Abstract
Aims
The aim of this study was to assess diagnostic and prognostic value of mid-regional pro-atrial natriuretic peptide (MR-proANP) and adrenomedullin (MR-proADM) for the evaluation of patients presenting to the emergency department with acute dyspnoea.
Methods and results
A total of 560 patients from the pro-B type natriuretic peptide Investigation of Dyspnoea in the Emergency Department were evaluated; 180 had acutely decompensated heart failure (ADHF). Concentrations of amino-terminal pro-B type natriuretic peptide (NT-proBNP), MR-proADM, and MR-proANP were measured, and patients were followed to 4 years for survival. Logistic regression evaluated utility of MR-proANP in ADHF diagnosis. Area under the curve (AUC), multivariate Cox regression, net reclassification improvement, and Kaplan–Meier survival analyses were used for mortality analyses. Mid-regional pro-atrial natriuretic peptide was higher in patients with ADHF (median 329 vs. 58 pmol/L; P < 0.001), and remained an independent predictor of HF diagnosis even when NT-proBNP was included as a covariate (odds ratio = 4.34, 95% CI = 2.11–8.92; P < 0.001). In time-dependent analyses, MR-proADM had the highest AUC for death during the first year; after 1 year, MR-proANP and NT-proBNP had a higher AUC. Both mid-regional peptides were independently prognostic and reclassified risk at 1 year [MR-proANP, hazard ratio (HR) = 2.99, MR-proADM, HR = 2.70; both P < 0.001] and at 4 years (MR-proANP, HR = 3.12, P < 0.001; MR-proADM, HR = 1.51, P = 0.03) and in Kaplan–Meier curves both mid-regional peptides were associated with death out to 4 years, individually or in a multimarker strategy.
Conclusion
Among patients with acute dyspnoea, MR-proANP is accurate for diagnosis of ADHF, while both MR-proANP and MR-proADM are independently prognostic to 4 years of the follow-up.
Keywords: Diagnosis, Prognosis, Biomarker, Heart failure
See page 2124 for the editorial comment on this article (doi:10.1093/eurheartj/ehs212)
Introduction
Among patients attending the emergency department (ED) with acute dyspnoea, a wide range of diagnostic possibilities are possible, each with its own specific prognosis; of the causes of acute dyspnoea in the ED setting, acutely decompensated heart failure (ADHF) represents a particularly important diagnosis given poor short- and longer-term post-discharge survival rates.1 To improve security of both ADHF diagnosis and prognostic assessment in dyspnoeic patients, the use of biomarkers has grown, with B-type natriuretic peptide (BNP) and its amino-terminal pro-B-type natriuretic peptide (NT-proBNP) having a prominent place in guideline statements.2 Beyond BNP and NT-proBNP, a wide array of newer biomarkers has been proposed for use in acutely dyspnoeic patients; because of this burgeoning range of choices, position statements call for thorough evaluation of novel biomarkers, suggesting such markers demonstrate at least comparable diagnostic or prognostic value over currently available assays to establish clinical utility.3
Recently, assays for the detection of mid-regional pro-atrial natriuretic peptide (MR-proANP) and mid-regional pro-adrenomedullin (MR-proADM; a fragment of adrenomedullin4) have been developed. Mid-regional pro-atrial natriuretic peptide has been shown to have potential diagnostic and prognostic utility in ADHF, comparable with other natriuretic peptides,5 while MR-proADM was recently reported to be additively prognostic to BNP and NT-proBNP in acute dyspnoea.5,6 Nonetheless, while early results suggest potential value of MR-proANP and/or MR-proADM for the evaluation of patients with acute dyspnoea, considerably more data are necessary before either would be considered validated. Here, we report the results of MR-proANP and MR-proADM for the diagnostic and prognostic evaluation of dyspnoeic patients (with and without ADHF) in the Pro-BNP Investigation of Acute Dyspnoea in the Emergency Department (PRIDE) study.
Methods
Patient population
The design and main results of the PRIDE study are described elsewhere.7 Patients were followed for vital status at 4 years; the follow-up for outcomes was available in 100% of the present cohort.8 For the purposes of this study, 560 subjects had available samples for the analysis of MR-proANP (180 with ADHF), and 565 had sample available for MR-proADM.
Biomarker assessment
NT-proBNP was measured on a Roche Elecsys 2010 platform (Roche Diagnostics, Indianapolis, IN, USA), with routine coefficient of variation (CV) <5%. Both MR-proANP and MR-proADM were measured on a Kryptor platform (Thermo-Fisher, BRAHMS, Germany). The CV for MR-proANP was <6.5%; for MR-proADM, the CV was variable based on detected concentration (<6% for 6 nmol/L and higher; between 6 and 11% for concentrations 0.5–6 nmol/L). For prognosis analyses, results for both estimated glomerular filtration rate (eGFR, utilizing the simplified modification of diet in renal disease equation) and galectin-3 (a macrophage-derived biomarker reflective of cardiovascular fibrosis) were included.9
Correlations
Given non-normality, correlations between NT-proBNP and both MR-proANP and MR-proADM were performed using the Spearman correlation.
