Abstract
Background:
A high-protein (HP), low-fat weight-loss diet may be advantageous for improving cardiometabolic health outcomes and body composition. To date, only limited research has been conducted in male participants.
Objective:
To evaluate the medium to long-term effects of two, low-fat, hypocaloric diets differing in carbohydrate:protein ratio on body composition and cardiometabolic health outcomes in overweight and obese males.
Design:
One hundred and twenty males (age 50.8±9.3 (s.d.) years, body mass index 33.0±3.9 kg m−2) were randomly assigned and consumed a low-fat, isocaloric, energy-restricted diet (7 MJ per day) with either HP (protein:carbohydrate:fat %energy, 35:40:25) or high carbohydrate (HC; 17:58:25). Body weight, body composition and cardiometabolic risk factors were assessed at baseline and after 12 and 52 weeks.
Results:
Sixty-eight participants completed the study (HP, n=33; HC, n=35). At 1 year both the groups experienced similar reductions in body weight (HP, −12.3±8.0 kg (−12%); HC, −10.9±8.6 kg (−11%); P=0.83 time × group interaction) and fat mass (−9.9±6.0 kg (−27%) vs −7.3±5.8 kg (−22%); P=0.11). Participants who consumed the HP diet lost less fat-free mass (−2.6±3.7 kg (−4%) vs −3.8±4.7 kg (−6%); P<0.01). Both groups experienced similar increases in high-density lipoprotein cholesterol (8%) and reductions in total cholesterol (−7%), low-density lipoprotein cholesterol (−9%), triglycerides (−24%), glucose (−3%), insulin (−38%), blood pressure (−7/−12%) and C-reactive protein (−29%), (P⩾0.14).
Conclusion:
In overweight and obese men, both a HP and HC diet reduced body weight and improved cardiometabolic risk factors. Consumption of a HP diet was more effective for improving body composition compared with an HC diet.
Keywords: caloric restriction, macronutrient composition, lifestyle intervention
Introduction
A growing body of evidence suggests that during caloric restriction, a low-fat diet (<30% fat), higher in protein (HP) and lower in carbohydrate, compared with a conventionally recommended high carbohydrate (HC), low-fat diet may offer a number of advantages.1 These include improving body composition by attenuating the loss of fat-free mass (FFM)2, 3, 4 and/or increasing body fat mass (FM) loss2, 5, 6 and reducing cardiovascular disease risk factors including insulin sensitivity and the blood lipid profile.5, 6, 7, 8, 9
Despite this evidence, the majority of studies available that evaluate the role of HP diets as a weight loss strategy have been conducted almost exclusively in female participants.3, 5, 6, 10, 11, 12, 13 While the limited number of mixed sex studies have either statistically adjusted for sex and/or were statistically underpowered to evaluate the between-sex differences.10, 14, 15, 16, 17, 18 Sex differences in protein kinetics have been previously documented;19, 20 hence, whether comparable effects of HP diet previously observed in females are also experienced by males remains largely unknown.21 This requires urgent investigation as males have a higher proportion of visceral adipose tissue,22 and a greater risk of cardiometabolic diseases.23
Therefore, the aim of the current study was to investigate the long-term effects of two, low-fat, hypocaloric diets differing in carbohydrate:protein ratio (HP vs HC), on body composition and cardiometabolic health in overweight and obese males.
Methods
Participants
One hundred and twenty three overweight or obese males were recruited by public advertisement. Participants were excluded if they had a body mass index <27 or >40 kg m−2, were aged <20 or >65 years, had diabetes or uncontrolled hypertension; a history of gastrointestinal, renal, coronary, metabolic or hepatic disease or a malignancy; were taking hypoglycaemic medication or drugs which affect insulin sensitivity, or were smokers. The study was approved by the Human Research Ethics committee of the Commonwealth Scientific and Industrial Research Organisation (CSIRO). Before study inclusion participants were screened at the CSIRO research clinic. All participants provided written informed consent before commencement.
Experimental protocol
In a parallel study design, participants were blocked, matched for age and body mass index, then randomised by the trial coordinators using computer-generated random number allocation to consume either an energy-restricted HP, low-fat diet (HP, n=59) or an isocaloric high carbohydrate, low-fat diet (HC, n=64) for 52 weeks.
At baseline, week 12 and at the end of the intervention (week 52), participants attended the clinic following an overnight fast for outcome assessment. At each assessment visit, participants had height, weight, waist circumference, blood pressure and body composition assessed, before a blood sample was drawn for assessment of blood lipids, creatinine, glucose, insulin and C-reactive protein. For 24 h immediately before the clinical assessments participants conducted a 24-h urine collection for assessment of urinary urea and creatinine. At each outcome assessment clinic visit participants completed a validated physical activity questionnaire.24, 25 Habitual physical activity levels were assessed using the Baecke physical activity questionnaires ‘total score'—an accumulation of the work, sports and non-sports leisure scores derived from the questionnaire. Participants were asked not to modify their lifestyle patterns during the study other than that required to comply with the study protocol. No specific prescription of physical activity was provided. On the basis of the previous studies examining the impact of dietary composition on the primary outcomes of interest,4, 26 it was estimated with an expected dropout of 25% that there would be sufficient power (80%, P<0.05) to detect a minimum difference between the HP and HC diet groups for changes in weight of 1.4 kg, FM of 1.1 kg and FFM of 0.7 kg.
Diets
The dietary patterns were isocaloric and moderate energy restricted (7 MJ per day energy intake). The planned macronutrient profiles of the diets were: HP diet; protein 35% (142 g, ∼1.30 g kg−1 per day), carbohydrate 40% (135 g), fat 25% (total 53 g, saturated 14 g). HC diet; protein 17% (88 g, ∼0.85 g kg−1 per day), carbohydrate 58% (198 g), fat 25% (total 51 g, saturated 14 g) that was designed to reflect current conventional dietary recommendations.27 Within the HP diet the prescribed daily protein distribution was approximately 20% (13 g) during the morning, 30% (39 g) at lunch time and 60% (78 g) of protein during the afternoon/evening period.
