Abstract
Objectives:
Visceral adipose tissue (VAT) is an independent risk factor in cardiometabolic diseases and is commonly measured by computed tomography (CT). It is measured clinically by waist circumference (WC). The L4/5 intervertebral space VAT (L4/5 VAT) is traditionally used to represent total VAT volume. We set out to determine (1) the level of intervertebral space on CT that best approximates the total VAT volume; (2) compare the association between WC and VAT in Singaporean Chinese and Indian; and (3) examine the correlation between VAT and cardiometabolic risk factors.
Subjects:
A total of 60 Chinese and 60 Asian Indian men older than 60 years were recruited. Their medical history was taken and anthropometry was measured. Fasting glucose, insulin, lipids, adipokines and inflammatory markers were measured. Insulin resistance was evaluated by homeostasis model assessment-insulin resistance. VAT was determined by CT. Total VAT volume was calculated in 22 patients from VAT areas at seven intervertebral levels. The optimal VAT area most representative of total VAT volume was determined and used for all patients to approximate total VAT volume.
Results:
The VAT area at L2/3 intervertebral space (L2/3 VAT) correlated almost perfectly with VAT volume (R2=0.974 and 0.946 for Chinese and Indians, respectively). Subjects from the two races had similar height, weight, body mass index (BMI), WC and L2/3 VAT but more Indian men had hypertension, hyperlipidemia and type 2 diabetes mellitus. WC was correlated with the L2/3 VAT area in both Chinese (r=0.484, P<0.001) and Indian subjects (r=0.366, P=0.004) without racial difference (P=0.2 for interaction term). L2/3 VAT also correlated better with cardiometabolic risk factors, adipokines and C-reactive protein than WC, BMI or L4/5 VAT.
Conclusion:
The L2-L3 intervertebral space was the best anatomic level for a single-slice CT cross-sectional area measurement of VAT to approximate total body visceral adipose volume in this population of Chinese and Asian Indian men older than 60 years. L2/3 VAT was better correlated with multiple cardiovascular risk factors, adipokines and inflammatory marker than either L4/5 VAT, WC or BMI.
Keywords: visceral adipose tissue, waist circumference, metabolic syndrome, Chinese, Asian Indian, Singapore
Introduction
Obesity is becoming more common1 and with it, so are diseases such as type 2 diabetes mellitus (DM),2, 3 hypertension3, 4 and hypercholesterolemia.3 Although increased body fat has an impact on health, body fat distribution, particularly fat stored in the visceral adipose tissue (VAT), is also important. VAT has been shown to be an independent risk factor for coronary heart disease,5 hypertension,6 DM7 and impaired glucose tolerance.8 VAT, but not subcutaneous adipose tissue, is correlated with intrahepatic fat and insulin resistance.9
The best way to assess VAT is not clear. In clinical practice, the waist circumference (WC) has been recommended as a marker for VAT.10 However, the relationship between WC and VAT varies between the ethnic groups. Japanese men had larger areas of VAT than Caucasians at the same levels of WC.11 The same is true of Filipinas compared with Caucasian women.12 An alternative means for assessing VAT (usually used in a research setting) is to use computed tomography (CT) or magnetic resonance imaging, but the utility of these modalities is limited by the amount of radiation exposure for CT and the high cost for magnetic resonance imaging. Therefore, most investigators used a single cross-sectional image as a representative measure of VAT volume as a compromise between accuracy and cost in their research. The VAT area at L4-L5 intervertebral space is usually the level used to estimate total VAT volume.13 Although the VAT area at L4-L5 intervertebral space has been used in studies conducted in Korean, Asian Indian and Chinese populations,14, 15, 16 it is not known whether that is indeed the best level at which to assess VAT in these populations. In fact, some recent studies had found the VAT area at L2-L3 intervertebral space to be more representative of total VAT in the Caucasian population.13, 17, 18
We set out to (1) identify the level of intervertebral space on CT that best approximates total VAT volume; (2) compare the association between WC and VAT in Chinese and Asian Indian men living in Singapore; and (3) examine the correlation between VAT and features of the metabolic syndrome, as well as selected inflammatory markers and adipokines in Chinese and Indian men living in Singapore.
Materials and methods
Institutional Review Board approval was obtained at the site. A total of 60 Chinese and 60 Indian men over the age of 60 were recruited. These subjects were primarily recruited from community-based health check fairs. We specifically recruited male subjects aged 60 years and above as they were more likely to have a higher cardiovascular burden. Informed consent was taken from the subjects. Where possible, we recruited healthy subjects who were not on medication for hypertension, hypercholesterolemia or diabetes. This proved to be harder for the Asian Indians and we ended up recruiting some who were on these medications. This is consistent with the previously published data that has shown a higher prevalence of diabetes, hypertension and dyslipidemia among Asian Indians compared with the Chinese.19, 20 We excluded those with known Cushing's syndrome, previous abdominal surgery, previous malignancies and those who were on antiviral medications, weight loss medications, corticosteroids, previous abdominal surgery or any investigational drugs for the past 3 months and those had excessive weight loss (>5% body weight) over the past 3 months.
