Abstract
We developed a paclitaxel-conjugated polymeric micelle, ABP-PEG3.5k-Paclitaxel (APP) consisting of poly (ethylene glycol) (PEG) and arginine-grafted poly(cystaminebisacrylamide-diaminohexane) (ABP) for the co-delivery of gene and drug. The APP polymer self-assembled into cationic polymeric micelles with a critical micelle concentration (CMC) value of approximately 0.062 mg/mL, which was determined from measurements of the UV absorption of pyrene. The micelles have an average size of about 3 nm and a zeta potential of about +14 mV. Due to the positive surface charge, APP micelles formed polyplexes with plasmid DNA approximately 200 nm in diameter. The luciferase gene and mouse interleukin-12 (IL-12) gene was used to monitor gene delivery potency. APP polyplexes showed increased gene delivery efficiency and cellular uptake with higher anticancer potency than paclitaxel alone. These results demonstrate that an APP micelle-based delivery system is well suitable for the co-delivery of gene and drug.
Keywords: Bioreducible polymer, Drug delivery, Gene therapy, Paclitaxel, Polymeric micelle, Interleukin-12
1. Introduction
Cancer is a disease in which abnormal and uncontrolled cells divide in the body. Cancer cells generally grow very fast, spreading and destroying other parts of the body. Cancer has remained hard to cure despite recent technical advances in surgery and other therapies, including radiation, chemo, and hormone therapy. Based on the Cancer Facts & Figures 2012, about 1,596,670 cancer cases and 571,950 cancer deaths are estimated to occur yearly in the U.S. Breast cancer is the most common type of cancer among females (29% of the estimated new cases) while in the case of males, prostate cancer is the leading cancer (29% of the estimated new cases) [1]. Chemotherapy is the most common therapeutic approach and paclitaxel, a microtubule-stabilizing and cell division-blocking anti-cancer chemotherapeutic, is one of the most commonly used drugs [2]. Paclitaxel binds to tubulin and inhibits the breakdown of microtubules. This paclitaxel-induced microtubule stabilization blocks cell cycle in the late G2/M-phase, and leads to cell death. Based on the efficacy of that, Paclitaxel is widely used to treat a variety of patients with varying forms of cancer such as ovarian, breast, lung, head and neck cancers [3, 4]. This drug is also used for the treatment of Kaposi’s sarcoma and for the prevention of restenosis of coronary stents [5–7]. However, clinical applications of paclitaxel are limited by disadvantages such as poor water solubility (0.3 μg/ml) and instability in aqueous solution [8, 9].
A co-solvent can increase the solubility of hydrophobic organic chemicals, such as paclitaxel. To solve solubility problem of paclitaxel, a co-solvent system, Cremophor® EL (a polyoxyethylated caster oil in 49.7% dehydrated alcohol), has been generally used in clinical applications. Still, this co-solvent system may cause other problems such as hypersensitivity reactions and neurotoxicity [10, 11].
Paclitaxel has numerous hydrolytically sensitive ester groups. Degradation of paclitaxel mainly occurs by hydrolysis under basic and neutral conditions in aqueous solution [12]. In order to increase the therapeutic efficiency and overcome the disadvantages of the current delivery system, various methods including emulsification, micellization, liposome formation, microencapsulation and formation of polymeric micelles have been developed [13, 14].
In cancer chemotherapy, long circulating carrier systems improve drug delivery potency. Polymeric micelles are one of the most promising candidates for cancer drug delivery [9, 15]. Due to their unique structure, formed by amphiphilic copolymers, polymeric micelles can solubilize poorly water soluble drugs including paclitaxel and protect hydrolytically sensitive drugs from the aqueous environment. In addition, polymeric micelles can stay in the bloodstream long enough to provide accumulation at the target area, thereby increasing therapeutic efficacy [16].
Previous work in our group demonstrated the effects of combination therapy of IL-12 and paclitaxel [17, 18]. The combined approach showed significant inhibition of tumor growth. Then, arginine-grafted poly (cystaminebisacrylamide-diaminohexane) (ABP) has been showed high transfection efficiency with low cytotoxicity. [19, 20] In this work, we designed a paclitaxel-conjugated polymeric micelle using the cationic bioreducible polymer ABP for the co-delivery of gene and drug. We evaluated physicochemical parameters of the micelle including particle size, surface charge, and functionality including cell transfection efficiency and anti-cancer potency. The luciferase gene and IL-12 gene were used to evaluate the efficacy for gene expression.