Participants met individually with a qualified dietician at baseline, and every 2 weeks during the first 12 weeks of the study and monthly thereafter. During these visits participants received detailed dietary prescription, meal planning advice and recipe information. To further facilitate dietary compliance, the dietary patterns were structured into quantities of daily foods and presented as a food checklist (Table 1). Throughout weeks 0–12, participants were supplied with a 2-week provision of diet-specific key foods, representing approximately 60% of the energy intake, to improve compliance and allow them to familiarise themselves with the food types and quantities utilised in the study. Participants were required to keep daily semi-quantitative food records in which foods consumed with a variable weight were weighed using kitchen scales before recording and foods with a standard unit (i.e., slice of bread) were recorded without pre-weighing. Dietary intake was assessed using a computerised database (Foodworks Professional Edition, version 4, 1998; Xyris Software, Highgate Hill, Australia) based on the analysis of 3 non-consecutive days (1 weekend day and 2 weeks days) of each 2-week period of diet-record data throughout the study. The composite value for dietary intake for weeks 0–12 and 12–52 (Table 2) was calculated as an average of the 2 week diet-record data blocks within each of the respective periods.
Table 1. Food profile of the treatment groups.
| HP | HC | |
|---|---|---|
| Dairy, low fat | 3 Serves | 1 Serve |
| Lean meata | 300 g (red meat 4 times weekly) | 100 g |
| Deli-sliced meat/canned fish | 100 g | 30 g |
| Fresh fruit | 300 g | 450 g |
| Pasta/rice/potatoa | Nil | 70 g dry weight |
| Bread wholegraina | 105 g | 140 g |
| Cheese, full fata | Nil | 30 g |
| Cereala | 20 g High fibreb+2 breakfast biscuitsc | 20 g high fibreb+2 breakfast biscuitsc |
| Salad | ½ cup | ½ cup |
| Vegetables | ⩾ 2.5 cups | ⩾2.5 cups |
| Oil/spread | 20 g | 20 g |
| Wine or equivalent | 750 ml per week | 750 ml per week |
Abbreviations: HC, high carbohydrate; HP, high protein.
The treatment groups were a high-protein, low-fat diet (HP) or an isocaloric high carbohydrate, low-fat diet (HC).
Food item provided to participants.
All Bran, Kellogg's, Michigan, USA.
Weet-Bix, Sanitarium Health and Wellbeing Company, NSW, Australia.
Table 2. Macronutrient composition of the treatment groups.
| HP (n=33) | HC (n=35) | P-valuea | |
|---|---|---|---|
| Energy (kJ) | |||
| Weeks 0–12 | 7134±771 | 7189±535 | 0.73 |
| Weeks 12–52 | 7629±1085 | 7243±739 | 0.09 |
| Protein (g) | |||
| Weeks 0–12 | 131.1±15.4 | 82.7±6.7 | <0.001 |
| Weeks 12–52 | 132.4±13.9 | 83.3±10.3 | <0.001 |
| Protein (% of energy) | |||
| Weeks 0–12 | 32.5±3.3 | 20.5±1.4 | <0.001 |
| Weeks 12–52 | 30.7±3.1 | 20.4±1.9 | <0.001 |
| Carbohydrate (g) | |||
| Weeks 0–12 | 154.4±31.8 | 208.4±16.3 | <0.001 |
| Weeks 12–52 | 157.9±28.1 | 195.2±23.4 | <0.001 |
| Carbohydrate (% of energy) | |||
| Weeks 0–12 | 37.4±3.8 | 51.0±3.6 | <0.001 |
| Weeks 12–52 | 35.9±3.4 | 47.3±3.9 | <0.001 |
| Fat (g) | |||
| Weeks 0–12 | 50.6±6.5 | 46.7±7.5 | 0.30 |
| Weeks 12–52 | 60.0±12.6 | 52.2±8.7 | <0.01 |
| Fat (% of energy) | |||
| Weeks 0–12 | 27.3±3.0 | 25.0±3.3 | <0.01 |
| Weeks 12–52 | 29.8±3.6 | 27.7±3.2 | 0.01 |
| Alcohol (g) | |||
| Weeks 0–12 | 6.5±4.7 | 8.4±7.2 | 0.20 |
| Weeks 12–52 | 9.4±8.8 | 11.2±8.1 | 0.40 |
| Alcohol (% of energy) | |||
| Weeks 0–12 | 2.7±2.0 | 3.5±2.7 | 0.21 |
| Weeks 12–52 | 3.6±3.1 | 4.6±3.2 | 0.17 |
| Saturated fat (g) | |||
| Weeks 0–12 | 15.4±2.2 | 14.3±2.3 | 0.06 |
| Weeks 12–52 | 18.4±5.0 | 16.5±3.5 | 0.06 |
| Saturated fat (% of total fat) | |||
| Weeks 0–12 | 34.3±4.1 | 34.8±4.8 | 0.60 |
| Weeks 12–52 | 34.0±4.1 | 35.3±4.8 | 0.23 |
Abbreviations: HC, high carbohydrate; HP, high protein.
Data are means±s.d. The treatment groups were a high-protein, low-fat diet (HP) or an isocaloric high carbohydrate, low-fat diet (HC).
Differences between groups (one way ANOVA).
Height, weight, blood pressure and body composition
Height was measured using a stadiometer (SECA, Hamburg, Germany) and body weight was measured using calibrated electronic digital scales (Mercury; AMZ 14, Tokyo, Japan). Dual-energy X-ray absorptiometry (Lunar Prodigy; General Electric, Madison, WI, USA) was used to measure body composition (total body FM and FFM). Waist circumference was measured on a horizontal plane 2 cm proximal to the uppermost lateral border of the right iliac crest. Seated blood pressure was measured using an automated sphygmomanometer (DYNAMAP 8100, Criticon, Tampa, FL, USA).