A detailed medical history was obtained through interviewing the subjects. These included information on smoking habits, alcohol ingestion, exercise frequency and the presence of DM, hypertension, hypercholesterolemia and ischemic heart disease. Family history of diabetes and hypertension were documented.
Height (to the nearest millimeter) was recorded in all subjects without shoes, and weight (in kilograms) was measured with subjects in light clothing using electronic weighing scales (seca 220 - seca deutschland, Hamburg, Germany). Body mass index (BMI) was computed using weight divided by the square of the height (kilograms per meter squared). WC was measured once at the midpoint between the costal margin and the iliac crest in the mid-clavicular line by a single trained technician. Hip circumference was measured at the level of the greater trochanter of the femur. The waist–hip ratio was computed as waist circumference divided by hip circumference. At least two readings of blood pressure (BP) were taken from subjects who had rested adequately before measurement, using a standard mercury sphygmomanometer. If the two readings differed by more than diastolic 5 mm Hg or systolic 10 mm Hg, a third reading was obtained. The mean value of the two closest readings was calculated and recorded as the subject's BP.
Fasting blood specimens for lipids, insulin (10 ml plain tubes) and glucose (2 ml fluoride oxalate tubes) were taken from all subjects after an overnight fast of 10 h. Plasma glucose was measured by the glucose oxidase method with an interassay CV of 3.3% (Beckman Coulter, Inc., Brea, CA, USA). Total cholesterol, high-density lipoprotein cholesterol (HDL-c) and triglyceride (TG) were measured by the 1-step immunoenzymatic assay with an interassay CV of between 1.6 and 1.7% (Beckman). Low-density lipoprotein cholesterol (LDL-c) was calculated by Friedewald's equation. Insulin resistance was evaluated by homeostasis model assessment-insulin resistance (HOMA-IR)21 using the formula: fasting serum insulin (μU ml−1) × fasting plasma glucose (mmol l−1)/22.5.
The adipokines leptin, resistin and adiponectin were measured using commercially available kits (Linco Research, Inc., St Charles, MO, USA). High-sensitivity C-reactive protein was measured using a highly sensitive near infrared particle immunoassay rate technology with intra- and interassay CV 1.3% and 4.1%, respectively (Beckman Coulter, Inc.). Interleukin-6 was measured by ELISA using commercially available kits Linco Research, Inc.
Abdominal CT
Abdominopelvic VAT was determined using a 64-slice multi-detector CT scanner (Somatom Definition, Siemens AG, Erlangen, Germany). Axial CT scan was performed with the subjects supine, from the dome of the diaphragm down to the bottom of the pelvis, using a 35 × 35-cm field of view. Non-contrast enhanced scans using routine scan parameters of kVp (120); effective mAs (210); slice collimation 0.6 mm; slice width 5.0 mm; pitch factor 1.4 and increment 5.0 mm were acquired. The thin-slice raw data was reconstructed into 1 mm sections at zero gap intervals.
All image analyses were performed by a single research assistant, under the guidance of the radiologist. The 3D CT image data sets were transferred to a PC workstation and volumes were measured using the commercial image analysis software, Analyze 9.0 (AnalyzeDirect, Inc., Overland Park, KS, USA).
Fat appears as areas of lower density on the CT images and was identified as areas of attenuation values of −195 to −45 Hounsfield units according to standardized protocol.22 The abdominopelvic VAT was defined as the area of adipose tissue within the inside edge of the abdominal wall, as proposed by Shen et al.23 All images were then checked and manually edited to ensure that fecal matter and bowel gas within the bowels were excluded from the volumetric analysis.
The cross-sectional VAT area at the seven intervertebral disk levels of T11/12, T12/L1, L1/2, L2/3, L3/4, L4/5 and L5/S1, and the total VAT volume at these levels in 22 randomly chosen subjects (11 Chinese and 11 Indians) were measured to determine which level was most representative of the total VAT volume. There were no differences in background characteristics between subjects who were chosen and those who were not chosen for this total VAT volume analysis. Subsequent analyses used only the VAT at the level which was most representative of total VAT volume and at the traditional L4/5 level for the full cohort of 120 subjects.
In addition, the cross-sectional VAT area at L2/3 was subdivided into intraperitoneal (IP) and retroperitoneal (RP) adipose tissue (AT). The areas of IP-AT and RP-AT were quantified by using a method similar to that of Baumgartner et al.,24 namely by drawing two diagonal lines from the anterior edge of the aorta and inferior vena cava tangentially across the anterior border of each kidney to their intersection with the line circumscribing the VAT area. RP-AT was defined as the VAT posterior to that line, and IP-AT was defined as the VAT anterior to it.
