Abstract
Some of the most striking symptoms after prefrontal damage are reduction of behavioral initiation and inability to suppress automatic behaviors. However, the relation between these 2 symptoms and the location of the lesions that cause them are not well understood. This study investigates the cerebral correlates of initiation and suppression abilities assessed by the Hayling Sentence Completion Test, using the human lesion approach. Forty-five patients with focal brain lesions and 110 healthy matched controls were examined. We combined a classical group approach with 2 voxel-based lesion methods. The results show several critical prefrontal regions to Hayling Test performance, associated with either common or differential impairment in “initiation” and “suppression” conditions. A crucial role for medial rostral prefrontal cortex (BA 10) in the initiation condition was shown by both group and lesion-mapping methods. A posterior inferolateral lesion provoked both initiation and suppression slowness, although to different degrees. An orbitoventral region was associated with errors in the suppression condition. These findings are important for clinical practice since they indicate that the brain regions required to perform a widely used and sensitive neuropsychological test but also shed light on the regions crucial for distinct components of adaptative behaviors, in particular, rostral prefrontal cortex.
Keywords: behavior, human, lesion study, neuropsychology, rostral prefrontal
Introduction
Behavioral adaptation results from a balance between automatically initiated behaviors (like those guided by strong stimulus–response associations) and the suppression of these automatic behaviors in order to elaborate new ones (when strong stimulus–response associations are not relevant anymore). These 2 aspects of behavior can be altered in patients with frontal lobe damage (Perret 1974). Frontal patients may show a reduced tendency to initiate behavior, often described by carers as appearing “apathetic” or “lethargic.” Another striking disorder after prefrontal damage is the expression of irrepressible automatic behaviors, such as in “utilization behavior” (Lhermitte et al. 1986; Shallice et al. 1989; Volle et al. 2002). Here, patients are unable to suppress automatic behaviors triggered by the external world. In real life, patients’ inability to stop themselves from doing or saying something inappropriate is commonly reported by families (Wilson et al. 1998).
The relationship between these 2 symptom clusters, and the regions of the brain which, when lesioned, cause them, are not well understood. Undoubtedly, these symptoms are frequent in neurological patients (Godefroy et al. 2010), they can be seen together in any one patient, and this observation has been a major contributor to the notion of a “dysexecutive syndrome.” However, the exact nature of the interrelations between these symptoms are not well understood. Consider for instance data from the DEX questionnaire, part of the Behavioral Assessment of the Dysexecutive Syndrome neuropsychological test battery (Wilson et al. 1996). The DEX is a 20-item questionnaire completed by family or carers. Three items of this questionnaire are particularly relevant to response initiation and suppression. Item 8 relates to initiation problems (seems lethargic or unenthusiastic about things), and items 2 and 16 relate to response suppression difficulties (“acts without thinking, doing the first thing that comes to mind”; “finds it difficult to stop doing something even if they know they shouldn’t”). Burgess et al. (1998) reported analysis of the relations between these symptoms in 92 mixed etiology neurological patients. Both symptoms were highly prevalent: 24% of the sample had frequent suppression problems, 30.5% had initiation problems. Subsequent analysis of these data showed that the correlation between the 2 response suppression items was high (0.56; P < 0.0001), supporting the construct validity of these 2 items. The correlation between these 2 suppression items (items 2 and 16) and the response initiation item (item 8) was lower (0.39; 0.36, respectively) but still highly significant (both P < 0.005). Principal component analysis showed a single common factor with similar loadings for all 3 items, and a second factor where the loadings for response initiation variable were very much higher. These patterns of symptoms, based on observations in everyday life, argue against the notion that response suppression and initiation problems always coexist (see also Godefroy et al.1996). However, neither do they suggest that these symptoms are usually seen completely independent from each other. The possibility most fitting with the data above is that response suppression and initiation problems may share some common causes, but initiation problems may also occur for quite different reasons also.
Unfortunately from an experimental viewpoint, response initiation and suppression are concurrent components of several neuropsychological tasks, such as verbal fluency and random item generation paradigms. Their individual contributions to performance are therefore difficult to separate, complicating task performance interpretation. One exception is the Stroop test (Stroop 1935). This is commonly used to measure the ability to suppress a prepotent response to words (word reading) in favor of an alternative one (ink color naming of the word). According to both functional neuroimaging (Bench et al. 1993; Milham et al. 2001; Brass et al. 2005) and lesion studies (Perret 1974; Golden 1976; Vendrell et al. 1995; Stuss et al. 2001), this task engages a well-known network including lateral prefrontal and cingulate regions. However, it is not clear whether the Stroop test performance relate to the kinds of everyday symptoms mentioned above (Burgess et al. 2006). The Stroop test measures suppression of overlearned stimulus–response association at the response level, but not at the conceptual or semantic level, while semantic and response conflict may be associated with distinct cerebral networks (Kan and Thompson-Schill 2004; van Veen and Carter 2005). Other studies that have explored the anatomy of motor action inhibition used the Go/No-go task, which is also a motor inhibition task (Godefroy et al. 1996; Picton et al. 2007; Simmonds et al. 2008). The anatomy of motor initiation have been explored separately using simple reaction time tasks (Godefroy et al. 2002; Stuss et al. 2005; Périn et al. 2010).
In the verbal domain, Burgess and Shallice (1996) designed a neuropsychological test, the Hayling Sentence Completion Test (HSCT or Hayling Test), allowing the assessment of response initiation and response suppression within the same verbal semantic task. The Hayling Test is composed of 2 conditions: response initiation (part A) and response suppression (part B). In both conditions, participants are read aloud by the experimenter a sentence that has the final word omitted. In the initiation condition, they are asked to provide a word that completes the sentence. For example, “London is a very busy … ?” (Where an appropriate answer is “city.”) By contrast, in the suppression condition, the participants are asked to provide a word that is completely “unrelated” to the sentence frame. In the previous example therefore, city would now be an inappropriate answer, whereas “banana” would be a good one. The Hayling test thus provides separate and comparable measures of verbal semantic initiation and suppression abilities.
The Hayling Test is known to be a particularly sensitive neuropsychological test for frontal functions (see, e.g., Hornberger et al. 2010). For instance, Burgess and Alderman (2004) report that only 4% of healthy age morbid and premorbid IQ-matched controls attained a scaled score of 2 (out of 10) on the Hayling Test, suppression subscore, but 77% of a group of patients with circumscribed bifrontal lesions attained that score or below. Moreover, 53% of them were worse than any control. This compares extremely favorably in terms of detection of abnormality with other neuropsychological tests of executive function (Burgess et al. 1998, 2006). Critically, however, while people with frontal lobe dysfunction may be impaired on either the initiation and suppression conditions, compared with patients with lesions more posterior, or to controls, these impairments can appear independently from each other (Burgess and Shallice 1996). This suggests that initiation and suppression may be subserved by, at least in part, distinct cerebral regions.
There have been few neuroimaging studies that have explored the cerebral correlates of the Hayling task or paradigms closely related to it. Mostly, they suggest involvement of lateral prefrontal cortex (PFC) in the suppression condition, while the initiation condition may involve ventromedial PFC (Collette et al. 2001; Nathaniel-James and Frith 2002). However, while functional imaging techniques may reveal brain regions that are engaged in a task, they need not indicate the regions that are “necessary” to perform the task (Coltheart 2006; Henson 2006; Kinkingnehun et al. 2007; Volle et al. 2008). Lesion studies are thus essential to complement functional imaging and in particular to be able to draw valid conclusions for clinical practice. Unfortunately however, lesion studies using the Hayling test are rare and do not clearly identify specific brains areas. Recently, Roca et al. (2010) gave a useful but rather indirect indication of Hayling function–structure brain correlates. In their lesion study of frontal patients, a deficit in a composite score of the Hayling test was found beyond that which could be explained by a deficit in “fluid intelligence,” whereas the variance in other widely used frontal neuropsychological tests could be (Fluency, Wisconsin card sorting test). Moreover, the maximum overlap of lesions from patients with greatest deficit unexplained by IQ was located in the right rostral PFC. This suggests that the rostral PFC plays a role in some cognitive processes that are assessed by the Hayling test but are not explored by common frontal tests. The exact location of brain areas crucial for the Hayling test however remains to be more directly addressed.
Recent techniques for lesion studies, such as voxel-by-voxel lesion-deficit mapping, allow precise clinical-radiological correlations by testing all damaged voxels and do not rely upon classifying patients into categorical groups or choosing a cutoff for pathology, in contrast with more classical methods. The use of these new methods has produced consistent results (Bates et al. 2003; Dronkers et al. 2004; Tyler et al. 2005; Committeri et al. 2007; Volle et al. 2008; Del Cul et al. 2009; Gläscher et al. 2009).
The aim of the present work was to examine the cerebral correlates of both initiation and suppression conditions of the Hayling test. We combined these recent voxel-based lesion methods and a more classical group approach, in 45 patients with focal brain lesions and 110 matched controls.
Materials and Methods
The experiment was approved by the local research ethics committee. All participants were able to provide written informed consent.
