Abstract
Infection with the human immunodeficiency virus-1 (HIV-1) and acquired immunodeficiency syndrome (AIDS) are often associated with severe and debilitating neurological problems that include behavioral abnormalities, motor dysfunction and frank dementia. HIV-1-infected peripheral immune cells, in particular macrophages, appear to infiltrate the CNS, release neurotoxins and provoke a neuropathological response involving all cell types in the brain. In the CNS, HIV-1 and its components initiate activation of chemokine receptors, inflammatory mediators and glutamate receptor-mediated excitotoxicity, all of which can activate numerous downstream signaling pathways and disturb neuronal and glial function. Recent experimental evidence suggests that disturbance by HIV-1 results not only in neuronal injury and death but also in impairment of neurogenesis. This article will review recently identified pathological mechanisms which potentially contribute to the development of neurocognitive impairment and dementia in association with HIV-1 infection.
Keywords: HIV-1, Neurotoxicity, Apoptosis, Neurodegeneration, Dementia, Stem Cell, Progenitor, Cell-Cycle, Proliferation, Neurogenesis
2. INTRODUCTION
Although human immunodeficiency virus-1 (HIV-1) initially targets and compromises cells of the immune system and eventually causes acquired immunodeficiency syndrome (AIDS), the virus can also cause a variety of neurological problems that can culminate in frank dementia. AIDS-related opportunistic infections may affect the central nervous system (CNS), in particular in the absence of treatment, but HIV-1 infection itself can also initiate a number of neurological syndromes (1). Neuropathological conditions directly triggered by HIV-1 include peripheral neuropathies, vacuolar myelopathy, and a clinical syndrome of cognitive and motor dysfunction that has been designated HIV-associated dementia (HAD) (2-5). A mild and lately more common form of HAD is termed minor cognitive motor disorder (MCMD) (3,5,6).
Although a high risk of neuropsychological impairment in HIV-1 infection seems to be indicated early on by anemia (7), the mechanisms contributing to the development of MCMD and HAD are still incompletely understood. However in recent years the discovery in the brain of cellular binding sites for HIV-1, the chemokine receptors, and progress in understanding neuroinflammation and neural stem cell biology provided new and surprising insights into the ways HIV-1 can compromise the CNS (3,8-14). In fact, recently accumulated evidence suggests that HIV-1 possibly strikes at the brain in two ways by not only causing neuronal injury and death but also by impairing neurogenesis (12,15-20). Therefore this article will review those developments regarding the understanding of HIV-1’s detrimental effect in the CNS.
3. NEUROLOGICAL DYSFUNCTION IN HIV-1 INFECTION AND AIDS
From the beginning of the AIDS epidemic until the advent of highly active antiretroviral therapy (HAART), the majority of severe neurological symptoms occurred in advanced stages of systemic HIV-1 disease and the prevalence of HAD was estimated to be 20-30% in individuals with low CD4 T cell counts (7). The introduction of HAART has increased the life span of people infected with HIV-1 and resulted in an at least temporary decrease in the incidence of HAD to as low as 10.5% (21). This transient effect suggests that the consequences of HIV-1 infection in the CNS are linked to the condition in the periphery. Apparently, a peripheral infection, its associated immune response and inflammatory processes can influence all cell types in the CNS (22,23). Nevertheless improved control of peripheral viral replication and the treatment of opportunistic infections continue to extend survival times, HAART fails to provide protection from MCMD or HAD, or to reverse the disease in most cases (24-27). While in the HAART era MCMD may be more prevalent than frank dementia, HAD remains a significant independent risk factor for death due to AIDS, and it is assumed to be the most common cause of dementia worldwide among people of age 40 or less, (6). Moreover, the proportion of new cases of HAD displaying a CD4 cell count greater then 200 μl-1 is growing (21), and another recent study found that in a group of 669 HIV patients who died between 1996 and 2001 more than 90% had been diagnosed with HAD as an AIDS-defining condition within the last 12 month of life (28). This situation and distinct patterns of viral drug resistance in plasma and cerebrospinal fluid (CSF) compartments might at least in part be due to poor penetration into the CNS of HIV protease inhibitors and several of the nucleoside analogues (10,12,24). Moreover, although HIV seems to penetrate into the CNS soon after infection in the periphery, and then resides primarily in perivascular macrophages and microglia (13,29,30), current therapeutic guidelines for AIDS suggest to start HAART only once the number of CD4+ T-cells begins to decline. Since this might occur up to years after peripheral infection, HAART is unlikely to prevent the entry of HIV-1 into the CNS (10). Therefore it is not very surprising that as people live longer with HIV-1 and AIDS the prevalence of dementia might be rising, and in recent years the incidence of HAD as an AIDS-defining illness has increased (3,10,12,14,21,24,31).
4. NEUROPATHOLOGY AND DEVELOPMENT OF DEMENTIA IN HIV-1 INFECTION
The blood-brain-barrier (BBB) may play an important role in the acute and chronic HIV infection of the CNS as it potentially controls the infiltration of virus-infected and uninfected peripheral immune cells, such as monocytes and macrophages (30,32). Astrocytes and microglia produce chemokines - cell migration/chemotaxis inducing cytokines - such as monocyte chemoattractant protein (MCP)-1, which appear to attract peripheral blood mononuclear cells across the BBB into the brain parenchyma (33). In fact, an increased risk of HAD has recently been connected to a mutant MCP-1 allele that causes increased infiltration of mononuclear phagocytes into tissues (34). Alternatively, it has been suggested that the inflammatory cytokine tumor necrosis factor (TNF)-alpha, promotes a paracellular route for the virus across the BBB (35).
Pathological features of HIV-1 infection in the brain are often referred to as HIV encephalitis (HIVE) and include widespread reactive astrocytosis, myelin pallor, microglial nodules, activated resident microglia, multinucleated giant cells, infiltration predominantly by monocytoid cells, including blood-derived macrophages, and decreased synaptic and dendritic density, combined with selective neuronal loss (1,36). Surprisingly, numbers of HIV-infected cells, multinucleated giant cells or viral antigens in CNS tissue do not correlate well with ante mortem measures of cognitive dysfunction (36-39). In contrast, increased numbers of microglia (37), decreased synaptic and dendritic density, selective neuronal loss (36,38,39), elevated TNF-alpha mRNA in microglia and astrocytes (40), and evidence of excitatory neurotoxins in CSF and serum (41) constitute the pathologic features most closely associated with the clinical signs of HAD. Furthermore, two recent studies found evidence that, in contrast to viral load, the amount of proviral HIV DNA in circulating monocytes and macrophages correlates well with HAD (42,43).
