Abstract
Aims
Threshold crossings of impedance trends detected by implanted devices have been associated with clinically relevant heart failure events, but long-term prognosis of such events has not been demonstrated. The aim of this study is to examine the relationship between alterations in intrathoracic impedance and mortality risk in patients with implantable devices.
Methods and results
We reviewed remote monitoring data in the de-identified Medtronic CareLink® Discovery Link that captured intrathoracic impedance trends for >6 months. The initial 6 months of the cardiac and impedance trends were used as the observation period to create the patient groups and cross-referenced with the Social Security Death Index for mortality data. In our study cohort of 21 217 patients, 36% experienced impedance threshold crossing within the initial 6 months of monitoring (defined as the ‘early threshold crossing’ group). Patients with early threshold crossings demonstrated an increased risk of age- and gender-adjusted all-cause mortality [hazard ratio (HR) 2.15, 95% confidence interval (CI) 1.95–2.38, P< 0.0001]. Increased mortality risk remained significant when analysed in subgroups of patients without defibrillator shock (HR 2.10, 95% CI 1.90–2.34, P< 0.0001, n= 1621) or within those patients without device-detectable atrial fibrillation (AF) during the initial 6 months of monitoring (HR 2.09, 95% CI 1.86–2.34, P< 0.0001, n= 17 235). Both the number and the duration of early threshold crossings of impedance trends detectable by implanted devices were associated with increased mortality risk. Furthermore, the improvement of altered impedance trends portends more favourable prognosis.
Conclusions
Threshold crossing of impedance trends detectable by implanted devices is associated with relatively increased mortality risk even after adjusted for demographic, device-detected AF, or defibrillator shocks.
Keywords: Intrathoracic impedance, Heart failure, Prognosis
Introduction
Despite major advances in drug and device therapies as well as better detection and management of heart failure (HF) at earlier disease stages, HF remains one of the major contributors to morbidity, mortality, and societal costs.1 This is in part due to the inability for health-care providers to adequately assess patient vulnerability or adequately monitor their responses to therapy. Frequently, signs and symptoms present late in the disease course, sometimes too late to avert hospitalizations, while at other times, the clinical manifestations may not be specific to the underlying cardiac dysfunction.
Broad adoption of implantable cardioverter defibrillator (ICD) and cardiac implanted resynchronization therapy with defibrillators (CRT-D) in patients with HF has provided a unique platform to develop ancillary sensor technologies to better understand longitudinal physiological alterations. The availability of intrathoracic impedance measurements in the clinical setting has opened an interesting debate regarding the clinical utility of such device-derived sensor information.2 Changes in these parameters have been associated with adverse clinical events such as HF hospitalizations in a variety of studies.3–9 However, strategies guided by such information have yet to be evaluated in prospective clinical trials in part because there are doubts regarding inaccuracies or potential variability in sensitivities of such prediction,10 which may lead to unnecessary treatments or inappropriate therapy. Such debate stems in part because of the lack of definitive outcomes data to support the clinical importance of these threshold crossings, the relatively small sample sizes of published studies in this topic, and the seemingly heterogeneous physiological responses in a clinical condition that lacks a gold standard. Herein, we examine the direct relationship between decreases in intrathoracic impedance and the subsequent prospective risk of mortality in HF patients treated with implantable devices.
Methods
Study population
The Medtronic CareLink® Discovery Link has been established as a de-identified repository of longitudinal data retrieved via a remote monitoring network of ICDs and CRT-Ds manufactured by Medtronic, Inc. Centers using CareLink have entered into a data use agreement that allows for the use of data for research purposes in accordance with regulations stipulated in the Health Insurance Portability and Accountability Act (HIPAA). We conducted a retrospective analysis of a representative sample of patients with devices implanted in the USA from June 2006 through September 2009. From the total number of CareLink patients implanted with either dual-chamber ICD or CRT-D devices during this time frame (n= 125 527), a one-third sample was randomly selected for analysis (n= 41 842). Given that intrathoracic impedance is needed for the study, we excluded those patients implanted with devices without this capability (in the device or in the CareLink® system, n= 9205) and those in whom impedance trend cardiac compass had missing data due to infrequent CareLink® data transmissions (longer than the 14-month cardiac compass storage, n= 8523). Furthermore, because analysis is based on the behaviour of the intrathoracic impedance during the first 6 months, patients who died within this period were excluded (n= 2897). These criteria were set a priori, driven solely by our ability to perform the analysis. After exclusions, 21 217 patients were included in the analysis.
