Scholar]
- 23.Belcher AM, et al. Impaired object recognition memory following methamphetamine, but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology. 2005;30:2026–2034. doi: 10.1038/sj.npp.1300771. [DOI] [PubMed] [Google Scholar]
- 24.Schwendt M, et al. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331:555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuczenski R, et al. Escalating dose-multiple binge methamphetamine exposure results in degeneration of the neocortex and limbic system in the rat. Exp Neurol. 2007;207:42–51. doi: 10.1016/j.expneurol.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krasnova IN, et al. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS One. 2010;5:e8790. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villemagne V, et al. Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neurosci. 1998;18:419–427. doi: 10.1523/JNEUROSCI.18-01-00419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Dell SJ, et al. Multiple methamphetamine injections induce marked increases in extracellular striatal dopamine which correlate with subsequent neurotoxicity. Brain Res. 1991;564:256–260. doi: 10.1016/0006-8993(91)91461-9. [DOI] [PubMed] [Google Scholar]
- 29.Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxymethamphetamine. Brain Res. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- 30.Mark KA, et al. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J Neurosci. 2004;24:11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berman SB, et al. Modification of dopamine transporter function: effect of reactive oxygen species and dopamine. J Neurochem. 1996;67:593–600. doi: 10.1046/j.1471-4159.1996.67020593.x. [DOI] [PubMed] [Google Scholar]
- 32.Wrona MZ, et al. Potential new insights into the molecular mechanisms of methamphetamine-induced neurodegeneration. NIDA Res Monogr. 1997;173:146–174. [PubMed] [Google Scholar]
- 33.Kuhn DM, et al. Dopamine quinones activate microglia and induce a neurotoxic gene expression profile: relationship to methamphetamine-induced nerve ending damage. Ann N Y Acad Sci. 2006;1074:31–41. doi: 10.1196/annals.1369.003. [DOI] [PubMed] [Google Scholar]
- 34.Dykens JA, et al. Mechanism of kainate toxicity to cerebellar neurons in vitro is analogous to reperfusion tissue injury. J Neurochem. 1987;49:1222–1228. doi: 10.1111/j.1471-4159.1987.tb10014.x. [DOI] [PubMed] [Google Scholar]
- 35.Dumuis A, et al. NMDA receptors activate the arachidonic acid cascade system in striatal neurons. Nature. 1988;336:68–70. doi: 10.1038/336068a0. [DOI] [PubMed] [Google Scholar]
- 36.Radi R, et al. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 37.Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. Aaps J. 2006;8:E337–347. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson’s disease. J Neurochem. 1999;73:1127–1137. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- 39.Brown JM, et al. Methamphetamine-induced inhibition of mitochondrial complex II: roles of glutamate and peroxynitrite. J Neurochem. 2005;95:429–436. doi: 10.1111/j.1471-4159.2005.03379.x. [DOI] [PubMed] [Google Scholar]
- 40.Casamenti F, et al. Interleukin-1beta activates forebrain glial cells and increases nitric oxide production and cortical glutamate and GABA release in vivo: implications for Alzheimer’s disease. Neuroscience. 1999;91:831–842. doi: 10.1016/s0306-4522(98)00680-0. [DOI] [PubMed] [Google Scholar]
- 41.Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Northrop NA, Yamamoto BK. Neuroimmune pharmacology from a neuroscience perspective. J Neuroimmune Pharmacol. 2011;6:10–19. doi: 10.1007/s11481-010-9239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staszewski RD, Yamamoto BK. Methamphetamine-induced spectrin proteolysis in the rat striatum. J Neurochem. 2006;96:1267–1276. doi: 10.1111/j.1471-4159.2005.03618.x. [DOI] [PubMed] [Google Scholar]
- 44.Straiko MM, et al. The effect of amphetamine analogs on cleaved microtubule-associated protein-tau formation in the rat brain. Neuroscience. 2007;144:223–231. doi: 10.1016/j.neuroscience.2006.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiyatkin EA, et al. Brain edema and breakdown of the blood-brain barrier during methamphetamine intoxication: critical role of brain hyperthermia. Eur J Neurosci. 2007;26:1242–1253. doi: 10.1111/j.1460-9568.2007.05741.x. [DOI] [PubMed] [Google Scholar]
- 46.Bowyer JF, et al. Neurotoxic-related changes in tyrosine hydroxylase, microglia, myelin, and the blood-brain barrier in the caudate-putamen from acute methamphetamine exposure. Synapse. 2008;62:193–204. doi: 10.1002/syn.20478. [DOI] [PubMed] [Google Scholar]
- 47.McCann UD, et al. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volkow ND, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 49.Wilson JM, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- 50.Moszczynska A, et al. Why is parkinsonism not a feature of human methamphetamine users? Brain. 2004;127:363–370. doi: 10.1093/brain/awh046. [DOI] [PubMed] [Google Scholar]