Neuronal damage and loss has been observed in distinct brain regions, including frontal cortex (44,45), substantia nigra (46), cerebellum (47), and putamen (48). Focal neuronal necrosis was reported for HAD brains earlier on (49), but more recently signs of neuronal apoptosis have been linked to HAD (50,51). A good correlation was observed of HAD with cellular DNA fragmentation in basal ganglia detected by terminal-deoxynucleotidyl-transferase-mediated dUTP nick-end labeling (TUNEL) in one study (52). Interestingly, signs of neuronal death were not clearly associated with viral burden or a history of dementia. However, the localization of apoptotic neurons was correlated with evidence of structural atrophy and closely associated with signs of microglial activation, especially within subcortical deep gray structures (50).
After the introduction of HAART, however, HIV neuropathology has begun shifting. Although the number of opportunistic infections appears to decline, two post mortem studies found increased macrophage/microglia infiltration and activation in hippocampus and basal ganglia of HAART-treated patients as compared to samples from the time before HAART and a higher prevalence of HIV-associated encephalitis at the time of autopsy (53,54). Post mortem specimens from HIV patients who failed HAART showed even more encephalitis and severe leukoencephalopathy (53). In line with these findings more recent neuropathological descriptions also reported various forms with severe HIVE and white matter injury, extensive perivascular lymphocytic infiltration, ‘burnt-out’ forms of HIVE and aging-associated beta-amyloid accumulation with Alzheimer’s-like neuropathology (55,56).
5. CHEMOKINE RECEPTORS AND HIV-1 INFECTION OF THE BRAIN
Chemokine receptors belong in the large category of G-protein coupled seven transmembrane-spanning domain receptors. Chemokine receptors and their ligands mediate leukocyte trafficking and contribute intimately to the organization of inflammatory responses of the immune system (57-60). In addition they are involved in the intricate control of organogenesis, including hematopoiesis, angiogenesis, and development of heart and brain (57,61-63). Chemokines and their receptors are in fact indispensable for maintenance, maturation and migration of hematopoietic and neural stem cells (60,64). However, the most prominent pathological function of certain chemokine receptors seems to be the mediation of HIV-1 infection (57-59).
Infection by HIV-1 of macrophages and lymphocytes in the periphery and microglia in the brain can occur after binding of the viral envelope protein gp120 to one of several possible chemokine receptors in conjunction with CD4. Depending on the exact variant of gp120, different HIV-1 strains may use CCR5 (CD195) and CCR3, or CXCR4 (CD184), or a combination of these chemokine receptors to enter target cells (58,59,65). Generally, T-cells are infected by ‘T-tropic’ viruses via the alpha-chemokine receptor CXCR4 and/or the beta-chemokine receptor CCR5. In contrast, macrophages and microglia are infected by ‘M-tropic’ HIV-1 primarily via CCR5 and CCR3, but the alpha-chemokine receptor CXCR4 may also be involved (65-68). In fact, usage of CCR5 is neither necessary nor sufficient for macrophage tropism (69,70). However, the crucial role of chemokine receptors on several levels in HIV-1 disease has become increasingly obvious in recent years (71). Usually CCR5-preferring HIV-1 strains (R5-tropic) are transmitted between humans, and individuals lacking CCR5 are highly resistant to primary HIV infection (72). CXCR4-using viruses (X4-tropic) occur in about 50% of infected individuals later in the course of HIV-1 disease and indicate progression to AIDS (66). However, in many HIV-1/AIDS patients the switch in coreceptor usage does not occur and R5-tropic viruses evolve over time into more cytopathic variants possessing higher CCR5 affinity combined with reduced CD4 dependence (69).
In the brain, neurons and astrocytes express among other chemokine receptors also CCR5 and CXCR4 (9,33), although these cells, in contrast to microglia, are not thought to harbor productive HIV-1 infection under in vivo conditions. However, several in vitro studies strongly suggest that CXCR4 is prominently involved in HIV-associated neuronal damage whereas CCR5 may play a dual role by being able to either serve a toxic or protective role (73-77).
Intact HIV-1, as well as picomolar concentrations of isolated viral envelope gp120, can induce neuronal death via CXCR4 and CCR5 receptors in cerebrocortical and hippocampal neurons and neuronal cell lines from humans and rodents (67,68,73,74,76-80). Recently, our group further investigated the role of chemokine receptors in the neurotoxicity of gp120 using mixed neuronal/glial cerebrocortical cultures from rat and mouse. We found that gp120 from CXCR4 (X4)-preferring as well as CCR5 (R5)-preferring and dual tropic HIV-1 strains all were able to trigger neuronal death. Neurotoxicity of X4-preferring gp120 was strongly reduced or absent in CXCR4-deficient cerebrocortical cultures while gp120 from a R5-preferring HIV-1 strain showed no longer neurotoxicity in the absence of CCR5. The gp120 of the dual tropic HIV-1SF2 exerted surprisingly even greater neurotoxicity in CCR5 knockout cultures compared to wild-type or CXCR4-deficient cerebrocortical cells but, like all other tested gp120s, was no longer toxic to double KO cerebrocortical cultures lacking both chemokine receptors (77). On the other hand, we observed earlier that the CCR5 ligands MIP-1beta and RANTES protect neurons against gp120-induced toxicity (76). Interestingly, this protective effect occurs no matter if gp120 prefers CXCR4 or CCR5 or both coreceptors. While the beta-chemokines may prevent CCR5-preferring gp120 from interacting with its receptor by competitive displacement, the blockade of CXCR4-mediated toxicity apparently involves heterologous cross-desensitization of this receptor via stimulation of CCR5 (77). These findings are consistent with a primarily neurotoxic activation of CXCR4 by gp120. In contrast, CCR5 might at least in part stimulate cytoprotective signals depending on the HIV-1 strain from which a given envelope protein originated.
6. HIV-1 INDUCED NEURONAL TOXICITY
While progress is being made in unraveling the pathologic processes, it remains a controversial issue how exactly HIV-1 infection provokes neuronal injury and death as well as neurocognitive and motor impairment (10,12,13,18). There is general agreement that HIV does not infect postmitotic, mature neurons, but the mechanism of neuronal damage is in question. There is ample evidence for neuronal injury by various viral proteins; including Tat, Nef, Vpr and the Env proteins gp120 and gp41 (3,12,18,81-85). All these observations have generated at least two different hypotheses on how HIV-1 initiates neuronal damage in the brain. The hypotheses can be described as the “direct injury” hypothesis and the “indirect’ or “bystander effect” hypothesis. These two hypotheses are by no means mutually exclusive, and while the available data support a role for both, an indirect form of neurotoxicity seems to predominate in a setting where glial and neuronal cells are present (3,12,13,18,30).