Biochemical analysis
Serum lipids (total cholesterol, high-density lipoprotein (HDL) cholesterol), triglycerides, plasma glucose, C-reactive protein and creatinine were measured using commercial enzymatic kits (Roche Diagnostics, Basel, Switzerland) on a Hitachi 902 autoanalyzer (Roche Diagnostics, Indianapolis, IN, USA). Low-density lipoprotein cholesterol was calculated using a modified Friedewald equation.28 Insulin was measured using a commercial enzyme immunoassay (Mercodia AB, Uppsala, Sweden). Analysis of 24-h urinary urea and creatinine was performed in a single assay at a commercial laboratory (IMVS, Adelaide, SA, Australia). Creatinine clearance was calculated as (urine creatinine (μmol l−1) × urine volume (ml))/(plasma creatinine (μmol l−1) × minutes) and corrected for body surface area.29
Statistical analysis
Statistical analyses were performed using SPSS for Windows (version 18.0, SPSS, Chicago, IL, USA). Data were examined for normality before analysis; non-normally distributed variables (triglycerides, C-reactive protein and insulin) were normalised for analysis using logarithmic transformation. Univariate analysis of variance (ANOVA) was used to assess differences in baseline characteristics and dietary data between groups. To evaluate the outcomes of this study, two separate analyses were performed. For the primary analysis (week 52 completers analysis) the effect of time and diet treatment on continuous variables was assessed using repeated measures ANOVA with time as the within subject factor and treatment (HP vs HC) as the between-subject factor. Baseline body mass index was included in the model as a co-variate for body composition outcomes. Where ANOVA showed a statistically significant time by treatment effect, a comparison of the change in each group for each time period (weeks 0–12 and weeks 0–52) was conducted using univariate ANOVA with Bonferroni adjustments for multiple comparisons. For the second analysis, an intention to treat evaluation was conducted using maximal likelihood mixed model analysis with fixed and random effects to analyse expected mean changes over time. The secondary analysis was based on the 120 participants that had outcomes assessed at week 0 and commenced the dietary programme. Between group differences for the proportion of dropouts, participants who lost >5% and/or 10% of their initial body weight were assessed using χ2 tests. Pearson's correlation coefficients were used to determine relationships between variables. One participant in the HP group was excluded from the dual-energy X-ray absorptiometry body composition analyses because they exceeded the equipment weight limit for scanning at baseline. A blood sample was unable to be obtained from two participants at week 52 (HP 1, HC 1) who were subsequently excluded from the biochemical blood analyses. Five participants (HP 3, HC 2) were excluded from the C-reactive protein results because they had C-reactive protein values>10 mg l−1. Statistical significance was set at P<0.05. Data are reported as means±s.d. unless otherwise specified.
Results
Participants
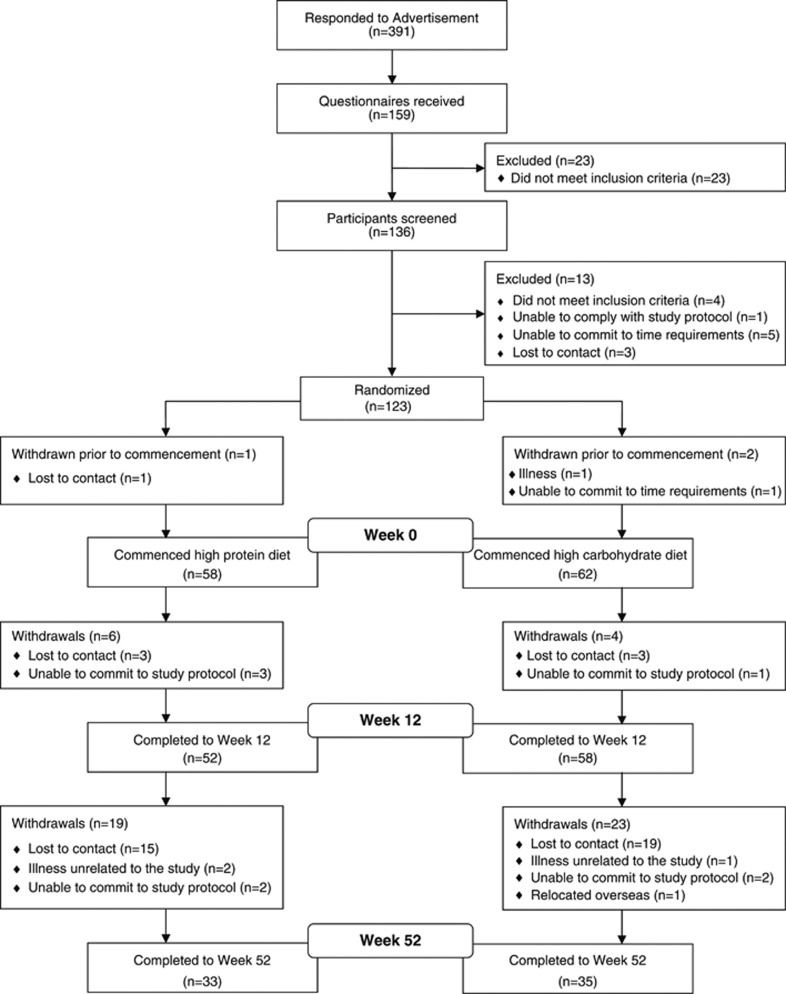
Three participants withdrew before the commencement of the study (HP 1, HC 2; Figure 1). One hundred and twenty participants had outcomes assessed at week 0 and commenced the study, 68 participants (57% HP 33, HC 35) completed the intervention and had outcome measurements assessed at week 52. Dropout rates were similar in both groups (HP 25, HC 27; P=0.96; Figure 1). At baseline, outcome variables were similar between completers and non-completers (P⩾0.14) except for triglycerides which were higher in the non-completers (2.07±1.01 vs 1.69±0.70 mmol l−1, P=0.03). Similarly, there were no significant differences between the diet groups at baseline for any outcome variable (P⩾0.08, Table 3) except for triglycerides, which were lower in HP (P=0.05). Physical activity levels were similar at baseline (P=0.80) and remained relatively constant throughout the intervention in both groups (P=0.21 time; P=0.07 time × group interaction).
Figure 1.
Participant flow.
