Abstract
Background:
Obesity is a major public health epidemic and is associated with increased risk of heart failure and mortality. We evaluated the impact of body mass index (BMI) on the prevalence of diastolic dysfunction (DD).
Methods:
We reviewed clinical records and echocardiogram of patients with baseline echocardiogram between 1996 and 2005 that showed normal left ventricular ejection fraction (LVEF). Diastolic function was labeled as normal, stage 1, stage 2 or stage 3/4 dysfunction. Patients were categorized as normal weight (BMI <25 kg m−2), overweight (25–29.9 kg m−2), obese (30–39.9 kg m−2) and morbidly obese (⩾40 kg m−2). Multivariable ordinal and ordinary logistic regression were performed to identify factors associated with DD, and evaluate the independent relationship of BMI with DD.
Results:
The cohort included 21 666 patients (mean (s.d.) age, 57.1 (15.1); 55.5% female). There were 7352 (33.9%) overweight, 5995 (27.6%) obese and 1616 (7.4%) morbidly obese patients. Abnormal diastolic function was present in 13 414 (61.9%) patients, with stage 1 being the most common. As BMI increased, the prevalence of normal diastolic function decreased (P<0.0001). Furthermore, there were 1733 patients with age <35 years; 460 (26.5%) and 407 (23.5%) were overweight and obese, respectively, and had higher prevalence of DD (P<0.001). Using multivariable logistic regression, BMI remained significant in both ordinal (all stages of diastolic function) and binary (normal versus abnormal). Also, obesity was associated with increased odds of DD in all patients and those aged <35 years.
Conclusions:
In patients with normal LVEF, higher BMI was independently associated with worsening DD.
Keywords: diastolic function, body mass index, outpatients, normal ejection function
Introduction
Obesity is a major public health epidemic and has been increasing at alarming rates worldwide and in all age groups.1, 2 It is associated with increasing risk of heart failure,3 and cardiac death.4 Excess body fat has been associated with inflammation,5 increase preload and afterload,6 insulin resistance,7 hypertension and left ventricular (LV) hypertrophy.8, 9 The precise mechanisms by which body mass index (BMI) affects cardiac function, nevertheless, are not well understood. Diastolic dysfunction (DD) and its progression are independent predictors of incident heart failure.10 A recent study showed that overweight and obesity were independent predictors of DD after adjustment for potential confounders.11 However, the study was performed in an elderly cohort of patients (mean age 72 years), the majority of which were hypertensive, and limited to mostly Hispanics from the North Manhattan Study. There are limited data, however, and with small-sample-size studies assessing the impact of BMI on DD in young normotensive patients.
We sought to evaluate the association between BMI and DD in a large cohort of patients with normal left ventricular ejection fraction (LVEF) undergoing outpatient echocardiograms.
Materials and methods
Study design
The study cohort consisted of patients who underwent an outpatient echocardiogram test with normal LV systolic function (LVEF ⩾55%), at the Cleveland Clinic or its satellite facilities between 1 January 1996 and 31 December 2005. For patients with more than one echocardiogram, only the first one was included and analyzed. These data were derived from a previous study of diastolic function and the original data set was used.12 Patients were excluded if diastolic function could not be assessed or was not reported, if severe mitral valve disease or prior mitral valve surgery were present, or if BMI could not be calculated. Inability to assess diastolic function was common in certain clinical scenarios such as in patients with tachycardia, atrial fibrillation, poor acoustic window and in limited echocardiogram performed on an urgent basis.
Using the above algorithm, 21 666 patients were included from a total of 65 696 echocardiograms performed.
Echocardiographic methods
Patients were imaged in the left lateral decubitus position with commercially available systems, and images were acquired per standard protocol. Diastolic function was assessed in our institution in a standardized method, and in accordance to the published and relevant guidelines by using a combination of echocardiographic variables including transmitral inflow pattern, pulmonary venous flow pattern, and beginning in the late 1990s and almost uniformly after 2001, mitral annular velocities assessed by tissue Doppler imaging.13 In patients with atrial fibrillation, if diastolic function was assessed, it was based on deceleration time of mitral E wave velocity14 and tissue Doppler imaging if available (that is, peak early mitral inflow velocity/diastolic early tissue velocity (E/e′)).15
Diastolic function was then labeled as normal or abnormal (DD). DD was then categorized as mild (stage 1, impaired relaxation), moderate (stage 2, pseudo-normal) and severe (stage 3/4, restrictive).16 Systolic function was assessed by quantitative and/or visual evaluation of the LVEF in accordance with published guidelines.17 LV mass and LV mass index were calculated based on the formula: LV mass (g): 0.8 × (1.04 ([LVIDD+PWTD+IVS]3−[LVIDD]3))+0.6g; LV mass index=LV mass/height2.7 (g m−2.7).18, 19
All echocardiograms were interpreted by experienced and board-certified readers who over-read and approved measurements made by the sonographers. The interobserver agreement of reproducibility and DD classification extrapolated from our ongoing quality assurance effort was on average 83%, and intraobserver agreement was 94%.
Clinical data
Clinical data were obtained from review of the electronic medical records from a period starting 6 months before the first echocardiographic testing and ending 6 months afterward. The indications for echocardiograms were previously published.12 The clinical diagnosis of conditions, including coronary artery disease, peripheral vascular disease, diabetes mellitus, hypertension, atrial fibrillation, congestive heart failure, hyperlipidemia, chronic obstructive pulmonary disease and chronic renal insufficiency, was established by reviewing records documented by a health-care provider in an electronic medical record system (EpicCare; Epic Systems Corporation, Madison, WI, USA) that was linked to relevant International Classification of Diseases, Ninth Revision (ICD-9) codes, as previously described.12 Patients' blood pressure, height and weight were obtained and recorded at the time the echocardiograms were performed.
