Abstract
Objective:
The mechanisms involved in early resolution of insulin resistance and type 2 diabetes mellitus after biliopancreatic diversion with duodenal switch (BPD-DS) surgery are still unknown. We evaluated early effects of BPD-DS on plasma acylation stimulating protein (ASP), an adipokine involved in lipid and glucose metabolism.
Subjects:
32 non-diabetic and 22 diabetic severely obese women (BMI⩾40 kg m−2) were evaluated for body composition and plasma parameters before, 24 h, 5 days, 6 and 12 months after surgery.
Results:
Within the early postoperative period (24 h), ASP decreased 25 and 30% in non-diabetic and diabetic women, respectively (P<0.001). Twenty-four hours after surgery, triglyceride, cholesterol, HDL-Chol, LDL-Chol and C3 also decreased, while glucose, insulin and high-sensitivity C-reactive protein (hsCRP) increased (all P<0.001). By 5 days, without significant weight loss, the decreases in ASP, cholesterol, HDL-Chol and LDL-Chol levels were all maintained. At this time, glucose, insulin and HOMA-IR also decreased 11 to 52% (all P<0.001). At 6 and 12 months, with pronounced weight loss and decreased per cent fat mass, there were further decreases in ASP (maximal −56% non-diabetic, −61% diabetic, P<0.001), as well as in glucose, insulin, HOMA-IR, triglyceride, cholesterol, LDL-Chol, HDL-Chol and hsCRP levels. Improved insulin resistance/diabetes at 5 days was predicted by 24 h changes as follows: per cent change ASP, HDL-Chol, hsCRP and total cholesterol predicted HOMA-IR (5 days) (r2=0.454, P<0.001), and per cent change ASP, HDL-Chol and hsCRP predicted change (5 days vs baseline) in HOMA-IR (r2=0.351, P<0.001).
Conclusion:
Acute postoperative decreases in ASP are associated with early improvement of insulin resistance/diabetes after BPD-DS surgery.
Keywords: C3adesArg, adipokine, complement C3, Insulin resistance, obesity
Introduction
Bariatric surgery has become a common strategy used in the treatment of severely obese patients with a body mass index (BMI) >40 or >35 kg m−2 with severe co-morbidities. The effectiveness in improving abnormalities in insulin and glucose metabolism ranges from 48% with gastric banding to 99% in biliopancreatic diversion (BPD) with or without duodenal switch (DS).1, 2 As early as 1995,3 RYGB bariatric surgery was recognized as a ‘cure' for diabetes. In restriction/malabsorption procedures, improved insulin sensitivity occurs before any significant weight loss, with studies reporting 80–100% remission rate within days (1 week) of surgery.4, 5 Even performing a duodenal-jejunal bypass in non-obese patients with type 2 diabetes (T2D) leads to disease remission in a majority of patients.6, 7 Information on factors to predict an improvement in insulin sensitivity would provide biomarkers and contribute to understanding the mechanisms involved.
Little is known regarding the exact mechanisms involved, but several have been proposed: (i) physical (anatomical) changes, (ii) hormonal changes (gastro-intestinal or other hormones) and, (iii) postoperative caloric restriction. The ‘foregut' hypothesis proposes that bypass of the proximal small intestine results in inhibition of a putative signal responsible for insulin resistance, and explains the more rapid diabetes resolution in those undergoing malabsorptive bariatric surgery vs procedures that are solely restrictive. The ‘hindgut' hypothesis suggests that early contact of the distal bowel with relatively undigested food enhances signals (such as hormones including incretins) that improve glucose metabolism. Postoperative caloric restriction may also contribute to the early resolution.9, 10 Changes in gastrointestinal and pancreatic hormones, including incretins as well as adipokines and cytokines, are reported over weeks to years, during which there are weight changes.8, 9 Few studies have examined changes within days, where there is no weight change. Most attention has focused on the incretins GLP-1 and GIP, which alter insulin secretion (entero-insular axis) and peripheral postprandial lipid and glucose clearance.11, 9, 12, 13 However, results are controversial with both early increases in GLP-1 and decreases in GIP (consistent with improved insulin sensitivity),14, 15 and the converse reported.16 Further, animal studies are inconclusive.11, 17
A proposed link to the gut-adipo-insular axis has also been suggested.18 The links between adipose tissue function, fatty acid metabolism, and glucose uptake into insulin-sensitive tissues (such as muscle) are now well established in healthy, obese and diabetic subjects. Adipose tissue is also an active endocrine organ secreting a variety of adipokines including leptin, adiponectin, acylation stimulating protein (ASP) and many others.19, 20 Over the past decade, the concept that hypertrophic adipose tissue is a promoter of type 2 diabetes progression owing to changes in adipokines and proinflammatory molecules, and their effects on impairment of insulin sensitivity has become accepted.21 With respect to bariatric surgery, 1 week after BPD, reductions of plasma leptin have been observed before any weight changes,14, 16 although not all agree.22 By contrast, there was no short-term change in adiponectin.23, 14, 16 However, neither of these hormones has been directly suggested to be responsible for the rapid improvement in insulin sensitivity in these studies.
ASP (aka C3adesArg) is a lipogenic adipokine produced by adipose tissue through the interaction of the precursor proteins of the alternative complement pathway C3, factor B, and adipsin.19 ASP is linked to obesity through its action to enhance triglyceride (TG) synthesis and storage in the adipocyte, via its receptor C5L2. ASP increases both glucose uptake as well as fatty acid esterification, independently of and additively to insulin.19 Plasma ASP levels are increased in a number of metabolic disorders associated with obesity including insulin resistance, hypothyroidism, type 2 diabetes, polycystic ovary syndrome and cardiovascular disease.19, 24, 25, 26, 27 With weight loss, either diet- or surgery-induced, plasma ASP decreases.19 Interestingly, obesity is not an essential feature of elevated ASP levels, as ASP is increased in subjects with type 2 diabetes, polycystic ovary syndrome, and lipoprotein lipase deficiency, even in the absence of obesity,24, 26, 27 suggesting that it may be a compensatory increase associated with adipose tissue dysfunction or insulin resistance.28
In the present study, we conducted a comprehensive investigation of short-term and long-term endocrine and metabolic changes following BPD-DS. Our aim was to evaluate potential acute changes in ASP levels, and the association with improvement of insulin resistance and diabetes before weight loss, (1 day and 5 days postoperatively) to evaluate ASP as a predictive factor for improved insulin sensitivity in the absence of weight loss. Further, we evaluated whether the short term and long-term ASP changes (6 months and 12 months) after BPD-DS surgery were present and similar in non-diabetic and diabetic women.