2. Materials and methods
2.1 Materials
Branched polyethylenimine (25kDa, bPEI), N-Boc-1,6-hexanediamine (N-Boc-DAH), trifluoroacetic acid (TFA), triisobutylsilane (TIS), piperidine, N,N′-dicyclohexylcarbodiimide (DCC), 4-(dimethylamino) pyridine (DMAP), ethylenediaminetetraacetic acid (EDTA), dimethyl thiazolyl blue tetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), N,N-dimethylformamide (DMF) and DL-buthionine-sulfoxamine (BSO) were purchased from Sigma-Aldrich (St. Louis, MO). N,N′--cystaminebisacrylamide (CBA) was purchased from Polysciences, Inc. (Warrington, PA). Luciferase assay kit was purchased from Promega (Madison, WI). Succinimidyl 3-(2-pyridyldithio) propionate (SPDP), mouse IL-12 p40 enzyme-linked immunosorbent assay (ELISA) kit and BCA protein assay kit were purchased from Pierce (Rocford, IL). Thiol PEG carboxyl (HS-PEG3.5k-COOH, Mw = 3.5 kDa) was purchased from JenKem Technology USA Inc. (Allen, TX). The reporter plasmids encoded luciferase (gWiz-Luci) was purchased from Aldevron (Fargo, ND). IL-12 plasmid used in this study previously was constructed and named p2CMVmIL-12 [21]. Dulbecco’s modified eagle medium (DMEM), Dulbecco’s phosphate-buffered saline (DPBS), fetal bovine serum (FBS), SYBR® safe DNA gel stain and YOYO®-1 iodide were purchased from Invitrogen (Carlsbad, CA). Paclitaxel (Genexol®) was kindly provided by Samyang Genex Co. (Seoul, Korea).
2.2 Synthesis of APP
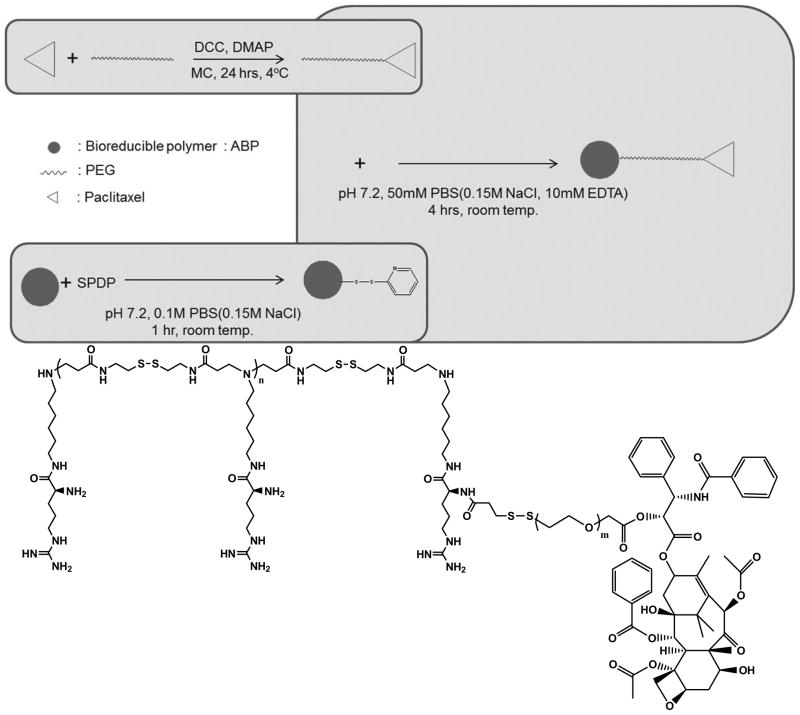
The polymer, arginine-grafted bioreducible poly (disulfide amine), ABP, was synthesized as previously reported [19]. ABP was dissolved in 0.1 M phosphate buffered saline (PBS, pH 7.2, 0.15 M NaCl). 1.2 equiv. of SPDP dissolved in DMF was added to the ABP solution and stirred for 1 h at room temperature and then the mixture was dialyzed and lyophilized. For the PEGylation of paclitaxel, HS-PEG3.5k-COOH was dissolved in anhydrous dichloromethane, and 2.6 equiv. of paclitaxel, 2.0 equiv. of DCC and 2.0 equiv. of DMAP were added into the paclitaxel solution at 0°C. The mixture was further reacted for 24 h at 4°C. The precipitate was filtered out and filtrate was extracted with methylene chloride/H2O. The organic phase was condensed and poured into ice-cold diethyl ether to precipitate the product. Then, ABP-SPDP (1.0 equiv.) and PEG3.5k-paclitaxel (2.5 equiv.) were dissolved in 50 mM PBS with 10 mM EDTA. The mixture was further reacted for 4 h at 4°C. The reaction was monitored by Thin-Layer Chromatography (TLC) with ninhydrin staining and UV spectroscopy for the released pyridine-2-thione. And then, the mixture was extracted, dialyzed and lyophilized. The structure of APP was verified by 1H NMR spectra.
2.3 Physical properties
The molecular weight of ABP and PEGylated paclitaxel was estimated by size-exclusion chromatography (SEC) (Superdex 75 column, calibrated with standard poly[N-(2-hydroxypropyl)methacrylamide] (pHPMA)) using AKTA FPLC system. ABP and PEGylated paclitaxel were dissolved at a concentration of 3 mg/mL. Acetate buffer (0.1 M ammonium acetate, 30% acetonitrile, pH 5.5) was used as eluent. Flow rate was 0.4 mL/min.
The critical micelle concentration (CMC) value of the APP was determined from the measurements of UV absorption (242, 262 and 272 nm) of pyrene [22]. An acetone solution of pyrene and APP was prepared and added to water. The acetone in the solution was then evaporated. The final concentration of pyrene in each sample solution was 1.2 μM and the final concentration of the APP micelles solutions were 0.01 to 0.30 mg/mL.
The average size and zeta-potential of the APP micelle was examined by using the Nano ZS (Malvern Instruments Ltd., Worcestershire, UK) with a He–Ne laser (633 nm) at 25°C. The concentration of the APP solution was 0.20 mg/mL.
