Abstract
Much progress has been made in the field studying the process of epileptogenesis via neuroimaging techniques. Conventional imaging methods include magnetic resonance imaging with morphometric analysis, magnetic resonance spectroscopy and positron emission tomography. Newer network-based methods such as diffusion tensor imaging and functional magnetic resonance imaging with resting functional connectivity are being developed and applied to clinical use. This review provides a brief summary of the major human and animal studies in both partial and generalized epilepsies that demonstrate the potential of these imaging modalities to serve as biomarkers of epileptogenesis.
Keywords: Epileptogenesis, Spike-wave seizure, Neuroimaging, Biomarker
1. Introduction
Epilepsy affects 2–3 million people in the United States [47] and exerts a huge toll on our society [117]. Currently, epilepsy can only be treated by medications or surgery upon diagnosis. Epileptogenesis is difficult to study in human patients because patients usually present with established epilepsy rather than at an early stage of the disease.
Neuroimaging has played an important role in measuring long-term changes in the brains of both partial and generalized epilepsy patients. Conventional imaging methods include magnetic resonance imaging (MRI) morphologic analysis, proton magnetic resonance spectroscopy (PMRS) and positron emission tomography (PET). Recently, network-based approaches, such as diffusion tensor imaging (DTI) and resting functional connectivity functional MRI (fMRI) are also being developed to measure structural and functional changes in the brain, even when seizures are not occurring.
The first section of this article reviews neuroimaging biomarkers of epileptogenesis in partial epilepsy and the second section, generalized epilepsy. We will review studies from both animal models and human patients, as each neuroimaging technique is discussed in turn. Partial onset seizures discussed in this review will include febrile seizures, posttraumatic epilepsy (PTE), tuberous sclerosis, and various forms of temporal lobe epilepsy (TLE) including hippocampal sclerosis and mesial temporal sclerosis, as well as others in both animal models and human patients. Our discussion of idiopathic generalized epilepsy is divided into the following subsyndromes: childhood and juvenile absence epilepsy, juvenile myoclonic epilepsy, and generalized tonic-clonic seizures.
2. Partial epilepsy
2.1. Magnetic resonance imaging
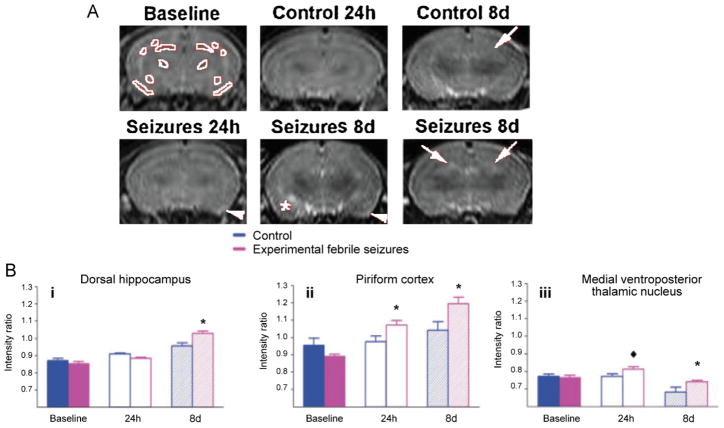
Magnetic resonance imaging (MRI) was first introduced in the diagnostic evaluation of epilepsy in the 1980s and has since become a mainstay of clinical care [33,103]. In addition to detecting structural abnormalities, by measuring the variations in signals produced by protons in the brain, MRI can detect pathological changes in certain type of seizures, such as febrile seizures (FSs). Associated with a sudden rise in body temperature, febrile seizures are the most common type of seizure seen in children [31]. While FSs often do not pose subsequent threat to an individual’s health, patients with prolonged FSs were demonstrated to be at a higher risk for developing TLE later in life [102]. In a rat model of experimentally induced prolonged FSs by way of hyperthermia, T2 signals taken from serial MRIs before and after seizure onset were abnormal in the dorsal hippocampus, piriform cortex and amygdala in 75% of these rats one day after seizures and in 87.5% of rats eight days after the induced febrile seizures [30] (Fig. 1). Although these abnormal MR signals in temporal lobe structures do not signify cell death, they may indicate underlying changes in neuronal integrity that promote epileptogenesis and provide early markers for subsequent TLE development.
Fig. 1.
T2-weighted MRI signal intensities after prolonged experimental febrile seizures (FSs). (A) The control and the seizure animal groups were imaged before the seizures on postnatal day 10, P10, to provide baseline intensities, and on P12 (24 h after seizure, seizure group) as well as 8 days after the seizures. Regions used for analysis are delineated in white in baseline image. Increased signal intensity is evident in the dorsal hippocampus (white arrows), piriform cortex (white arrowheads), and amygdala (asterisk). (B) T2 signal intensity modulation by prolonged experimental FSs: (i) a significant increase of T2 signal occurred in dorsal hippocampus, by 8 days after the seizures, (ii) in piriform cortex, signal intensity increased significantly at 24 h and 8 days, (iii) in the medial ventroposterior thalamic nucleus, signal intensity increased significantly at 8 days and there was a strong trend for increased signal at 24 h (diamond).
This figure is reproduced from Dube et al. [30] with permission.
In human patients with febrile status epilepticus, hyperintense hippocampal signal intensity on T2-weighted MRI and hippocampal volume changes were the most predictive indicators of subsequent development of mesial temporal sclerosis (MTS) [63,90,105,111]. In one study of 11 children with prolonged febrile seizures, seven cases developed severe hippocampal T2 signal abnormalities [63,111]. Five of these seven developed anatomical indicators of MTS, while none of the other 4 children with normal MRI signals demonstrated any evidence of MTS [90].
Posttraumatic epilepsy results from traumatic brain injury (TBI) caused by physical trauma to the brain and constitutes 5% of all cases of epilepsy [43]. In posttraumatic epileptogenesis, periods of enhanced abnormal plasticity occur, which mimic the critical period of development [83,89]. More recently, quantitative T2 signal evaluation has been used to analyze the severity of cortical damage and neuro-motor impairment soon after TBI. In a rat lateral fluid percussion injury (LFPI) model, T2 values were found not only to correlate with motor deficits and histological lesion volume, but also to successfully differentiate the moderate from the severe TBI groups [54]. Multiparametric quantitative MRI changes (such as T2, T1 ρ, diffusion tensor trace, the extent of hyperintense lesion and intracerebral hemorrhage) acquired shortly after TBI in the LFPI rat model were able to predict functional and histopathological changes 6–12 months post-LFPI [49]. These results demonstrate that early changes in the hippocampus and perifocal areas may be an indicator of the long-term outcome after TBI. These abnormal MRI changes can potentially serve as a useful clinical marker for identifying individuals with an elevated risk of developing PTE.
MRI has been used as a biomarker of epileptogenesis in other animal models of partial epilepsy. For instance, Roch et al. found that lithium-pilocarpine induced status epilepticus rats without MRI abnormalities or altered T2 relaxation times did not end up developing epilepsy, whereas those with increases in T2 relaxation times and abnormalities in the T2-weighted images, specifically at the level of piriform and entorhinal cortices, subsequently developed epilepsy [93]. Transient MRI changes in the hippocampus and piriform cortex after lithium-pilocarpine induced seizure have been shown to correctly predict the severity of later hippocampal damage [21]. With further study, these MRI signal changes have the potential to become a clinically useful prognostic biomarker for at-risk patients.
2.2. MRI morphometric analysis
MRI volumetry and morphometry are involved in comparing the size and shape of brain structures. In the case of voxel-based morphometry (VBM), this is done by spatially normalizing all images, segmenting gray matter from images, and then performing voxelwise parametric statistical tests to produce a parametric map of structural regions [2]. One of first studies using morphometric analysis that received mainstream media attention was the VBM analysis of hippocampi of London taxi drivers. The hippocampus has long been associated with spatial navigational skills. It was found that the posterior hippocampal regions of taxi drivers were larger than those of normal people, while the anterior hippocampal regions were smaller [69]. Subsequently, VBM analysis has been applied to epilepsy research and other brain disorders. In other forms of analysis, only the volume of regions of interest (ROIs) are evaluated.
MRI volumetry has revealed smaller ipsilateral thalamic volumes in TLE patients with febrile seizures than in those without [77]. Using VBM analysis, Labate et al., has concluded that patients with TLE exhibit gray matter volume reduction and other structural abnormalities in the hippocampus and thalamus [62]. These abnormalities were more severe in those who also had MTS [62]. However, there was no gray matter difference between refractory TLE and milder TLE with MTS [61]. These gray matter volume differences in patients with partial epilepsy makes morphometric analysis a potential biomarker to study changes in the brain during epileptogenesis.