Statistical analysis
Statistical analysis was done using SPSS 11.0 (SPSS Inc., Chicago, IL, USA). Student's t-test and the Mann–Whitney test were used to compare the means of normally and non-normally distributed parameters, respectively. Pearson correlation coefficients were computed to assess the relationship between VAT and WC in each ethnic group and the associations compared between ethnic groups. For hypertensive subjects with hypertension, we adjusted for antihypertensive medication use by adding a constant to the observed BP measurements, 10 mm Hg to systolic BP and 5 mm Hg to diastolic BP. This approach was suggested by Cui et al.25 and later validated by Tobin et al.26 Nineteen subjects who were on diabetic medication were excluded from the analysis of fasting glucose and insulin. Twenty subjects who were on lipid medication were excluded from the analysis of fasting lipids. Parameters that were not normally distributed underwent logarithmic transformation. Pearson's Correlations between VAT and WC and clinical and biochemical parameters were analyzed.
Multivariable regression using general linear models was performed separately for each ethnic group to assess the relationship between VAT and features of the metabolic syndrome. A regression model that included ethnicity, VAT and an ethnicity × VAT interaction term was used to determine whether the relationship between VAT and features of the metabolic syndrome differed by ethnicity.
Results
Table 1 shows the characteristics of our subjects. Subjects from the two groups were clinically similar. Any differences in height, weight, BMI, WC and L2/3 VAT area were not statistically significant. However, while the Chinese men were generally healthy, about two thirds of the Indian men had hypertension, one third had diabetes and one third had hypercholesterolemia. Despite the similarity in weight, WC and BMI, leptin and hs-CRP levels were higher in Asian Indians, whereas resistin levels were lower and adiponectin levels were similar.
Table 1. Baseline characteristics—mean (s.d.); n (%).
| Characteristic | Chinese (n=60) | Asian Indian (n=60) |
|---|---|---|
| Age | 66.8 (4.7) | 65.7 (7.0) |
| Weight (kg) | 65.3 (14.5) | 70.0 (13.5) |
| Height (cm) | 164 (6) | 165 (7) |
| BMI | 24.1 (4.3) | 26.3 (4.3) |
| Waist circumference (cm) | 84.7 (9.5) | 88.5 (14.1) |
| Hip circumference (cm) | 89.1 (17.0) | 92.7 (13.6) |
| Waist–hip ratio | 0.964 (0.105) | 0.958 (0.094) |
| L23 level VAT (mm3) | 78 213 (36 599) | 88 771 (41 980) |
| Systolic BP (mm Hg) | 136 (20) | 134 (22) |
| Diastolic BP (mm Hg) | 79 (9.8) | 76 (12) |
| Resistin (ng ml−1)a | 10.2 (5.82) | 7.79 (2.83) |
| Leptin (μg l−1)a | 4.51 (2.65) | 8.88 (6.13) |
| Adiponectin (ng ml−1) | 5999 (2992) | 6465 (3755) |
| Hs-CRP (mg l−1)a | 1.48 (1.74) | 2.88 (3.32) |
| Fasting glucose (mmol l−1)a | 5.43 (0.89) | 6.57 (2.54) |
| Fasting insulin (μU ml−1) | 6.87 (6.22) | 8.40 (4.45) |
| HOMA-IRa | 1.69 (1.72) | 2.61 (2.27) |
| Total cholesterol (mmol l−1)a | 5.39 (0.76) | 4.87 (0.99) |
| HDL-cholesterol (mmol l−1)a | 1.26 (0.27) | 1.10 (0.22) |
| Triglyceride (mmol l−1) | 1.51 (0.85) | 1.38 (0.72) |
| LDL-cholesterol (mmol l−1)a | 3.45 (0.88) | 3.13 (0.88) |
| Hypertension on medication | 0 (0%) | 37 (62%) |
| Diabetes on medication | 0 (0%) | 19 (32%) |
| Hyperlipidemia on medication | 0 (0%) | 20 (33%) |
Abbreviations: BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment-insulin resistance; LDL, low-density lipoprotein; VAT, visceral adipose tissue.
Chinese and Asian Indian between group difference significant at P<0.05.
The values presented in the above table represent mean (s.d.), except for the proportion of patients with hypertension, diabetes and hyperlipidemia on medications, for which the values represent n (%).
In 22 randomly selected subjects, we found that the L2/3 VAT area correlated almost perfectly with total VAT volume with r2 that is close to unity for both Chinese and Indian men (Table 2). The degree of correlation was much better than that for L4/5 VAT area and VAT volume (R2=0.974 and 0.946 for Chinese and Asian Indians, respectively).
Table 2. R 2 for correlation between VAT area and total VAT volume Chinese and Asian Indian.
| Level of VAT area | R2
between VAT area at that level and total VAT volume |
|
|---|---|---|
| Chinese | Asian Indian | |
| T11/12 | 0.886 | 0.682 |
| T12/L1 | 0.838 | 0.854 |
| L1/L2 | 0.967 | 0.942 |
| L2/L3 | 0.974 | 0.946 |
| L3/L4 | 0.952 | 0.944 |
| L4/L5 | 0.874 | 0.641 |
| L5/S1 | 0.785 | 0.563 |
Abbreviation: VAT, visceral adipose tissue.