2.4 Particle size and surface charge measurement of polyplexes
The average sizes and zeta-potentials of ABP and APP polyplexes at 25°C were examined using the Nano ZS. 100 μL of polyplex solutions (4 μg of plasmid DNA) were prepared in HEPES buffered saline (10 mM HEPES, 1 mM NaCl, pH 7.4) at various weight ratios ranging from 1 to 20. After 30 min of incubation, polyplex solutions were diluted to a final volume of 600 μL before measurement.
2.5 Gel retardation assay
10 μL of polyplexes were prepared in Hepes buffered saline (10 mM HEPES, 1 mM NaCl, pH 7.4) at various weight ratios ranging from 1 to 20, followed by incubation for 30 min. 0.7% agarose gel containing SYBR® safe DNA gel staining solution was prepared in TAE (10 mM Tris/HCl, 1% (v/v) acetic acid, 1mM EDTA) buffer. 2 μL of loading dye was added to each polyplex sample, and analyzed by electrophoresis in TAE buffer at 100V for 35 min. The migration of DNA bands was visualized by a UV illuminator using a Gel Documentation System (Bio-Rad, Hercules, CA).
2.6 Transfection experiments and luciferase assay
MCF-7 (human breast adenocarcinoma cell line) and A549 (human lung adenocarcinoma epithelial cell line) cells were seeded at 3.0×104 cells/well in 24-well plates in 500 μL of DMEM containing 10% FBS and incubated at 37°C for one day before transfection. Polyplexes were prepared with 1 μg of plasmid DNA at different weight ratios in 50 μL of FBS-free medium, and the mixtures were incubated for 30 min at room temperature. 25 kDa bPEI and ABP were used as controls. The medium was replaced by 450 μL FBS-free medium before transfection. Following 4 h treatment with polyplexes, the medium was replaced by 500 μL medium containing 10% FBS. Cells were incubated further for 2 days before measurement of luciferase activity.
To perform the luciferase assay, the cells were washed with DPBS and lysed for 30 min at room temperature with 150 μL of reporter lysis buffer (Promega, Madison, WI). The luciferase activity of 15 μL cell lysate was measured by using 100 μL of luciferase assay reagent on a Tecan Infinite M200 Pro (Tecan Group Ltd., Männedorf, Switzerland) and the protein content was measured by using a Micro BCA assay reagent kit (Pierce, Rockford, IL). All experiments were performed in triplicate.
2.7 ELISA for IL-12
MCF-7 cells were seeded at 3.0×104 cells/well in 24-well plates in 500 μL of DMEM containing 10% FBS and incubated at 37°C for one day before transfection. Polyplexes were prepared with 1 μg of plasmid DNA (p2CMVmIL-12) at weight ratio of 10:1 in 50 μL of FBS-free medium, and the mixtures were incubated for 30 min at room temperature. 25 kDa bPEI and ABP were used as controls. The medium was replaced by 450 μL FBS-free medium before transfection. Following 4 h treatment with polyplexes, the medium was replaced by 500 μL medium containing 10% FBS. Cells were incubated further for 1 day before measurement. The levels of IL-12 secreted in culture supernatants were determined by using mouse IL-12 p40 ELISA kit (Pierce, Rockford, IL).
To perform the ELISA, 50 μL sample diluent and 50 μL culture supernatants were added to each well of anti-mouse IL-12 p40 pre-coated 96-well strip plate and incubated for 1h at room temperature. The wells were washed three times with wash buffer and incubated for 1h at room temperature with 100 μL/well of biotinylated antibody reagent. The microplate wells were washed three times, 100 μL of streptavidin-HRP solution were added to each well and incubated for 30 min at room temperature. After being washed, ELISA plate was developed with 100 μL/well of substrate solution for 30 min at room temperature in the dark. Then, the reaction was stopped by adding 100 μL of stop solution to each well and the absorbance was measured at 450 nm with a reference of 550 nm using the Tecan Infinite M200 Pro.
2.8 Cellular uptake assay
MCF-7 and A549 cells were seeded at 1.0×105 cells/well in 12-well plates in 2 mL of DMEM containing 10% FBS and grown at 37°C for one day. Plasmid DNA was labeled with YOYO®-1 iodine (1 molecule of the YOYO®-1 per 50 base pair of nucleotide) for 30 min before use. Polyplexes were prepared with 0.5 μg of YOYO®-1 labeled plasmid DNA at weight ratio of 10:1 in 40 μL of FBS-free medium, and the mixtures were incubated for 30 min at room temperature. The polyplexes were added to the cells and incubated for 4 h at 37°C in serum-free medium. Then, medium was removed carefully from the wells. The cells were washed two times with ice-cold DPBS. After trypsinization, the cells were collected by centrifuge at 1500 rpm and suspended in 500 μL of 1% FBS in DPBS. The degree of cellular uptake was examined by using the BD FACScan analyzer (Becton Dickinson, San Jose, CA). And, the acquired data was analyzed using the CellQuest software. A minimum of 10,000 cells was assessed for each sample and all samples were evaluated in triplicate.
2.9 Cytotoxicity of polymers
To measure the cytotoxicity of the polymers, ABP, ABP-PEG3.5k and APP, MTT assays were performed. MCF-7 and A549 cells were seeded at 5000 cells/well in 96-well plates in 90 μL of DMEM containing 10% FBS and incubated at 37°C for one day. Then 10 μL of polymer solution at various concentrations was added and cells were incubated for 2 days before assay.