Subjects
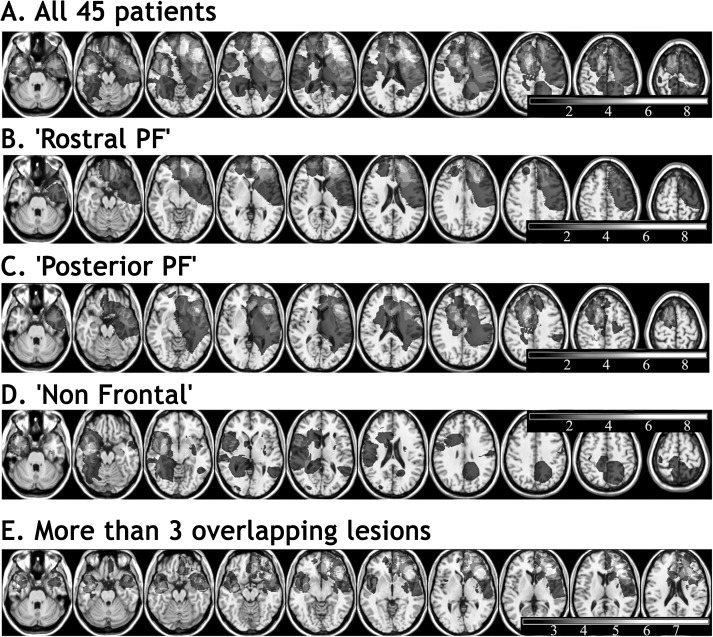
Patients were recruited mainly from the Neurosurgery and the Neurological Departments of King’s College Hospital, London, UK. Additional patients were recruited from 2 other London hospitals: the Regional Neurological and Rehabilitation Unit of the Homerton University Hospital and the Wolfson Rehabilitation Centre, St. George’s Healthcare Trust, Wimbledon. Sixty-seven patients were assessed (for background details, see Table 1), when attending for a full investigation of their lesion, if they met the following criteria. 1) The presence of a cerebral focal lesion was confirmed by an anatomical CT scan or magnetic resonance imaging (MRI), available for the current condition. 2) The lesion was acquired in adulthood (mostly haemorrhage, ischemic stroke, or brain tumor). 3) Participants were able to understand and perform the cognitive tasks. Patients who demonstrated gross disorientation, visual, memory, reading, naming, or instrumental impairments that would interfere with the Hayling tasks were not included (impairments detected on VOSP perception battery, on Shortened Revised Token Test; De Renzi and Faglioni 1978) on the National Adult Reading Test—NART (Nelson and O'Connell 1978; Bright et al. 2002), on McKenna confrontation naming test (McKenna and Warrington 1983), on Warrington's recognition memory test (Warrington 1984). 4) Patients were excluded if they had a prior history of neurological or psychiatric disease requiring hospitalization, of alcohol or other substance abuse or of developmental problems. 5) All included patients were right-hand dominant and had English as their first language. It is important to note that every patient who matched the above criteria was included, regardless of the location of the lesion and the pattern of the cognitive deficit. Full data including brain scans were eventually available for 45 patients. Figure 1A and Supplementary Figure S1 shows the location of lesions of these 45 patients.
Table 1.
Characteristics of the different populations included in the study
| All patients, N = 45 | Rostral PF, N = 8 | Posterior PF, N = 18 | Non Frontal, N = 19 | Controls, N = 110 | ||
| Mean (SD), min-max | ||||||
| Age (years) | 47.4 (10.7), 26–67 | 48.1 (12.5), 26–62 | 48.8 (10.6), 26–67 | 45.8 (10.4), 27–64 | 49.0 (14.7), 17–81 | |
| NART or WTAR (premorbid IQ) | 103.1 (14.0), 74–124 | 100.7 (19.2)b, 76–120 | 102.8 (16.2)a, 74–124 | 104.1 (10.4)a, 90–124 | 104.9 (11.6), 77–126 | |
| WAIS-FSIQ | 94.9 (14.6), 67–124 | 82.5 (16.4)b, 67–113 | 95.7 (14.0)b, 74–124 | 98.8 (12.6)c, 70–119 | ||
| Lesion volume (cm3) | 48.9 (83.1), 0.8–464.9 | 101.3 (148.5), 10.9–465 | 44.8 (75.5), 2.5–330 | 30.7 (36.4), 0.8–95.4 | ||
| Time interval # (months) | 9.0 (11.5), 1–69 | 5.1 (6.2), 1–19 | 14.1 (16.0), 1–69 | 5.7 (4.5)a, 1–19 | ||
| Frequencies | ||||||
| Gender | Male | 51% | 4 | 11 | 8 | 55%b |
| Female | 49% | 4 | 7 | 11 | 45%b | |
| Lesion side | Right | 20 | 4 | 10 | 6 | |
| Left | 21 | 2 | 7 | 12 | ||
| Bilateral | 4 | 2 | 1 | 1 | ||
| Lesion type | Vascular | 12 | 2 | 3 | 7 | |
| Tumoral | 31 | 5 | 15 | 9 | ||
| Other | 2 | 1 | 0 | 3 | ||
Note: In exponent are signaled the number of missing values for each test and group (a: 1 missing value; b: 2 missing values; c: 3 missing values). Among patients suffering for tumors, 18 presented with a glial tumor, 6 with a meningioma, and 5 with another or unknown etiology. Vascular patients had either ischemia (n = 3) or haemorrhage (n = 9) due to the rupture of a vascular malformation. Time interval corresponds to the period of time separating the neuropsychological evaluation and the brain imaging.
Figure 1.
Lesion overlaps of the 45 patients’ lesions, (A) pooled all together, (B), (C), (D) shown by group, and (E) regions where at least 3 lesions overlapped. The number of overlapping lesions is represented in a gray scale (the lightest, the more overlaps), in the MNI space (according to neurological convention, i.e., right is right).
Normative data for the Hayling test was acquired from a group of 110 healthy normal subjects (see Table 1) matched for age, gender and estimates of their basal (or premorbid for the patients) IQ, based on NART (Nelson and O'Connell 1978), or on the Wechsler test of adult reading—WTAR (Wechsler 2001). Control subjects were right-handed, native English speakers; they had no history of neurological or psychiatric disease.
Patients and controls were compared in both a classical group study and with a voxel-based approach. In addition to the comparison of patients to controls, we wanted to compare groups of patients with different lesion locations. Our a priori regions were determined as follows. Results obtained by functional MRI studies of the Hayling task (Collette et al. 2001; Nathaniel-James and Frith 2002) have shown activation in rostral prefrontal, dorsal and ventral lateral PFC, and in the lateral temporal and inferior parietal cortex. The lesion findings of Roca et al. (2010) described above also implicates damage to the frontal pole as a good candidate for a poor Hayling performance, at least under certain conditions. Thus, the most obvious subdivision one might employ is one that distinguishes between people with 1) rostral prefrontal damage; 2) prefrontal but nonrostral prefrontal damage; and 3) damage outside the frontal lobes. It is also consistent with classical rostrocaudal hierarchical models of brain functioning (Koechlin et al. 2003; Badre 2008; Fuster 2009). As a consequence, patients whose lesion did not involve the frontal lobes were pooled in the group called “Non Frontal” (n = 19). Two patients presented with a lesion that involved a small part of the inferior prefrontal area, but given that the overwhelming proportion of their lesion was non frontal, they were included in the “non frontal” group. Patients whose lesion involved the frontal lobes were either pooled in the group “Rostral PF” (rostral prefrontal; n = 8) if the rostral prefrontal region or frontal pole (approximately Brodmann area 10 [BA10]) was involved or in the group “Posterior PF” (n = 18) if BA10 was intact. The “Non Frontal” group was composed of 11 temporal, 5 parietal, and 3 subcortical lesions. The “Posterior PF” group consisted of 6 premotor, 4 dorsolateral prefrontal, 6 inferolateral, and 2 orbitofrontal lesions. The global overlap of the lesions and the overlapping lesions in each group are shown in Figure 1 and Supplementary Figure S1.
The Experimental Cognitive Tasks: the HSCT—Parts A and B
The Hayling test (Burgess and Shallice 1996, 1997) involves 2 conditions (see above, for examples). In both conditions, subjects were read 15 sentence frames in which the final word was omitted. In the first condition (“initiation”) subjects were asked to speak aloud a word that completed the sentence as quickly as possible. In the second condition (“suppression”), conducted after the first part, they were asked to give a word that was unrelated to the sentence frame in every way, that is, which made no sense in the context of the sentence. Responses that were semantically or contextually related to the frame of the sentence, as well as inappropriate language, were considered as errors.
One strong advantage the test has in interpretation of the results is that initiation and suppression constructs are measured with just a simple change of instruction—the rest of the demands of the 2 components of the task are identical.
For each condition, timing of response latencies were recorded and transformed into scaled scores. Measures of reaction times in the straightforward completion and in the unrelated completion conditions provided respectively an “initiation” and a “suppression” subscore. In “suppression,” the number and type of errors were also collected and transformed into a third subscore, the “suppression-errors” subscore. Each subscore (RT or number of errors) was converted into a scaled subscore, as is standard for the administration of this test, using a scale running from 1 to 10, the points on the scale corresponding with percentiles in the following way: 1: out of normal range, 2: percentile 1, 3: percentile 5, 4: percentile 10, 5: percentile 25; 6 percentile 50; 7: percentile 75; 8: percentile 90; 9: percentile 95, 10: percentile 99. The global Hayling score is a scaled summary of these 3 subscores (for a detailed description, see Burgess and Shallice 1996, 1997).
An additional simple reaction time task was performed in order to control for nonspecific slowing of responses. In this task, participants were shown pictures and words on a computer screen and were asked to press the space bar of a computer keyboard as soon as an item appeared. One hundred and twenty items were displayed, with a self-paced duration. Intervals between trials were 0.5 s (40 times), 1 s (40 times), or 2 s (40 times), randomly distributed.
Structural Imaging
Patients underwent either a structural MRI (n = 35) or a CT scan (n = 10), in the context of their clinical or neuropsychological evaluation or follow up. Images were acquired at the neuroradiology departments of the collaborating hospitals and were collected for clinical purposes only. They were used for research purposes here in accordance with the ethical approval. Accordingly, the scans were acquired using diverse acquisition sequences, depending on the machine and/or the patient’s pathology. The MR images used for further processing were T2-weighted MRI as they were available for all the patients who underwent an MRI. T2-weighted scans, although offering less contrast precision than T1-weighted scans between gray and white matter, give good pathological information, by highlighting regions of damage. However, all available sequences were used by the neurologist (E.V.) in order to identify the limits of each lesion. Structural MR and CT images were converted into the SPM format (Statistical Parametric Mapping; http://www.fil.ion.ucl.ac.uk/spm/) for further processing described below.