2.3. Proton magnetic resonance spectroscopy
Proton magnetic resonance spectroscopy (PMRS) and imaging (PMRSI) are noninvasive techniques for exploring the metabolic status of the brain in health and in disease [1,40,75]. The four major metabolites detected by PMRS at long echo times are N-acetylaspartate (NAA), creatine (Cr), choline-containing phospholipids (Cho) and lactate (Lac) [95]. NAA is a neuronal and axonal marker that decreases with neuronal loss or dysfunction; Cr, either alone or as phosphocreatine, is a marker for intact brain energy metabolism; Cho is a marker for membrane synthesis or repair, inflammation, or demyelination; Lac is a metabolite of anaerobic glycolysis [95]. Using PMRS to measure in vivo temporal lobe metabolite concentrations in patients with TLE attributable to unilateral hippocampal sclerosis (HS), Sinister et al. found a bilateral reduction of NAA + N-acetyl aspartyl-glutamate (NAAt) to creatine plus phosphocreatine (Cr) ratio (NAAt/Cr) in the temporal lobe; normalization of NAAt/Cr in the contralateral temporal lobe was seen following successful temporal lobe resection [104]. PMRS has also been used in the preoperative evaluation of patients with mesial temporal lobe epilepsy (mTLE): a decrease of NAA or more frequent presence of lactate provided diagnostic clues for the lateralization of the epileptogenic zone, while significant bilateral metabolic alterations in the mesial temporal lobe structures were associated with worse postoperative seizure control [20]. PMRSI in patients with medically intractable TLE was abnormal and predicted lateralization in 86% patients using PMRSI alone [17]. The combination of PMRSI and MRI volumetry can accurately and non-invasively lateralize TLE in the majority of patients [17]. Stefan et al. found that PMRS could detect subtle abnormalities that were not apparent on MRI [107]. Another study indicated that hippocampal structural damage may be depicted by PMRS [36]. In intractable TLE, correlation analysis showed significant linear correlation between the midtemporal NAA/Cr relative asymmetry ratio and surgical outcome; the greater the asymmetry, the better the outcome [64]. PMRS has provided new and important biological brain markers able to predict clinical outcomes and identify subjects at high risk for the poorer outcomes.
2.4. Positron emission tomography
Positron emission tomography (PET) was developed during the mid-1970s [88]. Various PET studies using several different radiotracers have been used to visualize and quantify changes in receptor density.
The most common PET radiotracer used is FDG (fludeoxyglucose (18F)), a glucose analogue. FDG-PET signal intensity is related to glucose uptake and metabolism in the brain. In a study of 11 children with focal epilepsy associated with tuberous sclerosis complex, Nishida et al. found that glucose hypometabolism was associated with increased delta wave-slowing and frequent spike activity on electrocorticography [80]. However, Liew et al. recently showed that (18) F-FCWAY-PET may show more specific binding reduction of serotonin receptor in seizure initiation regions [66]. It was found that when compared with controls, MRI-negative TLE patients had significantly reduced free fraction-corrected volume of distribution and significantly greater asymmetry indices in fusiform gyrus, hippocampus and parahippocampus ipsilateral to epileptic foci [66].
Various PET studies using several different radiotracers have been used to visualize and quantify changes in receptor density. Serotonin mediates an antiepileptic and anticonvulsant effect via 5-hydroxytryptamine-1A (5-HT1A) receptors, which are predominantly located in limbic areas [84]. PET studies using 5-HT1A receptor antagonist [18F] MPPF, [(11)C]WAY-100 635 or 18F-FCWAY have demonstrated that a decreased 5-HT1A receptor binding (or availability) correlated with the degree of epileptogenicity in regions involved in seizure onset and where discharges propagated (e.g., hippocampus and amygdala) [35,71,99,109]. In children with intractable partial epilepsy following cortical resection, Chugani et al. found an increased alpha[11C]methyl-L-tryptophan uptake in the lentiform nucleus ipsilateral to the resection as compared to the contralateral side (suggesting increased serotonin synthesis), and these asymmetries were higher than those measured in controls [22]. PET-detected changes in receptor binding or metabolite metabolism can be applied in the future to study the epileptogenic process in human patients with partial epilepsy.
2.5. Functional magnetic resonance imaging
In 1936, Pauling and Coryell found that deoxyhemoglobin was paramagnetic [86]. Ogawa et al. used this properties of hemoglobin to develop a new MRI image contrast named blood-oxygen-level dependent (BOLD) functional MRI (fMRI) [82]. BOLD fMRI provides a non-invasive measure of brain function and has been applied to epilepsy research. EEG-correlated fMRI has been used to investigate epileptogenic networks in patients with focal epilepsy. Several studies have looked at BOLD signal changes preceding focal EEG spikes [50,92]. Jacobs et al. were able to conclude that prespike BOLD fMRI responses in patients with focal epilepsy were more localized than late responses and were strongly related to the spike field [50].
EEG-fMRI has proven to be a valuable tool in the presurgical evaluation of patients with medically refractory epilepsy [120]. It also provides a noninvasive method for selecting tubers in patients with tuberous sclerosis complex [51]. These studies laid the foundation for its future use as a suitable imaging biomarker to track disease progression and treatment efficacy in partial epilepsy patients.
2.6. Resting functional connectivity with fMRI
Functional connectivity is the temporal correlation of a neuro-physiological index measured in different brain areas [34]. Using fMRI, Biswal et al. demonstrated correlation of low frequency (<0.1 Hz) fluctuations in the primary sensory cortex secondary to hand movement both within and between hemispheres [12]. mTLE is a neural network disorder involving widespread brain regions. Seeding the anterior hippocampal region of resection, Morgan et al. found increased negative connectivity across a network including thalamic, brainstem, frontal and parietal regions in left mTLE patients as compared to controls via full brain connectivity analysis [76]. This result supports theories of inhibited function in subcortical and cortical structures during ictal propagation.
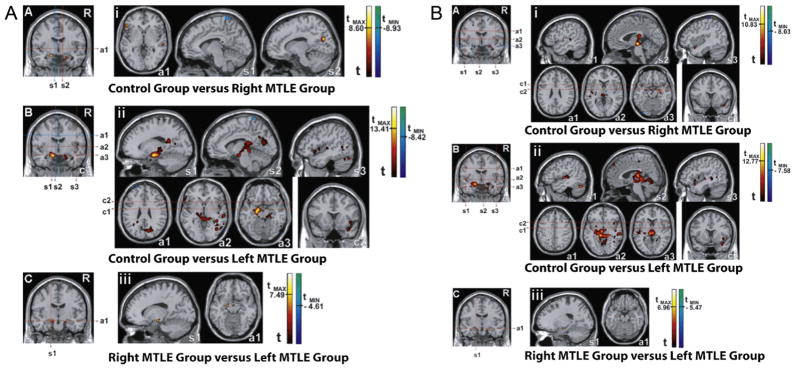
Using resting-state fMRI with independent component, Zhang et al. demonstrated that mTLE patients had decreased functional connectivity in the dorsal mesial prefrontal cortex, mesial temporal lobe and inferior temporal cortex [119]. In addition, only mTLE patients with right, but not left, hippocampal sclerosis had bilaterally decreased functional connectivity in the mesial temporal lobe and increased functional connectivity in the posterior cingulate cortex [119]. Looking at perceptual function, Zhang et al. found that compared with controls, mTLE patients presented with decreased functional connectivity in the auditory and sensorimotor networks, but increased functional connectivity in the primary visual cortex [118]. Left TLE patients also have reduced functional connectivity in the language areas [113]. Furthermore, in mTLE patients, left hippocampal sclerosis causes a greater reduction of functional connectivity in large parts of the limbic lobes (especially the mesial temporal lobe), than right hippocampal sclerosis [87] (Fig. 2).
Fig. 2.
Comparison of functional connectivity among right MTLE group, left MTLE group, and controls. Functional connectivity maps were generated by placing the seed in the left (A) and right hippocampus (B): (i) control group versus patients with right MTLE, (ii) control group versus patients with left MTLE, (iii) patients with right MTLE versus patients with left MTLE. Abbreviations—s1: first sagittal image; s2: second sagittal image; s3: third sagittal image; a1: first axial image; a2: second axial image; a3: third axial image; c1: first coronal image; c2: second coronal image. Statistical maps with t-scores higher than 6.
This figure is reproduced with permission from Pereira et al. [87].
fMRI with resting state functional connectivity may also be useful in the presurgical assessment of mTLE patients. Bettus et al. demonstrated that basal functional connectivity increases in the non-epileptic side was the most specific marker for lateralizing/localizing the epileptogenic zones [11]. These results suggest that fMRI-based resting functional connectivity is a powerful technique for detecting abnormal network function, and may be a useful biomarker for epileptogenesis and its prevention by early treatment.
2.7. Diffusion tensor imaging
Diffusion tensor imaging (DTI) measures diffusion properties of water protons in tissue [6] and can detect subtle white matter changes in the pathological state [18,52]. Apparent diffusion coefficient (ADC) is an average measure of water diffusion and fractional anisotropy (FA) measures the degree of alignment of cellular structures within a tissue (e.g., white matter fiber tracts), with 0 being the least anisotropic and 1 being highly anisotropic [6,7]. There have been many studies supporting DTI as a promising noninvasive biomarker. In animal studies, quantitative diffusion-weighted imaging (DWI) has been used in the prediction of increased seizure susceptibility [53] and in the diagnosis of subtle changes in brain water in other brain diseases [52,91] including epilepsy animal models [18]. In a rat model of moderate traumatic brain injury (TBI) induced by lateral fluid percussion, DWI correlated with mossy fiber sprouting density and EEG parameters such as spike count, epileptiform discharge number and first spike latency in the ipsilateral hippocampus at both early and chronic time points after TBI [53].