Materials and methods
Subjects
Subjects scheduled to undergo bariatric surgery (BPD-DS) were recruited through the bariatric surgery clinic of the Institut universitaire de cardiologie et de pneumologie de Québec (IUCPQ). Subjects were randomly selected (in chronologic order of surgeries, regardless of diabetic status or current medication) for participation based on inclusion criteria: women, >18 years of age, BMI⩾40 or BMI⩾35 kg m−2 with associated comorbidities, and were collected from surgeries performed between July 2006 to May 2008. Subjects who had previously undergone bariatric surgery or those bearing a pacemaker were excluded (patients with a pacemaker cannot undergo electrical bioimpedance assessment). Only subjects who completed the study (5 time periods, with blood samples collected and available) were included for subsequent biochemical analysis. Laboratory procedures were completed before statistical analysis was performed. The experimental protocol was approved by the ethics committee of the IUCPQ and all patients gave their written informed consent.
Anthropometric and biochemical measurements
Women were assessed preoperatively (within 3 months of surgery) and postoperatively (24 h, 5 days, 6 months and 12 months). Blood samples were collected between June 2006 to May 2009. Height was measured using a stadiometer (SECA, 216 1814009, Brooklyn, NY, USA). Total body mass, BMI, lean and fat masses were evaluated by electrical bioimpedance balance using standard formulas (Tanita TBF-310, Tokyo, Japan) following a 12-h fast. BMI was calculated as weight (kgs)/height (m2). Medical history was collected for diabetes, hypertension, coronary artery disease and dyslipidemia as well as the corresponding pharmacological therapy. The information provided by the patient was confirmed by consulting clinical files.
Venous blood was collected following a 12-h fast into EDTA containing tubes. Glycated hemoglobin (HbA1c) was evaluated in a fresh sample by turbidimetric inhibition immunoassay. All other tubes were rapidly placed on ice, centrifuged within 15 min, plasma collected and frozen in aliquots at -80 °C until analysis. Assays were measured in the hospital clinical biochemistry laboratory using standard methodology or in the research laboratory (CRP, C3, ASP and NEFA). High-sensitive C-reactive protein (hsCRP) and apolipoprotein B (apoB) levels were measured by immunoturbidimetric method (Integra 800 System, Roche Diagnostics, IN, USA). Complement C3 was measured by immunoturbidimetry (Kamiya Biochemical Company, Seattle, WA, USA). Plasma ASP was measured by ELISA.29 Non-esterified fatty acid (NEFA) was analyzed by colorimetric enzymatic assay (Wako Pure Chemicals, Richmond, VA, USA). All other biochemical assays were performed using a Modular system (Roche Diagnostics). LDL-cholesterol concentration was calculated with Friedewald's formula (no subjects had triglyceride values>4.5 mM).30 Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated from fasting plasma insulin and glucose levels as (insulin x glucose)/22.5, where insulin concentration is reported as milliunits per liter and glucose as millimolar concentrations.
Statistical analyses
All results are expressed as mean±s.d. or s.e.m. as indicated. The fasting data values were compared between non-diabetic and diabetic women using unpaired Student's t-test. Comparison across different times were analyzed by repeated measures ANOVA followed by Holm Sidak post-hoc test. Correlations of selected parameters were analyzed by linear regression (Forward Stepwise regression). GraphPad Prism (San Diego, CA, USA) and SigmaStat (San Rafaeal, CA, USA) software programs were used for graph and statistical analyses. Significance was set at P⩽0.05, where NS indicates ‘not significant'.
Results
Pre-operative body weight and composition
Subjects were randomly selected (in chronologic order of surgeries, regardless of diabetic status or current medication) for participation based on inclusion criteria: women, >18 years of age, BMI⩾40 or BMI⩾35 kg m−2 with associated comorbidities, and were collected from surgeries performed between July 2006 to May 2008. Only subjects who completed the study (5 time periods, with blood samples collected and available) were included for subsequent biochemical analysis. Of the 54 women, 32 were non-diabetic, and the women were separated into two groups for analysis based on diabetic status. Twenty-two women had been previously diagnosed as diabetic and were being treated accordingly with diet, biguanides, sulfonylureas, thiazolidinediones and/or insulin as indicated in Table 1. Eighteen diabetics were being treated with lipid-lowering therapy. Preoperative body composition and biochemical measurements are shown in Table 1. Diabetic and non-diabetic women were of similar age, body size and per cent fat mass. Plasma triglyceride levels and ASP were significantly higher in diabetic women compared with non-diabetic women (P<0.05) as were glucose, HbA1c and fructosamine. However, plasma C3 and hsCRP were not significantly different.
Table 1. Pre-operative characteristics of BPD-DS surgery women.
| Variables | Non-diabetic | Diabetic | |
|---|---|---|---|
| Demographics and treatment | 32 | 22 | P |
| Age (years) | 38.3±10.3 | 42.0±10.6 | NS |
| Diabetic medication (%) | 0/32 (0%) | 20/22 (91%) | <0.001 |
| Lipid medication | 4/32 (13%) | 18/22 (82%) | <0.001 |
| Body composition | |||
| BMI (kg m−2) | 48.6±7.4 | 49.8±7.9 | NS |
| Fat mass (kg) | 66.9±13.5 | 66.2±14.8 | NS |
| Lean mass (kg) | 59.3±8.1 | 61.0±9.5 | NS |
| Fat percentage (%) | 52.8±2.6 | 51.7±3.6 | NS |
| Biochemical measures | |||
| ASP (nmol l−1) | 30.0±15.7 | 38.4±21.0 | <0.05 |
| C3 (mg dl−1) | 1.99±0.34 | 2.09±0.4 | NS |
| hsCRP (mg dl−1) | 14.1±24.2 | 10.8±8.1 | NS |
| Glucose (mmol dl−1) | 5.76±1.67 | 8.28±3.28 | <0.001 |
| HbA1c (%) | 5.62±0.88 | 6.81±1.03 | <0.001 |
| Insulin (pmol l−1) | 149±71 | 193±119 | |
| Fructosamine (μmol l−1) | 202±33 | 230±47 | <0.01 |
| Lipid parameters | |||
| TG (mmol l−1) | 1.38±0.57 | 1.81±0.82 | <0.05 |
| Cholesterol (mmol l−1) | 4.80±0.68 | 4.53±1.04 | NS |
| HDL-Chol (mmol l−1) | 1.40±0.30 | 1.26±0.35 | NS |
| LDL-Chol (mmol l−1) | 2.78±0.59 | 2.45±0.77 | NS |
| Apo-B (mmol l−1) | 0.79±0.16 | 0.75±0.20 | NS |
| NEFA (mmol l−1) | 0.55±0.12 | 0.53±0.17 | NS |
Abbreviations: ASP acylation stimulating protein; BMI, body mass index; Chol, cholesterol; hsCRP, high-sensitivity C-reactive protein; NEFA, non-esterified fatty acid; TG, triglyceride; The values are presented as mean±s.d. and significant differences analyzed by Student's t-test for non-diabetic women vs diabetic women where pNS indicates not significant. For diabetic and lipid lowering medication, results were analyzed by χ2.