Table 3. Age, height, body weight and composition, cardiometabolic risk factors and renal function before and after 12 and 52 weeks consumption of either an energy-restricted HP or an isocaloric high carbohydrate, low fat diet (HC).
| N | HP | HC | P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| HP/HC | Baselinea | Timeb | Time × groupc | ITTd | ||||
| Age (years) | 33/35 | Week 0 | 51.3±9.4 | 50.2±9.3 | 0.63 | |||
| Height (m) | 33/35 | Week 0 | 1.77±0.06 | 1.77±0.08 | 0.71 | |||
| Body weight (kg) | 33/35 | Week 0 | 106.0±12.9 | 101.6±14.9 | 0.20 | <0.001 | 0.83 | 0.92 |
| Week 12 | 95.8±11.3 | 92.2±12.4 | ||||||
| Week 52 | 93.7±10.7 | 90.7±12.4 | ||||||
| Change 0–12 | −10.2±4.9 | −9.4±4.4 | ||||||
| Change 0–52 | −12.3±8.0 | −10.9±8.6 | ||||||
| Total body fat mass (kg) | 30/33 | Week 0 | 36.2±7.4 | 33.6±7.6 | 0.19 | <0.01 | 0.11 | 0.07 |
| Week 12 | 28.1±8.6 | 27.9±8.4 | ||||||
| Week 52 | 26.2±8.3 | 26.3±7.9 | ||||||
| Change 0–12 | −8.1±3.9 | −5.7±4.0 | ||||||
| Change 0–52 | −9.9±6.0 | −7.3±5.8 | ||||||
| Total body fat free mass (kg) | 30/33 | Week 0 | 68.3±7.1 | 68.2±9.9 | 0.97 | <0.001 | <0.01 | 0.17 |
| Week 12 | 66.1±5.9 | 64.4±7.3 | ||||||
| Week 52 | 65.6±5.9 | 64.3±7.8 | ||||||
| Change 0–12 | −2.1±3.7† | –3.8±4.3 | ||||||
| Change 0–52 | −2.6±3.7* | –3.8±4.7 | ||||||
| Body fat mass (%) | 30/33 | Week 0 | 34.4±5.1 | 32.9±4.3 | 0.19 | <0.001 | 0.02 | 0.07 |
| Week 12 | 29.4±6.9 | 29.8±5.9 | ||||||
| Week 52 | 28.2±7.3 | 28.6±5.6 | ||||||
| Change 0–12 | −5.0±3.3† | −3.1±3.7 | ||||||
| Change 0–52 | −6.2±4.2 | −4.2±4.2 | ||||||
| Waist circumference (cm) | 32/34 | Week 0 | 111.4±7.8 | 108.9±10.9 | 0.30 | 0.01 | 0.62 | 0.24 |
| Week 12 | 99.4±7.9 | 98.3±10.1 | ||||||
| Week 52 | 98.8±7.4 | 97.6±9.5 | ||||||
| Change 0–12 | −12.0±4.2 | −10.5±3.8 | ||||||
| Change 0–52 | −12.6±6.4 | −11.3±7.0 | ||||||
| Systolic blood pressure (mm Hg) | 31/34 | Week 0 | 133±12 | 137±13 | 0.24 | <0.001 | 0.73 | 0.78 |
| Week 12 | 122±12 | 124±12 | ||||||
| Week 52 | 122±12 | 127±15 | ||||||
| Change 0–12 | −11±10 | −12±9 | ||||||
| Change 0–52 | −10±10 | −10±15 | ||||||
| Diastolic blood pressure (mm Hg) | 31/34 | Week 0 | 83±9 | 85±12 | 0.55 | <0.001 | 0.18 | 0.79 |
| Week 12 | 73±7 | 71±10 | ||||||
| Week 52 | 74±8 | 74±8 | ||||||
| Change 0–12 | −10±6 | −14±8 | ||||||
| Change 0–52 | −9±7 | −11±11 | ||||||
| Total cholesterol (mmol l−1) | 32/34 | Week 0 | 5.11±0.99 | 5.32±0.78 | 0.32 | <0.001 | 0.14 | 0.46 |
| Week 12 | 4.55±0.92 | 4.45±0.79 | ||||||
| Week 52 | 4.81±0.89 | 4.90±0.69 | ||||||
| Change 0–12 | −0.55±0.73 | –0.88±0.80 | ||||||
| Change 0–52 | −0.29±0.52 | –0.42±0.64 | ||||||
| HDL cholesterol (mmol l−1) | 32/34 | Week 0 | 1.23±0.36 | 1.30±0.37 | 0.44 | <0.001 | 0.25 | 0.24 |
| Week 12 | 1.26±0.34 | 1.25±0.33 | ||||||
| Week 52 | 1.37±0.36 | 1.37±0.33 | ||||||
| Change 0–12 | 0.02±0.20 | –0.05±0.24 | ||||||
| Change 0–52 | 0.13±0.17 | 0.06±0.21 | ||||||
| LDL cholesterol (mmol l−1) | 32/34 | Week 0 | 3.20±0.94 | 3.19±0.62 | 0.94 | <0.001 | 0.34 | 0.39 |
| Week 12 | 2.80±0.85 | 2.59±0.72 | ||||||
| Week 52 | 2.94±0.81 | 2.88±0.63 | ||||||
| Change 0–12 | −0.40±0.61 | −0.60±0.71 | ||||||
| Change 0–52 | −0.27±0.60 | −0.31±0.58 | ||||||
| Triglycerides (mmol l−1) | 32/34 | Week 0 | 1.49±0.53 | 1.85±0.80 | <0.05 | <0.001 | 0.69 | 0.36 |
| Week 12 | 1.09±0.48 | 1.36±0.62 | ||||||
| Week 52 | 1.14±0.78 | 1.41±0.60 | ||||||
| Change 0–12 | −0.40±0.48 | −0.49±0.71 | ||||||
| Change 0–52 | −0.35±0.74 | −0.44±0.59 | ||||||
| Glucose (mmol l−1) | 32/34 | Week 0 | 5.84±0.61 | 5.77±0.73 | 0.71 | <0.01 | 0.66 | 0.72 |
| Week 12 | 5.58±0.43 | 5.46±0.53 | ||||||
| Week 52 | 5.63±0.39 | 5.64±0.76 | ||||||
| Change 0–12 | −0.26±0.52 | −0.32±0.64 | ||||||
| Change 0–52 | −0.21±0.51 | −0.13±0.87 | ||||||
| Insulin (mU l−1) | 32/34 | Week 0 | 10.82±8.26 | 9.29±4.66 | 0.30 | <0.001 | 0.49 | 0.51 |
| Week 12 | 6.47±2.80 | 5.89±2.75 | ||||||
| Week 52 | 5.41±2.53 | 7.03±13.29 | ||||||
| Change 0–12 | −4.34±6.83 | −3.40±3.02 | ||||||
| Change 0–52 | −5.41±8.31 | −2.26±12.40 | ||||||
| C-reactive protein (mg l−1) | 29/32 | Week 0 | 2.13±1.30 | 2.43±1.68 | 0.53 | <0.001 | 0.75 | 0.97 |
| Week 12 | 2.11±1.61 | 2.12±1.58 | ||||||
| Week 52 | 1.58±1.86 | 1.65±1.35 | ||||||
| Change 0–12 | −0.02±1.44 | −0.31±1.52 | ||||||
| Change 0–52 | −0.55±1.49 | −0.78±1.55 | ||||||
| Creatinine clearance (ml min−11.73 m−2) | 32/32 | Week 0 | 106.4±24.9 | 103.1±23.1 | 0.59 | 0.18 | 0.67 | 0.55 |
| Week 12 | 100.7±33.8 | 97.0±22.8 | ||||||
| Week 52 | 109.7±39.5 | 100.6±27.2 | ||||||
| Change 0–12 | −5.6±30.2 | −6.2±22.1 | ||||||
| Change 0–52 | 3.3±33.3 | −2.5±25.8 | ||||||
Abbreviations: HC, high carbohydrate; HDL, high-density lipoprotein; HP, high protein, low fat; ITT, Intention to Treat; LDL, low-density lipoprotein.