Data synthesis
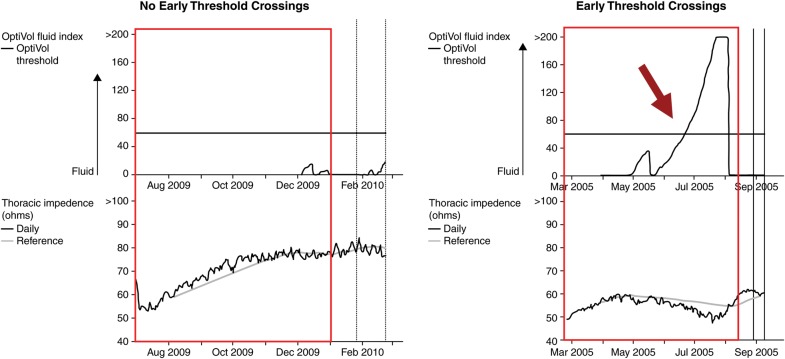
Detailed device upload information from remote monitoring of ICDs and CRTs were available for analyses. The mechanisms behind the detection of intrathoracic impedance in the specific devices monitored in this study have been described previously.11 Cardiac and impedance trends were used as the ‘observation period’ to identify patients with and without early threshold crossings during the initial 6 months (183 days) following the date of device implantation. ‘Early threshold crossing’ was defined as a reduction in intrathoracic impedance leading to a rise in the derived OptiVol® fluid index above the nominal threshold of 60 ohm-days (as previously determined and validated)6,9,11 within the observation period (Figure 1 illustrates an example with early threshold crossing and one without). The number of threshold crossings (none, 1, 2, or ≥3) and the duration in days spent above the threshold (none, 1–14, 15–28, or >28 days) during the observation period were also quantified. In a similar manner, we also performed a serial analysis of the impedance trends between the first 6 months (baseline to 6 months) and the next 6 months (from 6 to 12 months, termed ‘late threshold crossing’) following implantation to evaluate the impact of the development or resolution of altered impedance as determined by threshold crossings in a subset of patients, whereby 12 months or more of follow-up were available.
Figure 1.
Examples of intrathoracic impedance trends in patients with vs. without early threshold crossing.
The presence of device-detectable atrial fibrillation (AF) was defined as >6 h of mode switching due to presumed AF for at least 1 day. Mortality was evaluated from 6 months forward. Mortality data were obtained from the Medtronic Device Registry and cross-referenced with the Social Security Death Index up to 30 April 2010.
Statistical analysis
The Student's t-test for continuous variables and χ2 test for categorical variables were used to examine the differences in baseline characteristics between groups. The Kaplan–Meier analysis and the Cox proportional hazards regression were used for time-to-event analysis, with the starting date of follow-up after the observation period (6 months after implantation for baseline analysis and 12 months after implantation for serial analysis). Hazard ratios (HR) and 95% confidence intervals (95% CI) for all-cause mortality were adjusted for age and gender. The Cox proportional hazards models were also constructed within subsets of patients with or without arrhythmic events (AF, defibrillator shocks) and within subsets of increasing number as well as the duration of early threshold crossings. All analyses were performed using statistical software from SAS, Inc. (Version 9, Cary NC, USA).
Results
A total of 21 217 patients were identified in the CareLink® Discovery Link for analysis. These patients had at least 6 months of follow-up and OptiVol fluid index measurements available on all days starting from 34-day post-implant. The mean remote monitoring duration was 20 ± 9 months, representing a total of 34 690 patient-years of follow-up. During the 6-month observation period, 7623 (36%) patients experienced a total of 9577 early threshold crossings (mean 1.3 ± 0.5 crossings). Baseline characteristics of the study cohort are illustrated in Table 1 and stratified according to the presence or absence of early threshold crossings during the initial 6-month observation period. Similar results were observed when the observation period was extended to 12 months.
Table 1.
Baseline characteristics
| Overall population (n= 21 217) | Early threshold crossing (n= 7623) | No early threshold crossing (n= 13 594) | P-value | |
|---|---|---|---|---|
| Mean age (years) | 68 ±12 | 69 ± 12 | 68 ± 12 | <0.001 |
| Male gender (%) | 75 | 73 | 76 | <0.001 |
| CRT ± D (%) | 51 | 55 | 49 | <0.001 |
| Mean duration from implantation (months) | 20 ± 9 | 19 ± 9 | 20 ± 9 | <0.001 |
| Defibrillator shock for VT/VF (%)a | 8 | 10 | 6 | <0.001 |
| Device-detectable atrial fibrillation (%)a | 19 | 23 | 17 | <0.001 |
aDuring the observation period.
