Abstract
Clouds and inkblots often compellingly resemble something else—faces, animals, or other identifiable objects. Here, we investigated illusions of meaning produced by novel visual shapes. Individuals found some shapes meaningful and others meaningless, with considerable variability among individuals in these subjective categorizations. Repetition for shapes endorsed as meaningful produced conceptual priming in a priming test along with concurrent activity reductions in cortical regions associated with conceptual processing of real objects. Subjectively meaningless shapes elicited robust activity in the same brain areas, but activity was not influenced by repetition. Thus, all shapes were conceptually evaluated, but stable conceptual representations supported neural priming for meaningful shapes only. During a recognition memory test, performance was associated with increased frontoparietal activity, regardless of meaningfulness. In contrast, neural conceptual priming effects for meaningful shapes occurred during both priming and recognition testing. These different patterns of brain activation as a function of stimulus repetition, type of memory test, and subjective meaningfulness underscore the distinctive neural bases of conceptual fluency versus episodic memory retrieval. Finding meaning in ambiguous stimuli appears to depend on conceptual evaluation and cortical processing events similar to those typically observed for known objects. To the brain, the vaguely Elvis-like potato chip truly can provide a substitute for the King himself.
Keywords: conceptual priming, explicit memory, perceptual learning, perceptual recognition, semantic priming
Introduction
The common tendency to project something not actually present onto a nebulous object like a cloud, shadow, or inkblot is known as “pareidolia” (Fig. 1). In these cases, imagination can run wild as people conjure up a face, body part, animal, or some other real-world object. This phenomenon is captured by the Rorschach test (Rorschach 1921) and other projective psychological measures. In other circumstances, the illusion of meaning can be helpful, as when it is used to aid diagnosis of hard-to-decipher clinical magnetic resonance imaging (MRI) scans (Maranhao-Filho and Vincent 2009), and sometimes profitable, as when a 10-year-old cheese sandwich thought to resemble the face of a religious figure fetched $28 000 at auction (BBC 2004).
Figure 1.

Pareidolia experiences. (A) People claim to see the face of the Virgin Mary in an office window stain (Tisch 2004). (B) The same face is seen in a brain scan (Copsey 2008). (C) Is this a rabbit in the clouds or a dog? (D) Some claim that this pretzel is the Virgin Mary cradling a baby Jesus (Olson 2005). (E) Some see Elvis Presley in this potato chip (Oklahomaedge 2008). (F) Is this pulsar really a giant hand in space? (CNN 2009).
Consistent with the lay notion that pareidolia often involves the false perception of faces as opposed to other objects (discussed by Sagan and Druyan 1997), mechanisms of pareidolia have been most readily investigated in studies of face perception. Hadjikhani et al. (2009) showed that the N170 brain potential, which is a highly sensitive signal of face perception (Bentin et al. 1996), is elicited by objects configured to vaguely resemble faces but not to the same objects configured differently (see also Bentin et al. 2002). Likewise, face-sensitive neurons in nonhuman primate inferotemporal cortex show the same selectivity for face-like object configurations (Perrett et al. 1982). These findings provide some intriguing clues about the potential mechanisms of pareidolia but do not capture many aspects of the core phenomenon, especially for nonface illusions.
Some additional information regarding the possible mechanisms of pareidolia can be derived from the literature on neural processes involved in object identification. For instance, regions of object-sensitive cortex responsible for conceptual processing have been identified by comparing repetition-priming effects on neural activity (a.k.a. “neural adaptation” or “neural priming”) for novel “nonsense” stimuli to familiar “real-world” objects (reviewed in Martin 2007). Brain regions showing similar repetition effects for both novel and familiar stimuli are inferred to support perceptual information processing; regions showing unique repetition effects for familiar objects, which can activate stored conceptual representations, are linked to conceptual processing. Is it the case that pareidolia involves this sort of conceptual processing? If so, then we would expect to find repetition priming effects for nonsense stimuli that trigger pareidolia to differ from priming effects for other stimuli that are not experienced as meaningful. In other words, distinctions in conceptual and perceptual priming effects commonly identified between real-world and novel objects should generalize to situations in which meaning is purely subjective—to the extent that this subjective meaningfulness is associated with neural hallmarks of conceptual processing for familiar objects (a pervasive folk-psychological assumption is that finding meaning in an abstract or novel stimulus differs fundamentally from finding meaning in a real-world object, with the former depending on indirect inferences of meaning rather than on “genuine” conceptual processing per se [e.g., the proposal of Stenberg et al. 2010]. The current results provide evidence that casts doubt on this assumption).
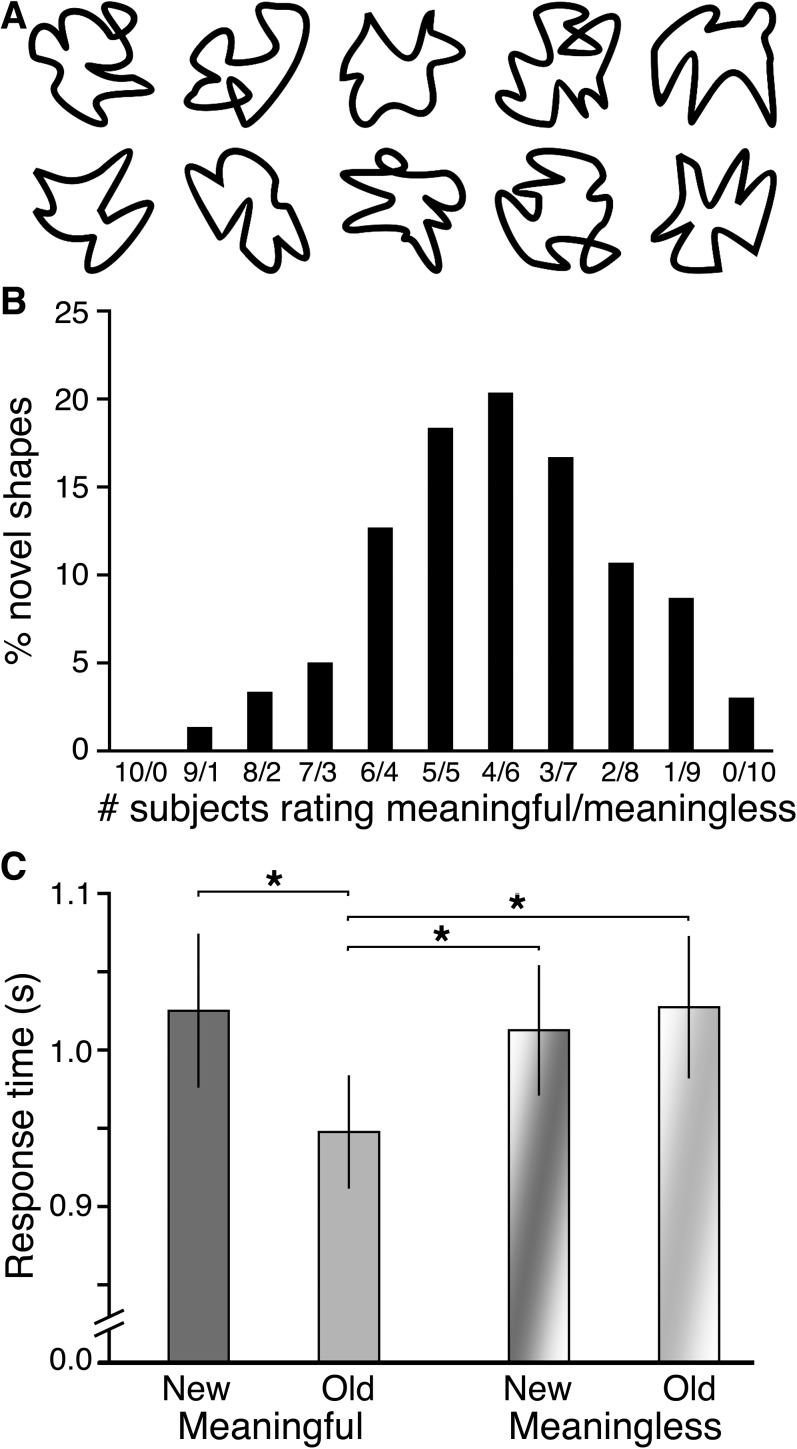
We therefore studied neural and behavioral responses to abstract visual stimuli that we refer to as “squiggles” due to their inclusion of curved line segments (Fig. 2). Which specific squiggles are seen as meaningful varies widely from person to person, but for each person these judgments are highly stable, even after 1 year (Voss and Paller 2007). Thus, we used each subject’s self-report to categorize squiggles as meaningful versus meaningless, with the former indicating the experience of pareidolia. On this basis, we analyzed behavioral and neural responses to meaningful and meaningless squiggles in tests of conceptual implicit (nondeclarative) memory and of explicit (declarative) memory (Richardson-Klavehn and Bjork 1988).