DTI is a sensitive method for detecting abnormalities in patients with partial epilepsy, even in structures without apparent changes on conventional MRI. For instance, in children with TLE, decreased FA and increased ADC were found in the hippocampi ipsilateral to the seizure focus, and significantly decreased FA was also found in the contralateral hippocampi of these patients [57]. In patients with mTLE accompanied by hippocampal sclerosis (HS), Thivard et al. showed that diffusion abnormalities were not restricted to the pathologic hippocampus but involved a larger network (i.e., the temporal lobe and extratemporal regions) [108]. DTI has also been used to evaluate thalamic differences between TLE patients with and without hippocampal sclerosis [55]. The mean diffusivity (MD) of the thalamus ipsilateral to the epileptogenic side was higher in TLE patients with HS than those without [55].
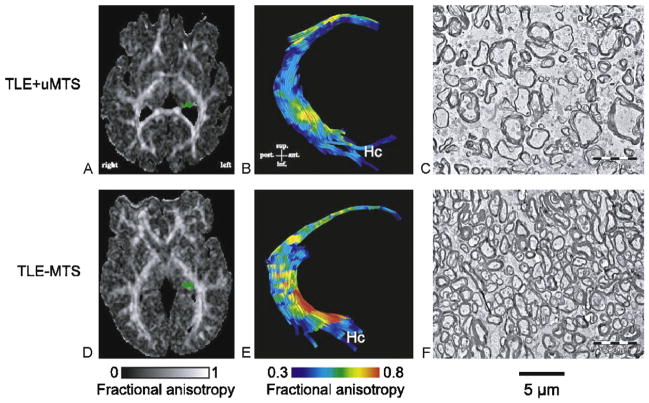
Many human studies have used DTI to study changes in the underlying connectivity of the epileptic brain. In TLE patients with mesial temporal sclerosis (MTS), DTI showed decreased FA in the genu of the corpus collosum and external capsule and increased MD in the genu and splenium of corpus callosum and external capsule [25,38]. Only TLE patients with unilateral MTS (relative to TLE patients without MTS) had abnormal DTI parameters of the fimbria-fornix and decreased anisotrophy in the cingulum [25]. On electron microscope, the fimbria-fornix of these patients showed increased extra-axonal fraction, and reduced myelin area and cumulative axonal membrane circumference, which correlated strongly with water diffusion anisotropy over the crus of the fimbria-fornix [26] (Fig. 3). This demonstrates a correlation between histology and human in vivo DTI and validates DTI as a noninvasive marker of white matter pathology.
Fig. 3.
DTI tractography and electron microscopy of the fimbria-fornix. Histological fields of the fimbria-fornix resected during surgery from two patients with TLE are shown with their corresponding FA maps (A and D, with the left fimbria-fornix marked as green) and tractography of the fimbria-fornix (B and E). The patient with MTS shows lower diffusion anisotropy of the fimbria-fornix (B) than the patient without MTS (E). This corresponds to lower axonal density and higher extra-axonal fraction for the patient with MTS (C) than for the patient without MTS (F).
This figure is reproduced from Concha et al. [26] with permission.
3. Generalized epilepsy
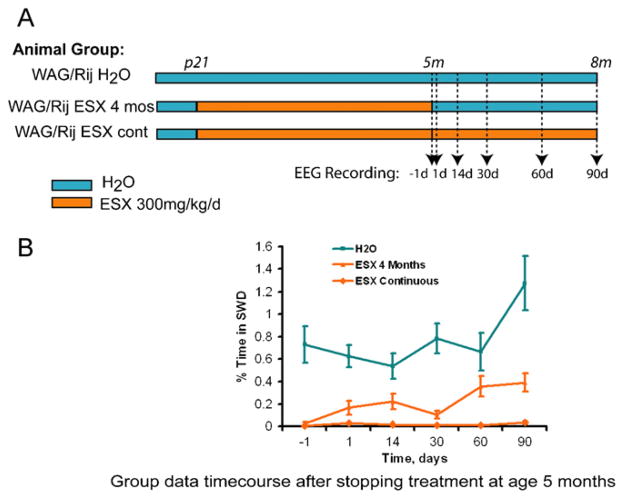
Blumenfeld et al. recently found that early treatment with ethosuximide given continuously in drinking water from P21 to age five months in a rat model of absence seizures blocked the expression of ion channels that are characteristic of seizure development [13,58] (Fig. 4). The treatment suppressed seizures, an effect that lasted 90 days after stopping the medication. These results suggested that there may be a “critical period” early in development during which treatment can result in both molecular and electrophysiological inhibition of epileptogenesis. This raises the hope that once genetics allows identification of those patients at risk, we can predict disease onset and eventually cure the underlying disease via primary prevention. In order to achieve this goal, non-invasive biomarkers to monitor disease progression, severity and efficacy of therapy, are urgently needed.
Fig. 4.
Early ethosuximide (ESX) treatment persistently suppressed the development of Spike-wave discharges (SWDs) in WAG/Rij rats, even after cessation of treatment. (A) Epileptic WAG/Rij rats were given either normal drinking water (H2O group), ESX 300 mg/kg/day from age p21 through age 5 months and then normal drinking water from age 5 to 8 months (ESX 4 month group), or ESX 300 mg/kg/day continuously from age p21 through 8 months (ESX continuous group). EEG was recorded 1 day before stopping ESX, and 1, 14, 30, 60, and 90 days after stopping ESX. (B) Quantification of effects of early ESX treatment on percent time in SWD. Even after stopping ESX, percent time in SWD remained markedly reduced in the treated rats (ESX 4 months group) when comparing all time points for days 1 through 90 to rats on normal H2O.
This figure is reproduced from Blumenfeld et al. [13] with permission.
3.1. MRI morphometric analysis: voxel-based morphometry
In idipopathic generalized epilepsy (IGE), MRI-VBM can reveal subtle structural abnormalities, even when MRI scans are normal on visual assessment. In patients with juvenile myoclonic epilepsy (JME), an increased gray matter volume in the mesiofrontal lobes and a reduced gray matter volume in the thalamus were detected by VBM analysis [10,56,67,114,115]. Reduced bilateral gray matter volume of visual cortices were found in JME patients with photosensitivity [67]. Using VBM, Helms et al. found decreased thalamic gray matter fraction and increased white matter fraction in brains of IGE patients [44] (Fig. 5A). These changes were greatest in the dorso-medial thalamus. In childhood absence epilepsy (CAE) patients, optimized voxel-based morphometry revealed gray matter decrease in both thalami and the subcallosal gyrus and white matter decrease in the extranuclear subcortical area and the basal forebrain [19,85]. Recently, Caplan et al. reported lower gray matter volumes in the left orbital frontal gyrus and bilateral temporal lobes of CAE patients [16]. These abnormal gray and white matter changes in the thalamus and frontal cortex emphasize the role of the thalamocortical network as the underlying mechanism of absence seizures and other generalized epilepsies [116].
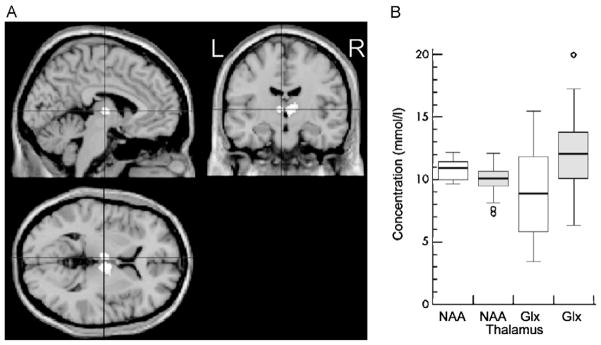
Fig. 5.
Decreased N-acetyl aspartate (NAA) and increased glutamate and glutamine (Glx) and reduced gray matter fraction in the thalamus of idiopathic generalized epilepsy (IGE) patients when compared with controls. (A) VBM showing decreased thalamus gray matter fraction in patients compared to controls. The white areas in the center of each image indicate z scores of ≥4. (B) Absolute concentrations of thalamic NAA and Glx in IGE (shaded) and controls (blank). The boxes indicate inner quartiles, the vertical lines the outer quartiles. Outliers are shown as circles.
This figure is reproduced from Helms et al. [44] with permission.
The ability to track these changes with VBM during epileptogenesis may make it a clinically useful biomarker to monitor the course of the disease. However, it has been pointed out that changes in VBM processing methods such as type and level of statistical correction, modulation, smoothing kernal, adjustment for brain size, subgroup analysis and software version can mimic biological differences and lead to misinterpretation [15,45]. For instance, a recent multi-site VBM study found site-specific differences between controls [85]. If VBM is to be used clinically as a biomarker, consistency in these user-specific parameters needs to be reinforced.
3.2. MRI morphometric analysis: surface-based morphometry
Other variants of morphometric analysis in neuroimaging include deformation-based morphometry and surface-based morphometry. Using surface-based morphometry, Tosun et al. showed that CAE children, when compared to normal subjects, did not undergo such normal age-related changes as a decrease in cortical thickness and an increase in the depth of sulci [110]. Lower IQ scores (although still in the average range) in CAE children when compared to controls were associated with less cortical thinning in the left middle frontal, left medial superior frontal gyrus, and right inferior temporal cortices, and smaller sulcal depth in the left superior temporal, middle frontal, superior frontal and parieto-occipital regions [110]. These morphometric differences between CAE and normal children demonstrate the diverse nature of neurodevelopmental abnormalities in CAE and their possible association with cognitive function.