Data are means±s.d.
The treatment groups were a HP or an isocaloric high carbohydrate, low-fat diet (HC).
*P<0.05 Significantly different to HC (univariate ANOVA).
†P<0.01 Significantly different to HC (univariate ANOVA).
Comparison of baseline characteristics at week 0 (one way ANOVA).
Changes over time in the groups from weeks 0, 12 and 52 (repeated measures ANOVA).
Treatment effect between groups for the change from weeks 0, 12 and 52 (repeated measures ANOVA).
Treatment effect between groups for the change from weeks 0, 12 and 52 (secondary maximal likelihood mixed model analysis—ITT (n=120)).
Week 52 completers analysis
Dietary composition
On the basis of food record data, participants in both groups showed good compliance to the prescribed diets (Table 2). The diets were similar in total energy (P⩾0.05) but participants in the HP diet group consumed less carbohydrate, and more protein and fat than those consuming the HC diet (P⩽0.01; Table 2). Participants achieved relative protein intakes of ∼1.24 and ∼0.82 g kg−1 per day in the HP and HC groups, respectively. In both groups overall, week 52 urinary urea correlated with reported protein intake (r=0.406, P=0.001). Twenty-four-hour urinary urea was significantly higher in the HP group at week 12 (HP 554±216 mmol/24 h vs HC 429±122 mmol/24 h, P<0.01) and week 52 (HP 570±221 mmol/24 h vs HC 460±138 mmol/24 h, P=0.02), verifying the higher reported protein intake.
Body weight and composition
After 52 weeks both groups had similar reductions in body weight (Table 3), FM and waist circumference (P⩽0.01 time; P⩾0.11 time × group interaction; Table 3). Both groups had a similar number of participants who achieved a weight loss of >5% (HP 85%, HC 83% P=0.82) and/or 10% (HP 61%, HC 43% P=0.14) of their initial body weight.
Participants who consumed the HP diet lost less FFM (P<0.001 time; P<0.01 time × group interaction; Table 3). The HP group had a greater reduction in percent body FM at week 12 (P<0.01 time × group interaction; Table 3). The percentage of weight loss attributed to FFM was HP 21% vs HC 40% at week 12, and HP 21% vs HC 35% at week 52.
Cardiometabolic risk factors and renal function
After 52 weeks both groups had a similar increase in HDL cholesterol, and reductions in total cholesterol, low-density lipoprotein cholesterol, triglycerides, glucose, insulin, blood pressure and C-reactive protein, (P<0.01 time; P⩾0.14 time × group interaction; Table 3). Creatinine clearance did not change in either group during the intervention (P=0.18 time; P=0.67 time × group interaction; Table 3). Overall, changes in body weight after 52 weeks correlated with changes in triglycerides (r=0.27, P=0.03), HDL cholesterol (r=−0.41, P<0.01), glucose (r=0.49, P<0.001) and insulin (r=0.51, P<0.001).
Intention to treat analysis
The secondary maximal likelihood mixed model analysis showed a similar pattern of results to the primary completer's analysis (Table 3). However, for total body FFM and percent total body FM the time × group effect which occurred in the primary completers analysis no longer reached statistical significance (P=0.17 and P=0.07, respectively; Table 3).
Overall, the participants who withdrew after week 12 were those who lost less weight during weeks 0–12 than those who completed the full 52 weeks (−7.4±3.0 vs −9.8±4.6 kg; P<0.01). Participants in HP who withdrew after week 12 lost less FM during weeks 0–12 than those participants in HP who completed the full 52 weeks (−5.6±3.3 vs −8.0±3.8 kg; P=0.03) and participants in HC who withdrew after week 12 tended to lose less FFM (−2.0±2.5 vs −3.7±4.2 kg; P=0.09) compared with the HC participants who completed the 52 weeks.
Discussion
This study showed in overweight and obese men, consuming of a low-fat, energy-restricted diet with a higher protein intake improves body composition to a greater extent compared with an isocaloric HC diet. A HP and HC diet similarly reduced body weight and improved a number of cardiometabolic risk factors.