Changes in BMI and body composition following BPD-DS surgery
Postoperatively, women were followed up at 24 h (1 day), 5 days, 6 months and 12 months. Acutely (5 days), there was no significant change in body composition (Table 2), but in both non-diabetic and diabetic women, respectively, at 6 months, there was a 26 and 25% decrease in BMI, reflecting a decrease in both fat mass (−39 and −40%) and lean mass (−12 and −10%), with an overall decrease in per cent body fat (Table 3). This was also true at 12 months, with further decreases in fat mass (−58 and −55%), although the patients still remained within the obese range based on BMI.
Table 2. Early changes in plasma values in non-diabetic and diabetic patients following BPD-DS surgery.
| Variables |
Non diabetic women n=32 |
Diabetic women n=22 |
||||||
|---|---|---|---|---|---|---|---|---|
| Pre-operative | 24 h | 5 days | P-value | Pre-operative | 24 h | 5 days | P-value | |
| BMI (kg m−2) | 48.6±7.4 | 48.6±7.4 | NS | 49.8±8 | 49.8±8.4 | NS | ||
| Fat mass (kg) | 66.9±13.5 | 66±14.9 | NS | 66.2±14.9 | 62.7±13.9 | NS | ||
| Lean mass (kg) | 59.3±8 | 60±7.9 | NS | 61±9.5 | 64.1±11.6* | <0.05 | ||
| Fat percentage (%) | 52.8±2.6 | 52±3.8 | NS | 51.7±3.6 | 49.3±3.9* | <0.05 | ||
| TG (mmol l−1) | 1.38±0.57 | 1.16±0.31* | 1.48±0.33 | <0.05 | 1.81±0.82 | 1.64±0.99 | 1.86±0.6 | <0.05 |
| Apo-B (mmol l−1) | 0.79±0.16 | 0.75±0.2 | ||||||
| NEFA (mmol l−1) | 0.55±0.12 | 0.67±0.3* | 0.65±0.23 | <0.05 | 0.53±0.17 | 0.56±0.18 | 0.63±0.18 | NS |
| Chol (mmol l−1) | 4.8±0.68 | 3.34±0.62* | 3.73±0.75* | <0.001 | 4.53±1.04 | 3.1±0.71* | 3.66±0.72* | <0.001 |
| HDL-Chol (mmol l−1) | 1.4±0.3 | 1.09±0.23* | 0.79±0.2* | <0.001 | 1.26±0.35 | 0.94±0.26* | 0.72±0.18* | <0.001 |
| LDL-Chol (mmol l−1) | 2.78±0.59 | 1.72±0.5* | 2.27±0.64* | <0.001 | 2.45±0.77 | 1.39±0.56* | 2.1±0.61* | <0.001 |
| Fructosamine (umol l−1) | 202±5.0 | 160±3.1*** | 165±3.3*** | <0.001 | 230±9.9 | 173±6.1*** | 181±7.1*** | <0.001 |
| hsCRP (g l−1) | 10.1±1.5 | 133±8.9*** | 68.4±6.9*** | <0.001 | 10.8±1.7 | 148±14.7*** | 86.5 ±16.4*** | <0.001 |
Abbreviations; BMI, body mass index; Chol, cholesterol; hsCRP, high sensitivity C-reactive protein; NEFA, non-esterified fatty acid; TG, triglyceride. The values are presented as mean±s.d. and significant differences were analyzed by repeated measures ANOVA followed by Holm–Sidak post-hoc test (*P<0.05) vs preoperative state.
Table 3. Long-term changes in plasma values in non-diabetic and diabetic patients following BPD-DS surgery.
| Variables |
Non-diabetic women n=32 |
Diabetic women n=22 |
||||||
|---|---|---|---|---|---|---|---|---|
| Pre-operative | 6 months | 12 months | P-value | Pre-operative | 6 months | 12 months | P-value | |
| BMI (kg m−2) | 48.6±7.4 | 35.8±6.2* | 29.5±6.2* | <0.001 | 49.8±8 | 37.2±6.6* | 32±5.6* | <0.001 |
| Fat mass (kg) | 66.9±13.5 | 40.5±11.3* | 28.1±11.1* | <0.001 | 66.2±14.9 | 39.6±11.1* | 29.9±9.7* | <0.001 |
| Lean mass (kg) | 59.3±8 | 52.4±7.2* | 50.3±6* | <0.001 | 61±9.5 | 55.1±9.3* | 50.9±9.2* | <0.001 |
| Fat percentage (%) | 52.8±2.6 | 43±5.3* | 34.8±7.4* | <0.001 | 51.7±3.6 | 41.4±5.9* | 36.5±7.1* | <0.001 |
| TG (mmol l−1) | 1.38±0.57 | 1.17±0.35* | 0.98±0.4* | <0.001 | 1.81±0.82 | 1.6±0.81 | 1.34±0.34* | <0.05 |
| Apo-B (mmol l−1) | 0.79±0.16 | 0.57±0.1* | 0.48±0.09* | <0.001 | 0.75±0.2 | 0.67±0.31 | 0.61±0.17 | NS |
| NEFA (mmol l−1) | 0.55±0.12 | 0.51±0.17 | 0.52±0.17 | NS | 0.53±0.17 | 0.64±0.24 | 0.58±0.24 | NS |
| Chol (mmol l−1) | 4.8±0.68 | 3.26±0.59* | 3.24±0.55* | <0.001 | 4.53±1.04 | 3.58±0.97* | 3.66±0.72* | <0.001 |
| HDL-Chol (mmol l−1) | 1.4±0.3 | 1.06±0.28* | 1.26±0.29* | <0.001 | 1.26±0.35 | 0.98±0.29* | 1.09±0.25* | <0.001 |
| LDL-Chol (mmol l−1) | 2.78±0.59 | 1.66±0.4* | 1.54±0.37* | <0.001 | 2.45±0.77 | 1.87±0.81* | 1.96±0.65* | <0.001 |
| Fructosamine (umol l−1) | 202±5.0 | 189±3.6 | 197±2.5 | NS | 230±9.9 | 203±5.1 | 201±4.0 | NS |
| hsCRP (g l−1) | 10.1±1.5 | 4.0±0.9 | 1.6±0.3 | NS | 10.8±1.7 | 6.4±1.6 | 2.5±0.5 | NS |
Abbreviations; BMI, body mass index; Chol, cholesterol; hsCRP, high sensitivity C-reactive protein; NEFA, non-esterified fatty acid; TG, triglyceride. The values are presented as mean±s.d. and significant differences were analyzed by repeated measures ANOVA followed by Holm–Sidak post-hoc test (*P<0.05) vs preoperative state.