Patients who experienced early threshold crossings were more likely to be younger, female, and implanted with a CRT-D device. Within the same 6-month period, a total of 1621 patients experience one or more defibrillator shocks and 3982 patients had device-detected AF. Patients with either of these arrhythmic events were more likely to experience early threshold crossing.
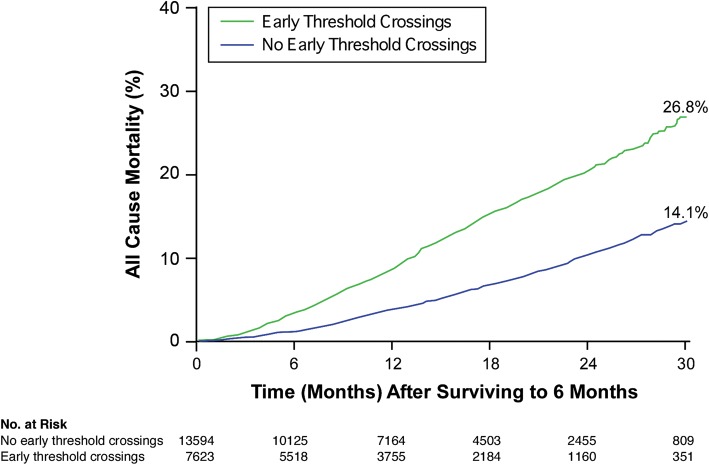
After the 6-month observation period, there were 852 deaths among 7623 patients in the group with early threshold crossing and 753 deaths out of 13 594 patients in the group without early threshold crossings (11.2 vs. 5.5%, respectively, P< 0.0001) with the threshold being programmed at 60 ohm-days. Figure 2 demonstrates the overall mortality trends stratified by the presence or absence of threshold crossing of intrathoracic impedance trends. Subsequent to the initial 6 months evaluation, the patients with early threshold crossings demonstrated a 2.15-fold increased risk of age- and gender-adjusted all-cause mortality (95% CI 1.95–2.38, P< 0.0001). The increased mortality risk associated with early threshold crossing remained significant when analysed within those who had received CRT-D (HR 2.23, 95% CI 1.97–2.52, P< 0.0001), as well as in subgroups of patients who did not received a defibrillation shock (HR 2.10, 95% CI 1.90–2.34, P< 0.0001) or in those patients without device-detectable AF during the initial 6 months of monitoring (HR 2.09, 95%CI 1.86–2.34, P< 0.0001; Table 2 and Figure 3). Even when the threshold for OptiVol® fluid index crossing was raised to 100 ohm-days, the increased mortality risk associated with early threshold crossing remained robust (HR 2.36, 95% CI 2.13–2.62, P< 0.0001).
Figure 2.
The Kaplan–Meier survival analysis for all-cause mortality stratified by the presence or absence of early intrathoracic impedance threshold crossings.
Table 2.
Cox's proportional hazards analyses for all-cause mortality in patient subgroups
| Unadjusted HR (95% CI) | P-value | Adjusted HRa (95% CI) | P-value | |
|---|---|---|---|---|
| Overall | 2.21 (2.00–2.44) | <0.001 | 2.15 (1.95–2.38) | <0.001 |
| Device type | ||||
| CRT ± D | 2.22 (1.96, 2.50) | <0.001 | 2.23 (1.97, 2.52) | <0.001 |
| ICD | 2.04 (1.73, 2.41) | <0.001 | 1.95 (1.65, 2.30) | <0.001 |
| Defibrillator shocks | ||||
| Present | 2.29 (1.68, 3.11) | <0.001 | 2.36 (1.74, 3.21) | <0.001 |
| Not present | 2.18 (1.96, 2.41) | <0.001 | 2.10 (1.90, 2.34) | <0.001 |
| Device-detectable AFb | ||||
| Present | 2.16 (1.79, 2.60) | <0.001 | 2.14 (1.77, 2.59) | <0.001 |
| Not present | 2.13 (1.90, 2.39) | <0.001 | 2.09 (1.86. 2.34) | <0.001 |
aCox's regression adjusted for age and gender.
bDevice-detectable AF defined as presence of AF >6 h of detection for at least 1 day.
