Abstract
Aims
The availability of new antithrombotic agents, each with a unique efficacy and bleeding profile, has introduced a considerable amount of clinical uncertainty with physicians. We have developed a clinical decision aid in order to assist clinicians in determining an optimal antithrombotic regime for the prevention of stroke in patients who are newly diagnosed with non-valvular atrial fibrillation.
Methods and results
The CHA2DS2-VASc and HAS-BLED scoring systems were used to assess patients’ baseline risks of stroke and major bleeding, respectively. The relative risks of stroke and major bleeding for each antithrombotic agent were then used to identify the agent associated with the lowest net risk. Individual patient factors such as the treatment threshold, bleeding ratio, and cost threshold modified the recommendations in order to generate a final recommendation. By considering both patient factors and clinical research concurrently, this clinical decision aid is able to provide specific advice to clinicians regarding an optimal stroke prevention strategy. The resulting treatment recommendation tables are consistent with the recommendations of the European Society of Cardiology and Canadian Cardiovascular Society Guidelines, which can be incorporated into either a paper-based or electronic format to allow clinicians to have decision support at the point of care.
Conclusion
The use of a clinical decision aid that considers both patient factors and evidence-based medicine will serve to bridge the knowledge gap and provide practical guidance to clinicians in the prevention of stroke due to atrial fibrillation.
Keywords: Atrial fibrillation, Stroke prevention, Decision aid, Antithrombotic, Anticoagulation
Introduction
Until recently the antithrombotic therapies available to patients for the prevention of stroke due to atrial fibrillation (PAF) have been fairly limited and have included: warfarin, an oral anticoagulant (OAC); acetylsalicylic acid (ASA), an antiplatelet agent; or no treatment. Accordingly, the ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation were relatively uncomplicated.1 These guidelines recommended treating patients with any high-risk factor or more than one moderate-risk factor for stroke with OAC, patients with one moderate-risk factor with either an OAC or ASA, and patients with no risk factors with ASA. Warfarin reduces the risk of stroke by 64%,2 but it has a narrow therapeutic window and an unpredictable response that requires routine coagulation monitoring and frequent dose adjustments.
Three new OAC agents have been introduced in the last year as alternative therapies for PAF. Dabigatran is an oral direct thrombin inhibitor that has recently been approved for PAF. In the RE-LY study,3 dabigatran 150 mg b.i.d. was associated with lower rates of stroke and systemic embolism (SSE) but a similar rate of major bleeding, while dabigatran 110 mg b.i.d. was associated with similar rates of SSE but lower rates of major bleeding, when compared with warfarin. Apixaban and rivaroxaban are oral direct factor Xa inhibitors. The ARISTOTLE study4 demonstrated that apixaban 5 mg b.i.d. was associated with lower rates of SSE and lower rates of major bleeding when compared with warfarin. In contrast, an intention to treat analysis of the ROCKET-AF study5 demonstrated that rivaroxaban 20 mg o.d., when compared with warfarin, was associated with similar rates of SSE and with similar rates of major bleeding. The recent or imminent approval of these medications, each with a unique efficacy and bleeding profile, expands the options available to physicians and patients.
At the same time, major bleeding has increasingly been recognized as an important factor that must be considered when determining an optimal stroke prevention strategy. Risk stratification schemas such as HEMORRHAGES6 and HAS-BLED7–9 have been developed to quantify patients’ risk of bleeding. The annual risk of bleeding, when combined with the annual risk of stroke as determined by scoring systems such as CHADS210 or CHA2DS2-VASc,11–13 contributes to the concept of net risk.
As such, the need to consider both the stroke risk and the bleeding risk (i.e. net risk), together with the availability of new antithrombotic agents, has introduced some uncertainty among clinicians as they attempt to formulate the most appropriate PAF strategy for each individual patient. Current guidelines14,15 now recommend an assessment of the bleeding risk as part of the overall patient assessment before starting anticoagulation, and some agencies are now calling for the development of clinical decision aids that consider individual patient's preferences regarding the relative importance of preventing strokes and risking bleeds,16 however, they provide little direction in terms of applying this information in clinical practice. Moreover, current guidelines provide little guidance on selecting a single OAC from the available options.
We have developed a clinical decision aid to assist clinicians in determining an optimal antithrombotic regime for the prevention of stroke in patients who are newly diagnosed with non-valvular atrial fibrillation (AF). The decision aid considers a patient's absolute risk of stroke, absolute risk of bleeding, the relative stroke risk reduction, and the relative bleeding risk increase associated with each antithrombotic agent to identify the agent associated with the lowest net risk. The treatment recommendations may be modified by the patient's treatment threshold, bleeding ratio, and cost threshold.
The aim of this article is to define the statistical methodology that is required to create the clinical decision aid, to present the resulting treatment recommendations that were derived from this analysis, and to discuss the practical implications of incorporating these treatment recommendations into clinical practice guidelines, continuing medical education (CME) activities, and clinical practice.
