Abstract
Tissue engineering-based approaches have the potential to improve stem cell engraftment by increasing cell delivery to the myocardium. Our objective was to develop and characterize a naturally-derived, autologous, biodegradable hydrogel in order to improve acute stem cell retention in the myocardium. HA-blood hydrogels(HA-Bl) were synthesized by mixing in a 1:1(v/v) ratio, lysed whole blood and hyaluronic acid(HA), whose carboxyl groups were functionalized with N-hydroxysuccinimide(NHS) to yield HA succinimidyl succinate(HA-NHS). We performed physical characterization and measured survival/proliferation of cardiosphere-derived cells(CDCs) encapsulated in the hydrogels. Hydrogels were injected intramyocardially or applied epicardially in rats. NHS-activated carboxyl groups in HA react with primary amines present in blood and myocardium to form amide bonds, resulting in a 3D hydrogel bound to tissue. HA-Bl hydrogels had a gelation time of 58±12s, swelling ratio of 10±0.5, compressive and elastic modulus of 14±3 and 1.75±0.6 kPa respectively. These hydrogels were not degraded at 4wks by hydrolysis alone. CDC encapsulation promoted their survival and proliferation. Intra-myocardial injection of CDCs encapsulated in these hydrogels greatly increased acute myocardial retention(p=0.001). Epicardial application of HA-blood hydrogels improved left ventricular ejection fraction following myocardial infarction (P=0.01). HA-blood hydrogels are highly adhesive, biodegradable, promote CDC survival and increase cardiac function following epicardial application after myocardial infarction.
Keywords: bioadhesive and biodegradable hydrogel, autologous blood hydrogel, modified hyaluronic acid, cardiac stem cell transplantation, molecular imaging, echocardiography
1. INTRODUCTION
Stem cell transplantation offers the promise of organ repair on demand in patients with heart failure resulting from myocardial infarction.[1–3] Unfortunately, the improvement in cardiac function derived from direct injection of isolated cells has been modest at best because of very low levels of engraftment. In vivo PET (positron emission tomography) studies, using cardiosphere-derived cells, also called CDCs (composed of cardiac-derived progenitor and supporting cells) revealed that ~20% of intra-myocardially injected cells are retained in the myocardium 1hr after transplantation[4] because cells rapidly escape from intra-myocardial injection sites via cardiac veins and enter the pulmonary circulation. Progressive cell death occurs in the first day post-transplantation, resulting in engraftment rates of <5% at 24hrs. Cell death following transplantation has been addressed by suspension of cells in a variety of materials, including polyethylene glycol, fibrin, a variety of synthetic hydrogels and ECM proteins like collagen [5, 6] and matrigel [7]. Hydrophilic hydrogel environments are advantageous for maintaining cell viability and achieving even cell distributions in engineered cardiac tissues.
In the body, stem cells reside in niches – the niche microenvironment, comprising of the extracellular matrix and chemical cues, regulates cell fate and function[8]. Recent studies indicate that polymers like hyaluronic acid (HA) can be used to design hydrogels which function as “cell niches,” promote angiogenesis and cardiac regeneration[9, 10]. HA is an important component of cardiac extracellular matrix, and can promote cell adhesion, motility, morphogenesis, regulate gene expression, signaling and proliferation[11] by interacting with HA receptors (CD44) widely present on cells[12].
The main goal of this study is to develop a bio-adhesive, biodegradable scaffold that increases acute cell retention in the myocardium following transplantation. Here, we report the synthesis, in vitro and in vivo testing of hydrogel scaffolds, synthesized from lysed blood and HA, whose carboxyl groups are functionalized with N-hydroxysuccinimide (NHS) to yield HA succinimidyl succinate (HA-NHS). The NHS-activated carboxyl groups react with primary amines which are abundant in blood and tissue to form amide bonds, resulting in hydrogels that entrap stem cells and covalently bind to transplanted tissue. Whole blood was chosen as a cross-linker because it can be easily obtained by venipuncture in an autologous fashion (in patients needing stem cell transplantation) and eliminates the risk of inducing immune reactions or transmission of pathogens. Additionally, whole blood contains fibronectin and vitronectin [13] as well as growth factors that could promote integrin activation, survival and proliferation of encapsulated stem cells.
2. METHODS
2.1 Synthesis of Hyaluronic Acid succinimidyl succinate (HA-NHS)
Chemical modification of carboxyl groups in hyaluronic acid (HA) to amine-reactive N-hydroxysuccinimide esters was achieved by reacting HA (MW 16 kDa; LifeCore Biomedical) with 1-ethyl-3-(3-diemthylaminopropyl) carbodiimide (EDC; Sigma), and N-hydroxyl succinimide (NHS; Sigma) in a ratio of 1:5.73:3.6 of HA:EDC:NHS dissolved in phosphate-buffered saline (PBS). After allowing the mixture to react, it was frozen at −80°C, precipitated using ethanol at −20°C, washed and dried. Dry HA-NHS is stable for at least 6 months at −20°C. Once dissolved in aqueo us buffers, the material begins to hydrolyze. However, if used within 10–15 min of dissolution, the effects due to hydrolysis are insignificant. The hydroxamate assay [14] was used to quantify the total NHS groups present in HA–NHS.
2.2 Hydrogel Synthesis
We synthesized HA-blood hydrogels and compared then to HA-PEG hydrogels for in vitro studies. We chose HA-PEG hydrogels as controls because PEG-amine has been extensively used for hydrogel synthesis in tissue engineering applications [19, 20]. HA-blood hydrogels were prepared by mixing in a 1:1 (v/v) ratio, 10 w/v% HA-NHS in PBS and lysed blood derived from human (1 normal volunteer) or rat (Wistar Kyoto). Whole blood was drawn into heparin-coated, vacuum sealed test-tubes (BD Bioscience) and lysed by freeze-thawing. HA-PEG hydrogels were prepared by mixing in a 1:1 (v/v) ratio, 10 w/v% HA–NHS in PBS and 10 w/v% PEG-(NH2)6 (15 kDa, Sunbio, Orinda, CA) in HEPES buffer.
All polymerization was carried out at pH 7–7.4 and room temperature. We chose a 1:1 (v/v) ratio for blood and HA in order to provide high adhesivity (HA-NHS) and a source of growth factors and adhesion motifs (lysed blood). In order to ensure functionality of the NHS groups, hydrogels were synthesized within 15 min of dissolving HA in PBS.