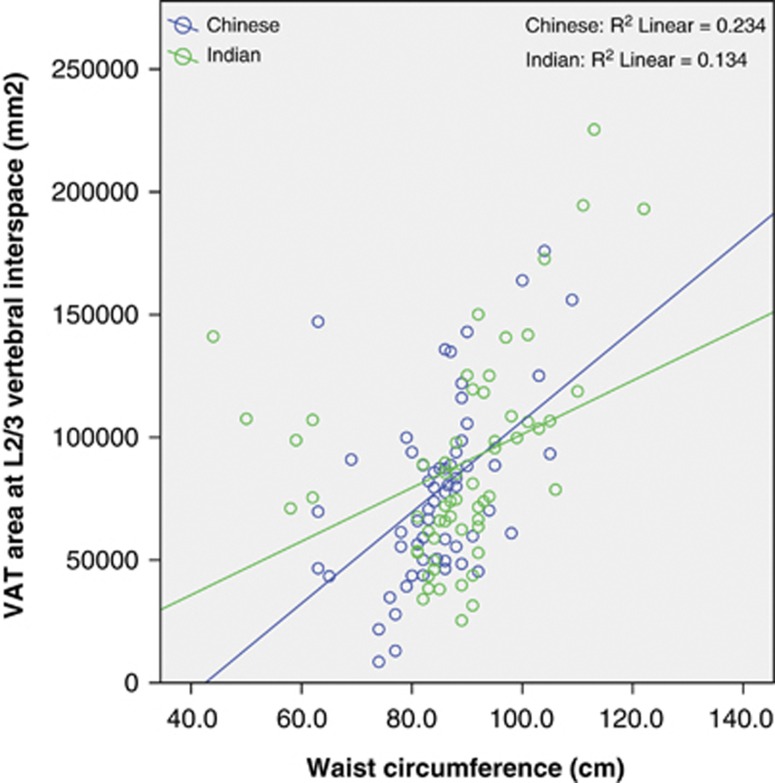
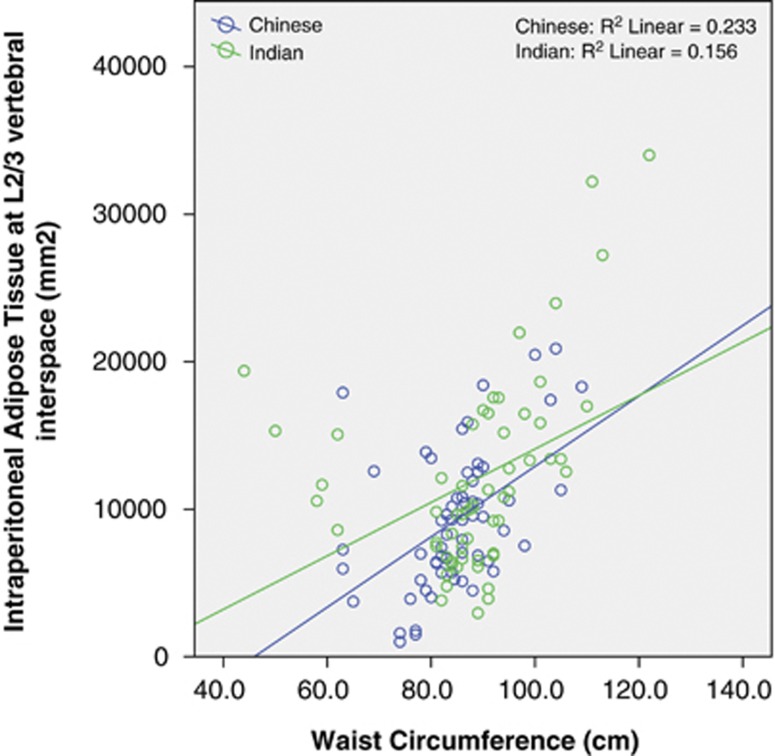
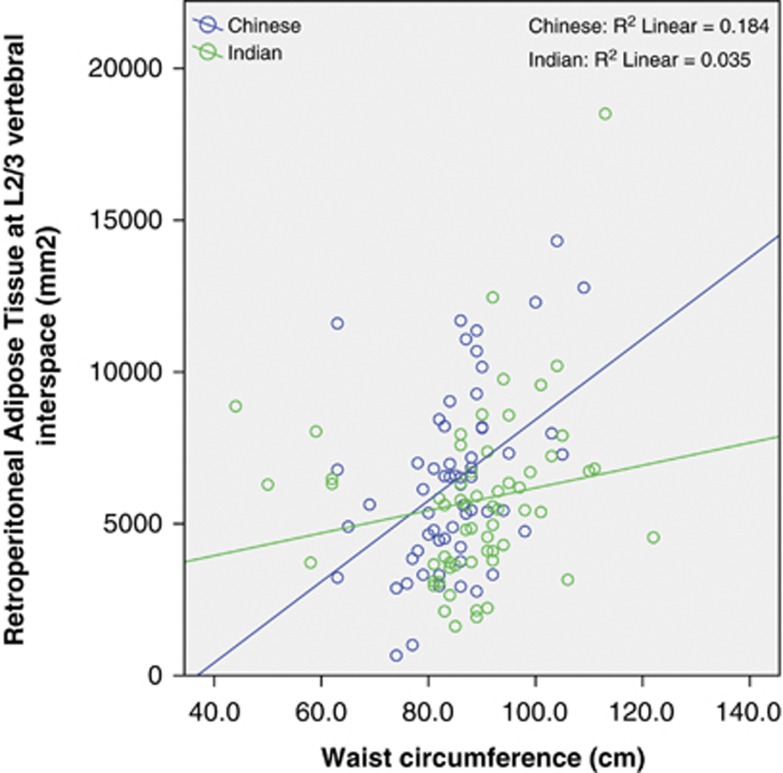
WC was correlated with the L2/3 VAT area in both Chinese (r=0.484, P<0.001) and Indian subjects (r=0.366, P=0.004; Figure 1). Thus, WC explained 23 and 13% of the variance of VAT area at L2/3. There was no significant difference between the two races, with P=0.2 for the interaction term race × WC. However, when we divided the VAT into IP-AT and RP-AT, some ethnic differences were observed. In both the ethnic groups, WC correlated with IP-AT in a similar manner (P-interaction=0.5, Figure 2), but increasing WC was associated with a greater increased in RP-AT in Chinese than in Indians (P-interaction 0.036, Figure 3). At low WC, Chinese men had lower RP-AT than Indian men; however, at high WC, Chinese men had higher RP-AT.