For cytotoxicity assay, 10 μL of filtered MTT solution (2 mg/mL in DPBS) were added to each well and incubated further for 2 h. After incubation, the medium was removed from the well and 100 μL DMSO was added to dissolve the insoluble formazan crystals. The absorbance was measured at 565 nm using the Tecan Infinite M200 Pro and the cell viability was calculated as a percentage relative to untreated control cells. All experiments were performed in quintuplicate. In addition, cytotoxicity of APP and paclitaxel was measured. In order to use the same amount of paclitaxel, the amount of paclitaxel in the APP polymer was determined by UV absorbance at 227 nm [23]. And then, paclitaxel dissolved in DMSO was used as a control.
2.10 Antitumor activity of polyplexes with inhibition of glutathione
APP has internal disulfide bonds, which are degraded in the reductive environment of the intracellular cytoplasm. To determine the impact of the reductive environment on the stability of the APP, the colorimetric MTT assay was performed with treatment of BSO (a glutathione synthesis inhibitor).
MCF-7 and A549 cells were seeded at 3.0×104 cells/well in 24-well plates in 500 μL of DMEM containing 10% FBS and incubated at 37°C for one day. For inhibition of glutathione, cells were treated by 200 μM BSO for 2 h before transfection. Polyplexes were prepared with 1 μg of plasmid DNA at weight ratio of 10:1 in 50 μL of FBS-free medium, and the mixtures were incubated for 30 min at room temperature. ABP, ABP-PEG3.5k and paclitaxel were used as controls. The medium was replaced by 450 μL FBS-free medium before transfection. The cells were treated with polyplexes for 4 h, after which the medium was replaced by 500 μL fresh medium containing 10% FBS and 100 μM BSO, and cells were incubated for 20 and 44 h.
To measure cell viability, 25 μL of filtered MTT solution (2 mg/mL in DPBS) were added to each well and incubated further for 2 h. The media was removed carefully and 500 μL of DMSO was added to each well to dissolve the insoluble formazan crystals. The absorbance was measured at 565 nm using the Tecan Infinite M200 Pro and the cell viability was calculated as a percentage relative to untreated control cells. All experiments were performed in quintuplicate.
2.11 Statistical analysis
Results are expressed as mean values ± standard deviation (SD). Statistical analysis and graphical presentation were performed using sigmaplot 10.0.
3. Results and discussion
3.1 Synthesis and features of APP
Paclitaxel-conjugated polymeric micelle consisting of PEG and ABP, ABP-PEG3.5k-Paclitaxel (APP), was synthesized following the synthetic procedure illustrated in Figure 1 (yield: 72.3%). For the PEGylation of paclitaxel, the 2′-hydroxyl of paclitaxel was esterified by carboxylic acids of HS-PEG3.5k-COOH (yield: 98.7%), band the thiol group of the PEGylated paclitaxel was reacted with SPDP-linked ABP (yield: 82.0%) [24, 25]. The reaction was monitored by thin layer chromatography (TLC) and UV spectroscopy. The molecular weight of ABP and PEGylated paclitaxel was also measured by FPLC-SEC system. The molecular weight of ABP was estimated to be 4.45×103 Da/mole and its PDI value was 1.49. And, the molecular weight of PEGylated paclitaxel was estimated to be 8.20×103 Da/mole (PDI value: 1.03). The structure of APP was confirmed by 1H-NMR, with unique peaks for PEG3.5k-paclitaxel (2.0~2.4, 7.2~7.6, 7.8 and 8.2 ppm) and ABP (2.5 ~ 2.7 ppm) (one paclitaxel per eight ABP units).
Figure 1.
The synthesis scheme and structure of APP
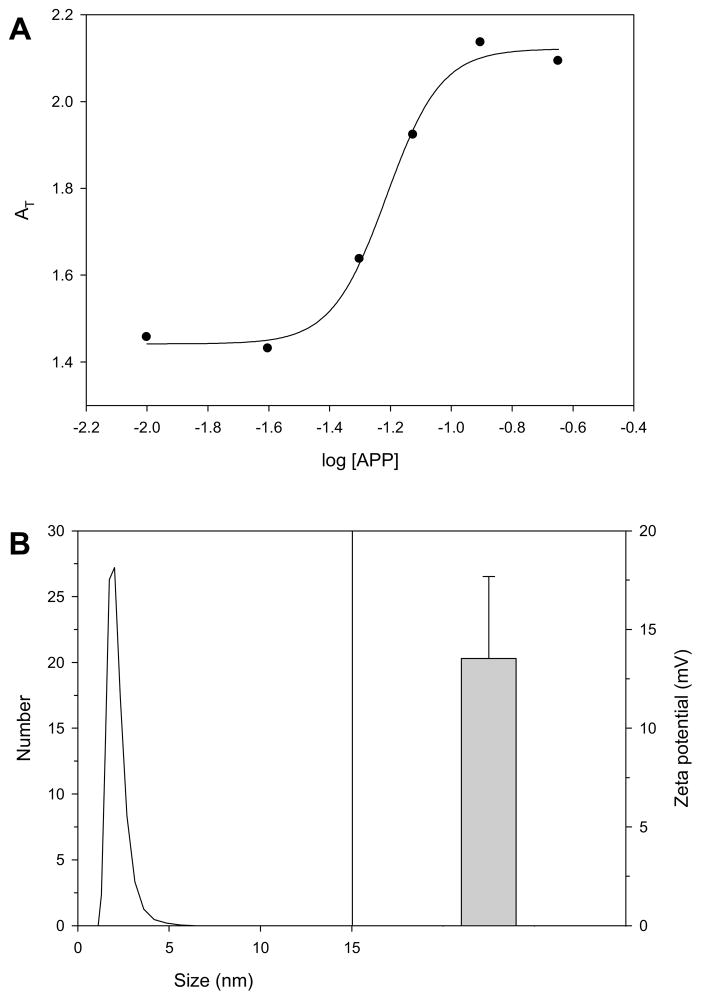
As shown in Figure 2, APP was found to have a CMC value of 0.062 mg/mL in deionized water by measuring UV absorbance. In addition, the average size and zeta-potential of the APP micelle was measured by using the Nano ZS at 0.20 mg/mL. The APP micelles showed an average particle size of 2.61 ± 0.98 nm and a surface charge of 13.5 ± 4.1 mV.