Figure 2.
Conceptual priming for meaningful novel visual shapes. (A) Example squiggle stimuli. (B) Interindividual differences in meaningfulness categorization are shown as the percentage of forms rated as meaningful versus meaningless by a given number of subjects. Squiggles rated by 5 subjects as meaningful and 5 as meaningless (5/5) are most variable, whereas 10/0 indicates 100% meaningful agreement across subjects and 0/10 100% meaningless agreement. (C) Response times are given for meaningful and meaningless old and new squiggles during the conceptual priming test. Error bars indicate SE. All significant pairwise differences are indicated. *P < 0.05.
Based on the aforementioned considerations, several predictions were made regarding neural processing during tests of conceptual implicit memory. To the extent that finding meaning in squiggles is due to the same kind of conceptual evaluation applied to real-world objects, we expected that repetition effects for meaningful squiggles would resemble extant descriptions of repetition effects for real-world objects (i.e., negative repetition effects in regions responsible for conceptual processing). To facilitate this comparison, we assessed brain activity in regions of interest (ROIs) that were selected a priori based on well-characterized and selective associations with conceptual processing in many previous studies using repetition paradigms with words and nameable pictures (Buckner et al. 2000; Donaldson et al. 2001; Martin and Chao 2001). Notably, these ROIs are selectively associated with conceptual processing and dissociated from, for example, perceptual processing and recognition memory (Donaldson et al. 2001). This ROI approach provides an unbiased means for assessing activity in areas sensitive to conceptual processing (i.e., regions were identified in other experiments, cf., Donaldson et al. 2001). We also included standard whole-brain analyses that permitted more exhaustive and exploratory assessment of the loci of perceptual and conceptual processing for squiggles. We reasoned that regions associated with conceptual priming for meaningful squiggles might also respond robustly upon viewing meaningless squiggles, reflecting relatively obligatory conceptual evaluation by these regions regardless of actual or perceived stimulus meaningfulness (cf., Federmeier and Laszlo 2009; Kutas and Federmeier 2011). However, we reasoned that only meaningful squiggles would be associated with stable conceptual representations suitable for supporting repetition priming effects.
An alternative explanation for any repetition-related effects on neural processing selective for meaningful squiggles is that they do not reflect conceptual processing per se but instead reflect retrieval of episodic information from study. Comparisons between neural responses during conceptual priming tests and recognition memory tests thus allowed us to determine whether similar or dissimilar neural processing events are associated with conceptual priming and episodic retrieval of squiggles.
Materials and Methods
Subjects
Behavioral and functional magnetic resonance imaging (fMRI) data were collected from 10 neurologically intact individuals recruited from the Northwestern University community (ages 20–31 years; 6 males).
Materials
Visual stimuli included 300 squiggles (Fig. 2A), which have been described in detail elsewhere (Groh-Bordin et al. 2006; Voss and Paller 2007; Voss, Schendan, et al. 2010).
Behavioral Methods
The experiment comprised 3 study–test blocks, the first 2 for testing priming and the third for testing recognition. Different stimuli were used in each block. During the study session for the priming blocks, participants viewed 30 squiggles individually (2000 ms duration; 1500–2500 ms randomized interstimulus interval [ISI]) and rated each using a 4-point meaningfulness scale (Voss and Paller 2007; Voss, Schendan, et al. 2010). Response speed was de-emphasized. The highest 2 ratings indicated any experience of pareidolia (strong or weak resemblance to a real-world face, animal, object, etc.) and were thus collapsed to form the “meaningful” category for analysis purposes. The lowest 2 ratings (vague feeling of meaning or no meaning) indicated no experience of pareidolia and were thus collapsed to form the “meaningless” category for analysis purposes. Despite these labels, the 2 categories should be considered to include objects that differ in relative meaningfulness along a continuum rather than a simple binary distinction. Still, the 2 categories differ systematically in meaningfulness, when averaging across category members, and as assessed according to the idiosyncratic criteria for finding meaning that each person brings to the task. A vast majority of squiggles tend to fall in one category for some subjects and in the other category for other subjects (Voss and Paller 2007; Voss, Schendan, et al. 2010).
After a break of approximately 90 s, subjects were administered the priming test. The same 30 squiggles were presented along with 15 new squiggles, in randomized order (500-ms duration; 3500 or 5500 ms ISI, for 60% and 40% of trials, respectively, assigned pseudorandomly) and subjects rated each stimulus for meaningfulness with the added emphasis of an explicit instruction to respond as quickly as possible. On average, only 1.9% (standard error [SE] = 1.2%) of squiggles were assigned to different meaningfulness categories at study versus test, and the test categorization was used for analysis in these instances. The assignment of response option to response finger was counterbalanced across subjects. All responses were made with the right hand.
The study session for the final block was identical to the previous blocks except that 120 squiggles were studied. Afterward, a surprise recognition memory test was administered. Subjects responded to the 120 old squiggles and 90 new squiggles using a 4-point modified “remember/know” scale (Gardiner and Java 1991), with response options including: remember (recollection), know (familiarity), guess, and new. Stimulus duration and ISIs were as in the priming tests. The stimuli comprising the old and new categories were counterbalanced across subjects for all tests. There were 2 different assignments of squiggle stimuli to test format (priming vs. recognition), alternated across subjects.
Following the final study–test block, subjects viewed each squiggle individually and rated meaningfulness. This permitted assessment of the stability of ratings over the experiment and allowed categorization of new squiggles from the recognition memory test, as these squiggles were not rated for meaningfulness previously. Prior to entering the scanner, subjects practiced a priming block using a separate set of 45 squiggle stimuli that did not appear subsequently in the experiment.
MRI Methods
The experiment was administered while subjects were situated in a Siemens Trio 3-T MRI scanner. Whole-brain, blood oxygen level–dependent sensitive, echo-planar images were collected continuously during the tests only (time repetition [TR] = 2 s; time echo = 25 ms; flip angle = 80°; 35 3-mm axial slices; 0-mm gap; field of view = 22 cm; 64 × 64 acquisition matrix; voxel size = 3.4 × 3.4 × 3.0 mm). Images were collected for an additional 10 s prior to the beginning of each test, and these were excluded from analysis. Images were collected 16 s after the final stimulus was presented. Stimulus onset times were locked to the scanner TR. A structural image (3D-magnetization prepared rapid gradient echo, voxel size = 0.9 × 0.9 × 1 mm; 160 axial slices) was collected while subjects made meaningfulness ratings following the last study–test block.
