Summary
C3H/HeJ mice have been reported to have relatively early onset of spike-wave discharges (SWD), and a defective AMPA receptor subunit Gria4 as the genetic cause. We investigated the time course of SWD development through serial EEG recordings in C3H/HeJ mice to better characterize this model. We found that at immature postnatal ages of 5–15 days, rare SWD-like events were observed at an average rate of 3 per hour, and with relatively broad spikes, irregular rhythm, slow frequency (5–6 Hz), and short duration (mean 1.75 s). This was followed by a transitional period of increasing SWD incidence, which then stabilized in mature animals at age 26–62 days, with SWD at an average rate of 45 per hour, narrower spike morphology, regular rhythm, higher frequency (7–8 Hz), and longer duration (mean 3.40 s). This sequence of maturational changes in SWD development suggests that effects of early intervention could be tested in C3H/HeJ mice over the course of a few weeks, rather than a few months as in rats, greatly facilitating future research on anti-epileptogenesis.
Keywords: Epilepsy, Electroencephalogram, Rodent, Absence seizure, Petit mal, Epileptogenesis
Introduction
Childhood absence epilepsy is characterized by brief lapses of consciousness and behavioral arrest, accompanied by bilateral 3 Hz spike-wave discharges observed on electroencephalogram (EEG) (Crunelli and Leresche, 2002; Blumenfeld, 2005a,b). Absence epilepsy is relatively common, affecting 10–15% of children with epilepsy, and can produce up to hundreds of seizures per day (Mirsky et al., 1986; Crunelli and Leresche, 2002). Childhood absence epilepsy is expressed in an age-dependent fashion, with seizures generally first emerging between the ages of 4 and 10 years (Hirsch et al., 2008). Most affected patients have their seizures spontaneously resolve in mid-adolescence, however, childhood absence epilepsy is not a benign disorder and a substantial number of patients suffer long-term psychosocial sequelae (Wirrell et al., 1997; Camfield and Camfield, 2002).
With recent advances in our understanding of the interplay between genetics and epilepsy, major efforts have been undertaken to achieve primary prevention of epilepsy (Pitkanen et al., 2007), as part of the recently proposed “Benchmarks for epilepsy research” (http://www.ninds.nih.gov/). An important step forward has been the finding that treatment of spike-wave discharges (SWD) early in development in rat models can suppress the later expression of SWD in adulthood even after medication was stopped (Dedeurwaerdere et al., 2005; Blumenfeld et al., 2008). However, substantial work remains to be done to further elucidate the molecular mechanisms of epileptogenesis, with the goal being total epilepsy prevention. Specifically, a detailed knowledge of the appropriate time course for interventions is needed to lay the groundwork for future studies. Although the development of SWD has been characterized in Wistar Albino Glaxo rats from Rijswijk (WAG/Rij) (Coenen and Van Luijtelaar, 1987) and in genetic absence epilepsy rats of Strasbourg (GAERS) (Vergnes et al., 1986), rat models are limited by their relative paucity of genetic characterization compared to mouse models. In addition, particularly in WAG/Rij rats, onset of SWD takes several months, which can make investigation of epileptogenesis a slow process.
Among mouse models for SWD, (Fletcher et al., 1996; Cox et al., 1997; Letts et al., 1998), the C3H/HeJ mouse (Frankel et al., 2005) has the advantage of relatively pure absence phenotype (brief episodes of SWD accompanied by behavioral immobility), without other kinds of seizures or neurological impairment such as cerebellar ataxia. A specific genetic defect, namely a mutation of the AMPA receptor subunit Gria4, was recently identified as the cause of SWD in C3H/HeJ mice (Beyer et al., 2008). In the interest of furthering progress in our understanding of epileptogenesis and its prevention, we sought to characterize the critical developmental period for SWD development in the C3H/HeJ mouse through serial EEG recordings.
Methods
Animals
All procedures were in full compliance with approved institutional animal care and use protocols. We used a total of 44 C3H/HeJ mice (strain #000659, The Jackson Laboratory, Bar Harbor, Maine, USA) for all experiments. Mice were housed according to institutional guidelines with free access to food and water on a 12 h light/dark cycle (lights on at 7:00 a.m.).