Figure 2.
(A) The sum of absorbance of all the major peaks (AT) vs. logarithm of the APP polymer concentration profile for pyrene (2.0 μM) at 300 K. (B) Zeta potential and average diameters of APP micelles
3.2 Particle size and surface charge measurement of polyplexes
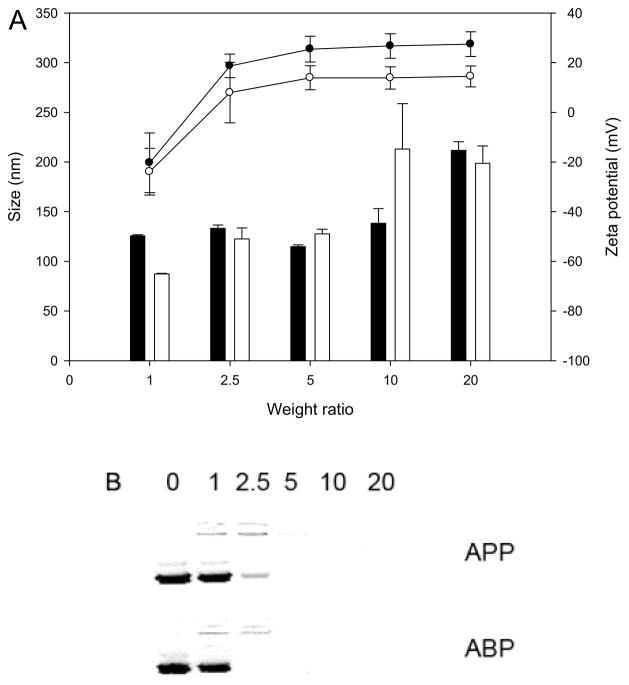
The average sizes and zeta potentials of ABP and APP polyplexes with plasmid DNA were measured using the Nano ZS (Figure 3A). The zeta potential of ABP polyplexes became positive from a weight ratio of 2.5:1 (N/P ratio of 3.7:1) and reached a plateau, +26 mV at a weight ratio of 20:1 (N/P ratio of 29.7:1). APP polyplexes reached a plateau at approximately +14 mV. The formation of polyplexes with the nanometer scale is one of the important considering factors in development of polymers for gene delivery [26, 27]. Due to the positive surface charge, the APP micelles can form polyplexes with plasmid DNA through electrostatic interaction. As shown in Figure 3A, the APP micelles efficiently condensed plasmid DNA into positively charged particles ranging from 125 to 210 nm at weight ratio 5:1 to 20:1 (N/P ratio 3.7 to 14.7). Interestingly, in spite of the lower surface charge of APP, the size pattern of APP polyplexes was almost similar to that of ABP polyplexes.
Figure 3.
Zeta potential and size analysis of ABP (black) and APP (white) polyplexes at various polymer/DNA ratios. Each data point represents the mean ± standard deviation (n = 3).
To confirm the formation of polyplex, agarose gel electrophoresis of the polyplexes was also performed at weight ratio 5:1 to 20:1 (figure 3B). The plasmid DNA showed complete retardation at weight ratio of 2.5 with ABP and 5 with APP. These results indicate that the APP micelle also may be used deliver plasmid DNA to cells.
3.3 In vitro transfection and cellular uptake assay with luciferase gene
The transfection efficiency of APP was evaluated in the MCF-7 and A549 cell line using luciferase gene. 25 kDa bPEI and ABP were used as controls. PEI polyplexes were prepared at a weight ratio 1:1. In order to determine the optimal condition of APP polymer for gene delivery, transfection experiments were performed at various weight ratios. At 2 days after treatment, APP polyplexes didn’t show a significant difference in transfection efficiency from that of ABP polyplexes at weight ratios of 10:1 and 20:1 (Figure 4A and C). ABP resulted in increasing transfection efficiency above a weight ratio 20:1, however, APP polyplexes showed reduced transfection efficiencies compared to ABP polyplexes due to the toxicity of paclitaxel in the APP polymer. (data not shown).
Figure 4.