Methods
Determination of baseline risks of systemic embolism and major bleeding
We utilized the Birmingham 2009 Stroke Risk Stratification Schema,11 also known by the acronym CHA2DS2-VASc (Congestive heart failure, Hypertension, Age ≥75 years, Diabetes, previous Stroke/TIA, Vascular disease, Age 65–74 years, and Sex category), to estimate the annual absolute risk of SSE.
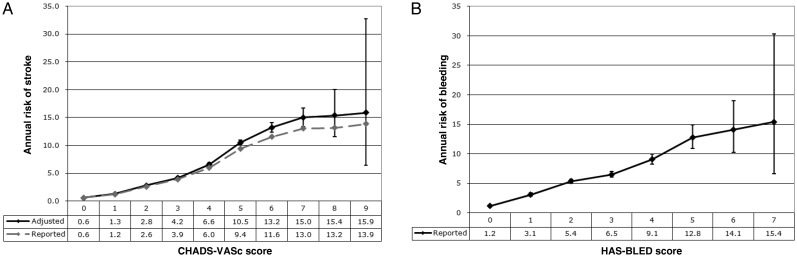
We derived a theoretical annual risk of stroke without treatment by adjusting SSE rates from a large cohort (n = 73 538) of ‘real world’ patients in the Danish National Patient Registry13 who have non-valvular AF and were not treated with warfarin. We chose to utilize the 10-year follow-up data from this study because the one-year follow-up data may overestimate the true event rate, given that all of the patients who were included in the analysis had recently been admitted to hospital. The rates in the Danish non-OAC cohort were adjusted to account for antiplatelet use within each group, assuming that antiplatelet use confers a 22% relative risk (RR) reduction.2 The adjusted rates were calculated by prorating the patient years according to the percentage of patients within each group who were taking antiplatelets, and then dividing the number of events by the adjusted patient years. The reported and adjusted annual risks of SSE by the CHA2DS2-VASc score are shown in Figure 1A. The event rates and exact confidence intervals were calculated independently for each score assuming a Poisson distribution.
Figure 1.
Annual risks of stroke and bleeding. (A) Annual risk (%/year) of stroke and systemic embolism (SSE) by the CHA2DS2-VASc score. Source: Danish National Patient Registry, 10-year follow-up rates.13 The data used included admission to hospital with or death from thromboembolic events such as ischaemic stroke and peripheral artery embolism. Pulmonary embolism events were excluded. The reported rates were adjusted to account for the use of antiplatelet agents. The decision aid utilizes the adjusted rates shown on the black line to determine patients’ annual risk of SSE with no treatment. (B) Annual risk (%/year) of major bleeding by the HAS-BLED score. Source: Danish National Patient Registry, 1-year incidence.9 Major bleeding was defined as any bleeding requiring hospitalization and/or causing a decrease in haemoglobin >2 g/L and/or requiring blood transfusion. The decision aid utilizes these values to determine patients’ annual risk of bleeding with no treatment.
We utilized the HAS-BLED risk stratification schema to determine the annual absolute risk of major bleeding (bleeding) in patients with AF.7,8 Once again we used incidence rates from an analysis of a cohort of AF patients in the Danish National Patient Registry who were not on OAC therapy.9 The risk of bleeding by the HAS-BLED score is shown in Figure 1B. Despite the large cohort (n = 73 813) there were insufficient data to provide a reliable estimate of the risk of bleeding for patients with HAS-BLED scores greater than seven.
More details about the CHA2DS2-VASc and HAS-BLED scoring systems, as well as the data used for the estimation of annual baseline risks of SSE and bleeding are available as Supplementary material online, Methods and results.
Analysis of antithrombotic therapies
The decision aid compares the following treatment options: no treatment, ASA, ASA plus clopidogrel 75 mg o.d. (ASA + clopidogrel), warfarin, dabigatran 110 mg b.i.d. (dabigatran 110), dabigatran 150 mg b.i.d. (dabigatran 150), apixaban 5 mg b.i.d. (apixaban), and rivaroxaban 20 mg o.d. (rivaroxaban).
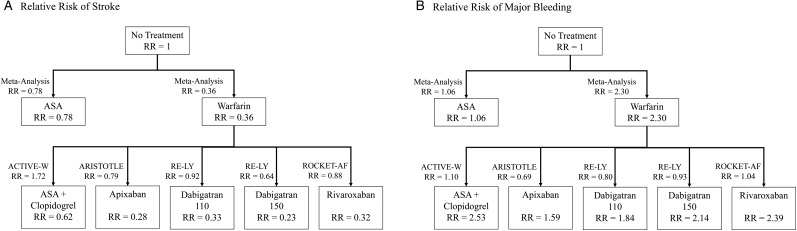
The 2007 meta-analysis of antithrombotic therapies by Hart et al.2 was the source for the annual RRs of stroke and major bleeding for ASA and warfarin. We reviewed the following randomized control trials to determine the annual RRs of stroke and major bleeding for the newer antithrombotic agents: ACTIVE-W17 (ASA + clopidogrel), RE-LY3 (dabigatran 110 and dabigatran 150), ARISTOTLE4 (apixaban), and ROCKET-AF5 (rivaroxaban). Because all of these trials used warfarin as the control, we also calculated the theoretical RR of stroke and the RR of major bleeding relative to no treatment by multiplying the reported RR of the intervention by the RR of warfarin. It was necessary to derive theoretical RRs in order to make comparisons between therapies and in order to estimate appropriately how a patient's risks change with the initiation of antithrombotic therapy.