Figure 1.
Correlation between WC and VAT at L2/3 intervertebral space by racial groups.
Figure 2.
Correlation between WC and IP-AT by racial groups.
Figure 3.
Correlation between WC and RP-AT by racial groups.
To further evaluate the biological relevance of the L2/3 VAT area compared with the L4/5 VAT area and WC, we examined the correlation between the VAT area at each level with CVD risk factors, the adipokines leptin and adiponectin, and the inflammatory marker hs-CRP, after correction for age and race (Table 3). The L2/3 VAT area showed a stronger correlation with virtually all the parameters. The correlations after additional adjustment for BMI remained statistically significant for L2/3 VAT for fasting glucose, fasting insulin, HOMA-IR, HDL-c, TG, leptin and adiponectin. In contrast, WC showed statistically significant associations only for fasting insulin, HOMA-IR, HDL-c and TG, and only TG remained statistically significant after further adjustment for BMI.
Table 3. Linear regression beta and P-values between clinical and biochemical parameters and L23 VAT, L45 VAT and waist, with or without adjustment for BMI.
|
L23 VAT (β, P) |
L45 VAT (β, P) |
Waist (β, P) |
BMI (β, P) | ||||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | |
| Adjusted systolic BP | 0.179, 0.054 | 0.091, 0.442 | 0.074, 0.431 | −0.058, 0.954 | 0.131, 0.161 | 0.041, 0.383 | 0.202, 0.033 |
| Adjusted diastolic BP | 0.163, 0.067 | 0.086, 0.448 | 0.012, 0.859 | −0.070, 0.471 | 0.133, 0.138 | 0.057, 0557 | 0.181, 0.046 |
| Ln (fasting glucose) | 0.420, <0.001 | 0.420, <0.001 | 0.185, 0.065 | 0.087, 0.424 | 0.405, <0.001 | 0.370, 0.001 | 0.265, 0.008 |
| Ln (fasting insulin) | 0.469, <0.001 | 0.391, 0.001 | 0.197, 0.050 | 0.044, 0.676 | 0.397, <0.001 | 0.279, 0.012 | 0.373, <0.001 |
| Ln (HOMA-IR) | 0.521, <0.001 | 0.451, <0.001 | 0.221, 0.027 | 0.060, 0.559 | 0.453, <0.001 | 0.339, 0.002 | 0.398, <0.001 |
| HDL-c | −0.250, 0.008 | −0.234, 0.045 | −0.173, 0.073 | −0.126, 0.235 | −0.250, 0.009 | −0.223, 0.041 | −0.167, 0.087 |
| Ln (TG) | 0.219, 0.030 | 0.342, 0.006 | 0.225, 0.027 | 0.279, 0.013 | 0.208, 0.040 | 0.283, 0.015 | −0.011, 0.916 |
| Ln (leptin) | 0.425, <0.001 | 0.241, 0.008 | 0.220, 0.008 | 0.054, 0.495 | 0.419, <0.001 | 0.261, 0.002 | 0.456, <0.001 |
| Ln (adiponectin) | −0.343, <0.001 | −0.288, 0.011 | −0.181, 0.054 | −0.091, 0.359 | −0.272, 0.003 | −0.185, 0.076 | −0.272, 0.004 |
| Ln (hs-CRP) | 0.259, 0.003 | 0.180, 0.106 | 0.073, 0.418 | −0.027, 0.781 | 0.210, 0.018 | 0.119, 0.240 | 0.244, 0.007 |
Abbreviations: BP, blood pressure; HDL-c, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment-insulin resistance; Hs-CRP, highly sensitive C-reactive protein; TG, triglyceride; VAT, visceral adipose tissue.
Model 1: adjusted for age and race; Model 2: adjusted for age, race and BMI.