AFNI software (Cox 1996) was used for image analysis. Preprocessing included coregistration through time (motion correction), removal of voxels with low signal (<30% of mean whole-brain signal), spatial smoothing (8-mm full-width at half-maximum Gaussian kernel), coregistration with the structural image, and transformation to Talairach–Tournoux stereotactic space (MNI-305). Stimulus-locked neural activity was estimated in each subject using hemodynamic response deconvolution with a general linear model, as quantified using average values from 5 to 9 s after stimulus onset to account for hemodynamic lag. The stimulus order and ISIs were pseudorandomly selected in order to maximize estimates of hemodynamic responses to old and new stimuli. Group-level activation differences between conditions were then identified, with subjects treated as a random factor. For the analysis of subjective experience of memory retrieval (see below), parameter estimates were obtained in each subject by including in the regression a vector that modeled the linear trend across the 4 response-type conditions, and group-level analyses were performed using these estimates. For the ROI analysis, estimated activity levels were averaged within 9-mm radius spheres centered on preselected stereotactic coordinates. Separate whole-brain contrasts used Monte Carlo simulations to determine combined voxel-wise and spatial-extent thresholds needed to achieve an overall reliability threshold of P = 0.01. The voxel-wise threshold was 0.05, and spatial-extent threshold was 567 mm3, as determined by the most stringent simulation and applied to all contrasts (the smallest cluster thus identified was 675 mm3).
Results
On average, 41% (SE = 2.9%) of the squiggles were categorized as meaningful, indicating the experience of pareidolia, with the remainder categorized as meaningless. As in our previous experiments (Voss and Paller 2007; Voss, Schendan, et al. 2010), there was a great deal of intersubject variability in the specific squiggles endorsed as meaningful (Fig. 2B), thus justifying the use of personalized ratings. Ratings given during study were highly consistent with ratings given at the end of the experiment, as 95% of the squiggles were categorized the same on both occasions (SE = 2.1%), supporting the notion that ratings characterized the experience of pareidolia in a stable way over time for each individual.
Priming was observed when meaningful squiggles were repeated during the conceptual priming test. Mean response times showed a significant main effect of repetition (old/new), F1,9 = 5.9, P = 0.03, = 0.40 and a significant interaction of repetition with meaningfulness (meaningful/meaningless), F1,9 = 5.8, P = 0.03, = 0.39. Response times for old meaningful squiggles were significantly faster than for new meaningful squiggles, old meaningless squiggles, and new meaningless squiggles, with no significant differences between any other 2 conditions (Fig. 2C). We infer that conceptual processing was more fluent than the second time meaningful squiggles were viewed relative to the first, leading to faster response times. No speedup was evident for meaningless squiggles, indicating no repetition-induced boost in fluency. Furthermore, the lack of priming for meaningless squiggles supports the notion that conceptual fluency was the cause for priming of meaningful squiggles rather than perceptual fluency or other nonspecific factors shared by both meaningless and meaningful squiggles (e.g., fluency of meaningfulness decisions or motor responses).
Response times during the study phase, when speed was not emphasized, did not differ significantly for meaningful and meaningless stimuli. Average response times were 2512 ms (SE = 145) and 2601 ms (SE = 171), respectively, across all study sessions (P = 0.69). Furthermore, there were no significant meaningful-versus-meaningless differences in response times within each individual study session (all P values > 0.31).
Neural Correlates of Conceptual Processing in ROIs Selected a Priori
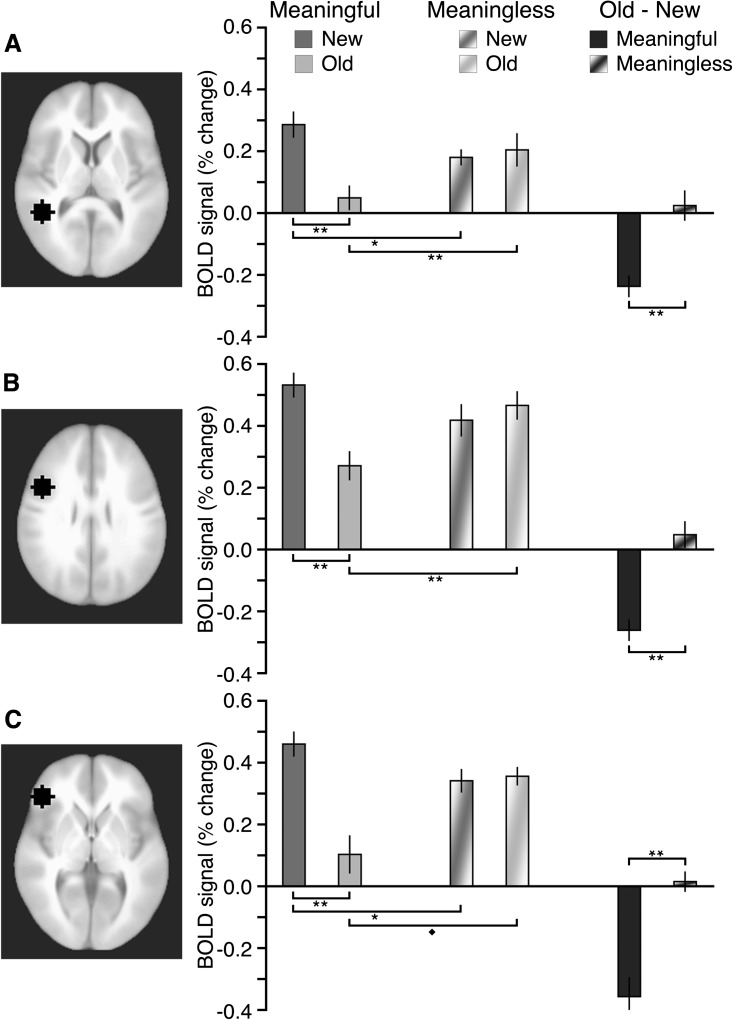
We scrutinized brain activity during the conceptual priming test in 3 regions consistently sensitive to conceptual processing for real-world objects in prior studies (Buckner et al. 2000; Donaldson et al. 2001). These 3 left-hemisphere regions (Fig. 3) included: inferior temporal cortex (Montreal Neurological Institute [MNI] coordinates: −43, −46, +6), dorsal inferior frontal cortex (−43, 9, 34), and ventral inferior frontal cortex (−43, 34, 3). Coordinates were taken directly from Donaldson et al. (2001). Our interests were 1) to determine whether these areas were more responsive to meaningful versus meaningless squiggles on initial viewing and 2) to determine how activity changed due to repetition of meaningful versus meaningless squiggles.
Figure 3.
Activity in conceptual brain regions in response to squiggles. Each of the 3 ROIs is shown at its middle axial slice, including: (A) left inferior temporal cortex, (B) left dorsal inferior frontal cortex, and (C) left ventral inferior frontal cortex. Estimated activity is shown for each ROI for meaningful and meaningless old and new squiggles, as well as for the old–new activity difference for meaningful and meaningless squiggles. Error bars indicate SE. All significant pairwise differences are highlighted. **P < 0.01. *P < 0.05. ♦P = 0.07.
In an analysis limited to new squiggles in the conceptual priming test, greater activity was observed for meaningful squiggles compared with meaningless squiggles, yielding a significant main effect of meaningfulness, F1,9 = 8.9, P = 0.02, = 0.49. Furthermore, there was a nonsignificant interaction with ROI, F2,18 = 0.01, not significant (ns), indicating that the meaningfulness effect was similarly present in all 3 left-hemisphere regions (Fig. 3). In sum, regions consistently linked to conceptual processing were more responsive to meaningful than meaningless squiggles the first time they were viewed.
With repetition, meaningful squiggles elicited less brain activity than they had initially, whereas activity did not change for meaningless squiggles (showing trends in the opposite direction, old > new). We formally compared old–new activity differences and found a significant main effect of meaningfulness, F1,9 = 31.2, P < 0.001, = 0.78 and a nonsignificant interaction with ROI, F2,18 = 0.4, ns. Thus, repetition effects computed as old–new activity differences were significantly more negative for meaningful than for meaningless squiggles, without significant variation across the 3 ROIs. Notably, reduced activity with repetition in these regions is a hallmark of the enhanced fluency of conceptual processing that occurs when real-world objects are viewed repeatedly (Schacter and Buckner 1998; Martin and Chao 2001; Henson 2003; Schacter et al. 2007).