Table 1 shows the data used to calculate the theoretical RRs of stroke and bleeding relative to no treatment. Figure 2 illustrates the method by which we used the reported RRs to derive the theoretical RRs of stroke and major bleeding for each antithrombotic therapy.
Table 1.
Relative risks of stroke and major bleeding of antithrombotic therapies
| Study | Intervention | Control | AR of stroke intervention (%/year) | AR of stroke control (%/year) | RR of stroke (95% CI) relative to control | RR of stroke relative to no treatment | AR of bleed intervention (%/year) | AR of bleed control (%/year) | RR of bleed (95% CI) relative to control | RR of bleed relative to no treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| Meta-analysis | ASA | No treatment or placebo | 5.22 | 6.33 | 0.78 (0.61–0.98) | 0.78 | N/A | N/A | 1.06 (0.48–1.98) | 1.06 |
| Meta-analysis | Warfarin | No treatment or placebo | 2.21 | 6.03 | 0.36 (0.26–0.51) | 0.36 | N/A | N/A | 2.30 (1.08–4.89) | 2.30 |
| ACTIVE-W | ASA + clopidogrel | Warfarin | 2.39 | 1.40 | 1.72 (1.24–2.37) | 0.62 | 2.42 | 2.21 | 1.10 (0.83–1.45) | 2.53 |
| RE-LY | Dabigatran 110 | Warfarin | 1.44 | 1.57 | 0.92 (0.74–1.13) | 0.33 | 2.71 | 3.36 | 0.80 (0.69–0.93) | 1.84 |
| RE-LY | Dabigatran 150 | Warfarin | 1.01 | 1.57 | 0.64 (0.51–0.81) | 0.23 | 3.11 | 3.36 | 0.93 (0.81–1.07) | 2.14 |
| ARISTOTLE | Apixaban | Warfarin | 1.27 | 1.60 | 0.79 (0.65–0.95) | 0.28 | 2.13 | 3.09 | 0.69 (0.60–0.80) | 1.59 |
| ROCKET-AF | Rivaroxaban | Warfarin | 2.12 | 2.42 | 0.88 (0.75–1.03) | 0.32 | 3.60 | 3.45 | 1.04 (0.90–1.20) | 2.39 |
AR, absolute risk; RR, relative risk; Bleed, major bleeding event. The ‘RR of stroke relative to no treatment’ values for newer agents (not ASA or warfarin) are calculated by multiplying the ‘RR of stroke relative to control’ by the RR of the control (warfarin) relative to no treatment or placebo (0.36). Similarly, the ‘RR of bleed relative to no treatment’ values for newer agents are calculated by multiplying the ‘RR of bleed relative to control’ by the RR of bleed of the control (warfarin) relative to no treatment or placebo (2.30). The RR of stroke relative to no treatment and the RR of bleed relative to no treatment are used by the decision aid to calculate patients’ risks of stroke and bleed with each treatment option.
Figure 2.
Derivation of relative risks of stroke and major bleeding relative to no treatment. (A) Derivation of the risk of stroke relative to no treatment. (B) Derivation of the risk of bleeding relative to no treatment. The values next to the arrows are the relative risk values reported in the relevant study. The relative risk values inside each child box are the product of the parent box and the study relative risk. The relative risk values inside each therapy box are utilized by the decision aid to calculate patients’ risks of stroke and bleeding with treatment.
Generation of treatment recommendation tables
For each combination of CHA2DS2-VASc and HAS-BLED scores, we calculated the net risk for every antithrombotic treatment option, and compared it with the net risk on no treatment in order to determine the antithrombotic regime with the lowest attributable net risk. Net risk is defined as the annual absolute risk of either SSE or Major Bleeding, and is calculated as 1 minus the risk of neither SSE nor Major Bleeding, where the risk of neither SSE nor Major Bleeding is equal to the product of the risk of no SSE and the risk of no Major Bleeding. For full details on how the net risk is calculated, see Supplementary material online, Methods and results.
The antithrombotic treatment option that was associated with the lowest attributable net risk for each combination of the CHA2DS2-VASc and the HAS-BLED scores was deemed to be the optimal antithrombotic regime for that particular cell in the treatment recommendation table. In the instance where all antithrombotic therapies have a net risk that is greater than or equal to the patient's net risk without treatment, no treatment was recommended.
The treatment recommendation tables may be modified according to individual patient factors such as the treatment threshold, bleeding ratio, and cost threshold.