6.1. Direct neuronal toxicity
The hypothesis that HIV proteins can directly injure neurons without any contribution of non-neuronal cells (microglia and/or astrocytes) is supported by experiments showing that viral envelope proteins are toxic in serum free primary neuronal cultures (74,75) and in neuroblastoma cell lines (73). The impact of neurotoxic factors secreted by non-neuronal cells is minimized in these experimental paradigms because serum-free neuronal cultures contain few if any non-neuronal cells, and neuroblastoma lines do not contain cells of other phenotypes. In case of HIV envelope protein gp120, which interacts with several members of the chemokine receptor family (see above), the direct form of HIV-induced neuronal injury may be mediated by chemokine receptor signaling in the absence of CD4 (73). Indeed, experiments employing an inhibitor of CXCR4 can in some cases prevent HIV/gp120-induced neuronal apoptosis (74-76,86). In a different study, gp120 was found to interact at nanomolar concentrations with the glycine binding site of the N-methyl-D-aspartate-type glutamate receptor (NMDAR) (87), suggesting an alternative mechanism by which HIV/gp120 may directly interfere with neuronal function and viability. The HIV-protein Tat (HIV/Tat) can be taken up into neuron-like PC12 cells by a receptor mediated mechanism (88) and may also have a direct effect on primary neurons by potentiating the response to excitotoxic stimuli (reviewed in (18)). Experiments using cultured hippocampal neurons suggested that the HIV-protein Vpr may cause direct neurotoxicity through formation of a cation-permeable channel (84). The absence of non-neuronal cells allows the study of potential direct effects of viral proteins on neurons, but one ought to bear in mind that because of the absence of non-neuronal cells a predominantly indirect effect cannot be detected. Notably, in the pathophysiological setting of the brain neurons always encounter the potential toxins in the presence of glial cells.
6.2. Indirect neuronal toxicity
In mixed neuronal/glial cerebrocortical cultures that model the cellular composition of the brain, HIV/gp120-induced apoptotic death appears to be mediated predominantly via the release of toxins from microglia and macrophages rather than by direct neuronal damage (68,76,78-81,89). In fact, both the absence or inactivation of macrophages and microglia basically abrogates the neurotoxicity of HIV/gp120 in mixed neuronal/glial cultures (76,80,89,90). Since at least in vitro inhibition of microglial activation suffices to prevent neuronal death after gp120 exposure, it seems likely that stimulation of CXCR4 or CCR5 in macrophages / microglia is a prerequisite for the neurotoxicity of gp120 (67,76). This hypothesis is further supported by the observation that introduction of HIV coreceptor-expressing macrophages completely restores neurotoxicity of gp120 in cerebrocortical cultures lacking both CXCR4 and CCR5 (M. Kaul, unpublished).
Macrophages and microglia can be infected by HIV-1, but they can also be stimulated by factors released from infected cells. These factors include cytokines, matrix metalloproteinases (MMPs) and shed viral proteins such as gp120 and Tat. Variations of the HIV-1 envelope protein gp120, in particular in its V1, V2 and V3 loop sequences, have been implicated in modulating the neurotoxicity of macrophages and microglia (91). Factors secreted by activated microglia affect all cell types in the CNS, resulting in upregulation of cytokines, chemokines and endothelial adhesion molecules (3,10,12,13,30). Some of these factors may directly or indirectly contribute to neuronal damage and apoptosis. Directly neurotoxic factors released from activated microglia and macrophages include excitatory amino acids (EAAs) and related substances, such as quinolinate, cysteine and a not completely characterized amine compound named ‘Ntox’ (3,10,12,13,41,89,92,93). EAAs can trigger neuronal apoptosis through a process known as excitotoxicity. This detrimental process involves excessive Ca2+ influx and free radical (nitric oxide and superoxide anion) formation by over-stimulation of glutamate receptors (92), activation of stress-associated protein kinases and caspases and production of proinflammatory lipids (3,10,13,14,18,94). Figure 1 shows a model of the HIV-1 associated neuropathology that may underlie the development of HAD. It is important to note that toxic viral proteins among factors released from microglia and glutamate set free by astrocytes may all act in concert to provoke neurodegeneration and dementia, even in the absence of extensive viral invasion of the brain. Furthermore, HAD might share the critical involvement of neuroinflammation and microglial activation with several other neurodegenerative diseases, such as Alzheimer’s disease, Multiple Sclerosis, Parkinson’s disease and even Frontotemporal Lobe Dementia (95).
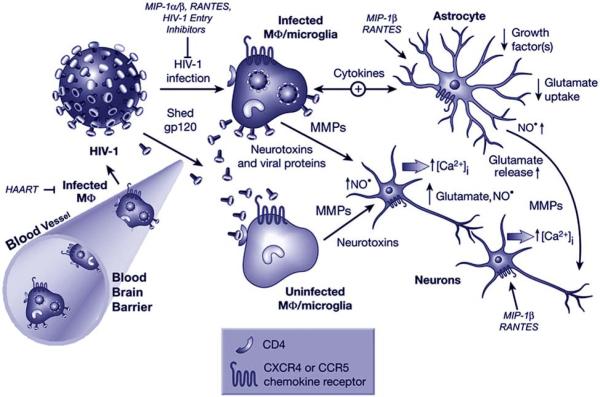
Figure 1.
Current model of HIV-1 neuropathology and potential sites for therapeutic intervention (protective factors are shown in italic): Immune-activated and HIV-infected, brain-infiltrating macrophages (Mphi) and microglia release potentially neurotoxic substances. These substances include quinolinic acid and other excitatory amino acids such as glutamate and l-cysteine, arachidonic acid, NTox, free radicals, TNF-alpha, and probably others. These factors from macrophages/microglia and also possibly from reactive astrocytes contribute to neuronal injury, dendritic and synaptic damage, and apoptosis as well as to astrocytosis. Entry of HIV-1 into macrophages/microglia occurs via gp120 binding, and therefore it is not surprising that gp120 (or a fragment thereof) is capable of activating uninfected macrophages/microglia to release similar factors to those secreted in response to productive HIV infection. Macrophages/microglia express CCR5 and CXCR4 chemokine receptors on their surface in addition to CD4 and viral gp120 binds via these receptors. Neurons and astrocytes also possess CXCR4 and CCR5 receptors on their surface, raising the possibility of direct interaction with gp120. Macrophages/microglia and astrocytes have mutual feed-back loops (bidirectional arrow). Cytokines participate in this multi-cellular network in several ways. For example, HIV-infection or gp120-stimulation of macrophages/microglia enhances their production of TNF-alpha and IL-1beta (cytokines-arrow). The TNF-alpha and IL-1beta produced by macrophages/microglia stimulate astrocytosis. Arachidonate released from Mphi/microglia impairs astrocyte clearing of the neurotransmitter glutamate and thus contributes to excitotoxicity. In conjunction with cytokines, the alpha-chemokine SDF-1 stimulates reactive astrocytes to release glutamate in addition to the free radical nitric oxide (NO·), which in turn may react with superoxide (O2·-) to form the neurotoxic molecule peroxynitrite (ONOO-). Neuronal injury is primarily mediated by overactivation of NMDARs with resultant excessive influx of Ca2+. This, in turn, leads to overactivation of a variety of potentially harmful signaling systems, the formation of free radicals, and release of additional neurotransmitter glutamate. Glutamate subsequently overstimulates NMDARs on neighboring neurons, resulting in further injury. This final common pathway of neurotoxic action can be blocked by NMDAR antagonists. For certain neurons, depending on their exact repertoire of ionic channels, this form of damage can also be ameliorated to some degree by calcium channel antagonists or non-NMDAR antagonists. HAART suppresses HIV-1 infection primarily in the periphery but has limited effect in the CNS. If present, MIP-1beta and RANTES, agonists of β-chemokine receptors, which are expressed in the CNS on neurons, astrocytes and microglia, can confer partial protection against neuronal apoptosis induced by HIV/gp120 or NMDA. Modified from (117).