Surgery and recordings
Surgical implants and recordings were performed using methods similar to those we used previously (Klein et al., 2004; Blumenfeld et al., 2008). Briefly, under ketamine (30 mg/kg), xylazine (6 mg/kg), and acepromazine (1 mg/kg) anesthesia, we implanted tripolar electrodes (Part #MS333/3-A, Tripolar electrode uncut untwisted 0.005; Plastics One Inc., Roanoke, VA; Internal control #8LMS3333XXXE, pedestal height: 8 mm) using a stereotactic frame (David Kopf Instruments, Tujunga, CA) in mice ranging in age from 5 d to 60 d. To provide good electrical contact, the ends of the recording electrodes were prepared before wrapping around skull screws by scraping off the polyimide insulation and exposing stainless steel wire up to 10 mm from the tip, leaving insulation intact proximally as verified under the microscope. Level of anesthesia was monitored by respiration, heart rate, glabrous skin perfusion, and response to foot pinch. To anesthetize mice at 5–6 d old, mice were placed on cold packs (Phifer and Terry, 1986; Danneman and Mandrell, 1997), and physiology and level of anesthesia was monitored as described above. In all animals, small burr holes (using Micro Drill Steel Burrs, 2.3 mm shaft diameter, 44 mm overall length; Item #19008-09, Fine Science Tools (USA), Inc.) were made in the skull without disturbing the dura. In mice over ten days old electrodes were secured to the skull using stainless steel screws (Small Parts, Inc., Part #MX-000120-01B-10, binding screw, with shaft length = 1/16 in., size = 000, thread = 120). For mice less than 10 days of age electrodes were secured with a smaller screw (Small Parts, Inc., Part #MX-0000160-01FL-10, fillister screw, with shaft length = 1/16 in., size = 0000, thread = 160). EEG recording electrodes were placed at frontal cortex (AP +2.0, ML +2.0 mm from bregma in adult mice, AP +1.0 to +2.0, ML +1.0 to +2.0 from bregma in younger animals), and parietal cortex (AP −6.0, ML +2.0 mm from bregma in adult mice, AP −4.0 to −6.0, ML +1.0 to +2.0 from bregma in younger animals) and a ground electrode was placed in the midline over the cerebellum. Dental acrylic (Cat #1255710; Henry Schein Inc., Indianapolis, IN; Lang Jet Denture Repair Acylic) was used to fix the electrode pedestal in place. Pups that had not yet been weaned (pups were typically weaned at 21 days) were implanted and returned to the dam’s cages by implanting the whole litter at the same time, with care taken to fully clean blood and debris from the implantation site. Pups and dams were observed to resume normal feeding and nesting patterns. Mice were given a post-operative analgesic of carprofen (5 mg/kg subcutaneous immediately and in drinking water for 48 h after surgery) and given a recovery period of at least 24 h after surgery. EEG signals were recorded via commutator (Plastics One, Inc.) using a Grass CP 511 amplifier (Grass-Telefactor, Astro Med, Inc., West Warwick, RI). Band pass frequency filter settings were 1–300 Hz. Signals were digitized at a sampling rate of 1 kHz with an NI USB-6008 A/D converter and LabView 7.1 software (National Instruments, Austin, TX), and analyzed using Spike 2 (Cambridge Electronic Design, Cambridge, UK). Continuous EEG data were recorded from awake-behaving mice starting at 12:00 p.m. and usually concluding before 6:00 p.m. Most of the recordings were obtained from each mouse for 3 h per day. Pups that were not yet weaned were removed from their mother’s cages just before recording sessions and returned promptly after recording. EEG recording were obtained at the following time points: 5–7 d (n = 9), 10–15 d (n = 6), 20–22 d (n = 8), 23–25 d (n = 5), 26–30 d (n = 7), 31–40 d (n = 11), 41–50 d (n = 5), and 51–62 d (n = 9).
Analysis of EEG data
SWDs were defined as large-amplitude (>2× the background EEG peak-to-peak amplitude) rhythmic 5–8 Hz discharges with spike-wave morphology lasting >1.0 s. We were more liberal in defining spike-wave morphology for the purposes of this study than in prior criteria (Coenen and Van Luijtelaar, 1987) so that any precursors of spike-wave activity could be identified at an early age. Intervals containing artifact or slow wave sleep were excluded from the analysis. Start and end time for all SWDs were manually marked and the number of seizures, and seizure durations were then calculated. Percent time in SWD was determined as (sum of SWD interval durations/total usable recording time) × 100%.
Power spectral analysis was performed for mice age 5–7 d (n = 9), 20–22 d (n = 8), and 51–62 (n = 9) on all marked SWD intervals included in the above analysis. Power spectra were calculated using Spike2 software, with scripts provided by Cambridge Electronic Design (Cambridge, U.K.). Bin size for the fast Fourier transform was 1.024 s.
Statistical analyses were performed using SPSS 16.0 (SPSS, Inc., Chicago, IL). Inflection points for time-course data were calculated by fitting a third-order polynomial to the data in Excel and then determining the point of sign change for the second derivative of this polynomial.