We then compared the correlations between IP-AT and RP-AT with CVD risk factors and inflammatory markers (Table 4). Both IP-AT and RP-AT were correlated with multiple metabolic traits both before and after adjustment for BMI. However, when both IP-AT and RP-AT were incorporated into the same model, only fasting insulin was correlated with RP-AT, whereas correlations for fasting glucose, HOMA-IR and TG remained statistically significant for IP-AT.
Table 4. Linear regression beta and P-values between clinical and biochemical parameters and IP-AT, RP-AT at L2/3, with or without adjustment for BMI.
|
IP-AT (β, P) |
RP-AT (β, P) |
|||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| Adjusted systolic BP | 0.202, 0.032 | 0.125, 0.297 | 0.102, 0.276 | 0.007, 0.949 |
| Adjusted diastolic BP | 0.155, 0.087 | 0.070, 0.543 | 0.148, 0.098 | 0.080, 0.430 |
| Ln (fasting glucose) | 0.471, <0.001 | 0.490, <0.001 | 0.278, 0.005 | 0.192, 0.100 |
| Ln (fasting insulin) | 0.413, <0.001 | 0.296, 0.012 | 0.514, <0.001 | 0.447, <0.001 |
| Ln (HOMA-IR) | 0.484, <0.001 | 0.383, 0.001 | 0.527, <0.001 | 0.446, <0.001 |
| HDL-c | −0.261, 0.007 | −0.243, 0.038 | −0.196, 0.040 | −0.152, 0.171 |
| Ln (TG) | 0.243, 0.018 | 0.363, 0.003 | 0.144, 0.156 | 0.203, 0.086 |
| Ln (leptin) | 0.410, <0.001 | 0.208, 0.026 | 0.375, <0.001 | 0.207, 0.012 |
| Ln (adiponectin) | −0.333, <0.001 | −0.267, 0.022 | −0.294, <0.001 | −0.215, 0.037 |
| Ln (hs-CRP) | 0.259, 0.004 | 0.175, 0.123 | 0.213, 0.017 | 0.126, 0.210 |
Abbreviations: BP, blood pressure; HDL-c, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment-insulin resistance; Hs-CRP, highly sensitive C-reactive protein; IP-AT, intraperitoneal adipose tissue; RP-AT, retroperitoneal adipose tissue; TG, triglyceride.
Model 1: adjusted for age and race; Model 2: adjusted for age, race and BMI.
Discussion
In this study, CT-based measures of VAT was more strongly correlated to metabolic parameters than WC, which is in line with the belief that VAT is an important fat depot in the pathogenesis of DM and CVD. Furthermore, WC was a rather poor measure of VAT in Chinese and Asian Indian men, explaining only 23% and 13% of the variance of VAT area, respectively, as compared with 60% in Caucasian men.27 In fact, the proportion of variance of VAT explained by WC was lower in our study than in other Asian populations. Based on previous studies, the variance of VAT area that was explained by WC was 33% in Indian men,15 39% in Japanese men,28 29% in Korean women,29 35% in Filipino American women30 and 54 to 56% in Japanese women.28, 31 We were unable to find any reported value of Chinese populations.
We also found that in Chinese and Asian Indian men, VAT at the L2/3 level was more strongly correlated to both total VAT volume as well as most clinical and laboratory markers for CVD than the VAT at L4/5, which is used most commonly for the assessment of visceral adiposity. These findings are in line with more recent studies in Caucasian populations that have shown the L2/3 VAT better represented visceral adiposity.13, 17, 18, 32 Further, in a study by Kuk et al.32 involving 85 Caucasian men of mean age about 50 years, the odds ratio (OR, per s.d.) for the metabolic syndrome based on the National Cholesterol Education Program (NCEP) criteria was much higher for the VAT at L1-L2 level (8.77) than at the L4-L5 level (3.94).
In our study, there was no significant difference of the relationship between WC and VAT between Chinese and Asian Indian men. In both races, the VAT increases as the WC increases. Several guidelines have suggested that we should use the same cutoff to define central obesity in Chinese and Asian Indians.33, 34 Although our findings would appear to support this recommendation, we caution that these results are limited to older men in these specific ethnic groups.
Lastly, although there was no difference between Chinese and Asian Indians in the correlation between WC and IP-AT, WC was strongly correlated with RP-AT in Chinese but not Asian Indians. In other words, as both Chinese and Asian Indian men become increasingly centrally obese, Chinese men would store more fat in the RP-AT, but both groups would also store more fat in the IP-AT. It had been recently reported in our population that the relationship between WC or BMI and insulin resistance were not different between Chinese and Asian Indian men.35 As IP-AT is the adipose depot that is more metabolically active as shown in other studies36 (and consistent with our own findings that IP-AT was correlated with more metabolic markers than RP-AT), our findings support the notion that both the groups are at similar risk for DM and CVD at similar degrees of central obesity.
