Abstract
Introduction:
Measuring adherence to smoking cessation pharmacotherapy is important to evaluating its effectiveness. Blood levels are considered the most accurate measure of adherence but are invasive and costly. Pill counts and self-report are more practical, but little is known about their relationship to blood levels. This study compared the validity of pill count and self-report against plasma varenicline concentration for measuring pharmacotherapy adherence.
Methods:
Data were obtained from a randomized pilot study of varenicline for smoking cessation among African American smokers. Adherence was measured on Day 12 via plasma varenicline concentration, pill count, 3-day recall, and a visual analogue scale (VAS; adherence was represented on a line with two extremes “no pills” and “all pills”).
Results:
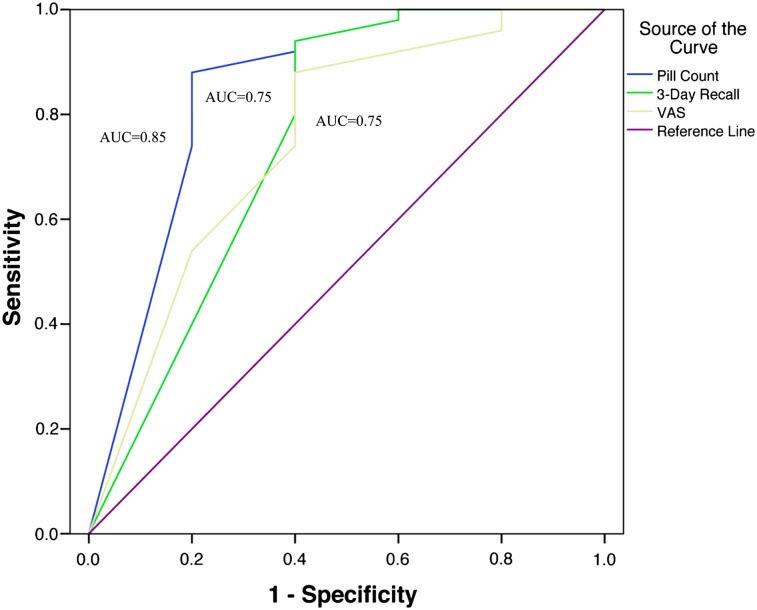
The sample consisted of 55 African American moderate to heavy smokers (average 16.8 cigarettes/day, SD = 5.6) and 63.6% were female. Significant correlations (p < .05) were found between plasma varenicline concentration and pill count (r = .56), 3-day recall (r = .46), and VAS (r = .29). Using plasma varenicline concentration of 2.0 ng/ml as the cutpoint for adherence, pill count demonstrated the largest area under the receiver operating characteristic curve (AUC = 0.85, p = .01) and had 88% sensitivity (95% CI = 75.0–95.0) and 80% specificity (95% CI = 30.0–99.0) for detecting adherence.
Conclusions:
Of 3 commonly used adherence measures, pill count was the most valid for identifying adherence in this sample of African American smokers. Pill count has been used across other health domains and could be incorporated into treatment to identify nonadherence, which, in turn, could maximize smoking cessation pharmacotherapy use and improve abstinence rates.
Introduction
One in five adults in the United States smokes cigarettes (Pleis, Ward, & Lucas, 2010). This rate remains high despite the fact that smoking greatly increases the risks of developing cancer, cardiovascular disorders, and pulmonary diseases and is responsible for an estimated 443,000 smoking-related deaths annually (Centers for Disease Control and Prevention [CDC], 2008). Although African Americans tend to be lighter smokers than Whites (CDC, 2008; Harris, Zang, Anderson & Wynder, 1993), African Americans are disproportionately affected by tobacco-related diseases as well as associated mortality rates (CDC, 2008; Harris et al., 1993; Fagan, Moolchan, Lawrence, Fernander, & Ponder, 2007). For example, the relative risk of tobacco-specific lung cancer is 43%–55% higher for African Americans than Whites (Haiman et al., 2006). Given the national health burden and health disparities associated with cigarette smoking, smoking cessation remains a public health priority.
African American ever-smokers are less likely to successfully quit than White ever-smokers (38% vs. 50%, respectively; Fu et al., 2008). Therefore, effective smoking cessation treatments for African American smokers are critical; however, few clinical trials have examined the efficacy of smoking cessation pharmacotherapy for this population, and African Americans are underrepresented in smoking cessation clinical trials (Webb, 2008). Seven pharmacologic agents, including nicotine replacement therapies, formulated as gum, patch, nasal spray, inhaler, and lozenge and two non-nicotine medications—bupropion and varenicline—have been approved by the U.S. Food and Drug Administration for treatment of tobacco dependence (Fiore et al., 2008). These pharmacotherapies roughly double or triple quit rates compared with placebo (Fiore et al., 2008). Despite the effectiveness of smoking cessation pharmacological agents, quit rates at 1 year are, on average, less than 30% (Eisenberg et al., 2008). Abstinence rates for African Americans enrolled in smoking cessation pharmacotherapy clinical trials are even lower (13%–21% at 26 weeks; Ahluwalia, Harris, Catley, Okuyemi, & Mayo, 2002; Ahluwalia et al., 2006; Cox et al., 2012).