In vitro transfection efficiency in MCF-7 (A) and A549 (C) cells with PEI (white bar), ABP (gray bars) and APP (black bars) polyplexes at varying weight ratios, which was determined by luciferase assays. Each data point represents the mean ± standard deviation (n = 3). Cellular uptake result by flow cytometer in MCF-7 (B) and A549 (D) cells at weight ratio of 10:1. Closed gray peak: cell only, dot line (•••): ABP polyplex and bold line (−): APP polyplex.
The cellular uptake efficiency of ABP and APP polyplexes using YOYO®-1 labeled plasmid DNA was determined by flow cytometry. MCF and A549 cells were transfected with the polyplexes under previously determined optimal conditions (weight ratio of 10:1) to generate the highest transfection efficiency. As shown in Figure 4B and D, in spite of the lower surface charge, APP polyplexes showed highly efficient cellular uptake (75.10 ± 3.23%, p < 0.01 for MCF-7 cells and 62.29 ± 2.11%, p < 0.05 for A549 cells) compared with that of ABP polyplexes (53.66 ± 3.01% for MCF-7 cells and 56.77 ± 2.64 % for A549 cells).
3.4 In vitro expression of IL-12
Cytokines can prevent tumor growth and activate immune responses, leading to remove cancer cells. Among various cytokine genes, IL-12 has been shown potent antitumor activities in a variety of mouse tumor models [28, 29]. Previous work in our group demonstrated the effects of IL-12 gene on in vivo. In addition, the combination therapy of IL-12 and paclitaxel showed significant inhibition of tumor growth [17, 18, 21]. To predict the possibility of in vivo, mouse IL-12 p40 unit of supernatants were analyzed by ELISA. As shown in Figure 5, the expression level of IL-12 p40 of APP polyplex treated group was higher than controls groups. This result demonstrated that APP is a good candidate for cytokine gene therapy.
Figure 5.
Mouse IL-12 levels in MCF-7 cells. Supernatants were analyzed by ELISA for IL-12 p40 unit.(* P < 0.05)
3.5 Cytotoxicity of polymers
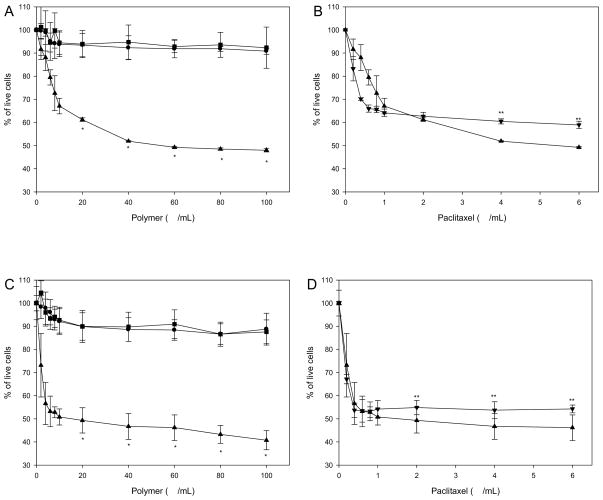
Cytotoxicity of the polymers in MCF-7 and A549 cells was monitored by using MTT assay (Figure 6A and C). Normally, cell viability tends to decrease with increasing concentrations of cationic polymers because the accumulation of the enhanced positive charge on the polymers may be toxic. However, ABP and ABP-PEG3.5k polymer were observed to exhibit no considerable cytotoxicity in MCF-7 and A549 cells at the weight ratios tested. It is noteworthy and interesting that APP polymer showed cytotoxicity. The half maximal inhibitory concentration (IC50) of APP micelle estimated to be approximately 40 μg/mL for MCF-7 cells and 10 μg/mL for A549 cells. These results indicate the antitumor activity of APP micelle.
Figure 6.
Cytotoxicity assay of polymers in MCF-7 (A) and A549 (C) cells by MTT assay. ABP (●), ABP-PEG3.5k (■) and APP (▲). (* P < 0.01 APP vs. ABP and ABP-PEG3.5k) Survival of MCF-7 (B) and A549 (D) cells after exposure for 48 h to paclitaxel (▼) and APP micelle (▲). (** P < 0.01 APP vs. paclitaxel) Each data point represents the mean ± standard deviation (n = 5).
The structural modification of paclitaxel usually occurs at 2′-hydroxyl position. However, modification of 2′-hydroxyl group reduces activity. In order to reduce the effect of the modification, paclitaxel was linked to ABP-PEG polymer via ester bond. To verify the efficiency of paclitaxel-conjugated APP on cell viability, paclitaxel dissolved in DMSO was used as a control. As shown in Figure 6B and D, there were no significant cell viability differences. These results indicate that APP has maintained the anticancer efficiency of paclitaxel.
3.6 Antitumor activity of polyplexes with inhibition of glutathione
Paclitaxel is linked to ABP-PEG polymer via ester bond which can undergo hydrolysis independent of reducing environment. However, APP polymer can make micelle above the CMC. Therefore, paclitaxel is segregated from the aqueous environment to form an inner core surrounded by bioreducible polymer. ABP has disulfide bonds and it is degraded in reducing environment such as cytoplasm. If paclitaxel sufficiently protected by ABP, the anticancer effect depend on reducing environment.