Imaging and Statistical Analyses
Imaging Preprocessing: Normalization and Lesion Segmentation
MRI images were preprocessed in SPM5 (http://www.fil.ion.ucl.ac.uk/; Wellcome Institute of Cognitive Neurology, London). The first step consisted of spatially normalizing MRIs to the Montreal Neurological Institute (MNI) template. As spatial normalization can be affected by the presence of a brain lesion, all signal abnormalities due to the lesion were first traced (first segmentation, using MRIcro, http://www.sph.sc.edu/comd/rorden/mricro.html) and were used as a mask during the normalization procedure to optimize the brain normalization. This masking procedure, “Cost Function Masking,” was used to weight the normalization to brain rather than nonbrain tissue or lesions (Brett et al. 2001). In fact both the Cost Function Masking method and the more recent “Unified Model” (Crinion et al. 2007) for normalizing brains were tested on our set of data. Visual inspection showed better results for the Cost Function Masking procedure. The “Unified segmentation” method produced more deformation around the brain damage. This is in accordance with recent results (Andersen et al. 2010) and may additionally be due to the fact that we used T2-weighted MRIs while gray and white matter differentiation (and thus segmentation) is greater on T1 images. The spatially normalized images were resliced with a final voxel size of 1 × 1 × 1 mm3. The normalized images were then compared with the MNI template to evaluate normalization accuracy. The normalizing procedure failed for 5 patients. For these 5 patients, the segmentation followed the same procedure as for CT scans, as described below. For the remaining 30 successful normalizations, brain lesions were manually segmented again, this time on the normalized anatomical MRI, in order to extract the normalized lesion volume. This second segmentation was used for further statistical analyses.
CT images (and also MRIs which failed to normalize) were preprocessed differently because the SPM normalization was not possible. Normalization and segmentation were performed in one step, by directly reconstructing the lesion onto the MNI template. Patients’ lesions were drawn on the MNI template by a neurologist (E.V.), who was at this time blind to the scores of the patients. This method has been used in other studies (Bates et al. 2003; Damasio et al. 2004). In order to facilitate the comparison of the patients’ space and the MNI space and to improve the lesion transfer, the patients’ structural image was reoriented to match the template orientation, in particular regarding the axial plan. This matching was performed using free rotations in the MRIcro software (http://www.sph.sc.edu/comd/rorden/mricro.html).
We used 3 distinct methods to test for the association between brain areas and deficits in the Hayling test: 1) level 1: a classical group comparison between patients with different lesion locations and 2) level 2: 2 distinct voxel-based lesion-deficit mapping approaches, AnaCOM (Anatomo-Clinical Overlapping Maps; Kinkingnehun et al. 2007), and VLSM (voxel-based lesion-symptom mapping; Bates et al. 2003; Rorden et al. 2007). We reasoned that where these methods gave converging results, the conclusions that could be drawn would be strengthened.
Level 1: A Priori Patient Groupings and Statistical Analyses
Once the volume of the lesion was obtained for all patients, each one was superimposed on an MRI template containing Brodmann areas (in MRIcro), and the anatomical region involved by the lesion were checked. This confirmed the classification of patients into their appropriate groups (classification based on native brain images) and revealed that BA10 was damaged in all “Rostral PF” patients but not in other groups of patients. The 4 groups (“Controls,” “Non Frontal,” “Posterior PF,” and “Rostral PF”) were then statistically compared.
Statistical tests were performed using SPSS software (SPSS for windows, version 16, SPSS Inc., Chicago IL). All demographic and behavioral data were tested for normality, and statistical tests were chosen consequently. When the assumption of normality was met, one-way analyses of variance (ANOVAs) and post hoc tests were used. Else, nonparametric tests were used: Kruskal–Wallis tests (and Mann–Whitney tests as post hoc). We checked for significant between-group differences in basic demographic, estimates of premorbid IQ (NART or WTAR), and lesion data (side, volume, etiology).
Then, we tested for between-group differences in Hayling scores and subscores (overall score, “initiation,” “suppression,” and “suppression-errors” subscores). Influence of age, IQ, premorbid IQ, and lesions characteristics on Hayling performances in patients was assessed by correlation analyses. A Kendall’s tau test was used because many scaled scores may have the same rank.
Level 2: Voxelwise Statistical Approaches
AnaCOM.
The principal analysis was performed using a recently developed voxel-by-voxel lesion mapping method, AnaCOM (for a full description of the method, see Kinkingnehun et al. 2007). AnaCOM permits statistical analysis of the voxels that explain the most variance in relation to a cognitive or behavioral deficit.
The previously described normalization and segmentation steps resulted in a 3D reconstruction of each patient’s lesion. The next step consisted of weighting each of these lesion volumes by the score obtained by each patient in a given task. This was performed by attributing, to all the voxels of each lesion volume, the value of the score of the corresponding patient (for instance, if a patient scored 3/10 in a given task, all the voxels included in his brain lesion were set at 3), while the rest of the image was set to zero, assuming that the brain lesion was responsible for the patient’s deficit. Volumes representing each patient’s lesion (n = 45) were then superimposed in order to build the “Maximum Overlap Map.” This map gave, for each voxel, the number of lesions that include this voxel. In these maps, the patterns of overlaps of the segmented lesions defined subregions (group of voxels covered by the same lesions). Statistical analyses were performed in the subregions that were composed of at least 3 lesions. For these subregions, a nonparametric Wilcoxon rank sum test was used, corrected for multiple comparisons (Bonferroni–Holm correction). This test compared performances of patients with damage to that subregion, with those of the control participants. Only regions where statistical significance at P < 0.05 was present after Holm correction were considered.
These steps were performed for the global score in the Hayling test and for each of the subscores: “initiation,” “suppression,” and “suppression-errors.” Statistical maps were thus obtained for each subscore. Each of these maps represented brain regions where the patients’ performance statistically differed from that of the control subjects for a given task. These results thus indicated the clusters of voxels within the areas covered by at least 3 overlaps that contributed the most to a given impairment.
Regions within the PFC where there were at least 3 overlaps included the left frontal pole, the right frontal pole mainly in its medial portion, the left inferolateral prefrontal, and the right dorsolateral and dorsomedial prefrontal region, the temporal pole extending to the lateral temporal cortex bilaterally (Fig. 1E). Consequently, conclusions are drawn from the analysis of these regions only.
VLSM.
A second lesion-deficit mapping method, VLSM, was applied to the same preprocessed lesions data. This method (Bates et al. 2003) differs from AnaCOM in terms of the statistical comparisons that are performed and in the way corrections for multiple comparisons are applied. First, while AnaCOM compares, in each subregion, the performances of patients with damage in that subregion to the performances of controls, VLSM compares the performances of patients’ with damage in a particular voxel to the performances of patients that do not have damage in that voxel. In other words, this method compares for each voxel a group of damaged patients to another group of patients. Using a group of patients as a control group is usual in classical lesion studies. Indeed, we also did so here, in the first approach described above (level 1), where we have distributed the patients on a priori grounds into 3 groups and compared them. It is a useful method in order to test if an observed difference between patients and controls is due to nonspecific effects of brain damage rather than to damage to a particular region. In voxel-based techniques, using “not damaged in that voxel” patients as a control group is appropriate when a deficit can result from only one lesion location (Godefroy et al. 1998). But when several regions are critical for the performance at a given task, the power of such a method is questionable. In this case, both “damaged in that voxel” patients and not damaged in that voxel patients might include one of the critical functional regions, in which case an informative structure–function relationship might be missed. This is especially critical when exploring PFC given its strong connective properties or when exploring executive or control functions that are distributed in a range of brain regions. Thus, if VLSM methods may have a better specificity, AnaCOM presents more power, in particular to detect several regions equally responsible for a deficit, by using normative performance values. The specificity of the behavioral deficit is then improved by the use of control tasks. Second, regarding the correction for multiple comparisons, AnaCOM uses Bonferroni and Holm thresholding based on the number of subregions (group of voxels where similar lesions overlap) instead of the number of voxels. This decreases the number of comparisons performed and thus increases dramatically the power of the statistical results. This method of correction has also been more recently implemented in VLSM via NPM (MRIcron software, http://www.cabiatl.com/mricro/npm/), as well as correction methods based on permutations. Thus, when using VLSM (as implemented in NPM), our data were analyzed at each of these thresholds (Bonferroni at the cluster level, FDR correction at the voxel level, and permutation correction) with the nonparametric Brunner Munzel test.