These regions thus demonstrated a pattern of activity consistent with their selective role in conceptual processing for squiggles. It was therefore possible to determine the extent to which conceptual evaluation occurs for stimuli without preexisting representations by evaluating activity in response to new meaningless squiggles in these regions. As shown in Figure 3, all 3 ROIs exhibited robust activity for new meaningless squiggles (P values = 0.002, 0.001, and 0.004, respectively for panels A–C), with significant cross-region variability, F2,18 = 3.7, P = 0.04, = 0.28, due to responses of smaller magnitude in inferior temporal cortex relative to the other 2 regions (pairwise P values < 0.05). Note that in each ROI, activity for new meaningless squiggles was reliably higher than for old meaningful squiggles, indicating that robust responses in these regions were not obligatory for all visual stimuli and instead reflected stimulus-evoked conceptual processing.
Whole -Brain fMRI Assessments of Conceptual Processing
To provide additional information regarding the nature of conceptual processing of squiggles, we next performed a whole-brain fMRI analysis for the conceptual priming test. Brain regions were first identified that showed significantly greater activity reductions (i.e., old < new) for meaningful squiggles than for meaningless squiggles, defined via a double subtraction [meaningful (old – new) – meaningless (old – new)]. Among the 5 regions thus identified as showing a significant meaning-by-repetition interaction, 2 overlapped to an appreciable extent with the ROIs described above (Fig. 4A and Table 1), whereas the other 3 were spatially distant. Two of the regions, left entorhinal/perirhinal cortex and bilateral anterior lingual gyrus (Fig. 4B and Table 1), have been associated with representation/storage of information regarding real-world objects (Murray et al. 2000) and show fMRI activity reductions with conceptual priming (Schacter and Buckner 1998; Henson 2003; O'Kane et al. 2005; Schacter et al. 2007; Voss et al. 2009). Thus, effects commonly observed for real-world objects were observed for meaningful squiggles in object-sensitive ventral visual cortex in addition to the ROIs described above. A region of left precentral (motor) cortex was also identified (Table 1), likely related to the right-hand execution of faster responses to meaningful squiggles.
Figure 4.
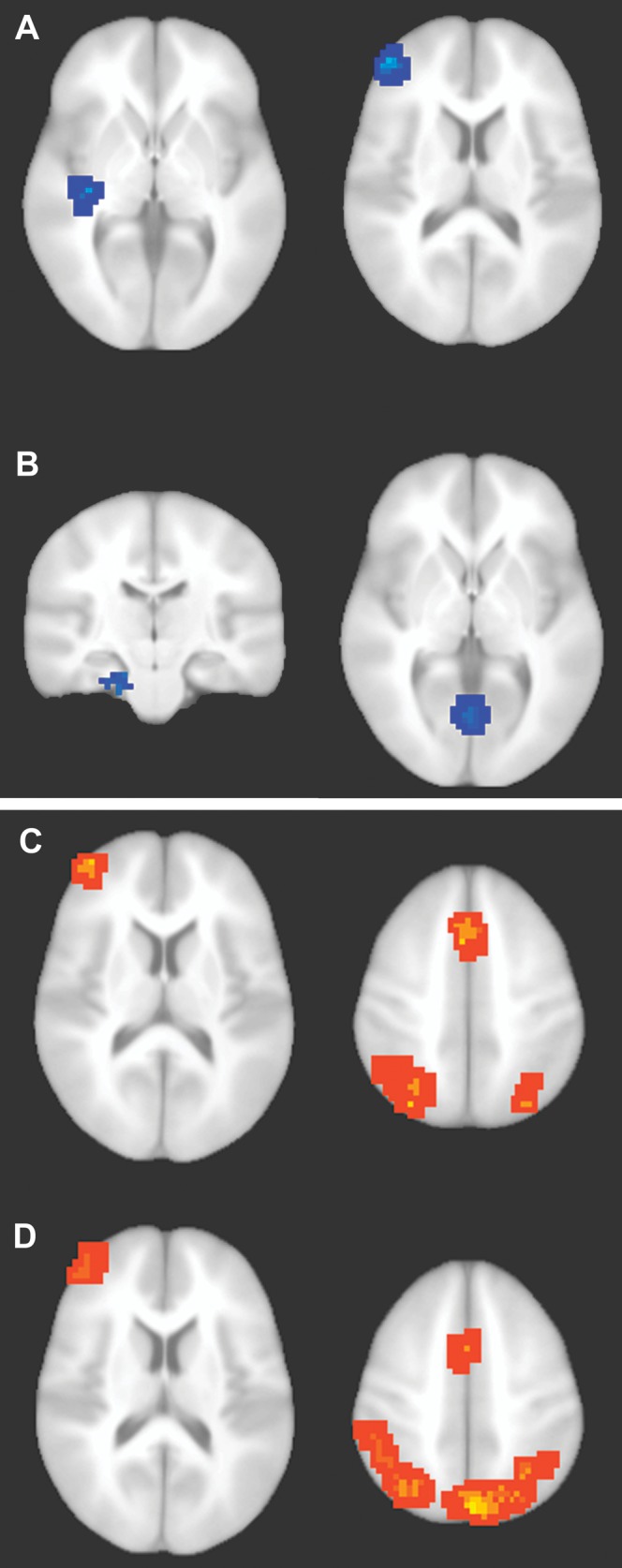
Neural correlates of conceptual priming and recognition. (A–B) Regions associated with conceptual priming were identified by the double subtraction [meaningful (old − new) − (meaningless (old − new)]. Blue coloration indicates significantly greater activity reductions for meaningful squiggles than for meaningless squiggles. (A) Left inferior temporal cortex (left side) and left inferior frontal cortex (right side). (B) Left anterior entorhinal/perirhinal cortex (left side) and bilateral anterior lingual gyrus (right side). Conceptual priming clusters are described in Table 1. (C–D) Regions associated with recognition for meaningful and meaningless squiggles. (C) Hot coloration indicates regions showing a linear trend of significantly more activity across the remember, know, guess, and new response types for old meaningful squiggles (remember > know > guess > new). Findings were typical of prior studies of recognition memory using words and nameable pictures (e.g., Fig. 3 of Donaldson et al. 2001). (D) Regions showing the same linear trend for meaningless squiggles. Recognition clusters are described in Table 3.
Table 1.
Regions showing more negative activity for meaningful old–new than for meaningless old–new (double subtraction) (Fig. 4A,B)
| Region | MNI coordinates | ||||
| mm3 | BA | X | Y | Z | |
| Bilateral anterior lingual gyrus | 675 | 28/30 | −1 | −66 | +1 |
| Left entorhinal/perirhinal cortex | 1134 | 28 | −22 | −17 | −28 |
| Left inferior/middle/superior temporal cortex | 4023 | 21/22 | −46 | −32 | 0 |
| Left inferior/middle/superior frontal cortex | 4266 | 10/46 | −43 | 51 | 18 |
| Left precentral gyrus | 13 581 | 4 | −59 | −5 | 25 |
Note: Note that the analysis was not signed and could have identified either positive or negative differences, but only more negative differences for meaningful were identified. Provided for each region is the volume (mm3), primary Brodmann’s areas (BA), and Talairach–Tournoux coordinates of the centroid (MNI-305).
In addition to the aforementioned interaction analysis, we also assessed the main effects of repetition and of stimulus meaning. The old/new contrast identified one fairly extensive region of visual cortex that showed less activity for old relative to new stimuli (Supplementary Fig. 1). Notably, this region encompassed the relatively anterior region of lingual gyrus that showed disproportionately greater negative repetition effects for meaningful compared with meaningless stimuli (Fig. 4B) and extended into widespread lingual/fusiform gyrus as well as more posterior occipital cortex. This region thus showed a repetition reduction for old versus new squiggles of both levels of meaningfulness, but in anterior aspects of the region the reduction was larger for meaningful stimuli, consistent with previous reports of an anterior-to-posterior gradient within object-sensitive visual cortex from processing that is more conceptual in nature to processing that is more perceptual/structural in nature (Martin 2007; Schacter et al. 2007).