Hypertension was defined as systolic blood pressure ⩾140 mm Hg or diastolic blood pressure ⩾90 mm Hg at the time of the echocardiogram, or self-reported history of hypertension, or the use of antihypertensive medications. Diabetes mellitus was defined as fasting glucose ⩾126 mg dl−1, or self-reported history or the use of diabetic medications. Hyperlipidemia was defined as lipid panel (low-density lipoprotein, high-density lipoprotein and non-high-density lipoprotein) greater than those recommended by ATP III guidelines, self-reported history or the use of antihyperlipidemic medications. Chronic renal insufficiency was defined as glomerular filtration rate <60 ml min−1. BMI was calculated using standard formula (weight (kg) divided by height-squared (m2)), and was classified as <25 kg m−2 (normal weight), 25–29.9 kg m−2 (overweight), 30–39.9 kg m−2 (obese) and ⩾40 kg m−2 (morbidly obese).20 The study was approved by the Cleveland Clinic Institutional Review Board with waiver of consent.
Statistical analysis
Continuous data were expressed as mean±s.d. or median (interquartile range). Kruskal–Wallis tests were used to analyze group differences for the continuous data. This non-parametric test accommodated for highly skewed variables, such as BMI. Categorical data were displayed as frequencies and percentages, and comparisons were made using χ2 tests or Fisher exact tests as appropriate.
Multivariable ordinal and ordinary logistic regression were performed to identify factors associated with DD, and to evaluate the independent relationship of BMI with DD. Variables that were considered in this analysis were age, gender, race, body size (height, weight, BMI and body surface area), atrial fibrillation, diabetes mellitus, hypertension, systolic and diastolic blood pressures, LV mass index, left atrial (LA) size, hyperlipidemia, chronic obstructive pulmonary disease, chronic renal insufficiency, congestive heart failure, serum creatinine, hemoglobin and date of the echocardiogram performed. Utilizing the final model, nomograms of the association between DD and BMI were plotted allowing the adjustment of other factors. Owing to the low numbers in the moderate and severe DD groups, the final logistic model combined these groups and the model predicted normal versus abnormal diastolic function. The model was also rerun using categorical BMI groups (that is, normal, overweight, obese and morbidly obese) for all patients and the subgroup of patients aged <35 years old (a group of patients generally not expected to have DD). Given the high number of patients with stage 1 DD that could overwhelm the logistic model, we preformed another binary logistic regression looking at the associations with DD stage 2 or greater compared with the remainder. Finally, because of possible attenuation effect of BMI, we categorized obesity class I (BMI 30–34.99 kg m−2), class II (BMI 35–39.99 kg m−2) and class III (BMI⩾40 kg m−2), and looked at the association with DD. Compared with normal weights, odds ratio for predicting abnormal diastolic function for the categorical BMI groups were expressed with 95% confidence interval. The discriminatory ability of the test was assessed using C-statistics.
All statistical tests were two sided. A P-value <0.05 was set a priori and considered statistically significant. All statistical analyses were performed using SAS statistical software, version 9 (SAS Institute Inc., Cary, NC, USA).
Results
Clinical data
The study population consisted of 21 666 patients (mean (s.d.) age, 57.1 (15.1); 55.5% female; 82.9% Caucasians; 13% with diabetes mellitus, mean (s.d.) BMI 29.1 (7.5) 25 kg m−2). There were 6703 (30.9%) normal weight (BMI <25 kg m−2), 7352 (33.9%) overweight (BMI 25–29.9 kg m−2), 5995 (27.6%) obese (BMI 30–39.9 kg m−2) and 1616 (7.4%) morbidly obese (BMI ⩾40 kg m−2) patients. The clinical, demographic and echocardiographic data of the study cohort stratified by BMI are described in Table 1.
Table 1. Baseline patient characteristics stratified by BMI.