Results
Level 1: A Priori Patient Groupings
Patients and Lesions Data
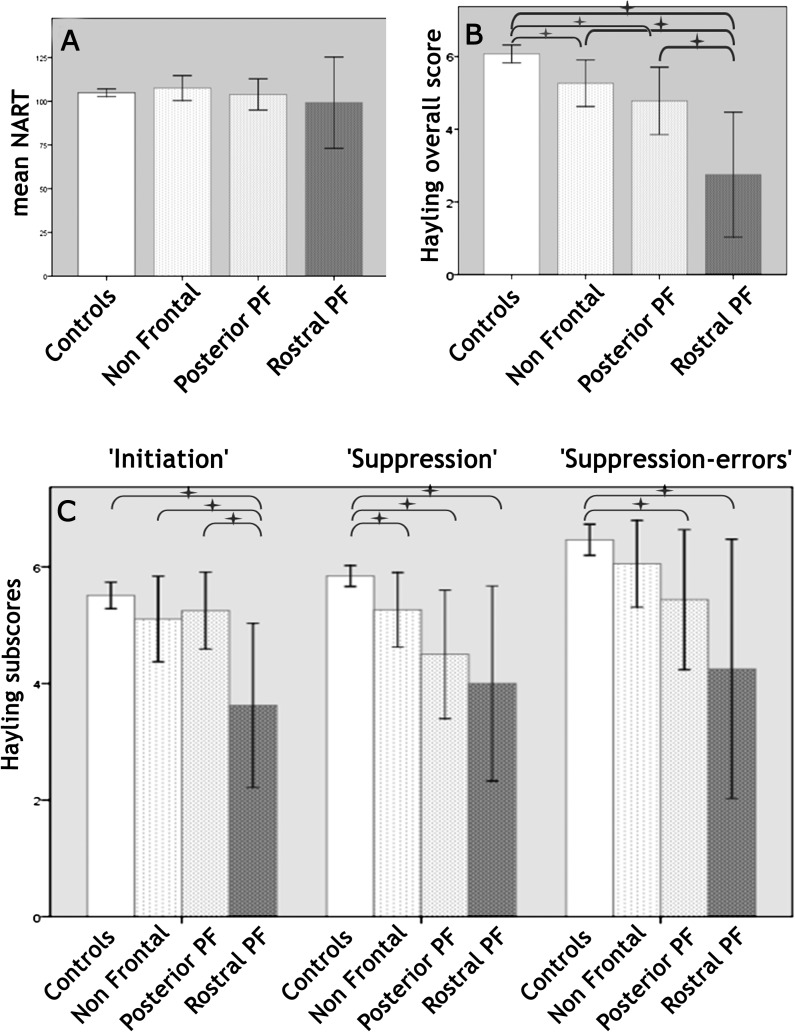
No significant difference was found between the 4 groups in terms of age at testing (one-way ANOVA: F3,151 = 0.29; P = 0.833), gender (Pearson chi-square: Chi2(3) = 1.51; P = 0.679), or premorbid IQ estimated by the NART (one-way ANOVA: F3,147 = 0.33; P = 0.802; Fig. 2A). Between the 3 patient groups determined a priori, lesion side was equally distributed (Pearson chi-square: χ2 (2) = 3.17; P = 0.205), as were the etiologies of the lesions (Pearson chi-square: χ2 (4) = 4.96; P = 0.291). The difference in lesion volume between the groups did not reach significance (H2 = 4.89; P = 0.087) nor did the FSIQ (F2,35 = 3.07; P = 0.059).
Figure 2.
Performances of the distinct groups on (A) the NART, (B) the overall Hayling score, and (C) Hayling subscores. Starred horizontal brackets indicate significant differences between groups.
Hayling Performances
Performances are summarized in Table 2. The following statistical results are reported in Table 2 and illustrated in Figure 2B,C.
Table 2.
Performance on the Hayling score and subscores for the 4 groups of participants and statistical comparisons between groups
| Hayling overall score | “Initiation’’ subscore | “Suppression” subscore | “Suppression-errors” subscore | |
| All patients, N = 45 | 4.62 (1.89)C, 1–7 | 4.88 (1.55)C, 1–7 | 4.74 (1.79)C, 1–6 | 5.49 (2.11)C, 1–7 |
| Rostral PF, N = 8 | 2.75 (2.05)C,N,P, 1–6 | 3.62 (1.69)C,N,P, 1–6 | 4.00 (2.00)C, 1–6 | 4.25 (2.66)C, 1–7 |
| Posterior PF, N = 18 | 4.78 (1.87)C,R, 1–6 | 5.25 (1.24)R, 3–7 | 4.50 (2.07)C, 1–6 | 5.44 (2.25)C, 1–7 |
| Non Frontal, N = 19 | 5.26 (1.33)C,R, 1–6 | 5.11 (1.52)R, 1–7 | 5.26 (1.33)C, 1–6 | 6.05 (1.55), 2–7 |
| Controls, N = 110 | 6.07 (1.30)N,P,R, 2–10 | 5.51 (1.19)R, 1–8 | 5.84 (0.94)N,P,R, 3–8 | 6.46 (1.40)P,R, 2–8 |
| Four groups effect (Kruskal–Wallis) | H (3) = 28.49; P < 0.001 | H (3) = 14.16; P = 0.003 | H (3) = 2.80; P < 0.001 | H (3) = 1.06; P = 0.018 |
| All patients versus “Controls” | U = 1382; z = −4.71; P < 0.001 | U = 1726.5; z = −2.74; P = 0.006 | U = 1496; z = −4.10; P < 0.001 | U = 1669; z = −2.81; P < 0.001 |
| “Rostral PF” versus “Controls” | U = 78; z = −4.20; P < 0.001 | U = 142; z = −3.59; P < 0.001 | U = 162; z = −3.59; P < 0.001 | U = 217; z = −2.44; P = 0.015 |
| “Rostral PF” versus “Non Frontal” | U = 24; z = −3.00; P = 0.004 | U = 35.5; z = −2.24; P = 0.025 | U = 43; z = −1.94; P = 0.052 | U = 47.5; z = −1.62; P = 0.106 |
| “Rostral PF” versus “posterior PF” | U = 32; z = −2.31; P = 0.026 | U = 27; z = −2.36; P = 0.018 | U = 49; z = −0.97; P = 0.330 | U = 51; z = −0.83; P = 0.405 |
| “Posterior PF” versus “Controls” | U = 597.5; z = −2.95; P = 0.003 | U = 741; z = −1.06; P = 0.292 | U = 545; z = −2.94; P = 0.003 | U = 604; z = −2.02; P = 0.043 |
| “Posterior PF” versus “Non Frontal” | U = 151.5; z = −0.67; P = 0.504 | U = 146; z = −0.21; P = 0.830 | U = 126.5; z = −0.98; P = 0.328 | U = 126; z = −0.93; P = 0.351 |
| “Non Frontal” versus “Controls” | U = 706.5; z = −2.50; P = 0.012 | U = 843.5; z = −1.41; P = 0.158 | U = 789; z = −2.02; P = 0.044 | U = 848; z = −1.26; P = 0.208 |
Note: Values are scaled scores out of 10. Mean are reported with standard deviation in brackets, followed by Min and Max values. For each raw, the superscript characters indicate the significance of a statistical comparison between the group described in this raw and “Rostral PF” (R), “posterior PF” (P), “Non Frontal” (N), and “Controls” (C). The actual statistical results of these comparisons are detailed in the lower columns of the table. In these latter columns, significant differences appear in bold (P < 0.05).
For the global Hayling score (Fig. 2B), a significant difference was found between the 4 groups “Rostral PF,” “Posterior PF,” “Non Frontal,” and “Controls.” Mann–Whitney tests were used to follow up this finding. It appeared that all patient groups pooled together were impaired compared with the “Control” group. The “Rostral PF” group was impaired compared with “Controls” and to each patient group. The “Posterior PF” group was also impaired compared with “Controls,” while the “Non Frontal” was not.
Between-group differences in performance were also observed in the Hayling “initiation,” “suppression,” and “suppression-errors” subscores (Fig. 2C) when performing Kruskal–Wallis tests. When pooling patient groups together, patients were significantly impaired compared with controls in all the subtests. More specific post hoc tests were conducted in order to investigate these results, using Mann–Whitney tests. First, only the “Rostral PF” group was impaired in all subscores compared with “Controls.” Compared with “Posterior PF” patients, “Rostral PF” patients were impaired only in the “initiation” subscore. Compared with “Non Frontal” patients, “Rostral PF” patients were impaired in the “initiation” subscore and marginally in the “suppression” subscore. Second, “Posterior PF” patients showed a distinct pattern: their performance was different from “Controls” in the “suppression” and “suppression-errors” subscores but not in the “initiation” subscore. Differences between the “Posterior PF” and the “Non Frontal” groups were not significant for any subscore. Finally, when comparing “Non Frontal” and “Control” groups, only the “suppression” subscore was significantly different.
For the simple reaction time control task, reaction times were scaled using the same method as for Hayling performance. Scaled scores (and corresponding mean reaction times) were 3.5 ± 3.1 (552 ± 242 ms) for “Rostral PF,” 4.0 ± 1.8 (507 ± 397 ms) for “Posterior PF,” 4.6 ± 1.5 (483 ± 408 ms) for “Non Frontal,” and 5.5 ± 1.7 (251 ± 85 ms) for “Controls.” There was a significant difference between groups (Kruskal–Wallis: H3 = 14.03; P = 0.003), patients being slower than controls (U = 1605; z = −3.56; P < 0.001) but frontal patients were not significantly different from non frontal ones (U = 182.5; z = −1,51; P = 0.131), and “Rostral PF” patients not different from “Posterior PF” patients (U = 58.5; z = −0.76; P = 0.447). Only frontal groups were significantly impaired compared with normal “Controls” (“Rostral PF”: U = 256; z = −2.03; P = 0.042; “Posterior PF”: U = 562.5; z = −3.02; P = 0.003; “Non Frontal”: U = 786.5; z = −1.8; P = 0.071). The correlation between Hayling “initiation” and simple RT task was not significant in controls (τ = 0.004; P = 0.966), but it was significant in patients (τ = 0.417; P = 0.001). We then looked at groups by tasks interactions, using the procedure described by Sawilowsky (1990) for nonparametric tests (Puri and Sen L statistic). We found a significant interaction between frontal groups (“Rostral PF” vs. “Posterior PF”) and tasks (Hayling “initiation” vs. simple RT task), with F1,22 = 6.52 and P = 0.018. This shows that Hayling “initiation” and simple RT tasks are differentially impaired in “Rostral” and in “Posterior PF” patients: simple RT task was impaired in both groups, while Hayling “initiation” was more specifically impaired in “Rostral PF” group.