Overall, both diet groups experienced similar weight loss at 1 year (∼11%); however, the HP diet group lost less FFM such that the FFM percentage contribution to overall weight loss in HP was only just over half that of the HC diet group (21% vs 35%). The mitigation of FFM reduction in HP was anticipated to some degree, given the level of protein intake in HP (∼1.24 g kg−1 of body weight vs 0.8 g kg−1 body weight in HC) was consistent with protein intakes reported in a meta-analysis by Kreiger et al.2 that were associated with attenuation of FFM loss following hypocaloric-induced weight loss. Leidy et al. has reported similar findings in preobese and obese women to those observed in this study, demonstrating during a 12-week hypocaloric weight loss intervention; despite achieving ∼9 kg weight loss across treatment groups, a HP diet (30% protein, 1.4 g kg−1per day) mitigated FFM reductions (−1.5 kg loss) compared with an isocaloric HC diet (18% protein, 0.8 g kg−1 per day; −2.8 kg). Farnsworth et al.4 also showed that a high-protein, low-fat (HP) diet preserved FFM in hyperinsulinemic females following 12 weeks of energy restriction and 4 weeks of energy balance, but in contrast to the current study, this effect was not evident in their male participants. In the study by Farnsworth et al.,4 females achieved a mean weight loss of 7 kg, FFM was reduced by −0.1 kg in the HP diet group (30% protein, ∼1.24 g kg−1 per day during weight loss phase) compared with −1.5 kg in the HC diet group (15% protein, ∼0.68 g kg−1 per day). For males, overall weight loss was 10.5 kg, but FFM reduced similarly with both diet treatments (HP diet group −2.5 kg, HC diet group −1.9 kg). However, male participants had higher baseline body weights than their female counterparts and subsequently relative protein intake on the HP diet was markedly less (males: ∼1.02 g kg−1 per day vs females: ∼1.23 g kg−1 per day). Hence, despite consuming a HP diet, actual protein intake in the males was below that identified in the meta-analysis by Krieger et al.2 as providing a benefit for FFM retention (1.05 g kg−1 per day). It is therefore possible that the higher relative protein intake achieved by the male participants in the current study (∼1.24 g kg−1 per day) may explain why participants were able to achieve a mitigation of FFM loss in HP compared with HC whereas the male participants in the Farnsworth et al.4 study were not. This suggests that the level of relative protein intake, rather than sex differences in protein metabolism, may be an important underlying determinant in regulating the body composition response to a HP diet.
Despite the differential changes we observed for changes in FFM and percent FM between the diet groups in the completers analysis, it is important to note that significant differences were no longer observed after conducting the secondary mixed model analysis. It is unclear as to why the effects were no longer significant; however, participants who withdrew after week 12 were generally those who lost less weight during weeks 0–12 and may therefore have been those who were less compliant to the diet. Subsequently, the inclusion of these data may have lessened the body composition effects at week 52 in the intention to treat analysis. Alternatively, it is possible the non-random dropout at week 12 relating to body composition (as indicated by the differences in changes in FM in HP and FFM in HC between completers and non-completers) may have biased the completer's analysis by favouring a treatment effect.
As FFM is strongly correlated with resting energy expenditure,30, 31, 32 accounting for ∼60–70% of daily energy expenditure,33 the lesser reduction of FFM observed in HP could have implication for achieving sustained weight control following the return to ad libitum dietary intake conditions.34 In addition, as skeletal muscle represents the largest mass of insulin-sensitive tissue,35 the mitigation of FFM reduction in HP may also provide benefits for metabolic health, particularly in this study population who is at increased risk of developing type 2 diabetes and/or cardiovascular disease.36 However, there remains a lack of long-term studies investigating the clinical benefits associated with FFM mitigation achieved during weight loss, making it difficult to determine the clinical significance of the changes in FFM observed.
In this study, a statistically significant difference was not observed between the treatment groups for the change in FM in the completers analysis (P=0.11) despite a 36% (2.6 kg) greater loss in absolute FM in HP compared with HC at week 52. A number of studies have previously reported a greater reduction in FM with a HP compared with a HC diet.5, 6 The mechanism/s whereby dietary protein may enhance FM reductions are not fully understood. It is plausible that HP diets have a reduced metabolic efficiency, as protein has a reduced energy efficiency for metabolism compared with an equivalent caloric intake of fat or carbohydrate.37
Marked reductions in cardiometabolic risk factors were observed in both groups. However, no differential changes between the groups were observed. This finding is, in part, consistent with results from the Diet, Obesity, and Genes (DiOGenes) Study, which showed following an initial very low calorie diet-induced weight loss (⩾8%), 26 weeks of consuming one of the 4 ad libitum diets varying in either protein:carbohydrate and/or glycemic index had similar effects on the blood lipid profile, blood pressure, fasting insulin and fasting glucose.38 Conversely, several studies have demonstrated that macronutrient composition of a hypocaloric diet can alter the blood lipid profile response to weight loss.5, 6, 7, 8 Clifton et al.8 conducted a pooled data analysis of three weight loss trials4, 5, 39 comparing an energy-restricted HP diet (30–40%, 110–136 g per day) with an isocaloric HC diet (15–20%, 60–67 g day−1). Although no differences were observed between dietary patterns for changes in glucose, insulin, total cholesterol, HDL cholesterol or low-density lipoprotein cholesterol, triacylglycerol levels, an independent risk factor for cardiovascular disease,40 decreased to a greater extent with a HP diet (−0.48 vs −0.27 mmol l−1). Although endogenous triglyceride synthesis has been demonstrated to increase in response to a chronic HC diet,41 a number of potential reasons could explain the lack of any observed between-group effects for triglycerides in the present study. As the HP group had lower triglyceride levels at baseline, participants in this group may not have had the same capacity for improvement compared with those in HC. Alternatively, in this study the absolute reported difference in carbohydrate intake between the groups (∼37 g) may not have been sufficient to induce a differential group effect on plasma triglycerides. It is also possible that the type of carbohydrate prescribed in the HC diet group that was generally from low glycemic index, high-fibre foods (Table 1) may not have induced the same undesirable effects on plasma triglycerides as contemporary high glycemic index low fat carbohydrate sources.41 Despite, the absence of any differential group effects, the magnitude of the overall changes in lipids and triglyceride are consistent with the expected per kg change identified in a meta-analysis examining the effect of weight reduction on blood lipids and lipoprotein.42 These changes represent a substantial reduction in cardiovascular disease risk.43 C-reactive protein is an inflammatory molecule implicated in the atherosclerotic process and also identified as a predictor of cardiovascular disease.44 In ad libitum conditions, the results of the DiOGenes study showed following weight loss, high-sensitivity C-reactive protein was further reduced when participants consumed a diet with low glycemic index and, to a lesser extent, reduced protein.38 Although we observed no effect of diet on C-reactive protein in the current study, participants achieved a reduction in C-reactive protein approximately twice that observed during the same duration of intervention with statin therapy.45
Creatinine clearance was measured as a marker of renal function.46 Although some concern remains about the impact of higher protein intakes on renal function,47 no changes in creatinine clearance occurred in either group. However, renal impairment risk to participants in this study was relatively low, given that participants had normal renal function at baseline,46 and that the reduced energy intake levels would have reduced the absolute quantity of protein ingested on a HP diet to a level equivocal to that consumed on a conventional energy-balanced diet.48 There is a need for long-term prospective studies to investigate the effect of HP weight loss diets on renal function in patients who have pre-existing renal dysfunction or who are at considerable risk, such as those with type 2 diabetes.49
This study had several limitations. This study provides a greater understanding of the role of HP and HC weight loss diets in men, but in the absence of a parallel evaluation of women, direct gender comparisons cannot be made and requires additional research. Moreover, while HC diets utilised in previous studies typically delivered relative protein intakes of ∼0.6–0.8 g kg−1 per day, considerably wide variability in absolute quantities can occur based on individual body weight, which could be particularly evident between men and women. How absolute vs relative protein intakes may influence the body composition response to weight loss, particularly between genders, remains an area for future research. The relatively high dropout rate (43%) may limit the generalisability of the findings for use in public health treatment programs. However, the attrition rate was comparable to other previous long-term dietary intervention studies50, 51 and the per protocol completers analysis provides a valuable contribution to the literature about the medium to long-term efficacy of a HP weight loss diet for men. There was also some degree of disparity between the protein intakes reported in the food records and the 24-h urinary urea values. Nevertheless, combined these outcomes provide evidence that irrespective of the precise quantity of protein consumed a substantial differential intake was achieved between the diet groups.