6.3. Downstream molecular mechanisms in HIV-1 neurotoxicity
HIV-1 infected or gp120-stimulated macrophages/microglia release neurotoxins some of which stimulate the NMDAR, an ionotropic glutamate and neurotransmitter receptor (68,96). NMDAR antagonists can ameliorate neuronal cell death in vitro due to HIV-infected macrophages or purified recombinant gp120 (68,96), and in vivo in gp120 transgenic mice (97). Under physiological conditions activation of ionotropic glutamate receptors in neurons initiates a transient depolarization and excitation. However, extended and/or excessive NMDAR activation causes excitotoxicity through a sustained elevation of the intracellular Ca2+ concentration and a subsequent compromise of mitochondrial function and cellular energy metabolism which in turn results in the production of free radicals (98-100). If the initial excitotoxic insult is fulminant, for example, in the ischemic core of a stroke, the cells die early from loss of ionic homeostasis, resulting in acute swelling and lysis (necrosis). If the insult is more mild, as seen in several neurodegenerative disorders including HAD, neurons enter a delayed death pathway known as apoptosis (51,94). Neuronal apoptosis due to gp120 toxicity or after a direct excitotoxic insult involves Ca2+ overload, activation of p38 MAPK and p53, release of cytochrome c and other molecules such as apoptosis-inducing factor (AIF) from mitochondria, activation of caspases, free radical formation, lipid peroxidation, and chromatin condensation (33,76,77,80,101,102). In line with these observations is a report that in human neurons, CXCR4 mediates the toxic effect of gp120 via a process involving the synthesis of ceramide and NADPH-dependent production of superoxide radicals (103). Oxidative processes and cellular distress are also reflected by alterations to the cellular lipid metabolism, and an increase in ceramide, sphingomyelin and hydroxynonenal has been implicated in the neurotoxic pathways associated with HAD (18).
HIV-infected or gp120-activated microglia also release inflammatory cytokines, such as TNF-alpha (40,104). TNF-alpha has direct effects on glutamate neurotransmission by increasing the synaptic expression of certain glutamate receptors (AMPAR) and inhibiting long-term potentiation (LTP) in a p38 MAPK-dependent manner (105,106). Moreover, TNF-alpha can promote neurotoxicity by facilitating glutamate excitotoxicity through inhibition of astroglial glutamate transporters (106) or even provoking neurotransmitter release from glial cells (107,108). Interestingly, TNF-alpha and HIV/Tat synergize to promote neuronal death, and this effect is prevented by antioxidants (67). Finally, it remains possible that TNF-alpha activates caspases within neurons via TNF-alpha receptor-1 (TNFR1), since TNFR1 is found on at least some neurons, and it can trigger caspase-8 activation. Indeed, antibody neutralization of TNF-alpha or inhibition of caspase-8 prevents the neurotoxicity of HIV/gp120 in cultured cerebrocortical neurons (102); and caspase-8 activity can trigger caspase-3 activation, leading to apoptosis.
Activated caspase-3 and p53 are prominently detected in neurons of brains from HAD patients (102). Furthermore, in vitro, neuronal caspases-3, -8 and -9 are involved and p53 is indispensable in neurons (and microglia) for HIV-1/gp120 to cause neurotoxicity (80,102,109). Separate studies suggested that CXCR4 and p53 are connected through signaling pathways that mediate toxic or protective mechanisms depending on whether gp120 or SDF-1, the receptor’s natural binding partner, acts as the ligand (110). In contrast to gp120IIIB, SDF-1 was found to have a neuroprotective effect. It activated Akt and MAPKs (111) and regulated the expression and localization of cell cycle proteins (110,112). SDF-1 increased acetylation of p53 and p21 as well as the expression of retinoblastoma protein (Rb) while reducing the amount of phosphorylated Rb in the nucleus. Together with a reduction of the activity of the transcription factor E2F1, an overall anti-apoptotic effect was observed. In contrast, envelope protein of HIV-1IIIB triggered activation of Apaf-1 and promoted cell death. Besides these in vitro findings, changes from the normal expression pattern have been observed for the same cell cycle proteins mentioned above in post mortem brains derived from non-human primates with SIV encephalitis and humans with HIV encephalitis (113). Interestingly, these changes in cell cycle proteins correlated with the presence of activated microglia and macrophages.
7. EFFECTS OF HIV-1 ON NEUROGENESIS
The CXCR4-SDF-1 receptor-ligand axis plays an important role in the physiological function of hematopoietic and neural stem cells (33,60,114,115). This fact indicates a potential of HIV-1 and its envelope protein to directly interfere with biological functions of neural stem and progenitor cells. Moreover, we found that engagement of CCR5 can cross-desensitize CXCR4 (77). Thus CCR5-preferring HIV/gp120, besides CXCR4-preferring viruses, can potentially indirectly interfere with CXCR4-dependent signaling.
Although it is widely accepted that HIV-1 fails to productively infect neurons, it has been reported that neural progenitor cells are permissive to the virus (15,17,18). However, even without viral infection HIV-1/gp120 and chemokines seem able to affect human or rodent neural progenitor cells (16,20,116). In one study, chemokines promoted the quiescence and survival of human neural progenitor cells via stimulation of CXCR4 and CCR3 and a mechanism that involves downregulation of extracellularly regulated kinase-1 and -2 (ERK-1/2) with simultaneous upregulation of the neuronal glycoprotein reelin (116). Exposure to HIV-1 caused quiescence of neural progenitors, again through engagement of CXCR4 and CCR3. The coat protein HIV-1/gp120 reportedly downregulated ERK-1/2 but had no effect on Reelin (16). Interestingly, the effects of both the chemokines and HIV-1/gp120 were reversible and could be inhibited with recombinant Apolipoprotein E3 (ApoE3), but not ApoE4. Furthermore, post mortem brain specimen from HAD patients displayed fewer adult neural progenitor cells in the dentate gyrus of the hippocampus than non-demented and uninfected controls (16).