Among all patients, correlation analyses showed no significant correlation between Hayling global score (the dependent variable) and 1) age at test (τ = −0.83, P = 0.471), 2) NART or WTAR as an estimation of the premorbid IQ (τ = −0.16, P = 0.205), 3) FSIQ-NART discrepancy as an estimation of general cognitive deterioration caused by the lesion (τ = −0.17, P = 0.195), 4) lesion volume (τ = −0.11, P = 0.341; although lesions seemed to be larger in Rostral PF group, statistical comparison between groups did not reach significance nor did correlations between Hayling test and lesion volume), 5) time interval (τ = 0.01, P = 0.939). In order to study the effect of the hemisphere of the lesion on task performance, we computed a Kruskal–Wallis test within each group of patients. There was no difference between performances in patients with left, right, or bilateral lesions (for the overall score: “Rostral PF” group: H2 = 1.20; P = 0.557; “Posterior PF” group: H2 = 5.90; P = 0.052; “Non Frontal” group: H2 = 2.57; P = 0.277; see Supplementary Table S1 for Hayling performance in each patient group according to lesion side).
In summary, this analysis showed that the performances of patients were lower than those of controls on all Hayling subscores. However, the “Rostral PF” patients were more impaired than any other group on the Hayling overall score. Moreover, on the initiation subtask, “Rostral PF” patients had significantly lower performances than either the “posterior PF” or the “non Frontal” patients. In other words, in the group study, “Rostral” patients appeared to be the only group associated with a deficit in the “initiation” condition. “Rostral” patients were also impaired in the “suppression” condition (at least compared with controls), and the condition by group interaction also was not significant (F1,22 = 0.312). Thus, we cannot conclude that a deficit in this patient group is specific to “initiation” processes. By contrast, “posterior PF” patients were impaired on the suppression subtask, on the simple RT task but not the initiation condition. These differences were unlikely to be due to differences in age, premorbid IQ, lesion volume, or lesion side since the groups did not differ on these variables.
Level 2: Voxelwise Approaches
AnaCOM Results
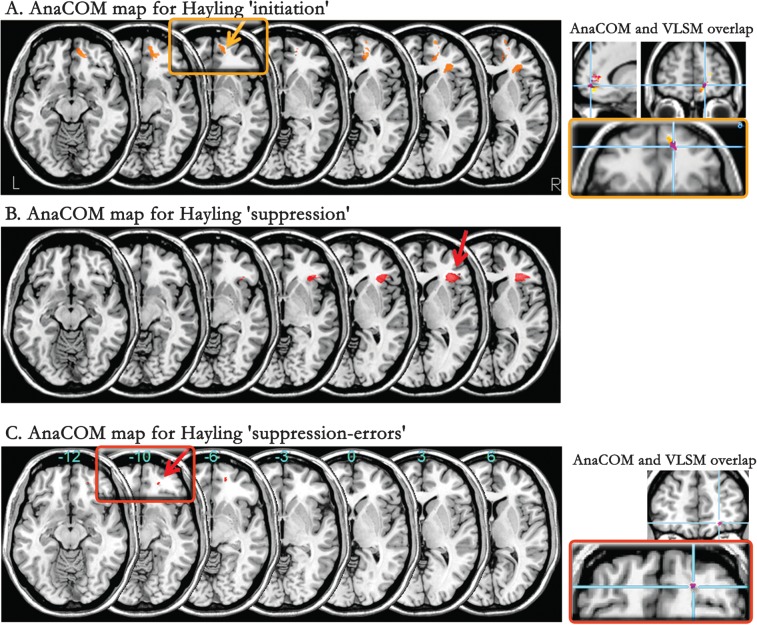
Voxelwise methods need no a priori hypothesis about lesion location, thus all the patients were pooled together in this analysis. After corrections for multiple statistical comparisons, AnaCOM maps of the Hayling global score revealed several clusters associated with a deficit within the covered prefrontal regions (Table 3). These clusters were grouped in 3 anatomical right-sided regions: 1) the frontal pole, more precisely the medial part of BA10, 2) the cingulate cortex and adjacent medial orbital and medial superior frontal gyrus, and 3) the inferior frontal gyrus.
Table 3.
Anatomical regions identified by AnaCOM as significantly associated with a deficit in the different Hayling scores
| Hayling | Anatomical regions | BA | MNI coordinates | P values (×10−4) | Mean scores | ||
| Overall score, H < 3.90 × 10−4 | Medial superior frontal/medial orbital G. | 10/11 | 13 | 53 | −7 | 1.3–3.9 | 2.5–2.8 |
| Anterior cingulate/medial superior frontal | 32/11 | 14 | 42 | 5 | 0.9–3.9 | 1–2.6 | |
| Inferior frontal G. | 47/45 | 39 | 32 | 4 | 1.9–4.0 | 1–2.9 | |
| ‘Initiation’’ subscore, H < 3.06 × 10−4 | Medial superior frontal/medial orbital G. | 10/11 | 12 | 56 | −9 | 0.5–3.1 | 3–3.8 |
| Medial superior frontal G. | 10 | 18 | 64 | 4 | 2.8 | 3 | |
| Inferior frontal G. | 47 | 34 | 32 | 4 | 0.5–2.7 | 3.4–3.8 | |
| Anterior cingulate/medial superior frontal | 32/11 | 17 | 46 | 3 | 0.6–2.8 | 2.2–3.3 | |
| Temporal pole | 38 | 42 | 12 | −19 | 3.1 | 3.8 | |
| “Suppression” subscore, H < 8.71 × 10−4 | Inferior frontal G. | 47/45 | 39 | 30 | 3 | 0.4–8.7 | 3.4–3.5 |
| “Suppression-errors” subscore, H < 23 × 10−4 | Anterior orbital G. | 11 | 22 | 47 | −11 | 23 | 1 |
Note: All the reported regions were significant after Holm correction for multiple comparisons (H: Holm threshold) and were right-sided. These regions were composed of groups of several significant clusters—that is patterns of lesion overlaps—each cluster having its own P value. Thus, the range of the P values for the clusters forming each region is given. The last column represents mean scores of patients damaged in each significant region. (BA, Brodmann area; G, gyrus).
When looking at the separate subscores of the Hayling test, we found that “initiation” and “suppression” were differentially associated with distinct brain regions. Areas associated with a deficit in the “initiation” subscore (Fig. 3A, yellow) were located in the right inferior medial frontal pole, involving 2 clusters in BA10, in a more posterior and orbital area (BA32/11) but also in the right inferior frontal gyrus (involving BA47) and in a small cluster of the superior temporal pole (BA 38). Areas associated with a speed deficit in the “suppression” condition (Fig. 3B) were observed within the right inferior frontal gyrus (involving BA 47 and 45). This region was also part of the “initiation” subscore map, but it was larger and contained more subregions (i.e., more overlapping patterns of lesions) in the “suppression” subscore map. Finally, areas associated with errors in the “suppression” task (“suppression-errors”) consisted of one small cluster located in the orbitoventral part of right rostral prefrontal region (BA11, Fig. 3C).
Figure 3.
AnaCOM maps. Statistical maps of the Hayling scores and subscores: (A) “initiation,” (B) “suppression,” (C) “suppression-errors,” are superimposed on serial axial sections of a normalized brain (left part of the figure). Framed regions are also emphasized on the right side of the figure, together with corresponding coronal and sagittal views. Figures on the right highlight the overlap between AnaCOM and VLSM regions (in purple) associated with a deficit on the “initiation” subscore (top right) and on the “suppression-errors” subscore (bottom right). Only significant areas at a Holm threshold are represented. Right side of the brain is on the right of each section.
In sum, this voxel-by-voxel lesion study showed that: 1) several right hemisphere brain regions were associated with a deficit on the Hayling test: mainly the frontal pole, the medial PFC, and anterior cingulate, an orbitoventral prefrontal area and the inferior frontal cortex; 2) deficits in “initiation” and “suppression” conditions involved differentially several cerebral areas: the right medial BA10 was associated with slowness in “initiation”, while the inferior frontal gyrus was associated with slowness in both conditions, though more strongly with the “suppression” subtask. An orbitoventral cluster in right BA11 was related to errors in the “suppression” condition.
Similar results were obtained when including only patients with an MRI in the AnaCOM analysis (and excluding CT scans from processing). Comparative coordinates of the regions observed in the whole group of patients versus the MRI subgroup are presented in a Supplementary Table S2.
VLSM Results
Using this method, a significant deficit in the “initiation” subscore of the Hayling test was associated with a lesion involving right medial BA10 and 11, after correction for multiple comparisons (permutation correction, P < 0.05; z < 3.785). This region partly overlapped the one found using the AnaCOM method (centered on MNI coordinates 5, 45, −15 and 12, 54, −6; z = 3.35; Fig. 3A). The “suppression-errors” subscore was associated with a lesion involving a small cluster within right BA11 (coordinates 22, 47, −11; z = 3.12; Fig. 3C) that overlapped the one observed with AnaCOM. No region was significantly associated with a deficit in the “suppression” subscore at a corrected threshold but at an uncorrected threshold, the inferolateral prefrontal region (BA45/47) observed with AnaCOM was also found. All results were not significant when using an FDR voxelwise correction or a Bonferroni correction at the cluster level.
Taken together, results from these 2 voxel-based method showed that several distinct regions are critical for the Hayling test and are differentially involved in “initiation,” “suppression,” and “suppression-errors” conditions. The largest impairment in “initiation” time was found in patients with a medial rostral prefrontal lesion, the strongest impairment in “suppression-errors” was found associated with lesions affecting the orbitofrontal cortex, and the biggest impairment in “suppression” time was related to lesions in the inferior frontal gyrus (only using AnaCOM). However, these methods do not allow us to test statistically the interactions between these conditions and these subregions, and a negative result for a given test in a given region should not be interpreted as an absence of involvement of this region in this test. Thus, we cannot conclude that each of these regions is specifically related to a given Hayling subtest.