For in vitro studies, CDCs were suspended in lysed blood or PEG-amine and mixed with HA in a 1:1 (v/v) ratio. For in vivo studies, HA and lysed blood were each aspirated into separate sterile 0.5 mL syringes connected by a sterile plastic tubing. The 2 components were mixed for 30–40s prior to intra-myocardial injection or epicardial application.
2.2.1 Stem cell isolation for encapsulation in hydrogel
Cardiosphere-derived cells (rCDCs) were isolated from hearts of male, 3 month old, Wistar Kyoto (syngeneic) rats (Harlan, Indianapolis, Indiana, USA) as previously described [7,10]. Cells were cultured in cardiac explant medium (CEM) which is composed of IMDM (Invitrogen), 10% fetal bovine serum, 1% L-Glutamine, and 0.1mmol 2-mercaptoethanol. CDCs were suspended in lysed blood or PEG-amine at a concentration of 10,000 cells/μl of hydrogel for all experiments.
2.3 Physical Characterization of Hydrogel
For physical characterization, all hydrogels were prepared as cylindrical blocks, 5 mm in diameter, with a total volume of 100 μL containing 1:1 (v/v) ratio of 10 w/v% HA-NHS in PBS and 10w/v% lysed blood or PEG-(NH2)6 in HEPES, using microcentrifuge-tube caps as molds.
2.3.1 Equilibrium swelling ratio analysis
The HA-PEG and HA-Blood hydrogels were incubated in PBS overnight in order to measure their wet weight at maximum saturation. They were subsequently transferred to eppendorf tubes and lyophilized for 48 hrs in order to measure dry weight.
2.3.2 Gelation time analysis
Using a 2–200μL pipetman, the HA–NHS and PEG–(NH2)6 or lysed blood were mixed and pipetted up and down until the solutions could no longer be pipetted. The time at which this happened was designated as the gelation time.
2.3.3 Compressive modulus analysis
To measure compressive modulus, hydrogel constructs were placed in between two parallel metal plates on an adjustable stage. The bottom plate was attached to a 250g loading weight and a force transducer connected to a computer. The gels were then deformed by 1% height in discrete 20sec intervals until 10% deformation was reached (electroforce 3200 testing instrument, Bose, Eden Prairie, MN). The best fit slope of stress-strain curve was used to calculate compressive modulus.
In order to determine the compressive moduli of rat myocardium, rats (n=5) were euthanized with 100% isoflurane right before the measurement. The heart was excised and langendorf perfusion was performed using 20mL of Ca2+/Mg2+ containing PBS for 5 min for contracted myocardium and Ca2+/Mg2+ -free PBS for relaxed myocardium. Myocardium from the anterior wall was cut in a cylindrical shape using a skin biopsy punch. Compressive and elastic moduli were measured as described above.
2.3.4 Degradation rate
Hydrogels can be degraded by hydrolysis, proteases present in tissue and/or secreted by encapsulated CDCs.
In order to evaluate degradation by hydrolysis, hydrogel constructs without cells (n=5) were incubated in PBS at 37°C for 0, 10, 20 a nd 30d. Hydrogels were lyophilized for 48 hrs and dry weight was measured. Change in gel dry weight was used to measure their 3D degradation rate.
Since cells can secrete matrix metallo-proteinases and hyaluronidases which could accelerate degradation of hydrogels, we also assessed hydrogel degradation in the presence of encapsulated cells. We incubated CDCs encapsulated in 50 μL hydrogel constructs (n=5) at 37°C in CEM for 1 week ; hydrogel dry weights were measured every 4 days for 8 days.
2.3.5 Protein release from HA-blood hydrogels
Proteins from lysed blood cells and soluble serum proteins from HA-blood hydrogels may be released over time to provide a source of nutrients for stem cells. Fifty microliters of HA-blood hydrogels (n=3) were incubated in PBS at 37°C. Sample aliquots 5–40 μL of PBS solution were obtained over 20d and protein concentration was measured using the Bradford assay (BioRad). The total volume of PBS was readjusted to 1mL after each sampling. Total blood protein concentration was determined from 25 μL of lysed blood suspended in 1mL PBS (equivalent to the hydrogel) in order to normalize results of protein estimation to the total protein content of lysed blood.
2.4 Hydrogel-Cell Interactions
For in vitro imaging studies, hydrogels of 50μL volume, containing 10,000 CDCs/μL of hydrogel were prepared and cultured in 35mm petri dishes, using CEM. Imaging was performed 24hrs and 7d following cell encapsulation, using 2-photon microscopy for assessment of cell viability.
2.4.1 Two photon Imaging
Two-photon microscopy was selected for imaging because it has the following advantages: 1) fluorescence excitation occurs only in the narrow focal plane of the objective lens, thus eliminating out of focus fluorescence in the collected image, 2) the long wavelength of the incident light permits deeper penetration of the tissue with less damaging effects outside the focal plane, 3) multiple fluorophores are stimulated by a single excitation wavelength with no cross- talk between excitation and emission bands. Two-photon excitation was provided by a Tsunami 3941 M1S femtosecond mode-locked TiSa pulsed laser (spectra Physics) pumped by a 10W Millenia X solid state laser. Cells were imaged using a Nikon 40X water lens. Image acquisitions were performed at 37°C, using the same laser power, gain and zoom and on the same day. Images of 512×512 pixels were collected, digitized at 8-bit resolution, and stored directly on the hard disk. Image analysis with the same processing parameters for all conditions was performed using Image J (NIH, http://rsb.info.nih.gov/ij/). For signal quantification, a region of interest was drawn and the mean fluorescence value was normalized to the fluorescent area. The Mean Gray Value (MGV) was determined using the region without cells, i.e. background noise, and subtracted from the value directly obtained from the region of interest. To analyze the data, 40–50 cells were taken from 10 different fields for each condition and arranged in ascending order. In order to eliminate outliers, we only used values in the inter-quartile range.
2.4.2 Cell Viability
We used the Live/Dead viability assay (Invitrogen) which assesses cell viability using Calcein (a cytoplasmic dye that is taken up and cleaved by cytoplasmic esterases, thus staining the cytoplasm green) and Ethidium homodimer-1 (a nuclear stain that only permeates cells with compromised cell membranes, i.e. cells that are dead or in the process of dying, staining the nucleus red). Samples were cultured in CEM media or Tyrode solution (HEPES 10 mM, NaCl 140 mM, KCl 5 mM, CaCl2 1 mM, MgCl2 1 mM and glucose 0.45 w/v%) for 1d and 1wk, before being labeled with 2 μM calcein and 2 μM Ethidium homodimer-1 for 20 minutes. The samples were then washed with PBS twice and imaged using 780nm laser excitation.