Notably, no other brain regions were identified as showing a main effect of repetition nor were any regions identified as showing a main effect of meaning (i.e., meaningful vs. meaningless). This finding highlights the selectivity of the regions identified as loci of conceptual processing in the aforementioned double subtraction (Table 1) in exhibiting repetition effects selectively for meaningful squiggles.
The regions shown in Figure 4A,B were thus specific in their association with conceptual processing of squiggles, and it was therefore possible to scrutinize their activity in response to new meaningless squiggles as a neural index of conceptual processing for meaningless stimuli seen for the first time. Anterior lingual gyrus, left rhinal cortex, left temporal cortex, and left frontal cortex showed robust responses to new meaningless squiggles (% signal change = 0.29, 0.17, 0.24, and 0.29, respectively, P values ≤ 0.005), without significant variation in activity across regions, F3,27 = 0.81, ns.
Behavioral and fMRI Correlates of Recognition Memory
We next turn to effects of meaning on behavioral and neural responses to squiggles during the recognition memory test. Accuracy is summarized in Table 2 for each response type. For meaningful squiggles, endorsement rates showed a significant interaction of repetition with response type (remember/know/guess/new), F3,27 = 13.9, P < 0.001, = 0.61, indicating differential use of response type based on repetition (i.e., successful old/new discrimination). Pairwise comparisons indicated that endorsement rates for old and new items differed only for remember (P < 0.001) and new (P = 0.002) response types. Meaningless squiggles also showed a significant interaction of repetition and response type, F3,27 = 12.7, P < 0.001, = 0.58, with pairwise comparisons indicating significant old/new discrimination only for remember (P = 0.002) and new (P < 0.001) responses. A trend for successful old/new discrimination with know responses (P = 0.02) did not survive correction for multiple comparisons. Thus, accurate recognition for both meaningful and meaningless squiggles appeared to be driven predominantly by remember responses, which occurred more frequently for meaningful old squiggles than for meaningless old squiggles, t9 = 5.8, P < 0.001, d = 2.4, potentially signaling recollection of conceptual details that were primarily available for meaningful items. Low accuracy was likely observed for know and guess responses due to the fact that encoding was incidental (i.e., a subsequent memory test was not anticipated during study). Indeed, in our prior studies, the accuracy of know responses was significantly above-chance for the same stimulus materials when encoding was intentional (Voss and Paller 2007; Voss, Schendan, et al. 2010).
Table 2.
Response rates for each response type and stimulus category
| Remember | Know | Guess | New | |
| Meaningful | ||||
| Old | 0.26 (0.03) | 0.20 (0.02) | 0.19 (0.03) | 0.35 (0.04) |
| New | 0.05 (0.02) | 0.19 (0.04) | 0.24 (0.03) | 0.52 (0.05) |
| Old–new | 0.21 (0.04)** | 0.01 (0.03) | −0.05 (0.04) | −0.17 (0.04)** |
| Meaningless | ||||
| Old | 0.07 (0.01) | 0.17 (0.03) | 0.22 (0.03) | 0.54 (0.05) |
| New | 0.03 (0.01) | 0.12 (0.02) | 0.23 (0.03) | 0.62 (0.05) |
| Old–new | 0.04 (0.01)** | 0.05 (0.02)* | −0.01 (0.01) | −0.09 (0.02)** |
Note: Parentheses indicate SE.
*P < 0.05 for the old versus new difference, **P < 0.01 for the old versus new difference.
Brain activity associated with recognition was assessed using 2 approaches that yielded similar findings. First, activity was assessed in relation to the subjective experience of memory retrieval, defined as activity that showed a linear trend across recognition response types (remember, know, guess, and new) for old squiggles during the recognition test. Activity for both meaningful squiggles (Fig. 4C) and meaningless squiggles (Fig. 4D) showed significant positive trends (remember > know > guess > new) in widespread, bilateral lateral/inferior parietal cortex, bilateral medial parietal cortex (precuneus), left anterior and dorsal frontal cortex, and medial frontal cortex (Table 3). This activity pattern has been one of the most reliable findings in fMRI studies of recognition memory over the last 2 decades (reviewed in Buckner and Koutstaal 1998; Buckner et al. 1999; Donaldson et al. 2001; Wagner et al. 2005).
Table 3.
Regions showing positive activity trends across recognition response types (Fig. 4C,D), identified separately for meaningful and meaningless squiggles
| MNI coordinates | |||||
| Region | mm3 | BA | X | Y | Z |
| Meaningful squiggles | |||||
| Superior parietal cortex (left centroid) | 19 170 | 7 | −33 | −65 | +44 |
| Superior parietal cortex (right centroid) | 7 | +30 | −71 | +54 | |
| Bilateral medial frontal gyrus | 6642 | 6 | −2 | +16 | +51 |
| Left superior frontal gyrus | 4293 | 46 | −40 | +53 | +16 |
| Meaningless squiggles | |||||
| Superior parietal cortex (left centroid) | 18 360 | 7 | −35 | −68 | +49 |
| Superior parietal cortex (right centroid) | 7 | +33 | −79 | +44 | |
| Bilateral medial frontal gyrus | 6102 | 6 | −2 | +6 | +55 |
| Left superior frontal gyrus | 5049 | 46 | −45 | +52 | +20 |
Note: Note that the analysis was not signed and could have identified either positive or negative trends, but only positive trends were identified. Provided for each region is the volume (mm3), primary Brodmann’s areas (BA), and Talairach–Tournoux coordinates of the centroid (MNI-305). Centroid coordinates are provided separately for each hemisphere for the large bilateral region of parietal cortex noted for both meaningfulness categories.
Activity was then assessed in relation to recognition accuracy by comparing responses to old squiggles given remember responses versus responses to correctly rejected new squiggles (i.e., the 2 response categories for which old and new squiggles were consistently discriminated). Resultant old–new effects for both meaningful and meaningless squiggles (Supplementary Fig. 2) were evident in similar regions as those in the analysis based on the subjective experience of memory retrieval (Fig. 4C,D), though they were somewhat more widespread. No significant differences were identified in a comparison between activations in the 2 analyses. Not only did the old–new analysis and the remember–know–guess–new analysis produce similar neural activations, but both analyses also showed that brain activity associated with recognition of abstract meaningful and meaningless stimuli was highly typical of recognition memory more generally (e.g., similar to that for words and nameable pictures).
Dissociation of Conceptual Priming and Recognition in fMRI Activity
We next sought to determine the extent to which processing related to conceptual priming and to explicit memory was unique to type of memory test and brain region. This was accomplished by juxtaposing old–new repetition effects for 3 sets of regions: 1) ROIs shown in Figure 3 that were selected a priori based on their association with conceptual processing, 2) regions shown in Figure 4A,B that were associated selectively with conceptual processing based on the double subtraction of repetition and meaningfulness in the conceptual priming test, and 3) regions shown in Figure 4C,D that were associated with memory retrieval based on responses in the recognition test. Following Donaldson et al. (2001), repetition effects were averaged over all regions within each set (i.e., across all ROIs or activation clusters) separately for each test format and for meaningful and meaningless squiggles.