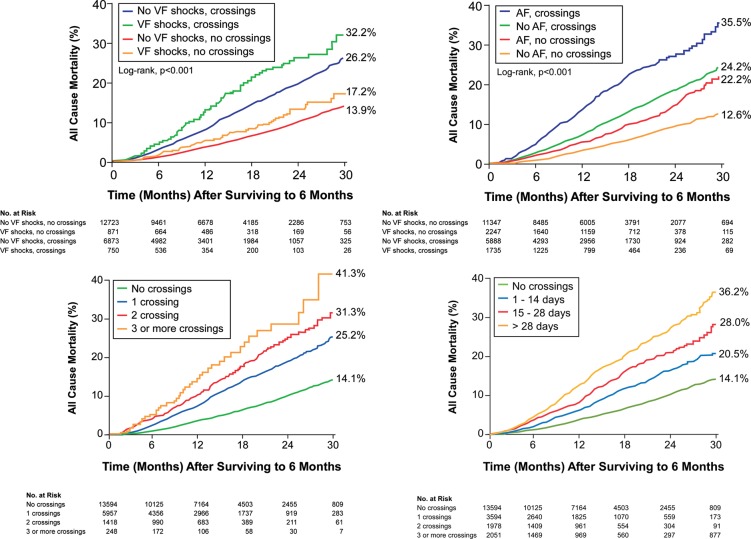
Figure 3.
The Kaplan–Meier survival analysis for all-cause mortality stratified by the time and the duration of early intrathoracic impedance threshold crossings and device characteristics (atrial fibrillation and defibrillator shocks).
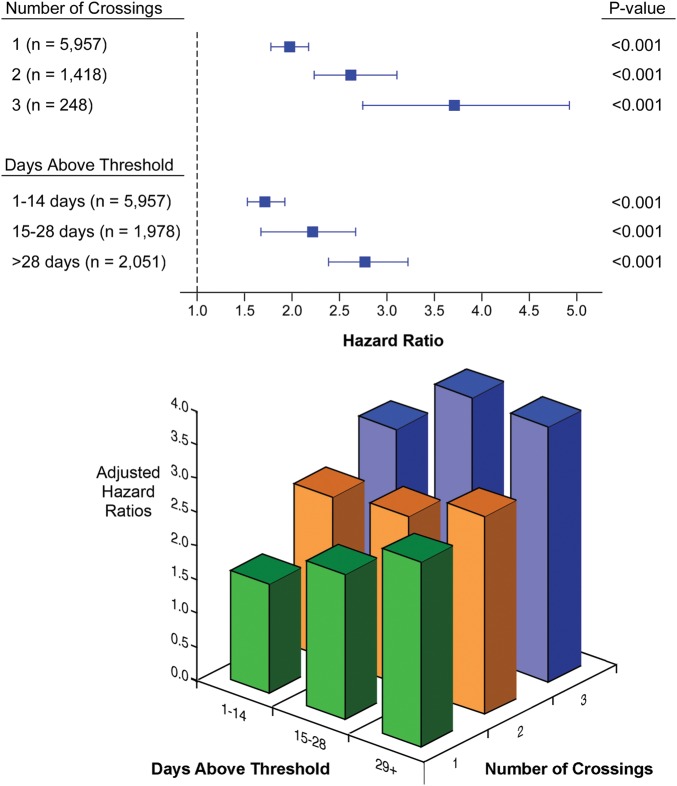
Figure 3 also shows the incremental mortality risks associated with a ‘dose-dependent’ increase in number of early threshold crossings as well as the number of days spent above the threshold over the first 6 months of monitoring. Patients with increased threshold crossings, either by the number of days above threshold or the number of threshold crossings, demonstrated incremental risk adjusted all-cause mortality subsequent to the initial 6-month evaluation period even when combined (all P< 0.001; Figure 4).
Figure 4.
Hazard ratios with an increasing number and duration of early threshold crossings compared with no early threshold crossings.