2.4.3 Cell Proliferation
Rat CDCs encapsulated in 50 μL HA-PEG or HA-BL hydrogels (n=6) were cultured in CEM. Lyophilized hydrogels were homogenized and digested with 500μL proteinase K (0.1 mg/mL proteinase K, 10 mM Tris, 1 mM EDTA, and 0.1% Triton X-100) at 60°C for 15h after culturing for days 0, 4 and 8. An aliquot (5 μL) was used to quantify the double-stranded DNA concentration using the PicoGreen assay (Invitrogen). Since nuclei from lysed blood cells can produce a signal, results were normalized to the signal obtained at the time of cell encapsulation. PicoGreen is a flurochrome that selectively binds dsDNA and has an excitation/emission maxima at 480/520 nm. When bound to dsDNA, fluorescence enhancement of PicoGreen is exceptionally high; little background occurs since the unbound dye has virtually no fluorescence [15].
2.4.4 Scanning electron microscopy
We utilized FEI Quanta 200 Environmental SEM (ESEM) to examine the surface structure of HA-blood hydrogels. For this purpose, 100μL of HA-blood hydrogel samples were prepared as described above, with and without CDCs (2million CDCs in each hydrogel) and cultured for 24hrs. Samples were incubated in PBS for 2hrs before imaging. Samples were placed in an ESEM chamber at 4°C and 100% humidity and excited using an electron beam strength of 20–25 kV @ 500–550 Pa. Humidity of the chamber was reduced in 5% increments until optimal resolution was achieved and samples could be adequately imaged.
2.5 In Vivo Studies and Histology
In vivo studies were performed using HA-lysed blood hydrogels based on results of in vitro studies comparing HA-PEG with HA-lysed blood hydrogels.
All procedures were performed with prior approval from the Johns Hopkins Animal Care and Use Committee.
2.5.1 Cell injection
Rat CDCs (5×106) were labelled with the positron emitter, 18FDG in order to measure acute cell retention in the myocardium following intra-myocardial cell-hydrogel transplantation [16]. Media was changed to glucose-free DMEM (Invitrogen) containing 10% FBS one hour prior to labelling with 18FDG. For cell labelling, rCDCs were subsequently incubated with 2 μCi/ml of 18FDG in glucose-free DMEM for 30 min. Subsequently, labelling media was removed by 2 washes in PBS, CDCs were trypsinized, pelleted by centrifugation, suspended in 90μL of lysed human blood and transferred to a 27.5 gauge 0.5 mL injection syringe. CDCs suspended in lysed blood were mixed with 90 μL HA immediately prior to intra-myocardial injection.
2.5.2 Animal surgery - cell injection
Immuno-deficient, RNU nude female rats (Charles River) and syngeneic WK rats weighing 200–250 gm were used as cell recipients to permit quantification of cell retention in the heart and lungs following (18FDG-labeled) CDC encapsulation in hydrogels containing lysed human and rat blood respectively. Syngeneic WK rats transplanted with HA-blood (lysed rat blood) were used for echocardiography and pathology studies.
Myocardial infarction was induced in animals used for in vivo PET imaging and experiments. Rats whose hearts were explanted for pathology did not have induction of MI, which permitted assessment of the effects of hydrogel injection on tissue architecture and inflammation without the possibly confounding effects of myocardial infarction.
Rats were intubated, anesthesia was induced with 4% isoflurane inhalation and maintained with 2% inhalation. The heart was exposed through a left lateral thoracotomy and a small anterior myocardial infarction was induced by distal ligation of the left anterior descending artery (only for ECHO imaging experiments). We subsequently either injected a total of 70–90 μL of the HA-blood hydrogel containing 2 million rCDCs, intra-myocardially into 2 sites in the anterior left ventricular wall or applied the HA-blood hydrogel alone, epicardially on the anterior wall of the beating heart following treatment of the visceral pericardium with 2.5% trypsin for 5min. Subsequently, the chest was closed and the animal was placed in the PET scanner for in vivo PET imaging. Animals with intra-myocardial injection of hydrogel were mechanically ventilated until sacrifice. Animals treated with intra-myocardial injection of cell suspensions in IMDM and epicardial applications of hydrogel were extubated.
We have previously shown that measured radioactivity in the heart and lung is an excellent measure of cell retention in these organs [16]. We performed ex vivo measurements of radioactivity using a gamma counter and in vivo PET-CT imaging of hydrogel-encapsulated 18FDG-labeled CDCs and compared it to intra-myocardial injection of 18FDG-labeled CDCs suspended in IMDM (serum-free).
2.5.3 PET-CT imaging
PET images were acquired on a GE Healthcare Vista (GE Healthcare, Piscataway, New Jersey, USA) small animal PET system. Animals were anaesthetized and placed in a supine, head first position into the PET scanner. 18FDG images for quantification of CDC engraftment were obtained as dynamic, list mode acquisitions for 60min reconstructed in 10min frames. For myocardial delineation and accurate quantification of activity exclusively derived from rCDCs retained in the myocardium, a perfusion PET scan (20 min static acquisition) was performed following injection of 37MBq of 13NH3 via the tail vein at the end of the FDG acquisition. Free 18F (37mBq) was injected after completion of the 13NH3 acquisition. After a period of 10min, required for adequate uptake of fluoride by the bones, a 15 min static acquisition was obtained. The purpose of this scan is to obtain images with distinguished radioactivity uptake in bones that serve as co-registration landmarks and permits signal quantification. All images were acquired at exactly the same position and the animals’ body’s temperature was monitored and controlled by a heating lamp.
After completion of the PET acquisitions, the animal (restrained on the same bed) was moved into a CT scanner and CT images were obtained. A separate micro-CT scanner was used because a dual modality PET/CT scanner is not available at our institution. We used the bi-module SPECT/CT live small animal imaging system, Gamma Medica X-SPECT (Gamma Medica, Northridge, CA, USA) to perform X-ray computed tomography. An X-ray tube of tube voltage 75 kVp with 512 projections was used to acquire over a 360 degree range. Projections containing 1184×1120 isotropic pixels (100μm) were reconstructed into a CT volume of 5123 isotropic voxels (170μm3).
Co-registration of PET and CT images were generated using rigid body transformation by manually identifying bones as landmarks. All images were analyzed using AMIDE software [17]. For quantification of signal in the heart, a volume of interest (VOI) was drawn to include the bright spot at the site of cell injection. For quantification of activity in the lungs, 2 VOIs were drawn, one for each lung.