To determine the impact of the reductive environment on the antitumor activity of the bioreducible APP, the colorimetric MTT assay was performed with treatment of BSO, a glutathione synthesis inhibitor. Glutathione depletion is a hallmark of apoptosis. The toxicity test of BSO was performed, since intracellular glutathione depletion can lead to cell death [30, 31]. However, cytotoxicity of MCF-7 and A549 cells showed low sensitivity to the concentration of BSO. As shown in Figure 7, there were no significant cell viability differences in the presence or absence of BSO for ABP and ABP-PEG3.5k polyplexes. APP polyplexes showed low cytotoxicity in the presence of BSO suggesting paclitaxel is not released from APP under non-reductive conditions. In addition, APP polyplexes showed higher anticancer potency than paclitaxel in the absence of BSO. This result demonstrated that the release of paclitaxel might follow the reduction of APP in the cytoplasm, indicating that APP micelle-based delivery system is well suited for the co-delivery of gene and drug.
Figure 7.
The antitumor activity result with (black bar) or without (white bars) DL-buthionine-sulfoxamine (BSO) in MCF-7 (A) and A549 (B) cells. Each data point represents the mean ± standard deviation (n = 5). (* P < 0.01)
4. Conclusion
In this paper, we have described the development of paclitaxel conjugated polymeric micelle consisting of poly (ethylene glycol) and arginine-grafted bioreducible poly (disulfide amine), ABP-PEG3.5k-Paclitaxel (APP). The potential of polymeric micelle was evaluated by particle size, surface charge, cell transfection efficiency and anticancer potency. As a result, APP polyplex showed increased cellular uptake efficiency compared to ABP polyplex, and higher anticancer potency than paclitaxel alone. In addition, APP polyplex showed low cytotoxicity in non-reductive conditions compared to normal reductive conditions. This result demonstrates that the release of paclitaxel might follow the reduction of APP in the cytoplasm. Therefore, APP polyplex may stay in the bloodstream long enough to accumulate in the target area in vivo, and cause the increase of therapeutic efficacy. APP can be a promising candidate for a greater range of clinically important disease states. The above results lead us to conclude that APP micelle-based delivery system is well suited for gene and drug delivery. Our next goal is to determine the effects of APP activity on in vivo.
Supplementary Material
Table 1.
The comparison of the physicochemical characteristics
| ABP | Paclitaxel | PEG | PEGylated paclitaxel | PEGylated ABP | APP | |
|---|---|---|---|---|---|---|
| Molecular weight | 4.45×103 a,d | 853.9 b | 3.56×103 b,(c) | 4.39×103 a,d | 8.20×103 a,d | 9.01×103 d |
| No. of (+)/1 μg | 2.71×1015 | - | - | - | 1.47×1015 | 1.34×1015 |
Molecular weight was estimated by size-exclusion chromatography.
Molecular weight was provided by the manufacturer.
Molecular weight was determined by MALDI.
Molecular weight was estimated by 1H NMR.
Acknowledgments
This work was financially supported by NIH grants CA107070. The authors would like to thank Samyang Genex Co. (Seoul, Korea) for providing paclitaxel.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American cancer society cancer facts & figures 2012. 2012 Online. Available from URL: http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/ACSPC-031941.
- 2.Xiao H, Verdier-Pinard P, Fernandez-Fuentes N, Burd B, Angeletti R, Fiser A, et al. Insights into the mechanism of microtubule stabilization by Taxol. Proc Natl Acad Sci U S A. 2006;103(27):10166–73. doi: 10.1073/pnas.0603704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowinsky EK, Donehower RC. Paclitaxel (taxol) N Engl J Med. 1995;332(15):1004–14. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 4.Wall ME, Wani MC. Camptothecin and taxol: from discovery to clinic. J Ethnopharmacol. 1996;51:239–54. doi: 10.1016/0378-8741(95)01367-9. [DOI] [PubMed] [Google Scholar]
- 5.Sgadari C, Toschi E, Palladino C, Barillari G, Carlei D, Cereseto A, et al. Mechanism of paclitaxel activity in Kaposi’s sarcoma. J Immunol. 2000;165(1):509–17. doi: 10.4049/jimmunol.165.1.509. [DOI] [PubMed] [Google Scholar]
- 6.Heldman AW, Cheng L, Jenkins GM, Heller PF, Kim DW, Ware M, Jr, et al. Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation. 2001;103(18):2289–95. doi: 10.1161/01.cir.103.18.2289. [DOI] [PubMed] [Google Scholar]
- 7.Herdeg C, Oberhoff M, Baumbach A, Blattner A, Axel DI, Schröder S, et al. Local paclitaxel delivery for the prevention of restenosis: biological effects and efficacy in vivo. J Am Coll Cardiol. 2000;35(7):1969–76. doi: 10.1016/s0735-1097(00)00614-8. [DOI] [PubMed] [Google Scholar]
- 8.Goldspiel BR. Clinical overview of the taxanes. Pharmacotherapy. 1997;17:110S–25S. [PubMed] [Google Scholar]