| Variable | Normal weight BMI <25 kg m−2 (n=6703) | Overweight BMI 25–29.9 kg m−2 (n=7352) | Obese BMI 30–39.9 kg m−2 (n=5995) | Morbidly obese BMI ⩾40 kg m−2 (n=1616) | P |
|---|---|---|---|---|---|
| Demographic/clinical dataa | |||||
| Age, mean (s.d.), years | 56.1 (17.3) | 58.5 (14.6) | 57.1 (13.2) | 54.6 (12.7) | <0.0001 |
| Male sex | 2373 (35.4%) | 3931 (53.5%) | 2825 (47.1%) | 512 (31.7%) | <0.0001 |
| Caucasians | 4343 (82.9%) | 4774 (83.0%) | 3858 (82.9%) | 1009 (82.1%) | 0.89 |
| Coronary artery disease | 35 (0.5%) | 37 (0.5) | 30 (0.5%) | 8 (0.5%) | 0.99 |
| Atrial fibrillation | 356 (5.3%) | 425 (5.8%) | 367 (6.1%) | 88 (5.4%) | 0.25 |
| Diabetes mellitus | 356 (5.3%) | 777 (10.6%) | 1154 (19.3%) | 441 (27.3%) | <0.0001 |
| Hypertension | 729 (10.9%) | 1086 (14.8%) | 1102 (18.4%) | 319 (19.8%) | <0.0001 |
| Hyperlipidemia | 1806 (27.0%) | 3176 (43.2%) | 2803 (46.8%) | 685 (42.4%) | <0.0001 |
| Peripheral vascular disease | 93 (0.9%) | 89 (1.2%) | 65 (1.1%) | 15 (0.9%) | 0.43 |
| Congestive heart failure | 192 (2.9%) | 206 (2.8%) | 275 (4.6%) | 91 (5.6%) | <0.0001 |
| COPD | 249 (3.7%) | 246 (3.3%) | 176 (2.9%) | 60 (3.7%) | 0.090 |
| Chronic renal insufficiency | 101 (1.5%) | 123 (1.7%) | 114 (1.9%) | 29 (1.8%) | 0.38 |
| Creatinine, mean (s.d.), mg dl−1 | 0.96 (0.39) | 1.01 (0.37) | 1.01 (0.39) | 0.99 (0.41) | <0.0001 |
| Hemoglobin, mean (s.d.), g dl−1 | 12.1 (2.7) | 12.7 (2.6) | 12.7 (2.6) | 12.3 (2.4) | <0.0001 |
| Systolic BP, mean (s.d.), mm Hg | 136 (22) | 141 (21) | 144 (20) | 147 (20) | <0.0001 |
| Diastolic BP, mean (s.d.), mm Hg | 80 (11) | 83 (10) | 85 (11) | 86 (11) | <0.0001 |
| Echocardiographic datab | |||||
| End-diastolic diameter/height, mean (s.d.), cm m−1 | 2.61 (0.40) | 2.59 (0.40) | 2.61 (0.40) | 2.66 (0.42) | <0.0001 |
| End-systolic diameter/height, mean (s.d.), cm m−1 | 1.60 (0.36) | 1.59 (0.35) | 1.60 (0.35) | 1.63 (0.37) | <0.001 |
| Relative wall thickness, mean (s.d.) | 0.51 (0.13) | 0.51 (0.14) | 0.51 (0.14) | 0.51 (0.13) | 0.70 |
| Left ventricular mass/height2.7, mean (s.d.), kg m−2.7 | 40.9 (14.5) | 41.4 (14.1) | 42.2 (14.6) | 43.9 (14.8) | <0.0001 |
| Left atrium size, mean (s.d.), cm | 3.44 (0.65) | 3.71 (0.61) | 3.88 (0.58) | 3.92 (0.63) | <0.0001 |
Abbreviations: BMI, body mass index; BP, blood pressure; COPD, chronic obstructive pulmonary disease.
P-value comparing means or proportions of baseline characteristics across different BMI ranges.
Could be measured in 16 876 patients for race; 21 653 for coronary artery disease, atrial fibrillation, diabetes mellitus, hypertension, hyperlipidemia, peripheral vascular disease, congestive heart failure, COPD and chronic renal insufficiency; 15 673 for serum creatinine and hemoglobin; 15 795 for systolic blood pressure; and 15 954 for diastolic blood pressure.
Could be measured in 20 672 patients for end-diastolic and end-systolic diameter/height; 19 397 for relative wall thickness; 19 397 for left ventricular mass index; and 20 731 for left atrial size.
BMI and cardiovascular risk factors
Obese and morbidly obese patients were younger, and had higher prevalence of cardiovascular risk factors such as hypertension, diabetes mellitus, hyperlipidemia, congestive heart failure, LV hypertrophy (LV mass index) and elevated blood pressure as compared with those with normal or overweight (Table 1).
BMI and DD
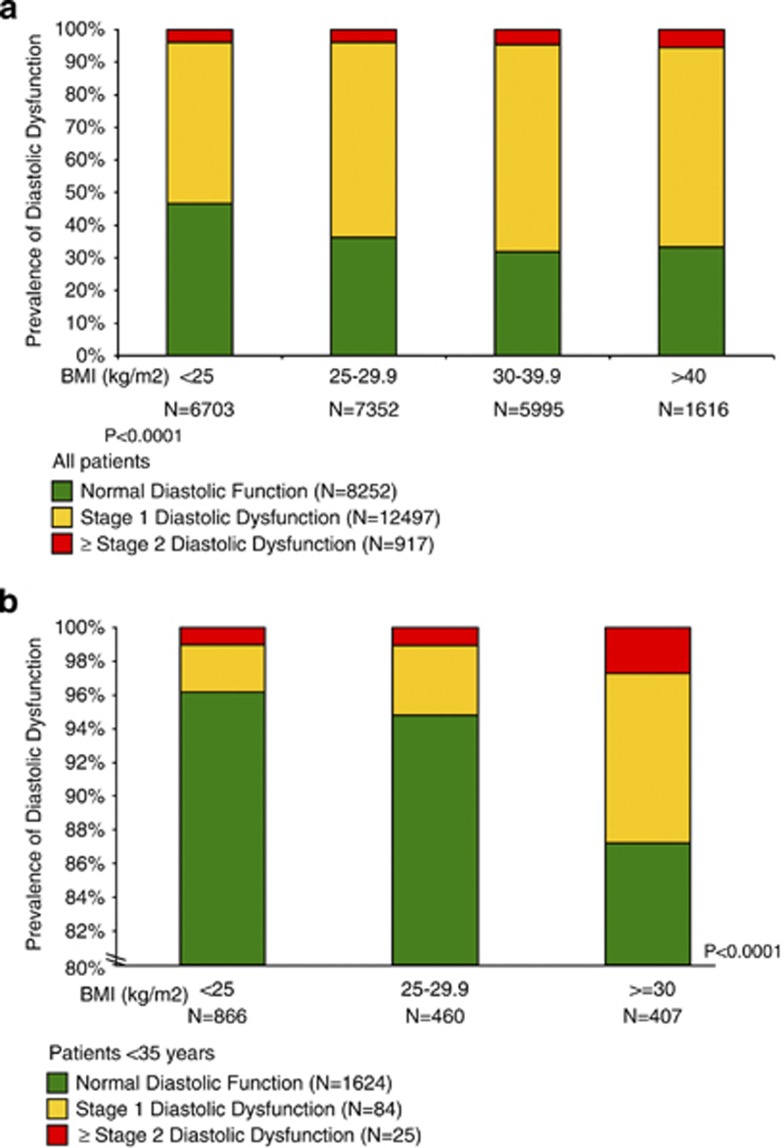