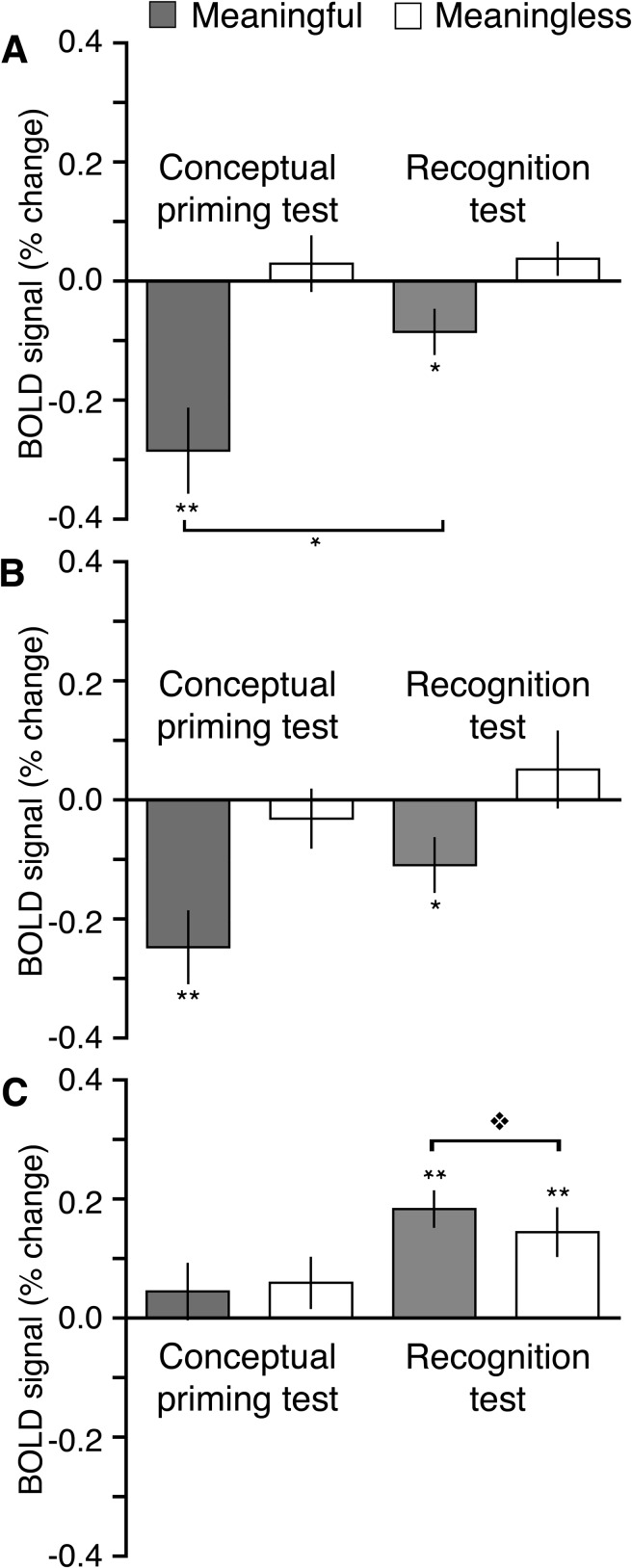
Figure 5A shows old–new difference values averaged over the a priori conceptual ROIs. Analyses showed a main effect of meaningfulness, F1,9 = 27.9, P < 0.001, = 0.76 and an interaction between meaningfulness and memory test (priming vs. recognition), F1,9 = 12.5, P < 0.01, = 0.60. These effects reflected more negative repetition effects for meaningful squiggles compared with meaningless squiggles and greater repetition effects for meaningful squiggles in the conceptual priming test relative to the recognition test. A similar pattern was shown in for the regions associated with conceptual processing based on the whole-brain double subtraction of repetition and meaningfulness (Fig. 5B). Although direct statistical comparisons between activity in the priming test and the recognition test are not warranted because this region was defined based on the conceptual priming test, and results could therefore be biased toward finding between-test differences, reliably negative repetition effects selective for meaningful squiggles were identified for both test formats.
Figure 5.
Functional dissociation of brain regions sensitive to conceptual priming versus recognition. Differences in estimated activity between old and new squiggles (i.e., repetition effects, old–new) are shown separately for the meaningful and meaningless categories averaged over all regions found to be sensitive to either conceptual priming or to recognition in aforementioned analyses. (A) Repetition effects in the a priori defined conceptual ROIs shown in Figure 3. (B) Repetition effects in the regions identified as selective for conceptual priming via the double subtraction of meaningful and meaningless repetition effects shown in Figure 4A,B. (C) Repetition effects in the regions identified as selective for recognition memory judgments shown in Figure 4C,D. Error bars indicate SE. **P < 0.01. *P < 0.05.  P = 0.06.
P = 0.06.
Figure 5C shows repetition effects for regions associated with recognition memory. Positive repetition effects were reliable for the recognition test, which is unsurprising given that this region was defined based on these effects, and statistical comparisons between test formats were not conducted for this reason (as for Fig. 5B). Positive repetition effects in the conceptual priming test were not reliable. Notably, the negative repetition effects for meaningful squiggles characteristic of conceptual priming in regions summarized in Figure 5A,B were not identified in this region. Repetition effects in the recognition test were marginally more positive for meaningful than for meaningless squiggles, P = 0.06, potentially reflecting the small behavioral recognition confidence differences between these conditions. Overall, the pattern of results shows a high degree of independence between neural correlates of conceptual priming and recognition. Negative repetition effects were identified for both test formats only in regions associated with conceptual priming and only for meaningful squiggles, whereas positive repetition effects were identified for both meaningfulness categories only in the recognition test and only in regions associated with recognition memory.
Associations between Conceptual Processing and Recognition
In addition to testing for dissociations between conceptual processing and recognition, we also sought evidence for potential associations. Given that conceptual fluency can lead subjects to incorrectly claim that a new stimulus is old (i.e., to false alarm; reviewed in Voss and Paller 2008; Voss and Federmeier 2011), we assessed false alarms for new squiggles in the recognition test. False alarm rates for remember, know, and guess responses (Table 2) showed a significant main effect of meaningfulness, F1,9 = 8.1, P = 0.02, = 0.47, and nonsignificant interaction of meaningfulness with response type, F2,18 = 0.82, ns, indicating that false alarms were more prevalent for meaningful than for meaningless squiggles independent of response type. The overall false-alarm rate collapsed across response type was 0.48 (SE = 0.05) for meaningful squiggles and 0.38 (SE = 0.04) for meaningless squiggles.
Brain activation for false alarms (collapsed across remember, know, and guess responses, which showed no interaction with meaningfulness behaviorally) was then assessed in comparison to brain activation for correct rejections. For meaningful squiggles, false alarms elicited significantly less activity than correct rejections in left inferior frontal cortex and in bilateral superior frontal gyrus (Supplementary Fig. 3). This finding suggests that conceptual processing was operative in generating false alarms for meaningful squiggles, as the left inferior frontal region overlapped considerably with the same region used in the ROI analysis (Fig. 3C) and to some extent with the frontal regions identified as selectively associated with conceptual processing via the whole-brain analysis (Fig. 4A). In contrast, no significant activations were identified for the same comparison for meaningless squiggles.
Discussion
The pareidolia experience occurred preferentially in this experiment for a subset of squiggle stimuli perceived to carry meaning. The particular stimuli that were rated as meaningful versus meaningless varied greatly from individual to individual. Yet, the validity of these ratings is supported by their high consistency in each individual over time, as well as by the strikingly different results obtained in conceptual priming and recognition memory tests.
Repetition of meaningful squiggles produced conceptual priming, which took the form of faster conceptual judgments for repeated squiggles compared with new ones. Furthermore, brain regions linked to conceptual processing in studies with words and nameable pictures produced greater activity for meaningful than for meaningless squiggles (Fig. 3). When meaningful squiggles were repeated, these conceptual regions exhibited less activity, just as the same regions show reduced activity when words and nameable pictures are repeated (Schacter and Buckner 1998; Buckner et al. 2000; Donaldson et al. 2001; Henson 2003; Schacter et al. 2007). In contrast, meaningless squiggles were not associated with repetition-related activity reductions in these regions. Repetition-related activity reductions for meaningful squiggles were also identified in anterior ventral visual regions and perirhinal/entorhinal cortex (Fig. 4B), regions implicated in the storage and representation of information regarding highly familiar stimulus categories and where activity reductions are shown in tests of conceptual priming for these stimulus categories (Schacter and Buckner 1998; Murray et al. 2000; Henson 2003; O'Kane et al. 2005; Schacter et al. 2007; Voss et al. 2009). In contrast, repetition effects for meaningless squiggles were confined within more posterior visual cortical regions that generally show negative repetition effects related to perceptual processing (Martin 2007).