Results
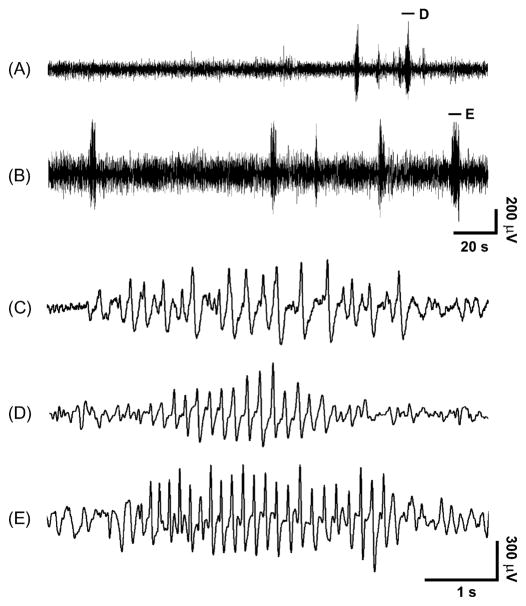
We found that SWD were very uncommon at age 5 through age 15 days, then gradually increased, and appeared to plateau after age 25 days. Examples of EEG recordings at different ages are shown in Fig. 1. At the youngest ages, the few SWD that occurred had a somewhat irregular morphology, and the spikes tended to have a relatively broad appearance (Fig. 1C). As the animals matured, SWD morphology became more regular in rhythm, and the spikes appeared more narrow (Fig. 1D and E). The shape, and different phases of the spikes and slow waves in C3H/HeJ mice were similar to those reported in other rodent models (Sitnikova and van Luijtelaar, 2007), with a large negative-going spike, and a relatively small negative slow wave in each cycle.
Figure 1.
Examples of EEG recordings showing SWD in C3H/HeJ mice at different ages. SWD were observed less often at age 23 d (A) than at age 59 d (B). Morphology of SWDs also changed in mice as they matured, with examples shown on more expanded time scale at age 5 d (C), 23 d (D), and 59 d (E). As mice increased in age, the spike-wave morphology became more regular, spikes became narrower, and SWD frequency increased. SWD shown in (D) and (E) are from intervals marked by horizontal lines in (A) and (B). The corresponding lower time resolution trace for (C) is not shown. EEG was recorded in a bipolar montage (frontal minus parietal) with negative voltages displayed as upgoing.
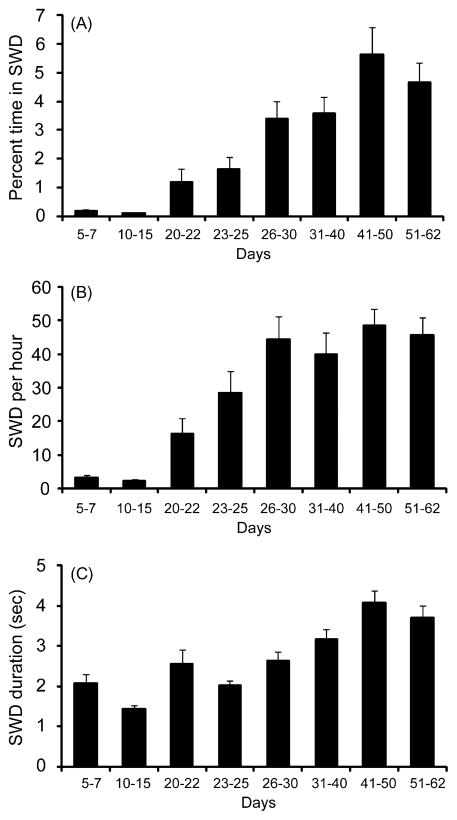
To investigate the progression of EEG changes during development, we analyzed repeated EEG samples at different ages ranging from 5 d through 62 d (Fig. 2). We found that the percentage of time in SWD and the number of SWD per hour were very low below age 15 d, but then progressively increased, and appeared to level off after age 26–30 d (Fig. 2A and B). Percent time in SWD increased from a mean of 0.1% in immature animals (age 5–15 d) to 4% in adulthood (ages 26–62 d) (p = 0.006, two-tailed t-test), with an inflection point at 33.3 days. Comparing percent time in SWD for all 8 groups (Fig. 2A) by ANOVA (F = 15.82, p < 0.001) with post hoc Bonferroni correction yielded significant differences between all individual groups below 15 d and above 26 d (p < 0.05). Similarly, the mean SWD per hour increased from 3 per hour in immature mice (5–15 d) to 45 per hour in mature mice (26–62 d) (p = 0.0001), with an inflection point at 22.4 days. ANOVA (F = 18.59, p < .001) again yielded significant differences between all individual groups below 15 d and above 26 d (p < 0.05, Bonferroni corrected). Seizure duration also showed a progressive, though less dramatic increase during development, with a mean seizure duration of 1.75 s at 5–15 d, and 3.40 s at 26–62 d (p = 0.03) (Fig. 2C); inflection point at 29.3 days. ANOVA (F = 14.00, p < .001) once again yielded significant differences between all groups ≤15 d and ≥26 d (p < 0.05, Bonferroni corrected).