In cultures of primary mouse and human neural progenitor cells obtained from fetal or adult tissue, cells stain positively for the neural stem cell marker nestin and readily undergo cell division. After several rounds of proliferation, the progenitors exit the cell cycle and express neuronal markers such as βIII-tubulin (TuJ1). Our immunocytochemical studies showed that the progenitors expressed CXCR4 and CCR5. Treatment with HIV-1/gp120 in vitro reduced the proliferation of adult progenitors without producing apoptosis. Comparable to what had been observed by others in brain specimen from HAD patients, we also found a reduction of proliferating neural progenitors in the hippocampal dentate gyrus of transgenic mice that express HIV/gp120 in the brain in comparison to non-transgenic controls (20). Accounting for these effects, we found that gp120 inhibited proliferation of neural progenitor cells through activation of a pathway that involved p38MAPK, MAPK-activated protein kinase 2, a cell-cycle check-point kinase, and Cdc25B/C which in turn caused the arrest of the cell cycle in the G1 phase. The resulting decrease in neural progenitor proliferation caused by gp120 also meant that there were fewer progenitor cells present to differentiate into neurons, thus impairing neurogenesis (20). Neurogenesis in the dentate gyrus of the hippocampus has been implicated in learning and memory formation, neurocognitive functions which are impaired in HAD. Thus the apparent ability of HIV-1/gp120 to interfere with the normal function of neural progenitor cells suggested the possibility that HAD might develop as a consequence not only of injury and death of existing neurons but also due to virus-induced disturbance of potential homeostasis and renewal mechanisms in the CNS (Figure 2).
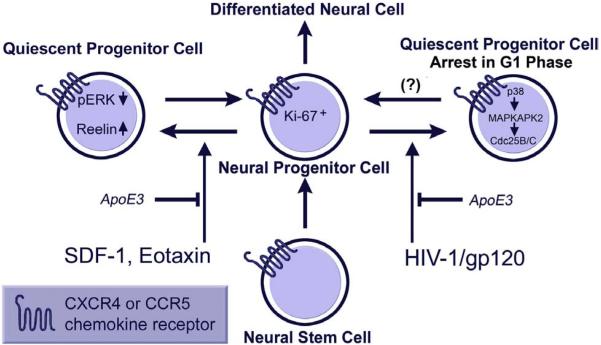
Figure 2.
Current model of HIV-1 interference with the function of neural progenitor cells and potential sites for therapeutic intervention (protective factors are shown in italic): Exposure to chemokines, SDF-1 and Eotaxin, or HIV-1/gp120 of mouse or human neural progenitor cells (NPCs) reduces proliferation and promotes quiescence. ApoE3 inhibits these effects on NPCs. NPCs express nestin and show decreased proliferation as judged by decreased BrdU incorporation. However, NPCs do not undergo apoptosis, as evidenced by lack of TUNEL staining and nuclear condensation under the same conditions (16,20,116). HIV/gp120 impairs proliferation of progenitors through activation of a pathway consisting of p38MAPK, MAPK-activated protein kinase 2 and Cdc25B/C which results in cell-cycle arrest in the G1 phase. It is not clear yet in how far this effect is reversible. Modified from (117).
8. PERSPECTIVE
While our understanding of HIV-1 disease and its associated neuropathology continues to grow and HAART has tremendously improved the treatment of HIV-1 infection in the periphery, an effective pharmacotherapy for HAD is still not available. However, recognizing that HIV-1 affects the brain not only through neurotoxicity but also by slowing potential homeostasis maintenance and repair mechanisms is an important step to identify and develop targets for future therapeutic approaches.
ACKNOWLEDGEMENTS
M.K. is supported by the National Institutes of Health, R01 NS050621.
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- HIV-1
human immunodeficiency virus-1
- HAD
HIV-associated dementia
- IL-1
interleukin-1
- MMPs
matrix metalloproteinases
- MCMD
minor cognitive-motor deficiency
- MAPK
mitogen-activated protein kinase
- NMDA
N-methyl-D-aspartate
- TNF
tumor necrosis factor
REFERENCES
- 1.Petito CK, Cho ES, Lemann W, Navia BA, Price RW. Neuropathology of acquired immunodeficiency syndrome (AIDS): an autopsy review. J Neuropathol. Exp. Neurol. 1986;45:635–646. doi: 10.1097/00005072-198611000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Glass JD, Wesselingh SL, Selnes OA, McArthur JC. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology. 1993;43:2230–2237. doi: 10.1212/wnl.43.11.2230. [DOI] [PubMed] [Google Scholar]
- 3.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 4.Power C, Gill MJ, Johnson RT. Progress in clinical neurosciences: the neuropathogenesis of HIV infection: host-virus interaction and the impact of therapy. Can. J Neurol Sci. 2002;29:19–32. doi: 10.1017/s0317167100001682. [DOI] [PubMed] [Google Scholar]
- 5.Gendelman HE, Grant I, Lipton SA, Everall I, Swindells S. The neurology of AIDS. 2nd ed. Oxford University Press; London: 2005. [Google Scholar]
- 6.Ellis RJ, Deutsch R, Heaton RK, Marcotte TD, McCutchan JA, Nelson JA, Abramson I, Thal LJ, Atkinson JH, Wallace MR, Grant I, San Diego HIV Neurobehavioral Research Center Group Neurocognitive impairment is an independent risk factor for death in HIV infection. Arch Neurol. 1997;54:416–424. doi: 10.1001/archneur.1997.00550160054016. [DOI] [PubMed] [Google Scholar]
- 7.McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Selnes OA, Jacobson LP. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- 8.Lavi E, Kolson DL, Ulrich AM, Fu L, Gonzalez-Scarano F. Chemokine receptors in the human brain and their relationship to HIV infection. J Neurovirol. 1998;4:301–311. doi: 10.3109/13550289809114531. [DOI] [PubMed] [Google Scholar]
- 9.Miller RJ, Meucci O. AIDS and the brain: Is there a chemokine connection? Trends Neurosci. 1999;22:471–479. doi: 10.1016/s0166-2236(99)01408-3. [DOI] [PubMed] [Google Scholar]
- 10.Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Minghetti L. Role of inflammation in neurodegenerative diseases. Curr. Opin. Neurol. 2005;18:315–321. doi: 10.1097/01.wco.0000169752.54191.97. [DOI] [PubMed] [Google Scholar]
- 12.Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat. Rev. Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 14.Jones G, Power C. Regulation of neural cell survival by HIV-1 infection. Neurobiol. Dis. 2006;21:1–17. doi: 10.1016/j.nbd.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J. Virol. 2004;78:7319–7328. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krathwohl MD, Kaiser JL. HIV-1 promotes quiescence in human neural progenitor cells. J Infect. Dis. 2004;190:216–226. doi: 10.1086/422008. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz L, Major EO. Neural progenitors and HIV-1-associated central nervous system disease in adults and children. Curr. HIV. Res. 2006;4:319–327. doi: 10.2174/157016206777709438. [DOI] [PubMed] [Google Scholar]