- 9.Lee SC, Huh KM, Lee J, Cho YW, Galinsky RE, Park K. Hydrotropic polymeric micelles for enhanced paclitaxel solubility: in vitro and in vivo characterization. Biomacromolecules. 2007;8(1):202–8. doi: 10.1021/bm060307b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohler DR, Goldspiel BR. Paclitaxel (taxol) Pharmacotherapy. 1994;14(1):3–34. doi: 10.1002/j.1875-9114.1994.tb02785.x. [DOI] [PubMed] [Google Scholar]
- 11.Kuppens IE. Current state of the art of new tubulin inhibitors in the clinic. Curr Clin Pharmacol. 2006;1(1):57–70. doi: 10.2174/157488406775268200. [DOI] [PubMed] [Google Scholar]
- 12.Tian J, Stella VJ. Degradation of paclitaxel and related compounds in aqueous solutions II: Nonepimerization degradation under neutral to basic pH conditions. J Pharm Sci. 2008;97(8):3100–8. doi: 10.1002/jps.21214. [DOI] [PubMed] [Google Scholar]
- 13.Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm. 2002;235:179–92. doi: 10.1016/s0378-5173(01)00986-3. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Hao J, Li Y, Zhang Z, Sha X, Han L, et al. Poly(caprolactone)-modified Pluronic P105 micelles for reversal of paclitaxcel-resistance in SKOV-3 tumors. Biomaterials. 2012;33(18):4741–51. doi: 10.1016/j.biomaterials.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Kim SC, Yoon HJ, Lee JW, Yu J, Park ES, Chi SC. Investigation of the release behavior of DEHP from infusion sets by paclitaxel-loaded polymeric micelles. Int J Pharm. 2005;293:303–10. doi: 10.1016/j.ijpharm.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Control Release. 2001;73(2–3):137–72. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 17.Janát-Amsbury MM, Yockman JW, Lee M, Kern S, Furgeson DY, Bikram M, et al. Combination of local, nonviral IL12 gene therapy and systemic paclitaxel treatment in a metastatic breast cancer model. Mol Ther. 2004;9(6):829–36. doi: 10.1016/j.ymthe.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Janát-Amsbury MM, Yockman JW, Anderson ML, Kieback DG, Kim SW. Combination of local, non-viral IL12 gene therapy and systemic paclitaxel chemotherapy in a syngeneic ID8 mouse model for human ovarian cancer. Anticancer Res. 2006;26(5A):3223–8. [PubMed] [Google Scholar]
- 19.Kim TI, Ou M, Lee M, Kim SW. Arginine-grafted bioreducible poly(disulfide amine) for gene delivery systems. Biomaterials. 2009;30(4):658–64. doi: 10.1016/j.biomaterials.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nam HY, Lee Y, Lee M, Shin SK, Kim TI, Kim SW, et al. Erythropoietin gene delivery using an arginine-grafted bioreducible polymer system. J Control Release. 2012;157(3):437–44. doi: 10.1016/j.jconrel.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahato RI, Lee M, Han S, Maheshwari A, Kim SW. Intratumoral delivery of p2CMVmIL-12 using water-soluble lipopolymers. Mol Ther. 2001;4(2):130–8. doi: 10.1006/mthe.2001.0425. [DOI] [PubMed] [Google Scholar]
- 22.Basu Ray G, Chakraborty I, Moulik SP. Pyrene absorption can be a convenient method for probing critical micellar concentration (cmc) and indexing micellar polarity. J Colloid Interface Sci. 2006;294(1):248–54. doi: 10.1016/j.jcis.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Yang T, Choi MK, Cui FD, Kim JS, Chung SJ, Shim CK, et al. Preparation and evaluation of paclitaxel-loaded PEGylated immunoliposome. J Control Release. 2007;120(3):169–77. doi: 10.1016/j.jconrel.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Shi Q, Wang HK, Bastow KF, Tachibana Y, Chen K, Lee FY, et al. Antitumor agents 210. Synthesis and evaluation of taxoid-epipodophyllotoxin conjugates as novel cytotoxic agents. Bioorg Med Chem. 2001;9(11):2999–3004. doi: 10.1016/s0968-0896(01)00206-1. [DOI] [PubMed] [Google Scholar]
- 25.Dhanikula AB, Panchagnula R. Preparation and characterization of water-soluble prodrug, liposomes and micelles of Paclitaxel. Curr Drug Deliv. 2005;2(1):75–91. doi: 10.2174/1567201052772861. [DOI] [PubMed] [Google Scholar]
- 26.Choi JS, Choi YH, Park JS. Characterization of linear polymer-dendrimer block copolymer/plasmid DNA complexes: formation of core-shell type nanoparticles with DNA and application to gene delivery in vitro. Bull Korean Chem Soc. 2004;25(7):1025–30. [Google Scholar]
- 27.Pack DW. Encyclopedia of polymer science and technology. John Wiley & Sons, Inc; 2002. Gene-delivery polymers. [Google Scholar]
- 28.Rakhmilevich AL, Janssen K, Turner J, Culp J, Yang NS. Cytokine gene therapy of cancer using gene gun technology: superior antitumor activity of interleukin-12. Hum Gene Ther. 1997;8(11):1303–11. doi: 10.1089/hum.1997.8.11-1303. [DOI] [PubMed] [Google Scholar]
- 29.Smyth MJ, Taniguchi M, Street SE. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000;165(5):2665–70. doi: 10.4049/jimmunol.165.5.2665. [DOI] [PubMed] [Google Scholar]
- 30.Franco R, Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 2009;16(10):1303–14. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- 31.Ortega AL, Mena S, Estrela JM. Glutathione in cancer cell death. Cancers. 2011;3(1):1285–310. doi: 10.3390/cancers3011285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.