For ex vivo radioactivity measurements, rats were euthanized 1hr following injection of 18FDG-labeled cells encapsulated in the hydrogel. The heart and lungs were excised and transferred to scintillation vials. Counts from heart, lung and blood were recorded in a gamma-counter (Perkin Elmer, Waltham, MA). We calculated the ratio of cells in the heart to cells in the lung by dividing the signal in the heart to the signal in the lung.
2.5.4 Echocardiography
We performed echocardiograms at 2 time points, namely, 24hr and 3 wks post-MI in 2 groups, namely the control group that only underwent myocardial infarction (n=3), and the epicardial hydrogel group that underwent induction of myocardial infarction followed by epicardial application of HA-blood hydrogels (n=5).
For imaging, rats were anesthetized with 1.5% isoflurane (using a nose cone) for the duration of imaging and placed supine on an electrical heating pad and a tensor lamp was used to provide additional heat. The rat core temperature was monitored with a rectal probe. ECG signals were obtained by contacting the mouse limbs, coupled with electrically conductive gel to ECG electrodes integrated itno the heating pad. Ultrasound imaging was performed using the VEVO 2100 system (VisualSonics, Toronto, On, Canada). Using B-mode imaging, the MS250 scanhead (fc=21MHz, 256 elements) was positioned and immobilized using the VisualSonics Vevo Integrated Rail System. Two dimensional long axis images were used for the measurement of ejection fraction (calculated as the difference between end-diastolic and end-systolic volumes normalized to end-diastolic volume, expressed as a percentage). The trans-mitral valve flow was measured using pulse-wave Doppler (PW-mode). Following a brief stabilization period, a baseline recording of pulse-wave Doppler blood flow was obtained. The short-axis image was not acquired because of distortion of anatomy resulting from a left sided pneumothorax and sterna adhesions in the post-operative period. Image processing was performed off-line.
2.5.5 Histology
Hydrogel scaffolds containing 2×106 rCDCs were injected intra-myocardially or applied epicardially in syngeneic, immune-competent Wistar Kyoto rats in order to assess the effects of the hydrogels on inflammation in the heart. Following euthanasia at different time points (24hrs, 1d and 1 wk), the tissue was extracted and fixed in 4% paraformaldehyde overnight. Tissue sections were stained with hematoxylin and Eosin (H&E), Masson’s trichrome or Saffranin-O.
2.6 Statistics
Continuous variables were expressed as means +/− SE. Intergroup differences were assessed using Student’s t-test or ANOVA. P value of <0.05 was considered statistically significant. All statistical analysis was performed using JMP software.
3. RESULTS
3.1 Physical characterization of HA-blood hydrogels and comparison with HA-PEG hydrogels
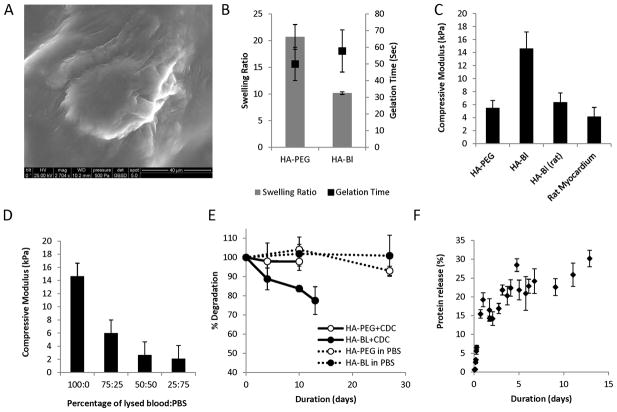
HA-blood and HA-PEG hydrogels resulted from the reaction of NHS groups in HA with free amine groups present on blood proteins and in PEG-amine to form amide bonds. SEM showed evidence of cell encapsulation in HA-blood hydrogels (Fig 1A). The hydroxamate assay revealed that 1 of 6 carboxyl groups in HA was modified with NHS.
Figure 1. Characterization of HA-blood (human, lysed) and HA-PEG hydrogels.
A. Scanning electron microscopy image of CDC encapsulated in HA-blood hydrogel. (Calibration bar is 40μm).
B. Gelation time of HA-PEG and HA blood hydrogels was similar but equilibrium swelling ratio of HA-PEG hydrogels was greater than HA-blood hydrogels
C. Young’s moduli of HA-blood hydrogels using human blood was higher than the modulus of rat myocardium and HA-blood hydrogels composed of lysed rat blood (WK rats)
D. Young’s moduli of HA-blood hydrogels decreased proportionately as the concentration of lysed blood in the hydrogels decreased by diluting with PBS
E. Minimal degradation of HA-blood and HA-PEG hydrogels by hydrolysis (dashed lines). Presence of CDCs accelerates degradation of HA-blood hydrogels (solid lines).
F. Protein release from HA-blood hydrogels in PBS
The optimal gelation time depends on the specific clinical application: a very short gelation time would preclude injection through a 28G needle, and a very long gelation time would result in egress of the cell-hydrogel mixture from the injection site and into the lungs, possibly resulting in pulmonary embolism/infarction. The gelation times of HA-PEG and HA-Blood hydrogels were similar (50±10.5 seconds for HA-PEG vs. 58±12 seconds for HA-Blood, p=0.12) Fig 1B.
A higher swelling ratio is related to larger average pore size of the scaffold allowing greater diffusion of metabolites into the hydrogel. The swelling ratio of HA-PEG hydrogels was 2.1 times greater than the swelling ratio for HA-Blood hydrogels (21± 2.6 for HA-PEG vs. 10± 0.5 for HA-Blood, p=0.007) -Fig 1B.
Uniaxial compressive modulus measures deformation in the x and y direction from compression in the z direction, and is an indicator of a gel’s ability to resist compressive force, which is very important for cardiac applications. We found the compressive modulus of contracted rat myocardium to be 4.18±1.39kPa (Fig 1C) and relaxed rat myocardium to be 1.5± 0.68kPa. Injection of a significant volume of a stiff material into the myocardium could disrupt cardiac architecture and impair contraction, whereas a very soft material would be expelled into the coronary venous system and into the pulmonary circulation as a result of cardiac contraction. The compressive modulus of HA-blood hydrogels was 2.55 times higher than HA-PEG hydrogels (14± 3 kPa for HA-blood vs. 5.5± 1.1 kPa for HA-PEG)-Fig 1C; compressive modulus decreased with increased dilution of blood by PBS (Fig 1D). We also observed variation in the moduli of HA-blood hydrogels depending upon the source of blood used (Fig 1C). The compressive modulus of HA-blood hydrogels composed of whole (non-lysed) human blood was 3.31±1.39 kPa and with lysed rat blood was 6.38±1.4 kPa. It is possible that variations in blood cell count, serum and intracellular protein content between human and rat blood [18–25] explain these differences.