Figure 2.
Quantification of seizure development in C3H/HeJ mice. Very few SWD were seen in 5–15 d animals. SWD then progressively increased and appeared to level off above about 26 d. (A) Percent time spent in SWD (=100 × time in SWD/total recording time). (B) Number of SWD per hour. (C) SWD duration. Number of C3H/HeJ mice recorded at each time point were as follows: 5–7 d (n = 9), 10–15 d (n = 6), 20–22 d (n = 8), 23–25 d (n = 5), 26–30 d (n = 7), 31–40 d (n = 11), 41–50 d (n = 5), and 51–62 d (n = 9). Values are mean ± SEM.
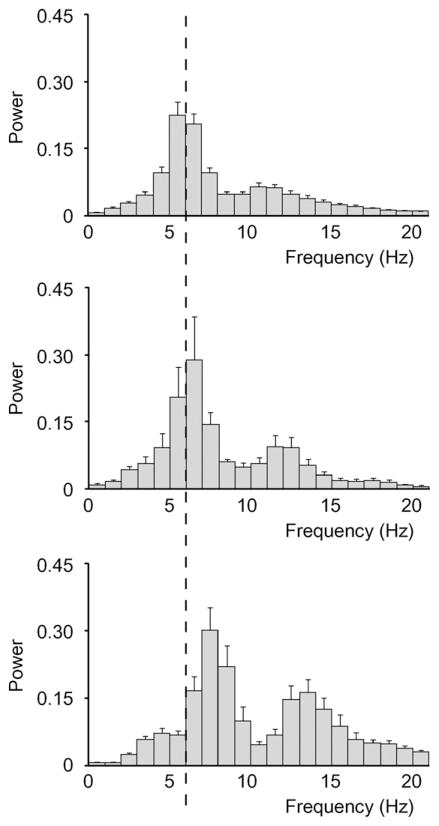
As was already mentioned, seizure morphology also changed during development, with older animals exhibiting SWD that had a higher frequency, more regular rhythm, and narrower spikes (Fig. 1C and D). To quantify these changes, we performed power spectral analysis of all SWD at three time points corresponding to immature (5–7 d), transitional (20–22 d), and mature (51–62 d) stages of SWD development observed above (Figs. 1 and 2). Based on this analysis, we found that the peak SWD frequency shifted from 5 to 6 Hz in mice age 5–7 d (Fig. 3A), to 6–7 Hz in mice age 20–22 d (Fig. 3B), and finally to 7–8 Hz in mice age 51–62 d (Fig. 3C). We also observed a second, smaller harmonic peak in the power spectrum, which increased progressively with age from 10 to 11 Hz (5–7 d), to 11–12 Hz (20–22 d), and finally to 13–14 Hz (51–62 d) (Fig. 3). In contrast to the changes in SWD peak frequencies, we did not detect a change in SWD peak amplitude between the three groups (F = 1.00, p = 0.40). These results confirmed the observations based on visual read (Fig. 1) that SWD shift towards higher frequencies as C3H/HeJ mice reach maturity.
Figure 3.
Power spectral analysis showed an increase in SWD frequency as mice increase in age. (A) Power spectrum of mice 5–7 d shows a major peak at 5–6 Hz, with a minor harmonic peak at 10–11 Hz (n = 9 mice, 146 SWD). (B) Power spectrum of mice 20–22 d shows a major peak at 6–7 Hz, with a minor harmonic peak at 11–12 Hz (n = 8 mice, 292 SWD). (C) Power spectrum of mice 51–62 d shows a major peak at 7–8 Hz, with a minor harmonic peak at 13–14 Hz (n = 9 mice, 823 SWD). Vertical dashed line indicates 6 Hz for ease of comparison between spectra. Values are mean ± SEM.
Discussion
We observed the emergence and development of SWD in C3H/HeJ mice, with the most rapid changes occurring over a time period from approximately 15–25 days of age. This developmental pattern was most apparent in the number of SWD per hour (Fig. 2B). The total percent time in SWD also was very low before day 15 and subsequently increased rapidly, but the plateau after day 26 was not as apparent, possibly because SWD duration showed a trend towards continued slight increases after day 26 (Fig. 2A and C).