These findings show that conceptual processing is selectively influenced by stimulus repetition in largely the same way for meaningful squiggles as for real-world objects. After just one encounter with a meaningful squiggle, its representation in object-sensitive cortex is sufficiently stable so as to support standard repetition priming effects as observed for real-world objects. These findings thus contradict the commonsense notion that conceptual processing of abstract objects is somewhat of an oxymoron—that finding meaning in a nonsense object does not rely on “true” conceptual processing (e.g., as claimed by Stenberg et al. (2010)) and show that the experience of pareidolia is associated with the neural hallmarks of conceptual processing identified for real-world objects.
The current findings converge with results from electrophysiological experiments with the same stimulus set and procedure for determining meaningfulness. In these previous experiments, we found that repetition produced conceptual priming and amplitude reductions of N400 event-related brain potentials (ERPs) for meaningful squiggles but not for meaningless squiggles (Voss and Paller 2007; Voss, Schendan, et al. 2010). The N400 is elicited by meaningful stimuli such as words and nameable pictures and is widely acknowledged as a neural correlate of conceptual evaluation (Kutas and Federmeier 2011). However, N400 effects in Voss and Paller (2007) and in Voss, Schendan, et al. (2010) were more frontally distributed than prototypical N400 effects and were therefore termed “FN400.” Although recent evidence indicates that N400 and FN400 can reflect the same sort of conceptual processing (Voss and Federmeier 2011), it is conceivable that FN400 effects for abstract stimuli differ functionally from standard N400 effects (FN400 potentials have been suggested by some to reflect explicit familiarity [Rugg and Curran 2007], but there are strong counterarguments against this point of view [e.g., Paller et al. 2007]. In particular, we have shown selective associations between FN400 and conceptual implicit memory that highlight their functional equivalence to N400 correlates of conceptual processing [reviewed in Voss and Federmeier 2011]). It is notable that FN400 potentials did not vary as a function of meaningfulness for squiggles seen for the first time (Voss and Paller 2007; Voss, Schendan, et al. 2010), and only meaningful squiggles showed FN400 repetition effects as correlates of conceptual priming. A similar N400 pattern was also observed for pseudowords found to be either meaningful or meaningless (Voss, Lucas, et al. 2010). This parallels the current findings that activity levels in conceptual regions were matched for meaningful and meaningless squiggles seen for the first time, with repetition priming effects in these regions only for meaningful squiggles. Thus, both ERP and fMRI findings converge in suggesting that conceptual processing occurs obligatorily for all stimuli, irrespective of preexisting representation (Federmeier and Laszlo 2009; Laszlo and Federmeier 2011) and that repetition priming effects occur only for stimuli with suitably stable conceptual representations. The current findings provide the anatomical specificity needed to unambiguously relate processing of abstract stimuli to activity in conceptual regions, including regions in ventral visual and parahippocampal cortex that are putative generators of the N400 (Kutas and Federmeier 2011), suggesting that FN400 potentials reflect genuine conceptual processing of squiggles, just as do N400 potentials for words and real objects.
Findings from the recognition memory test are also relevant for interpreting the neural correlates of conceptual processing for novel shapes. Regions that showed negative repetition effects for meaningful squiggles did so in both a priming test and a recognition test (although effects were larger in the priming test for some regions). In contrast, processing related to episodic memory retrieval was observed only during the recognition test. Furthermore, there was a striking dissociation of neural correlates of conceptual priming and of recognition. Repetition effects in the recognition test were positive (greater responses for old than new) and occurred in brain regions largely separate from those identified in the conceptual priming test. Furthermore, positive repetition effects in the recognition test were of roughly the same magnitude for meaningful and meaningless squiggles, whereas negative repetition effects in the priming test were present only for meaningful squiggles. Thus, conceptual priming and recognition were distinct in the location of the relevant activity, the sign of the repetition effects (i.e., negative vs. positive, respectively), and the sensitivity to stimulus meaningfulness (sensitive vs. insensitive, respectively). These distinctions between neural correlates of conceptual priming and recognition arose even though the same type of encoding was engaged during study, as subjects did not expect a different type of test for priming versus recognition blocks. Unlike priming/recognition contrasts based on using multiple blocks of each type of memory test, these findings cannot be attributed to differential encoding operations.
This dissociation supports the interpretation that the conceptual priming effects on behavior and neural activity were not secondary to episodic retrieval and recognition. These results add to the small number of previously reported dissociations between conceptual priming and recognition memory in fMRI activity (Donaldson et al. 2001; Voss et al. 2008; Wimber et al. 2010) and between perceptual priming and recognition memory (Schott et al. 2005, 2006), which are often interpreted as evidence for distinct implicit and explicit memory processes (Voss and Paller 2008). Conceptual priming can also be dissociated from recognition memory in ERP repetition effects for squiggles (Voss and Paller 2007) and for other stimulus materials (Voss and Paller 2006; Voss, Lucas, et al. 2010). Notably, late-onset positive-polarity ERPs related to recognition memory are fairly insensitive to stimulus meaningfulness, both for squiggles (Voss and Paller 2007) as well as for pseudowords (Voss, Lucas, et al. 2010), thus showing the same pattern reported here in fMRI activity.
An unpredicted but nonetheless noteworthy finding from the current experiment concerns false-alarm responses in the recognition memory test. False alarms were significantly more prevalent for meaningful than meaningless squiggles, and this bias to respond “old” for meaningful new squiggles was associated with reduced activity in left inferior frontal cortex and bilateral superior frontal cortex (Supplementary Fig. 3). This novel result is interesting because, whereas conceptual processing fluency is widely thought to increase false alarms in recognition studies with words and nameable pictures (discussed in Voss and Federmeier 2011), to our knowledge no previous experiment has identified the neural events responsible for this fluency-related response bias. Importantly, the brain activations related to the bias were highly similar to those related to conceptual fluency due to stimulus repetition (i.e., reduced activity in left inferior and superior frontal gyrus). One possibility is that new stimuli showed some variability in terms of conceptual processing fluency, and this fluency was sometimes attributed to repetition (oldness), leading to a selective response bias for a subset of conceptually meaningful stimuli. This interpretation will require additional direct evidence, but our results nonetheless suggest that the pareidolia experience might also provide a useful model for investigating mechanisms of fluency-based biases in recognition memory judgments.
To conclude, based on the observed patterns of neural repetition effects across tasks, we suggest that pareidolia might be such a compelling experience because the process of identifying conceptual meaning in novel or nonsense figures is essentially the same as identifying meaning in familiar real-world objects. Future studies of the neural processing relevant to pareidolia and to meaning more generally may provide novel insights into how the organization of conceptual processing differs across individuals (see also Pizzagalli et al. 2001), thereby addressing the question of what neurocognitive architecture is necessary to see a potato chip not just as a tasty snack but as the embodiment of Elvis.
Supplementary Material
Supplementary material can be found at:
Funding
Beckman Institute Postdoctoral Fellowship award; US National Institute of Health Pathway to Independence award (K99-NS069788 to J.L.V.); National Science Foundation grant BCS-0818912; Center for Advanced Magnetic Resonance Imaging at the Northwestern University Feinberg School of Medicine.
Supplementary Material
Acknowledgments
Conflict of Interest : None declared.