Mid-regional pro-atrial natriuretic peptide and mid-regional pro-adrenomedullin in the diagnosis of acutely decompensated heart failure
To evaluate the diagnostic value of MR-proANP and MR-proADM for identifying patients given a gold-standard diagnosis of ADHF, operating characteristics of both for diagnosis were evaluated using receiver operator curve analyses [ROC, with area under the curve (AUC) determination] and compared with NT-proBNP.
Following, concentrations of MR-proANP in those with and without ADHF were compared using the Wilcoxon rank sum test; for those with ADHF, concentrations were also expressed as a function of the New York Heart Association (NYHA) symptom severity classification.
The performance of MR-proANP to identify ADHF was then evaluated using age-adjusted cut-offs [≥104 pmol/L (for age <65 years) and ≥214 pmol/L for age ≥65 years]5 and compared with age-adjusted thresholds for NT-proBNP-based ADHF diagnosis,10 with odds ratio and 95% confidence intervals (CI) generated. A second value for MR-proANP to exclude (or ‘‘rule out’’) ADHF was determined to maximize negative predictive value (NPV), constrained by at least 25% of the overall population in the rule-out group defined by this cut-off.
To identify whether MR-proANP was an independent predictor of the final ADHF diagnosis compared with other variables, a multivariable logistic regression model was constructed using covariates previously found to be significantly associated with HF diagnosis from this cohort7 and fitted with results for MR-proANP. Furthermore, to further test the hypothesis that MR-proANP improves diagnosis of ADHF beyond NT-proBNP, we calculated reclassification statistics for the diagnosis of ADHF and improved model discrimination (using ROC analysis) when MR-proANP was added to a model containing NT-proBNP. Net reclassification improvement (NRI) was calculated using the category-free (or continuous) approach11 with 999 bootstrap replications to estimate the 95% CI.
As the accuracy of both BNP and NT-proBNP has been questioned in specific subgroups such as those with grey zone values,12 the elderly, those with atrial fibrillation, obese patients, and those with renal failure, we specifically examined the diagnostic performance of MR-proANP in such subjects.
Prognostic value of mid-regional pro-atrial natriuretic peptide and mid-regional pro-adrenomedullin in prognosis in acute dyspnoea
Time-dependent AUC analyses were performed to assess the ability of MR-proANP and MR-proADM to discriminate mortality at various time points after index hospitalization (30, 90 days, 1, 4 years). From the ROC curves, optimal cut-points for prognosis were identified, and used to examine the prognostic value of MR-proANP and MR-proADM at 1 and 4 years. Both biomarkers were added to multivariate Cox proportional hazards models containing covariates already known to predict death at each point, as previously described.8,13 Hazard ratio (HR) and 95% CI were generated. In these analyses, MR-proANP and MR-proADM were entered as dichotomous variables, using the ROC-optimal value for each; following, we then entered NT-proBNP, in order to evaluate the individual or additive value of the novel markers for prognosis. C-statistics for Cox models with and without the novel markers were also calculated and compared using the likelihood ratio test. Net reclassification improvement was calculated using the category-free approach11 as above. Kaplan–Meier curves for survival at different time points were constructed and compared using the log-rank test, with MR-proANP and MR-proADM considered separately and in composite; in composite modelling, we once again examined the merits of various combinations of MR-proANP and MR-proADM. We then calculated the HR and 95% CI using the first category as referent. Following, we examined the change in C-statistic from adding the combination of markers, as well as NRI, as described above.
Finally, we performed a subgroup survival analysis of those with ADHF using the same methods as we did for the entire dyspnoeic population.
SAS version 9.2 (Cury, NC, USA) or R version 2.5.1 (http://www.r-project.org, library Design, Hmisc, ROCR) were used for analysis. All P-values are two-sided, with a value <0.05 considered significant.
Results
Baseline patient characteristics
The baseline clinical characteristics for patients stratified by the final diagnosis of ADHF vs. non-ADHF cause of dyspnoea are demonstrated in Table 1.
Table 1.