Although the results of varenicline randomized clinical trials (RCTs) have been promising (Garrison & Dugan, 2009), varenicline has a range of adverse effects including nausea, headache, and insomnia (Garrison & Dugan, 2009; Jimenez-Ruiz, Berlin, & Hering, 2009) that may decrease adherence. Less than optimal adherence may limit the effectiveness of cessation medications; however, adherence to pharmacotherapy has received little attention in smoking cessation RCTs. In the limited varenicline studies conducted to date, greater adherence predicts higher quit rates (Hays, Leischow, Lawrence, & Lee, 2010). In a pooled analysis of two RCTs, the effect of pharmacotherapy at 12 weeks was greater for those who had higher levels of adherence relative to each active drug treatment group as a whole for varenicline (quit rates of 59% for participants with >80% adherence vs. 44% among those with <80% adherence) and bupropion (43% vs. 30%) (Hays et al., 2010). In the COMPASS smoking cessation intervention trial, good adherence to varenicline, defined as ≥80% of days taken, was associated with doubling in Month 6 quit rates (52% vs. 25%) compared with poor adherence (Catz et al., 2011). Similarly, in the parent trial to the current study, adherence was significantly associated with smoking abstinence such that participants who were quit at Month 3 had higher varenicline adherence rates than those who continued to smoke (Nollen et al., 2011). These differences in quit rates by adherence level suggest that adherence is an important factor in the effectiveness of smoking cessation pharmacotherapy.
Adherence to smoking cessation pharmacotherapy has been associated with higher abstinence rates at end of treatment (bupropion and nicotine patch, Killen et al., 2004; nicotine lozenge, Shiffman, 2007; nicotine patch, Shiffman, Sweeney, Ferguson, Sembower, & Gitchell, 2008) and 52 weeks (bupropion and nicotine patch, Killen et al., 2006). Killen et al. (2004) assessed pharmacotherapy adherence using self-reported patch use and urinalysis of bupropion metabolites; however, abstinence was associated with using more nicotine patches, and smoking reduction was associated with nicotine patch use and detectable levels of bupropion metabolites in this sample. Participants assigned to active nicotine patch and naltrexone who were adherent on purposeful nonadherence items (i.e., not intentionally stopping taking their medications) had higher abstinence rates at end of treatment than those who were nonadherent (61.36% vs. 49.12%, respectively; Toll, McKee, Martin, Jatlow, & O’Malley, 2007). Toll et al. also reported convergent validity for self-reported adherence with patch count, electronic drug exposure monitor cap data, and plasma naltrexone metabolite concentrations.
Interventions to improve medication adherence may result in greater treatment effectiveness. Two studies utilized the Medication Event Monitoring System (MEMS), an electronic pill bottle cap, and found that participants receiving weekly graphical feedback on their pill taking demonstrated greater adherence (Mooney, Sayre, Hokanson, Stotts, & Schmitz, 2007; Schmitz, Sayre, Stotts, Rothfleisch, & Mooney, 2005). Moreover, greater adherence to bupropion was associated with higher cessation rates (Mooney et al., 2007). These findings indicate that smoking cessation medication adherence may be a critical secondary intervention target and warrants further study.
Biological tests such as blood levels of medication metabolites are not subject to response bias or misreporting and, therefore, are generally deemed the most accurate measure of adherence (Vermeire, Hearnshaw, Van Royen, & Denekens, 2001). These tests reflect both compliance and rate of elimination of the drug and are more likely than other measures to represent medication actually taken by allowing researchers to directly determine the extent to which the medication is present in a participant’s blood stream. However, they are invasive and costly, may not be available for all medication types (Vermeire et al., 2001), may be influenced by individual differences in pharmacokinetics (Bosworth, 2006), and involve samples being collected within a relatively narrow window of time.
Self-report has been utilized as an indicator of adherence and is convenient and easy to administer (Bosworth, 2006). The self-report measures utilized in this study have been supported in HIV adherence research; higher medication adherence using 3-day recall and visual analogue scale (VAS) was associated with lower HIV viral load (Giordano, Guzman, Clark, Charlebois, & Blangsberg, 2004; Walsh, Madalia, & Gazzard, 2002). Several authors (Haynes, Ackloo, Sahota, McDonald, & Yao, 2008; McDonald, Garg, & Haynes, 2002) have recommended objective adherence measures such as pill count and MEMS because participants tend to overestimate their medication adherence via self-report. While these methods are more objective than self-report, they do not measure actual ingestion of the prescribed medications. Thus, each assessment method offers relative advantages accompanied by its respective limitations.
The paucity of research on adherence to smoking cessation pharmacotherapy may be partly attributed to a lack of consensus on the best methods for measuring adherence. Given the effectiveness of varenicline for smoking cessation and the importance of adherence to its effectiveness, this study compared the validity of three commonly used adherence measures, pill count, 3-day recall, and VAS, against plasma varenicline concentration for measuring medication adherence among African American smokers enrolled in a pilot trial of varenicline.
Methods
Participants
Data are from a randomized pilot study of varenicline in combination with adherence support or standard care counseling for smoking cessation among African American smokers (Nollen et al., 2011). All participants in this study received varenicline. Of 72 enrolled participants, 11 were lost to follow-up at Day 12, 5 did not have pill count data for the 3 days prior to Day 12, and 1 took twice the prescribed dose of varenicline, leaving a final sample of 55 participants in the current study. Day 12 was selected as the timepoint of interest because participants were titrated to the full dose beginning on Day 8 and were expected, based on a half-life of 24 hr, to have reached varenicline steady state by Day 12.
Procedures
Approval for the procedures was obtained from the University of Kansas Medical Center’s Human Subjects Committee. Participants were recruited from a community-based clinic that serves a predominantly African American population. Written informed consent was obtained from each participant during the first study visit.
Participant eligibility for the study was based on the following criteria: (a) self-identified as African American or Black, (b) at least 18 years of age, (c) smoked greater than 10 cigarettes/day (cpd) on at least 5 days/week for the past 30 days, (d) had home address and working telephone, (e) interested in quitting smoking, (f) willing to take varenicline for 12 weeks, and (g) willing to provide a blood sample and complete study visits. Participants were excluded if they had conditions that medically contraindicated the use of varenicline (e.g., cardiovascular event in the past month, pregnancy) or had received or were receiving other smoking cessation treatments.