The degradation rate represents the time it takes for a hydrogel to fully degrade into microscopic size particles that can potentially be removed from the site of injection by the body. In the heart, it is important for the polymer to degrade naturally in order to eliminate non-excitable barriers to electrical propagation, which can be pro-arrhythmic [26]. HA-Blood and HA-PEG cannot be degraded over 4 weeks with hydrolysis alone. In the presence of encapsulated CDCs, HA-blood hydrogels degrade significantly faster than HA-PEG (16±1.6% vs. 2.6±2.3% on day 10, p=0.008) - Fig 1E.
Analysis of proteins released from the hydrogel revealed a two phase release: we saw a rapid release of 10–15% of the total protein content within the first 12 hours (~1.1% per hr), which probably represents unbound proteins, followed by a slower protein release phase (0.04% per hr) which is likely to result from hydrolysis.
3.2 Hydrogel-Cell Interactions
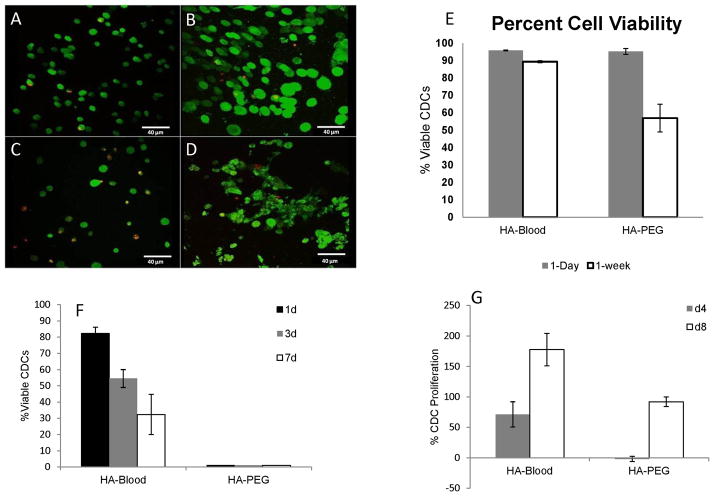
Cell viability is an important indication of the success of a hydrogel in supporting cells by providing structural support, binding domains, and substrates. On day 1, >95% of cells were viable in both HA/Blood (n=186, n-alive=178) and HA/PEG hydrogels (n=151, n-alive=144-Fig 2A, B, E), but cells were larger in HA-blood hydrogels probably because of cell adhesion to hydrogels. However, after 1 week, 89% of CDC’s in HA/Blood hydrogels were still viable while the viability of cells in HA/PEG hydrogels decreased to 56% (Fig 2C, D, E). Furthermore, cells in HA-PEG hydrogels were still small and rounded (Fig 2C), while cells in HA-blood hydrogels showed evidence of spreading (Fig 2D) at 1 week. Of note, when CDCs were cultured in HA-blood and HA-PEG hydrogels in Tyrode solution (in the absence of serum), CDC viability in HA-blood hydrogels was 82.2±3.7% on d1, 54.5±5.5% on d3, and 32.4±12.4% on d7, compared to 0% for HA-PEG hydrogels (p<0.001) -Fig 2F, suggesting that HA-blood hydrogels provide adhesion motifs and growth factors for cell survival.
Figure 2. HA-blood hydrogels promote cell spreading and proliferation.
Representative 2-photon microscopy images illustrating live (green) and dead CDCs (red) on day 1 in HA-PEG (A) and HA-blood (human, lysed) hydrogels (B) and day 7 in HA-PEG (C) and HA-blood (D) hydrogels cultured in CEM
E. Bar graphs summarize CDC survival at 1d and 1wk in HA-blood and HA-PEG hydrogels cultured in CEM
F. HA-blood hydrogels, but not HA-PEG hydrogels permit CDC survival when cultured in Tyrode solution (containing glucose and electrolytes, but no serum)
G. Picogreen assay revealed CDC proliferation on d4 and d8 in HA-blood hydrogels, but only on d8 in HA-PEG hydrogels
HA-blood hydrogels promoted CDC proliferation in the hydrogels. The signal, reflecting dsDNA content of the hydrogels increased by 71±20% over baseline in HA-blood hydrogels but decreased by −1.9±4.2% in HA PEG hydrogels on d4 post-encapsulation (p<0.001). On d8 post-encapsulation, when CDCs have presumably had an opportunity to secrete their own extracellular matrix, the signal increased in both hydrogels, but the increase in HA-blood hydrogels (177.7±26.5%) was greater than that observed in HA-PEG hydrogels (91.9±7.9%, p<0.001) – Fig 2G.
3.3 In Vivo Hydrogel Application
Cell encapsulation in HA-blood hydrogels greatly increased acute myocardial retention (1hr) following intra-myocardial injection: the ratio of activity (resulting from 18FDG-labeling of CDCs) in the heart to lungs was 4.7±1.6 (n=5) with hydrogel and 0.9±0.6 (n=5) with CDCs suspended in serum-free medium (controls).
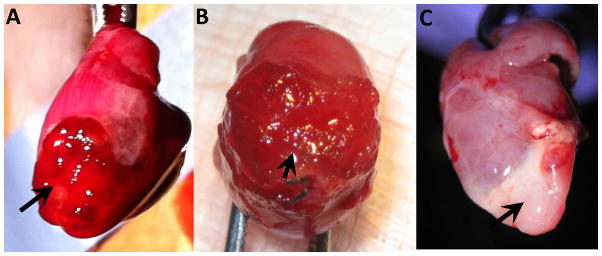
Intra-myocardial injection of HA-blood hydrogel (derived from rat and human blood) resulted in 100% mortality (n=20) following extubation (discontinuation of mechanical ventilation); this result was independent of the rat strain, source of blood (human versus rat), presence/absence of cells in the hydrogel and presence/absence of myocardial infarction. In contrast, 90% of animals treated with epicardial application of hydrogel (Fig 3A, B, C) or intra-myocardial injection of CDCs suspended in IMDM survived.
Figure 3. Epicardial hydrogel application.
Explanted rat hearts at 1d (A), 3d (B) and 7d (C) following epicardial application of HA-blood (WK rat, lysed) hydrogel (black arrow).