Baseline characteristics of the study population, stratified by the presence or absence of acutely decompensated heart failure
| Characteristic | Patients without ADHF (n = 380) | Patients with ADHF (n = 180) | P-value |
|---|---|---|---|
| Age | 57 ± 16.3 | 72.8 ± 13.6 | <0.001 |
| Past history (%) | |||
| Diabetes mellitus | 69 (17.7) | 88 (42.1) | <0.001 |
| Prior heart failure | 37 (9.5) | 113 (54.1) | <0.001 |
| Atrial arrhythmias | 35 (9) | 67 (32.1) | <0.001 |
| Obstructive airway disease | 46 (11.8) | 25 (12) | 1.00 |
| Hypertension | 159 (40.8) | 133 (63.6) | <0.001 |
| Coronary artery disease | 79 (20.3) | 88 (42.1) | <0.001 |
| Prior myocardial infarction | 35 (9) | 43 (20.6) | <0.001 |
| Medications on presentation (%) | |||
| Beta-blocker | 112 (28.7) | 116 (55.5) | <0.001 |
| Loop diuretic | 61 (15.6) | 116 (55.5) | <0.001 |
| Digoxin | 17 (4.4) | 47 (22.5) | <0.001 |
| ACE inhibitor | 57 (14.6) | 68 (32.5) | <0.001 |
| Aspirin | 91 (23.3) | 92 (44) | <0.001 |
| Nitrate | 29 (7.4) | 31 (14.8) | 0.006 |
| Physical examination | |||
| Body mass index, kg/m2 | 28.5 ± 7.1 | 27.9 ± 6.3 | 0.40 |
| S3 gallop | 1 (0.3) | 4 (1.9) | 0.053 |
| Heart rate, b.p.m. | 88.2 ± 22.4 | 86.5 ± 23.5 | 0.15 |
| Systolic blood pressure, mmHg | 134.7 ± 26 | 140.1 ± 30 | 0.08 |
| Rales | 56 (14.4) | 100 (47.8) | <0.001 |
| Wheezing | 110 (28.2) | 38 (18.2) | 0.007 |
| Murmur | 27 (6.9) | 40 (19.1) | <0.001 |
| Chest radiography | |||
| Interstitial oedema | 14 (3.6) | 87 (41.6) | <0.001 |
| Infiltrate | 53 (13.6) | 32 (15.3) | 0.62 |
| Cardiomegaly | 15 (3.8) | 35 (16.7) | <0.001 |
| Pleural effusion | 31 (7.9) | 72 (34.4) | <0.001 |
| Laboratory results | |||
| GFR (mL/min/1.73 m2) median (IQR) | 97.5 (67.3–119.9) | 58.8 (35.3–74.1) | <0.001 |
| BUN (mg/dL), median (IQR) | 17.4 (11.2–21) | 29.6 (19–34) | <0.001 |
| Haemoglobin (g/dL) | 13.2 (12.2–14.8) | 12.2 (10.6–13.8) | <0.001 |
| Troponin T > 0.01 ng/mL, n (%) | 28 (7.3) | 89 (42.6) | <0.001 |
| NT-proBNP (pg/mL), median (IQR) | 630.5 (46.6–430) | 8136 (1689–9970) | <0.001 |
| MR-proANP (pmol/L), median (IQR) | 96.8 (32.8–111.1) | 420.3 (204.9–558.4) | <0.001 |
| MR-pro ADM (nmol/L), median (IQR) | 0.50 (0.20–0.70) | 1.20 (0.60–1.60) | <0.001 |
HF, heart failure; GFR, glomerular filtration rate; IQR, inter-quartile range; BUN, blood urea nitrogen; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Correlations between mid-regional peptides and amino-terminal pro-B type natriuretic peptide
Strong correlation between NT-proBNP and MR-proANP was found (ρ = 0.81, 95% CI = 0.77–0.83; P < 0.001). Modest correlations existed between NT-proBNP and MR-proADM (ρ = 0.48, 95% CI = 0.42–0.55; P < 0.001) and MR-proANP and MR-proADM (ρ = 0.56, 95% CI = 0.50–0.61; P < 0.001).
Mid-regional peptides in acutely decompensated heart failure diagnosis
For the diagnosis of ADHF, MR-proANP had an AUC of 0.90 (95% CI = 0.87–0.93; P < 0.001), which was lower than NT-proBNP (AUC = 0.94, 95% CI = 0.92–0.96; P < 0.001; P = 0.001 for difference). Mid-regional pro-adrenomedullin had an AUC of 0.80 for ADHF (95% CI = 0.77–0.84; P < 0.001), significantly lower than either MR-proADM or NT-proBNP (both P < 0.001); accordingly, we focused on MR-proANP for subsequent diagnosis analyses.
Patients with a final diagnosis of ADHF had significantly higher MR-proANP concentrations, compared with those without [329 (100–558) vs. 58 (33–112)pmol/L; P < 0.001]. Additionally, worse NYHA symptom severity was associated with higher MR-proANP concentrations: class II = 287 (226–428)pmol/L, class III = 303 (149–343)pmol/L, and class IV = 393 (234–640)pmol/L (P < 0.001 across categories).
Using an age-adjusted cut-point strategy to diagnose ADHF (age < 65 years ≥104 pmol/L; age ≥ 65 years ≥ 214 pmol/L), MR-proANP had a sensitivity of 82%, specificity of 86%, positive predictive value (PPV) of 73%, and a NPV of 91%. These operating characteristics differed somewhat with NT-proBNP at its own optimal cut-points (sensitivity 90%, specificity 84%, PPV of 88% and NPV of 66%; 10). An MR-proANP < 57 pmol/L had 97% NPV to exclude the diagnosis of ADHF, comparable with an NT-proBNP cut-point <300 pg/mL.14
The operating characteristics of MR-proANP for the diagnosis of ADHF were similar as in the overall cohort in the pre-specified subgroup of older subjects (see Supplementary material online, Table S1); on the other hand, the sensitivity of MR-proANP was reduced in patients with a grey zone NT-proBNP12 and in those with a body mass index ≥30 kg/m2. Atrial fibrillation and eGFR ≤60 mL/min/1.73 m2 both reduced specificity.
In multivariate logistic regression models with and without NT-proBNP, an elevated age-adjusted MR-proANP was an independent predictor of a final diagnosis of ADHF (Table 2). When MR-proANP was added to a model containing NT-proBNP for the diagnosis of ADHF, there was a significant improvement in the AUC of 0.044 (P < 0.001), and led to reclassification of 63% of patients with ADHF and 72% of patients without (total NRI 135%, 95% CI = 1.22–1.48, P < 0.001).