Acute and long-term lipid, lipoprotein and liver enzyme responses
Preoperatively, Plasma cholesterol, HDL-cholesterol and LDL-cholesterol decreased at 1 and 5 days in both non-diabetic and diabetic women, at which point none were on lipid-lowering therapy (Table 2). Over the long-term, there was a significant reduction of fasting cholesterol, HDL-cholesterol and LDL-cholesterol in both non-diabetic and diabetic women, while plasma TG and apoB levels decreased only in the non-diabetic women (Table 3).
ASP, complement C3 and hsCRP before and after BPD-DS surgery
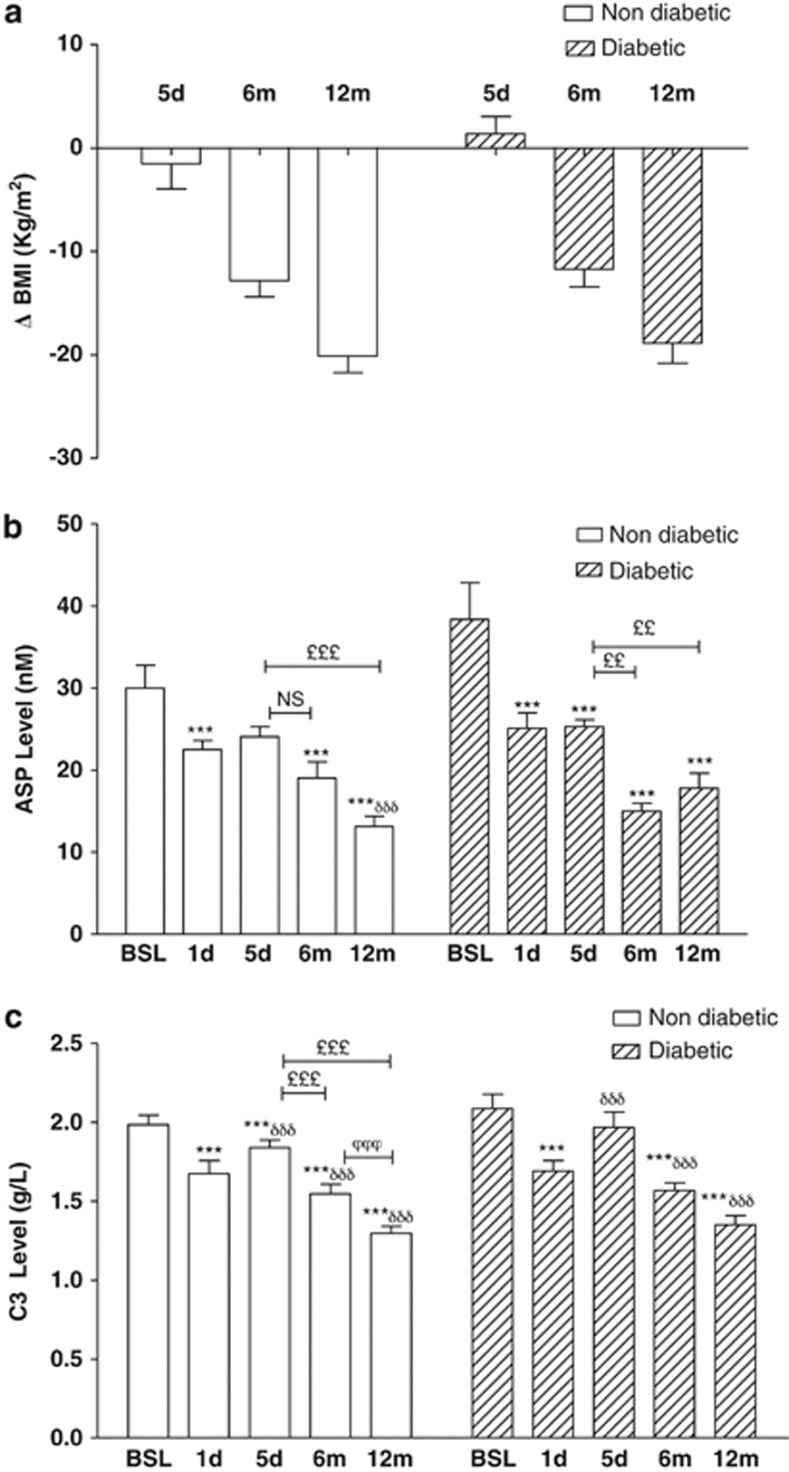
Circulating post-operative ASP levels (Figure 1b) decreased significantly and comparably in both cohorts (−25% in non-diabetics and −35% in diabetics at 1 day, and −20% in non-diabetics and −34% in diabetics at 5 days), with no difference between non-diabetics and diabetics, while BMI did not change significantly over this time period (Figure 1a). Plasma ASP decreased further over the next 12 months (maximal −56%, P<0.001), with concomitant weight loss. More than half the maximal decrease over the 12 months, occurred within the first day, and was maintained thereafter.