Based on our findings we propose that SWD development in this model can be described in three stages. In the “immature stage,” up to age 15 days, SWD-like events, possibly larval SWD or a related developmental phenomenon, were observed on average only 3 times per hour. These events may indicate an early predisposition to later development of fully realized SWD in C3H/Hej mice, or may represent other related rhythmic brain activity. SWD-like event morphology in the immature stage was characterized by irregular rhythm, relatively broad spikes, slow fundamental frequency (5–6 Hz), and brief duration (mean 1.75 s). In the “transitional stage” from 16 through 25 days, SWD incidence, duration, and fundamental frequency gradually increased. In the “mature stage,” after approximately day 26 (or perhaps somewhat later with regard to SWD duration) SWD were observed an average of 45 times per hour. Mature SWD had a more regular rhythm, narrower spikes, higher fundamental frequency (7–8 Hz), and longer duration (mean 3.4 s).
While EEG recordings from implanted skull electrodes allowed for good characterization of SWD events in young and adult mice, more refined electrophysiology techniques would better localize regional brain involvement during SWD in this mouse model. One limitation of our study then is the lack of microelectrode recordings from specific cortical and subcortical brain areas during SWD. Future investigations will focus on refining the spatial characterization of SWD in C3H/Hej mice through more invasive electrophysiology techniques. In addition, we assumed that the SWD were bilaterally symmetrical, and we recorded from only one side, however further studies with bilateral recordings may be beneficial. Furthermore, the evolution of epileptogenesis in this model may be better characterized through improved analysis methods such as averaged time-frequency wavelet analysis at different ages.
While no previous studies have analyzed the development of epilepsy in the C3H/HeJ mouse in detail, extensive characterization has been completed in two other rodent models of absence epilepsy, rats of the WAG/Rij strain (Coenen and Van Luijtelaar, 1987; Coenen and Van Luijtelaar, 2003) and GAERS (Vergnes et al., 1982, 1986; Marescaux and Vergnes, 1995; Danober et al., 1998). Researchers have previously concluded that the seizures in these strains of rats are morphologically, behaviorally, and pharmacologically similar to the absence seizures experienced by human patients (Micheletti et al., 1985; Van Luijtelaar and Coenen, 1988; Coenen et al., 1992; Marescaux et al., 1992; Marescaux and Vergnes, 1995; van Luijtelaar et al., 2002; Coenen and Van Luijtelaar, 2003).
WAG/Rij rats exhibit almost no spike-wave activity at age 75 d, approximately 5–7 SWD per hour at 140 d, and 16–18 SWD per hour at 245 d (Coenen and Van Luijtelaar, 1987). The cumulative duration of the spike-wave complexes increases in parallel, from close to zero at 75 d, to approximately 25 and 75 seconds per hour at the ages of 140 days and 245 days, respectively (Coenen and Van Luijtelaar, 1987). GAERS show essentially no SWD activity through 30 days of age, after which point SWD activity increases until the age of four months, when all tested GAERS show SWD activity, and SWD become even more severe at age six months (Vergnes et al., 1986; Marescaux et al., 1992; Marescaux and Vergnes, 1995).
The C3H/HeJ mouse model for human absence epilepsy was described relatively recently (Frankel et al., 2005) and is particularly promising for studying epileptogenesis because of the earlier age of SWD onset, and the known genetic defect in this model (Beyer et al., 2008). Like the rat models, epileptic activity in C3H/HeJ mice is morphologically, behaviorally, and pharmacologically similar to human absence epilepsy (Frankel et al., 2005). Previous characterization of the C3H/HeJ model revealed SWD activity with burst frequencies of 7–8 Hz and epileptiform activity in mice as young as 3.5 weeks. We observed that the age of SWD development in C3H/HeJ mice was between age 15 and 25 d, which was substantially earlier than in either GAERS or WAG/Rij rats. We also observed that the morphology and power spectra of SWD in mature C3H/HeJ mice were similar to rat models (Drinkenburg et al., 1993; Blumenfeld et al., 2008), but that at earlier ages, the power spectrum peaked at lower frequencies.
Backcross studies in C3H/HeJ mice have shown a recessive, non-Mendelian mode of inheritance of the absence epilepsy phenotype. It was recently found that the absence seizures in C3H/HeJ mice are due to a mutation of the AMPA receptor subunit Gria4, which is predominantly present in the thalamic reticular nucleus (Beyer et al., 2008). Gria4 mutants display enhanced synaptic excitation of inhibitory thalamic reticular neurons, with increased duration of synaptic responses. Seizure genesis in Gria4 mutants may then occur because of stronger inhibition of thalamic relay cells and the promotion of rebound burst firing responses (Beyer et al., 2008). Enhanced firing of thalamic reticular neurons has previously been implicated in triggering the transition from normal activity to SWD generation (Blumenfeld and McCormick, 2000).