Table 2.
Multivariable logistic regression models (A) without and (B) with amino-terminal pro-B type natriuretic peptide using covariates predictive of acutely decompensated heart failure diagnosis in the PRIDE cohort
| Covariate | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|
| (A) | |||
| Elevated age-adjusted MR-proANPa | 13.75 | 7.51–25.16 | <0.001 |
| Radiographic pulmonary oedema | 10.13 | 4.42–23.25 | <0.001 |
| Orthopnoea | 4.86 | 2.31–10.20 | <0.001 |
| Use of loop diuretic on presentation | 2.96 | 1.62–5.41 | <0.001 |
| Presence of rales on exam | 2.48 | 1.27–4.86 | 0.008 |
| Age (per decade) | 1.68 | 1.35–2.10 | <0.001 |
| Cough | 0.56 | 0.30–1.05 | 0.07 |
| Fever | 0.16 | 0.05–0.48 | 0.001 |
| (B) | |||
| Elevated age-adjusted MR-proANPa | 4.34 | 2.11–8.92 | <0.001 |
| Elevated age-adjusted NT-proBNPb | 9.73 | 4.63–20.43 | <0.001 |
| Radiographic pulmonary oedema | 7.28 | 3.11–17.04 | <0.001 |
| Orthopnoea | 6.60 | 2.87–15.17 | <0.001 |
| Use of loop diuretic on presentation | 2.87 | 1.50–5.49 | <0.001 |
| Presence of rales on exam | 2.26 | 1.11–4.60 | 0.03 |
| Age (by decade) | 1.74 | 1.37–2.21 | <0.001 |
| Cough | 0.47 | 0.24–0.91 | 0.03 |
| Fever | 0.13 | 0.04–0.40 | <0.001 |
NT-proBNP, N-terminal pro-B-type natriuretic peptide.
aElevated age-adjusted MR-proANP was defined as ≥104 pmol/L (for age <65 years), and ≥214 pmol/L for age ≥65 years).
bElevated NT-proBNP was defined as ≥450 pg/mL for age < 50 years, ≥900 pg/mL for ages 50–75 years, and ≥1800 pg/mL for age >75 years.
Mid-regional pro-atrial natriuretic peptide and mid-regional pro-adrenomedullin as prognostic markers for patients presenting with acute dyspnoea
Time-dependent ROC curves for death to 4 years for various biomarkers are shown in Figure 1. At 1 and 4 years, there were 107 and 189 deaths in the overall cohort. Concentrations of MR-proADM had consistently superior ability to discriminate mortality across the initial year from enrolment (Figure 1A), while after 1 year, both NT-proBNP and MR-proANP discriminated mortality better than MR-proADM (Figure 1B). The estimated glomerular filtration rate showed strong ability to predict risk early on after presentation, but this value waned over time, while galectin-3 showed consistently lower AUC across the entire time horizon.
Figure 1.
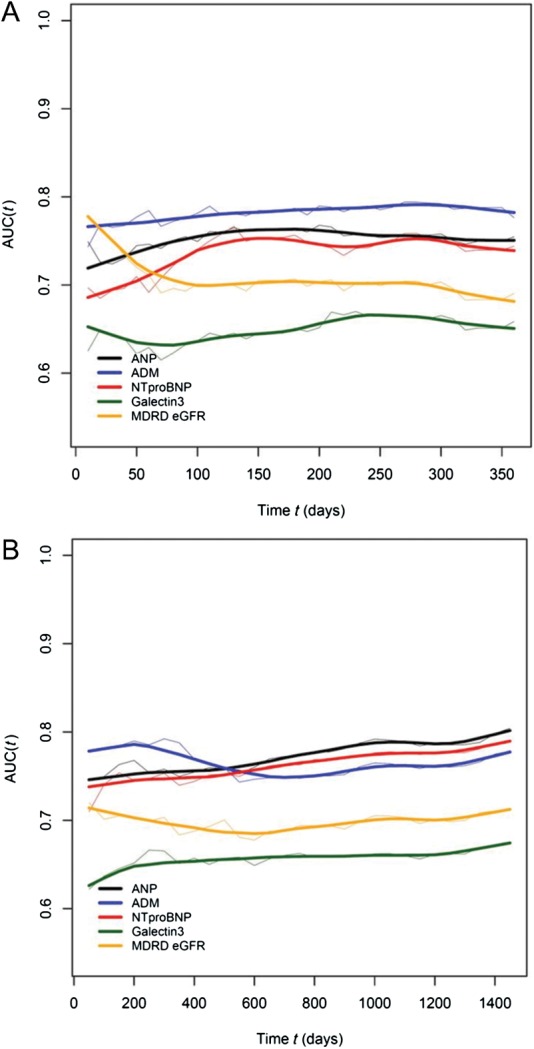
Time dependent changes in the area under the curve for multiple biomarkers in acutely dyspnoeic patients for discrimination of mortality at (A) 1-year and (B) 4-year follow-up. Grey lines reflect 95% confidence intervals.
