Abstract
Purpose of review
Acute graft vs. host disease (GVHD) is a considerable source of morbidity and mortality following allogeneic hematopoietic cell transplantation (HCT). Accordingly, progress in the prevention and primary therapy of this complication is needed to improve patient outcomes.
Recent findings
Guided by insights into acute GVHD pathogenesis, investigators have explored novel cellular and pharmacologic approaches to acute GVHD prevention that demonstrates promise. While pan-T cell depletion has reduced GVHD, novel strategies that selectively deplete alloreactive T cells or modulate the balance of effector T cells and regulatory T cells offer promise to selectively abrogate acute GVHD while retaining protection from primary disease relapse and infectious complications.
Summary
Divergent approaches in the primary therapy of acute GVHD have explored both combination approaches with standard dose glucocorticoids and additional immunosuppressive agents and conversely steroid-sparing approaches including topical agents such as beclomethasone or sirolimus as a steroid-free approach to acute GVHD therapy. Mature results of high quality clinical trials are needed to determine the optimal therapy that results in effective control of the syndrome and limited toxicity. These complementary outcomes represent the therapeutic goal for future investigation in acute GVHD therapy.
Keywords: Graft-versus-Host Disease (GVHD), Hematopoietic Cell Transplantation (HCT), regulatory T cells (Tregs)
Introduction: Prevention of acute graft-versus-host disease
Acute graft vs. host disease (GVHD) is a major source of morbidity and mortality following allogeneic hematopoietic cell transplantation (HCT). As current pharmacologic strategies are insufficient to prevent acute GVHD, 1,2 investigators continue to exploit approaches that affect GVHD immunobiology to improve patient outcomes. A triphasic conceptual model of GVHD pathogenesis introduced 20 years ago simplifies a complex network: Tissue damage from conditioning therapy, activation of host antigen presenting cells and donor T cells resulting in differentiation, migration, and an effector phase in which T cells mediate tissue damage by releasing inflammatory cytokines including Tumor necrosis factor (TNF)-α and Interleukin (IL)-1, and cytotoxic moieties. More recent investigation demonstrated the importance of regulatory mechanisms, including regulatory T cells (Tregs). Pre-clinical models demonstrated their potential for abrogating acute GVHD, and clinical correlative data has suggested a relationship between the incidence and severity of GVHD and circulating Tregs. Hence, there is great interest in the clinical translation of such potential for the prevention of acute GVHD.
Brunstein, et al have expanded umbilical cord blood donor Treg using anti-CD3/CD28 beads and IL-2, and have examined the safety of infusion of these cells in a phase I study (n = 23).3 Median expansion was 211-fold, and the median post-expansion proportion of CD4+CD127-FoxP3+ cells was 64% (range 31%–96%). Dose escalation was performed up to 30 × 105 Treg/kg. Patients received initially cyclosporine (CSA)/mycophenolate mofetil (MMF), and later sirolimus (SIR)/MMF. Grade II–IV acute GVHD was 43%, compared to historical control of 61% (p = 0.05). These data substantiate the feasibility of ex-vivo Treg expansion. Further work is needed to examine the efficacy of this approach.
Sirolimus promotes peripheral Treg expansion while suppressing effector T cells. Rodriguez, et al have expanded available data on tacrolimus (TAC)/SIR in GVHD prevention with the publication of a phase II study (n = 85) after one of three conditioning regimens in matched sibling HCT. Grade II–IV acute GVHD was 43% (37–50%), and III–IV was 19%. The 2 year incidence of chronic GVHD was 46%. NRM was low at 4.8% (2–12%) at 100 days, and 10.2% (6–18%) at 2 years. These data provide further evidence in support of TAC/SIR for GHVD prevention. More conclusive evidence for the benefit of TAC/SIR compared to TAC/MTX will result from the national Blood and Marrow Transplant Clinical Trials Network (BMT-CTN) trial (http://clinicaltrials.gov/ct2/show/NCT01106833).