Abnormal diastolic function was prevalent in 13 414 (61.9%) patients with stage 1 being the most common with 12 497 (93.2%), 867 (6.5%) stage 2 DD, 50 (0.4%) stage 3, 4 DD. The BMI increased across diastolic stages, with a median of 26.6 kg m−2 in normal diastolic function and 28.7 kg m−2 in stage 3, 4 (P<0.001)(Table 2). Furthermore, more patients with normal diastolic function had normal weight (37.8%), while those with DD were predominately either overweight (35.1% and 32.6% for stage 1 and 2, respectively) or obese (42% for stage 3, 4) (P<0.0001) (Table 2). On the other hand, as BMI increased the prevalence of normal diastolic function decreased (47% in normal weight patients; 36% in overweight; and 32% in obese and morbidly obese), while the prevalence of DD increased (P<0.0001) (Figure 1). In fact, the prevalence of stage 1 DD increased from 50% in normal weight to 60% in overweight, 63% in obese and 61% in morbidly obese patients (P<0.0001). Similarly, the prevalence of stage ⩾2 DD were 3.9%, 4.0%, 4.5% and 5.5% for normal, overweight, obese and morbidly obese patients, respectively (P<0.0001) (Figure 1a).
Table 2. Diastolic function and BMI.
|
Diastolic function |
||||
|---|---|---|---|---|
| Normal (n=8252) | Stage 1 (n=12 497) | Stage 2 (n=867) | Stage 3, 4 (n=50) | |
| BMI, mean (s.d.), kg m−2 | 28.26 (7.58) | 29.67 (7.43) | 29.84 (7.87) | 29.59 (6.10) |
| BMI, median (25th, 75th), kg m−2 | 26.6 (23.1, 31.0) | 28.3 (24.8, 32.7) | 28.2 (24.5, 33.4) | 28.7 (24.2, 33.6) |
| BMI <25 kg m−2 | 3123 (37.8%) | 3320 (26.6%) | 246 (28.4%) | 14 (28.0%) |
| BMI 25–29.9 kg m−2 | 2674 (32.4%) | 4381 (35.1%) | 283 (32.6%) | 14 (28.0%) |
| BMI 30–39.9 kg m−2 | 1917 (23.2%) | 3806 (30.5%) | 251 (29%) | 21 (42%) |
| BMI ⩾40 kg m−2 | 538 (6.5%) | 990 (7.9%) | 87 (10.0%) | 1 (2.0%) |
Abbreviation: BMI, body mass index.
P<0.0001.
Figure 1.
Prevalence of DD stratified by BMI. Bar histograms illustrating the prevalence of DD with higher BMI in all patients (a) and those <35 years old (b). As BMI increased, the prevalence of normal diastolic function decreased while the prevalence of DD and grade severity increased (P<0.0001 for all patients (a) and those <35 years old (b)).
Normotensive patients without diabetes
There were 16 482 normotensive patients and without diabetes mellitus; 5685 (34.5%) were overweight, 4088 (24.8%) obese and 992 (6.0%) morbidly obese. DD was presented in 9357 (56.8%) patients, and was more prevalent in overweight and obese patients versus those with normal weights (58.8% and 62.6%, respectively, versus 49.5%, P<0.001).
DD in patients <35 years old
Analysis of patients <35 years old included 1733 patients (mean (s.d.) age 27.5 (4.8) years; 2.7% hypertensive; 0% coronary artery disease; 0.5% with heart failure; and 2.8% diabetes mellitus). 866 (50%) had normal weight, 460 (26.5%) were overweight and 407 (23.5%) were obese. Obese patients had significantly higher prevalence of hypertension (5.2% versus 1.3%, P<0.001), diabetes (6.2% versus 1.3%, P<0.001), mean systolic (s.d.) and diastolic (s.d.) blood pressure (133.1 (17.9) versus 120.1 (15.3) mm Hg, and 83.6 (11.1) versus 75.8 (10.4) mm Hg, respectively, P<0.001 for both) and mean LA size (s.d.) (3.6 (0.5) versus 3.1 (0.5) cm, P<0.001) as compared with those with normal weights. 109 (6.3%) had DD. The prevalence of DD increased from 3.8% in normal weight to 5.2% in overweight and 12.8% in obese patients (P<0.001) (Figure 1b).