Base models for death at 1 year containing NT-proBNP had a C-statistic of 0.760; the addition of MR-proANP results resulted in significant improvement in the C-statistic to 0.774 (P = 0.02). In a similar fashion, addition of MR-proADM considerably increased the C-statistic to 0.792 (P < 0.001). In a multivariate Cox proportional hazards model that included previously described variables predictive of death at 1 year in the PRIDE,13 at optimal cut-points, both MR-proANP (HR = 2.99; P < 0.001) and MR-proADM (HR = 2.70; P < 0.001) were predictive of death (see Supplementary material online, Table S2) and reclassified risk (see Supplementary material online, Table S3).
At 4 years, base models containing NT-proBNP had a C-statistic of 0.776; addition of MR-proANP and MR-proADM to this model resulted in significant improvement in the C-statistic to 0.796 (P < 0.001). In a multivariate Cox proportional hazards model that included previously described variables predictive of death at 4 years in the PRIDE,8 both MR-proANP (HR = 3.12, 95% CI = 1.85–5.27; P < 0.001) and MR-proADM (HR = 1.51, 95% CI = 1.03–2.20; P = 0.03) were predictive of death (Table 3). Moreover, addition of NT-proBNP results to the model did not attenuate the predictive value of MR-proANP or MR-proADM at 4 years (Table 3). We then calculated continuous NRI for a model containing MR-proADM and MR-proANP on the top of our previously established clinical risk model for death at 4 years. A model containing both mid-regional peptides in addition to NT-proBNP had a continuous NRI of 75% (95% CI = 60–93%; P < 0.0001), driven by correct reclassification of mortality in 22% (P = 0.004) and survival in 53% (P < 0.0001; Table 4A). These results were similar to models without NT-proBNP at 4 years (Table 4B).
Table 3.
Multivariable Cox proportional hazards analysis for 4-year survival for both mid-regional pro-atrial natriuretic peptide and mid-regional pro-adrenomedullin with (A) and without (B) amino-terminal pro-B type natriuretic peptide in the model
| Covariate | Hazard ratio | 95% Confidence interval | P-value |
|---|---|---|---|
| (A) | |||
| Age ≥75 years | 1.55 | 1.11–2.17 | 0.01 |
| NT-proBNP ≥986 pg/mL | 1.34 | 0.82–2.22 | 0.25 |
| Haemoglobin (per 20 g/L increase) | 0.88 | 0.82–0.95 | 0.0006 |
| Active tobacco use | 0.78 | 0.57–1.08 | 0.13 |
| Aldosterone use on presentation | 2.04 | 1.17–3.56 | 0.01 |
| MR-proANP ≥194 pmol/L | 3.12 | 1.85–5.27 | <0.0001 |
| MR-proADM ≥0.77 nmol/L | 1.51 | 1.03–2.20 | 0.03 |
| (B) | |||
| Age ≥75 years | 1.55 | 1.11–2.16 | 0.01 |
| Haemoglobin (per 20 g/L increase) | 0.88 | 0.82–0.95 | 0.0005 |
| Active tobacco use | 0.79 | 0.57–1.09 | 0.15 |
| Aldosterone use on presentation | 2.05 | 1.18–3.56 | 0.01 |
| MR-proANP >194 pmol/L | 3.86 | 2.56–5.82 | <0.0001 |
| MR-proADM >0.77 nmol/L | 1.57 | 1.09–2.28 | 0.02 |
Table 4.
Reclassification analyses for a model containing mid-regional pro-atrial natriuretic peptide and mid-regional pro-adrenomedullin in addition to a clinical risk model with (A) and without (B) amino-terminal pro-B type natriuretic peptide for death at 4 years, utilizing the continuous version of the net reclassification improvement approach
| MR-proANP and MR-proADM with NT-proBNP | All | Reclassified upwards | Reclassified downwards | NRI |