3.3.1 Echocardiography
There was no significant difference in left ventricular ejection fraction (LVEF) between animals treated with epicardial application of HA-lysed blood hydrogels and controls (no treatment) at 24hrs post-myocardial infarction, indicating that animals from both groups suffered a similar ischemic insult (57±1.8 in controls vs 53±3.9 for hydrogel group at 24hrs; p=0.16). However 3 weeks post-MI, left ventricular systolic function increased significantly in the hydrogel group, when compared to the baseline at 24hrs (53±3.9 vs 62±2.1, p=0.01), but not in controls (57±1.8 vs 61±8.3, p=0.50), suggesting that epicardial application of HA-blood hydrogels promotes regeneration. Diastolic function as evaluated by E/A ratio was within the normal range at both time points for the control (1.7±0.01 vs 1.7±0.25, p=0.81) and hydrogel group (1.6±0.3 vs 1.5±0.3, p=0.68) indicating that epicardial application of the hydrogel did not elicit a constrictive pericarditis-type physiology.
3.3.2 Pathology
For intra-myocardially injected hydrogels, we found a disruption of cardiac architecture at the injection site, but no evidence of inflammation 1hr post-injection (Fig 4A, B). H&E staining revealed an acute inflammatory response with neutrophil infiltration at the hydrogel-epicardial interface 24hr after epicardial application of the HA-blood hydrogel (Fig 4C). At 1 wk, multi-nuclear giant cells and macrophages were seen infiltrating the hydrogel. Masson trichrome staining revealed no evidence of myocardial fibrosis in the epicardial layer adjacent to the hydrogel at 1 week. H&E staining showed persistence of the hydrogel at 1week post- application (Fig 4D).
Figure 4. Histology of HA-blood hydrogels (lysed WK rat) following in vivo application.
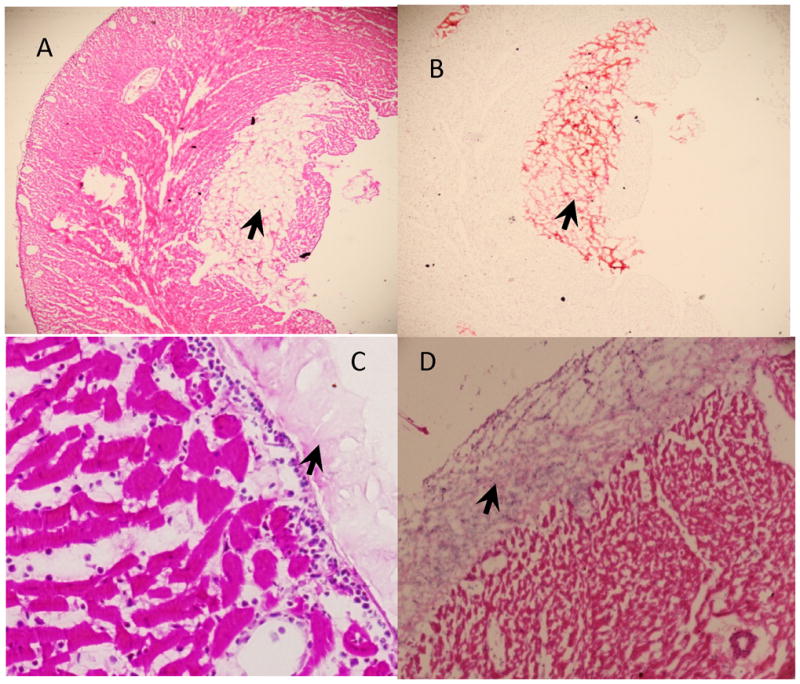
A. H&E staining of myocardium 1hr after intra-myocardial injection of hydrogel (black arrow).
B. Saffranin-O staining reveals presence of intra-myocardial hydrogel (black arrow) 1hr after intra-myocardial injection
C. H&E staining of myocardium 24hrs after epicardial application of hydrogel (black arrow) reveals neutrophil infiltration in the epicardium at the hydrogel-myocardial interface
D. H&E staining of myocardium 7d after epicardial application of hydrogel (black arrow) reveals presence of epicardial hydrogel
4. DISCUSSION
HA-blood hydrogels are easy to synthesize, promote stem cell survival and proliferation and show promise for cell delivery by epicardial application. Previous studies aimed at improving engraftment have used agents such as fibrin glue which is pro-thrombotic, could be immunogenic and may increase mortality when used during coronary artery bypass grafting[27], polyethylene glycol (PEG), a polymer that lacks adhesion motifs and is not degraded by naturally occurring enzymes, and HA alone. The combination of autologous blood and HA has the following advantages: a) both components provide adhesion motifs that can activate pro-survival pathways: blood contains vitronectin and fibronectin with RGD(arginine-glycine-aspartate)[28] motifs that can activate integrins, while HA receptors (CD44) are present on several stem cells; b) blood can provide growth factors to transplanted cells prior to establishment of angiogenesis, c) HA and/or its degradation products can promote angiogenesis[29], vasculogenesis, cardiogenesis; c) hydrogels can be degraded by enzymes (hyaluronidases, proteases) present in the heart and by hydrolysis; d) our method of covalent cross-linking allows hydrogel synthesis and adhesion to transplanted tissue without the use of ultraviolet light, heat or sutures, facilitating clinical translation.
For cellular cardiomyoplasty, hydrogel scaffolds need to have enough mechanical strength (rigidity) to withstand cardiac contraction without fracturing, as well as sufficient elasticity to not impede systolic and diastolic function. A study by Ifkovits et al [30], in the ovine model of myocardial infarction indicated that use of HA-based hydrogels with higher rigidity/compressive moduli (43 kPa) resulted in greater functional improvement than hydrogels with compressive modulus similar to cardiac muscle (8kPa). Our preliminary studies using various human blood components (plasma, serum, whole blood, lysed blood, albumin) revealed that only lysed blood resulted in hydrogels with compressive modulus which was greater than twice the compressive modulus of rat myocardium. Hence, we used lysed blood-HA hydrogels for in vitro and in vivo testing. Another advantage of HA-blood hydrogels is their porosity, which is represented by a high swelling ratio that permits exchange of electrolytes, metabolites and substrates, as well as infiltration of circulating cells and migration of transplanted cells into native tissue. Most importantly, HA-blood hydrogels adhere to the beating myocardium and improve ejection fraction following myocardial infarction.
Animals treated with intra-myocardial injection of HA-blood hydrogels died following extubation, but could survive for several hours while mechanically ventilated. Possible mechanisms underlying this phenomenon include lung embolization/infarction and impaired myocardial mechanics resulting from hydrogel-induced disruption of myocardial architecture. We speculate that impaired myocardial mechanics is the culprit based on the results of in vivo PET imaging, and the fact that this effect was seen even following hydrogel injection into normal myocardium; extubation from the ventilator would be expected to increase cardiac pre-load and after-load [31], which reduce cardiac output and hence can induce cardiogenic shock and death in the setting of impaired heart function. In contrast, mortality in animals treated with epicardial application of the HA-blood hydrogel was similar to controls, both in the presence and absence of myocardial infarction. Importantly, epicardial application of the hydrogel did not induce a constrictive pericarditis-like physiology at any time point following application, as assessed by echocardiography; pathology confirmed lack of an exuberant inflammatory response and fibrosis at 7 days.