- 51.Sekine Y, et al. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry. 2006;63:90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- 52.Kish SJ, et al. Brain serotonin transporter in human methamphetamine users. Psychopharmacology (Berl) 2009;202:649–661. doi: 10.1007/s00213-008-1346-x. [DOI] [PubMed] [Google Scholar]
- 53.Sekine Y, et al. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- 55.Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kettenmann H, et al. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 57.Simon SL, et al. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- 58.Boileau I, et al. Increased vesicular monoamine transporter binding during early abstinence in human methamphetamine users: Is VMAT2 a stable dopamine neuron biomarker? J Neurosci. 2008;28:9850–9856. doi: 10.1523/JNEUROSCI.3008-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ornstein TJ, et al. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- 60.Kim SJ, et al. Frontal glucose hypometabolism in abstinent methamphetamine users. Neuropsychopharmacology. 2005;30:1383–1391. doi: 10.1038/sj.npp.1300699. [DOI] [PubMed] [Google Scholar]
- 61.Chung A, et al. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2007;10:765–775. doi: 10.1017/S1461145706007395. [DOI] [PubMed] [Google Scholar]
- 62.Kim YT, et al. Dose-dependent frontal hypometabolism on FDG-PET in methamphetamine abusers. J Psychiatr Res. 2009;43:1166–1170. doi: 10.1016/j.jpsychires.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 63.Salo R, et al. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Res. 2002;111:65–74. doi: 10.1016/s0165-1781(02)00111-7. [DOI] [PubMed] [Google Scholar]
- 64.Nordahl TE, et al. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci. 2003;15:317–325. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- 65.Salo R, et al. Drug abstinence and cognitive control in methamphetamine-dependent individuals. J Subst Abuse Treat. 2009;37:292–297. doi: 10.1016/j.jsat.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoffman WF, et al. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berl) 2006;188:162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- 67.Hoffman WF, et al. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology (Berl) 2008;201:183–193. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology (Berl) 2012;219:607–620. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rendell PG, et al. Prospective memory impairment in former users of methamphetamine. Psychopharmacology (Berl) 2009;203:609–616. doi: 10.1007/s00213-008-1408-0. [DOI] [PubMed] [Google Scholar]
- 70.Meredith CW, et al. Implications of chronic methamphetamine use: a literature review. Harv Rev Psychiatry. 2005;13:141–154. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- 71.Bisagno V, et al. Short toxic methamphetamine schedule impairs object recognition task in male rats. Brain Res. 2002;940:95–101. doi: 10.1016/s0006-8993(02)02599-4. [DOI] [PubMed] [Google Scholar]
- 72.Schroder N, et al. Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse. 2003;49:89–96. doi: 10.1002/syn.10210. [DOI] [PubMed] [Google Scholar]
- 73.O’Dell SJ, et al. A neurotoxic regimen of methamphetamine impairs novelty recognition as measured by a social odor-based task. Behav Brain Res. 2011;216:396–401. doi: 10.1016/j.bbr.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 74.Chapman DE, et al. Long-term changes in basal ganglia function after a neurotoxic regimen of methamphetamine. J Pharmacol Exp Ther. 2001;296:520–527. [PubMed] [Google Scholar]
- 75.Herring NR, et al. Effect of +-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology (Berl) 2008;199:637–650. doi: 10.1007/s00213-008-1183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herring NR, et al. (+)-Methamphetamine-induced monoamine reductions and impaired egocentric learning in adrenalectomized rats is independent of hyperthermia. Synapse. 2010;64:773–785. doi: 10.1002/syn.20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kesner RP, et al. Memory for spatial locations, motor responses, and objects: triple dissociation among the hippocampus, caudate nucleus, and extrastriate visual cortex. Exp Brain Res. 1993;93:462–470. doi: 10.1007/BF00229361. [DOI] [PubMed] [Google Scholar]
- 78.Schenk F, Morris RG. Dissociation between components of spatial memory in rats after recovery from the effects of retrohippocampal lesions. Exp Brain Res. 1985;58:11–28. doi: 10.1007/BF00238949. [DOI] [PubMed] [Google Scholar]
- 79.Brown MW, et al. Recognition memory: material, processes, and substrates. Hippocampus. 2010;20:1228–1244. doi: 10.1002/hipo.20858. [DOI] [PubMed] [Google Scholar]
- 80.Feinberg LM, et al. Recognition memory for social and non-social odors: differential effects of neurotoxic lesions to the hippocampus and perirhinal cortex. Neurobiol Learn Mem. 2012;97:7–16. doi: 10.1016/j.nlm.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 81.Friedman SD, et al. Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacol Biochem Behav. 1998;61:35–44. doi: 10.1016/s0091-3057(98)00066-5. [DOI] [PubMed] [Google Scholar]