Discussion
This is, to our knowledge, the first lesion study involving a large group of patients that examines the crucial brain regions for Hayling Test performance. We studied performances in both the “initiation” and “suppression” conditions in 45 patients with circumscribed cerebral lesions. We subjected the data to 3 types of analysis. The first was a classical approach, using patient groupings based on a priori hypotheses about what the likely structure–function relationships should be. Patients were divided into 3 groups: “Rostral PF,” “Posterior PF,” and “Non Frontal”. Statistical comparisons between the patient groups and between patients and controls showed that “Rostral PF” patients were more impaired than any other group on the Hayling overall score. Compared with controls, rostral patients presented a deficit in both the “initiation” and the “suppression” conditions. This deficit consisted of both slower responses (“initiation” and “suppression” conditions) and more errors (“suppression-errors” subscore). Nevertheless, while “Rostral PF” patients had lower performances than “Posterior PF” and “Non Frontal” patients in the “initiation” condition, it was not the case for the “suppression” condition. In the latter condition, “Posterior PF” and “Non Frontal” patients were also impaired compared with controls. In other words, the group study showed that a deficit in the “initiation” and “suppression” conditions appeared to be differentially related to rostral or posterior prefrontal damage. A deficit in the “initiation” condition appeared associated with rostral prefrontal damage but not to other brain damage.
These results were confirmed and extended by using 2 modern lesion-deficit mapping approaches: AnaCOM and VLSM. These methods highlighted several distinct regions involved in Hayling performance located within the right rostral prefrontal region (medial BA10), the right inferolateral prefrontal region (BA45 and 47), and a right orbitoventral region (BA11 and 32). Converging results from AnaCOM and VLSM showed that a deficit in the straightforward completion time (“initiation” subscore) was associated with right medial rostral PFC. This region was not associated with a significant deficit in the “suppression” subtask in these voxel-based methods. These findings are in line with the previous classical group approach, which also showed that rostral patients are more impaired than any other patients in the “initiation” condition. For the “suppression” condition explored with AnaCOM, slow unrelated completion was associated with involvement of the right inferolateral prefrontal region. To a lesser extent, this region was also associated with slower responses in the straightforward completion (“initiation”) condition. The involvement of right inferolateral PFC was consistent with the group approach that showed an impairment of “Posterior PF” patients in the “suppression” condition, though a deficit in this condition was not specific to this patient group. Furthermore, damage to one cluster in the orbitoventral PFC was associated with errors in the “suppression” condition with both voxel-based methods. Patients with involvement of this region tended to produce words that were connected in some way to the sentence (or were socially inappropriate), when they should have been giving unrelated words. These results are broadly consistent with the classical approach that showed less group-specific impairment in the “suppression” condition.
The current results replicate those of Burgess and Shallice (1996), in the sense that patients with damage in PFC performed worse than either controls or patients with more posterior lesions in the Hayling test and which also suggested that impairments on each condition are dissociable (see also Burgess et al. 1998). In addition, the present findings suggest that the right medial frontal pole, a right orbitofrontal, and a right inferolateral prefrontal region may have distinct roles in the different components of the Hayling test.
Contributions of the Medial Rostral Region to Performance in the Straightforward Completion (“Initiation” Condition)?
The strongest effect in our study is the association between damage to structures within the right medial rostral frontal lobe and slowness in straightforward completions. In previous initiation and suppression studies, the initiation condition was usually considered as a reference task, and attention was focused on additional processes that occurred in the suppression condition as compared with the initiation condition (as detailed below). Less interest has been given to the initiation task by itself (but see Stuss et al. 2005; Shallice et al. 2008). The current findings from both the group study and the voxel-based approaches however suggest that some cognitive processes that depend on rostral PFC are better examined by the “initiation” task. This is highly consistent with behavioral results from Burgess et al. (1998), showing that suppression and initiation problems may share some common causes, but initiation problems may also occur for separate reasons. Previous functional imaging studies that used the Hayling or other verbal completion tasks have also shown activation in medial rostral PFC in conditions equivalent to the “initiation” condition, that is, when the response was strongly related to the presented cue (Desmond et al. 1998; Seger et al. 2000; Nathaniel-James and Frith 2002). What could these specific cognitive processes behaviorally measured by the “initiation” time and that depend on the medial rostral prefrontal region be?
In several lesion studies, right medial frontal regions have been associated with the maintenance of vigilance, of intentions and/or preparation to respond, also called “energization” (Stuss et al. 2005) and measured by motor initiation tasks. It is likely that this energizing system is required by the Hayling task. However, the medial BA10 region (as well as the cingulate region in BA32/11) we identified here is somewhat inferior and anterior to the medial prefrontal region observed in previous studies (Alexander et al. 2005; Stuss et al. 2005; Shallice et al. 2008). In addition, “Rostral PF” patients were not significantly slower at our simple reaction time task, and the interaction between task (Hayling “initiation” vs. simple RT task) and group (“Rostral PF” vs. “Posterior PF”) was significant, which rules out an alternative interpretation in terms of a pure response speed deficit (since a deficit of this kind would affect both conditions). It also suggests that verbal initiation and motor initiation, assessed by the Hayling “initiation” and the “basic RT” motor tasks, respectively, may be impaired independently or may rely in part on distinct brain subregions. A recent functional imaging study also found BA10 involvement in a task requiring overcoming learned avoidance and initiation of a new response and low BA10 involvement in inhibiting a prepotent response (Greening et al. 2011).
It is nevertheless likely that a simple reaction time task (deficits on which has been associated with a more posterior dorsomedial region) emphases elementary motor initiation, and this differs from the form of initiation assessed by the Hayling test. Among the possible processes that differentiate the Hayling “initiation” condition from simple motor initiation tasks, one can consider the linguistic (e.g., latency in word finding) and/or semantic components. The sentences chosen for the test (see Burgess and Shallice 1996) deliberately cue a restricted set of responses, with a strong semantic association between one sentence and its completion. In this case, sentence completion is likely to trigger a relatively automatic retrieval of a straightforward completion, that is, activation of close semantic associates. The Hayling “initiation” subscore could reflect in part this automatic activation within a semantic network delimited by the sentence frame, activation that could be slower in rostral patients. There are several arguments that make this hypothesis a possibility worth exploring. Rostral medial BA10 is part of a semantic memory network, according to functional neuroimaging methods (Saffran 2000; Patterson et al. 2007; Buckner et al. 2008; Binder et al. 2009; Lambon Ralph et al. 2010) and has been also associated with contextual association (Bar et al. 2007; Bar 2009) and with spontaneous cognition (in contrast to controlled cognition, Lieberman et al. 2004; Gilbert et al. 2007; Andrews-Hanna et al. 2010). Besides, medial rostral PFC regions may mediate not only semantic but also social event knowledge (Krueger et al. 2009). According to this view, it is possible that since the Hayling sentences typically describe situational contexts, the rostral patients might have problems completing them because they may have “lost” the event knowledge of what usually happens when X and Y occur.
Regions Associated with the Hayling “Suppression” Condition
The “suppression” condition requires suppressing automatic activation of related completions, in order to generate and select an unrelated one. This condition was found to be associated with both distinct and shared regions with the “initiation” condition using the voxel-based methods.
The inferior and lateral prefrontal region (including BA45/47) was associated with a slowness in both “initiation” and “suppression” conditions, suggesting that these 2 conditions can be commonly impaired in posterior frontal patients. To a certain extent, the current findings converge with the few functional imaging studies that used the Hayling test and showed the involvement of the lateral PFC in both conditions (Collette et al. 2001; Nathaniel-James and Frith 2002; Allen et al. 2008). This may suggest that these conditions require some processes that depend commonly on the inferolateral prefrontal region. Again, this is highly consistent with behavioral results from Burgess et al. (1998), showing that initiation and suppression can be commonly impaired and may share common processes and/or crucial anatomical regions. At the theoretical level, the demands made by sentence comprehension on verbal working memory in both conditions, the maintenance of the task sets, and the selection and evaluation of a response, are candidate processes to explain this finding. It is also possible that this common involvement of the inferolateral prefrontal region in different tasks reflects nonspecific attention or speed deficits. Furthermore, this finding of a common region may also question whether initiation and suppression processes are captured entirely independently by the “initiation” and “suppression” subscores of the Hayling task. For instance, in the “suppression” condition, participants may also need to initiate a response (as in the “initiation” condition) after suppressing the prepotent one.
Nevertheless, AnaCOM showed more significant clusters associated with the “suppression” than the “initiation” condition. In addition, the “posterior PF” group was impaired in the “suppression” condition but not in the “initiation” condition in the group study, a profile that was also observed in individual patients from our group. Though it is not direct evidence, this could mean that additional processes, more specific to the “suppression” condition (or better captured by this condition), are dependent on these additional inferolateral prefrontal subregions. This interpretation is in accordance with functional imaging, showing activation in the inferior (BA 47/45) and superior (BA 46 and 9) frontal gyri, as well as in the rostrolateral PFC (BA10) when the “suppression” was contrasted to the “initiation” condition (Collette et al. 2001; Nathaniel-James and Frith 2002). There are several processes that have been associated with the inferior frontal gyrus and that theoretically are more important for the “suppression” than the “initiation” condition. First, there is inhibition of prepotent but inappropriate responses and switching to an alternative response, as suggested by functional MRI (Garavan et al. 1999; Konishi et al. 1999; Liddle et al. 2001; Menon et al. 2001; Buchsbaum et al. 2005; Xue et al. 2008; Kenner et al. 2010; Walther et al. 2010), as well as the lesion approach (Aron et al. 2003; Rieger et al. 2003; Picton et al. 2007). Second, there are the processes that relate to the “episodic cognitive control” that is needed to withhold an automatic completion given a past instruction while no stop signal is provided for each sentence (Koechlin et al. 2003; Koechlin and Summerfield 2007). Finally, when using tasks that shared many similarities with the Hayling test, the inferolateral prefrontal region has been associated with controlled retrieval of semantic memory (Martin et al. 1995; Wagner et al. 2001; Badre and Wagner 2004, 2007; Martin and Cheng 2006), and/or in the selection of semantic responses among competitive alternatives (Thompson-Schill 2003; Kan and Thompson-Schill 2004; Thompson-Schill et al. 2005, 1997; Thompson-Schill and Botvinick 2006), though these studies focused on the left PFC. It is also likely that “suppression” slowness captures various cognitive processes, possibly supported by distinct anatomical regions (including the inferolateral prefrontal region). This hypothesis might explain why there was no significant region associated with this condition using the VLSM method, which compares patients damaged in one voxel to patients not damaged in that voxel but who may be damaged in other functional areas involved in the task.