- 18.Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12:893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz L, Civitello L, Dunn-Pirio A, Ryschkewitsch S, Berry E, Cavert W, Kinzel N, Lawrence DM, Hazra R, Major EO. Evidence of human immunodeficiency virus type 1 infection of nestin-positive neural progenitors in archival pediatric brain tissue. J. Neurovirol. 2007;13:274–283. doi: 10.1080/13550280701344975. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto S, Kang Y, Brechtel C, Siviglia E, Russo R, Clemente A, Harrop A, McKercher S, Kaul M, Lipton SA. HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell cycle withdrawal and G1 arrest. Cell Stem Cell. 2007;1:230–236. doi: 10.1016/j.stem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 21.McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- 22.Turrin NP, Rivest S. Unraveling the molecular details involved in the intimate link between the immune and neuroendocrine systems. Exp. Biol Med (Maywood.) 2004;229:996–1006. doi: 10.1177/153537020422901003. [DOI] [PubMed] [Google Scholar]
- 23.Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci. 2005;25:1788–1796. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunningham PH, Smith DG, Satchell C, Cooper DA, Brew B. Evidence for independent development of resistance to HIV-1 reverse transcriptase inhibitors in the cerebrospinal fluid. AIDS. 2000;14:1949–1954. doi: 10.1097/00002030-200009080-00010. [DOI] [PubMed] [Google Scholar]
- 25.Cysique LA, Maruff P, Brew BJ. Variable benefit in neuropsychological function in HIV-infected HAART-treated patients. Neurology. 2006;66:1447–1450. doi: 10.1212/01.wnl.0000210477.63851.d3. [DOI] [PubMed] [Google Scholar]
- 26.Giancola ML, Lorenzini P, Balestra P, Larussa D, Baldini F, Corpolongo A, Narciso P, Bellagamba R, Tozzi V, Antinori A. Neuroactive antiretroviral drugs do not influence neurocognitive performance in less advanced HIV-infected patients responding to highly active antiretroviral therapy. J. Acquir. Immune. Defic. Syndr. 2006;41:332–337. doi: 10.1097/01.qai.0000197077.64021.07. [DOI] [PubMed] [Google Scholar]
- 27.Nath A, Sacktor N. Influence of highly active antiretroviral therapy on persistence of HIV in the central nervous system. Curr. Opin. Neurol. 2006;19:358–361. doi: 10.1097/01.wco.0000236614.51592.ca. [DOI] [PubMed] [Google Scholar]
- 28.Welch K, Morse A. The clinical profile of end-stage AIDS in the era of highly active antiretroviral therapy. AIDS Patient. Care STDS. 2002;16:75–81. doi: 10.1089/10872910252806126. [DOI] [PubMed] [Google Scholar]
- 29.Ho DD, Rota TR, Schooley RT, Kaplan JC, Allan JD, Groopman JE, Resnick L, Felsenstein D, Andrews CA, Hirsch MS. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. N Engl J Med. 1985;313:1493–1497. doi: 10.1056/NEJM198512123132401. [DOI] [PubMed] [Google Scholar]
- 30.Gartner S. HIV infection and dementia. Science. 2000;287:602–604. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- 31.Lipton SA. Treating AIDS dementia (letter; comment) Science. 1997;276:1629–30. doi: 10.1126/science.276.5319.1629b. [DOI] [PubMed] [Google Scholar]
- 32.Nottet HS, Persidsky Y, Sasseville VG, Nukuna AN, Bock P, Zhai QH, Sharer LR, McComb RD, Swindells S, Soderland C, Gendelman HE. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996;156:1284–95. [PubMed] [Google Scholar]
- 33.Asensio VC, Campbell IL. Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci. 1999;22:504–512. doi: 10.1016/s0166-2236(99)01453-8. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez E, Rovin BH, Sen L, Cooke G, Dhanda R, Mummidi S, Kulkarni H, Bamshad MJ, Telles V, Anderson SA, Walter EA, Stephan KT, Deucher M, Mangano A, Bologna R, Ahuja SS, Dolan MJ, Ahuja SK. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci U S A. 2002;99:13795–13800. doi: 10.1073/pnas.202357499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiala M, Looney DJ, Stins M, Way DD, Zhang L, Gan X, Chiappelli F, Schweitzer ES, Shapshak P, Weinand M, Graves MC, Witte M, Kim KS. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol Med. 1997;3:553–564. [PMC free article] [PubMed] [Google Scholar]
- 36.Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- 37.Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann. Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 38.Achim CL, Wang R, Miners DK, Wiley CA. Brain viral burden in HIV infection. Journal of Neuropathology and Experimental Neurology. 1994;53:284–294. doi: 10.1097/00005072-199405000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Wiley CA, Masliah E, Achim CL. Measurement of CNS HIV burden and its association with neurologic damage. Adv Neuroimmunol. 1994;4:319–325. doi: 10.1016/s0960-5428(06)80272-x. [DOI] [PubMed] [Google Scholar]
- 40.Wesselingh SL, Takahashi K, Glass JD, McArthur JC, Griffin JW, Griffin DE. Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV-infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry. J Neuroimmunol. 1997;74:1–8. doi: 10.1016/s0165-5728(96)00160-9. [DOI] [PubMed] [Google Scholar]
- 41.Heyes MP, Brew BJ, Martin A, Price RW, Salazar AM, Sidtis JJ, Yergey JA, Mouradian MM, Sadler AE, Keilp J, Rubinow D, Markey SP. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann Neurol. 1991;29:202–209. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- 42.Shiramizu B, Gartner S, Williams A, Shikuma C, Ratto-Kim S, Watters M, Aguon J, Valcour V. Circulating proviral HIV DNA and HIV-associated dementia. AIDS. 2005;19:45–52. doi: 10.1097/00002030-200501030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiramizu B, Ratto-Kim S, Sithinamsuwan P, Nidhinandana S, Thitivichianlert S, Watt G, Desouza M, Chuenchitra T, Sukwit S, Chitpatima S, Robertson K, Paul R, Shikuma C, Valcour V. HIV DNA and Dementia in Treatment-Naive HIV-1-Infected Individuals in Bangkok, Thailand. Int. J. Med. Sci. 2006;4:13–18. doi: 10.7150/ijms.4.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ketzler S, Weis S, Haug H, Budka H. Loss of neurons in the frontal cortex in AIDS brains. Acta Neuropathol. (Berl) 1990;80:92–94. doi: 10.1007/BF00294228. [DOI] [PubMed] [Google Scholar]
- 45.Everall IP, Luthert PJ, Lantos PL. Neuronal loss in the frontal cortex in HIV infection. Lancet. 1991;337:1119–21. doi: 10.1016/0140-6736(91)92786-2. [DOI] [PubMed] [Google Scholar]
- 46.Reyes MG, Faraldi F, Senseng CS, Flowers C, Fariello R. Nigral degeneration in acquired immune deficiency syndrome (AIDS) Acta Neuropathol. (Berl) 1991;82:39–44. doi: 10.1007/BF00310921. [DOI] [PubMed] [Google Scholar]
- 47.Graus F, Ribalta T, Abos J, Alom J, Cruz-Sanchez F, Mallolas J, Miro JM, Cardesa A, Tolosa E. Subacute cerebellar syndrome as the first manifestation of AIDS dementia complex. Acta Neurol Scand. 1990;81:118–120. doi: 10.1111/j.1600-0404.1990.tb00945.x. [DOI] [PubMed] [Google Scholar]