A full description of the open-label trial is provided elsewhere (Nollen et al., 2011). In brief, all participants received a 1-month supply of varenicline and were randomized into a standard care condition or an adherence support condition. All participants received counseling at baseline to create a quit plan for Day 8. Participants initiated varenicline on Day 1 and were titrated to the full dose using the following schedule: 0.5 mg every day for 3 days, followed by 0.5 mg twice a day for 4 days, and then 1 mg twice a day for the remaining 7 weeks. The adherence support treatment group received two sessions of phone counseling and three in-person counseling sessions based on the Information–Motivation–Behavioral Skills Model of adherence behavior change (Fisher, Fisher, Amico, & Harman, 2006). All participants were given identical pamphlets on varenicline use as well as a culturally targeted smoking cessation guide for African Americans. Participants received $20 gift cards at the randomization visit and at Week 12 in appreciation of their time.
Measures
To address the potential range of literacy among participants, all self-report measures were read to the participants by a trained research assistant.
Adherence Self-report
The VAS and 3-day recall were administered at Day 12 to assess self-reported adherence to varenicline. The VAS is a single-item measure adapted from research assessing HIV antiretroviral adherence (Walsh et al., 2002). The VAS instructed respondents to place an X on a line between “no pills’ and “all pills” to show how many pills of the study medication had been taken since starting varenicline. Placement of the X corresponded with a predetermined adherence percentage (e.g., an X at 0 inches equaled 0% adherence, an X at 0.5 inches equaled 10% adherence, an X at 1.0 inches equaled 20% adherence, etc.). The 3-day recall adherence measure (Chesney et al., 2000) instructed respondents to indicate how many pills they took at three time periods (yesterday, 2 days ago, and 3 days ago). The responses for these three items were summed and calculated as a percentage of the pills prescribed. The VAS and 3-day recall are both reliable and validated measures of medication adherence (Amico et al., 2006; Chesney et al., 2000; Giordano et al., 2004; Lu et al., 2008).
Pill Count
Medication was dispensed in a 1-month pill box at randomization and at Months 1 and 2. In-person pill counts were completed at monthly medication visits by research staff. Using a standardized protocol adapted from Bangsberg, Hecht, Charlebois, Chesney, and Moss (2001), research staff opened and recorded the number of pills observed (i.e., 0, 1, or 2 pills) in each compartment of the pill box. For the primary analyses in this study, in-person pill count was counted for the 3 days prior to the Day 12 blood draw for varenicline analysis. Given that varenicline has a 24-hr half-life (Garrison & Dugan, 2009), this pill count timeframe was selected to capture adherence to varenicline over the same 3-day window detected by plasma varenicline concentration level analyses. Pill count adherence was scored as the percentage of pills taken divided by the number of pills prescribed. For example, given varenicline dosing of twice daily for 3 days, participants who took 6 out of 6 pills had 100% pill count, and this was considered perfect adherence.
Plasma Varenicline Concentration Levels
Varenicline levels were used as the reference standard because biological tests of medication metabolites are not subject to issues of response bias or misreporting and, therefore, are generally deemed the most accurate method for assessing medication adherence (Vermeire et al., 2001). Blood was collected from each participant on Day 12. Participants’ most recent dose of the study medication and the exact time and date of the blood collection were recorded. Each blood specimen (20 ml) was drawn into a tube containing ethylenediaminetetraacetic acid, immediately iced, and centrifuged at 4 °C to separate the plasma.
Concentrations of varenicline in plasma were determined using liquid chromatography–tandem mass spectrometry. The method used for these analyses is a modification of a published method for determination of varenicline in plasma and urine (Faessel et al., 2006) and is described in detail in the Supplementary Material. Using this procedure, the limit of quantitation was 0.1 ng/ml.
Baseline Measures
Participants reported their age, gender, education, marital status, and monthly household income. The metric height and weight of each participant was measured at the first visit in order to calculate body mass index (BMI). Participants reported average number of cigarettes smoked per day in the last 7 days, whether they smoked mentholated or non-mentholated cigarettes, and age when they started smoking regularly (California Department of Health and Human Services, 2002). Number of years smoking regularly was calculated from the difference between current age and age at which regular use began. Nicotine dependence was assessed using the single item of time to first cigarette after waking taken from the Fagerström Test for Nicotine Dependence (Baker et al., 2007; Heatherton, Kozlowski, Frecker, & Fagerström, 1991). Participants’ expired carbon monoxide levels were measured at baseline.
Analyses
Preliminary analyses using t tests found no significant differences between the adherence support (n = 30) and standard care (n = 25) groups (both received varenicline) on plasma varenicline concentration (means were 5.9 [SD = 3.3] and 6.5 [SD = 2.7], respectively, p = .45), pill count (83.3% [SD = 29.7] and 90.0% [SD = 22.0], p = .34), and self-reported adherence (90.0 [SD = 23.4] and 94.7 [SD = 10.4] for 3-day recall, p = .33; 90.8 [SD = 19.2] and 94.6 [SD = 9.4] for VAS, p = .35). Therefore, we collapsed across treatment group for the remainder of the analyses. Summary statistics were calculated for participant characteristics. Partial correlations were calculated to examine the relationship between varenicline plasma concentration, pill count, 3-day recall, and VAS. Gender and BMI have been shown to influence the rate of elimination of varenicline (Ravva, Gastonguay, Tensfeldt, & Faessel, 2009); therefore, we controlled for gender and BMI.