The strength of our study was that we had comprehensive data, including CT assessment for VAT with its IP-AT and RP-AT compartments, together with clinical and laboratory markers of obesity and cardiovascular disease. Singapore is a multi-racial country with a population that comprises 74.1% Chinese and 9.2% Asian Indians37 that has seen a recent rapid increase in the rates of both DM38 and cardiovascular mortality.39 Similar trends have been observed in China and India,40, 41, 42, 43 two of the most populous countries in the world. As such, we believe our findings will have great relevance to other countries in Asia. However, the main limitation of our study was that our Chinese and Asian Indian subjects were rather different. The Chinese were mostly healthy, whereas most of the Asian Indians had one or more chronic diseases. As such, we would be cautious about extrapolating these results to a different population.
In conclusion, the L2-L3 intervertebral space was the best anatomic level for a single-slice CT cross-sectional area measurement of VAT to approximate total body visceral adipose volume in this population of Chinese and Asian Indian men older than 60 years. L2/3 VAT was better correlated with multiple cardiovascular risk factors, adipokines and inflammatory marker than either L4/5 VAT, WC or BMI.
Acknowledgments
We would like to thank the National Medical Research Council of Singapore in the funding of this research.
The authors declare no conflict of interest.
References
- Ford ES, Ford ES, Li C, Zhao G, Tsai J. Trends in obesity and abdominal obesity among adults in the United States from, 1999–2008. Int J Obes (Lond) 2011;35:736–743. doi: 10.1038/ijo.2010.186. [DOI] [PubMed] [Google Scholar]
- Lee JW, Brancati FL, Yeh HC. Trends in the prevalence of type 2 diabetes in Asians versus whites: results from the United States National Health Interview Survey, 1997–2008. Diabetes Care. 2011;34:353–357. doi: 10.2337/dc10-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estoppey D, Paccaud F, Vollenweider P, Marques-Vidal P. Trends in self-reported prevalence and management of hypertension, hypercholesterolemia and diabetes in Swiss adults, 1997–2007. BMC Public Health. 2011;11:114. doi: 10.1186/1471-2458-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L. Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care. 1999;22:1808–1812. doi: 10.2337/diacare.22.11.1808. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, et al. Visceral adiposity is an independent predictor of incident hypertension in Japanese Americans. Ann Intern Med. 2004;140:992–1000. doi: 10.7326/0003-4819-140-12-200406150-00008. [DOI] [PubMed] [Google Scholar]
- Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23:465–471. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, et al. Visceral adiposity and the risk of impaired glucose tolerance: a prospective study among Japanese Americans. Diabetes Care. 2003;26:650–655. doi: 10.2337/diacare.26.3.650. [DOI] [PubMed] [Google Scholar]
- Westerbacka J, Cornér A, Tiikkainen M, Tamminen M, Vehkavaara S, Häkkinen AM, et al. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47:1360–1369. doi: 10.1007/s00125-004-1460-1. [DOI] [PubMed] [Google Scholar]
- Pouliot MC, Després JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Sekikawa A, Murata K, Maegawa H, Okamura TakamiyaT, et al. Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population-basedstudy. Int J Obes (Lond) 2006;30:1163–1165. doi: 10.1038/sj.ijo.0803248. [DOI] [PubMed] [Google Scholar]
- Rosario M, Schrimshaw EW, Hunter J. Ethnic/racial differences in the coming-out process of lesbian, gay, and bisexual youths: a comparison of sexual identity development over time. Cultur Divers Ethnic Minor Psychol. 2004;10:215–228. doi: 10.1037/1099-9809.10.3.215. [DOI] [PubMed] [Google Scholar]
- Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80:271–278. doi: 10.1093/ajcn/80.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JA, Kim BG, Cho H, Kim HS, Park J, Baik SH, et al. The cutoff values of visceral fat area and waist circumference for identifying subjects at risk for metabolic syndrome in elderly Korean: Ansan Geriatric (AGE) cohort study. BMC Public Health. 2009;9:443. doi: 10.1186/1471-2458-9-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjana M, Sandeep S, Deepa R, Vimaleswaran KS, Farooq S, Mohan V, et al. Visceral and central abdominal fat and anthropometry in relation to diabetes in Asian Indians. Diabetes Care. 2004;27:2948–2953. doi: 10.2337/diacare.27.12.2948. [DOI] [PubMed] [Google Scholar]
- Ye Y, Bao Y, Hou X, Pan X, Wu H, Li H, et al. Identification of waist circumference cutoffs for abdominal obesity in the Chinese population: a 7.8-year follow-up study in the Shanghai urban area. Int J Obes (Lond) 2009;33:1058–1062. doi: 10.1038/ijo.2009.134. [DOI] [PubMed] [Google Scholar]