3.3. Proton magnetic resonance spectroscopy
PMRS has evolved from a technique in chemistry (i.e., NMR spectroscopy) to determine the structure of molecules to one that measures the level of different metabolites in the brain in vivo [94]. Using quantitative single volume PMRS in IGE patients, two groups found increased absolute concentration of glutamate and glutamine (Glx) and decreased NAA in the thalamus [9,44] (Fig. 5B). The concentration of these metabolites can be predictive of progressive thalamic dysfunction in these patients. In photosensitive IGE, PMRS showed decreased NAA in the right frontal lobe and left thalamus, decreased NAA/creatine (Cr) ratio in the left thalamus, increased choline (Cho)/Cr ratio in the right frontal lobe and increased NAA/Cr ratio in the left occipital lobe [3]. Using MRS, Savic et al. found decreased thalamic NAA, Cho and myo-inositol in both JME and generalized tonic clonic epilepsy (GTCS) patients, but decreased frontal lobe NAA only in JME patients. This showed the involvement of the thalamus in GTCS and differentiated alterations within the thalamocortical loop between the two types of seizure [98,100]. All of these studies indicate that there could potentially be a role for MRS to evaluate the type, severity and development of epilepsy in individual patients.
3.4. Positron emission tomography
As discussed earlier, PET was applied to epilepsy research several years after it was first developed in the 1970s [59]. One advantage of PET is the large number of radioactive tracers that can be used to study various metabolic processes in the brain. Using FDG-PET, Hikima et al. demonstrated that there was significant interictal glucose hypometabolism in bilateral basal ganglia only during generalized epilepsy but not during localization-related epilepsy [46]. In children with continuous spike-and-wave activity during slow-wave sleep, Luat et al. found a concordance between lateralized glucose hypermetabolism and the presumed origin of generalized interictal spike activity delineated by quantitative EEG analysis in five of the six patients studied [68].
PET and a dopamine (DA) transporter ligand [(11)C]PE2l have been used to investigate the regional binding potential (BP) to dopamine transporter in JME and GTCS patients. It was found that JME patients had lower tracer binding in the midbrain and substantial nigra, whereas GTCS patients had reduced tracer binding in the putamen [23,24]. In addition, these changes in BP were correlated with performance in neuropsychological tests [23]. Furthermore, the serotonin system was also altered in JME patients, with a reduced BP in the dorsolateral prefrontal cortex, raphe nuclei, and hippocampus [72]. The ability of PET to detect glucose hypometabolism in IGE patients and to differentiate among different IGE subsyndromes based on changes in receptor binding could make it useful as a biomarker to study these diseases and their epileptogenic processes.
3.5. Functional magnetic resonance imaging
Using BOLD fMRI to study spike-wave discharges of WAG/Rij rats, a genetic absence model of epilepsy, it has been shown that increases were most prominent in focal regions of somatosensory cortex, motor cortex and thalamus, while the occipital region was spared [70,74,79]. These regions were shown to have both increased neuronal firing and increased CBF during SWD [78]. During bicuculline-induced generalized tonic-clonic seizures, the BOLD fMRI increases were still largest in somatosensory cortex, while decreases were seen in the hippocampus [29,79,101].
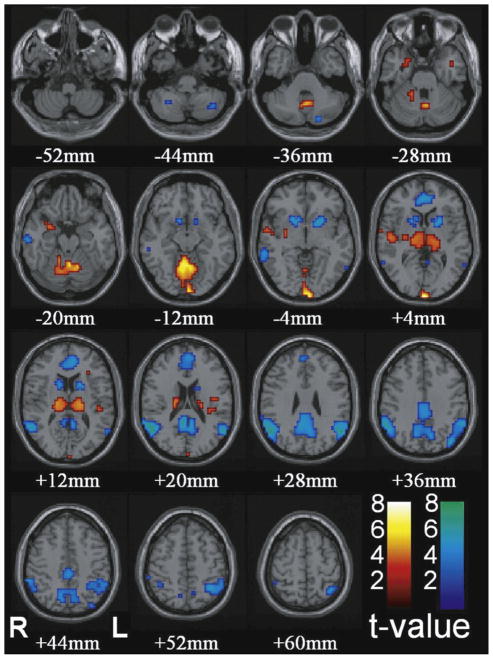
In human CAE patients, fMRI increases were observed in the bilateral thalamus and occipital cortex, while decreased fMRI signals were observed in the bilateral lateral parietal cortex, precuneus, cingulate gyrus, and basal ganglia [4,8,37,41,60] (Fig. 6). The fMRI time course of human absence seizure is characterized by small early fMRI increases in the orbital/medial frontal and medial/lateral parietal cortex >5 s before seizure onset, followed by profound fMRI decreases continuing >20 s after seizure offset [5]. This dynamic sequence of fMRI changes offered a view of the temporal complexity of seizure-related fMRI changes, which was not detected by hemodynamic response function modeling. Furthermore, identification of a seizure network via these fMRI changes provides potential targets for therapy, and for monitoring of therapeutic efficacy.
Fig. 6.
Increases in thalamus and decreases cortical regions are the most prominent changes with conventional HRF modeling in CAE patients. Functional data are superimposed on the MNI brain template “colin27” (sing subj T1 in SPM2) displaced in radiological right–left convention. In total, 54 seizures in nine patients were analyzed using GLM with canonical HRF in SPM2. fMRI increases were seen in bilateral thalamus, occipital cortex, and to a lesser extent the midline cerebellum, anterior and lateral temporal lobes, insula, and adjacent to the lateral ventricles. fMRI decreases were seen in the bilateral parietal, medial parietal, and cingulate cortex and basal ganglia.
This figure is reproduced from Bai et al. [5] with permission.
3.6. fMRI with resting functional connectivity
fMRI-based resting functional connectivity is a noninvasive method for assessing connectivity [12,113], which can readily be translated from animal studies to use in human epilepsy patients. Since generalized epilepsy such as absence seizure involves the thalamocortical network, neuroimaging methods geared toward long-range interactions, such as DTI and resting functional connectivity, may be robust methods for measuring long-term changes, even when seizures are not occurring or are blocked by medication. In WAG/Raj rats, the regions most intensely involved by seizures (i.e., bilateral cortical regions including the somatosensory cortex) showed markedly increased resting functional connectivity when compared with controls [73]. Further studies are needed to determine whether preventing epileptogenesis will also prevent abnormal functional connectivity in epileptic networks in this rat model [13]. In a study of human CAE patients, there was a significantly increased interhemispheric correlation between orbitofrontal cortex regions [4]. These abnormalities were observed in both animal and human subjects, even when seizures were not occurring, and have the potential to serve as an interictal biomarker of disease severity in childhood absence epilepsy and other generalized epilepsies.
3.7. Diffusion tensor imaging
Water diffusion is highly anisotropic (i.e., have a directional dependence) in nerves and white matter structures [7]. Water molecules preferentially diffuse along the length of the densely packed axons. They are hindered in the perpendicular direction by the axonal membrane and myelin sheath, which modulate the degree of anisotropy. DTI measures diffusion properties of water protons in tissue and can detect subtle white matter changes in different pathological states including epilepsy [14,81,106]. In children and adults with malformations of cortical development and different forms of epilepsy, there is often reduced white matter FA [32,96,108] and DTI may detect abnormalities in these patients even when conventional MRI is normal. Several studies using DTI in human patients with idiopathic generalized epilepsy have now been published. Investigating the white matter abnormalities underlying IGE, Li et al. found significantly decreased FA values in the cerebellum of 14 young male patients with GTCS only [65]. A decreased FA was found in the anterior thalamic radiations (i.e., fibers connecting the anterior thalamus with prefrontal cortical areas) of patients with JME when compared to both healthy controls and patients with cryptogenic partial epilepsy [28]. The FA reductions in these patients correlated with the frequency of generalized tonic-clonic seizures, but not with the duration of antiepileptic medication [28,112].
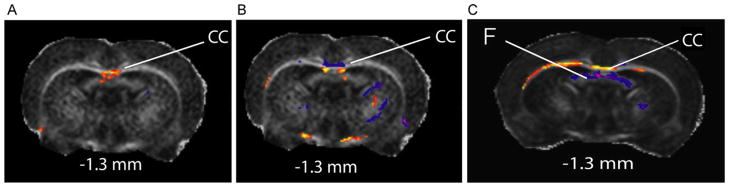
In WAG/Rij rats, there was reduced FA and increased perpendicular diffusivity in the anterior corpus callosum, which was not observed in young rats before seizure onset or nonepileptic controls [18] (Fig. 7). Genetic absence epilepsy rats from Strasbourg (GAERS) has more pronounced changes [18]. The white matter abnormalities can be due to reduction in myelin [39,42] and/or decreased axon fiber density [27,48] or due to changes in the density and orientation of crossing fibers in pathways connecting regions of seizure activity. The animal models will enable histological studies to determine the mechanisms for these observed DTI changes. DTI can also be a potential noninvasive biomarker for monitoring treatment by visualizing structural recovery in affected brain regions.