In conclusion, in overweight and obese men both a HP and HC diet reduced body weight and improved cardiometabolic risk factors. Consumption of a HP diet was more effective for improving body composition compared with an HC diet.
Acknowledgments
We thank the volunteers who made the study possible through their participation. We gratefully acknowledge Julia Weaver, Anne McGuffin and Kathryn Bastiaans for co-ordinating this trial; Jennifer Keogh and Gemma Williams for assisting in delivering of the dietary intervention; Rosemary McArthur for providing nursing expertise. Alison Hill and Kade Davison for conducting the body composition scans; Mark Mano, Julie Turner and Candita Sullivan for assisting with the biochemical assays; Julie Syrette for data management of the study; Kylie Lange for assisting with the statistical analysis. This work was supported with a project grant from Meat and Livestock Australia. Trial Registration: Australian New Zealand Clinical Trials Registry (http://www.anzctr.org.au) ACTR No: ACTRN12606000002583.
AUTHOR CONTRIBUTIONS
MN, PC, GB designed research, GB conducted research, TW analysed data, TW, GB wrote the paper. TW had primary responsibility for the final content. All authors read and approved the final manuscript.
The authors declare no conflict of interest.
References
- Abete I, Astrup A, Martinez JA, Thorsdottir I, Zulet MA. Obesity and the metabolic syndrome: role of different dietary macronutrient distribution patterns and specific nutritional components on weight loss and maintenance. Nutr Rev. 2010;68:214–231. doi: 10.1111/j.1753-4887.2010.00280.x. [DOI] [PubMed] [Google Scholar]
- Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: a meta-regression 1. Am J Clin Nutr. 2006;83:260–274. doi: 10.1093/ajcn/83.2.260. [DOI] [PubMed] [Google Scholar]
- Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity. 2007;15:421–429. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]
- Farnsworth E, Luscombe ND, Noakes M, Wittert G, Argyiou E, Clifton PM. Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am J Clin Nutr. 2003;78:31–39. doi: 10.1093/ajcn/78.1.31. [DOI] [PubMed] [Google Scholar]
- Noakes M, Keogh JB, Foster PR, Clifton PM. Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am J Clin Nutr. 2005;81:1298–1306. doi: 10.1093/ajcn/81.6.1298. [DOI] [PubMed] [Google Scholar]
- Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, et al. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003;133:411–417. doi: 10.1093/jn/133.2.411. [DOI] [PubMed] [Google Scholar]
- Wolfe BM, Giovannetti PM. Short-term effects of substituting protein for carbohydrate in the diets of moderately hypercholesterolemic human subjects. Metabolism. 1991;40:338–343. doi: 10.1016/0026-0495(91)90142-j. [DOI] [PubMed] [Google Scholar]
- Clifton PM, Bastiaans K, Keogh JB. High protein diets decrease total and abdominal fat and improve CVD risk profile in overweight and obese men and women with elevated triacylglycerol. Nutr Metab Cardiovasc Dis. 2009;19:548–554. doi: 10.1016/j.numecd.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Layman DK, Shiue H, Sather C, Erickson DJ, Baum J. Increased dietary protein modifies glucose and insulin homeostasis in adult women during weight loss. J Nutr. 2003;133:405–410. doi: 10.1093/jn/133.2.405. [DOI] [PubMed] [Google Scholar]
- Flechtner-Mors M, Boehm BO, Wittmann R, Thoma U, Ditschuneit HH. Enhanced weight loss with protein-enriched meal replacements in subjects with the metabolic syndrome. Diabetes Metab Res Rev. 2010;26:393–405. doi: 10.1002/dmrr.1097. [DOI] [PubMed] [Google Scholar]
- Layman DK, Evans E, Baum JI, Seyler J, Erickson DJ, Boileau RA. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr. 2005;135:1903–1910. doi: 10.1093/jn/135.8.1903. [DOI] [PubMed] [Google Scholar]
- Stamets K, Taylor DS, Kunselman A, Demers LM, Pelkman CL, Legro RS. A randomized trial of the effects of two types of short-term hypocaloric diets on weight loss in women with polycystic ovary syndrome. Fertil Steril. 2004;81:630–637. doi: 10.1016/j.fertnstert.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Campbell WW, Tang M. Protein intake, weight loss, and bone mineral density in postmenopausal women. J Gerontol Series A Biol Sci Med Sci. 2010;65:1115–1122. doi: 10.1093/gerona/glq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;85:1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- Johnston CS, Tjonn SL, High-protein SwanPD. low-fat diets are effective for weight loss and favorably alter biomarkers in healthy adults. J Nutr. 2004;134:586–591. doi: 10.1093/jn/134.3.586. [DOI] [PubMed] [Google Scholar]
- Kleiner RE, Hutchins AM, Johnston CS, Swan PD. Effects of an 8-week high-protein or high-carbohydrate diet in adults with hyperinsulinemia. Med Gen Med. 2006;8:39. [PMC free article] [PubMed] [Google Scholar]
- Layman DK, Evans EM, Erickson D, Seyler J, Weber J, Bagshaw D, et al. A moderate-protein diet produces sustained weight loss and long-term changes in body composition and blood lipids in obese adults. J Nutr. 2009;139:514–521. doi: 10.3945/jn.108.099440. [DOI] [PubMed] [Google Scholar]
- Luscombe ND, Clifton PM, Noakes M, Farnsworth E, Wittert G. Effect of a high-protein, energy-restricted diet on weight loss and energy expenditure after weight stabilization in hyperinsulinemic subjects. Int J Obes Relat Metab Disord. 2003;27:582–590. doi: 10.1038/sj.ijo.0802270. [DOI] [PubMed] [Google Scholar]