Several possible maturational changes could affect the time course of seizure onset in this model of absence epilepsy. Differential production of hormones throughout the life of the organism has been shown to affect neural circuits and epileptic phenomena (Mattson and Cramer, 1985; Morrell, 1992), however, sexual maturity in mice occurs at about 7 weeks (Suckow et al., 2001), substantially after the time of SWD onset. Developmental changes in the expression of voltage-gated ion channels have been reported previously in SWD models and could play a role (Klein et al., 2004; Strauss et al., 2004; Blumenfeld et al., 2008), as could changes in GABA receptor expression (Brooks-Kayal et al., 2001). Another age-related change which occurs with appropriate timing to participate in the emergence of SWD in this model, is the switch of the GABA receptor’s activity from excitatory to inhibitory, dependent on the chloride transporter (Plotkin et al., 1997; Dzhala et al., 2005; Ben-Ari, 2006; Huberfeld et al., 2007; Munoz et al., 2007; Kahle and Staley, 2008). While these are a few possible mechanisms for the development of SWD in the C3H/HeJ model as it ages, numerous other possibilities exist (Jensen and Baram, 2000; Ben-Ari and Holmes, 2006; Dube et al., 2007; Scharfman, 2007).
In conclusion, we observed the early appearance of SWD activity in C3H/HeJ mice, precursors of which are possibly seen on the fifth postnatal day. Between age 15 and 25 d there is a marked increase in the number of SWD per hour, and a progressive maturational development of SWD morphology, frequency, and duration. Future investigations of the C3H/HeJ model should include the assessment of early interventions in this model, to determine if early treatment can suppress epileptogenesis as was observed in rats (Blumenfeld et al., 2008). This model could then be used to determine the critical periods for intervention, the appropriate treatment window, and eventually further elucidate the molecular mechanisms of epileptogenesis. Therefore, the C3H/HeJ model provides substantial hope for future epilepsy research, including investigations of primary prevention.
Acknowledgments
We thank Dr. Wayne Frankel for advice regarding the C3H/HeJ model, Steven Clifford of CED for Spike2 scripts used in the EEG analysis, and Dr. Ulrich Schridde for initial behavioral observations in C3H/HeJ mice. This work was supported by NIH R01 NS049307, and by the Betsy and Jonathan Blattmachr family.
References
- Ben-Ari Y. Basic developmental rules and their implications for epilepsy in the immature brain. Epileptic Disord. 2006;8:91–102. [PubMed] [Google Scholar]
- Ben-Ari Y, Holmes GL. Effects of seizures on developmental processes in the immature brain. Lancet Neurol. 2006;5:1055–1063. doi: 10.1016/S1474-4422(06)70626-3. [DOI] [PubMed] [Google Scholar]
- Beyer B, Deleuze C, Letts VA, Mahaffey CL, Boumil RM, Lew TA, Huguenard JR, Frankel WN. Absence seizures in C3H/HeJ and knockout mice caused by mutation of the AMPA receptor subunit Gria4. HMG Advance Access. 2008 doi: 10.1093/hmg/ddn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia. 2005a;46 (Suppl 9):21–33. doi: 10.1111/j.1528-1167.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H. Consciousness and epilepsy: why are patients with absence seizures absent? Prog Brain Res. 2005b;150:271–286. doi: 10.1016/S0079-6123(05)50020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, McCormick DA. Corticothalamic inputs control the pattern of activity generated in thalamocortical networks. J Neurosci. 2000;20:5153–5162. doi: 10.1523/JNEUROSCI.20-13-05153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, Klein JP, Schridde U, Vestal M, Rice T, Khera DS, Bashyal C, Giblin K, Paul-Laughinghouse CP, Wang F, Phadke A, Mission J, Agarwal RK, Englot DJ, Motelow J, Nersesyan H, Waxman SG, Levin AR. Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia. 2008;49 (3):400–409. doi: 10.1111/j.1528-1167.2007.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Kelly ME, Coulter DA. Gamma-aminobutyric acid(A) receptor subunit expression predicts functional changes in hippocampal dentate granule cells during postnatal development. J Neurochem. 2001;77:1266–1278. doi: 10.1046/j.1471-4159.2001.00329.x. [DOI] [PubMed] [Google Scholar]