Preclinical studies have demonstrated that the transfer of cells treated with extra-corporeal phototherapy (ECP) with ultraviolet A radiation reverses established GVHD by increasing donor Tregs.4 Shaughnessy, et al have aimed to exploit the effect of ECP on host antigen-presenting cells: Their phase II multicenter trial tested 2 consecutive days of ECP administered before HCT. CSA and methotrexate (MTX) were administered following ablative conditioning and infusion of peripheral blood stem cells (PBSC) or bone marrow (BM) from matched related (MRD) or unrelated donors (MUD) (n = 66).5 Grade II–IV acute GVHD was 35% (23–48%), chronic GVHD at one year was 38% (21–47%), and overall survival (OS) at one year was 77% (64–86%). In comparison to historical controls not treated with ECP, there was no significant difference in outcomes. While these data do not support that pre-transplant ECP prevents acute GVHD, modulation of host antigen presentation remains a key area of investigation with potential for clinical translation.
As evidence supports a central role for donor T cells in acute GVHD pathogenesis, investigators have refined protocols for T cell depletion. Jakubowski, et al published a phase II trial of ex-vivo T cell depletion employing CD34 enrichment by the Miltenyi device in 35 unrelated donor transplants (PBSC 29, BM 6).6 The median CD3+ cell dose was 1.52 × 103/kg. With no pharmacologic prophylaxis, the grade II-IV acute GVHD was 6%, chronic GVHD 29%, NRM 20% at 100 days and 29% and 1 year. Epstein-Barr Virus (EBV)-associated post-transplant lymphoproliferative disease (PTLD) occurred in 8.5% of the cases. With the highly intense conditioning, the relapse incidence was low, 6% at 4 years, despite a largely advanced disease cohort. Devine, et al have confirmed the efficacy of this protocol in HLA-matched sibling donor transplantation (n = 44) for acute myeloid leukemia (AML) in complete remission (CR)1 or CR2 in the BMT CTN 0303 trial.7 T cell depleted allografts contained a median CD3+ dose of 6.6 × 103/kg. Without pharmacologic prophylaxis, grade II–IV acute GVHD was 22.7% (10.2–35.3%), and grade III–IV was 4.5% (0–10.8%). Extensive chronic GVHD was 6.8% (0–14.4%) at 24 months. With median follow up of 34 months, the 36 month DFS was 58%, which is in keeping with DFS reported for AML in CR1 with comparable myeloablative approaches.8 NRM was 14% (3.4–24%) by 12 months, and 23.2% (9.3–37.1%) by 36 months. These results demonstrate that subtotal depletion of donor T cells provides protection against GVHD. Risks inherent in this approach, including increased infectious risk and EBV associated PTLD, support alternate strategies to mitigate the GVHD risk.
Others have examined alternative pharmacologic prophylaxis strategies to improve on outcomes achieved with a calcineurin inhibitor and methotrexate. Parmar, et al have reported the results of a novel phase I/II, controlled, Bayesian adaptively randomized study employing a regimen of TAC/MTX (5 mg/m2 on days +1, +3, +6, and only on day +11 in controls) and pentostatin (dose levels 0, 0.5, 1, 1.5, and 2 mg/m2 administered on days +8, +15, +22, and +30) in mismatched related donors (n = 10) and MUD (n = 137).9 Pentostatin doses of 1.0 and 1.5 mg/m2 had the greatest success rates. However, grade II–IV acute GHVD incidence (35.7% vs. 55.6%, p = 0.085), chronic GVHD and OS did not significantly differ compared to control. It is not clear from these data that the addition of pentostatin has significantly improved protection from acute GVHD.
Our group conducted a phase II trial of TAC/MMF vs. TAC/MTX in recipients of MRD and MUD PBSC transplants to test the hypothesis that MMF administered for a year is more effective than a short course of MTX on days +1, +3, +6 and +11 in depleting allo-activated T cells.10 There was no significant difference in the incidence of grade II–IV acute GVHD between the study arms (78 vs. 79%, p = 0.8). No significant differences were observed in depletion of replicating T cells. In total these findings suggest that substitution of MMF for MTX alleviates MTX-associated toxicity, but is not more effective than MTX in the prevention of severe acute GVHD, especially in unrelated donors.