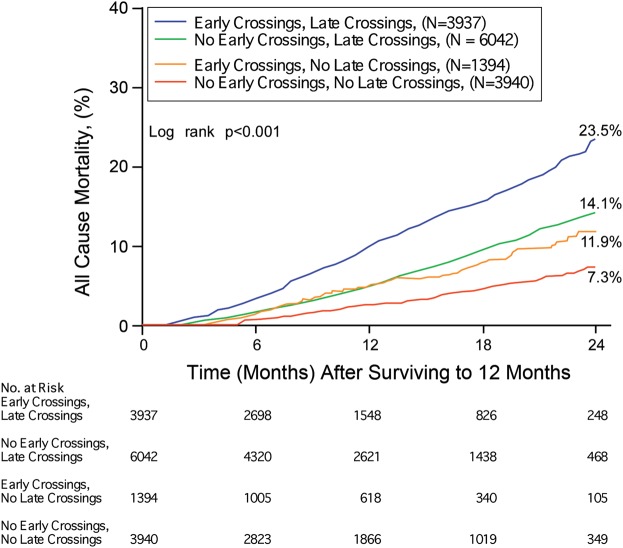
In the subset of 15 313 patients with 12 months or more of follow-up data, patients were stratified according to the presence or absence of early threshold crossings coupled with late threshold crossings. Within the subgroup of patients with early threshold crossings, a subsequent stabilization of the impedance trends (i.e. no late threshold crossings) was associated with a mortality risk reduction (Figure 5, HR 0.48, P< 0.001). Similar behaviour was also observed within the subgroup of patients without early threshold crossings; in this case, a subsequent de-stabilization of impendence trends (i.e. late threshold crossings) was associated with a mortality risk increase (HR 2.10, P< 0.001).
Figure 5.
The Kaplan–Meier survival analysis for all-cause mortality stratified by the presence and absence of ‘Early’ (0–6 months) as well as ‘Late’ (6–12 months) intrathoracic impedance threshold crossing.
Discussion
Much of the debate surrounding the clinical development of sensor technologies for implanted devices has focused on the precision and accuracy of specific measure(s) or algorithm(s) to detect conditions that are considered clinically relevant, and the ability recognizes such alterations early enough to change the course of events with appropriate interventions.2 Hence, the demonstration of a link between conditions defined by such measures or algorithms and adverse consequences is an important validation to the approach. The key finding of this analysis is that an impedance threshold crossing detected by an implanted device is associated with relatively increased age- and gender-adjusted mortality risk. Increased risks were consistent within subgroups of patients with and without device-detected arrhythmic events (device-detectable AF and defibrillator shocks). Furthermore, we also observed that the improvement of impedance trends portends more favourable prognosis (patients with early crossings and no late crossings). This finding implies that physiological derangements detected by intrathoracic impedance algorithms can be prognostically relevant and independent of arrhythmic events.
The presence of persistent or recurrent clinical congestion is a major determinant of long-term outcomes in patients with HF. A wide range of clinical signs (e.g. third heart sound, jugular venous distention)12 and invasive haemodynamic measurements (e.g. intracardiac filling pressures and cardiac output)13 of congestion have supported this notion. However, the concept of ‘congestion’ is relatively subjective and is usually reserved for overt fluid retention or symptomatic deterioration—it is rarely identified beyond traditional modes of clinical encounter. It is important to emphasize that most published work in the literature has assumed that intrathoracic impedance is equivalent to congestion. We acknowledged that intrathoracic impedance itself is not a haemodynamic measurement and not directly measuring blood volume. In this way, it may be a surrogate similar to the ability of brain natriuretic peptide to identify a subpopulation of HF patients with an increased mortality risk. There may also be other underlying reasons for changes in intrathoracic impedance beyond overt congestion (such as pneumonia or lead dislodgement), and changes in impedance may also reflect underlying tissue oedema. Nevertheless, there have been several demonstrations of concordance between intrathoracic impedance trends and direct measurements of intracardiac pressures.14–16 Similarly, changes in impedance trends are correlated with echocardiographic and biochemical surrogates of decompensation.16–18 Based on previous registries that have documented the presence of congestive symptoms at the time period of intrathoracic impedance threshold crossings in the majority of patients,3,6,19 our findings indirectly support the association between impedance trends and underlying physiological derangements that are often associated with congestion. In other words, such changes may identify potentially destabilized individuals (whether there is presence or absence of self-reported overt congestion) based on the review of historical impedance trends. Conversely, it is reassuring to identify individuals without significant changes in intrathoracic impedance leading to threshold crossings, since these individuals may be in a lower mortality risk category.