P-value | |
|---|---|---|---|---|---|---|
| (A) | ||||||
| Kaplan–Meier rate of event | 31.1% | 54.2% | 18.6% | |||
| Expected number of event subjects | 170 | 104 | 66 | Among event subjects | 22% | 0.004 |
| Expected number of non-event subjects | 376 | 88 | 288 | Among non-event subjects | 53% | <0.001 |
| Overall (95% bootstrap CI) | 75.5% (59.5–92.8%) | <0.001 | ||||
| MR-proANP and MR-proADM without NT-proBNP | All | Reclassified upwards | Reclassified downwards | NRI | P-value | |
| (B) | ||||||
| Kaplan–Meier rate of event | 30.9% | 55.2% | 17.9% | |||
| Expected number of event subjects | 170 | 106 | 64 | Among event subjects | 25% | 0.0013 |
| Expected number of non-event subjects | 380 | 86 | 294 | Among non-event subjects | 55% | <0.001 |
| Overall (95% bootstrap CI) | 79.4% (62.6–96.3%) | <0.001 | ||||
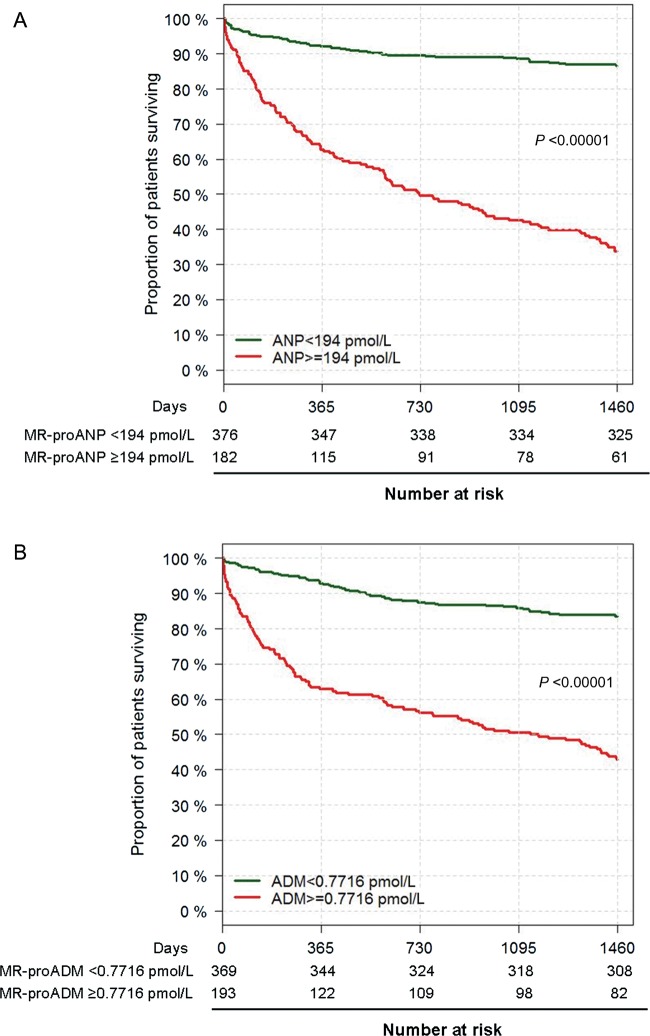
In Kaplan–Meier analysis for survival based on ROC-determined cut-offs for both biomarkers, patients with above-cut-off concentrations of both MR-proANP and MR-proADM had dramatically reduced survival for patients with acute dyspnoea (Figure 2). Left censoring of the data for MR-proADM beginning at 1 year showed persistent divergence of the Kaplan–Meier curves (P < 0.001).
Figure 2.
Kaplan–Meier survival curves for patients with acute dyspnoea with follow-up to 4 years for (A) mid-regional pro-atrial natriuretic peptide and (B) mid-regional pro-adrenomedullin.
Given the independent value of both MR-proANP and MR-proADM for prognosis, we examined their additive value to predict risk. There was significant divergence of Kaplan–Meier curves out to 4 years, particularly in those with both peptides elevated (Figure 3). Given this, we constructed multivariable Cox models that examined the combination of ‘both markers low’’, ‘one marker elevated’’, and ‘both markers elevated’’ to examine the incremental risk predicted by these combinations. Compared with the risk of both markers low (referent), one elevated marker predicted intermediate risk (HR = 2.82, 95% CI = 1.76–4.54; P < 0.001), and with both markers elevated, the highest risk was observed (HR = 6.07, 95% CI = 3.84–9.60; P < 0.001). Adding the information of multimarker testing on the top of clinical variables increased the C-statistic from 0.74 to 0.79 (P < 0.001), and considering patients with this multimarker approach considerably improved the continuous NRI (86%; due to correct reclassification of events by 31% and correct reclassification of non-events by 55%).
Figure 3.
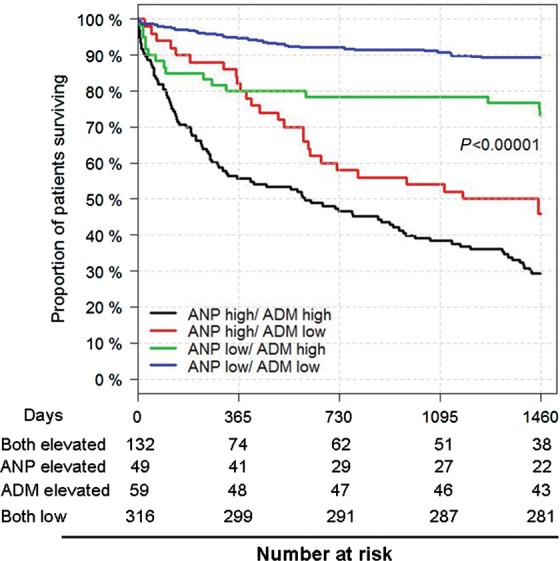
Combination of mid-regional pro-atrial natriuretic peptide and mid-regional pro-adrenomedullin for multimarker risk assessment in dyspnoea. Patients with mid-regional pro-atrial natriuretic peptide >194 pmol/L and mid-regional pro-adrenomedullin >0.77 nmol/L had the highest risk.
Mid-regional pro-atrial natriuretic peptide and mid-regional pro-adrenomedullin as prognostic markers for patients presenting with acutely decompensated heart failure
The 180 ADHF patients with both MR-proANP and MR-proADM results were studied in a similar fashion as the entire cohort, with comparable prognostic importance as in the cohort as a whole (see Supplementary material online, Figures S1 and S2).