Fig. 7.
Epileptic adult WAG/Rij (8 months) and GAERS (1.7 months) rats, but not young WAG/Rij rats (1.7 months) have decreased fractional anisotropy in anterior corpus callosum compared to controls. t-Maps at −1.3 mm are shown, with warm colors representing decreased FA when compared with nonepileptic controls, and cool colors the opposite. (A) In adult epileptic WAG/Rij rats, decreased FA was observed in the anterior corpus callosum (CC). (B) In young WAG/Rij rats, anterior corpus callosum does not show decreased FA when compared with controls. (C) In adult epileptic GAERS, extensive decreased FA was observed in the anterior corpus callosum. Unlike the WAG/Rij rats, the fornix showed increased FA in GAERS when compared with controls, which may represent a strain difference not directly related to seizures. t value threshold = 2.00, extent threshold = 50 voxels (voxel dimension 0.234 mm × 0.234 mm × 0.5 mm), and FA threshold = 0.30.
This figure is reproduced from Chahboune et al. [18] with permission.
4. Conclusions
MRI with morphometric analysis, MR spectroscopy and PET are established imaging methods to study chronic changes in partial and generalized epilepsy. Newer methods such as DTI and fMRI with resting functional connectivity emphasize the network interaction in the thalamocortical circuitry and are currently being tested in both animal models and human subjects. These noninvasive biomarkers allow for the investigation of treatment effects on epileptogenesis and present the possibility of early intervention and predictive potential in the future management of epilepsy patients.
5. Summary of implications and future directions
It was recently shown that early treatment can beneficially alter the phenotype of genetic epilepsy in rodents [13,97]. These animal models appear to demonstrate a critical period in epileptogenesis, during which spike-and-wave seizures can be suppressed, leading to long-term changes in the brain. It is important to know if such a “critical period” exists in human epilepsy patients and if so, whether intervening before disease onset could lead to suppression of symptoms later in life. Once our genetic knowledge grows, primary prevention by therapy will soon be a realistic goal. Prevention of epilepsy in individuals susceptible to epilepsy would have a major impact on patient quality of life. The neuroimaging biomarkers of epileptogenesis reviewed in this paper may be useful to monitor disease progression and treatment effectiveness, allowing important proof-of-principle animal and human studies to be carried out. Studies in animals and humans with these new neuroimaging biomarkers of epileptogenesis will pave the way for future treatment trials in human patients.
Acknowledgments
This work was supported by NIH R01 NS049307(HB), P30 NS052519(HB), the Betsy and Jonathan Blattmachr family (HB), and by Epilepsy Foundation Award ID 123505 (AMM). We also thank Samantha Balakirsky for helpful comments on the manuscript.
References
- 1.Arnold DL, Matthews PM. Practical aspects of clinical application of MRS in the brain. In: Young IR, editor. MR Spectroscopy: Clinical Applications and Techniques. Martin Dunitz; London: 1996. pp. 139–159. [Google Scholar]
- 2.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 3.Aydin-Ozemir Z, Terzibasioglu E, Altindag E, Sencer S, Baykan B. Magnetic resonance spectroscopy findings in photosensitive idiopathic generalized epilepsy. Clin EEG Neurosci. 2010;41:42–49. doi: 10.1177/155005941004100109. [DOI] [PubMed] [Google Scholar]
- 4.Bai X, Guo J, Killory B, Vestal M, Berman R, Negishi M, Danielson N, Novotny EJ, Constable RT, Blumenfeld H. Resting functional connectivity between the hemispheres in childhood absence epilepsy. Neurology. doi: 10.1212/WNL.0b013e31821e54de. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai X, Vestal M, Berman R, Negishi M, Spann M, Vega C, Desalvo M, Novotny EJ, Constable RT, Blumenfeld H. Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. J Neurosci. 2010;30:5884–5893. doi: 10.1523/JNEUROSCI.5101-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 7.Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 8.Berman R, Negishi M, Vestal M, Spann M, Chung MH, Bai X, Purcaro M, Motelow JE, Danielson N, Dix-Cooper L, Enev M, Novotny EJ, Constable RT, Blumenfeld H. Simultaneous EEG, fMRI, and behavior in typical childhood absence seizures. Epilepsia. 2010;51:2011–2022. doi: 10.1111/j.1528-1167.2010.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernasconi A, Bernasconi N, Natsume J, Antel SB, Andermann F, Arnold DL. Magnetic resonance spectroscopy and imaging of the thalamus in idiopathic generalized epilepsy. Brain. 2003;126:2447–2454. doi: 10.1093/brain/awg249. [DOI] [PubMed] [Google Scholar]
- 10.Betting LE, Mory SB, Li LM, Lopes-Cendes I, Guerreiro MM, Guerreiro CA, Cendes F. Voxel-based morphometry in patients with idiopathic generalized epilepsies. Neuroimage. 2006;32:498–502. doi: 10.1016/j.neuroimage.2006.04.174. [DOI] [PubMed] [Google Scholar]
- 11.Bettus G, Guedj E, Joyeux F, Confort-Gouny S, Soulier E, Laguitton V, Cozzone PJ, Chauvel P, Ranjeva JP, Bartolomei F, Guye M. Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp. 2009;30:1580–1591. doi: 10.1002/hbm.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 13.Blumenfeld H, Klein JP, Schridde U, Vestal M, Rice T, Khera DS, Bashyal C, Giblin K, Paul-Laughinghouse C, Wang F, Phadke A, Mission J, Agarwal RK, Englot DJ, Motelow J, Nersesyan H, Waxman SG, Levin AR. Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia. 2008;49:400–409. doi: 10.1111/j.1528-1167.2007.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boska MD, Hasan KM, Kibuule D, Banerjee R, McIntyre E, Nelson JA, Hahn T, Gendelman HE, Mosley RL. Quantitative diffusion tensor imaging detects dopaminergic neuronal degeneration in a murine model of Parkinson’s disease. Neurobiol Dis. 2007;26:590–596. doi: 10.1016/j.nbd.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruggemann JM, Wilke M, Som SS, Bye AM, Bleasel A, Lawson JA. Voxel-based morphometry in the detection of dysplasia and neoplasia in childhood epilepsy: limitations of grey matter analysis. J Clin Neurosci. 2009;16:780–785. doi: 10.1016/j.jocn.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Caplan R, Levitt J, Siddarth P, Wu KN, Gurbani S, Sankar R, Shields WD. Frontal and temporal volumes in Childhood Absence Epilepsy. Epilepsia. 2009;50:2466–2472. doi: 10.1111/j.1528-1167.2009.02198.x. [DOI] [PubMed] [Google Scholar]
- 17.Cendes F, Caramanos Z, Andermann F, Dubeau F, Arnold DL. Proton magnetic resonance spectroscopic imaging and magnetic resonance imaging volumetry in the lateralization of temporal lobe epilepsy: a series of 100 patients. Ann Neurol. 1997;42:737–746. doi: 10.1002/ana.410420510. [DOI] [PubMed] [Google Scholar]
- 18.Chahboune H, Mishra AM, DeSalvo MN, Staib LH, Purcaro M, Scheinost D, Papademetris X, Fyson SJ, Lorincz ML, Crunelli V, Hyder F, Blumenfeld H. DTI abnormalities in anterior corpus callosum of rats with spike-wave epilepsy. Neuroimage. 2009;47:459–466. doi: 10.1016/j.neuroimage.2009.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan CH, Briellmann RS, Pell GS, Scheffer IE, Abbott DF, Jackson GD. Thalamic atrophy in childhood absence epilepsy. Epilepsia. 2006;47:399–405. doi: 10.1111/j.1528-1167.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 20.Chernov MF, Ochiai T, Ono Y, Muragaki Y, Yamane F, Taira T, Maruyama T, Tanaka M, Iseki H, Kubo O, Okada Y, Hori T, Takakura K. Role of proton magnetic resonance spectroscopy in preoperative evaluation of patients with mesial temporal lobe epilepsy. J Neurol Sci. 2009;285:212–219. doi: 10.1016/j.jns.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Choy M, Cheung KK, Thomas DL, Gadian DG, Lythgoe MF, Scott RC. Quantitative MRI predicts status epilepticus-induced hippocampal injury in the lithium-pilocarpine rat model. Epilepsy Res. 2010;88:221–230. doi: 10.1016/j.eplepsyres.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Chugani HT, Juhasz C, Chugani DC, Lawrenson L, Muzik O, Chakraborty PK, Sood S. Increased striatal serotonin synthesis following cortical resection in children with intractable epilepsy. Epilepsy Res. 2008;78:124–130. doi: 10.1016/j.eplepsyres.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciumas C, Wahlin TB, Espino C, Savic I. The dopamine system in idiopathic generalized epilepsies: identification of syndrome-related changes. Neuroimage. 2010;51:606–615. doi: 10.1016/j.neuroimage.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 24.Ciumas C, Wahlin TB, Jucaite A, Lindstrom P, Halldin C, Savic I. Reduced dopamine transporter binding in patients with juvenile myoclonic epilepsy. Neurology. 2008;71:788–794. doi: 10.1212/01.wnl.0000316120.70504.d5. [DOI] [PubMed] [Google Scholar]
- 25.Concha L, Beaulieu C, Collins DL, Gross DW. White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:312–319. doi: 10.1136/jnnp.2007.139287. [DOI] [PubMed] [Google Scholar]
- 26.Concha L, Livy DJ, Beaulieu C, Wheatley BM, Gross DW. In vivo diffusion tensor imaging and histopathology of the fimbria-fornix in temporal lobe epilepsy. J Neurosci. 2010;30:996–1002. doi: 10.1523/JNEUROSCI.1619-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Concha L, Livy DJ, Gross DW, Wheatley BM, Beaulieu C. Direct correlation between diffusion tensor imaging and electron microscopy of the fornix in humans with temporal lobe epilepsy. Proc. Intl. Soc. Mag. Reson. Med; Toronto, Canada. 2008. p. 107. [Google Scholar]
- 28.Deppe M, Kellinghaus C, Duning T, Moddel G, Mohammadi S, Deppe K, Schiffbauer H, Kugel H, Keller SS, Ringelstein EB, Knecht S. Nerve fiber impairment of anterior thalamocortical circuitry in juvenile myoclonic epilepsy. Neurology. 2008;71:1981–1985. doi: 10.1212/01.wnl.0000336969.98241.17. [DOI] [PubMed] [Google Scholar]
- 29.DeSalvo MN, Schridde U, Mishra AM, Motelow JE, Purcaro MJ, Danielson N, Bai X, Hyder F, Blumenfeld H. Focal BOLD fMRI changes in bicuculline-induced tonic-clonic seizures in the rat. Neuroimage. 2010;50:902–909. doi: 10.1016/j.neuroimage.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dube C, Yu H, Nalcioglu O, Baram TZ. Serial MRI after experimental febrile seizures: altered T2 signal without neuronal death. Ann Neurol. 2004;56:709–714. doi: 10.1002/ana.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dube CM, Brewster AL, Richichi C, Zha Q, Baram TZ. Fever, febrile seizures and epilepsy. Trends Neurosci. 2007;30:490–496. doi: 10.1016/j.tins.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eriksson SH, Rugg-Gunn FJ, Symms MR, Barker GJ, Duncan JS. Diffusion tensor imaging in patients with epilepsy and malformations of cortical development. Brain. 2001;124:617–626. doi: 10.1093/brain/124.3.617. [DOI] [PubMed] [Google Scholar]
- 33.Filler A. The History, Development and Impact of Computed Imaging in Neurological Diagnosis and Neurosurgery: CT, MRI, and DTI. Natureprecedings. 2009 http://precedings.nature.com/documents/3267/version/5.