The treatment threshold is defined as the minimum absolute risk reduction (ARR) of SSE that a patient expects to realize in order to agree to initiate antithrombotic therapy. An individual's treatment threshold is dependent upon many factors, including the patient's baseline annual absolute risk of SSE, quality of life priorities and attitudes about medication. The decision aid eliminates antithrombotic therapies that have an ARR of SSE less than the treatment threshold.
The bleeding ratio is defined as the maximum number of major bleeding events that a patient would be willing to endure in order to prevent one SSE. A low bleeding ratio indicates a high priority on safety. A high bleeding ratio indicates a higher priority on efficacy. This concept was introduced because recent evidence suggests that stroke is a much more important outcome to most patients than bleeding,18 and therefore these two outcomes should be weighed accordingly when determining the net risk. The details about how the bleeding ratio affects the net risk calculation and therefore the decision aid's final recommendation are presented in Supplementary material online, Methods and results.
Finally, the cost threshold is defined as the maximum amount of money that a patient is willing/able to pay per day in order to realize the treatment threshold. The decision aid eliminates antithrombotic therapies that cost more than the cost threshold.
Results
Using the methodology outlined above, we developed a website19 and an iPAD Application20 that generate treatment recommendation tables according to user-specified criteria. The treatment recommendation tables could also be incorporated into a printed format (printable clinical decision aid available as Supplementary material online), such as a pocket card, note pad, or slide rule, or incorporated into CME activities. Here and in Supplementary material online, we provide examples of treatment recommendation tables for some common clinical scenarios.
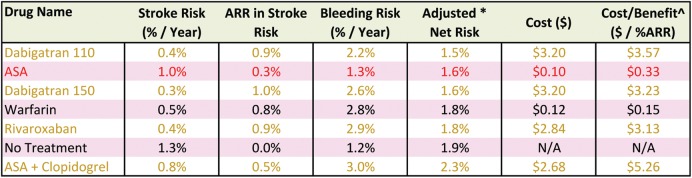
The electronic versions of the decision aid provide clinicians with validation tables showing the detailed calculations underlying the determination of the optimal treatment option for any given cell in the treatment recommendation table. Figure 3 is an example of the validation table for the CHA2DS2-VASc = 1 and HAS-BLED = 0 cell of the treatment recommendation table in which the treatment threshold = 0.5% [Number needed to treat (NNT) = 200], the bleeding ratio = 2:1, and the cost threshold = $0.50. The seven options available in Ontario are listed in order of the net risk. In this case, warfarin is the recommended treatment because it has a lower net risk than no treatment. Although ASA's net risk is lower than warfarin, it was excluded because its ARR did not reach the treatment threshold of 0.5%. Both Dabigatran 110 and Dabigatran 150 were associated with a lower net risk than warfarin, but they were excluded because their cost exceeded the cost threshold of $0.50.
Figure 3.
Validation table for a single cell in the treatment recommendation table. Sample validation table from the website version of the clinical decision aid, which is available for each cell of the treatment recommendation table. This validation table is for the CHA2DS2-VASc score = 1, HAS-BLED score = 0 cell of the treatment recommendation table with settings: treatment threshold = 0.5%, bleeding ratio = 2:1, cost threshold = $0.50, and jurisdiction = Ontario, Canada. ARR, absolute risk reduction. Adjusted* net risk, net risk adjusted for a bleeding ratio of 2:1. See the Supplementary material online, Methods and results for details about the calculation of the adjusted net risk. Cost/Benefit^: The daily cost ($) of therapy divided by the absolute risk reduction (%) of stroke. The rows are colour-coded to indicate which do not meet the user-specified criteria. Tan: the medication cost exceeds the cost threshold. Red: the absolute risk reduction of stroke does not meet the treatment threshold.