Sensitivity, specificity, and the area under the receiver operating characteristic curve (AUC) were calculated for pill count, 3-day recall, and VAS to assess the validity of these measures as compared with the reference standard, plasma varenicline concentration. A definitive cut point does not currently exist for determining adherence using plasma varenicline concentration. In order to determine the optimal plasma level cutpoint, we adopted an exploratory strategy utilized by Breslau and Johnson (2000) and conducted a series of receiver operating characteristic (ROC) analyses across the range of plasma varenicline concentrations observed in this study using the following cutpoints: 2.0, 4.0, 6.0, 8.0, 10.0, 12.0, and 14.0 ng/ml. Based on the AUC values for varenicline levels plotted against pill count adherence, 3-day recall, and VAS (see Table 3), 2.0 ng/ml was selected as the cutoff that would best distinguish between participants with high and low adherence and is the reference point used in the remainder of the analyses. We selected this cutpoint because it yielded the highest AUC values across the three measure of adherence. ROC curves are paired estimates of specificity and sensitivity across the range of all values on a measure for the condition of interest and are used to determine the accuracy and/or discriminatory power of a test (Zweig & Campbell, 1993). In this study, the AUC provided an estimate of the probability that a participant with a high rate of adherence as per the pill count, 3-day recall, and VAS would have higher plasma varenicline concentrations compared with a participant with a low rate of adherence on these measures. An AUC of 1.0 indicates perfect discrimination, while an AUC of 0.70 indicates good discrimination, and an AUC of 0.50 or less indicates poor discrimination.
Table 3.
Exploratory Analyses of the AUC for Pill Count, 3-Day Recall, and Visual Analogue Scale Using Various Cutpoints for Plasma Varenicline Concentrationa
| Plasma varenicline concentration cutpoints (ng/ml) | |||||||
| ≥2.0 | ≥4.0 | ≥6.0 | ≥8.0 | ≥10.0b | ≥12.0b | ≥14.0b | |
| AUC (p value) | |||||||
| Pill count | 0.85 (0.01) | 0.74 (0.006) | 0.73 (0.01) | 0.71 (0.86) | 0.67 (0.89) | 0.66 (0.92) | 0.66 (0.92) |
| 3-day recall | 0.75 (0.07) | 0.59 (0.07) | 0.66 (0.03) | 0.66 (0.89) | 0.63 (0.91) | 0.62 (0.93) | 0.62 (0.93) |
| Visual analogue scale | 0.75 (0.07) | 0.69 (0.12) | 0.60 (0.22) | 0.58 (0.23) | 0.68 (0.86) | 0.39 (0.87) | 0.39 (0.87) |
Note. AUC = area under the receiver operating characteristic curve.
Cut-points were selected based on the distribution of plasma varenicline concentrations in our sample (mean [SD] = 6.2 (3.0), range = 0.0–14.1)
Model fit unstable because of the small number of participants meeting this plasma level cutoff (≥10 ng/ml, n = 2; ≥12 ng/ml, n = 1; ≥14 ng/ml, n = 1).
Because of early reported adverse effects, two participants were not yet titrated to the standard 2 mg/day of varenicline on the 3 days prior to the blood draw. Plasma varenicline concentrations for these two participants were dose normalized to 2 mg/day for the analyses. Statistical analyses were conducted using SPSS version 18.
Results
Participant Characteristics
Baseline characteristics of the sample are reported in Table 1. The sample was predominantly female (63.6%) and average age was 46.6 (SD = 11.4) years. Participants smoked an average of 16.8 cpd, and the majority were nicotine dependent as indicated by smoking within 30 min of waking (89.1%).
Table 1.
Participant Baseline Characteristics
| No. (%) or mean (SD) | |
| Demographic variables | |
| Age in years, mean (SD) | 46.6 (11.4) |
| Female, N (%) | 35 (63.6) |
| Married/living with partner, N (%) | 20 (36.4) |
| Monthly income <$1,800, N (%) | 28 (50.9) |
| <High school graduate, N (%) | 10 (18.2) |
| Body mass index (kg/m2), mean (SD) | 32.5 (7.2) |
| Tobacco-related variables | |
| Cigarettes/day, mean (SD) | 16.8 (5.7) |
| Duration of smoking in years, mean (SD) | 28.6 (12.0) |
| Smoke menthol cigarettes, N (%) | 44 (80.0) |
| Exhaled carbon monoxide in ppm, mean (SD) | 39.0 (20.7) |
| Time to first cigarette, N (%) | |
| <5 min | 22 (40) |
| 6–30 min | 27 (49.1) |
| 31–60 min | 3 (5.5) |
| >60 min | 3 (5.5) |
Correlations Between Adherence Measures and Plasma Varenicline Concentrations
Summary statistics and the correlations between plasma varenicline concentration and adherence measures are displayed in Table 2. Adherence was high across all measures. Plasma varenicline concentrations ranged from 0.0 to 14.1 ng/ml, with 9.1% (5/55) having levels between 0.0 and 1.9.ng/ml and 90.9% (50/55) of participants having levels between 2.0 and 14.1 ng/ml. Adherence on the other measures was also high, ranging from a mean of 86.4% adherence for pill count to a mean of 92.5% for VAS.
Table 2.
Intercorrelations and Summary Statistics for Adherence Measures
| 3-day recall | VAS | Pill count | Mean (SD) | Minimum | Maximum | |
| 3-day recall | — | 92.1% (18.7) | 0% | 100% | ||
| VAS | 0.50** | — | 92.5% (15.5) | 0% | 100% | |
| 3-Day pill count | 0.73** | 0.47** | — | 86.4% (26.5) | 0% | 100% |
| Varenicline levels (ng/ml) | 0.46** | 0.29* | 0.56** | 6.2 (3.0) | 0 | 14.1 |
Note. VAS = visual analogue scale. Spearman correlations for intercorrelations among adherence self-report measures and pill count. Correlations with plasma varenicline concentration were calculated using partial correlations controlling for body mass index and gender. N = 55.