While there is limited clinical information to determine the indications of device implantation or the underlying cardiac phenotypes of the participating patients, the prevalence of threshold crossings based on intrathoracic impedance trends were similar among patients with ICDs and CRT-D devices, even though patients fulfilling criteria for CRT-D are often encountered at more advanced stages. There may be several explanations for this observation. First, the therapeutic benefits of CRT-D may stabilize patients with advanced HF and may even reverse ongoing cardiac remodelling, resulting in a more favourable disease course. It is likely that these individuals (predominantly in the New York Heart Association Class III-IV range at the time of their implant) may have sought medical attention from health-care providers that were more likely to care for patients with HF. In contrast, patients who received an ICD, particularly those fulfilling implantation criteria for prophylactic purposes (i.e. ‘MADIT-II criteria’) may not have overt congestive symptoms at the time of implantation but progressed over time. These patients may not have the same level of HF disease management or even awareness of their disease severity as their CRT-D counterparts. While there have been reports suggesting that the burden of arrhythmic events may be associated with alterations in impedance trends,20–22 our analyses suggested that threshold crossing, either by mere occurrence or by degree based on the number or duration of crossings, may portend poor prognosis, regardless of the presence or absence of interim arrhythmic events.
What are the implications of these findings? Prior studies have suggested that subtle changes in clinical status or self-care behaviour could be identified with patient interviews at the time of intrathoracic impedance threshold crossings.19,23 Therefore, the ability to identify the heightened risk of an individual patient based on information remotely collected beyond surveillance of arrhythmic events or device function may open an opportunity to identify patients in a vulnerable period beyond the traditional clinical encounter. Strategies to triage these patients for intensification of HF disease management prescription in a timely fashion may provide opportunities to avert clinical deterioration—a hypothesis that still warrants further testing. Demonstrating the ability for a measure such as intrathoracic impedance to provide risk stratification does not automatically imply our better understanding of how to care for these at-risk individuals. What these findings have illustrated is the fact that patients with early threshold crossings are at a relatively higher mortality risk category, regardless of what they represent. For patients with implanted devices already observed in a remote monitoring infrastructure, the occurrence of early or multiple threshold crossings may focus the need for intensification of HF disease management or a more in-depth evaluation of his or her clinical status—all independent of the traditional clinical encounters or additional testing. These additional encounters could, in principle, drive up the cost of care but in combination with proper actions they should in turn reduce the overall financial burden by preventing hospitalizations and improving outcomes. Although makes logical sense, the clinical benefits of such remote monitoring strategies remain to be proven, and the prognostic significance of such monitoring parameters has to be demonstrated in the first place. With more and more continuous data from physiological sensors (previously unknown) being available, the appropriate design of treatment strategies incorporating this proactive device-derived information is needed in order to test whether they may impact on clinical outcomes. Further studies are warranted to determine how to better manage such high-risk population by recognizing their underlying intrathoracic impedance trends.24
Study limitations
Despite a very large and unbiased view of the device-derived data from a remote monitoring portal, there was limited clinical information or covariates that were readily available to enhance our understanding of the underlying mechanisms leading to (or confounding) such observations. Due to de-identification of the data set, there was no information regarding whether these threshold crossings had been associated with subsequent HF hospitalizations or therapeutic interventions, even though in the large majority of patients identified, the frequency of device interrogation was consistent with standard 3-month intervals by electrophysiology practices. We were also unable to determine the exact cause of death for individual patients, nor a variety of clinical covariates that may influence the prognostic value of threshold crossings. Our observations were based on analysis using a single threshold of 60 ohm-days, and individual variability of threshold sensitivity may affect our findings. The exclusion from the primary analysis of patients who died during the first 6 months of follow-up, as well as the exclusion from the long-term analysis of patients who died during the first 12 months of follow-up, introduces a survival bias in these analyses. It is important to recognize that the mortality rate in this ‘real-world’ population remains high even in the non-threshold crossing group of patients. Hence, the presence of impedance threshold crossing may identify relative rather than absolute risk differences, and there were likely many other factors (both cardiac and non-cardiac) that may have influenced the relatively high mortality rates of patients with implanted devices.
Conclusion
In patients with implanted devices, threshold crossing of intrathoracic impedance trends detectable by implanted devices is associated with relatively increased mortality risk even after adjusted for age and gender and within prior device-detectable AF and defibrillator shock subgroups. Both the number and the duration of threshold crossing of impedance trends detectable by implanted devices are also associated with relatively increased mortality risk, and resolution of impedance trends over time was associated with a relatively more favourable prognosis.