Figure 1.
Changes in body weight, plasma ASP and complement C3 after BPD-DS bariatric surgery occur before changes in BMI. Changes in BMI (a), and plasma levels of ASP (b),and complement C3 (c) before and after BPD-DS surgery at 1 and 5 days (d), 6 and 12 months (m) are presented for non-diabetic (n=32) and diabetic (n=22) women. Significance was determined by one-way repeated measures ANOVA following by Holm–Sidak post-hoc test. Values are presented as mean±s.e.m., where *P<0.05, **P<0.01 and ***P<0.001 vs baseline, and δindicates significance vs 1 day, £significance vs 5 days and ϕsignificance vs 6 months.
Complement C3, the precursor to ASP, as well as a central immune protein, was also evaluated (Figure 1c). C3 decreased acutely at 1 day, returning towards initial values at 5 days. By contrast, the inflammatory marker hsCRP was significantly increased at both 1 and 5 days (Table 2). Both C3 and CRP decreased comparably at 6 and 12 months in both non-diabetic and diabetic women.
Rapid improvement in diabetes and insulin resistance
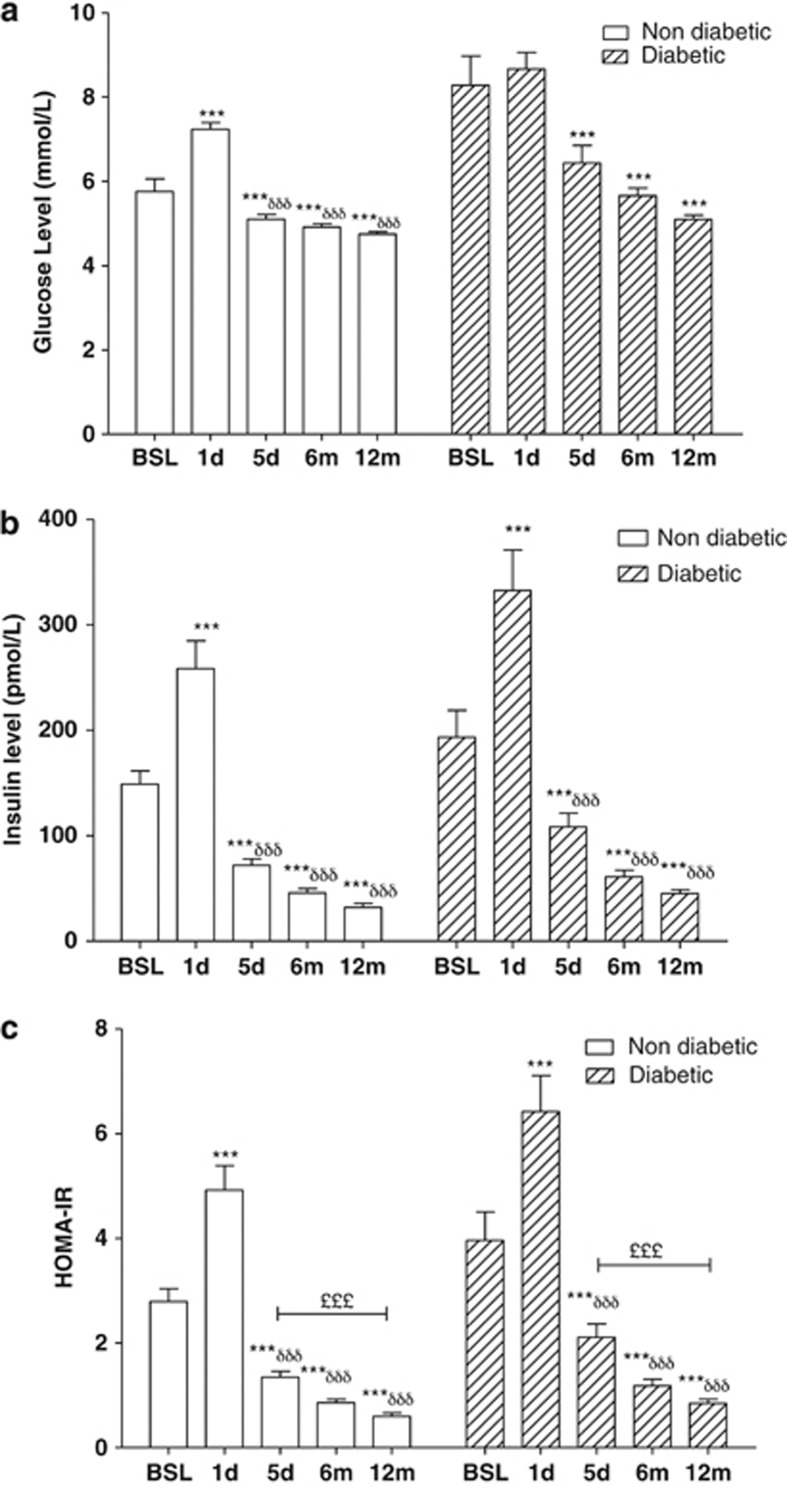
At 1 day after BPD-DS surgery, fasting glucose increased significantly in non-diabetics, with insulin increases in both groups of women (Figure 2a and b). By 5 days after surgery, only 14 of 22 diabetics were still receiving medication (primarily biguanides) and fasting plasma glucose and insulin were within normal ranges (although diabetics were higher than non-diabetics). There was a decrease in HOMA-IR at 5 days (Figure 2c). Fasting fructosamine levels decreased at 1 and 5 days post-bariatric surgery (Table 2).
Figure 2.
Changes in glucose, insulin, and HOMA-IR after BPD-DS bariatric surgery occur before changes in BMI. Results are presented for glucose (a), insulin (b), and HOMA-IR (c) before and at 1 and 5 days (d), 6 and 12 months (m) after BPD-DS in non-diabetic (n=32) and diabetic (n=22) women. Significance was determined by one-way repeated measures ANOVA following by Holm–Sidak post-hoc test. Values are presented as mean±s.e.m., where *P<0.05, **P<0.01 and ***P<0.001 vs baseline, δsignificance vs 1 day, £significance vs 5 days.