*p < .05; **p < .01.
Significant positive associations were found between the three adherence measures and plasma varenicline concentration, with the strength of association ranging from r = .29 for VAS to r = .56 for pill count (p < .05). Pill count had the strongest relationship with plasma varenicline concentration, accounting for 31.4% of the variance in the concentration of varenicline detected in participants’ plasma after controlling for the effects of gender and BMI.
Validity of the Adherence Measures Compared With Plasma Varenicline Concentrations
Using a plasma varenicline concentration cutoff of 2.0 ng/ml as determined through the exploratory analyses shown in Table 3, pill count had the largest AUC (AUC = .85, p = .01) compared with an AUC of 0.75 (p = .07) and 0.75 (p = .07) for 3-day recall and VAS, respectively. Sensitivity, specificity, and the AUC for pill count, 3-day recall, and VAS as compared with the reference standard, plasma varenicline concentrations are displayed in Figure 1 and Table 4. Examination of the coordinates of the ROC curve provided an estimate of the optimal adherence rate cut point for maximizing the detection of adherence by each of the three measures compared with the reference standard, plasma concentration. The optimal cutpoint for both pill count and 3-day recall was 75% adherence—that is, taking 75% or more of the prescribed dose—whereas the optimal cutpoint for VAS was 87.5%. Using these adherence cut points, sensitivity was comparable across measures (see Table 4); however, pill count specificity (80% specificity, 95% CI = 30.0–99.0) was markedly greater than that of 3-day recall and VAS (60% specificity, 95% CI = 17.0–93.0 for both measures). The probability that adherence—plasma varenicline levels greater than or equal to 2.0 ng/ml—was correctly identified across measures (i.e., positive predictive value) was high and ranged from 0.96 to 0.98, whereas the probability that pill count or self-report indicating nonadherence—plasma varenicline levels less than 2.0 ng/ml—was accurate (i.e., negative predictive value) was lower, ranging from 0.33 to 0.55.
Figure 1.
Area under the receiver operating characteristic curve for pill count, 3-day recall, and VAS.
Table 4.
Optimum Adherence Cutpoints and Related Sensitivity, Specificity, PPV, and NPV of Pill Count, 3-Day Recall, and Visual Analogue Scale for Detecting Adherence When Compared With Plasma Varenicline Concentrationa
| Cutpoint, % | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV (95% CI) | NPV (95% CI) | |
| Pill count | 75.0 | 0.88 (0.75–0.95) | 0.80 (0.30–0.99) | 0.98 (0.87–1.0) | 0.4 (0.14–0.73) |
| 3-Day recall | 75.0 | 0.94 (0.82–0.98) | 0.60 (0.17–0.93) | 0.96 (0.85–0.99) | 0.50 (0.14–0.86) |
| Visual analogue scale | 87.5 | 0.88 (0.75–0.95) | 0.60 (0.17–0.93) | 0.96 (0.84–0.990 | 0.33 (0.09–0.69) |
Note. NPV = negative predictive values; PPV = positive predictive values.
Using the cutpoint of 2.0 ng/ml.
Discussion
In this study, we found significant concordance among the varenicline adherence self-report measures, pill count, and plasma varenicline concentration. Of the pill count and two commonly used self-report adherence measures (3-day recall and VAS), pill count had the strongest association with plasma varenicline concentration, the largest area under the ROC curve, and also demonstrated the best combined sensitivity and specificity. Specifically, among participants categorized as adherent by their plasma varenicline concentration, pill count correctly classified them 88% of the time (sensitivity). While 3-day recall and VAS also demonstrated high sensitivity, the specificity of pill count was greater. Specifically, for participants categorized as non adherent by their plasma varenicline concentration, pill count correctly classify them 80% of the time compared with only 60% of the time with 3-day recall and VAS (specificity). Across all measures, the probability that a result indicating adherence would meet the threshold of 2.0 ng/ml for varenicline plasma adherence was uniformly high (positive predictive value). However, given the high rate of adherence in our sample, the probability that participants categorized as nonadherent on pill count, 3-day recall, or VAS had varenicline plasma concentrations of less than 2.0 ng/ml was lower (ranging from 33% for VAS to 50% for 3-day recall; negative predictive value).
Few studies have examined pill count as a method for assessing adherence to smoking cessation pharmacotherapy, although it has been used widely across other health domains and could be reasonably incorporated into tobacco dependence treatment (Bangsberg et al., 2001; Kalichman et al., 2007, 2008; van Onzenoort et al., 2009). The use of pill boxes in this study may have simplified and improved the accuracy of the pill count protocol. Specifically, trained research assistants opened each pill box compartment and recorded the number of pills remaining. They did not have to remove varenicline from pill bottles, sort, and count the medication, an approach that may be prone to lost pills and counting errors. Pill boxes may have an added advantage of facilitating adherence, with one study finding that pill box organizers improved patient adherence by 4.1%–4.5% compared with no pill box organizers (Petersen et al., 2007) and could have contributed to the high rates of adherence seen in this study, although future research is needed to confirm this speculation.