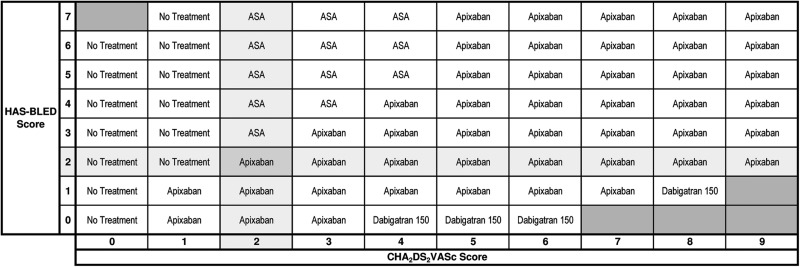
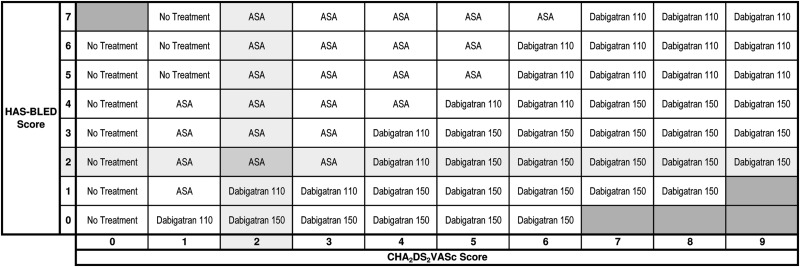
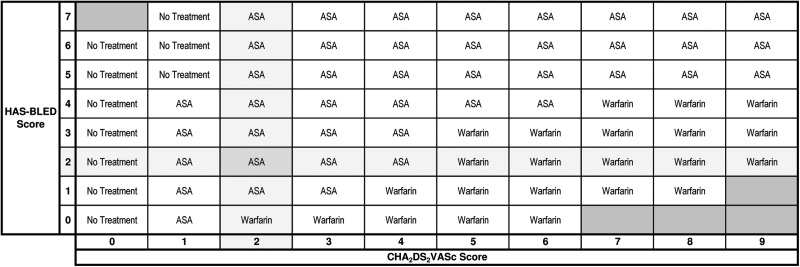
The treatment recommendation table for all patients with treatment threshold = 0.5% (NNT = 200), bleeding ratio = 2:1, and cost threshold = null is shown in Figure 4. Blacked out cells represent impossible combinations of CHA2DS2-VASc and HAS-BLED scores due to overlapping risk factors. We set a default bleeding ratio of 2:1 on the basis of the quality of life utility scores of stroke (0.39) and major bleeding (0.8) in a recent study on the cost-effectiveness of stroke prophylaxis in patients with AF.18 The highlighted cell shows that apixaban is recommended for a patient with CHA2DS2-VASc = 2 and HAS-BLED = 2. Elsewhere on the table no treatment is recommended for patients with CHA2DS2-VASc = 0. Acetylsalicylic acid is recommended for patients at a lower risk of SSE with a high risk of major bleeding, and dabigatran 150 is recommended for patients at a higher risk of SSE (CHA2DS2-VASc > 3) with a low risk of major bleeding. The treatment recommendation table for currently approved therapies at the time of the writing of this manuscript is shown in Figure 5.
Figure 4.
The treatment recommendation table including new agents. The treatment recommendation table with settings: treatment threshold = 0.5%, bleeding ratio = 2:1, cost threshold = null, and jurisdiction = null. The stroke risk increases from the left to the right along with the CHA2DS2-VASc scores at the bottom. The bleeding risk increases from the bottom to the top along with the HAS-BLED scores on the left. The black boxes represent impossible combinations of the stroke and bleed risk due to overlapping risk factors. Apixaban is recommended for a patient with a CHA2DS2-VASc score 2 and HAS-BLED score 2.
Figure 5.
The treatment recommendation table with therapies available in Canada. The treatment recommendation table with settings: treatment threshold = 0.3%, bleeding ratio = 2:1, cost threshold = $4.00, and jurisdiction = Ontario, Canada. The stroke risk increases from the left to the right along with the CHA2DS2-VASc scores at the bottom. The bleeding risk increases from the bottom to the top along with the HAS-BLED scores on the left. The black boxes represent impossible combinations of the stroke and bleed risk due to overlapping risk factors. Acetylsalicylic acid is recommended for a patient with a CHA2DS2-VASc score 2 and HAS-BLED score 2. At the time of the writing, the therapies available in Canada are: ASA, clopidogrel, dabigatran 110, dabigatran 150, rivaroxaban, and warfarin. Not available: apixaban.
The treatment recommendation table for all patients with treatment threshold = 0.3% (NNT = 333), bleeding ratio = 2:1, and cost threshold = $0.50 is shown in Figure 6. Lowering the cost threshold favours less expensive, more cost-effective antithrombotic therapies. The highlighted cell now recommends ASA for a patient with the same baseline risks (CHA2DS2-VASc = 2, HAS-BLED = 2). Elsewhere in the table warfarin is recommended for patients with a higher risk of SSE and/or with a lower risk of major bleeding. No treatment is again recommended for patients with CHA2DS2-VASc = 0.
Figure 6.
The treatment recommendation table with the cost threshold = $0.50. The treatment recommendation table with settings: treatment threshold = 0.3%, bleeding ratio = 2:1, cost threshold = $0.50, and jurisdiction = Ontario, Canada. The stroke risk increases from the left to the right along with the CHA2DS2-VASc scores at the bottom. The bleeding risk increases from the bottom to the top along with the HAS-BLED scores on the left. The black boxes represent impossible combinations of the stroke and bleed risk due to overlapping risk factors. Acetylsalicylic acid is recommended for a patient with a CHA2DS2-VASc score 2 and HAS-BLED score 2.