Multivariable analysis
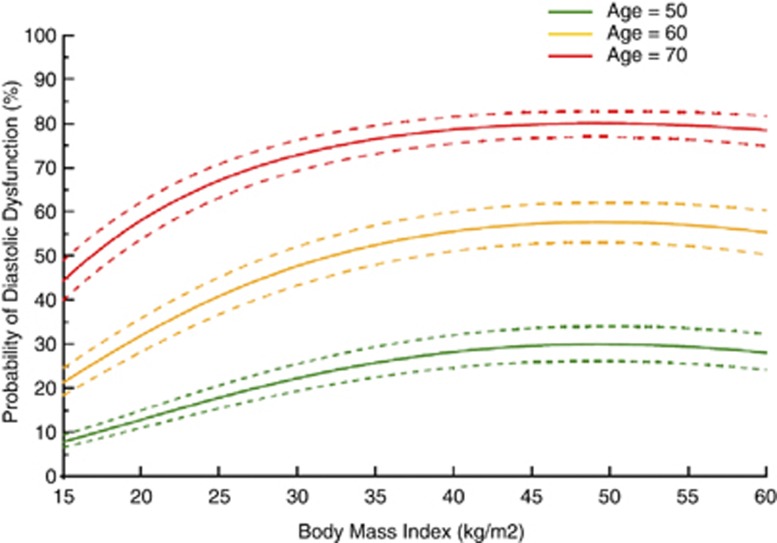
Using multivariable logistic regression to assess for the effect of BMI on diastology and after accounting for the factors mentioned above, BMI remained significant in both ordinal (all stages of diastolic function: normal, stage 1, 2 and 3/4) and binary (normal versus abnormal) with a nonlinear association. The model had good discriminatory power between normal and abnormal groups with C-statistic of 0.86 (Table 3). DD was associated with patient's age, BMI (shaped with terms for both older and younger age, greater and lower BMI), diabetes mellitus, hypertension, chronic obstructive lung disease, creatinine, diastolic blood pressure and atrial fibrillation. LV mass and LA size were not independent predictors and did not influence the strength of the observed association. Using this model, a normogram was produced depicting the probability of DD across values of BMI and stratified based on three hypothetical age groups for illustration purposes (Figure 2). At any given BMI, the probability of DD was higher for older patients. Conversely, for any given age group, the probability of DD increased with higher BMI.
Table 3. Predictors of abnormal diastolic function in all patients using BMI.
| Analysis of maximum likelihood estimates | ||||
|---|---|---|---|---|
|
Parameter |
Estimate |
s.e. |
Wald χ2 |
P-value |
| Age | 0.1499 | 0.0108 | 193 | <0.0001 |
| Age (exp) | −0.5687 | 0.1717 | 11 | 0.0009 |
| BMI (log) | 2.1880 | 0.1946 | 126 | <0.0001 |
| BMI (squared) | −0.7170 | 0.1219 | 34 | <0.0001 |
| AFib | −0.3559 | 0.0779 | 21 | <0.0001 |
| Diabetes | 0.3044 | 0.0586 | 27 | <0.0001 |
| Hypertension | 0.2765 | 0.0542 | 26 | <0.0001 |
| COPD | 0.5478 | 0.1175 | 22 | <0.0001 |
| Creatinine (log) | 0.4907 | 0.0601 | 67 | <0.0001 |
| BP diastolic | 0.0192 | 0.00194 | 98 | <0.0001 |
| Intercept | −14.6015 | 0.6086 | 576 | <0.0001 |
Abbreviations: AFib, atrial fibrillation; BMI, body mass index; BP, blood pressure; COPD, chronic obstructive pulmonary disease.
C=0.86.
Figure 2.
Probability of DD by BMI. Utilizing the final model in Table 3, nomograms with 95% confidence intervals of the association between DD and BMI are plotted and stratified based on three hypothetical age groups. For any given age, the prevalence of DD increased with increasing BMI. For example, the probability of DD for a 50-year-old patient with normal systolic function is ≈15% if he has a normal weight, 20% if overweight, 25% if obese and 28% if morbidly obese.
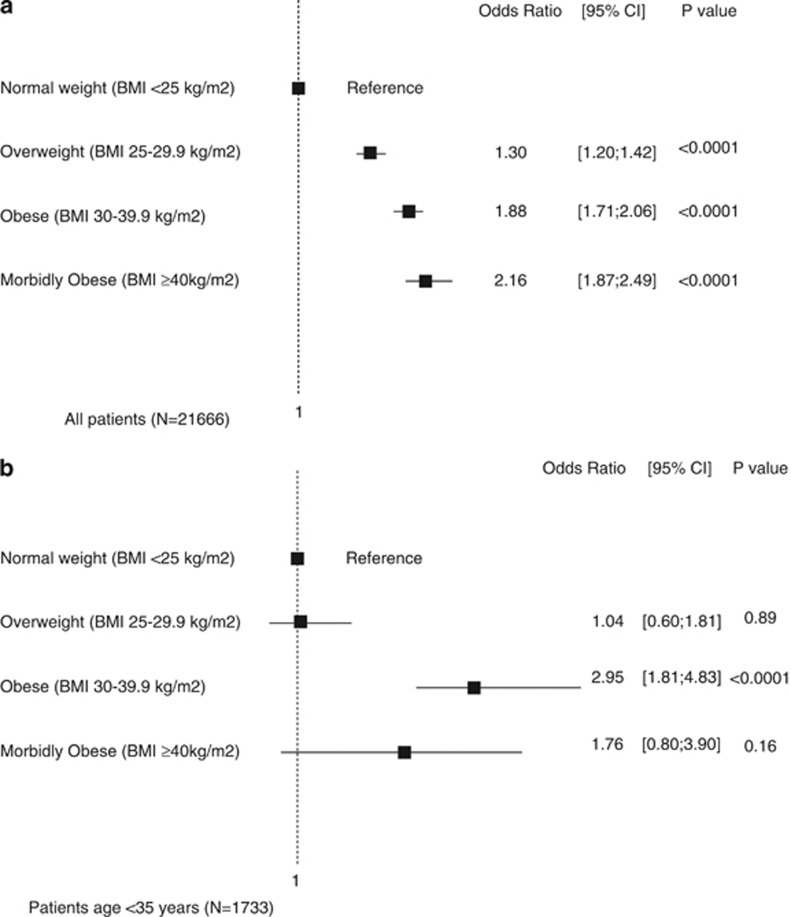
Using BMI as categorical groups (Supplementary Appendix A), being overweight, obese and morbidly obese were associated with increased odds of DD as compared with those with normal weights (odds ratio (95% confidence interval) 1.30 (1.20–1.42), 1.88 (1.71–2.06), 2.16 (1.87–2.49), respectively, P<0.0001 for all) (Figure 3a). Reclassifying obesity into class I, II and III yielded similar results. Also, after rerunning the model and looking at predictors of DD stage ⩾2, obesity (1.39 (1.04–1.86), P=0.026) and morbid obesity (2.07 (1.37–3.14), P=0.001) were independent predictors, as well as class II and class III obesity.