This is one of the first smoking cessation clinical studies to collect and report plasma varenicline concentrations. The mean and range of plasma varenicline concentrations in this study were similar to values reported by Ravva et al. (2009) for adults taking a 1 mg twice daily dose. Ravva et al. reported varenicline levels averaging 7.7 ng/ml and predicted population 95% CIs of 2.5–15 ng/ml. In addition, the novel analytical method used in the present study for measurement of varenicline (see Supplementary Material) required a smaller sample volume (0.5 ml compared with 1 ml) and achieved the same lower limit of quantitation (0.1 ng/ml) as published procedures (Faessel et al., 2006). Measurement of plasma varenicline concentration allows researchers to directly determine the extent to which the medication is present in a participant’s blood stream. Plasma varenicline concentration reflects both compliance and rate of elimination of the drug. As research supporting varenicline’s efficacy as a smoking cessation agent continues to build, more studies like this one are needed to quantify plasma levels and improve its clinical utility.
Strengths of this study include the use of plasma varenicline concentration and examination of the concordance of multiple adherence measures. Additionally, we provide validity evidence for measures of smoking cessation pharmacotherapy with a sample of African American smokers. More study is needed to explain lower cessation rates among African American smokers, and researchers should explore the role of menthol in relation to pharmacotherapy adherence as well as genetic factors related to varenicline elimination. An important limitation is that we did not assess renal function, a factor that has been shown to influence varenicline levels (Ravva et al., 2009). Variability in renal function in this sample could impact the magnitude of relationship found between the adherence measures and plasma varenicline concentration; therefore, future research is needed to confirm our findings while adjusting for renal function. A second limitation is the small sample size that was comprised of mostly African American women. Finally, the varenicline cutpoint used as the reference point for the pill count, 3-day recall, and VAS ROC analyses was selected based on analysis of the study data. Therefore, this cutpoint may differ for other samples. While this study provides preliminary evidence of the accuracy of pill count for assessing smoking cessation pharmacotherapy adherence, findings should be replicated in a larger and more diverse sample. Additionally, the association between varenicline adherence self-report, pill count, and plasma varenicline concentrations and smoking abstinence should be examined in a larger clinical trial. This study compared pill count, self-report, and plasma varenicline concentrations at Day 12—the earliest timepoint that participants were titrated to the full dose and varenicline steady state was reached. Over the duration of a clinical trial or in practice settings, we anticipate that assessing adherence at earlier timepoints will be particularly important as this is predictive of later smoking abstinence (Fish et al., 2009; Leischow, Ranger-Moore, Muramoto, & Matthews, 2004; Nollen et al., 2011; Toll et al., 2007).
To conclude, in addition to evaluation of the effectiveness of pharmacological treatments for smoking cessation, researchers should assess the extent to which participants utilize the smoking cessations agents as prescribed. Of three commonly used noninvasive adherence measures, pill count was the most accurate for identifying adherence in this sample of African American smokers. Pill count has been widely used across other health domains and could be reasonably incorporated into research and/or clinical practice as a way to identify and target non-adherence early in treatment, thereby maximizing smoking cessation pharmacotherapy use and improving abstinence rates.
Supplementary Material
Supplementary Material can be found online at http://www.ntr.oxfordjournals.org
Funding
This work was funded by the University of Kansas Cancer Center and Pfizer Global Pharmaceuticals. Pfizer Global Pharmaceuticals provided study medication but played no role in the design, conduct of the study, or interpretation and analysis of the data. Development and performance of the varenicline plasma analyses were supported in part by grant P30 DA012393 from the National Institute on Drug Abuse. J.S.A. is supported in part by 1P60MD003422 from the National Institute for Minority Health and Health Disparities at the National Institutes of Health.
Declaration of Interests
J.S.A. and N.L.B. serve as paid consultants to Pfizer Inc. which markets varenicline. N.L.B. also is a paid consultant for other pharmaceutical companies that are developing or market smoking cessation medications and has served as a paid expert witness in litigation against tobacco companies.
Supplementary Material
Acknowledgments
The authors would like to thank Swope Health Central, the recruitment site for the study, as well as the volunteers who participated in this research.
References
- Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained-release bupropion for smoking cessation in African Americans. Journal of the American Medical Association. 2002;288:468–474. doi: 10.1001/jama.288.4.468. doi:10.1001/jama.288.4.468. [DOI] [PubMed] [Google Scholar]
- Ahluwalia JS, Okuyemi K, Nollen N, Choi WS, Kaur H, Pulvers K, et al. The effects of nicotine gum and counseling among African American light smokers: A 2 × 2 factorial design. Addiction. 2006;101:883–891. doi: 10.1111/j.1360-0443.2006.01461.x. doi:10.1111/j.1360-0443.2006.01461.x. [DOI] [PubMed] [Google Scholar]