- Camfield P, Camfield C. Epileptic syndromes in childhood: clinical features, outcomes, and treatment. Epilepsia. 2002;43 (Suppl 3):27–32. doi: 10.1046/j.1528-1157.43.s.3.3.x. [DOI] [PubMed] [Google Scholar]
- Coenen AM, Van Luijtelaar EL. The WAG/Rij rat model for absence epilepsy: age and sex factors. Epilepsy Res. 1987;1:297–301. doi: 10.1016/0920-1211(87)90005-2. [DOI] [PubMed] [Google Scholar]
- Coenen AM, Van Luijtelaar EL. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav Genet. 2003;33:635–655. doi: 10.1023/a:1026179013847. [DOI] [PubMed] [Google Scholar]
- Coenen AM, Drinkenburg WH, Inoue M, van Luijtelaar EL. Genetic models of absence epilepsy, with emphasis on the WAG/Rij strain of rats. Epilepsy Res. 1992;12:75–86. doi: 10.1016/0920-1211(92)90029-s. [DOI] [PubMed] [Google Scholar]
- Cox GA, Lutz CM, Yang CL, Biemesderfer D, Bronson RT, Fu A, Aronson PS, Noebels JL, Frankel WN. Sodium/hydrogen exchanger gene defect in slow-wave epilepsy mutant mice. Cell. 1997;91:139–148. doi: 10.1016/s0092-8674(01)80016-7. (Erratum appears in Cell, 1997, 91 (December(6)), 861) [DOI] [PubMed] [Google Scholar]
- Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3:371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- Danneman PJ, Mandrell TD. Evaluation of five agents/methods for anesthesia of neonatal rats. Lab Anim Sci. 1997;47:386–395. [PubMed] [Google Scholar]
- Danober L, Deransart C, Depaulis A, Vergnes M, Marescaux C. Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog Neurobiol. 1998;55:27–57. doi: 10.1016/s0301-0082(97)00091-9. [DOI] [PubMed] [Google Scholar]
- Dedeurwaerdere S, Boon P, De Smedt T, Claeys P, Raedt R, Bosman T, Van Hese P, Van Maele G, Vonck K. Chronic levetiracetam treatment early in life decreases epileptiform events in young GAERS, but does not prevent the expression of spike and wave discharges during adulthood. Seizure. 2005;14:403–411. doi: 10.1016/j.seizure.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Drinkenburg WH, van Luijtelaar EL, van Schaijk WJ, Coenen AM. Aberrant transients in the EEG of epileptic rats: a spectral analytical approach. Physiol Behav. 1993;54:779–783. doi: 10.1016/0031-9384(93)90092-t. [DOI] [PubMed] [Google Scholar]
- Dube CM, Brewster AL, Richichi C, Zha Q, Baram TZ. Fever, febrile seizures and epilepsy. Trends Neurosci. 2007;30:490–496. doi: 10.1016/j.tins.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. (See comment) [DOI] [PubMed] [Google Scholar]
- Fletcher CF, Lutz CM, O’Sullivan TN, Shaughnessy JD, Jr, Hawkes R, Frankel WN, Copeland NG, Jenkins NA. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell. 1996;87:607–617. doi: 10.1016/s0092-8674(00)81381-1. [DOI] [PubMed] [Google Scholar]
- Frankel WN, Beyer B, Maxwell CR, Pretel S, Letts VA, Siegel SJ. Development of a new genetic model for absence epilepsy: spike-wave seizures in C3H/He and backcross mice. J Neurosci. 2005;25:3452–3458. doi: 10.1523/JNEUROSCI.0231-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch ET, Pierre, Panayiotopoulos CP. Childhood and juvenile absence epilepsies. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. 2. Chapter 239 Lippincott Williams & Wilkins; Philadelphia, PA: 2008. pp. 2397–2411. [Google Scholar]
- Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, Rivera C. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen FE, Baram TZ. Developmental seizures induced by common early-life insults: short- and long-term effects on seizure susceptibility. Mental Retard Dev Disabilities Res Rev. 2000;6:253–257. doi: 10.1002/1098-2779(2000)6:4<253::AID-MRDD4>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Staley K. Altered neuronal chloride homeostasis and excitatory GABAergic signaling in human temporal lobe epilepsy. Epilepsy Curr. 2008;8:51–53. doi: 10.1111/j.1535-7511.2008.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JP, Khera DS, Nersesyan H, Kimchi EY, Waxman SG, Blumenfeld H. Dysregulation of sodium channel expression in cortical neurons in a rodent model of absence epilepsy. Brain Res. 2004;1000:102–109. doi: 10.1016/j.brainres.2003.11.051. [DOI] [PubMed] [Google Scholar]
- Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett FS, 2nd, Mori Y, Campbell KP, Frankel WN. The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat Genet. 1998;19:340–347. doi: 10.1038/1228. (See comment) [DOI] [PubMed] [Google Scholar]
- Marescaux C, Vergnes M. Genetic absence epilepsy in rats from Strasbourg (GAERS) Ital J Neurol Sci. 1995;16:113–118. doi: 10.1007/BF02229083. [DOI] [PubMed] [Google Scholar]
- Marescaux C, Vergnes M, Depaulis A. Genetic absence epilepsy in rats from Strasbourg–a review. J Neural Transmission Suppl. 1992;35:37–69. doi: 10.1007/978-3-7091-9206-1_4. [DOI] [PubMed] [Google Scholar]
- Mattson RH, Cramer JA. Epilepsy, sex hormones, and antiepileptic drugs. Epilepsia. 1985;26 (Suppl 1):S40–51. doi: 10.1111/j.1528-1157.1985.tb05723.x. [DOI] [PubMed] [Google Scholar]
- Micheletti G, Vergnes M, Marescaux C, Reis J, Depaulis A, Rumbach L, Warter JM. Antiepileptic drug evaluation in a new animal model: spontaneous petit mal epilepsy in the rat. Arzneimittel-Forschung. 1985;35:483–485. [PubMed] [Google Scholar]
- Mirsky AF, Duncan CC, Myslobodsky MS. Petit mal epilepsy: a review and integration of recent information. J Clin Neurophysiol. 1986;3:179–208. [PubMed] [Google Scholar]
- Morrell MJ. Hormones and epilepsy through the lifetime. Epilepsia. 1992;33 (Suppl 4):S49–61. doi: 10.1111/j.1528-1157.1992.tb06227.x. [DOI] [PubMed] [Google Scholar]
- Munoz A, Mendez P, DeFelipe J, Alvarez-Leefmans FJ. Cation-chloride cotransporters and GABA-ergic innervation in the human epileptic hippocampus. Epilepsia. 2007;48:663–673. doi: 10.1111/j.1528-1167.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- Phifer CB, Terry LM. Use of hypothermia for general anesthesia in preweanling rodents. Physiol Behav. 1986;38:887–890. doi: 10.1016/0031-9384(86)90058-2. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Kharatishvili I, Karhunen H, Lukasiuk K, Immonen R, Nairismagi J, Grohn O, Nissinen J. Epileptogenesis in experimental models. Epilepsia. 2007;48 (Suppl 2):13–20. doi: 10.1111/j.1528-1167.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na–K–2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA’s excitatory role in immature brain. J Neurobiol. 1997;33:781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. The neurobiology of epilepsy. Curr Neurol Neurosci Rep. 2007;7:348–354. doi: 10.1007/s11910-007-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnikova E, van Luijtelaar G. Electroencephalographic characterization of spike-wave discharges in cortex and thalamus in WAG/Rij rats. Epilepsia. 2007;48:2296–2311. [PubMed] [Google Scholar]
- Strauss U, Kole MH, Brauer AU, Pahnke J, Bajorat R, Rolfs A, Nitsch R, Deisz RA. An impaired neocortical Ih is associated with enhanced excitability and absence epilepsy. Eur J Neurosci. 2004;19:3048–3058. doi: 10.1111/j.0953-816X.2004.03392.x. [DOI] [PubMed] [Google Scholar]
- Suckow MA, Brayton C, Danneman P. The Laboratory Mouse. CRC Press; Boca Raton, FL: 2001. [Google Scholar]
- Van Luijtelaar EL, Coenen AM. Circadian rhythmicity in absence epilepsy in rats. Epilepsy Res. 1988;2:331–336. doi: 10.1016/0920-1211(88)90042-3. [DOI] [PubMed] [Google Scholar]
- van Luijtelaar EL, Drinkenburg WH, van Rijn CM, Coenen AM. Rat models of genetic absence epilepsy: what do EEG spike-wave discharges tell us about drug effects? Methods Find Exp Clin Pharmacol. 2002;24 (Suppl D):65–70. [PubMed] [Google Scholar]
- Vergnes M, Marescaux C, Micheletti G, Reis J, Depaulis A, Rumbach L, Warter JM. Spontaneous paroxysmal electroclinical patterns in rat: a model of generalized non-convulsive epilepsy. Neurosci Lett. 1982;33:97–101. doi: 10.1016/0304-3940(82)90136-7. [DOI] [PubMed] [Google Scholar]
- Vergnes M, Marescaux C, Depaulis A, Micheletti G, Warter JM. Ontogeny of spontaneous petit mal-like seizures in Wistar rats. Brain Res. 1986;395:85–87. doi: 10.1016/s0006-8993(86)80011-7. [DOI] [PubMed] [Google Scholar]
- Wirrell EC, Camfield CS, Camfield PR, Dooley JM, Gordon KE, Smith B. Long-term psychosocial outcome in typical absence epilepsy. Sometimes a wolf in sheeps’ clothing. Arch Pediatrics Adolescent Med. 1997;151:152–158. doi: 10.1001/archpedi.1997.02170390042008. [DOI] [PubMed] [Google Scholar]