- Volpi E, Lucidi P, Bolli GB, Santeusanio F, De Feo P. Gender differences in basal protein kinetics in young adults. J Clin Endocrinol Metab. 1998;83:4363–4367. doi: 10.1210/jcem.83.12.5330. [DOI] [PubMed] [Google Scholar]
- Tipton KD. Gender differences in protein metabolism. Curr Opin Clin Nutr Metab Care. 2001;4:493–498. doi: 10.1097/00075197-200111000-00005. [DOI] [PubMed] [Google Scholar]
- Lovejoy JC, Sainsbury A. Sex differences in obesity and the regulation of energy homeostasis. Obes Rev. 2009;10:154–167. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6 (Suppl 1:60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkhasov RM, Shteynshlyuger A, Hakimian P, Lindsay GK, Samadi DB, Shabsigh R. Are men shortchanged on health? Perspective on life expectancy, morbidity, and mortality in men and women in the United States. Int J Clin Pract. 2010;64:465–474. doi: 10.1111/j.1742-1241.2009.02289.x. [DOI] [PubMed] [Google Scholar]
- Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- Philippaerts RM, Westerterp KR, Lefevre J. Doubly labelled water validation of three physical activity questionnaires. Int J Sports Med. 1999;20:284–289. doi: 10.1055/s-2007-971132. [DOI] [PubMed] [Google Scholar]
- Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, et al. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003;133:411–417. doi: 10.1093/jn/133.2.411. [DOI] [PubMed] [Google Scholar]
- National Health and Medical Research Council . Nutrient Reference Values for Australia and New Zealand. Commonwealth of Australia: Canberra; 2006. [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Du Bois D, Du Bois EF.A formula to estimate the approximate surface area if height and weight be known. 1916 Nutrition 19895303–311.discussion 312–313. [PubMed] [Google Scholar]
- Miller AT, Blyth CS. Lean body mass as a metabolic reference standard. J Appl Physiol. 1953;5:311–316. doi: 10.1152/jappl.1953.5.7.311. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Burnand B, Schutz Y, Jequier E. Energy expenditure before and during energy restriction in obese patients. Am J Clin Nutr. 1985;41:753–759. doi: 10.1093/ajcn/41.4.753. [DOI] [PubMed] [Google Scholar]
- Webb P. Energy expenditure and fat-free mass in men and women. Am J Clin Nutr. 1981;34:1816–1826. doi: 10.1093/ajcn/34.9.1816. [DOI] [PubMed] [Google Scholar]
- Stiegler P, Cunliffe A. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss. Sports Med. 2006;36:239–262. doi: 10.2165/00007256-200636030-00005. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318:467–472. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- Maiorana A, O'Driscoll G, Goodman C, Taylor R, Green D. Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diabetes Res Clin Pract. 2002;56:115–123. doi: 10.1016/s0168-8227(01)00368-0. [DOI] [PubMed] [Google Scholar]
- Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third national health and nutrition examination survey. J Clin Endocrinol Metab. 2011;96:2898–2903. doi: 10.1210/jc.2011-0435. [DOI] [PubMed] [Google Scholar]
- Fine EJ, Feinman RD. Thermodynamics of weight loss diets. Nutr Metab. 2004;1:15. doi: 10.1186/1743-7075-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogebakan O, Kohl A, Osterhoff MA, van Baak MA, Jebb SA, Papadaki A, et al. Effects of Weight Loss and Long-Term Weight Maintenance With Diets Varying in Protein and Glycemic Index on Cardiovascular Risk Factors: The Diet, Obesity, and Genes (DiOGenes) Study: A Randomized, Controlled Trial. Circulation. 2011;124:2829–2838. doi: 10.1161/CIRCULATIONAHA.111.033274. [DOI] [PubMed] [Google Scholar]
- Luscombe-Marsh ND, Noakes M, Wittert GA, Keogh JB, Foster P, Clifton PM. Carbohydrate-restricted diets high in either monounsaturated fat or protein are equally effective at promoting fat loss and improving blood lipids. Am J Clin Nutr. 2005;81:762–772. doi: 10.1093/ajcn/81.4.762. [DOI] [PubMed] [Google Scholar]
- Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–219. [PubMed] [Google Scholar]
- Sacks FM, Katan M. Randomized clinical trials on the effects of dietary fat and carbohydrate on plasma lipoproteins and cardiovascular disease. Am J Med. 2002;113:13S–24S. doi: 10.1016/s0002-9343(01)00987-1. [DOI] [PubMed] [Google Scholar]
- Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56:320–328. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- Levine GN, Keaney JF, Vita JA. Cholesterol reduction in cardiovascular disease. Clinical benefits and possible mechanisms. N Engl J Med. 1995;332:512–521. doi: 10.1056/NEJM199502233320807. [DOI] [PubMed] [Google Scholar]
- Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138:460–467. doi: 10.7326/0003-4819-138-6-200303180-00009. [DOI] [PubMed] [Google Scholar]
- Eisenstein J, Roberts SB, Dallal G, Saltzman E. High-protein weight-loss diets: are they safe and do they work? A review of the experimental and epidemiologic data. Nutr Rev. 2002;60 (7 Part 1:189–200. doi: 10.1301/00296640260184264. [DOI] [PubMed] [Google Scholar]
- Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. 2004;23:373–385. doi: 10.1080/07315724.2004.10719381. [DOI] [PubMed] [Google Scholar]
- Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C. Mohammed BS et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348:2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]