- Abate N, Garg A, Coleman R, Grundy SM, Peshock RM. Prediction of total subcutaneous abdominal, intraperitoneal, and retroperitoneal adipose tissue masses in men by a single axial magnetic resonance imaging slice. Am J Clin Nutr. 1997;65:403–408. doi: 10.1093/ajcn/65.2.403. [DOI] [PubMed] [Google Scholar]
- Irlbeck T, Massaro JM, Bamberg F, O'Donnell CJ, Hoffmann U, Fox CS. Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. Int J Obes (Lond) 34:781–787. doi: 10.1038/ijo.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo KK, Tai BC, Heng D, Lee JM, Ma S, Hughes K, et al. Ethnicity modifies the association between diabetes mellitus and ischaemic heart disease in Chinese, Malays and Asian Indians living in Singapore. Diabetologia. 2006;49:2866–2873. doi: 10.1007/s00125-006-0469-z. [DOI] [PubMed] [Google Scholar]
- Sabanayagam C, Lim SC, Wong TY, Lee J, Shankar A, Tai ES. Ethnic disparities in prevalence and impact of risk factors of chronic kidney disease. Nephrol Dial Transplant. 2010;25:2564–2570. doi: 10.1093/ndt/gfq084. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Kvist H, Chowdhury B, Grangård U, Tylén U, Sjöström L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48:1351–1361. doi: 10.1093/ajcn/48.6.1351. [DOI] [PubMed] [Google Scholar]
- Shen W, Wang Z, Punyanita M, Lei J, Sinav A, Kral JG, et al. Adipose tissue quantification by imaging methods: a proposed classification. Obes Res. 2003;11:5–16. doi: 10.1038/oby.2003.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner RN, Heymsfield SB, Roche AF, Bernardino M. Abdominal composition quantified by computed tomography. Am J Clin Nutr. 1988;48:936–945. doi: 10.1093/ajcn/48.4.936. [DOI] [PubMed] [Google Scholar]
- Cui JS, Hopper JL, Harrap SB. Antihypertensive treatments obscure familial contributions to blood pressure variation. Hypertension. 2003;41:207–210. doi: 10.1161/01.hyp.0000044938.94050.e3. [DOI] [PubMed] [Google Scholar]
- Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–2935. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- Kashihara H, Lee JS, Kawakubo K, Tamura M, Akabayashi A. Criteria of waist circumference according to computed tomography-measured visceral fat area and the clustering of cardiovascular risk factors. Circ J. 2009;73:1881–1886. doi: 10.1253/circj.cj-09-0183. [DOI] [PubMed] [Google Scholar]
- Hyun YJ, Kim OY, Jang Y, Ha JW, Chae JS, Kim JY, et al. Evaluation of metabolic syndrome risk in Korean premenopausal women: not waist circumference but visceral fat. Circ J. 2008;72:1308–1315. doi: 10.1253/circj.72.1308. [DOI] [PubMed] [Google Scholar]
- Araneta MR, Barrett-Connor E. Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and white women. Obes Res. 2005;13:1458–1465. doi: 10.1038/oby.2005.176. [DOI] [PubMed] [Google Scholar]
- Miyatake N, Takenami S, Fujii M. Evaluation of visceral adipose accumulation in Japanese women and establishment of a predictive formula. Acta Diabetol. 2004;41:113–117. doi: 10.1007/s00592-004-0153-z. [DOI] [PubMed] [Google Scholar]
- Kuk JL, Church TS, Blair SN, Ross R. Does measurement site for visceral and abdominal subcutaneous adipose tissue alter associations with the metabolic syndrome. Diabetes Care. 2006;29:679–684. doi: 10.2337/diacare.29.03.06.dc05-1500. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- Khoo CM, Sairazi S, Taslim S, Gardner D, Wu Y, Lee J, et al. Ethnicity modifies the relationships of insulin resistance, inflammation, and adiponectin with obesity in a multiethnic Asian population. Diabetes Care. 34:1120–1126. doi: 10.2337/dc10-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest. 1995;96:88–98. doi: 10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Statistics, M.o.T.I., Republic of Singapore, Census of Population 2010, Singapore. 2010.
- Hoe Y.Singapore's adult diabetes rate at 12-year high. (Internet) 2010 (cited 26 November 2010). . http://www.channelnewsasia.com/stories/singaporelocalnews/view/1095709/1/.html . (accessed 26 July 2012).
- Molina JA, Heng BH. Global trends in cardiology and cardiothoracic surgery—an opportunity or a threat. Ann Acad Med Singapore. 2009;38:541–545. [PubMed] [Google Scholar]
- Zhang YX, Wang SR. Distribution of body mass index and the prevalence changes of overweight and obesity among adolescents in Shandong, China from 1985 to 2005. Ann Hum Biol. 2008;35:547–555. doi: 10.1080/03014460802334239. [DOI] [PubMed] [Google Scholar]
- Balarajan Y, Villamor E. Nationally representative surveys show recent increases in the prevalence of overweight and obesity among women of reproductive age in Bangladesh, Nepal, and India. J Nutr. 2009;139:2139–2144. doi: 10.3945/jn.109.112029. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Stein R, Kwan T, Yu C, Kwan J, Chen SL, et al. Evolving cardiovascular disease prevalence, mortality, risk factors, and the metabolic syndrome in China. Clin Cardiol. 2009;32:491–497. doi: 10.1002/clc.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. Recent trends in coronary heart disease epidemiology in India. Indian Heart J. 2008;60 (Suppl B:B4–18. [PubMed] [Google Scholar]