With weight loss (6 and 12 months), glucose, insulin and HOMA-IR continued to decrease. At 6 and 12 months, fructosamine was decreased compared with preoperative values. During this time, the number of diabetics receiving treatment decreased (5 patients at 6 months and only 2 still being treated at 12 months).
ASP and complement C3 correlate with diabetes and insulin resistance improvement
The rapid changes in plasma ASP and C3 at 1 day precede the improvement in glucose- treatment insulin homeostasis that was only evident by 5 days. Using HOMA-IR as an index of glucose-insulin homeostasis, we evaluated which early changes in parameters at 1 day (including all those listed in Tables 1 and 2 and Figure 1) could account for the improvement in HOMA-IR. Similar results were obtained when all subjects were analyzed together, or for diabetics and non-diabetics analyzed separately. For all subjects together, as shown in Table 4, a combination of acute change in ASP, HDL-cholesterol, total cholesterol and hsCRP explained 45% of the variation in HOMA-IR at 5 days (P<0.001), and a combination of acute change in ASP, HDL cholesterol and hsCRP explained 35% of the variation in delta-HOMA-IR (change from baseline to 5 days, P<0.001).
Table 4. Correlation of acute changes with improvement in HOMA-IR at 5 days.
| Model 1 | Model 2 | |
|---|---|---|
| Dependent variable | HOMA-IR (5 days) | ΔHOMA-IR (5 days) |
| R2 (P-value) | 0.454 (P<0.001) | 0.351 (P<0.001) |
| Independent variables (24 h) | %Δ-ASP (P=0.001) | %Δ-ASP (P=0.001) |
| Δ-HDL (P<0.001) | HDL (P=0.009) | |
| hsCRP (P=0.020) | hsCRP (P=0.036) | |
| Total cholesterol (P<0.001) |
For dependent variables, HOMA-IR at 5 days and ΔHOMA-IR (5-day value —preop. value) were used. Independent variables included all variables shown in Tables 1 and 2 and Figure 1 at 24 h, or the change in these variables at 24 h (24-h value—preop. value). Forward stepwise regression analysis was used, with the model explaining the greatest variability presented.
Discussion
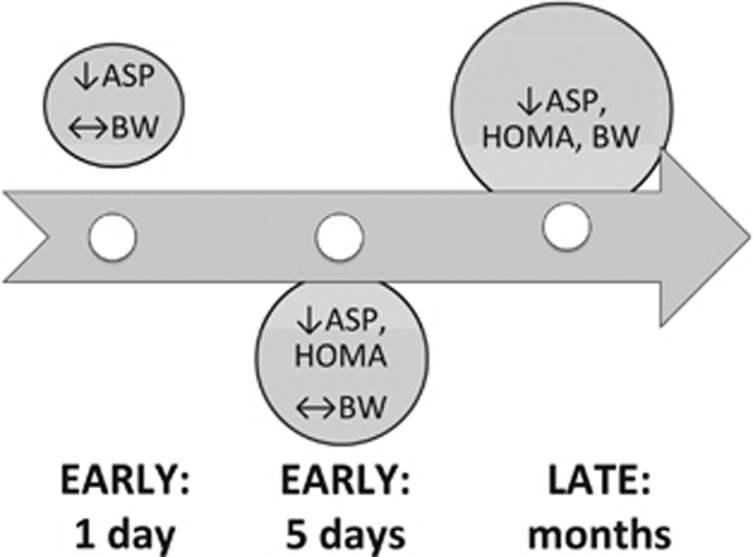
The significant findings of the present investigation are that at a very early time point (1 day) after BPD-DS surgery, when no significant changes in body weight and fat mass were measurable, the non-diabetic and diabetic women experienced marked reductions of fasting ASP and complement C3 levels. By 5 days post-surgery, all patients studied showed a marked decrease in fasting glucose, insulin, HOMA-IR and fructosamine levels, with a consistent maintenance of decreased ASP. At this time, there was as yet no change in body weight. This is shown graphically in Figure 3. By 6 and 12 months, with the large decreases in body weight and fat mass, there were further decreases in plasma ASP, C3, insulin, glucose, hsCRP levels, and various lipid parameters, consistent with other studies,31, 32 and as demonstrated with other adipokines such as leptin.32
Figure 3.
Graphical representation of time frame of changes in ASP, HOMA-IR and bodyweight. Changes in ASP initiate at an early time point (1 day), before changes in HOMA-IR (5 days), and in the absence of any change in body weight, which only occurs in the months following bariatric surgery. ↓decrease, ↔no change, BW body weight.
The strengths and limitations of this study should be noted. In the present study, there was no comparison to a weight loss group induced by non-surgery means, although diet studies rarely achieve weight losses of this magnitude (>50% loss of fat mass). Further, changes within the first 24 h may relate to post-surgery stress (discussed below). On the other hand, these subjects were thoroughly evaluated at multiple time points, allowing intra-individual comparison of changes over 1 year.
It should be noted that the acute decreasess in plasma ASP and its precursor, immune protein complement C3, do not parallel the post-surgery increases in hsCRP that may be inflammatory in nature, and a direct consequence of the surgery. This contrasts with acute changes in leptin, which also increase within the 24 h period, along with increases in other inflammatory factors such as TNFα, sIL2R and IL6.22 At 2–3 days postoperative, leptin levels are not different from preoperative levels, although inflammatory factors are still increased.22 This suggests that the changes in ASP may be more related to metabolic improvement than to an inflammatory response.
Interestingly, the rapid decreases in ASP that are maintained at 5 days, as with the improved insulin sensitivity by 5 days, are independent of body weight changes (which only occur later). It has been suggested that the mechanism of rapid resolution of diabetes may be due to physical (anatomical) changes, hormonal changes (gastro-intestinal or other) or postoperative caloric restriction. Data from this study support the concept that hormonal changes, such as changes in ASP, may contribute to improved insulin sensitivity, but also raise the question of what mediates the changes in plasma ASP.