Adjustment of the treatment threshold, bleeding ratio, and cost threshold modifies the treatment recommendations in ways that accord with clinical intuitions. An increased treatment threshold favours no treatment in patients at a low risk of SSE, and favours antithrombotic therapies with greater efficacy in patients at a higher risk of SSE. An increased bleeding ratio favours antithrombotic therapies with greater efficacy over therapies with greater safety. Specifically, as the bleeding ratio was raised from 2:1 to 5:1, dabigatran 150 was more likely to be recommended over apixaban for patients with higher CHA2DS2-VASc scores, and apixaban was more likely to be recommended over ASA for patients with lower CHA2DS2-VASc scores. The Supplementary material online includes treatment recommendation tables for these threshold adjustment scenarios (see Supplementary material online, Figures S2 and S3).
Discussion
The availability of a relative abundance of new antithrombotic agents has created a knowledge gap between emerging research and clinical practice. Guideline committees have wrestled with the implications of this new research, and it would appear that there remains a great deal of indecision among clinical experts and between organizations and jurisdictions.
For instance, while the Canadian Cardiovascular Society (CCS) guidelines14 continue to recommend the CHADS2 score for the assessment of the thromboembolic risk, the European Society of Cardiology (ESC) guidelines15 have incorporated the CHA2DS2-VASc score into their guidelines. The ESC guidelines recommend no antithrombotic therapy for patients with a CHA2DS2-VASc score of 0, but the CCS guidelines recommend ASA for patients with a CHADS2 score of 0. And while the ESC guidelines suggest that dabigatran could be considered as an alternative to warfarin, the CCS guidelines recommend that dabigatran be used preferentially over warfarin. The 2011 ACCF/AHA/HRS focused update on dabigatran states that dabigatran is ‘useful as an alternative to warfarin’ for select patients.21 In contrast to Health Canada's regulatory body that approved both the 150 mg b.i.d. and 110 mg b.i.d. doses of dabigatran, the United States’ Food and Drug Administration (US FDA) recently declined to approve the 110 mg b.i.d. dose of dabigatran for PAF. None of the guidelines has yet to provide guidance in terms of incorporating either apixaban or rivaroxaban into clinical practice.
Likewise, uncertainty remains as to how to incorporate the concept of net risk of antithrombotic therapy into clinical practice. Both the CCS and the ESC guidelines now recommend that bleeding risk assessment should be part of the overall patient assessment before starting antithrombotic therapy. The ESC guidelines suggest that dabigatran 150 could be considered as an alternative to warfarin for patients at a low risk of bleeding (HAS-BLED score ≤2) and dabigatran 110 as an alternative for patients with ‘a measurable risk of bleeding’ (HAS-BLED score ≥3).15 In contrast, the CCS guidelines do not provide any concrete direction on how to apply this information in clinical practice, only suggesting caution in the use of antithrombotics and closer monitoring and follow-up for patients at a high risk of major bleeding.14 However, there appears to be a growing consensus that clinical decision aids need to be developed that considers both the stroke risk and the bleeding risk, and at the same time takes into consideration patient's attitudes towards stroke and major bleeding.16 An example of a paper version of the clinical decision aid is available as Supplementary material online, printable clinical decision aid.
The decision aid's treatment recommendation tables are generally consistent with current practice guidelines while providing more detailed guidance than has previously been available. By integrating both patient factors (SSE risk, bleeding risk, treatment threshold, cost threshold, and bleeding ratio) and the totality of clinical research (efficacy and safety), this decision aid provides the clinician with the necessary perspective in order to determine the optimal antithrombotic strategy for an individual patient. This results in the generation of treatment recommendation tables that have a number of notable features.
First, when assuming a treatment threshold of 0.5% and a bleeding ratio of 2:1, the treatment recommendation table is consistent with ESC Guidelines in that it recommends no treatment for patients with a CHA2DS2-VASc score of 0.
Secondly, when no cost threshold is set, only three antithrombotic therapies are recommended: ASA, apixaban, and dabigatran 150. These recommendations are in accordance with the US FDA decision not to approve the dabigatran 110 dose, and the CCS guidelines’ recommendation to preferentially utilize the dabigatran 150 dose over dabigatran 110 and warfarin. While both dabigatran 150 (superior efficacy to warfarin) and apixaban (superior efficacy and safety to warfarin) have distinctive efficacy/bleeding profiles compared with warfarin, neither ASA + clopidogrel, dabigatran 110 nor rivaroxaban is sufficiently distinct from warfarin to result in their inclusion in the treatment recommendation table.
Thirdly, warfarin is excluded from the treatment recommendation table unless the cost threshold is low (see Figure 6), in which case the decision aid generates a treatment recommendation table that is remarkably similar to the ACC/AHA/ESC 2006 guidelines that recommend only ASA and warfarin,1 provided that one is willing to accept the relatively modest efficacy of ASA.