Funding
This work was supported by Medtronic Inc. Funding to pay the Open Access publication charges for this article was provided by Medtronic Inc.
Conflict of interest: W.H.W.T., R.S.S., and J.T.H. are consultants with Medtronic Inc., and E.N.W. and J.W.J. are employees of Medtronic Inc. This manuscript was prepared independent of the sponsor.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J American Heart Association Statistics Committee and Stroke Statistics S. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merchant FM, Dec GW, Singh JP. Implantable sensors for heart failure. Circ Arrhythm Electrophysiol. 2010;3:657–667. doi: 10.1161/CIRCEP.110.959502. [DOI] [PubMed] [Google Scholar]
- 3.Whellan DJ, Ousdigian KT, Al-Khatib SM, Pu W, Sarkar S, Porter CB, Pavri BB, O'Connor CM. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study. J Am Coll Cardiol. 2010;55:1803–1810. doi: 10.1016/j.jacc.2009.11.089. [DOI] [PubMed] [Google Scholar]
- 4.Soga Y, Ando K, Arita T, Hyodo M, Goya M, Iwabuchi M, Nobuyoshi M. Efficacy of fluid assessment based on intrathoracic impedance monitoring in patients with systolic heart failure. Circ J. 2010;75:129–134. doi: 10.1253/circj.cj-10-0730. [DOI] [PubMed] [Google Scholar]
- 5.Catanzariti D, Lunati M, Landolina M, Zanotto G, Lonardi G, Iacopino S, Oliva F, Perego GB, Varbaro A, Denaro A, Valsecchi S, Vergara G. Monitoring intrathoracic impedance with an implantable defibrillator reduces hospitalizations in patients with heart failure. Pacing Clin Electrophysiol. 2009;32:363–370. doi: 10.1111/j.1540-8159.2008.02245.x. [DOI] [PubMed] [Google Scholar]
- 6.Small RS, Wickemeyer W, Germany R, Hoppe B, Andrulli J, Brady PA, Labeau M, Koehler J, Sarkar S, Hettrick DA, Tang WH. Changes in intrathoracic impedance are associated with subsequent risk of hospitalizations for acute decompensated heart failure: clinical utility of implanted device monitoring without a patient alert. J Card Fail. 2009;15:475–481. doi: 10.1016/j.cardfail.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Perego GB, Landolina M, Vergara G, Lunati M, Zanotto G, Pappone A, Lonardi G, Speca G, Iacopino S, Varbaro A, Sarkar S, Hettrick DA, Denaro A. Implantable CRT device diagnostics identify patients with increased risk for heart failure hospitalization. J Interv Card Electrophysiol. 2008;23:235–242. doi: 10.1007/s10840-008-9303-5. [DOI] [PubMed] [Google Scholar]
- 8.Maines M, Catanzariti D, Cemin C, Vaccarini C, Vergara G. Usefulness of intrathoracic fluids accumulation monitoring with an implantable biventricular defibrillator in reducing hospitalizations in patients with heart failure: a case–control study. J Interv Card Electrophysiol. 2007;19:201–207. doi: 10.1007/s10840-007-9155-4. [DOI] [PubMed] [Google Scholar]
- 9.Yu CM, Wang L, Chau E, Chan RH, Kong SL, Tang MO, Christensen J, Stadler RW, Lau CP. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841–848. doi: 10.1161/CIRCULATIONAHA.104.492207. [DOI] [PubMed] [Google Scholar]
- 10.Conraads VM, Tavazzi L, Santini M, Oliva F, Gerritse B, Yu CM, Cowie MR. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: the SENSE-HF trial. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr050. doi:10.1093/eurheartj/ehr1050. [DOI] [PubMed] [Google Scholar]
- 11.Wang L. Fundamentals of intrathoracic impedance monitoring in heart failure. Am J Cardiol. 2007;99:3G–10G. doi: 10.1016/j.amjcard.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Drazner MH, Rame JE, Stevenson LW, Dries DL. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med. 2001;345:574–581. doi: 10.1056/NEJMoa010641. [DOI] [PubMed] [Google Scholar]