- 48.Everall I, Luthert P, Lantos P. A review of neuronal damage in human immunodeficiency virus infection: its assessment, possible mechanism and relationship to dementia. J Neuropathol. Exp. Neurol. 1993;52:561–566. doi: 10.1097/00005072-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Ho DD, Bredesen DE, Vinters HV, Daar ES. The acquired immunodeficiency syndrome (AIDS) dementia complex. Ann. Intern. Med. 1989;111:400–410. doi: 10.7326/0003-4819-111-5-400. [DOI] [PubMed] [Google Scholar]
- 50.Adle-Biassette H, Chretien F, Wingertsmann L, Hery C, Ereau T, Scaravilli F, Tardieu M, Gray F. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol. Appl. Neurobiol. 1999;25:123–133. doi: 10.1046/j.1365-2990.1999.00167.x. [DOI] [PubMed] [Google Scholar]
- 51.Petito CK, Roberts B. Evidence of apoptotic cell death in HIV encephalitis. Am. J Pathol. 1995;146:1121–1130. [PMC free article] [PubMed] [Google Scholar]
- 52.Rostasy K, Monti L, Yiannoutsos C, Wu J, Bell J, Hedreen J, Navia BA. NFkappaB activation, TNF-alpha expression, and apoptosis in the AIDS-Dementia-Complex. J Neurovirol. 2000;6:537–543. doi: 10.3109/13550280009091954. [DOI] [PubMed] [Google Scholar]
- 53.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol. Exp. Neurol. 2005;64:529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- 54.Langford TD, Letendre SL, Larrea GJ, Masliah E. Changing patterns in the neuropathogenesis of HIV during the HAART era. Brain Pathol. 2003;13:195–210. doi: 10.1111/j.1750-3639.2003.tb00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Everall IP, Hansen LA, Masliah E. The shifting patterns of HIV encephalitis neuropathology. Neurotox. Res. 2005;8:51–61. doi: 10.1007/BF03033819. [DOI] [PubMed] [Google Scholar]
- 56.Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19:407–411. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- 57.Locati M, Murphy PM. Chemokines and chemokine receptors: biology and clinical relevance in inflammation and AIDS. Annu. Rev. Med. 1999;50:425–440. doi: 10.1146/annurev.med.50.1.425. [DOI] [PubMed] [Google Scholar]
- 58.Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 59.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 60.Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat. Rev. Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- 61.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 62.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 63.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp. Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 65.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay CR, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 66.Michael NL, Moore JP. HIV-1 entry inhibitors: evading the issue (news) (see comments) Nat. Med. 1999;5:740–742. doi: 10.1038/10462. [DOI] [PubMed] [Google Scholar]
- 67.Ohagen A, Ghosh S, He J, Huang K, Chen Y, Yuan M, Osathanondh R, Gartner S, Shi B, Shaw G, Gabuzda D. Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. J Virol. 1999;73:897–906. doi: 10.1128/jvi.73.2.897-906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen W, Sulcove J, Frank I, Jaffer S, Ozdener H, Kolson DL. Development of a human neuronal cell model for human immunodeficiency virus (HIV)-infected macrophage-induced neurotoxicity: apoptosis induced by HIV type 1 primary isolates and evidence for involvement of the Bcl-2/Bcl-xL-sensitive intrinsic apoptosis pathway. J Virol. 2002;76:9407–9419. doi: 10.1128/JVI.76.18.9407-9419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gorry PR, Churchill M, Crowe SM, Cunningham AL, Gabuzda D. Pathogenesis of macrophage tropic HIV-1. Curr. HIV. Res. 2005;3:53–60. doi: 10.2174/1570162052772951. [DOI] [PubMed] [Google Scholar]
- 70.Li S, Juarez J, Alali M, Dwyer D, Collman R, Cunningham A, Naif HM. Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates. J Virol. 1999;73:9741–9755. doi: 10.1128/jvi.73.12.9741-9755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verani A, Lusso P. Chemokines as natural HIV antagonists. Curr. Mol. Med. 2002;2:691–702. doi: 10.2174/1566524023361862. [DOI] [PubMed] [Google Scholar]
- 72.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 73.Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr. Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- 74.Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meucci O, Fatatis A, Simen AA, Miller RJ. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci U S A. 2000;97:8075–8080. doi: 10.1073/pnas.090017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaul M, Lipton SA. Chemokines and activated macrophages in gp120-induced neuronal apoptosis. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA. HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection. Cell Death. Differ. 2007;14:296–305. doi: 10.1038/sj.cdd.4402006. [DOI] [PubMed] [Google Scholar]
- 78.Iskander S, Walsh KA, Hammond RR. Human CNS cultures exposed to HIV-1 gp120 reproduce dendritic injuries of HIV-1-associated dementia. J Neuroinflammation. 2004;1:7. doi: 10.1186/1742-2094-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walsh KA, Megyesi JF, Wilson JX, Crukley J, Laubach VE, Hammond RR. Antioxidant protection from HIV-1 gp120-induced neuroglial toxicity. J Neuroinflammation. 2004;1:8. doi: 10.1186/1742-2094-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garden GA, Guo W, Jayadev S, Tun C, Balcaitis S, Choi J, Montine TJ, Moller T, Morrison RS. HIV associated neurodegeneration requires p53 in neurons and microglia. FASEB J. 2004;18:1141–1143. doi: 10.1096/fj.04-1676fje. [DOI] [PubMed] [Google Scholar]
- 81.Brenneman DE, Westbrook GL, Fitzgerald SP, Ennist DL, Elkins KL, Ruff MR, Pert CB. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988;335:639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- 82.New DR, Ma M, Epstein LG, Nath A, Gelbard HA. Human immunodeficiency virus type 1 tat protein induces death by apoptosis in primary human neuron cultures. J Neurovirol. 1997;3:168–73. doi: 10.3109/13550289709015806. [DOI] [PubMed] [Google Scholar]
- 83.Adamson DC, Wildemann B, Sasaki M, Glass JD, McArthur JC, Christov VI, Dawson TM, Dawson VL. Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science. 1996;274:1917–1921. doi: 10.1126/science.274.5294.1917. [DOI] [PubMed] [Google Scholar]
- 84.Piller SC, Jans P, Gage PW, Jans DA. Extracellular HIV-1 virus protein R causes a large inward current and cell death in cultured hippocampal neurons: implications for AIDS pathology. Proc Natl Acad Sci U S A. 1998;95:4595–4600. doi: 10.1073/pnas.95.8.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koedel U, Kohleisen B, Sporer B, Lahrtz F, Ovod V, Fontana A, Erfle V, Pfister HW. HIV type 1 Nef protein is a viral factor for leukocyte recruitment into the central nervous system. J Immunol. 1999;163:1237–1245. [PubMed] [Google Scholar]