Discussion
In patients with acute dyspnoea, establishing an early and certain cause for the presence or absence of ADHF is crucially important, while establishing severity of presentation and ascertainment of risk stratification may be useful for treatment decision-making and planning early follow-up; such risk assessment is recommended by clinical practice guidelines.2
In the present study, we found MR-proANP to be accurate for the identification or exclusion of ADHF in dyspnoeic patients. Intriguingly, addition of MR-proANP results to NT-proBNP improved diagnostic accuracy by correctly reclassifying patients with and without ADHF. In addition, results from both MR-proANP and MR-proADM (the latter secreted by a variety of organs in response to oxidative stress and tissue hypoxia) were independent predictors of short- to longer-term prognosis in patients with acute dyspnoea with or without ADHF. Measurement of both MR-proANP and MR-proADM reclassified risk individually, with their conjoint measurement adding even more reclassification. Our analysis—performed with a rigour recommended by current standards3—provides good evidence for the diagnostic and prognostic importance of these novel biomarkers.
In the largest published study of MR-proANP in undifferentiated dyspnoea [the Biomarkers in Acute Heart Failure (BACH) study],5 An MR-proANP was independently predictive of a diagnosis of ADHF beyond BNP concentration. Utilizing age-adjusted cut-offs, we found that MR-proANP had a lower sensitivity but a higher specificity and PPV, compared with the results in BACH. Interestingly, even in the context of NT-proBNP results, MR-proANP remained independently predictive of ADHF. It is intriguing that two natriuretic peptides from separate methods of synthesis and sites of origin (ANP is synthesized mainly in the atrium and stored as granules, while BNP is synthesized and released de novo mainly in the ventricles) add independent diagnostic information for diagnosis and raise the possibility that these two classes of peptides may occasionally impart different clinical information.
In BACH, it was suggested that MR-proANP might assist in clarifying diagnostic uncertainty in the context of areas of vulnerability for BNPs, such as with grey zone results,12 obesity, or in patients with impaired renal function. Our results do not support this contention, however. Importantly, the effects of atrial fibrillation were particularly negative on MR-proANP; given the primarily atrial site for MR-proANP synthesis, it seems plausible that arrhythmias arising in the atrium may lead to excessive release of ANP, independent of ADHF status. Indeed, intense ANP-mediated diuresis following termination of supraventricular tachyarrhythmia has been described.14 Clinicians should be aware that while MR-proANP has generally similar vulnerabilities as NT-proBNP, the effects of atrial fibrillation are particularly significant on MR-proANP.
The prognostic value of both MR-proANP and MR-proADM was substantial in our analysis, and our results considerably extend the findings of the BACH study, which followed patients out to 90 days. Short-term prognostic value is probably most impactful from a decision-making perspective; however, the longer-term data provided in our analysis are certainly novel, extending well beyond current time horizons studied for these markers, and suggest that the combination of MR-proADM together with a natriuretic peptide would be expected to provide important information regarding risk in acute dyspnoea across short, intermediate, and longer term. Our data do not inform whether the measurement of MR-proANP or MR-proADM may be used for therapeutic monitoring or guidance; given their prognostic value, MR-proADM and MR-proANP should now be scrutinized for this application as has been done for NT-proBNP.15
As clinicians have numerous biomarker tools to evaluate risk, our results are informative; in PRIDE, the information afforded from the combination of a natriuretic peptide and MR-proADM was above and beyond renal function assessment or galectin-3. The latter, a biomarker of fibrosis was found in a prior study from this data set to be additively prognostic to natriuretic peptides for risk stratification;9 in the present analysis, galectin-3 did not remain in the model in the presence of MR-proADM, suggesting MR-proADM to be superior for risk assessment in PRIDE.
Our study has several limitations. Our hazard modelling at 30 and 90 days was insufficiently powered, and thus hypothesis generating. However, the relationships we observed in the short term were largely consistent with other time points. We did not examine relationships between cardiac structure and function and MR-proADM or MR-proANP, although such analyses are planned. We do not have serial biomarker measurements, such as following ADHF treatment, which should be considered an important study to follow analyses such as ours and the BACH study, which focused on baseline values only. This limitation not withstanding, the biorepository from the PRIDE study is well-recognized for the evaluation of novel biomarkers in acute dyspnoea and ADHF.
In conclusion, we found firm evidence of diagnostic value for MR-proANP, and prognostic value for both MR-proANP and MR-proADM in patients with acute dyspnoea. Future work studying the relationships between these mid-regional pro-peptide fragments and cardiac structure and function, as well as studies of their value for biomarker-guided HF therapy is planned.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by a grant from BRAHMS GmBH. R.V.S. is supported by an American Heart Association Post-Doctoral Fellowship Award (11POST000002) and a training grant from the Heart Failure National Institutes of Health Clinical Research Network (U01-HL084877). H.K.G. is supported by the Dennis and Marilyn Barry Clinical Research Fellowship. Q.A.T. is supported by National Institutes of Health grant K23HL098370 and L30HL093896. J.L.J. is supported by a grant from the Balson Clinical Scholar Fund.
Conflict of interest: J.L.J. has received grants from Roche Diagnostics, Siemens Diagnostics, Critical Diagnostics, and BRAHMS, GmBH.