In addition to this result, errors in the “suppression” condition were associated with a specific area within the orbitofrontal cortex (BA 11). Although the identified subregion was restricted to a small cluster, its location was consistent in both AnaCOM and VLSM and also with results from functional MRI (de Zubicaray et al. 2000; Collette et al. 2001). Similarly, damage to this region had been associated with problems in suppressing inappropriate behaviors, impulsivity, perseverative responses (Horn et al. 2003; Viskontas et al. 2007). The exact processes that depend on this region (Zald and Andreotti 2010) and those that permit the performance of the “suppression” task however remain to be determined.
Of course, interpretation of our results is limited to the regions where a sufficient amount of lesions overlapped, and it is possible that there are other critical regions that have not been discovered here. As mentioned above, the left inferior and anterior lateral frontal region and the right superior lateral PFC were not well represented in our patients’ lesions. Despite this limitation, it is important to note that the significant regions observed in AnaCOM do not correspond to the regions of maximum overlap, as illustrated in Supplementary Figure S2.
In sum, in accordance with families’ reports on frontal patients’ behavior, the current study identified both common and differential impairments on “initiation” and “suppression” tasks, depending on the location of the prefrontal damage. “Initiation” slowness was significantly impaired in rostral prefrontal patients, compared with other patients or to controls, in the group, VLSM and AnaCOM studies, suggesting a role for rostral PFC in verbal initiation, possibly in relation to automatic activation of semantic responses. An area in orbitofrontal region was associated with “suppression” errors in voxel-based analyses, which is consistent with a deficit in inhibiting inappropriate behaviors in orbitofrontal patients. Finally, responses in “initiation” and “suppression” conditions can be commonly slowed down in right inferolateral prefrontal patients, questioning the functional specificity and/or organization of this region or perhaps suggesting that the ”initiation” and “suppression” conditions share common processes. These findings also suggest that RT and accuracy measures may depend on different anatomical substrates and capture distinct processes required by the “suppression” of related completion in a sentence frame.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
Wellcome Trust (grant 061171 to P.W.B.); “Agence Nationale de la Recherche” (grant ANR-09-RPDOC-004-01 to E.V.); “Institut Lilly” (grant Neurology to E.V.); Royal Society University Research Fellowship (to S.J.G.).
Acknowledgments
The authors thank the participants who generously gave their time for this study. Conflict of Interest: None declared.
References
- Alexander MP, Stuss DT, Shallice T, Picton TW, Gillingham S. Impaired concentration due to frontal lobe damage from two distinct lesion sites. Neurology. 2005;65:572–579. doi: 10.1212/01.wnl.0000172912.07640.92. [DOI] [PubMed] [Google Scholar]
- Allen P, Mechelli A, Stephan KE, Day F, Dalton J, Williams S, McGuire PK. Fronto-temporal Interactions during overt verbal initiation and suppression. J Cogn Neurosci. 2008;20:1656–1669. doi: 10.1162/jocn.2008.20107. [DOI] [PubMed] [Google Scholar]
- Andersen SM, Rapcsak SZ, Beeson PM. Cost function masking during normalization of brains with focal lesions: still a necessity? Neuroimage. 2010;53:78–84. doi: 10.1016/j.neuroimage.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network's role in spontaneous cognition. J Neurophysiol. 2010;104:322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Selection, integration, and conflict monitoring. Assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron. 2004;41:473–487. doi: 10.1016/s0896-6273(03)00851-1. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bar M. The proactive brain: memory for predictions. Philos Trans R Soc Lond B Biol Sci. 2009;364:1235–1243. doi: 10.1098/rstb.2008.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Mason M, Fenske M. The units of thought. Hippocampus. 2007;17:420–428. doi: 10.1002/hipo.20287. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RS, Dolan RJ. Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia. 1993;31:907–922. doi: 10.1016/0028-3932(93)90147-r. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, von Cramon DY. The inhibition of imitative and overlearned responses: a functional double dissociation. Neuropsychologia. 2005;43:89–98. doi: 10.1016/j.neuropsychologia.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14:486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Bright P, Jaldow E, Kopelman MD. The National Adult Reading Test as a measure of premorbid intelligence: a comparison with estimates derived from demographic variables. J Int Neuropsychol Soc. 2002;8:847–854. doi: 10.1017/s1355617702860131. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang W, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin Card-Sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Alderman N. Executive dysfunction. In: Goldstein LH, McNeil JE, editors. Clinical neuropsychology: a practical guide to assessment and management for clinicians. Chichester (UK): John Wiley: 2004. pp. 185–209. [Google Scholar]
- Burgess PW, Alderman N, Evans J, Emslie H, Wilson BA. The ecological validity of tests of executive function. J Int Neuropsychol Soc. 1998;4:547–558. doi: 10.1017/s1355617798466037. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Alderman N, Forbes C, Costello A, Coates L, Dawson D, Anderson ND, Gilbert SJ, Dumontheil I, Channon S. The case for the development and use of “ecologically valid” measures of executive function in experimental and clinical neuropsychology. J Int Neuropsychol Soc. 2006;12:194–209. doi: 10.1017/S1355617706060310. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. Response suppression, initiation and strategy use following frontal lobe lesions. Neuropsychologia. 1996;34:263–272. doi: 10.1016/0028-3932(95)00104-2. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. The Hayling and Brixton Tests. Bury St Edmunds (UK): Thames Valley Test Company; 1997. [Google Scholar]
- Collette F, Van der Linden M, Delfiore G, Degueldre C, Luxen A, Salmon E. The functional anatomy of inhibition processes investigated with the Hayling task. Neuroimage. 2001;14:258–267. doi: 10.1006/nimg.2001.0846. [DOI] [PubMed] [Google Scholar]
- Coltheart M. What has functional neuroimaging told us about the mind (so far)? Cortex. 2006;42:323–331. doi: 10.1016/s0010-9452(08)70358-7. [DOI] [PubMed] [Google Scholar]
- Committeri G, Pitzalis S, Galati G, Patria F, Pelle G, Sabatini U, Castriota-Scanderbeg A. Neural bases of personal and extrapersonal neglect in humans. Brain. 2007;130:431–441. doi: 10.1093/brain/awl265. [DOI] [PubMed] [Google Scholar]
- Crinion J, Ashburner J, Leff A, Brett M, Price C, Friston K. Spatial normalization of lesioned brains: performance evaluation and impact on fMRI analyses. Neuroimage. 2007;37:866–875. doi: 10.1016/j.neuroimage.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zubicaray GI, Zelaya FO, Andrew C, Williams SC, Bullmore ET. Cerebral regions associated with verbal response initiation, suppression and strategy use. Neuropsychologia. 2000;38:1292–1304. doi: 10.1016/s0028-3932(00)00026-9. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Faglioni P. Normative data and screening power of a shortened version of the Token Test. Cortex. 1978;14:41–49. doi: 10.1016/s0010-9452(78)80006-9. [DOI] [PubMed] [Google Scholar]
- Del Cul A, Dehaene S, Reyes P, Bravo E, Slachevsky A. Causal role of prefrontal cortex in the threshold for access to consciousness. Brain. 2009;132:2531–2540. doi: 10.1093/brain/awp111. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Glover GH. Dissociation of frontal and cerebellar activity in a cognitive task: evidence for a distinction between selection and search. Neuroimage. 1998;7:368–376. doi: 10.1006/nimg.1998.0340. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Cortex and memory: emergence of a new paradigm. J Cogn Neurosci. 2009;21:2047–2072. doi: 10.1162/jocn.2009.21280. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Nat Acad Sci U S A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW. Comment on “Wandering minds: the default network and stimulus-independent thought”. Science. 2007;317:43. doi: 10.1126/science.317.5834.43. Author reply 43. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Tranel D, Paul LK, Rudrauf D, Rorden C, Hornaday A, Grabowski T, Damasio H, Adolphs R. Lesion mapping of cognitive abilities linked to intelligence. Neuron. 2009;61:681–691. doi: 10.1016/j.neuron.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy O, Azouvi P, Robert P, Roussel M, LeGall D, Meulemans T. Dysexecutive syndrome: diagnostic criteria and validation study. Ann Neurol. 2010;68:855–864. doi: 10.1002/ana.22117. [DOI] [PubMed] [Google Scholar]
- Godefroy O, Duhamel A, Leclerc X, Saint Michel T, Henon H, Leys D. Brain-behaviour relationships. Some models and related statistical procedures for the study of brain-damaged patients. Brain. 1998;121(Pt 8):1545–1556. doi: 10.1093/brain/121.8.1545. [DOI] [PubMed] [Google Scholar]
- Godefroy O, Lhullier C, Rousseaux M. Non-spatial attention disorders in patients with frontal or posterior brain damage. Brain. 1996;119:191–202. doi: 10.1093/brain/119.1.191. [DOI] [PubMed] [Google Scholar]