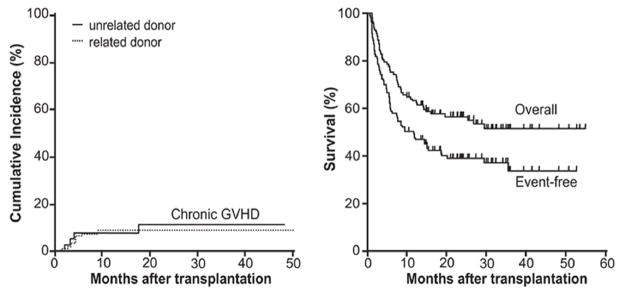
Luznik et al have pioneered GVHD prophylaxis with post-transplant high dose cyclophosphamide (CY, 50 mg/kg/d on days +3 and +4) based on its potent and selective activity against allo-activated donor T cells.11 Based on murine preclinical data, Johns Hopkins investigators have first demonstrated the effectiveness of post-transplant CY in preventing GVHD after haploidentical marrow transplant. In a recent phase I–II Bayesian design trial (n = 117) including MRD and MUD, T replete marrow was transplanted following myeloablative busulfan (BU) and CY. With sole post-transplant CY prophylaxis, 43% developed grade II–IV acute GVHD, and 10% had grade III–IV. Impressively, the cumulative incidence of chronic GVHD was only 10% with median follow up of 26.3 months (Figure 1). NRM was only 9% at 100 days and 17% at 2 years. These data suggest that pharmacologic strategies targeting alloreactive donor T cells can mitigate acute and chronic GVHD risk and facilitate transplantation tolerance.
Figure 1.
Cumulative incidence of chronic GVHD and survival outcomes following post-HCT high dose cyclophosphamide (reprinted from Luznik, et al, Blood 2010)
The performance of high quality clinical trials that modulate immunobiology of acute GVHD offer promise to advance the field and spare patients morbidity and mortality associated with the syndrome.
Therapy of acute graft-versus-host disease
Glucocorticoids (prednisone at ≥ 1–2 mg/kg for 7 to 14 days, followed by gradual dose reduction) have been considered the standard initial treatment for acute GVHD.12 CRs occur in approximately 35% to 50% of the patients at day 28 of therapy.12–14 The likelihood of GVHD treatment response decreases with increasing severity of the disease.15,16 The response to primary therapy is of critical importance as it correlates with survival post transplant.17
If the manifestations of GVHD in any organ worsen over 3 days of treatment, or if the skin does not improve by 5 days while other organ manifestations are present, secondary therapy should be considered.12 An additional immunosuppressive agent should be added,18–20 as attempts to use higher initial doses of glucocorticoids 21 or prolonged steroid tapering failed to improve responses rates.22 No consensus exists on the optimal treatment of patients with steroid refractory or dependent GVHD.
New immunosuppressive agents and/or strategies are required to improve management of GVHD and decrease its toxicities. Effective therapy for acute GVHD might improve CR rates and result in better survival after allogeneic HCT.23 Few controlled studies have been conducted testing initial treatment of acute GVHD with novel agents in addition to glucocorticoids to improve GVHD response rates and survival. Initial attempts using anti-T cell antibodies in addition to standard GVHD therapy failed to improve response.24–26 A randomized trial comparing prednisone (2 mg/kg/day) plus a humanized monoclonal antibody against the interleukin-2 receptor (daclizumab) against prednisone plus placebo for primary treatment of acute GVHD did not improve response rates. Furthermore, the combination resulted in significantly worse 100-day survival and 1-year OS due to increased relapse and GVHD related mortality.27
Levine et al tested the combination of methylprednisolone (2 mg/kg/day) plus a tumor necrosis factor α inhibitor, etanercept, as initial therapy in a pilot study 28 followed by a Phase II clinical trial.29 Etanercept and glucocorticoids were significantly more likely to achieve CR after 4 weeks of treatment compared to an external control group treated with glucocorticoids alone (69% vs. 33%; P <.001), and response benefits persisted at 12 weeks (77% vs. 50%; P <.001). Difference in results was observed regardless of stem cell donor (related vs. unrelated), or conditioning regimen (myeloablative vs. reduced intensity) and/or organ involved (skin vs. liver vs. gastro-intestinal tract). Incidence of infections, malignancy relapse and/or flare of GVHD did not vary among compared groups. Combination therapy translated into a significantly improved survival at 6 months for unrelated recipients. 29