Supplementary Material
References
- 1.Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, Witteman JC, Stricker BH. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004;25:1614–1619. doi: 10.1016/j.ehj.2004.06.038. doi:10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 2.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–989. doi: 10.1016/j.ejheart.2008.08.005. doi:10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 3.van Kimmenade RR, Januzzi JL., Jr Emerging biomarkers in heart failure. Clin Chem. 2012;58:127–138. doi: 10.1373/clinchem.2011.165720. doi:10.1373/clinchem.2011.165720. [DOI] [PubMed] [Google Scholar]
- 4.Jougasaki M, J B. Adrenomedullin: potential in physiology and pathophysiology. Life Sci. 2000;66:885–872. doi: 10.1016/s0024-3205(99)00358-6. doi:10.1016/S0024-3205(99)00672-4. [DOI] [PubMed] [Google Scholar]
- 5.Maisel A, Mueller C, Nowak R, Peacock WF, Landsberg JW, Ponikowski P, Mockel M, Hogan C, Wu AH, Richards M, Clopton P, Filippatos GS, Di Somma S, Anand I, Ng L, Daniels LB, Neath SX, Christenson R, Potocki M, McCord J, Terracciano G, Kremastinos D, Hartmann O, von Haehling S, Bergmann A, Morgenthaler NG, Anker SD. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J Am Coll Cardiol. 2010;55:2062–2076. doi: 10.1016/j.jacc.2010.02.025. doi:10.1016/j.jacc.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Maisel A, Mueller C, Nowak R, Peacock WF, Ponikowski P, Mockel M, Hogan C, Wu AH, Di Somma S, Anand I, Ng L, Daniels LB, Neath SX, Christenson R, Potocki M, McCord J, Hartmann O, Morgenthaler N, A S. Midregion prohormone adrenomedullin and prognosis in patients presenting with acute dyspnea: results from the BACH study. J Am Coll Cardiol. 2011;58:1057–1067. doi: 10.1016/j.jacc.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Januzzi JL, Camargo C, Anwaruddin S, Baggish A, Chen A, Krauser D, Tung R, Cameron R, Nagurney J, Chae C, Lloyd-Jones D, Brown D, Foran-Melanson S, Sluss P, Lee-Lewandrowski E, L K. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95:948–954. doi: 10.1016/j.amjcard.2004.12.032. doi:10.1016/j.amjcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 8.Januzzi JL, Jr, Rehman S, Mueller T, van Kimmenade RR, Lloyd-Jones DM. Importance of biomarkers for long-term mortality prediction in acutely dyspneic patients. Clin Chem. 2010;56:1814–1821. doi: 10.1373/clinchem.2010.146506. doi:10.1373/clinchem.2010.146506. [DOI] [PubMed] [Google Scholar]
- 9.Shah R, Chen-Tournoux A, Picard M, van Kimmenade R, Januzzi J. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12:826–832. doi: 10.1093/eurjhf/hfq091. doi:10.1093/eurjhf/hfq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Januzzi J, van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, Pinto Y, Richards M. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2005;27:330–337. doi: 10.1093/eurheartj/ehi631. doi:10.1093/eurheartj/ehi631. [DOI] [PubMed] [Google Scholar]
- 11.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Statistics in medicine. 2011;30:11–21. doi: 10.1002/sim.4085. doi:10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Kimmenade RR, Pinto YM, Bayes-Genis A, Lainchbury JG, Richards AM, Januzzi JL., Jr Usefulness of intermediate amino-terminal pro-brain natriuretic peptide concentrations for diagnosis and prognosis of acute heart failure. Am J Cardiol. 2006;98:386–390. doi: 10.1016/j.amjcard.2006.02.043. doi:10.1016/j.amjcard.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 13.Januzzi J, Sakhuja R, O'Donoghue M, Baggish A, Anwaruddin S, Chae C, Cameron R, Krauser D, Tung R, Camargo C, Lloyd-Jones D. Utility of amino-terminal pro-brain natriuretic peptide testing for prediction of 1-year mortality in patients with dyspnea treated in the emergency department. Arch Intern Med. 2006;166:315–320. doi: 10.1001/archinte.166.3.315. doi:10.1001/archinte.166.3.315. [DOI] [PubMed] [Google Scholar]
- 14.Abe H, Nagatomo T, Kobayashi H, Miura Y, Araki M, Kuroiwa A, Nakashima Y. Neurohumoral and hemodynamic mechanisms of diuresis during atrioventricular nodal reentrant tachycardia. Pacing Clin Electrophysiol. 1997;20:2783–2788. doi: 10.1111/j.1540-8159.1997.tb05436.x. doi:10.1111/j.1540-8159.1997.tb05436.x. [DOI] [PubMed] [Google Scholar]
- 15.Januzzi JL, Jr, Rehman SU, Mohammed AA, Bhardwaj A, Barajas L, Barajas J, Kim HN, Baggish AL, Weiner RB, Chen-Tournoux A, Marshall JE, Moore SA, Carlson WD, Lewis GD, Shin J, Sullivan D, Parks K, Wang TJ, Gregory SA, Uthamalingam S, Semigran MJ. Use of amino-terminal pro-B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol. 2011;58:1881–1889. doi: 10.1016/j.jacc.2011.03.072. doi:10.1016/j.jacc.2011.03.072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.