- 34.Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- 35.Giovacchini G, Toczek MT, Bonwetsch R, Bagic A, Lang L, Fraser C, Reeves-Tyer P, Herscovitch P, Eckelman WC, Carson RE, Theodore HW. 5-HT 1A receptors are reduced in temporal lobe epilepsy after partial-volume correction. J Nucl Med. 2005;46:1128–1135. [PMC free article] [PubMed] [Google Scholar]
- 36.Goncalves Pereira PM, Oliveira E, Rosado P. Relative localizing value of amygdalo-hippocampal MR biometry in temporal lobe epilepsy. Epilepsy Res. 2006;69:147–164. doi: 10.1016/j.eplepsyres.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci USA. 2005;102:15236–15240. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gross DW, Concha L, Beaulieu C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia. 2006;47:1360–1363. doi: 10.1111/j.1528-1167.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 39.Gulani V, Webb AG, Duncan ID, Lauterbur PC. Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magn Reson Med. 2001;45:191–195. doi: 10.1002/1522-2594(200102)45:2<191::aid-mrm1025>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Gupta RK, Cloughesy TF, Sinha U, Garakian J, Lazareff J, Rubino G, Rubino L, Becker DP, Vinters HV, Alger JR. Relationships between choline magnetic resonance spectroscopy, apparent diffusion coefficient and quantitative histopathology in human glioma. J Neurooncol. 2000;50:215–226. doi: 10.1023/a:1006431120031. [DOI] [PubMed] [Google Scholar]
- 41.Hamandi K, Laufs H, Noth U, Carmichael DW, Duncan JS, Lemieux L. BOLD and perfusion changes during epileptic generalised spike wave activity. Neuroimage. 2008;39:608–618. doi: 10.1016/j.neuroimage.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Harsan LA, Poulet P, Guignard B, Steibel J, Parizel N, de Sousa PL, Boehm N, Grucker D, Ghandour MS. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res. 2006;83:392–402. doi: 10.1002/jnr.20742. [DOI] [PubMed] [Google Scholar]
- 43.Hauser WA, Annegers JF, Kurland LT. Prevalence of epilepsy in Rochester, Minnesota: 1940–1980. Epilepsia. 1991;32:429–445. doi: 10.1111/j.1528-1157.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- 44.Helms G, Ciumas C, Kyaga S, Savic I. Increased thalamus levels of glutamate and glutamine (Glx) in patients with idiopathic generalised epilepsy. J Neurol Neurosurg Psychiatry. 2006;77:489–494. doi: 10.1136/jnnp.2005.074682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henley SM, Ridgway GR, Scahill RI, Kloppel S, Tabrizi SJ, Fox NC, Kassubek J. Pitfalls in the use of voxel-based morphometry as a biomarker: examples from huntington disease. Am J Neuroradiol. 2010;31:711–719. doi: 10.3174/ajnr.A1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hikima A, Mochizuki H, Oriuchi N, Endo K, Morikawa A. Semiquantitative analysis of interictal glucose metabolism between generalized epilepsy and localization related epilepsy. Ann Nucl Med. 2004;18:579–584. doi: 10.1007/BF02984579. [DOI] [PubMed] [Google Scholar]
- 47.Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68:326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 48.Hui ES, Fu QL, So KF, Wu EX. Diffusion tensor MR study of optic nerve degeneration in glaucoma. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:4312–4315. doi: 10.1109/IEMBS.2007.4353290. [DOI] [PubMed] [Google Scholar]
- 49.Immonen RJ, Kharatishvili I, Grohn H, Pitkanen A, Grohn OH. Quantitative MRI predicts long-term structural and functional outcome after experimental traumatic brain injury. Neuroimage. 2009;45:1–9. doi: 10.1016/j.neuroimage.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 50.Jacobs J, Levan P, Moeller F, Boor R, Stephani U, Gotman J, Siniatchkin M. Hemodynamic changes preceding the interictal EEG spike in patients with focal epilepsy investigated using simultaneous EEG-fMRI. Neuroimage. 2009;45:1220–1231. doi: 10.1016/j.neuroimage.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 51.Jacobs J, Rohr A, Moeller F, Boor R, Kobayashi E, LeVan Meng P, Stephani U, Gotman J, Siniatchkin M. Evaluation of epileptogenic networks in children with tuberous sclerosis complex using EEG-fMRI. Epilepsia. 2008;49:816–825. doi: 10.1111/j.1528-1167.2007.01486.x. [DOI] [PubMed] [Google Scholar]
- 52.Kale RA, Gupta RK, Saraswat VA, Hasan KM, Trivedi R, Mishra AM, Ranjan P, Pandey CM, Narayana PA. Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology. 2006;43:698–706. doi: 10.1002/hep.21114. [DOI] [PubMed] [Google Scholar]
- 53.Kharatishvili I, Immonen R, Grohn O, Pitkanen A. Quantitative diffusion MRI of hippocampus as a surrogate marker for post-traumatic epileptogenesis. Brain. 2007;130:3155–3168. doi: 10.1093/brain/awm268. [DOI] [PubMed] [Google Scholar]
- 54.Kharatishvili I, Sierra A, Immonen RJ, Grohn OH, Pitkanen A. Quantitative T2 mapping as a potential marker for the initial assessment of the severity of damage after traumatic brain injury in rat. Exp Neurol. 2009;217:154–164. doi: 10.1016/j.expneurol.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 55.Kim CH, Koo BB, Chung CK, Lee JM, Kim JS, Lee SK. Thalamic changes in temporal lobe epilepsy with and without hippocampal sclerosis: a diffusion tensor imaging study. Epilepsy Res. 2010;90:21–27. doi: 10.1016/j.eplepsyres.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Kim JH, Lee JK, Koh SB, Lee SA, Lee JM, Kim SI, Kang JK. Regional grey matter abnormalities in juvenile myoclonic epilepsy: a voxel-based morphometry study. Neuroimage. 2007;37:1132–1137. doi: 10.1016/j.neuroimage.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 57.Kimiwada T, Juhasz C, Makki M, Muzik O, Chugani DC, Asano E, Chugani HT. Hippocampal and thalamic diffusion abnormalities in children with temporal lobe epilepsy. Epilepsia. 2006;47:167–175. doi: 10.1111/j.1528-1167.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 58.Klein JP, Khera DS, Nersesyan H, Kimchi EY, Waxman SG, Blumenfeld H. Dysregulation of sodium channel expression in cortical neurons in a rodent model of absence epilepsy. Brain Res. 2004;1000:102–109. doi: 10.1016/j.brainres.2003.11.051. [DOI] [PubMed] [Google Scholar]
- 59.Kuhl DE, Engel J, Jr, Phelps ME, Kowell AP. Epileptic patterns of local cerebral metabolism and perfusion in man: investigation by emission computed tomography of 18F-fluorodeoxyglucose and 13N-ammonia. Trans Am Neurol Assoc. 1978;103:52–53. [PubMed] [Google Scholar]
- 60.Labate A, Briellmann RS, Abbott DF, Waites AB, Jackson GD. Typical childhood absence seizures are associated with thalamic activation. Epileptic Disord. 2005;7:373–377. [PubMed] [Google Scholar]
- 61.Labate A, Cerasa A, Aguglia U, Mumoli L, Quattrone A, Gambardella A. Voxel-based morphometry of sporadic epileptic patients with mesiotemporal sclerosis. Epilepsia. 2010;51:506–510. doi: 10.1111/j.1528-1167.2009.02310.x. [DOI] [PubMed] [Google Scholar]
- 62.Labate A, Cerasa A, Gambardella A, Aguglia U, Quattrone A. Hippocampal and thalamic atrophy in mild temporal lobe epilepsy: a VBM study. Neurology. 2008;71:1094–1101. doi: 10.1212/01.wnl.0000326898.05099.04. [DOI] [PubMed] [Google Scholar]
- 63.Lewis DV, Barboriak DP, MacFall JR, Provenzale JM, Mitchell TV, VanLandingham KE. Do prolonged febrile seizures produce medial temporal sclerosis? Hypotheses, MRI evidence and unanswered questions. Prog Brain Res. 2002;135:263–278. doi: 10.1016/s0079-6123(02)35025-8. [DOI] [PubMed] [Google Scholar]