The BMT CTN reported the results of a randomized, phase 2 multicenter trial to evaluate the efficacy of 4 agents, each in combination with glucocorticoids as initial therapy for acute GVHD.30 Patients were randomized to methylprednisolone 2 mg/kg/day plus either etanercept, MMF, denileukin diftitox (denileukin), or pentostatin. Day-28 CR rates were 26%, 60%, 53%, and 38%, respectively. The corresponding rates of severe infections were 48%, 44%, 62%, and 57%, and the 9-month OS rates were 47%, 64%, 49%, and 47%, respectively. Patients who received MMF for GVHD prophylaxis (24%) were randomized only to a non-MMF arm, creating an allocation bias. Non-MMF arms included 30%–34% patients previously treated with MMF as GVHD prophylaxis. Since pre-treatment with MMF affects GVHD responsiveness,31 the allocation bias raised the concern that patients with less responsive acute GVHD were preferentially allocated to non-MMF arms and biased the results in favor of MMF. Despite this caveat, efficacy and toxicity data of this BMT CTN trial 30 indicated that MMF plus glucocorticoids might be the most promising of the four regimens, and therefore it was selected for comparison against glucocorticoids alone in a phase 3 trial that is currently open to accrual (BMT CTN Protocol 0802- http://clinicaltrials.gov/ct2/show/NCT01002742).
Primary treatment of GHVD using glucocorticoids as backbone for acute GVHD treatment provides also a template to test investigational (non-FDA approved) agents. Our group is currently testing the efficacy of a novel histone deacetylase inhibitor, panobinostat, in addition to glucocorticoids in a prospective phase I/II clinical trial (http://clinicaltrials.gov/ct2/show/NCT01111526).
Prolonged exposure to glucocorticoids is associated with complications that impair quality of life and increase risk of infections. In addition, methylprednisolone used in combination with CSA for GVHD prophylaxis resulted in a higher incidence of chronic GVHD (44% vs. 21%; P=.02) vs. CSA alone.32 These data indicate that despite effectiveness in suppressing GVHD in some patients, glucocorticoids may interfere with signals required for development of immune tolerance.
Glucocorticoid dose-finding, prospective controlled clinical trials for the treatment of GVHD are few in the literature. A prospective randomized trial has shown that glucocorticoids at doses higher than 2 mg/kg/day do not offer benefits for treatment of acute GVHD. 21 Mielcarek et al have conducted a retrospective analysis to evaluate the efficacy of lower glucocorticoid doses for the treatment of acute GHVD. 33 Outcomes were compared between low-dose (1 mg/kg/day; n=347) and standard dose (2 mg/kg/day; n=386) prednisone or equivalent. Groups differed in degree of donor/recipient HLA matching, stem cell sources, timing of GVHD therapy and GVHD grading among others. Multivariate analysis after adjusting for GVHD-associated factors revealed no differences in OS, relapse, secondary GVHD therapy and non-relapse mortality for patients with grades I–II GVHD, and reduced risk of invasive fungal infections in the low-dose prednisone group.
Systemic glucocorticoid-sparing approaches have been initially tested by McDonald et al in the treatment of upper intestinal GVHD with nausea, vomiting and diarrhea ≤1 L/day. Beclomethasone dipropionate (BDP), a topically active non-absorbable glucocorticoid, was administered for 30 days in a single center trial, achieving GVHD control without recurrence and allowing faster taper of systemic glucocorticoids than usual.34 Follow up randomized placebo-controlled multicenter trial testing oral BDP (8 mg for 50 days) in addition to short course of glucocorticoids (1–2 mg/kg/day, tapered on day 10 to a physiological dose by day 16), has confirmed that BDP allows a rapid steroid taper. Primary endpoint time to treatment failure by study day 50 was not reached but day 80 efficacy, day 200 and 1 year survival was significantly better in the BDP group. 35 Ongoing Phase III confirmatory trial is currently accruing subjects testing the primary endpoint of occurrence GVHD treatment failure during the 80 study period, encompassing a 50-day treatment and a 30-day observation period http://clinicaltrials.gov/ct2/show/NCT00926575). Additional glucocorticoid-sparing strategies have tested low dose MTX combined with low dose methylprednisolone (0.5 mg/Kg/day followed by taper on day 5 and cessation at about day 30) for the treatment of acute GVHD with encouraging results.36 In summary, these studies established the proof of principle that steroids-sparing approaches are feasible and should be further explored to reduce morbidity and improve OS after HCT.