- 82.Izquierdo A, et al. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kosheleff AR, et al. Work aversion and associated changes in dopamine and serotonin transporter after methamphetamine exposure in rats. Psychopharmacology (Berl) 2012;219:411–420. doi: 10.1007/s00213-011-2367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Belcher AM, et al. A sensitizing regimen of methamphetamine causes impairments in a novelty preference task of object recognition. Behav Brain Res. 2006;170:167–172. doi: 10.1016/j.bbr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 85.Rogers JL, et al. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl) 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reichel CM, et al. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36:782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reichel CM, et al. Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology. 2012;62:1119–1126. doi: 10.1016/j.neuropharm.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cass WA, Manning MW. Recovery of presynaptic dopaminergic functioning in rats treated with neurotoxic doses of methamphetamine. J Neurosci. 1999;19:7653–7660. doi: 10.1523/JNEUROSCI.19-17-07653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cass WA. Attenuation and recovery of evoked overflow of striatal serotonin in rats treated with neurotoxic doses of methamphetamine. J Neurochem. 2000;74:1079–1085. doi: 10.1046/j.1471-4159.2000.0741079.x. [DOI] [PubMed] [Google Scholar]
- 90.Melega WP, et al. Recovery of striatal dopamine function after acute amphetamine- and methamphetamine-induced neurotoxicity in the vervet monkey. Brain Res. 1997;766:113–120. doi: 10.1016/s0006-8993(97)00548-9. [DOI] [PubMed] [Google Scholar]
- 91.Harvey DC, et al. Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain Res. 2000;871:259–270. doi: 10.1016/s0006-8993(00)02439-2. [DOI] [PubMed] [Google Scholar]
- 92.Volkow ND, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang GJ, et al. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- 94.Berman SM, et al. Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse. Mol Psychiatry. 2008;13:897–908. doi: 10.1038/sj.mp.4002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCann UD, et al. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- 96.Cass WA, et al. Restorative effects of GDNF on striatal dopamine release in rats treated with neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2000;914:127–136. doi: 10.1111/j.1749-6632.2000.tb05190.x. [DOI] [PubMed] [Google Scholar]
- 97.O’Dell SJ, et al. Running wheel exercise ameliorates methamphetamine-induced damage to dopamine and serotonin terminals. Synapse. 2012;66:71–80. doi: 10.1002/syn.20989. [DOI] [PubMed] [Google Scholar]
- 98.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 99.Smith AD, Zigmond MJ. Can the brain be protected through exercise? Lessons from an animal model of parkinsonism. Exp Neurol. 2003;184:31–39. doi: 10.1016/j.expneurol.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 100.Navaratna D, et al. Mechanisms and targets for angiogenic therapy after stroke. Cell Adh Migr. 2009;3:216–223. doi: 10.4161/cam.3.2.8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma Y, et al. Vascular endothelial growth factor in cerebral ischemia. J Neurosci Res. 2011;89:969–978. doi: 10.1002/jnr.22628. [DOI] [PubMed] [Google Scholar]
- 102.Warburton EC, Brown MW. Findings from animals concerning when interactions between perirhinal cortex, hippocampus and medial prefrontal cortex are necessary for recognition memory. Neuropsychologia. 2010;48:2262–2272. doi: 10.1016/j.neuropsychologia.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 103.Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McFadden LM, et al. Methamphetamine self-administration causes persistent striatal dopaminergic alterations and mitigates the deficits caused by a subsequent methamphetamine exposure. J Pharmacol Exp Ther. 2012;340:295–303. doi: 10.1124/jpet.111.188433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lominac KD, et al. Distinct neurochemical adaptations within the nucleus accumbens produced by a history of self-administered vs non-contingently administered intravenous methamphetamine. Neuropsychopharmacology. 2012;37:707–722. doi: 10.1038/npp.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Belcher AM, et al. Methamphetamine influences on recognition memory: comparison of escalating and single-day dosing regimens. Neuropsychopharmacology. 2008;33:1453–1463. doi: 10.1038/sj.npp.1301510. [DOI] [PubMed] [Google Scholar]
- 107.Walsh SL, Wagner GC. Motor impairments after methamphetamine-induced neurotoxicity in the rat. J Pharmacol Exp Ther. 1992;263:617–626. [PubMed] [Google Scholar]
- 108.Callaghan RC, et al. Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend. 2012;120:35–40. doi: 10.1016/j.drugalcdep.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 109.Volkow ND, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]