- 64.Li LM, Cendes F, Antel SB, Andermann F, Serles W, Dubeau F, Olivier A, Arnold DL. Prognostic value of proton magnetic resonance spectroscopic imaging for surgical outcome in patients with intractable temporal lobe epilepsy and bilateral hippocampal atrophy. Ann Neurol. 2000;47:195–200. [PubMed] [Google Scholar]
- 65.Li Y, Du H, Xie B, Wu N, Wang J, Wu G, Feng H, Jiang T. Cerebellum abnormalities in idiopathic generalized epilepsy with generalized tonic-clonic seizures revealed by diffusion tensor imaging. PLoS One. 2010;5:e15219. doi: 10.1371/journal.pone.0015219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liew CJ, Lim YM, Bonwetsch R, Shamim S, Sato S, Reeves-Tyer P, Herscovitch P, Dustin I, Bagic A, Giovacchini G, Theodore WH. 18F-FCWAY and 18F-FDG PET in MRI-negative temporal lobe epilepsy. Epilepsia. 2009;50:234–239. doi: 10.1111/j.1528-1167.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin K, Jackowski AP, Carrete H, Jr, de Araujo Filho GM, Silva HH, Guaranha MS, Guilhoto LM, Bressan RA, Yacubian EM. Voxel-based morphometry evaluation of patients with photosensitive juvenile myoclonic epilepsy. Epilepsy Res. 2009;86:138–145. doi: 10.1016/j.eplepsyres.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 68.Luat AF, Asano E, Juhasz C, Chandana SR, Shah A, Sood S, Chugani HT. Relationship between brain glucose metabolism positron emission tomography (PET) and electroencephalography (EEG) in children with continuous spike-and-wave activity during slow-wave sleep. J Child Neurol. 2005;20:682–690. doi: 10.1177/08830738050200081001. [DOI] [PubMed] [Google Scholar]
- 69.Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meeren HK, Pijn JP, Van Luijtelaar EL, Coenen AM, Lopes da Silva FH. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22:1480–1495. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Merlet I, Ostrowsky K, Costes N, Ryvlin P, Isnard J, Faillenot I, Lavenne F, Dufournel D, Le Bars D, Mauguiere F. 5-HT1A receptor binding and intracerebral activity in temporal lobe epilepsy: an [18F]MPPF-PET study. Brain. 2004;127:900–913. doi: 10.1093/brain/awh109. [DOI] [PubMed] [Google Scholar]
- 72.Meschaks A, Lindstrom P, Halldin C, Farde L, Savic I. Regional reductions in serotonin 1A receptor binding in juvenile myoclonic epilepsy. Arch Neurol. 2005;62:946–950. doi: 10.1001/archneur.62.6.946. [DOI] [PubMed] [Google Scholar]
- 73.Mishra AM, Bai X, Purcaro MJ, Motelow JE, DeSalvo MN, Hyder F, Blumenfeld H. Interictal resting functional connectivity in Wag/Rij rats: a possible biomarker of epilepsy. Epilepsia. 2009;50:362. [Google Scholar]
- 74.Mishra AM, Ellens DJ, Schridde U, Motelow JM, Purcaro M, Hyder F, Blumenfeld H. Spatio-temporal Dynamics of the BOLD fMRI Signal Changes and Physiology During Spike-wave Seizures in WAG/Rij Rats. Society for Neuroscience; Washington, DC: 2008. [Google Scholar]
- 75.Mishra AM, Gupta RK, Jaggi RS, Reddy JS, Jha DK, Husain N, Prasad KN, Behari S, Husain M. Role of diffusion-weighted imaging and in vivo proton magnetic resonance spectroscopy in the differential diagnosis of ringenhancing intracranial cystic mass lesions. J Comput Assist Tomogr. 2004;28:540–547. doi: 10.1097/00004728-200407000-00017. [DOI] [PubMed] [Google Scholar]
- 76.Morgan VL, Gore JC, Abou-Khalil B. Functional epileptic network in left mesial temporal lobe epilepsy detected using resting fMRI. Epilepsy Res. 2010;88:168–178. doi: 10.1016/j.eplepsyres.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Natsume J, Bernasconi N, Andermann F, Bernasconi A. MRI volumetry of the thalamus in temporal, extratemporal, and idiopathic generalized epilepsy. Neurology. 2003;60:1296–1300. doi: 10.1212/01.wnl.0000058764.34968.c2. [DOI] [PubMed] [Google Scholar]
- 78.Nersesyan H, Herman P, Erdogan E, Hyder F, Blumenfeld H. Relative changes in cerebral blood flow and neuronal activity in local microdomains during generalized seizures. J Cereb Blood Flow Metab. 2004;24:1057–1068. doi: 10.1097/01.WCB.0000131669.02027.3E. [DOI] [PubMed] [Google Scholar]
- 79.Nersesyan H, Hyder F, Rothman DL, Blumenfeld H. Dynamic fMRI and EEG recordings during spike-wave seizures and generalized tonic-clonic seizures in WAG/Rij rats. J Cereb Blood Flow Metab. 2004;24:589–599. doi: 10.1097/01.WCB.0000117688.98763.23. [DOI] [PubMed] [Google Scholar]
- 80.Nishida M, Asano E, Juhasz C, Muzik O, Sood S, Chugani HT. Cortical glucose metabolism correlates negatively with delta-slowing and spike-frequency in epilepsy associated with tuberous sclerosis. Hum Brain Mapp. 2008;29:1255–1264. doi: 10.1002/hbm.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Obenaus A, Jacobs RE. Magnetic resonance imaging of functional anatomy: use for small animal epilepsy models. Epilepsia. 2007;48(Suppl 4):11–17. doi: 10.1111/j.1528-1167.2007.01237.x. [DOI] [PubMed] [Google Scholar]
- 82.Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993;64:803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pagni CA, Zenga F. Prevention and treatment of post-traumatic epilepsy. Expert Rev Neurother. 2006;6:1223–1233. doi: 10.1586/14737175.6.8.1223. [DOI] [PubMed] [Google Scholar]
- 84.Palchaudhuri M, Flugge G. 5-HT1A receptor expression in pyramidal neurons of cortical and limbic brain regions. Cell Tissue Res. 2005;321:159–172. doi: 10.1007/s00441-005-1112-x. [DOI] [PubMed] [Google Scholar]
- 85.Pardoe H, Pell GS, Abbott DF, Berg AT, Jackson GD. Multi-site voxel-based morphometry: methods and a feasibility demonstration with childhood absence epilepsy. Neuroimage. 2008;42:611–616. doi: 10.1016/j.neuroimage.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pauling L, Coryell CD. The magnetic properties and structure of the hemochromogens and related substances. Proc Natl Acad Sci USA. 1936;22:159–163. doi: 10.1073/pnas.22.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pereira FR, Alessio A, Sercheli MS, Pedro T, Bilevicius E, Rondina JM, Ozelo HF, Castellano G, Covolan RJ, Damasceno BP, Cendes F. Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC Neurosci. 2010;11:66. doi: 10.1186/1471-2202-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Phelps ME, Hoffman EJ, Mullani NA, Ter-Pogossian MM. Application of annihilation coincidence detection to transaxial reconstruction tomography. J Nucl Med. 1975;16:210–224. [PubMed] [Google Scholar]
- 89.Pitkanen A, McIntosh TK. Animal models of post-traumatic epilepsy. J Neurotrauma. 2006;23:241–261. doi: 10.1089/neu.2006.23.241. [DOI] [PubMed] [Google Scholar]
- 90.Provenzale JM, Barboriak DP, VanLandingham K, MacFall J, Delong D, Lewis DV. Hippocampal MRI signal hyperintensity after febrile status epilepticus is predictive of subsequent mesial temporal sclerosis. Am J Roentgenol. 2008;190:976–983. doi: 10.2214/AJR.07.2407. [DOI] [PubMed] [Google Scholar]
- 91.Ranjan P, Mishra AM, Kale R, Saraswat VA, Gupta RK. Cytotoxic edema is responsible for raised intracranial pressure in fulminant hepatic failure: in vivo demonstration using diffusion-weighted MRI in human subjects. Metab Brain Dis. 2005;20:181–192. doi: 10.1007/s11011-005-7206-z. [DOI] [PubMed] [Google Scholar]