Summary
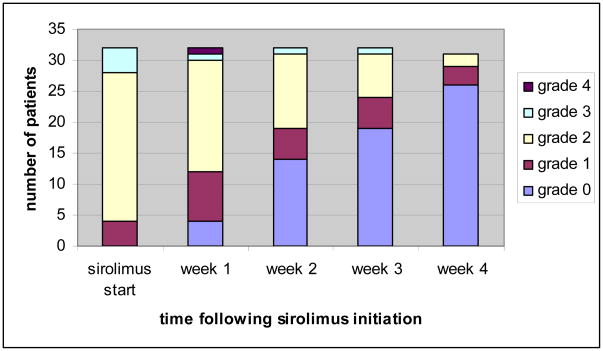
Our group has experience using solely SIR, an inhibitor of mammalian mTOR, as primary treatment of acute GVHD in patients deemed high risk for steroid toxicity.37 SIR is quite effective in GVHD prevention and its anti-tumor activity might decrease relapse after transplantation.38 Treatment of grades I–III acute GVHD affecting primarily skin and gut (n=32) resulted in a CR rate of 50% with a favorable toxicity profile.39 Response to treatment was achieved at median of 14 days (range 5–28 days) (Figure 2). Those patients requiring glucocorticoids achieved CR with prednisone doses of only 0.5–1 mg/kg/day suggesting a potential steroid-sparing effect. Prospective clinical trials are needed to address the definitive role of SIR alone for acute GVHD treatment.
Figure 2.
Response to sirolimus as sole primary therapy of acute GVHD (reprinted from Pidala, et al, Haematologica, 2011)
With divergent efforts in acute GVHD therapy, namely combination therapy with traditional glucocorticoid doses vs. steroid-sparing approaches, mature results from high quality trials are needed to direct best practice that optimizes efficacy while sparing toxicity.
Key points.
Animal models predict that GVHD prevention and operational tolerance require tipping the balance in favor of regulatory T cells, against effector T cells.
Adoptive Treg transfer and sirolimus both favor regulatory T cells and have clinical activity in GVHD prevention and treatment.
Post-transplant high-dose cyclophosphamide is effective in eliminating alloreactive effector T cells and has clinical activity in GVHD prevention.
Glucocorticoids prevent or least delay transplantation tolerance.
Glucocorticoid-sparing approaches, such as non-absorbable enteric steroids, have improved patient survival.
Acknowledgments
This work was supported in part by NIH grants CA132197, AI082498, CA076292
References
- 1.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 2.Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–2314. [PubMed] [Google Scholar]
- 3*.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–1070. doi: 10.1182/blood-2010-07-293795. Brunstein, et al have demonstrated the feasibility of donor Treg expansion and safety of infusion of such cells in the setting of umbilical cord blood transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatza E, Rogers CE, Clouthier SG, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112:1515–1521. doi: 10.1182/blood-2007-11-125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaughnessy PJ, Bolwell BJ, van Besien K, et al. Extracorporeal photopheresis for the prevention of acute GVHD in patients undergoing standard myeloablative conditioning and allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:1068–1076. doi: 10.1038/bmt.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Jakubowski AA, Small TN, Kernan NA, et al. T Cell-Depleted Unrelated Donor Stem Cell Transplantation Provides Favorable Disease-Free Survival for Adults with Hematologic Malignancies. Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.01.005. Jakubowskik, et al demonstrate marked protection from GVHD with aggressive T cell depletion in unrelated donor transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Devine SM, Carter S, Soiffer RJ, et al. Low Risk of Chronic Graft-versus-Host Disease and Relapse Associated with T Cell-Depleted Peripheral Blood Stem Cell Transplantation for Acute Myelogenous Leukemia in First Remission: Results of the Blood and Marrow Transplant Clinical Trials Network Protocol 0303. Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.02.002. Devine, et al report mature results from CTN 0303 in which there is a notably low risk of chronic GVHD with T cell depletion as sole GVHD prophylaxis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litzow MR, Perez WS, Klein JP, et al. Comparison of outcome following allogeneic bone marrow transplantation with cyclophosphamide-total body irradiation versus busulphan-cyclophosphamide conditioning regimens for acute myelogenous leukaemia in first remission. Br J Haematol. 2002;119:1115–1124. doi: 10.1046/j.1365-2141.2002.03973.x. [DOI] [PubMed] [Google Scholar]