Ultimately a patient's decision to accept or reject a treatment is based on the perceived benefits, risks, costs and impact on the quality of life, and so a clinical decision aid is more robust when it can incorporate an individual's attitudes, values, and circumstances. Recent studies have found remarkable variation in patients’ attitudes towards stroke,22 with many patients perceiving a moderate or major stroke to be equal to or worse than death. Those patients may be willing to endure more major bleeding, even for a very modest ARR in stroke risk, and even if this comes at an increased cost. Accordingly, the default bleeding ratio of 2:1 is likely an underestimate for many patients, and in such instances, it may be more appropriate to employ a treatment recommendation table with a higher bleeding ratio, as per Supplementary material online, Figure S3, Methods and results. In contrast, someone who is at a relatively low risk of stroke (CHA2DS2-VASc score of 1) might reasonably opt for no treatment if he or she does not consider a 0.8% ARR in stroke risk to be enough to justify the cost, side effects, or inconvenience of realizing warfarin's 64% RRR in stroke risk. People with a low bleeding ratio (such as Jehovah's Witnesses) or people with a low cost threshold (such as people without a drug plan) may also require a more significant ARR in stroke risk in order to justify the assumed risks and/or costs of antithrombotic therapy.
It is important to note that an individual's treatment threshold, bleeding ratio, and cost threshold are neither fixed nor independent values; these values are inter-related and they may change over time. For instance, it is quite likely that those patients who demand a greater efficacy (i.e. a higher treatment threshold) also require a less hazardous safety profile (i.e. lower bleeding ratio) from a therapy. Other factors, such as individual circumstances (ability to pay for medications), and health status (advancing age, recent stroke) may change over time, and therefore affect their decisional balance with respect to the determination of the treatment threshold and bleeding ratio. For example, patients who present to a stroke prevention clinic with a recent TIA and newly diagnosed AF are generally more likely to have a lower treatment threshold than otherwise healthy patients who present to the family physician with newly diagnosed AF. Further, it is not clear if patients are primarily interested in relative or absolute risk reductions from potential therapies. In other words, as their absolute risk of SSE or Major Bleeding increases, does their treatment threshold or bleeding ratio adjust accordingly?
An electronic format (website or iPAD Application) of the decision aid has several advantages over paper-based formats of the decision aid, such as pocket cards or slide rules. Internet-based versions can be quickly updated as new agents gain approval for PAF in specific jurisdictions and as costs and availability change over time. Once studies have been done directly comparing the new agents or as new studies may become available about baseline risks of SSE or major bleeding, the electronic formats can quickly incorporate the best available efficacy, safety, and risk data. An electronic format also has the potential to prompt for and consider additional patient characteristics that might constitute absolute or relative contraindications for some therapies, or that might require dose adjustments of other therapies. For example, in the ARISTOTLE study the investigators decreased the dose of apixaban to 2.5 mg b.i.d. for patients who had two of the three following criteria: age >80 years, body weight <60 kg, or renal dysfunction (serum creatinine >133 μmol/L).4 Similarly, a post hoc analysis of the RE-LY study has led to the recommendation that the lower 110 mg b.i.d. dose of dabigatran be used for patients >80 years old and/or patients at an increased risk of bleeding.23
Limitations
This decision aid is intended to complement, and not replace, clinical judgment. The decision aid does not yet consider absolute or relative contraindications to therapies such as allergies/intolerances, advanced age, low body weight, or the presence of stage IV or V chronic kidney disease. Nor does it consider concomitant conditions that may affect the choice of antithrombotic therapy, such as recent percutaneous coronary intervention, or the presence of rheumatic fever or artificial heart valves.
The cost–benefit analyses that this decision aid performs are fairly modest. A more comprehensive model for performing cost–benefit analyses is available as Supplementary material online, Methods and results. However, given the dichotomy in pricing between ASA/warfarin and the new OAC agents, more detailed models are unlikely to provide substantively different results. Further, in order to accurately reflect the true cost of new OAC agents relative to warfarin and relative to no treatment, one must consider the costs associated with anticoagulation management24 and the costs of medical care associated with strokes or major bleeding events, all of which are substantial.18
The trials of new OACs were very similar in terms of design, patient population, definitions of stroke and major bleeding, and statistical analysis, which enabled us to make comparisons across studies. The one exception was the ROCKET-AF trial, for which the patient population had a higher absolute risk of stroke: mean CHADS2 score 3.5 vs. 2.0–2.2 in the other trials. However, given our use of RRs rather than absolute risks, we determined that this would have minimal impact on the results. In all cases we used data from the intention to treat analyses.
The scenarios presented here and in the Supplementary material online make definite assumptions about patients’ treatment threshold and bleeding ratio based on the best available evidence. Recognizing that stroke is a highly undesirable outcome for patients who are considering antithrombotic therapy, a bleeding ratio of 2:1 is likely an underestimate for many patients. Further research should be performed to directly ascertain patients’ attitudes towards tolerance of the bleeding risk in the setting of PAF, so that a representative bleeding ratio can be applied to create a treatment recommendation table that accurately reflects the views of the average patient. Given the significant inter-patient variability, we have created an iPad application20 that utilizes probability trade-off techniques to determine the treatment threshold and bleeding ratio for each individual patient. This allows for the creation of treatment recommendation tables that consider a patient's personal attitudes and values regarding the risk of stroke and bleeding.