- 86.Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng YC, Gelbard HA, Shepard RB, Swartz JM, Gendelman HE. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- 87.Fontana G, Valenti L, Raiteri M. gp120 can revert antagonism at the glycine site of NMDA receptors mediating GABA release from cultured hippocampal neurons. J Neurosci. Res. 1997;49:732–738. doi: 10.1002/(SICI)1097-4547(19970915)49:6<732::AID-JNR7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 Tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- 89.Giulian D, Vaca K, Noonan CA. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- 90.Giulian D, Wendt E, Vaca K, Noonan CA. The envelope glycoprotein of human immunodeficiency virus type 1 stimulates release of neurotoxins from monocytes. Proc Natl Acad Sci U S A. 1993;90:2769–2773. doi: 10.1073/pnas.90.7.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Power C, McArthur JC, Nath A, Wehrly K, Mayne M, Nishio J, Langelier T, Johnson RT, Chesebro B. Neuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differs between demented and nondemented AIDS patients. J Virol. 1998;72:9045–9053. doi: 10.1128/jvi.72.11.9045-9053.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lipton SA, Sucher NJ, Kaiser PK, Dreyer EB. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991;7:111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- 93.Yeh MW, Kaul M, Zheng J, Nottet HS, Thylin M, Gendelman HE, Lipton SA. Cytokine-stimulated, but not HIV-infected, human monocyte-derived macrophages produce neurotoxic levels of L-cysteine. J. Immunol. 2000;164:4265–4270. doi: 10.4049/jimmunol.164.8.4265. [DOI] [PubMed] [Google Scholar]
- 94.Nicotera P, Ankarcrona M, Bonfoco E, Orrenius S, Lipton SA. Neuronal necrosis and apoptosis: two distinct events induced by exposure to glutamate or oxidative stress. Adv Neurol. 1997;72:95–101. [PubMed] [Google Scholar]
- 95.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog. Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 96.Dreyer EB, Kaiser PK, Offermann JT, Lipton SA. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- 97.Toggas SM, Masliah E, Mucke L. Prevention of HIV-1 gp120-induced neuronal damage in the central nervous system of transgenic mice by the NMDA receptor antagonist memantine. Brain Res. 1996;706:303–307. doi: 10.1016/0006-8993(95)01197-8. [DOI] [PubMed] [Google Scholar]
- 98.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders (see comments) N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 99.Doble A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol Ther. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- 100.Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 101.Tenneti L, D’Emilia DM, Troy CM, Lipton SA. Role of caspases in N-methyl-D-aspartate-induced apoptosis in cerebrocortical neurons. J Neurochem. 1998;71:946–959. doi: 10.1046/j.1471-4159.1998.71030946.x. [DOI] [PubMed] [Google Scholar]
- 102.Garden GA, Budd SL, Tsai E, Hanson L, Kaul M, D’Emilia DM, Friedlander RM, Yuan J, Masliah E, Lipton SA. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci. 2002;22:4015–4024. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jana A, Pahan K. Human immunodeficiency virus type 1 gp120 induces apoptosis in human primary neurons through redox-regulated activation of neutral sphingomyelinase. J Neurosci. 2004;24:9531–9540. doi: 10.1523/JNEUROSCI.3085-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Persidsky Y, Buttini M, Limoges J, Bock P, Gendelman HE. An analysis of HIV-1-associated inflammatory products in brain tissue of humans and SCID mice with HIV-1 encephalitis. J Neurovirol. 1997;3:401–416. doi: 10.3109/13550289709031186. [DOI] [PubMed] [Google Scholar]
- 105.Butler MP, O’Connor JJ, Moynagh PN. Dissection of tumor-necrosis factor-alpha inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early-but not late-phase LTP. Neuroscience. 2004;124:319–326. doi: 10.1016/j.neuroscience.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 106.Pickering M, Cumiskey D, O’Connor JJ. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp. Physiol. 2005;90:663–670. doi: 10.1113/expphysiol.2005.030734. [DOI] [PubMed] [Google Scholar]
- 107.Benveniste EN, Benos DJ. TNF-alpha- and IFN-gamma-mediated signal transduction pathways: effects on glial cell gene expression and function. FASEB J. 1995;9:1577–1584. doi: 10.1096/fasebj.9.15.8529837. [DOI] [PubMed] [Google Scholar]
- 108.Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat. Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 109.Tun C, Guo W, Nguyen H, Yun B, Libby RT, Morrison RS, Garden GA. Activation of the extrinsic caspase pathway in cultured cortical neurons requires p53-mediated down-regulation of the X-linked inhibitor of apoptosis protein to induce apoptosis. J. Neurochem. 2007;102:1206–1219. doi: 10.1111/j.1471-4159.2007.04609.x. [DOI] [PubMed] [Google Scholar]
- 110.Khan MZ, Shimizu S, Patel JP, Nelson A, Le MT, Mullen-Przeworski A, Brandimarti R, Fatatis A, Meucci O. Regulation of neuronal P53 activity by CXCR4. Mol. Cell Neurosci. 2005;30:58–66. doi: 10.1016/j.mcn.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Khan MZ, Brandimarti R, Patel JP, Huynh N, Wang J, Huang Z, Fatatis A, Meucci O. Apoptotic and antiapoptotic effects of CXCR4: is it a matter of intrinsic efficacy? Implications for HIV neuropathogenesis. AIDS Res. Hum. Retroviruses. 2004;20:1063–1071. doi: 10.1089/aid.2004.20.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khan MZ, Brandimarti R, Musser BJ, Resue DM, Fatatis A, Meucci O. The chemokine receptor CXCR4 regulates cell-cycle proteins in neurons. J Neurovirol. 2003;9:300–314. doi: 10.1080/13550280390201010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jordan-Sciutto KL, Wang G, Murphey-Corb M, Wiley CA. Cell cycle proteins exhibit altered expression patterns in lentiviral-associated encephalitis. J Neurosci. 2002;22:2185–2195. doi: 10.1523/JNEUROSCI.22-06-02185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–148. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- 115.Belmadani A, Tran PB, Ren D, Assimacopoulos S, Grove EA, Miller RJ. The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors. J Neurosci. 2005;25:3995–4003. doi: 10.1523/JNEUROSCI.4631-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Krathwohl MD, Kaiser JL. Chemokines promote quiescence and survival of human neural progenitor cells. Stem Cells. 2004;22:109–118. doi: 10.1634/stemcells.22-1-109. [DOI] [PubMed] [Google Scholar]
- 117.Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr. HIV Res. 2006;4:307–318. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]