Epicardial cell delivery via scaffolds is very attractive during open heart surgery because it would permit the transplantation of large numbers of cells which are retained in the heart, in contrast to intra-myocardial injections where back leak of cells from the injection site and egress via the coronary venous system result in only a small proportion of cells remaining at the transplantation site [16, 32]. An important advantage of HA-blood hydrogels, over HA [33, 34] or PEG-based [35, 36] hydrogels for clinical applications is that they can be synthesized using autologous blood (which can be easily obtained by venipuncture from the patient undergoing cell transplantation 24hrs prior) and they provide adhesion motifs as well as growth factors that promote stem cell proliferation. This could permit transplantation of small numbers of autologous cells (that typically take several weeks to expand) early following myocardial infarction (possibly via percutaneous catheter delivery to the epicardium), resulting in reduction of scar formation and improved cardiac regeneration.
Currently, most clinical studies employ multiple needle-based injections for intra-myocardial transplantation in order to maximize the number of transplanted cells. This results in an inhomogeneous distribution of cells and disruption of the myocardial architecture (resulting in fibrosis) at the injection site, which can be pro-arrhythmic [26, 37]. HA-blood hydrogel synthesis and epicardial application are technically facile and hence would be easy to implement in the operating room or a cardiac catheterization laboratory for percutaneous delivery of large numbers of cells, in contrast to cell sheets [38–40] that need careful handling and transfer.
5. LIMITATIONS
In this study, we only evaluated survival and proliferation of CDCs in vitro. However, we believe that similar results would be obtained from other adherent stem cell types such as mesenchymal stem cells, endothelial progenitor cells and cardiac progenitors derived from embryonic stem cells or induced pluripotent cells. Further studies are needed using other stem cell types, embedded in these hydrogels, in small and large animal models, prior to clinical translation. The high mortality observed with intra-myocardial hydrogel injection in our study, may be related to using the rat model, where the volume of injected hydrogel is high, relative to left ventricular mass and infarct size. Studies in large animal models, (porcine or ovine [30]) where the volume of injected hydrogel is small relative to myocardial mass and infarct size may be preferable to the rat model for in vivo testing of hydrogels for cardiac applications.
Growth factors in blood that promote cell proliferation could prevent spontaneous cardiac differentiation in the hydrogel. This could be an advantage for in vivo applications because it could permit induction of angiogenesis[41] by the HA component while cells are still encapsulated in the hydrogel. Hydrogel degradation following the establishment of angiogenesis would permit the transplanted stem cells to respond to local cues that promote differentiation into cardiac myocytes, whose metabolic profile and ATP demands are higher than undifferentiated stem cells.
Here, we report results using blood derived from a single healthy volunteer. Since cell count and blood protein levels differ in individuals, this could have a significant effect on the mechanical properties of hydrogels incorporating this material. Future studies using blood from patients would be required prior to clinical translation, since therapeutic levels of common cardiac medications such as statins, ACE-inhibitors, beta blockers may influence stem cell function.
6. CONCLUSION
HA-Blood hydrogels are a addition to the field of cardiac tissue engineering for the treatment of myocardial infarction. HA-blood hydrogels are self-polymerizing, highly adhesive, biodegradable and increase cardiac function following epicardial application after myocardial infarction. Combining autologous blood and HA in a hydrogel provides adhesion motifs and growth factors that promote cell proliferation in the hydrogel matrix. These hydrogels have a high potential for clinical translation in studies of cell transplantation in the heart and other organs.
Acknowledgments
This work was funded by be the American heart association (AHA-BGIA), NIH RO1 HL092985 and NIH 5UL1RR025005-05. Dr. Angel Chan was supported by NIH T32HL07227 Training Grant. Dr. Stella Vakrou was supported by Hellenic Society of Cardiology. Sophia Brown, MS was supported by a NIH Diversity supplement to RO1 HL092985. Dr. Xiaoping Lin was partially supported by the China Scholarship Council. We are grateful to James Fox and Jim Engles for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 2.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 106:971–80. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonios M, Chang CY, Pinheiro A, Dimaano VL, Higuchi T, Melexopoulou C, et al. Cardiac resynchronization by cardiosphere-derived stem cell transplantation in an experimental model of myocardial infarction. J Am Soc Echocardiogr. 2011;24:808–14. doi: 10.1016/j.echo.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonios M, Terrovitis J, Chang CY, Engles JM, Higuchi T, Lautamaki R, et al. Myocardial substrate and route of administration determine acute cardiac retention and lung bio-distribution of cardiospherederived cells. J Nucl Cardiol. 18:443–50. doi: 10.1007/s12350-011-9369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schussler O, Coirault C, Louis-Tisserand M, Al-Chare W, Oliviero P, Menard C, et al. Use of arginineglycine- aspartic acid adhesion peptides coupled with a new collagen scaffold to engineer a myocardiumlike tissue graft. Nat Clin Pract Cardiovasc Med. 2009;6:240–9. doi: 10.1038/ncpcardio1451. [DOI] [PubMed] [Google Scholar]
- 6.Schussler O, Coirault C, Louis-Tisserand M, Al-Chare W, Oliviero P, Menard C, et al. Use of arginineglycine- aspartic acid adhesion peptides coupled with a new collagen scaffold to engineer a myocardiumlike tissue graft. Nature Clinical Practice Cardiovascular Medicine. 2009;6:240–9. doi: 10.1038/ncpcardio1451. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann WH, Didie M, Wasmeier GH, Nixdorff U, Hess A, Melnychenko I, et al. Cardiac grafting of engineered heart tissue in syngenic rats. Circulation. 2002;106:I151–7. [PubMed] [Google Scholar]