- 13.Mullens W, Abrahams Z, Skouri HN, Taylor DO, Starling RC, Francis GS, Young JB, Tang WH. Prognostic evaluation of ambulatory patients with advanced heart failure. Am J Cardiol. 2008;101:1297–1302. doi: 10.1016/j.amjcard.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Rademaker MT, Charles CJ, Melton IC, Richards AM, Frampton CM, Siou J, Qu F, Eigler NL, Gutfinger D, Troughton RW. Monitoring of heart failure: comparison of left atrial pressure with intrathoracic impedance and natriuretic peptide measurements in an experimental model of ovine heart failure. Clin Sci (Lond) 2011;120:207–217. doi: 10.1042/CS20100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanderheyden M, Houben R, Verstreken S, Stahlberg M, Reiters P, Kessels R, Braunschweig F. Continuous monitoring of intrathoracic impedance and right ventricular pressures in patients with heart failure. Circ Heart Fail. 2010;3:370–377. doi: 10.1161/CIRCHEARTFAILURE.109.867549. [DOI] [PubMed] [Google Scholar]
- 16.Maines M, Catanzariti D, Cirrincione C, Valsecchi S, Comisso J, Vergara G. Intrathoracic impedance and pulmonary wedge pressure for the detection of heart failure deterioration. Europace. 2010;12:680–685. doi: 10.1093/europace/eup419. [DOI] [PubMed] [Google Scholar]
- 17.Tomasi L, Zanotto G, Zanolla L, Golia G, Ometto R, Bonanno C, Vergara G, Maines M, Lonardi G, Visentin E, Rauhe W, Latina L, Perrone C, Varbaro A, De Santo T. Physiopathologic Correlates of Intrathoracic Impedance in Chronic Heart Failure Patients. Pacing Clin Electrophysiol. 2011;34:407–413. doi: 10.1111/j.1540-8159.2010.02979.x. [DOI] [PubMed] [Google Scholar]
- 18.Luthje L, Vollmann D, Drescher T, Schott P, Zenker D, Hasenfuss G, Unterberg C. Intrathoracic impedance monitoring to detect chronic heart failure deterioration: relationship to changes in NT-proBNP. Eur J Heart Fail. 2007;9:716–722. doi: 10.1016/j.ejheart.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Mullens W, Oliveira LP, Verga T, Wilkoff BL, Tang WH. Insights from internet-based remote intrathoracic impedance monitoring as part of a heart failure disease management program. Congest Heart Fail. 2010;16:159–163. doi: 10.1111/j.1751-7133.2010.00149.x. [DOI] [PubMed] [Google Scholar]
- 20.Ip JE, Cheung JW, Park D, Hellawell JL, Stein KM, Iwai S, Liu CF, Lerman BB, Markowitz SM. Temporal Associations Between Thoracic Volume Overload and Malignant Ventricular Arrhythmias: A Study of Intrathoracic Impedance. J Cardiovasc Electrophysiol. 2011;22:293–299. doi: 10.1111/j.1540-8167.2010.01924.x. [DOI] [PubMed] [Google Scholar]
- 21.Moore HJ, Peters MN, Franz MR, Karasik PE, Singh SN, Fletcher RD. Intrathoracic impedance preceding ventricular tachyarrhythmia episodes. Pacing Clin Electrophysiol. 2010;33:960–966. doi: 10.1111/j.1540-8159.2010.02746.x. [DOI] [PubMed] [Google Scholar]
- 22.Andriulli J. Device monitoring of intrathoracic impedance: clinical observations from a patient registry. Am J Cardiol. 2007;99:23G–28G. doi: 10.1016/j.amjcard.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 23.Rathman LD, Lee CS, Sarkar S, Small RS. A critical link between heart failure self-care and intrathoracic impedance. J Cardiovasc Nurs. 2010 doi: 10.1097/JCN.0b013e3181ee28c8. doi:2010.1097/JCN.2010b2013e3181ee2028c2018. [DOI] [PubMed] [Google Scholar]
- 24.Brachmann J, Bohm M, Rybak K, Klein G, Butter C, Klemm H, Schomburg R, Siebermair J, Israel C, Sinha AM, Drexler H on behalf of the OptiLink HFSEBaI. Fluid status monitoring with a wireless network to reduce cardiovascular-related hospitalizations and mortality in heart failure: rationale and design of the OptiLink HF Study (Optimization of Heart Failure Management using OptiVol Fluid Status Monitoring and CareLink) Eur J Heart Fail. 2011;13:796–804. doi: 10.1093/eurjhf/hfr045. [DOI] [PMC free article] [PubMed] [Google Scholar]