- Amico KR, Fisher WA, Cornman DH, Shuper PA, Redding CG, Konkle-Parker D.J.,, et al. Visual analog scale of ART adherence: Association with 3-day self-report and adherence barriers. Journal of Acquired Immune Deficiency Syndromes. 2006;42:455–459. doi: 10.1097/01.qai.0000225020.73760.c2. doi:10.1097/01.qai.0000225020.73760.c2. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith, Su-Young K, et al. Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine & Tobacco Research. 2007;9(Suppl. 4):S555–S570. doi: 10.1080/14622200701673480. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsberg DR, Hecht FM, Charlebois ED, Chesney M, Moss A. Comparing objective measures of adherence to HIV antiretroviral therapy: Electronic medication monitors and unannounced pill counts. AIDS and Behavior. 2001;5:275–281. doi:http://dx.doi.org/10.1023/A:1011396711486. [Google Scholar]
- Bosworth HB. Medication treatment adherence. In: Bosworth HB, Oddone EZ, Weinberger M, editors. Patient treatment adherence: Concepts, interventions, and measurement. New York, NY: Routledge; 2006. pp. 147–194. [Google Scholar]
- Breslau N, Johnson EO. Predicting smoking cessation and major depression in nicotine-dependent smokers. American Journal of Public Health. 2000;90:1122–1127. doi: 10.2105/ajph.90.7.1122. doi:10.2105/AJPH.90.7.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Department of Health and Human Services, Tobacco Control Section. California tobacco survey: 1999. La Jolla, CA: Cancer Prevention and Control Unit; 2002. [Google Scholar]
- Catz SL, Jack LM, McClure JB, Javitz HS, Deprey M, Zbikowski SM, et al. Adherence to varenicline in the COMPASS smoking cessation intervention trial. Nicotine & Tobacco Research. 2011;1:361–368. doi: 10.1093/ntr/ntr003. doi: 10.1093/ntr/ntr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses—2000–2004. Morbidity and Mortality Weekly Report. 2008;57:1226–1228. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5745a3.htm. [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. Patient care committee & adherence working group of the outcomes committee of the adult AIDS clinical trials group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. doi:10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Cox LS, Nollen NL, Mayo MS, Choi WS, Faseru B, Benowitz NL, et al. Bupropion for smoking cessation in African American light smokers: A randomized controlled trial. Journal of the National Cancer Institute. 2012;104:1–9. doi: 10.1093/jnci/djr513. doi: 10.1093/jnci/djr513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg MJ, Filion KB, Yavin D, Belisle P, Mottillo S, Joseph L, et al. Pharmacotherapies for smoking cessation: A meta-analysis of randomized controlled trials. Canadian Medical Association Journal. 2008;179:135–144. doi: 10.1503/cmaj.070256. doi:10.1503/cmaj.070256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan P, Moolchan ET, Lawrence D, Fernander A, Ponder PK. Identifying health disparities across the tobacco continuum. Addiction. 2007;102(Suppl. 2):5–29. doi: 10.1111/j.1360-0443.2007.01952.x. doi:10.1111/j.1360-0443.2007.01952.x. [DOI] [PubMed] [Google Scholar]
- Faessel HM, Smith BJ, Gibbs MA, Gobey JS, Clark DJ, Burstein AH. Single-dose pharmacokinetics of varenicline, a selective nicotinic receptor partial agonist, in healthy smokers and nonsmokers. Journal of Clinical Pharmacology. 2006;46:991–998. doi: 10.1177/0091270006290669. doi:10.1177/0091270006290669. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry S. J.,, et al. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- Fish LJ, Peterson BL, Namenek Brouwer RJ, Lyna P, Oncken CA, Swamy GK, et al. Adherence to nicotine replacement therapy among pregnant smokers. Nicotine & Tobacco Research. 2009;11:514–518. doi: 10.1093/ntr/ntp032. doi: 10.1093/ntr/ntp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychology. 2006;25:462–473. doi: 10.1037/0278-6133.25.4.462. doi:10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- Fu SS, Burgess DJ, Hatsukami DK, Noorbaloochi S, Clothier BA, Nugent S, et al. Race and nicotine replacement treatment outcomes among low-income smokers. American Journal of Preventive Medicine. 2008;35(Suppl. 6):S442–S448. doi: 10.1016/j.amepre.2008.09.009. doi:10.1016/j.amepre.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Garrison GD, Dugan SE. Varenicline: A first-line treatment option for smoking cessation. Clinical Therapeutics. 2009;31:463–491. doi: 10.1016/j.clinthera.2009.03.021. doi:10.1016/j.clinthera.2009.03.021. [DOI] [PubMed] [Google Scholar]
- Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clinical Trials. 2004;5:74–79. doi: 10.1310/JFXH-G3X2-EYM6-D6UG. doi:10.1310/JFXH-G3X2-EYM6-D6UG. [DOI] [PubMed] [Google Scholar]
- Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. New England Journal of Medicine. 2006;354:333–342. doi: 10.1056/NEJMoa033250. doi:10.1056/NEJMoa33250. [DOI] [PubMed] [Google Scholar]
- Harris R, Zang E, Anderson J, Wynder E. Race and sex differences in lung cancer risk associated with cigarette smoking. International Journal of Epidemiology. 1993;22:592–599. doi: 10.1093/ije/22.4.592. doi:10.1093/ije/22.4.592. [DOI] [PubMed] [Google Scholar]
- Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews. 2008 doi: 10.1002/14651858.CD000011.pub3. (2), CD000011. doi:10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- Hays JT, Leischow SJ, Lawrence D, Lee TC. Adherence to treatment for tobacco dependence: Association with smoking abstinence and predictors of adherence. Nicotine & Tobacco Research. 2010;12:574–581. doi: 10.1093/ntr/ntq047. doi:10.1093/ntr/ntq047. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström tolerance questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. doi:10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Jimenez-Ruiz C, Berlin I, Hering T. Varenicline: A novel pharmacotherapy for smoking cessation. Drugs. 2009;69:1319–1338. doi: 10.2165/00003495-200969100-00003. doi:10.2165/00003495-200969100-00003. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Amaral CM, Cherry C, Flanagan J, Pope H, Eaton L, et al. Monitoring medication adherence by unannounced pill counts conducted by telephone: Reliability and criterion-related validity. HIV Clinical Trials. 2008;9:298–308. doi: 10.1310/hct0905-298. doi: 10.1310/hct0905-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Amaral CM, Stearns H, White D, Flanagan J, Pope H, et al. Adherence to antiretroviral therapy assessed by unannounced pill counts conducted by telephone. Journal of General Internal Medicine. 2007;22:1003–1006. doi: 10.1007/s11606-007-0171-y. doi:10.1007/s11606-007-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Murphy GM, Hayward C, Arredondo C, Cromp D, et al. Extended treatment with bupropion SR for cigarette smoking cessation. Journal of Consulting and Clinical Psychology. 2006;74:286–294. doi: 10.1037/0022-006X.74.2.286. doi:10.1037/0022-006X.74.2.286. [DOI] [PubMed] [Google Scholar]
- Killen JD, Robinson TN, Ammerman S, Hayward C, Rogers J, Stone C, et al. Randomized clinical trial of the efficacy of bupropion combined with nicotine patch in the treatment of adolescent smokers. Journal of Consulting and Clinical Psychology. 2004;72:729–735. doi: 10.1037/0022-006X.72.4.729. doi: 10.1037/0022-006X.72.4.729. [DOI] [PubMed] [Google Scholar]
- Leischow SJ, Ranger-Moore J, Muramoto ML, Matthews E. Effectiveness of the nicotine inhaler for smoking cessation in an OTC setting. American Journal of Health Behavior. 2004;28:291–301. doi: 10.5993/ajhb.28.4.1. doi:10.5993/AJHB.28.4.1. [DOI] [PubMed] [Google Scholar]
- Lu M, Safren SA, Skolnik PR, Rogers WH, Coady W, Hardy H, et al. Optimal recall period and response task for self-reported HIV medication adherence. AIDS and Behavior. 2008;12:86–94. doi: 10.1007/s10461-007-9261-4. doi:10.1007/s10461-007-9261-4. [DOI] [PubMed] [Google Scholar]
- McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: Scientific review. Journal of the American Medical Association. 2002;288:2868–2879. doi: 10.1001/jama.288.22.2868. doi:10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Sayre SL, Hokanson PS, Stotts AL, Schmitz JM. Adding MEMS feedback to behavioral smoking cessation therapy increases compliance with bupropion: A replication and extension study. Addictive Behaviors. 2007;32:875–880. doi: 10.1016/j.addbeh.2006.06.022. doi:10.1016/j.addbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Nollen NL, Cox LS, Nazir N, Ellerbeck EF, Owen A, Pankey S, et al. A pilot clinical trial of varenicline for smoking cessation in Black smokers. Nicotine & Tobacco Research. 2011;13:868–873. doi: 10.1093/ntr/ntr063. doi:10.1093/ntr.ntr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen ML, Wang Y, van der Laan MJ, Guzman D, Riley E, Bangsberg DR. Pillbox organizers are associated with improved adherence to HIV antiretroviral therapy and viral suppression: A marginal structural model analysis. Clinical Infectious Diseases. 2007;45:908–915. doi: 10.1086/521250. doi: 10.1086/521250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleis JR, Ward BW, Lucas JW. Summary health statistics for U.S. adults: National health interview survey, 2009. Vital Health Statistics. 2010;10:1–259. Retrieved from http://www.cdc.gov/nchs/data/series/sr_10/sr10_249.pdf. [PubMed] [Google Scholar]
- Ravva P, Gastonguay MR, Tensfeldt TG, Faessel HM. Population pharmacokinetic analysis of varenicline in adult smokers. British Journal of Clinical Pharmacology. 2009;68:669–681. doi: 10.1111/j.1365-2125.2009.03520.x. doi:10.1111/j.1365-2125.2009.03520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Sayre SL, Stotts AL, Rothfleisch J, Mooney ME. Medication compliance during a smoking cessation clinical trial: A brief intervention using MEMS feedback. Journal of Behavioral Medicine. 2005;28:139–147. doi: 10.1007/s10865-005-3663-4. doi:10.1007/s10865-005-3663-4. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Use of more nicotine lozenges leads to better success in quitting smoking. Addiction. 2007;102:809–814. doi: 10.1111/j.1360-0443.2007.01791.x. doi:10.111/j.1360-0443.2007.01791.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: Secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clinical Therapeutics. 2008;30:1852–1858. doi: 10.1016/j.clinthera.2008.09.016. doi:10.1016/j.clinthera.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Toll BA, McKee SA, Martin DJ, Jatlow P, O’Malley SS. Factor structure and validity of the Medication Adherence Questionnaire (MAQ) with cigarette smokers trying to quit. Nicotine & Tobacco Research. 2007;9:597–605. doi: 10.1080/14622200701239662. doi:777521519 [pii] 10.1080/14622200701239662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Onzenoort HAW, Verberk WJ, Kessels AGH, Kroon AA, Neef C, van der Kuy PH, et al. Assessing medication adherence simultaneously by electronic monitoring and pill count in patients with mild-to-moderate hypertension. American Journal of Hypertension. 2009;23:149–154. doi: 10.1038/ajh.2009.207. doi:10.1038/ajh.2009.207. [DOI] [PubMed] [Google Scholar]
- Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: Three decades of research. A comprehensive review. Journal of Clinical Pharmacy and Therapeutics. 2001;26:331–342. doi: 10.1046/j.1365-2710.2001.00363.x. doi:10.1046/j.1365-2710.2001.00363.x. [DOI] [PubMed] [Google Scholar]
- Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS (London, England) 2002;16:269–277. doi: 10.1097/00002030-200201250-00017. doi:10.1097/00002030-200201250-00017. [DOI] [PubMed] [Google Scholar]
- Webb MS. Treating tobacco dependence among African Americans: A meta-analytic review. Health Psychology. 2008;27(Suppl.):S271–S282. doi: 10.1037/0278-6133.27.3(suppl.).s271. doi:10.1037/0278-6133.27.3(Suppl.).S271. [DOI] [PubMed] [Google Scholar]
- Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clinical Chemistry. 1993;39:561–577. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.