Conclusion
Recent changes in the antithrombotic landscape as well as increasing recognition of the importance of balancing stroke and bleeding risks among AF patients have made selecting the antithrombotic therapy more complicated. This decision aid assists clinicians in determining an optimal antithrombotic regime for the prevention of stroke due to AF, and can be incorporated into either a paper-based (note pad, pocket card, or slide rule) or electronic format so that clinicians have decision support at the point of care. Pending the approval of apixaban for the prevention of stroke due to AF, it would be reasonable to employ the treatment recommendation table for currently available therapies (Figure 5) for patients who are not cost-constrained, and to utilize the more fiscally conservative Treatment Recommend Table (Figure 6) for patients who do have cost restrictions.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
Funding to pay the Open Access publication charges for this article was provided by Dr Stephen LaHaye.
Conflict of interest: A.S.: Honoraria from Boehringer Ingelheim for speaking engagements. S.G., S.L., D.B., A.G.D., J.B.O.: none declared.
Supplementary Material
References
- 1.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, LeHeuzey J-Y, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 2.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 3.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L RE-LY Steering Committee and Investigators. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 4.Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FWA, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Eng J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KAA, Califf RM the ROCKET AF Sterring Committee. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Eng J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 6.Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, Radford MJ. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial fibrillation (NRAF) Am Heart J. 2006;151:713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. CHEST. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 8.Lip GYH, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2011;57:173–180. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Olesen JB, Lip GYH, Hansen PR, Lindharsen J, Ahlehoff O, Andersson C. Bleeding risk in ‘real world’ patients with atrial fibrillation: comparison of two established bleeding prediction schemes in a nationwide cohort. J Thromb Haemost. 2011;9:1460–1467. doi: 10.1111/j.1538-7836.2011.04378.x. [DOI] [PubMed] [Google Scholar]
- 10.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 11.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. CHEST. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 12.Lip GYH, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation. Stroke. 2010;41:2731–2738. doi: 10.1161/STROKEAHA.110.590257. [DOI] [PubMed] [Google Scholar]
- 13.Olesen JB, Lip GYH, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124. doi:10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cairns JA, Connolly S, McMurtry S, Stephenson M, Talajic M. Canadian Cardiovascular Society Atrial Fibrillation Guidelines 2010: prevention of stroke and systemic thromboembolism in atrial fibrillation and flutter. Can J Cardiol. 2011;27:74–90. doi: 10.1016/j.cjca.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Camm AJ, Kirchof P, Lip GYH, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohnloser SH, Kohl P, Le Heuzey J-Y, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation. Europace. 2010;12:1360–1420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 16.Canadian Agency for Drugs and Technology for Health. Recommendations for optimal warfarin management for prevention of thromboembolic events in patients with atrial fibrillation. http://cadth.ca/en/products/optimal-use/warfarin-management/reports. (9 December 2011) [PubMed] [Google Scholar]
- 17.Connolly SJ, Pogue J, Hart RG, Pfeffer M, Hohnloser SH, Chrolavicius S, Yusuf S. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the atrial fibrillation Clopidogrel Trial with Irbesartran for prevention of Vascular Events (ACTIVE W): a randomized control trial. Lancet. 2006;367:1903–1912. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 18.Shah SV, Gage BF. Cost effectiveness of dabigatran for stroke prophylaxis in patients with atrial fibrillation. Circulation. 2011;123:2562–2570. doi: 10.1161/CIRCULATIONAHA.110.985655. [DOI] [PubMed] [Google Scholar]
- 19.Clinical Support Systems. AFib.ca: a clinical decision aid for the selection of antithrombotic therapy for the prevention of stroke due to atrial fibrillation. http://www.afib.ca. (23 February 2012) [DOI] [PMC free article] [PubMed]
- 20.Clinical Support Systems. ‘AFib CDA’ iPad Application available in the iTunes App Store. (23 February 2012)
- 21.Wann LS, Curtis AB, Ellenbogen KA, Estes NAM, III, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Jacobs AK. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran) Circulation. 2011;123:1144–1150. doi: 10.1161/CIR.0b013e31820f14c0. [DOI] [PubMed] [Google Scholar]
- 22.Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. 1996;156:1829–1836. [PubMed] [Google Scholar]
- 23.Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, Yang S, Alings M, Kaatz S, Hohnloser SH, Diener HC, Franzosi MG, Huber K, Reilly P, Varrone J, Yusuf S. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulation therapy (RE-LY) trial. Circulation. 2011;123:2363–2372. doi: 10.1161/CIRCULATIONAHA.110.004747. [DOI] [PubMed] [Google Scholar]
- 24.Schulman S, Anderson DR, Bungard TJ, Jaeger T, Kahn SR, Wells P, Wilson SJ. Direct and indirect costs of management of long-term warfarin therapy in Canada. J Thromb Haemost. 2010;8:2192–200. doi: 10.1111/j.1538-7836.2010.03989.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.