Figure 3.
Odds ratio of having DD. Forest plot illustrating the odds ratios (95% confidence interval) of having DD in all patients (a) and those aged <35 years (b) based on categorical BMI groups. Patients with normal weights were used as reference.
In patient's age <35 years, the odds of abnormal DD were higher for obese (2.95 (1.81–4.83), P<0.0001), morbidly obese (1.76 (0.80–3.90), P=0.16), but not for overweight (1.04 (0.60–1.81), P=0.89) (Supplementary Appendix B, Figure 3b).
Discussion
This is the largest study to our knowledge to assess the impact of BMI on diastolic function, and focused only on patients with normal LVEF. After adjusting for age, hypertension, diabetes, LA size, LV mass index and other covariates, BMI remains an independent and strong predictor of DD with a continuous relationship. Also, being overweight, obese and morbidly obese was associated with increased odds of DD for all patients. In those aged <35 years, obesity was associated with ∼3-fold increase in odds of having DD.
Obesity has become an epidemic.1, 2 In our cohort, less than one-third of all patients and less than half of those younger than 35 years old had normal weights. These numbers reflect a cohort from a decade ago, and are probably worse now. There is also increased prevalence of cardiovascular risk factors such as hypertension, diabetes mellitus and LV hypertrophy with higher BMI (Table 1). In addition, obesity is associated with heart failure,4 cardiovascular complications and mortality3, but so is DD.10, 12 The latter has been proposed as one of the pathophysiological links between obesity and heart failure.11 With increasing body adiposity, changes in cardiac metabolism occur, leading to myocardial fatty infiltration, inflammation and cardiac toxicity, with the end result of DD.5 Indeed, Kuznetsova et al.21 Showed that BMI was associated with DD in 539 patients from the general community in Europe. However, the prevalence of DD was much lower (only 25% had DD), the mean BMI was significantly smaller than our cohort (mean (s.d.), 26 kg m−2 (ref. 4) versus 29 kg m−2,8 P<0.0001) and with virtually no patients with BMI⩾35 kg m−2 while our cohort had >3400 patients. Another study showed that BMI was associated with DD independent of LV mass and other confounders.11 However, the studied cohort consisted of elderly patients (mean age 72 years) and with >72% having hypertension and high prevalence of coronary artery disease. Our current cohort was on average 15 years younger (mean age 57 years), had a much lower prevalence of hypertension (14.9%) and included a large number of morbidly obese patients (N=1616) who are in most studies under-represented. Also, the cohort studied by Russo et al.11 was from the North Manhattan Study, a predominately Hispanic community, while our cohort was more representative of the general US population. Furthermore, 1733 patients of our cohort were younger than 35 years old. The epidemic of obesity is now affecting younger age groups. Hence, the recognition of DD at such young age group is of great interest from a cardiovascular epidemiological standpoint.
There have been several studies evaluating DD in young patient cohorts showing the association between BMI and DD; however, all these studies were of small sample size, and none of them graded the severity of diastolic function. Furthermore, the causation has often been confounded by other processes found in obesity such as hypertension. In 1999, Carroll et al.22 showed in an animal model of obesity that diastolic compliance is reduced early in the development of obesity, and may contribute to reduced cardiac reserve. DD has clinical, prognostic and therapeutic ramifications.23, 24, 25, 26, 27 Hypertension, LV hypertrophy, diabetes mellitus and insulin resistance are complications of obesity and have all been associated with worsening of DD.10 In fact, these cardiovascular risk factors were much more prevalent in the obese and morbidly obese patients than those with normal BMI for all patients and those <35 years old (Table 1). After adjusting for these confounders, BMI was still independently associated with DD (Table 3). Our results were further strengthened by the fact that the majority of patients did not have hypertension, and that the cohort was much younger than presented by Russo et al. Of particular interest are patients who are <35 years old. In this group of patients with normal LVEF and minimal cardiovascular disease, DD should be quite uncommon. Yet, DD was three times more prevalent in obese versus normal weight patients (12.8% versus 3.8%, P<0.0001) (Figure 1b).
In addition, data on DD in morbidly obese patients are limited to few studies, the largest with <100 patients as compared with ∼1600 patients in our cohort.28, 29, 30 The financial impact of morbid obesity on society is overwhelming. Not only was the prevalence of hypertension and diabetes 2 and 5 times, respectively, higher than those with normal weights (Table 1), but so was DD, particularly in those <35 years old (Figure 1).