Plasma ASP increases in obesity and decreases with weight loss, correlating with various indices of body size (such as BMI).19 Further, plasma ASP correlates in a number of studies with indices of insulin-glucose metabolism (such as HOMA-IR) and various lipid parameters.19 However, independent of obesity, plasma ASP is increased in non-obese subjects, including those with diabetes, polycystic ovary syndrome, hypothyroidism, cardiovascular disease and dyslipidemia,6, 27, 25, 19, 26 suggesting other regulatory mechanisms. In particular, dietary intake, especially the dietary lipoproteins chylomicrons, stimulate production of ASP in vitro in adipocytes,33, 34 and in vivo in human studies.35, 36, 37 In the present context, while acute caloric restriction at 1 day (fasting state) may explain a decreased ASP level, at 5 days, fasting TG and NEFA are comparable to preoperative values, suggesting another mechanism. It has been proposed that alterations in the gastrointestinal tract can influence the gut-adipo-insular axis, as demonstrated by changes in adipose tissue metabolic cycling in post-colonic surgery patients.18 Potentially, changes in adipose tissue function post-BPD-DS surgery, could impact on ASP production. Thiazolidinedione treatment in type 2 diabetics, which alters adipose tissue function, decreases ASP production in adipose tissue in humans,37 which might explain why diabetics overall did not have significantly different ASP levels at the pre-operative time point. However, at 5 days, only two subjects were receiving thiazolidinedione treatment. Whether incretins themselves (GLP-1 or GIP) have any effect on ASP is unknown, although GLP-1 affects adipose tissue function.12, 13
Decreased plasma ASP suggests a change in subsequent ASP function. Increased insulin and glucose are indicative of insulin resistance, while a decrease indicates improved insulin function. By analogy, an increased plasma ASP may suggest compensation, indicative of an ‘ASP resistant' state.28 ASP resistance is demonstrated in vitro by reduced specific binding and response to ASP in cells from subjects with high ASP levels.38 Hypothetically, a decrease in plasma ASP could be reflective of increased ASP sensitivity, although this remains to be demonstrated experimentally. ASP has effects on TG storage and glucose transport in adipocytes, effects that are both additive and independent to those of insulin.19 ASP has been shown to stimulate insulin secretion.39 The rapid decreases in plasma ASP may contribute to the decreased insulin, having a supportive role, as with the incretins.
In conclusion, acute down regulation of ASP and C3 levels are associated with early improvement of insulin resistance and diabetes after BPD-DS surgery evidenced by a normalization of both glucose and insulin levels. Whether the increased ASP levels contribute to the insulin resistance, or are increased to compensate for the insulin resistance remains to be determined.
Acknowledgments
This work was supported in part by following grants: Quebec Heart and Lung Institute Foundation (PP), Canadian Institute for Health Research (KC). PP is a senior clinical researcher of the Fonds de recherche en santé du Québec (FRSQ). Katherine Cianflone holds a Canada Research Chair in Adipose Tissue. MNM is supported by a doctoral scholarship from Canadian Institutes for Health Research (CIHR) and JM is a recipient of a graduate scholarship from the CIHR Training program in Obesity/Healthy Body Weight Research. We appreciate the graphics and text assistance of Mélanie Cianflone in preparation of the manuscript.
References
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2005;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- Poirier P, Cornier MA, Mazzone T, Stiles S, Cummings S, Klein S, et al. Bariatric surgery and cardiovascular risk factors: a scientific statement from the American Heart Association. Circulation. 2011;123:1683–1701. doi: 10.1161/CIR.0b013e3182149099. [DOI] [PubMed] [Google Scholar]
- Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. doi: 10.1097/00000658-199509000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandagopal R, Brown RJ, Rother KI. Resolution of type 2 diabetes following bariatric surgery: implications for adults and adolescents. Diabetes Technol Ther. 2010;12:671–677. doi: 10.1089/dia.2010.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Schauer P. Bariatric surgery and the gut hormone response. Nutr Clin Pract. 2010;25:175–182. doi: 10.1177/0884533610361739. [DOI] [PubMed] [Google Scholar]
- Yang J, Li C, Liu H, Gu H, Chen P, Liu B. Effects of subtotal gastrectomy and Roux-en-Y gastrojejunostomy on the clinical outcome of type 2 diabetes mellitus. J Surg Res. 2010;164:e67–e71. doi: 10.1016/j.jss.2010.07.004. [DOI] [PubMed] [Google Scholar]
- DePaula AL, Stival AR, DePaula CC, Halpern A, Vêncio S. Impact on dyslipidemia of the laparoscopic ileal interposition associated to sleeve gastrectomy in type 2 diabetic patients. J Gastrointest Surg. 2010;14:1319–1325. doi: 10.1007/s11605-010-1252-5. [DOI] [PubMed] [Google Scholar]
- Brandt ML, Harmon CM, Helmrath MA, Inge TH, McKay SV, Michalsky MP. Morbid obesity in pediatric diabetes mellitus: surgical options and outcomes. Rev Endocrinol. 2010;6:637–645. doi: 10.1038/nrendo.2010.167. [DOI] [PubMed] [Google Scholar]
- Lifante JC, Inabnet WB. Early improvement in Type 2 diabetes in obese patients following gastric bypass and bilio-pancreatic diversion: the role of the entero-insular axis. J Chir (Paris) 2008;145:549–555. doi: 10.1016/s0021-7697(08)74685-4. [DOI] [PubMed] [Google Scholar]
- Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RS, Kini S. GIP and bariatric surgery. Obes Surg. 2011;21:244–252. doi: 10.1007/s11695-010-0305-x. [DOI] [PubMed] [Google Scholar]
- Beck B. Gastric inhibitory polypeptide: a gut hormone with anabolic functions. J Mol Endocrinol. 1989;2:169–174. doi: 10.1677/jme.0.0020169. [DOI] [PubMed] [Google Scholar]
- Sancho V, Trigo MV, Martín-Duce A, Gonz Lez N, Acitores A, Arnés L, et al. Effect of GLP-1 on D-glucose transport, lipolysis and lipogenesis in adipocytes of obese subjects. Int J Mol Med. 2006;17:1133–1137. [PubMed] [Google Scholar]