- 9*.Parmar S, Andersson BS, Couriel D, et al. Prophylaxis of graft-versus-host disease in unrelated donor transplantation with pentostatin, tacrolimus, and mini-methotrexate: a phase I/II controlled, adaptively randomized study. J Clin Oncol. 2011;29:294–302. doi: 10.1200/JCO.2010.30.6357. Parmar, et al employ a novel endpoint (composite of patients being alive, engrafted, in remission, without GVHD at 100 days post HCT, and with no ≥ grade 3 GVHD at any time) in this trial. Risk for GVHD in pentostatin-treated cohorts is not significantly different compared to control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Perkins J, Field T, Kim J, et al. A randomized phase II trial comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate for acute graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2010;16:937–947. doi: 10.1016/j.bbmt.2010.01.010. Perkins, et al demonstrate that mycophenolate mofetil spares toxicity associated with methotrexate, but no significant differences in acute or chronic GVHD are observed. [DOI] [PubMed] [Google Scholar]
- 11*.Luznik L, Bolanos-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115:3224–3230. doi: 10.1182/blood-2009-11-251595. Luznik, et al demonstrate that single agent prophylaxis with high-dose post-transplantation cyclophosphamide effectively prevents acute and chronic GVHD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109:4119–4126. doi: 10.1182/blood-2006-12-041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hings IM, Severson R, Filipovich AH, et al. Treatment of moderate and severe acute GVHD after allogeneic bone marrow transplantation. Transplantation. 1994;58:437–442. doi: 10.1097/00007890-199408270-00008. [DOI] [PubMed] [Google Scholar]
- 14.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 15.Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990;76:1464–1472. [PubMed] [Google Scholar]
- 16.Weisdorf D, Haake R, Blazar B, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75:1024–1030. [PubMed] [Google Scholar]
- 17*.Saliba RM, Couriel DR, Giralt S, et al. Prognostic value of response after upfront therapy for acute GVHD. Bone Marrow Transplant. 2011 doi: 10.1038/bmt.2011.41. Saliba, et al demonstrate the prognostic significance of response to therapy in determining acute GVHD outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pidala J, Anasetti C. Glucocorticoid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:1504–1518. doi: 10.1016/j.bbmt.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Busca A. The use of monoclonal antibodies for the treatment of graft-versus-host disease following allogeneic stem cell transplantation. Expert Opin Biol Ther. 2011 doi: 10.1517/14712598.2011.566852. [DOI] [PubMed] [Google Scholar]
- 20.Horwitz EM, Maziarz RT, Kebriaei P. MSCs in hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:S21–29. doi: 10.1016/j.bbmt.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Van Lint MT, Uderzo C, Locasciulli A, et al. Early treatment of acute graft-versus-host disease with high- or low-dose 6-methylprednisolone: a multicenter randomized trial from the Italian Group for Bone Marrow Transplantation. Blood. 1998;92:2288–2293. [PubMed] [Google Scholar]
- 22.Hings IM, Filipovich AH, Miller WJ, et al. Prednisone therapy for acute graft-versus-host disease: short- versus long-term treatment. A prospective randomized trial. Transplantation. 1993;56:577–580. doi: 10.1097/00007890-199309000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Van Lint MT, Milone G, Leotta S, et al. Treatment of acute graft-versus-host disease with prednisolone: significant survival advantage for day +5 responders and no advantage for nonresponders receiving anti-thymocyte globulin. Blood. 2006;107:4177–4181. doi: 10.1182/blood-2005-12-4851. [DOI] [PubMed] [Google Scholar]
- 24.Cahn JY, Labopin M, Mandelli F, et al. Autologous bone marrow transplantation for first remission acute myeloblastic leukemia in patients older than 50 years: a retrospective analysis of the European Bone Marrow Transplant Group. Blood. 1995;85:575–579. [PubMed] [Google Scholar]