- 8.Schenke-Layland K, Nsair A, Van Handel B, Angelis E, Gluck JM, Votteler M, et al. Recapitulation of the embryonic cardiovascular progenitor cell niche. Biomaterials. 32:2748–56. doi: 10.1016/j.biomaterials.2010.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon SJ, Fang YH, Lim CH, Kim BS, Son HS, Park Y, et al. Regeneration of ischemic heart using hyaluronic acid-based injectable hydrogel. J Biomed Mater Res B Appl Biomater. 2009;91:163–71. doi: 10.1002/jbm.b.31386. [DOI] [PubMed] [Google Scholar]
- 10.Ifkovits JL, Tous E, Minakawa M, Morita M, Robb JD, Koomalsingh KJ, et al. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci U S A. 107:11507–12. doi: 10.1073/pnas.1004097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:11298–303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita Y, Kitagawa M, Nakamura S, Azuma K, Ishii G, Higashi M, et al. CD44 signaling through focal adhesion kinase and its anti-apoptotic effect. FEBS Lett. 2002;528:101–8. doi: 10.1016/s0014-5793(02)03262-3. [DOI] [PubMed] [Google Scholar]
- 13.McFarland CD, Thomas CH, DeFilippis C, Steele JG, Healy KE. Protein adsorption and cell attachment to patterned surfaces. J Biomed Mater Res. 2000;49:200–10. doi: 10.1002/(sici)1097-4636(200002)49:2<200::aid-jbm7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Strehin I, Nahas Z, Arora K, Nguyen T, Elisseeff J. A versatile pH sensitive chondroitin sulfate-PEG tissue adhesive and hydrogel. Biomaterials. 2010;31:2788–97. doi: 10.1016/j.biomaterials.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn SJ, Costa J, Emanuel JR. PicoGreen quantitation of DNA: effective evaluation of samples pre- or post-PCR. Nucleic Acids Res. 1996;24:2623–5. doi: 10.1093/nar/24.13.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonios M, Terrovitis J, Chang CY, Engles JM, Higuchi T, Lautamaki R, et al. Myocardial substrate and route of administration determine acute cardiac retention and lung bio-distribution of cardiospherederived cells. J Nucl Cardiol. 2011;18:443–50. doi: 10.1007/s12350-011-9369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging. 2003;2:131–7. doi: 10.1162/15353500200303133. [DOI] [PubMed] [Google Scholar]
- 18.Lerner AJ, Schoenberg MR. In: Deconstructing the Medical Chart: The Little Black Book of Neuropsychology. Schoenberg MR, Scott JG, editors. Springer; US: 2011. pp. 39–58. [Google Scholar]
- 19.Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26:72–6. [PubMed] [Google Scholar]
- 20.Zaias J, Mineau M, Cray C, Yoon D, Altman NH. Reference values for serum proteins of common laboratory rodent strains. J Am Assoc Lab Anim Sci. 2009;48:387–90. [PMC free article] [PubMed] [Google Scholar]
- 21.Busher J. Serum Albumin and Globulin. In: Walker HKHW, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3. Boston: 1990. [PubMed] [Google Scholar]
- 22.Schmid-Schonbein GW, Seiffge D, DeLano FA, Shen K, Zweifach BW. Leukocyte counts and activation in spontaneously hypertensive and normotensive rats. Hypertension. 1991;17:323–30. doi: 10.1161/01.hyp.17.3.323. [DOI] [PubMed] [Google Scholar]
- 23.Cecil RL, Goldman L, Bennett JC, Drazen JM. Cecil textbook of medicine. 21. Philadelphia: W.B. Saunders; 2000. [Google Scholar]
- 24.Wada M, Kumagai K, Murata M, YSY, Shindo H. Warfarin reduces the incidence of osteonecrosis of the femoral head in spontaneously hypertensive rats. J Orthop Sci. 2004;9:585–90. doi: 10.1007/s00776-004-0829-9. [DOI] [PubMed] [Google Scholar]
- 25.Daly ME. Determinants of platelet count in humans. Haematologica. 2011;96:10–3. doi: 10.3324/haematol.2010.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abraham MR, Henrikson CA, Tung L, Chang MG, Aon M, Xue T, et al. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ Res. 2005;97:159–67. doi: 10.1161/01.RES.0000174794.22491.a0. [DOI] [PubMed] [Google Scholar]
- 27.Goerler H, Oppelt P, Abel U, Haverich A. Safety of the use of Tissucol (R) Duo S in cardiovascular surgery: retrospective analysis of 2149 patients after coronary artery bypass grafting. European Journal of Cardio-Thoracic Surgery. 2007;32:560–6. doi: 10.1016/j.ejcts.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Lai Y, Yu L, Ding J. Effects of immobilizing sites of RGD peptides in amphiphilic block copolymers on efficacy of cell adhesion. Biomaterials. 2010;31:7873–82. doi: 10.1016/j.biomaterials.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, et al. Hyaluronan-mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biology. 2007;26:58–68. doi: 10.1016/j.matbio.2006.08.261. [DOI] [PubMed] [Google Scholar]
- 30.Ifkovits JL, Tous E, Minakawa M, Morita M, Robb JD, Koomalsingh KJ, et al. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci U S A. 2010;107:11507–12. doi: 10.1073/pnas.1004097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shekerdemian L, Bohn D. Cardiovascular effects of mechanical ventilation. Arch Dis Child. 1999;80:475–80. doi: 10.1136/adc.80.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terrovitis J, Lautamaki R, Bonios M, Fox J, Engles JM, Yu J, et al. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol. 2009;54:1619–26. doi: 10.1016/j.jacc.2009.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 34.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23:H41–56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–51. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 36.Almany L, Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26:2467–77. doi: 10.1016/j.biomaterials.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 37.Chang MG, Zhang Y, Chang CY, Xu L, Emokpae R, Tung L, et al. Spiral waves and reentry dynamics in an in vitro model of the healed infarct border zone. Circ Res. 2009;105:1062–71. doi: 10.1161/CIRCRESAHA.108.176248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Memon IA, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Taketani S, et al. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg. 2005;130:1333–41. doi: 10.1016/j.jtcvs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 39.Kondoh H, Sawa Y, Miyagawa S, Sakakida-Kitagawa S, Memon IA, Kawaguchi N, et al. Longer preservation of cardiac performance by sheet-shaped myoblast implantation in dilated cardiomyopathic hamsters. Cardiovasc Res. 2006;69:466–75. doi: 10.1016/j.cardiores.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Furuta A, Miyoshi S, Itabashi Y, Shimizu T, Kira S, Hayakawa K, et al. Pulsatile cardiac tissue grafts using a novel three-dimensional cell sheet manipulation technique functionally integrates with the host heart, in vivo. Circ Res. 2006;98:705–12. doi: 10.1161/01.RES.0000209515.59115.70. [DOI] [PubMed] [Google Scholar]
- 41.Ware JA, Simons M. Angiogenesis in ischemic heart disease. Nature medicine. 1997;3:158–64. doi: 10.1038/nm0297-158. [DOI] [PubMed] [Google Scholar]