- 92.Rathakrishnan R, Moeller F, Levan P, Dubeau F, Gotman J. BOLD signal changes preceding negative responses in EEG-fMRI in patients with focal epilepsy. Epilepsia. 2010;51:1837–1845. doi: 10.1111/j.1528-1167.2010.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roch C, Leroy C, Nehlig A, Namer IJ. Predictive value of cortical injury for the development of temporal lobe epilepsy in 21-day-old rats: an MRI approach using the lithium-pilocarpine model. Epilepsia. 2002;43:1129–1136. doi: 10.1046/j.1528-1157.2002.17802.x. [DOI] [PubMed] [Google Scholar]
- 94.Rosen Y, Lenkinski RE. Recent advances in magnetic resonance neurospectroscopy. Neurotherapeutics. 2007;4:330–345. doi: 10.1016/j.nurt.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. Anat Rec. 2001;265:54–84. doi: 10.1002/ar.1058. [DOI] [PubMed] [Google Scholar]
- 96.Rugg-Gunn FJ, Eriksson SH, Symms MR, Barker GJ, Duncan JS. Diffusion tensor imaging of cryptogenic and acquired partial epilepsies. Brain. 2001;124:627–636. doi: 10.1093/brain/124.3.627. [DOI] [PubMed] [Google Scholar]
- 97.Sarkisova KY, Kuznetsova GD, Kulikov MA, van Luijtelaar G. Spike-wave discharges are necessary for the expression of behavioral depression-like symptoms. Epilepsia. 2010;51:146–160. doi: 10.1111/j.1528-1167.2009.02260.x. [DOI] [PubMed] [Google Scholar]
- 98.Savic I, Lekvall A, Greitz D, Helms G. MR spectroscopy shows reduced frontal lobe concentrations of N-acetyl aspartate in patients with juvenile myoclonic epilepsy. Epilepsia. 2000;41:290–296. doi: 10.1111/j.1528-1157.2000.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 99.Savic I, Lindstrom P, Gulyas B, Halldin C, Andree B, Farde L. Limbic reductions of 5-HT1A receptor binding in human temporal lobe epilepsy. Neurology. 2004;62:1343–1351. doi: 10.1212/01.wnl.0000123696.98166.af. [DOI] [PubMed] [Google Scholar]
- 100.Savic I, Osterman Y, Helms G. MRS shows syndrome differentiated metabolite changes in human-generalized epilepsies. Neuroimage. 2004;21:163–172. doi: 10.1016/j.neuroimage.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 101.Schridde U, Khubchandani M, Motelow JE, Sanganahalli BG, Hyder F, Blumenfeld H. Negative BOLD with large increases in neuronal activity. Cereb Cortex. 2008;18:1814–1827. doi: 10.1093/cercor/bhm208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shinnar S, Berg AT, Treiman DM, Hauser WA, Hesdorffer DC, Sackellares JC, Leppik I, Sillanpaa M, Sommerville KW. Status epilepticus and tiagabine therapy: review of safety data and epidemiologic comparisons. Epilepsia. 2001;42:372–379. doi: 10.1046/j.1528-1157.2001.01600.x. [DOI] [PubMed] [Google Scholar]
- 103.Shorvon SD. A history of neuroimaging in epilepsy 1909–2009. Epilepsia. 2009;50(Suppl 3):39–49. doi: 10.1111/j.1528-1167.2009.02038.x. [DOI] [PubMed] [Google Scholar]
- 104.Simister RJ, McLean MA, Barker GJ, Duncan JS. Proton MR spectroscopy of metabolite concentrations in temporal lobe epilepsy and effect of temporal lobe resection. Epilepsy Res. 2009;83:168–176. doi: 10.1016/j.eplepsyres.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 105.Sokol DK, Demyer WE, Edwards-Brown M, Sanders S, Garg B. From swelling to sclerosis: acute change in mesial hippocampus after prolonged febrile seizure. Seizure. 2003;12:237–240. doi: 10.1016/s1059-1311(02)00195-4. [DOI] [PubMed] [Google Scholar]
- 106.Song SK, Kim JH, Lin SJ, Brendza RP, Holtzman DM. Diffusion tensor imaging detects age-dependent white matter changes in a transgenic mouse model with amyloid deposition. Neurobiol Dis. 2004;15:640–647. doi: 10.1016/j.nbd.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 107.Stefan H, Feichtinger M, Pauli E, Schafer I, Eberhardt KW, Kasper BS, Hopp P, Buchfelder M, Huk J, Paulus W. Magnetic resonance spectroscopy and histopathological findings in temporal lobe epilepsy. Epilepsia. 2001;42:41–46. doi: 10.1046/j.1528-1157.2001.080873.x. [DOI] [PubMed] [Google Scholar]
- 108.Thivard L, Lehericy S, Krainik A, Adam C, Dormont D, Chiras J, Baulac M, Dupont S. Diffusion tensor imaging in medial temporal lobe epilepsy with hippocampal sclerosis. Neuroimage. 2005;28:682–690. doi: 10.1016/j.neuroimage.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 109.Toczek MT, Carson RE, Lang L, Ma Y, Spanaki MV, Der MG, Fazilat S, Kopylev L, Herscovitch P, Eckelman WC, Theodore WH. PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology. 2003;60:749–756. doi: 10.1212/01.wnl.0000049930.93113.20. [DOI] [PubMed] [Google Scholar]
- 110.Tosun D, Siddarth P, Toga AW, Hermann B, Caplan R. Effects of childhood absence epilepsy on associations between regional cortical morphometry and aging and cognitive abilities. Hum Brain Mapp. 2010 doi: 10.1002/hbm.21045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.VanLandingham KE, Heinz ER, Cavazos JE, Lewis DV. Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann Neurol. 1998;43:413–426. doi: 10.1002/ana.410430403. [DOI] [PubMed] [Google Scholar]
- 112.Vulliemoz S, Vollmar C, Koepp MJ, Yogarajah M, O’Muircheartaigh J, Carmichael DW, Stretton J, Richardson MP, Symms MR, Duncan JS. Connectivity of the supplementary motor area in juvenile myoclonic epilepsy and frontal lobe epilepsy. Epilepsia. 2010 Nov 3; doi: 10.1111/j.1528-1167.2010.02770.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 113.Waites A, Briellmann R, Saling M, Abbott D, Jackson G. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol. 2006;59:335–343. doi: 10.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- 114.Woermann FG, Free SL, Koepp MJ, Ashburner J, Duncan JS. Voxel-by-voxel comparison of automatically segmented cerebral gray matter—a rater-independent comparison of structural MRI in patients with epilepsy. Neuroimage. 1999;10:373–384. doi: 10.1006/nimg.1999.0481. [DOI] [PubMed] [Google Scholar]
- 115.Woermann FG, Free SL, Koepp MJ, Sisodiya SM, Duncan JS. Abnormal cerebral structure in juvenile myoclonic epilepsy demonstrated with voxel-based analysis of MRI. Brain. 1999;122(Pt 11):2101–2108. doi: 10.1093/brain/122.11.2101. [DOI] [PubMed] [Google Scholar]
- 116.Yasuda CL, Betting LE, Cendes F. Voxel-based morphometry and epilepsy. Expert Rev Neurother. 2010;10:975–984. doi: 10.1586/ern.10.63. [DOI] [PubMed] [Google Scholar]
- 117.Yoon D, Frick KD, Carr DA, Austin JK. Economic impact of epilepsy in the United States. Epilepsia. 2009;50:2186–2191. doi: 10.1111/j.1528-1167.2009.02159.x. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Z, Lu G, Zhong Y, Tan Q, Liao W, Chen Z, Shi J, Liu Y. Impaired perceptual networks in temporal lobe epilepsy revealed by resting fMRI. J Neurol. 2009;256:1705–1713. doi: 10.1007/s00415-009-5187-2. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Z, Lu G, Zhong Y, Tan Q, Liao W, Wang Z, Wang Z, Li K, Chen H, Liu Y. Altered spontaneous neuronal activity of the default-mode network in mesial temporal lobe epilepsy. Brain Res. 2010;1323:152–160. doi: 10.1016/j.brainres.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 120.Zijlmans M, Huiskamp G, Hersevoort M, Seppenwoolde JH, van Huffelen AC, Leijten FS. EEG-fMRI in the preoperative work-up for epilepsy surgery. Brain. 2007;130:2343–2353. doi: 10.1093/brain/awm141. [DOI] [PubMed] [Google Scholar]