- 25.Martin PJ, Nelson BJ, Appelbaum FR, et al. Evaluation of a CD5-specific immunotoxin for treatment of acute graft-versus-host disease after allogeneic marrow transplantation. Blood. 1996;88:824–830. [PubMed] [Google Scholar]
- 26.Cragg L, Blazar BR, Defor T, et al. A randomized trial comparing prednisone with antithymocyte globulin/prednisone as an initial systemic therapy for moderately severe acute graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6:441–447. doi: 10.1016/s1083-8791(00)70036-x. [DOI] [PubMed] [Google Scholar]
- 27.Lee SJ, Zahrieh D, Agura E, et al. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood. 2004;104:1559–1564. doi: 10.1182/blood-2004-03-0854. [DOI] [PubMed] [Google Scholar]
- 28.Uberti JP, Ayash L, Ratanatharathorn V, et al. Pilot trial on the use of etanercept and methylprednisolone as primary treatment for acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:680–687. doi: 10.1016/j.bbmt.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111:2470–2475. doi: 10.1182/blood-2007-09-112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Alousi AM, Weisdorf DJ, Logan BR, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114:511–517. doi: 10.1182/blood-2009-03-212290. Alousi, et al report the results of the CTN trial testing combination therapies employing standard dose glucocorticoids and additional immune suppressive agents. This study informs the current CTN trial examining standard glucocorticoids vs. glucocorticoids with mycophenolate mofetil for primary therapy of acute GVHD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perkins J, Field T, Kim J, et al. A randomized phase II trial comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate for acute graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2010;16:937–947. doi: 10.1016/j.bbmt.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Deeg HJ, Lin D, Leisenring W, et al. Cyclosporine or cyclosporine plus methylprednisolone for prophylaxis of graft-versus-host disease: a prospective, randomized trial. Blood. 1997;89:3880–3887. [PubMed] [Google Scholar]
- 33*.Mielcarek M, Storer BE, Boeckh M, et al. Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood. 2009;113:2888–2894. doi: 10.1182/blood-2008-07-168401. Mielcarek, et al examine the impact of initial steroid dosing on patient outcomes in this analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald GB, Bouvier M, Hockenbery DM, et al. Oral beclomethasone dipropionate for treatment of intestinal graft-versus-host disease: a randomized, controlled trial. Gastroenterology. 1998;115:28–35. doi: 10.1016/s0016-5085(98)70361-0. [DOI] [PubMed] [Google Scholar]
- 35*.Hockenbery DM, Cruickshank S, Rodell TC, et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood. 2007;109:4557–4563. doi: 10.1182/blood-2006-05-021139. Hockenbery, et al demonstrate the activity of oral beclomethasone dipropionate in this randomized trial. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Xu LP, Liu KY, et al. Low-dose MTX combined with low-dose methylprednisolone as a first-line therapy for the treatment of acute GVHD: safety and feasibility. Bone Marrow Transplant. 2010 doi: 10.1038/bmt.2010.197. [DOI] [PubMed] [Google Scholar]
- 37.Pidala J, Kim J, Anasetti C. Sirolimus as primary treatment of acute graft-versus-host disease following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:881–885. doi: 10.1016/j.bbmt.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cutler C, Antin JH. Sirolimus immunosuppression for graft-versus-host disease prophylaxis and therapy: an update. Curr Opin Hematol. 2010;17:500–504. doi: 10.1097/MOH.0b013e32833e5b2e. [DOI] [PubMed] [Google Scholar]
- 39*.Pidala J, Tomblyn M, Nishihori T, et al. Sirolimus demonstrates activity in the primary therapy of acute graft-versus-host disease without systemic glucocorticoids. Haematologica. 2011 doi: 10.3324/haematol.2011.041236. Pidala, et al report that sirolimus has activity as a sole immune suppressive agent in the primary therapy of acute GVHD. [DOI] [PMC free article] [PubMed] [Google Scholar]