References
- BBC. ‘Virgin Mary’ toast fetches $28,000.[Internet] 2004. [Retrieved 2010 October 8]. Available from: [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cogn Neurosci. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Sagiv N, Mecklinger A, Friederici A, von Cramon YD. Priming visual face-processing mechanisms: electrophysiological evidence. Psychol Sci. 2002;13:190–193. doi: 10.1111/1467-9280.00435. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Kelley WM, Petersen SE. Frontal cortex contributes to human memory formation. Nat Neurosci. 1999;2:311–314. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W. Functional neuroimaging studies of encoding, priming, and explicit memory retrieval. Proc Natl Acad Sci U S A. 1998;95:891–898. doi: 10.1073/pnas.95.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Rosen BR. Functional MRI evidence for a role of frontal and inferior temporal cortex in amodal components of priming. Brain. 2000;123:620–640. doi: 10.1093/brain/123.3.620. [DOI] [PubMed] [Google Scholar]
- CNN. NASA photos show giant 'cosmic hand' [Internet] 2009. [Retrieved 2010 October 8]. Available from: [Google Scholar]
- Copsey H. Do you see the Virgin Mary in this brain scan? TC Palm [Internet] 2008. [Retrieved 2010 October 8]. Available from: [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Buckner RL. Dissociating memory retrieval processes using fMRI: evidence that priming does not support recognition memory. Neuron. 2001;31:1047–1059. doi: 10.1016/s0896-6273(01)00429-9. [DOI] [PubMed] [Google Scholar]
- Federmeier KD, Laszlo S. Time for meaning: electrophysiology provides insights into the dynamics of representation and processing in semantic memory. In: Ross BH, editor. Psychology of learning and motivation. Vol. 51. Burlington (MA): Academic Press; 2009. pp. 1–44. [Google Scholar]
- Gardiner JM, Java RI. Forgetting in recognition memory with and without recollective experience. Mem Cognit. 1991;19:617–623. doi: 10.3758/bf03197157. [DOI] [PubMed] [Google Scholar]
- Groh-Bordin C, Zimmer HD, Ecker UK. Has the butcher on the bus dyed his hair? When color changes modulate ERP correlates of familiarity and recollection. Neuroimage. 2006;32:1879–1890. doi: 10.1016/j.neuroimage.2006.04.215. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Kveraga K, Naik P, Ahlfors SP. Early (M170) activation of face-specific cortex by face-like objects. Neuroreport. 2009;20:403–407. doi: 10.1097/WNR.0b013e328325a8e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN. Neuroimaging studies of priming. Prog Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP) Annu Rev Psychol. 2011;62:621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo S, Federmeier KD. The N400 as a snapshot of interactive processing: evidence from regression analyses of orthographic neighbor and lexical associate effects. Psychophysiology. Forthcoming 2011 doi: 10.1111/j.1469-8986.2010.01058.x. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranhao-Filho P, Vincent MB. Neuropareidolia: diagnostic clues apropos of visual illusions. Arq Neuropsiquiatr. 2009;67:1117–1123. doi: 10.1590/s0004-282x2009000600033. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: structure and processes. Curr Opin Neurobiol. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Hampton RR, Saksida LM. The parahippocampal region and object identification. Ann N Y Acad Sci. 2000;911:166–174. doi: 10.1111/j.1749-6632.2000.tb06725.x. [DOI] [PubMed] [Google Scholar]
- O'Kane G, Insler RZ, Wagner AD. Conceptual and perceptual novelty effects in human medial temporal cortex. Hippocampus. 2005;15:326–332. doi: 10.1002/hipo.20053. [DOI] [PubMed] [Google Scholar]
- Oklahomaedge. Oklahoma woman selling Elvis potato chip [Internet] 2008. [Retrieved 2010 October 8]. Available from: [Google Scholar]
- Olson E. Internet casino buys Virgin Mary snack for $10,600 [Internet] 2005. [Retrieved 2010 October 8]. Available from: [Google Scholar]
- Paller KA, Voss JL, Boehm SG. 2007. Validating neural correlates of familiarity. Trends Cogn Sci. 11:243–250. [DOI] [PubMed]
- Perrett DI, Rolls ET, Caan W. Visual neurones responsive to faces in the monkey temporal cortex. Exp Brain Res. 1982;47:329–342. doi: 10.1007/BF00239352. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D, Lehmann D, Brugger P. Lateralized direct and indirect semantic priming effects in subjects with paranormal experiences and beliefs. Psychopathology. 2001;34:75–80. doi: 10.1159/000049284. [DOI] [PubMed] [Google Scholar]
- Richardson-Klavehn A, Bjork RA. Measures of memory. Annu Rev Psychol. 1988;39:475–543. [Google Scholar]
- Rorschach H. Psychodiagnostik. Bern (Switzerland): Bircher; 1921. [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends Cogn Sci. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Sagan C, Druyan A. The Demon-Haunted World: Science as a candle in the dark. New York: Ballantine Books; 1997. [Google Scholar]
- Schacter DL, Buckner RL. Priming and the brain. Neuron. 1998;20:185–195. doi: 10.1016/s0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Curr Opin Neurobiol. 2007;17:171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Schott BH, Henson RN, Richardson-Klavehn A, Becker C, Thoma V, Heinze HJ, Duzel E. Redefining implicit and explicit memory: the functional neuroanatomy of priming, remembering, and control of retrieval. Proc Natl Acad Sci U S A. 2005;102:1257–1262. doi: 10.1073/pnas.0409070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Richardson-Klavehn A, Henson RN, Becker C, Heinze HJ, Duzel E. Neuroanatomical dissociation of encoding processes related to priming and explicit memory. J Neurosci. 2006;26:792–800. doi: 10.1523/JNEUROSCI.2402-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg G, Johansson M, Hellman J, Rosen I. “Do you see yonder cloud?”—on priming concepts, a new test, and a familiar outcome. Reply to Lucas et al.: “Familiarity or conceptual priming? Good question! Comment on Stenberg, Hellman, Johansson, and Rosen (2009)”. J Cogn Neurosci. 2010;22:618–620. doi: 10.1162/jocn.2009.21268. [DOI] [PubMed] [Google Scholar]
- Tisch C. For Mary's faithful, a shattering loss [Internet] 2004. [Retrieved 2010 October 8]. Available from: [Google Scholar]
- Voss JL, Federmeier KD. FN400 potentials are functionally identical to N400 potentials and reflect semantic processing during recognition testing. Psychophysiology. 2011;48:532–546. doi: 10.1111/j.1469-8986.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Hauner KK, Paller KA. Establishing a relationship between activity reduction in human perirhinal cortex and priming. Hippocampus. 2009;19:773–778. doi: 10.1002/hipo.20608. [DOI] [PubMed] [Google Scholar]
- Voss JL, Lucas HD, Paller KA. Conceptual priming and familiarity: different expressions of memory during recognition testing with distinct neurophysiological correlates. J Cogn Neurosci. 2010;22:2638–2651. doi: 10.1162/jocn.2009.21341. [DOI] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Fluent conceptual processing and explicit memory for faces are electrophysiologically distinct. J Neurosci. 2006;26:926–933. doi: 10.1523/JNEUROSCI.3931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Neural correlates of conceptual implicit memory and their contamination of putative neural correlates of explicit memory. Learn Mem. 2007;14:259–267. doi: 10.1101/lm.529807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Brain substrates of implicit and explicit memory: the importance of concurrently acquired signals of both memory types. Neuropsychologia. 2008;46:3021–3029. doi: 10.1016/j.neuropsychologia.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Reber PJ, Mesulam MM, Parrish TB, Paller KA. Familiarity and conceptual priming engage distinct cortical networks. Cereb Cortex. 2008;18:1712–1719. doi: 10.1093/cercor/bhm200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Schendan HE, Paller KA. Finding meaning in novel geometric shapes influences electrophysiological correlates of repetition and dissociates perceptual and conceptual priming. Neuroimage. 2010;49:2879–2889. doi: 10.1016/j.neuroimage.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wimber M, Heinze HJ, Richardson-Klavehn A. Distinct frontoparietal networks set the stage for later perceptual identification priming and episodic recognition memory. J Neurosci. 2010;30:13272–13280. doi: 10.1523/JNEUROSCI.0588-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.