DD is a dynamic phenomenon. Not only can it progress and worsen with time, but can also improve.31 Recent data from our group have shown that in patients with normal LVEF, diastolic function changed in 27% of patients (1.1 years mean time between echoes) (16% had worsening and 11% had improvement). Worsening of DD was an independent predictor of all-cause mortality with similar hazard ratio to worsening of systolic function, irrespective of baseline diastolic function.31 In a similar study, worsening of DD was associated with new incidence of heart failure.10 Furthermore, Achong et al.32 showed that improvement in DD has been associated with trend toward better survival. The effect of weight loss on DD in morbidly obese patients has been evaluated in small studies with evidence of improvement of diastolic parameters,33, 34 although other studies showed no significant change.35 It is of interest to evaluate in a large cohort of patients whether reduction of BMI is associated with improvement in DD after adjusting for improvement in blood pressure, diabetes and LV mass.
Strengths and limitations
This is the largest study to our knowledge that evaluates the association between obesity and DD across different age groups, and underlines the unexpectedly high prevalence of DD even in young obese patients (age <35 years), who are not spared. With the growing epidemic of obesity, additional research is warranted to evaluate whether significant weight loss may indeed reverse DD early on. However, we acknowledge several limitations. This is a retrospective study from a single tertiary center with selection and referral bias, particularly given the large number of patients who were excluded for various reasons; hence, a large study validating these findings in the general US community is warranted. Given the cross-sectional nature of the study, the direction of the relationship between BMI and DD needs to be interpreted cautiously; perhaps DD leads to less exercise tolerance, sedentary lifestyle and weight gain. A prospective study is warranted to validate these findings, assess cause–effect relationship and perform a causal modeling to determine to what extent some of the known factors might mediate this effect. In addition, the readers were unblinded to the medical history of patients, and LV systolic function was assessed using quantitative and/or visual LVEF (cutoff LVEF ⩾55% was used for normal systolic function), which is not perfect, but universally accepted. Furthermore, there was no systematic review of the echocardiograms to reassess diastolic function or concordance between different methods that were available or evolved over the 10-year study period, but rather the data were extracted from prior reads, which is comparable to what is done in clinical practice. Although the assessment of diastology is performed in a standard manner at our institution, the use of tissue Doppler imaging only began in the late 1990s. Also, the proper archiving of the echocardiographic measurements, particularly diastolic parameters, did not start until recently; hence most of the diastolic parameters (LA volumes, transmitral filling and tissue velocities) were not available in the database. The echo-Doppler indices of LV filling pressures that were measured and intrinsic myocardial DD are not universally accepted as interchangeable; yet, they are still fairly accepted and adopted. While the intra- and interobserver agreements were not ideal, there were a large number of patients, and a lack of precision in the determination of DD would only tend to underestimate the magnitude of the observed association. In addition, the list of medications was not available; however, there has been no single proven medication that impacts directly diastolic function. Other unmeasured or unobserved variables, including rate of weight gain, chronicity of obesity, waist circumference, insulin levels, malignancy and pulmonary hypertension, were not accounted for. Finally, while several pathophysiological links between obesity and DD have been proposed, a clear mechanism still needs to be identified.
Conclusion
In our study of relatively young and middle age patients with normal LVEF, higher BMI was independently associated with worsening DD. Patients aged <35 years were not spared either. While the exact mechanism linking obesity to DD is still not well understood, further studies are warranted, particularly given the obesity epidemic and the likely expected increase in the incidence of DD. It will be interesting to evaluate whether significant weight loss may indeed reverse DD early on, and if so affects outcomes.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Nutrition and Diabetes website (http://www.nature.com/nutd)
Author contributions
Drs AlJaroudi, Houghtaling and Jaber had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: AlJaroudi, Halley, Thomas and Jaber. Acquisition of data: AlJaroudi and Jaber. Analysis and interpretation: AlJaroudi, Houghtaling, Agarwal and Jaber. Drafting of the manuscript: AlJaroudi, Agarwal, Halley, Grimm, Rodriguez, Menon, Thomas and Jaber. Critical revision of the manuscript for important intellectual content: AlJaroudi, Rodriguez, Halley, Agarwal, Grimm, Menon, Thomas and Jaber. Administrative, technical and material support: AlJaroudi, Rodriguez, Grimm, Thomas and Jaber. Study supervision: AlJaroudi and Jaber.