- Godefroy O, Lhullier-Lamy C, Rousseaux M. SRT lengthening: role of an alertness deficit in frontal damaged patients. Neuropsychologia. 2002;40:2234–2241. doi: 10.1016/s0028-3932(02)00109-4. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Identification of brain disorders by the Stroop color—word test. J Clin Psychol. 1976;32:654–658. doi: 10.1002/1097-4679(197607)32:3<654::aid-jclp2270320336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Greening SG, Finger EC, Mitchell DGV. Parsing decision making processes in prefrontal cortex: response inhibition, overcoming learned avoidance, and reversal learning. Neuroimage. 2011;54(2):1432–1441. doi: 10.1016/j.neuroimage.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Henson R. What has (neuro)psychology told us about the mind (so far)? A reply to Coltheart 2006. Cortex. 2006;42:387–392. doi: 10.1016/s0010-9452(08)70365-4. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JFW, Woodruff PWR. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Hornberger M, Savage S, Hsieh S, Mioshi E, Piguet O, Hodges J. Orbitofrontal dysfunction discriminates behavioral variant frontotemporal dementia from Alzheimer’s disease. Dement Geriatr Cogn Disord. 2010;30:547–552. doi: 10.1159/000321670. [DOI] [PubMed] [Google Scholar]
- Kan IP, Thompson-Schill SL. Selection from perceptual and conceptual representations. Cogn Affect Behav Neurosci. 2004;4:466–482. doi: 10.3758/cabn.4.4.466. [DOI] [PubMed] [Google Scholar]
- Kenner NM, Mumford JA, Hommer RE, Skup M, Leibenluft E, Poldrack RA. Inhibitory motor control in response stopping and response switching. J Neurosci. 2010;30:8512–8518. doi: 10.1523/JNEUROSCI.1096-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkingnehun S, Volle E, Pelegrini-Issac M, Golmard JL, Lehericy S, du Boisgueheneuc F, Zhang-Nunes S, Sosson D, Duffau H, Samson Y, et al. A novel approach to clinical-radiological correlations: Anatomo-Clinical Overlapping Maps (AnaCOM): method and validation. Neuroimage. 2007;37:1237–1249. doi: 10.1016/j.neuroimage.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11:229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Krueger F, Barbey AK, Grafman J. The medial prefrontal cortex mediates social event knowledge. Trends Cogn Sci. 2009;13(3):103–109. doi: 10.1016/j.tics.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Sage K, Jones RW, Mayberry EJ. Coherent concepts are computed in the anterior temporal lobes. Proc Nat Acad Sci. 2010;107:2717–2722. doi: 10.1073/pnas.0907307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhermitte F, Pillon B, Serdaru M. Human autonomy and the frontal lobes. Part I: imitation and utilization behavior: a neuropsychological study of 75 patients. Ann Neurol. 1986;19:326–334. doi: 10.1002/ana.410190404. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Hum Brain Mapp. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Jarcho JM, Satpute AB. Evidence-based and intuition-based self-knowledge: an FMRI study. J Pers Soc Psychol. 2004;87:421–435. doi: 10.1037/0022-3514.87.4.421. [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG. Discrete cortical regions associated with knowledge of color and knowledge of action. Science. 1995;270:102–105. doi: 10.1126/science.270.5233.102. [DOI] [PubMed] [Google Scholar]
- Martin RC, Cheng Y. Selection demands versus association strength in the verb generation task. Psychon Bull Rev. 2006;13:396–401. doi: 10.3758/bf03193859. [DOI] [PubMed] [Google Scholar]
- McKenna PJ, Warrington EK. Graded naming test. Windsor (UK): Nelson; 1983. [Google Scholar]
- Menon V, Adleman NE, White C, Glover G, Reiss A. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res Cogn Brain Res. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Nathaniel-James DA, Frith CD. The role of the dorsolateral prefrontal cortex: evidence from the effects of contextual constraint in a sentence completion task. Neuroimage. 2002;16:1094–1102. doi: 10.1006/nimg.2002.1167. [DOI] [PubMed] [Google Scholar]
- Nelson HE, O'Connell A. Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex. 1978;14:234–244. doi: 10.1016/s0010-9452(78)80049-5. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Périn B, Godefroy O, Fall S, de Marco G. Alertness in young healthy subjects: an fMRI study of brain region interactivity enhanced by a warning signal. Brain and Cognition. 2010;72:271–281. doi: 10.1016/j.bandc.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Perret E. The left frontal lobe of man and the suppression of habitual responses in verbal categorical behaviour. Neuropsychologia. 1974;12:323–330. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S. Effects of focal frontal lesions on response inhibition. Cereb Cortex. 2007;17:826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- Rieger M, Gauggel S, Burmeister K. Inhibition of ongoing responses following frontal, nonfrontal, and basal ganglia lesions. Neuropsychology. 2003;17:272–282. doi: 10.1037/0894-4105.17.2.272. [DOI] [PubMed] [Google Scholar]
- Roca M, Parr A, Thompson R, Woolgar A, Torralva T, Antoun N, Manes F. Executive function and fluid intelligence after frontal lobe lesions. Brain. 2010;133:234–247. doi: 10.1093/brain/awp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath H, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Saffran EM. The organization of semantic memory: in support of a distributed model. Brain Lang. 2000;71:204–212. doi: 10.1006/brln.1999.2251. [DOI] [PubMed] [Google Scholar]
- Sawilowsky SS. Nonparametric tests of interaction in experimental design. Rev Educ Res. 1990;60(1):91–126. [Google Scholar]
- Seger CA, Desmond J, Glower G, Gabrieli JD. Functional magnetic resonance imaging evidence for right-hemisphere involvement in processing unusual semantic relationships. Neuropsychology. 2000;14:361–369. doi: 10.1037//0894-4105.14.3.361. [DOI] [PubMed] [Google Scholar]
- Shallice T, Stuss DT, Alexander MP, Picton TW, Derkzen D. The multiple dimensions of sustained attention. Cortex. 2008;44:794–805. doi: 10.1016/j.cortex.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW, Schon F, Baxter DM. The origins of utilization behaviour. Brain. 1989;112(Pt 6):1587–1598. doi: 10.1093/brain/112.6.1587. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop J. Studies of interferences in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- Stuss DT, Alexander MP, Shallice T, Picton TW, Binns MA, Macdonald R, Borowiec A, Katz DI. Multiple frontal systems controlling response speed. Neuropsychologia. 2005;43:396–417. doi: 10.1016/j.neuropsychologia.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Floden D, Alexander MP, Levine B, Katz D. Stroop performance in focal lesion patients: dissociation of processes and frontal lobe lesion location. Neuropsychologia. 2001;39:771–786. doi: 10.1016/s0028-3932(01)00013-6. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL. Neuroimaging studies of semantic memory: inferring “how” from “where”. Neuropsychologia. 2003;41:280–292. doi: 10.1016/s0028-3932(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol. 2005;15:219–224. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Botvinick MM. Resolving conflict: a response to Martin and Cheng 2006. Psychon Bull Rev. 2006;13:402–408. doi: 10.3758/bf03193860. discussion 409–411. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Nat Acad Sci U S A. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Marslen-Wilson W, Stamatakis EA. Dissociating neuro-cognitive component processes: voxel-based correlational methodology. Neuropsychologia. 2005;43:771–778. doi: 10.1016/j.neuropsychologia.2004.07.020. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. Separating semantic conflict and response conflict in the Stroop task: a functional MRI study. Neuroimage. 2005;27:497–504. doi: 10.1016/j.neuroimage.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Vendrell P, Junqué C, Pujol J, Jurado MA, Molet J, Grafman J. The role of prefrontal regions in the Stroop task. Neuropsychologia. 1995;33:341–352. doi: 10.1016/0028-3932(94)00116-7. [DOI] [PubMed] [Google Scholar]
- Viskontas IV, Possin KL, Miller BL. Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Ann N Y Acad Sci. 2007;1121:528–545. doi: 10.1196/annals.1401.025. [DOI] [PubMed] [Google Scholar]
- Volle E, Beato R, Levy R, Dubois B. Forced collectionism after orbitofrontal damage. Neurology. 2002;58:488–490. doi: 10.1212/wnl.58.3.488. [DOI] [PubMed] [Google Scholar]
- Volle E, Kinkingnéhun S, Pochon J, Mondon K, Thiebaut de Schotten M, Seassau M, Duffau H, Samson Y, Dubois B, Levy R. The functional architecture of the left posterior and lateral prefrontal cortex in humans. Cereb Cortex. 2008;18:2460–2469. doi: 10.1093/cercor/bhn010. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Paré-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Walther S, Goya-Maldonado R, Stippich C, Weisbrod M, Kaiser S. A supramodal network for response inhibition. Neuroreport. 2010;21:191–195. doi: 10.1097/WNR.0b013e328335640f. [DOI] [PubMed] [Google Scholar]
- Warrington EK. Recognition memory test manual. Windsor (UK): Nfer-Nelson; 1984. [Google Scholar]
- Wechsler D. Wechsler test of adult reading (WTAR) San Antonio (TX): Psychological Corp: 2001. [Google Scholar]
- Wilson B, Alderman N, Burgess PW, Emslie H, Evans J. Behavioural assessment of the dysexecutive syndrome. Bury St Edmunds (UK): Thames Valley Test Company; 1996. [Google Scholar]
- Wilson BA, Gomar JJ, Emslie H, Alderman N, Burgess PW. The development of an ecologically valid test for assessing patients with a dysexecutive syndrome. Neuropsychol Rehabil. 1998;8:213–228. [Google Scholar]
- Xue G, Aron AR, Poldrack RA. Common neural substrates for inhibition of spoken and manual responses. Cereb Cortex. 2008;18:1923–1932. doi: 10.1093/cercor/bhm220. [DOI] [PubMed] [Google Scholar]
- Zald DH, Andreotti C. Neuropsychological assessment of the orbital and ventromedial prefrontal cortex. Neuropsychologia. 2010;48(12):3377–3391. doi: 10.1016/j.neuropsychologia.2010.08.012. [DOI] [PubMed] [Google Scholar]