- Guidone C, Manco M, Valera-Mora E, Iaconelli A, Gniuli D, Mari A, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55:2025–2031. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- Kashyap SR, Daud S, Kelly KR, Gastaldelli A, Win H, Brethauer S, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 2010;34:462–471. doi: 10.1038/ijo.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33:1438–1442. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco D, de Luis DA, Romero A, González Sagrado M, Conde R, Izaola O, et al. The effects of duodenal-jejunal exclusion on hormonal regulation of glucose metabolism in Goto-Kakizaki rats. Am J Surg. 2007;194:221–224. doi: 10.1016/j.amjsurg.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Robertson MD, Bickerton AS, Dennis AL, Vidal H, Jewell DP, Frayn KN. Enhanced metabolic cycling in subjects after colonic resection for ulcerative colitis. J Clin Endocrinol Metab. 2005;90:2747–2754. doi: 10.1210/jc.2004-2108. [DOI] [PubMed] [Google Scholar]
- Cianflone K, Xia Z, Chen LY. Critical review of acylation-stimulating protein physiology in humans and rodents. Biochim Biophys Acta. 2003;1609:127–143. doi: 10.1016/s0005-2736(02)00686-7. [DOI] [PubMed] [Google Scholar]
- Lago F, Gómez R, Gómez-Reino JJ, Dieguez C, Gualillo O. Adipokines as novel modulators of lipid metabolism. Trends Biochem Sci. 2009;34:500–510. doi: 10.1016/j.tibs.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Athyros VG, Tziomalos K, Karagiannis A, Anagnostis P, Mikhailidis DP. Should adipokines be considered in the choice of the treatment of obesity-related health problems. Curr Drug Targets. 2010;11:122–135. doi: 10.2174/138945010790030992. [DOI] [PubMed] [Google Scholar]
- Maruna P, Gürlich R, Fried M, Frasko R, Chachkhiani I, Haluzik M. Leptin as an acute phase reactant after non-adjustable laparoscopic gastric banding. Obes Surg. 2001;11:609–614. doi: 10.1381/09608920160556814. [DOI] [PubMed] [Google Scholar]
- Couce ME, Cottam D, Esplen J, Schauer P, Burguera B. Is ghrelin the culprit for weight loss after gastric bypass surgery? A negative answer. Obes Surg. 2006;16:870–878. doi: 10.1381/096089206777822151. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lu HL, Zhang J, Yu HY, Wang HW, Zhang MX, et al. Relationships among acylation stimulating protein, adiponectin and complement C3 in lean vs obese type 2 diabetes. Int J Obes (Lond) 2006;30:439–446. doi: 10.1038/sj.ijo.0803173. [DOI] [PubMed] [Google Scholar]
- Yu H, Yang Y, Zhang M, Lu H, Zhang J, Wang H, et al. Thyroid status influence on adiponectin, acylation stimulating protein (ASP) and complement C3 in hyperthyroid and hypothyroid subjects. Nutr Metab (Lond) 2006;3:13. doi: 10.1186/1743-7075-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglialunga S, Julien P, Tahiri Y, Cadelis F, Bergeron J, Gaudet D, et al. Lipoprotein lipase deficiency is associated with elevated acylation stimulating protein plasma levels. J Lipid Res. 2009;50:1109–1119. doi: 10.1194/jlr.M800430-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhang J, Wen Y, Wang H, Zhang M, Cianflone K. Increased acylation-stimulating protein, C-reactive protein, and lipid levels in young women with polycystic ovary syndrome. Fertil Steril. 2009;91:213–219. doi: 10.1016/j.fertnstert.2007.11.031. [DOI] [PubMed] [Google Scholar]
- St-Pierre DH, Cianflone K, Smith J, Coderre L, Karelis AD, Imbeault P, et al. Change in plasma acylation stimulating protein during euglycaemic-hyperinsulinaemic clamp in overweight and obese postmenopausal women: a MONET study. Clin Endocrinol (Oxf) 2009;70:539–546. doi: 10.1111/j.1365-2265.2008.03353.x. [DOI] [PubMed] [Google Scholar]
- Maslowska M, Vu H, Phelis S, Sniderman AD, Rhode BM, Blank D, et al. Plasma acylation stimulating protein, adipsin and lipids in non-obese and obese populations. Eur J Clin Invest. 1999;29:679–686. doi: 10.1046/j.1365-2362.1999.00514.x. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Faraj M, Havel PJ, Phélis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594–1602. doi: 10.1210/jc.2002-021309. [DOI] [PubMed] [Google Scholar]
- Swarbrick MM, Stanhope KL, Austrheim-Smith IT, Van Loan MD, Ali MR,, Wolfe BM, et al. Longitudinal changes in pancreatic and adipocyte hormones following Roux-en-Y gastric bypass surgery. Diabetologia. 2008;51:1901–1911. doi: 10.1007/s00125-008-1118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowska M, Scantlebury T, Germinario R, Cianflone K. Acute in vitro production of acylation stimulating protein in differentiated human adipocytes. J Lipid Res. 1997;38:1–11. [PubMed] [Google Scholar]
- Scantlebury T, Maslowska M, Cianflone K. Chylomicron-specific enhancement of acylation stimulating protein and precursor protein C3 production in differentiated human adipocytes. J Biol Chem. 1998;273:20903–20909. doi: 10.1074/jbc.273.33.20903. [DOI] [PubMed] [Google Scholar]
- Saleh J, Summers LK, Cianflone K, Fielding BA, Sniderman AD, Frayn KN. Coordinated release of acylation stimulating protein (ASP) and triacylglycerol clearance by human adipose tissue in vivo in the postprandial period. J Lipid Res. 1998;39:884–891. [PubMed] [Google Scholar]
- Kalant D, Phélis S, Fielding BA, Frayn KN, Cianflone K, Sniderman AD. Increased postprandial fatty acid trapping in subcutaneous adipose tissue in obese women. J Lipid Res. 2000;41:1963–1968. [PubMed] [Google Scholar]
- Tahiri Y, Karpe F, Tan GD, Cianflone K. Rosiglitazone decreases postprandial production of acylation stimulating protein in type 2 diabetics. Nutr Metab (Lond) 2007;4:11. doi: 10.1186/1743-7075-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XJ, Cianflone K, Genest J, Sniderman AD. Plasma acylation stimulating protein (ASP) as a predictor of impaired cellular biological response to ASP in patients with hyperapoB. Eur J Clin Invest. 1998;28:730–739. doi: 10.1046/j.1365-2362.1998.00359.x. [DOI] [PubMed] [Google Scholar]
- Ahrén B, Havel PJ, Pacini G, Cianflone K. Acylation stimulating protein stimulates insulin secretion. Int J Obes Relat Metab Disord. 2003;27:1037–1043. doi: 10.1038/sj.ijo.0802369. [DOI] [PubMed] [Google Scholar]