Supplementary Material
References
- Eckel RH, York DA, Rossner S, Hubbard V, Caterson I, Jeor ST, et al. Prevention Conference VII: obesity, a worldwide epidemic related to heart disease and stroke: executive summary. Circulation. 2004;110:2968–2975. doi: 10.1161/01.CIR.0000140086.88453.9A. [DOI] [PubMed] [Google Scholar]
- Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- Murphy NF, MacIntyre K, Stewart S, Hart CL, Hole D, McMurray JJ. Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15 000 middle-aged men and women (the Renfrew-Paisley study) Eur Heart J. 2006;27:96–106. doi: 10.1093/eurheartj/ehi506. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–236. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer MS, Anderson KM, Levy D. Separate and joint influences of obesity and mild hypertension on left ventricular mass and geometry: the Framingham Heart Study. J Am Coll Cardiol. 1992;19:130–134. doi: 10.1016/0735-1097(92)90063-s. [DOI] [PubMed] [Google Scholar]
- Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, et al. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA) JACC Cardiovasc Imaging. 2010;3:266–274. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane G, Karon B, Mahoney D, Redfield M, Roger V, Burnett J, et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, et al. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–1374. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halley CM, Houghtaling PL, Khalil MK, Thomas JD, Jaber WA. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171:1082–1087. doi: 10.1001/archinternmed.2011.244. [DOI] [PubMed] [Google Scholar]
- Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- Hurrell DG, Oh JK, Mahoney DW, Miller FA, Seward JB. Short deceleration time of mitral inflow E velocity: prognostic implication with atrial fibrillation versus sinus rhythm. J Am Soc Echocardiogr. 1998;11:450–457. doi: 10.1016/s0894-7317(98)70025-2. [DOI] [PubMed] [Google Scholar]
- Okura H, Takada Y, Kubo T, Iwata K, Mizoguchi S, Taguchi H, et al. Tissue Doppler-derived index of left ventricular filling pressure, E/E', predicts survival of patients with non-valvular atrial fibrillation. Heart. 2006;92:1248–1252. doi: 10.1136/hrt.2005.082594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician's Rosetta Stone. J Am Coll Cardiol. 1997;30:8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998;158:1855–1867. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- Kuznetsova T, Herbots L, López B, Jin Y, Richart T, Thijs L, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112. doi: 10.1161/CIRCHEARTFAILURE.108.822627. [DOI] [PubMed] [Google Scholar]
- Carroll JF, Summers RL, Dzielak DJ, Cockrell K, Montani JP, Mizelle HL. Diastolic compliance is reduced in obese rabbits. Hypertension. 1999;33:811–815. doi: 10.1161/01.hyp.33.3.811. [DOI] [PubMed] [Google Scholar]
- Pascual M, Pascual DA, Soria F, Vicente T, Hernandez AM, Tebar FJ, et al. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89:1152–1156. doi: 10.1136/heart.89.10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmala W, Wong C, Kuliczkowska J, Leano R, Przewlocka-Kosmala M, Marwick TH. Use of body weight and insulin resistance to select obese patients for echocardiographic assessment of subclinical left ventricular dysfunction. Am J Cardiol. 2008;101:1334–1340. doi: 10.1016/j.amjcard.2007.12.034. [DOI] [PubMed] [Google Scholar]
- Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, et al. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43:1399–1404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- Leggio M, Cruciani G, Sgorbini L, Mazza A, Bendini MG, Pugliese M, et al. Obesity-related adjunctive systo-diastolic ventricular dysfunction in patients with hypertension: echocardiographic assessment with tissue Doppler velocity and strain imaging. Hypertens Res. 2011;34:468–473. doi: 10.1038/hr.2010.266. [DOI] [PubMed] [Google Scholar]
- Libhaber CD, Norton GR, Majane OH, Libhaber E, Essop MR, Brooksbank R, et al. Contribution of central and general adiposity to abnormal left ventricular diastolic function in a community sample with a high prevalence of obesity. Am J Cardiol. 2009;104:1527–1533. doi: 10.1016/j.amjcard.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Barbosa MM, Beleigoli AM, de Fatima Diniz M, Freire CV, Ribeiro AL, Nunes MC. Strain imaging in morbid obesity: insights into subclinical ventricular dysfunction. Clin Cardiol. 2011;34:288–293. doi: 10.1002/clc.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willens HJ, Chakko SC, Lowery MH, Byers P, Labrador E, Gallagher A, et al. Tissue Doppler imaging of the right and left ventricle in severe obesity (body mass index >35 kg/m2) Am J Cardiol. 2004;94:1087–1090. doi: 10.1016/j.amjcard.2004.06.076. [DOI] [PubMed] [Google Scholar]
- Di Bello V, Santini F, Di Cori A, Pucci A, Palagi C, Delle Donne MG, et al. Relationship between preclinical abnormalities of global and regional left ventricular function and insulin resistance in severe obesity: a Color Doppler Imaging Study. Int J Obes (Lond) 2006;30:948–956. doi: 10.1038/sj.ijo.0803206. [DOI] [PubMed] [Google Scholar]
- AlJaroudi W, Alraies M, Halley C, Rodriguez L, Grimm RA, Thomas JD, et al. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation. 2012;125:782–788. doi: 10.1161/CIRCULATIONAHA.111.066423. [DOI] [PubMed] [Google Scholar]
- Achong N, Wahi S, Marwick TH. Evolution and outcome of diastolic dysfunction. Heart. 2009;95:813–818. doi: 10.1136/hrt.2008.159020. [DOI] [PubMed] [Google Scholar]
- Willens HJ, Chakko SC, Byers P, Chirinos JA, Labrador E, Castrillon JC, et al. Effects of weight loss after gastric bypass on right and left ventricular function assessed by tissue Doppler imaging. Am J Cardiol. 2005;95:1521–1524. doi: 10.1016/j.amjcard.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Alpert MA, Lambert CR, Terry BE, Cohen MV, Mulekar M, Massey CV, et al. Effect of weight loss on left ventricular diastolic filling in morbid obesity. Am J Cardiol. 1995;76:1198–1201. doi: 10.1016/s0002-9149(99)80339-7. [DOI] [PubMed] [Google Scholar]
- Syed M, Rosati C, Torosoff MT, El-Hajjar M, Feustel P, Alger S, et al. The impact of weight loss on cardiac structure and function in obese patients. Obes Surg. 2009;19:36–40. doi: 10.1007/s11695-008-9645-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.